Abstract
In this article, we provide information on patient-reported side effects from a cross-section of real-world patients. Specifically, data on side effects were tabulated for patients taking one of the following attention deficit hyperactivity disorder medications: amphetamine and dextroamphetamine; atomoxetine; dexmethylphenidate; isdexamfetamine; and methylphenidate. Forty-eight percent of the approximately 325 patients surveyed reported having experienced a side effect as a result of taking an attention deficit hyperactivity disorder medication. Most common side effects mentioned included loss of appetite, sleep problems, and mood disturbances. Only 21 percent of side effects were considered very bothersome or extremely bothersome. Regardless of how bothersome the side effects were, only 20 percent of patients mentioned the side effects to their prescribing physicians.
Keywords: Attention deficit hyperactivity disorder, ADHD, side effect, patient-reported side effect, amphetamine, dextroamphetamine, atomoxetine, dexmethylphenidate, isdexamfetamine, methylphenidate
Introduction
Recognizing that time for patient care is limited, it is important for practicing physicians to understand which issues to prioritize in their patient interactions. In this article, we provide information on patient-reported side effects from a cross-section of real-world patients.
Methods
iGuard.org, a medication monitoring service, randomly surveys enrolled members on a continuous basis to obtain data on treatment satisfaction, efficacy, and side effects using a validated patient-reported outcomes instrument called the Treatment Satisfaction Questionnaire for Medications (TSQM). Data on side effects were tabulated for patients taking one of the following attention deficit hyperactivity disorder (ADHD) medications: amphetamine and dextroamphetamine; atomoxetine; dexmethylphenidate; isdexamfetamine; and methylphenidate.
Results
Forty-eight percent of the approximately 325 members surveyed experienced a side effect as a result of taking an ADHD medication. Figure 1 displays the most commonly mentioned side effects. Of the 135 patients who listed at least one side effect, loss of appetite, sleep problems (typically difficulty falling asleep), and mood disturbance (often mood swings) were most frequently reported by patients. Only 21 percent of these patients experiencing an issue with an ADHD medication indicated that the side effects were very bothersome or extremely bothersome (Figure 2).
Figure 1.
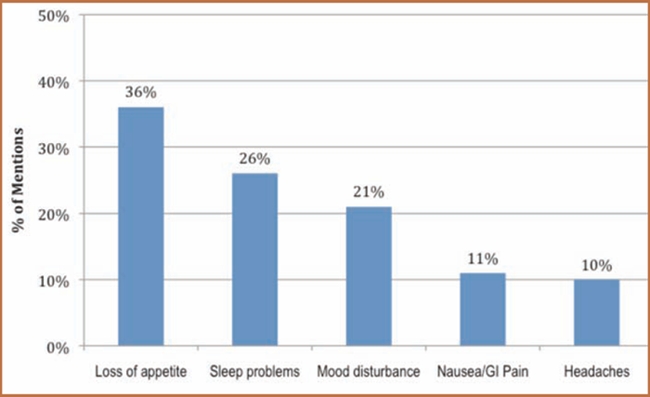
Most commonly mentioned side effects (n=135 patients listing at least 1 side effect). All other mentions are <10% prevalence.
Source: Analysis of www.iGuard.org data for amphetamine and dextroamphetamine; atomoxetine; dexmethylphenidate; isdexamfetamine; and methylphenidate
Figure 2.
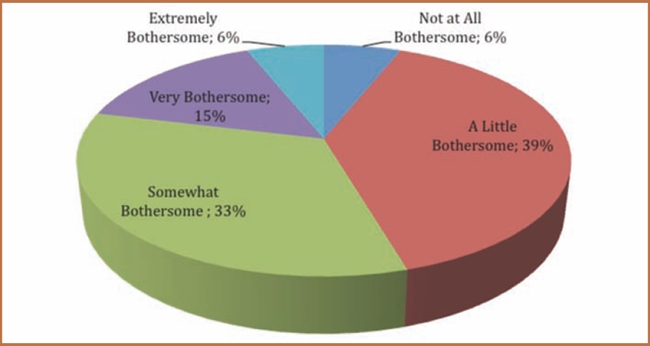
Impact of side effects
Source: Analysis of www.iGuard.org data for amphetamine and dextroamphetamine; atomoxetine; dexmethylphenidate; isdexamfetamine; and methylphenidate
With respect to stimulants versus nonstimulants, the proportion of individuals reporting side effects was somewhat similar (48% for stimulants vs. 46% in nonstimulant users), but the side effects reported differed slightly, with mood disturbance more prevalent among stimulant users and nausea/gastrointestinal problems more common among those taking nonstimulants.
Figure 3 shows the proportion of patients who discussed side effects with their prescribing physician. As seen in Figure 3, only 21 percent of patients who experienced a side effect reported it to their physician. Interestingly, the proportion of patients reporting side effects to their physician was the same for all patients, even those within the subset whose side effects were very bothersome or extremely bothersome (21% vs. 22%).
Figure 3.
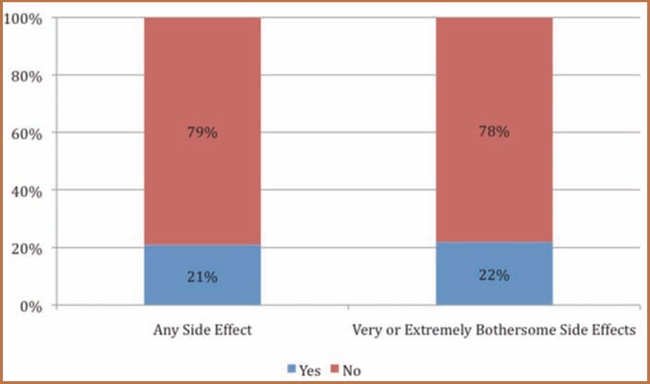
Communication of side effects to the prescribing physician
Source: Analysis of www.iGuard.org data for amphetamine and dextroamphetamine; atomoxetine; dexmethylphenidate; isdexamfetamine; and methylphenidate
Expert Commentary
Pharmacological treatment of ADHD symptoms is typically characterized by significant therapeutic outcomes across multiple domains. Also evident with these clinical benefits is the associated pattern of relatively common adverse events (AEs) that may impact, and even impair, short- and long-term outcomes.1 Such AEs may vary with the age of the individual treated. For instance, preschool-aged children tend to have more marked adverse events than older age groups.2 Some treatment-induced AEs usually subside within the first 1 to 2 weeks of treatment or when medication dose or timing is altered. AEs are typically dose-dependent and mild-to-moderate in severity, yet they often may be the reason why patients discontinue treatment.3 For instance, greater than five percent weight loss can affect medical outcomes,4 and therefore typically is a criterion for termination from treatment.
Several keys issues related to AEs should be addressed more fully in future a priorias well as well as post-hoc data analyses, as follows: 1) those problems documented as AEs that relate to pre-existing medical issues coexistent with ADHD prior to stimulant or nonstimulant treatment; and 2) the naïve or previous-treatment exposure status of patients. For the former, documentation of baseline presentation of medical conditions, such as sleep dysfunction, is critical to establish whether sleep issues following medication treatment is related to the disorder or AEs. For the latter, it may be extremely useful to “borrow” the epidemiological term person-time to describe the sum of individual units of time that the persons in the study population actually have been exposed to specific pharmacological treatments and/or doses actual exposure time.5 This may provide a more objective way of comparing AEs, such as in a treatment study in which AEs appear more common at the lowest dosage level due to the fact that optimal dose titration leads to greatest exposure at the lowest doses.
Contributor Information
Elisa Cascade, Ms. Cascade is Vice President, Quintiles Inc./iGuard, Falls Church, Virginia.
Amir H. Kalali, Dr. Kalali is Vice President, Global Therapeutic Group Leader CNS, Quintiles, Inc., and Professor of Psychiatry, University of California, San Diego, California.
Sharon B. Wigal, Dr. Wigal is Clinical Professor of Pediatrics University of California; Irvine Director of Clinical Trials, Child Development Center, Irvine, California.
References
- 1.Wigal SB. Efficacy and safety limitations of attention deficit hyperactivity disorder pharmacotherapy in pediatric patients. J Pediatrics. 2009;154:S13–S21. doi: 10.2165/00023210-200923000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Wigal T, Greenhill L, Chuang S, et al. Safety and tolerability of methylphenidate in preschool children with ADHD. J Am Acad Child Adolesc Psychiatry. 2006;45(11):1294–1303. doi: 10.1097/01.chi.0000235082.63156.27. [DOI] [PubMed] [Google Scholar]
- 3.Efron D, Jarman F, Barker M. Side effects of methylphenidate and dexamphetamine in children with attention deficit hyperactivity disorder: a double-blind, crossover trial. Pediatrics. 1997;100(4):662–666. doi: 10.1542/peds.100.4.662. [DOI] [PubMed] [Google Scholar]
- 4.Sermet-Caudelus I, Poisson-Salomon A, Colomb V, et al. Simple pediatric nutritional risk score to identify children at risk of malnutrition. Am J Clin Nutr. 2000;72:64–7. doi: 10.1093/ajcn/72.1.64. [DOI] [PubMed] [Google Scholar]
- 5.University of Michigan. epiCentral: Michigan center for public health preparedness “Person-Time.”. [1 April 2010]. https://practice.sph.umich.edu/micphp/epicentral/person_time.php Office of Public Health Practice, School of Public Health, University of Michigan.


