Abstract
An efficient synthesis of a derivative of monophosphoryl lipid A suitable for coupling to various structures for the construction of glycoconjugate vaccines, and its conjugation with an N-modified form of the tumor-associated antigen GM3, is presented.
Lipid A (1) is the hydrophobic domain of lipopolysaccharides (LPS) of the outer membranes of Gram-negative bacteria.1 Its structure consists of a β-1,6-linked di-glucosamine with 1,4′-di-O-phosphorylation and 2,2′-N- and 3,3′-O-acylation (Figure 1). Lipid A of different bacterial origins can vary significantly in terms of the number and structure of the fatty acids.1
Figure 1.
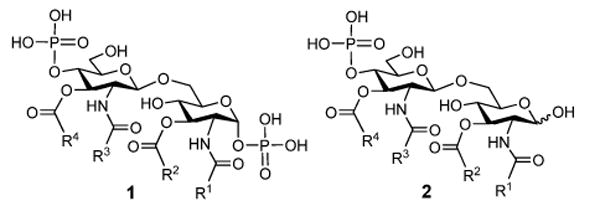
Generic structures of lipid A (1) and MPLA (2)
Lipid A is a strong immunostimulator. It functions through Toll-like receptor 4 (TLR4), the activation of which triggers a cascade of immunological responses, including the production of various cytokines and chemokines, such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), IL-6, and interferon-β (IFN-β).2 Therefore, lipid A is an important target for the development of useful immunomodulators,3, 4 such as vaccine adjuvants, and for the development of new conjugate vaccines.5 However, a serious problem associated with lipid A is that it is pro-inflammatory and can cause fatal septic shock.6 Recent structure-activity relationship (SAR) studies have disclosed that both the fatty acid structure and the phosphorylation degree of lipid A can affect its activity and toxicity.7–10 Most notably, it was found that monophosphoryl lipid A (MPLA 2), which has only one phosphate group linked to the 4′-OH, had drastically reduced toxicity but retained immunostimulatory activity.11 MPLA has been proved to be clinically safe.12
Meanwhile, carbohydrate-based vaccines for cancer and for various infectious diseases is currently a hot topic.13 However, a crucial issue related to carbohydrate antigens is that they are poorly immunogenic, so to form effective vaccines, they have to be covalently linked to an immunologically active carrier.14 In this regard, MPLA can be of great potential. First, owing to its bacterial origins, MPLA could be a “danger signal” to the immune system to significantly enhance the immunogenicity of a carbohydrate antigen once they are covalently coupled, as observed by Boons et al15 with a different conjugate. Second, as noted above, MPLA is an immunostimulator and adjuvant; this can further help to stimulate the immune system.
To probe MPLA for the development of effective conjugate vaccines, we prepared a monophosphoryl form 3 (Scheme 1) of Neisseria memingitidis lipid A.16 SAR studies showed that modification of the reducing end of MPLA had little influence on its immunological activities.10 The syntheses of lipid A and MPLA have been reported17–23 (incomplete), but 3 is designed to have a functionality that will facilitate its coupling to other structures. For example, the azido group of 3 will enable the coupling of 3 to other molecules by click chemistry. The azido group can also be readily reduced to form a primary amine to facilitate the introduction of other functionalities and, therefore, MPLA coupling to various structures by other methods.
Scheme 1.
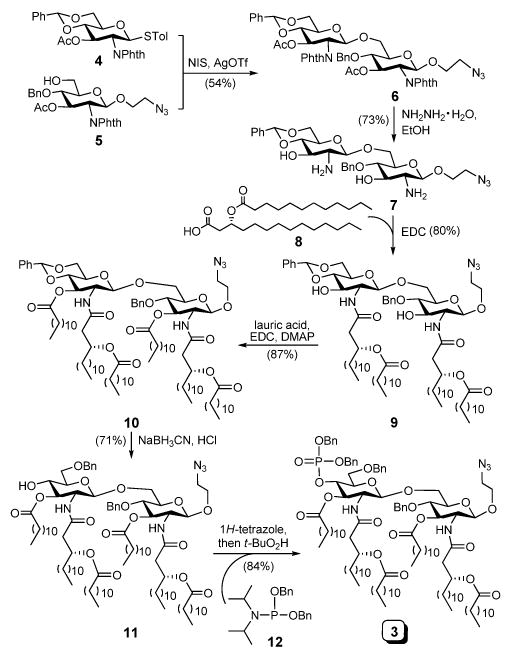
Synthesis of the target molecule 3
Our synthesis of 3 is outlined in Scheme 1. Monosaccharide blocks 4 and 5 were synthesized from commercially available glucosamine following a series of established transformations. The glycosylation reaction between 4 and 5 was achieved with N-iodosuccinimide (NIS) and silver trifluoromethanesulfonate (AgOTf) as promoters. This reaction was unexpectedly slow, and it took about 2 days at room temperature to complete, but it was stereoselective to afford the desired β-anomer 6 (J1′,2′ = 8.8 Hz) in a 54% isolated yield. Then, the phthalyl and acetyl groups in 6 were simultaneously removed upon treatment with hydrazine in refluxing ethanol to give 7. Selective acylation of the free amino groups of 7 by (R)-3-dodecanoyl-tetradecanoic acid 8 using 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) as the condensation reagent went smoothly to furnish 9 in a yield of 80%. Acylation of both C-3 and C-3′ hydroxyl groups of 9 by lauric acid was slow, and it required a higher temperature (45 °C), but the reaction was clean to produce 10 in an excellent yield. Thereafter, regioselective opening of the benzylidene acetal ring of 10 was accomplished using sodium cyanoborohydride (NaCNBH3) and HCl in dry diethyl ether to produce 11, which has a free hydroxyl group on C-4′, and the regiochemistry was confirmed via an acetylation experiment. Finally, 11 was phosphorylated in a two-step-one-pot manner to afford the synthetic target 3.
To demonstrate that 3 can be useful for the development of conjugate vaccines, we then examined the coupling of 3 to N-phenylacetyl GM3 (NPhAcGM3), a neoantigen investigated in our lab for cancer immunotherpay.24 As outlined in Scheme 2, after the azido group of 3 was reduced with Zn under acidic conditions, the resultant free amine was acylated with succinic anhydride to form 13, which was then transformed into active ester 14 to facilitate the coupling to 15. The reaction between 14 and 15 afforded the desired MPLA-NPhAcGM3 conjugate 16 in a good yield.
Scheme 2.
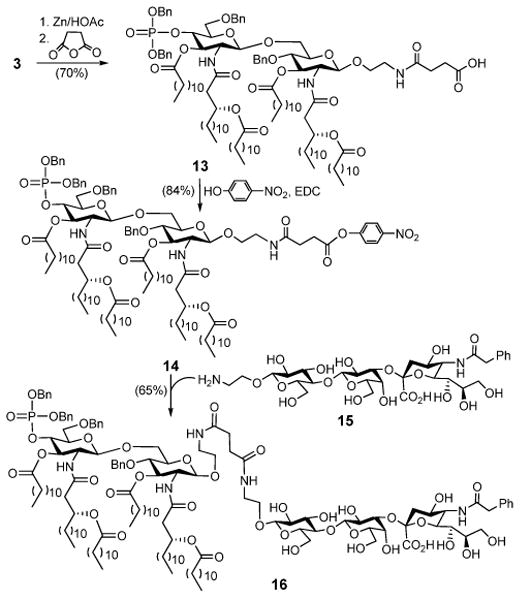
Synthesis of MPLA-NPhAcGM3 conjugate 16
In summary, a highly convergent and efficient method was established for the synthesis of an MPLA derivative 3, which was derived from monosaccharides 4 and 5 in 6 separate steps and a 17% overall yield. This MPLA derivative is suitable for coupling with various structures. As a demonstration, 3 was effectively coupled with a modified tumor-associated antigen to afford 16, which was readily deprotected via Pd-catalyzed hydrogenolysis. The immunological property of conjugate 16 and its deprotected product as cancer vaccines are now under investigation in our laboratory.
The authors thank NIH (CA95142) for financial supports of this work and thank Drs. B Shay and L Hryhorczuk for HRMS measurements and Dr. B Ksebati for NMR measurements.
Supplementary Material
Footnotes
Electronic Supplementary Information (ESI) available: Experimental procedures and data; NMR and MS spectra of the intermediates and final products. See DOI: 10.1039/b000000x/
Notes and references
- 1.Erridge C, Bennett-Guerrero E, Poxton IR. Microbes Infect. 2002;4:837. doi: 10.1016/s1286-4579(02)01604-0. [DOI] [PubMed] [Google Scholar]
- 2.Dobrovolskaia MA, Vogel SN. Microbes Infect. 2002;4:903. doi: 10.1016/s1286-4579(02)01613-1. [DOI] [PubMed] [Google Scholar]
- 3.Persing DH, Coler RN, Lacy MJ, Johnson DA, Baldridge JR, Hershberg RM, Reed SG. Trends Microbiol. 2002;10(Suppl):S32. doi: 10.1016/s0966-842x(02)02426-5. [DOI] [PubMed] [Google Scholar]
- 4.Baldridge J, Myers K, Johnson D, Persing D, Cluff C, Hershberg R. In: Vaccine Adjuvants. Hackett CJ, Harn DAJ, editors. Humana Press Inc.; Totowa, N.J.: 2006. p. 235. [Google Scholar]
- 5.Baldridge JR, Crane RT. Methods. 1999;19:103–107. doi: 10.1006/meth.1999.0834. [DOI] [PubMed] [Google Scholar]
- 6.E. S. Van Amersfoort, T. J. C. Van Berkel and J. Kuiper, 2003, pp. 379.
- 7.Qureshi N, Kutuzova G, Takayama K, Rice PA, Golenbock DT. J Endotoxin Res. 1999;5:147. [Google Scholar]
- 8.Morrison DC, Silverstein R, Luchi M, Shnyra A. Infect Dis Clin N Am. 1999;13:313. doi: 10.1016/s0891-5520(05)70077-5. [DOI] [PubMed] [Google Scholar]
- 9.Darveau RP. Curr Opin Microbiol. 1998;1:36. doi: 10.1016/s1369-5274(98)80140-9. [DOI] [PubMed] [Google Scholar]
- 10.Rietschel ET, Kirikae T, Schade FU, Mamat U, Schmidt G, Loppnow H, Ulmer AJ, Zahringer U, S U, Di Padova F, Schreier M, Brade H. FASEB J. 1994;8:217. doi: 10.1096/fasebj.8.2.8119492. [DOI] [PubMed] [Google Scholar]
- 11.Ribi E, Cantrell J, Feldner T, Myers K, Peterson J. Microbiol. 1986:9. [Google Scholar]
- 12.Casella CR, Mitchell TC. Cell Mol Life Sci. 2008;65:3231. doi: 10.1007/s00018-008-8228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Danishefsky SJ, Allen JR. Angew Chem Int Ed. 2000;39:837. doi: 10.1002/(sici)1521-3773(20000303)39:5<836::aid-anie836>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 14.Jennings HJ, Sood RK. In: Neoglycoconjugates: Preparation and Applications. Lee YC, Lee RT, editors. Academic Press; San Diego: 1994. p. 325. [Google Scholar]
- 15.Buskas T, Ingale S, Boons GJ. Angew Chem Int Ed. 2005;44:5985. doi: 10.1002/anie.200501818. [DOI] [PubMed] [Google Scholar]
- 16.V. A. Kulshin, U. Zahringer, B. Lindner, C. E. Frasch, C. M. Tsai, B. A. Dmitriev and E. T. Rietschel, 1992, pp. 1793.
- 17.Mochizuki T, Iwano Y, Shiozaki M, Kurakata S, Kanaic S, Nishijima M. Tetrahedron. 2000;56:7691. doi: 10.1016/s0008-6215(99)00326-2. [DOI] [PubMed] [Google Scholar]
- 18.Rembold H, Schmidt RR. Carbohydr Res. 1993;246:137. doi: 10.1016/0008-6215(93)84029-6. [DOI] [PubMed] [Google Scholar]
- 19.Demchenko AV, Wolfert MA, Santhanam B, Moore JN, Boons GJ. J Am Chem Soc. 2003;125:6103. doi: 10.1021/ja029316s. [DOI] [PubMed] [Google Scholar]
- 20.Yin N, Marshall RL, Matheson S, Savage PB. J Am Chem Soc. 2003;125:2426. doi: 10.1021/ja0284456. [DOI] [PubMed] [Google Scholar]
- 21.Jiang Z, Budzynski WA, Qiu D, Yalamati D, Koganty RR. Carbohydr Res. 2007;342:784. doi: 10.1016/j.carres.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Gaekwad J, Wolfert MA, Boons GJ. J Am Chem Soc. 2007;129:5200. doi: 10.1021/ja068922a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fukase Y, Fujimoto Y, Adachi Y, Suda Y, Kusumoto S, Fukase K. Bull Chem Soc Jpn. 2008;81:796. [Google Scholar]
- 24.Pan YB, Chefalo P, Nagy N, Harding CV, Guo Z. J Med Chem. 2005;48:875. doi: 10.1021/jm0494422. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


