Prologue
The era of deductive analysis of the electrocardiogram was prolific in its ability to yield accurate inferences regarding the pathophysiology of a vast number of heart rhythm disorders. However, occasionally there was a tendency to infer hypothetical electrophysiological mechanisms without the benefit of direct cellular information. This turned out to be misleading in some cases in which suggested mechanisms were subsequently found to be incorrect, and it also unintentionally hampered the search for more basic mechanisms. Two cases in point are the use of the terms “phase 3” and “phase 4” block in reference to tachycardia-dependent (TD) and pause-dependent (PD) paroxysmal atrioventricular block (PAVB),1 respectively. Not only did basic studies definitely show that TD-PAVB is not related to phase 3 block, but also significant basic studies demonstrated that PD-PAVB need not depend on a phase 4 depolarization mechanism. Here we revisit the problems of TD- and PD-PAVB and illustrate by clinical and basic science examples possible pathophysiological mechanisms.
Keywords: Tachycardia-dependent AV block, Pause-dependent AV Block, Syncope, Electrophysiology
Introduction
Paroxysmal atrioventricular block (PAVB), defined as the sudden and unexpected repetitive block of the atrial impulse on its way to the ventricles, is an important etiology of syncope primarily caused by the delayed emergence of an adequate escape rhythm.1 The majority of cases of PAVB develop in conjunction with increased rate of the atrial input to the atrioventricular (AV) conduction system, that is, TD-PAVB.2,3 On the other hand, there are published examples in which PAVB was initiated by a supraventricular pause.1,4–6 These cases have been labeled as either “bradycardia- dependent PAVB” or “phase 4 PAVB.” However, as demonstrated below, they are more accurately described as “pause-dependent PAVB” (PD-PAVB). In an unpublished series of 42 cases seen by one of us (NE-S), tachycardia-dependent PAVB (TD-PAVB) occurred in 37 patients (88%), while PD-PAVB occurred in the remaining five patients (12%). In many of these patients, the presenting symptoms were presyncope or syncope, depending on the duration of ventricular asystole. Yet in a few patients the early emergence of a stable escape rhythm prevented the development of significant symptoms.
Although PAVB is a serious arrhythmia, its association with sudden cardiac death in some cases is difficult to ascertain. In a study of 22 patients with syncope, an implantable loop recorder revealed episodes of PAVB in association with syncope in 17 patients.7 None of these patients suffered injury attributable to syncopal relapse, and a majority of patients eventually received a permanent pacemaker. However, in some patients, syncope associated with PAVB can result in serious injury.
TD-PAVB
Since early clinical observations by Mobitz,8 type II second-degree AV block has been associated with PAVB and Stokes-Adams syndrome. Atrial rate acceleration was postulated as a possible cause of intermittent complete AV block and Stokes-Adams attacks as early as 1905.9 Repetitive concealed conduction has been suggested as the mechanism of prolonged ventricular asystole in cases of second-degree AV block.10–12
Examples of TD-PAVB in patients with acute myocardial infarction have been reported.1,13 The increase in the atrial rate occurred either spontaneously or in response to pharmacological agents. In those patients, PAVB occurred in close association with Mobitz type II AV block. On the other hand, cases of TD-PAVB have been described in patients with Stokes-Adams syndrome who do not have acute myocardial ischemia.14 In those patients, there is also the close association of Mobitz type II block and PAVB.15 TD-PAVB occurred in association with a spontaneous gradual increase of the atrial rate or with the abrupt occurrence of supraventricular tachycardia (Figure 1). Several patients showed what has been described as “labile” AV conduction, which, at least in some cases, represented a TD 2:1 AV block. Schwartz and Schwartz reported few cases in which isoprenaline given to treat transient heart block in patients with Stokes-Adams syndrome resulted in marked sinus tachycardia and PAVB.16
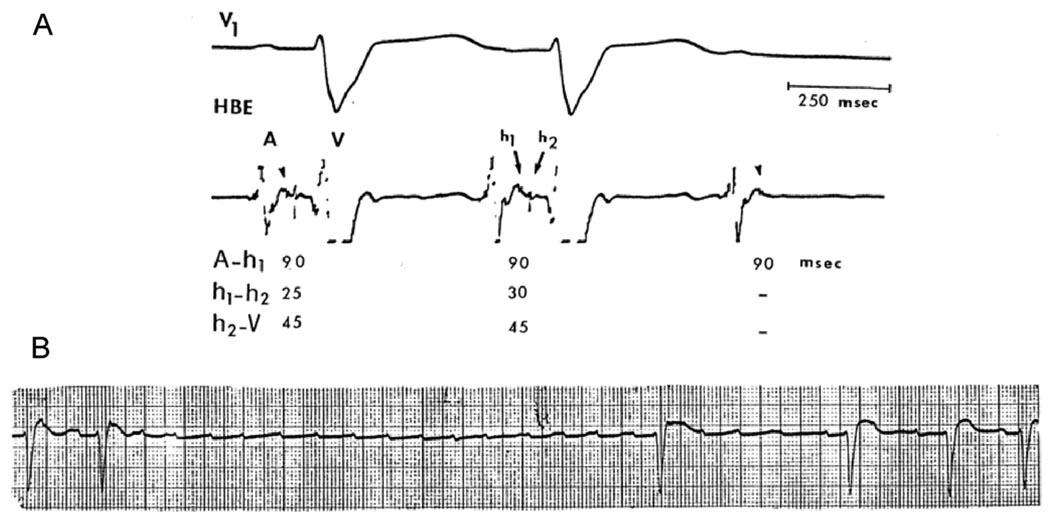
Figure 1.
Electrocardiograms (ECGs) from a 65-year-old woman with a history of recurrent syncopal episodes showed normal sinus rhythm, complete left BBB pattern, and periods of Mobitz type II AV block. A: Electrophysiological study (EPS) showed intra-His bundle block with split His bundle potential (h1, h2) and a 5-ms increment of the h1, h2 interval before block. Intra-His bundle block was initially unrecognized because the h1 deflection was overlooked. B: Rhythm strip 1 day later shows atrial tachycardia (rate 150–170 bpm) leading to TD-PAVB with 5.6-second ventricular asystole. AV conduction improved when the tachycardia slowed down (probably with a period of 2:1 AV block). Note the different time scale of the EPS recording.
Some cases of PAVB occur abruptly and without a perceptible increase in the atrial rate (Figure 2). In such cases, some subtle variation in the degree of electrophysiological disorder in the AV conduction system secondary to slight changes in coronary perfusion, autonomic tone, or circulating catecholamine could be invoked.3 The latter may act through further impairment of conduction in already depressed Purkinje fibers.17 These factors, rather than a change in the heart rate, may explain the episodes of PAVB that may develop abruptly during a stable atrial rhythm, while at other times AV conduction may be maintained during a higher atrial rate (Figure 2).
Figure 2.
Recordings from a 73-year-old woman with recurrent syncope. A: Selected recordings from a Holter ECG. The second and third strips are continuous recordings of lead I showing the development of PAVB with no apparent change in the atrial rate. Several such episodes developed during the 24-hour recording, while 1:1 AV conduction could be seen at other times at a faster atrial rate (top rhythm strip). B: Conduction system study obtained a week later when the patient presented with a stable 2:1 AVB. The recording shows a split His potential (H and H’) and intra-Hisian block on the His bundle electrogram (HBE). Notice the normal QRS duration.
Although the site of AV block in clinical cases cannot usually be ascertained in the absence of a His bundle recording, the presence of bundle branch block (BBB) in many cases may suggest that conduction block occurred in the remaining conducting bundle. However, some clinical observations suggest that the favorite site of PAVB is the His bundle even in the presence of BBB.12 This was the case in our unpublished series of PAVB. Of the 42 cases, 10 underwent a conduction system study; in seven patients, the block was localized in the His bundle (Figures 1 and 2). In the remaining three cases, the block occurred below the His bundle recording. However, as shown in Figure 1, it is not uncommon that intra-His bundle block is underdiagnosed during an electrophysiological study and is labeled instead as infra-His bundle block or even as AV nodal block.
It is important to note, however, that most clinical examples with chronic “stable” lesions in the His-Purkinje system respond to rapid atrial pacing with 2:1 or higher degree block in the AV node, His-Purkinje system, or both, but not by PAVB. In those patients, the propensity for repetitive concealed conduction in the His-Purkinje system may be absent. An alternative explanation would be that the critical short cycle necessary for repetitive block in the His-Purkinje system could not be achieved because of physiological or pathological AV nodal refractoriness.12
In some patients in whom TD AV block in the His-Purkinje system could be demonstrated by incremental atrial pacing, rapid ventricular pacing may also induce a high-degree AV block.12,18,19 Similar observations have been reported in an experimental model of acute ischemia of the proximal His-Purkinje system, in which PAVB could be induced by rapid atria or ventricular pacing.20 The AV block induced by ventricular pacing has been attributed to a “fatigue” phenomenon caused by invasion and repetitive depolarization of Purkinje fibers at the critical site of AV block.18
Electrophysiological mechanism of TD-PAVB
Figure 3 illustrates diagrammatically the difference in electrophysiological properties of normal versus diseased cardiac myocytes and His-Purkinje fibers. Normal cells are characterized by a more negative resting potential, larger action potential amplitude, and fast depolarizing sodium current. Refractoriness depends on a normal voltage-time course of the sodium current, and the end of repolarization usually signals full recovery of excitability.21 This is the mechanism of phase 3 AVB, which is correctly referred to as voltage-dependent block (see the upper panel of Figure 3). On the other hand, diseased cells have a less negative resting potential, smaller action potential amplitude of shorter duration, and a much slower depolarizing current that could still be carried by a sodium current with depressed kinetics. Because recovery of excitability depends on the balance between inward depolarizing currents (i.e., INa and ICa) and outward repolarizing potassium currents (IK1, IKr, and IKs), in these cells the reduced sodium current increases the relative importance of repolarizing currents during the early phase of diastole, despite full repolarization.22 These outward currents oppose depolarization, and thus recovery of excitability extends beyond the end of repolarization, as shown in the lower panel of Figure 3 by the inability of the cell to reach threshold when a stimulus is applied at the earliest premature interval. This is referred to as “postrepolarization refractoriness” and implies that, contrary to a normal cell, a premature stimulus has to fall at some point later in diastole before it can result in a propagating action potential. The depolarized action potential has slower conduction properties and is very susceptible to conduction block. At a more depolarized resting potential, the sodium current cannot be activated, but a slow depolarizing current through the calcium channel could still be induced by a strong stimulus. This action potential is usually referred to as the “slow response” and is also characterized by slow conduction properties and postrepolarization refractoriness. 23
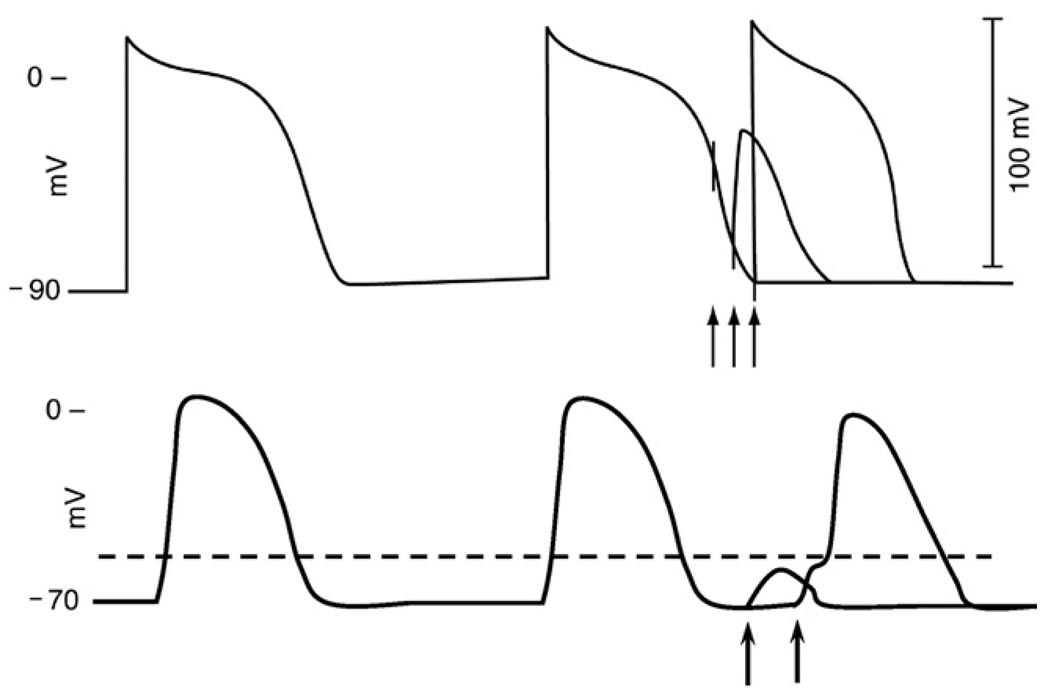
Figure 3.
Diagrammatic illustration of the responses of a normal ventricular myocyte action potential (AP, top) and a depressed AP (bottom) to premature stimulation. Top, so-called phase 3 or voltage-dependent refractoriness. The first stimulus (arrow) falls on an early phase 3 and fails to elicit a response. The second stimulus falls in late phase 3 and results in an abbreviated, slowly rising AP of low amplitude. The third stimulus that falls at the end of repolarization results in a full AP. The bottom recording illustrates postrepolarization refractoriness (arrows) in a depressed fiber. Because of delayed recovery of excitability, despite full repolarization after the second action potential, a depolarizing stimulus applied very early in diastole is incapable of bringing the cell to the threshold potential (broken horizontal line). Thus the stimulus fails, and only a subthreshold depolarization is seen. When a similar stimulus is applied later in diastole, the cell is activated after a substantial delay.
An important property of depressed cells that is relevant to the mechanism of TD-PAVB is the phenomenon of concealed conduction.10–12,24 Probably the most commonly recognized clinical condition in which concealed conduction plays an important role is in patients with atrial flutter when the rhythm suddenly converts to atrial fibrillation. An atrial flutter with an atrial rate of approximately 300/min could present with 2:1 AV conduction and a fast ventricular response of 150/min. During atrial fibrillation, characterized by a faster atrial input to the AV conduction system (approximately 550 impulses/min), the ventricular response can suddenly slow down. This seemingly paradoxical situation is due to concealed conduction in the AV node, where an atrial impulse that blocks in the AV node leaves in its wake enough alterations of refractoriness so that the next one or several atrial impulses will also block. A similar phenomenon of rate-dependent concealed conduction in the diseased His-Purkinje system can explain the repetitive conduction block. The phenomenon has been clearly demonstrated in excised bundles of canine Purkinje fibers exposed to a high potassium concentration, which depolarizes the normal resting membrane potential.23 The same mechanism was later demonstrated in a more clinically relevant model of TD-PAVB.3 Figure 4 illustrates one of these experiments. The top panel shows intracardiac electrode recordings demonstrating TD-PAVB in the ischemic His bundle of a dog, a recording very much reminiscent of the clinical situation. The bottom panel illustrates in vitro microelectrode recordings in an ischemic His-Purkinje preparation showing more clearly the characteristics of a depressed His bundle action potential including postrepolarization refractoriness and rate-dependent repetitive conduction block. Of interest is the observation that TD intermittent block occurred in close proximity to the development of second degree AVB associated with subthreshold foot potentials preceding each action potential and minimal increment of conduction delay before block (the so-called, Mobitz type II second block). Figures 1 and 2 represent the clinical counterpart of the experimental recordings shown in Figure 4. In fact, when a greater degree of incremental conduction delay occurred, with progressively longer foot potentials before block (the equivalent of the so-called Wenckebach type I, second-degree block in the His-Purkinje system), faster rates usually resulted in 2:1 or higher grade AVB but not the repetitive failure of conduction associated with TD-PAVB.
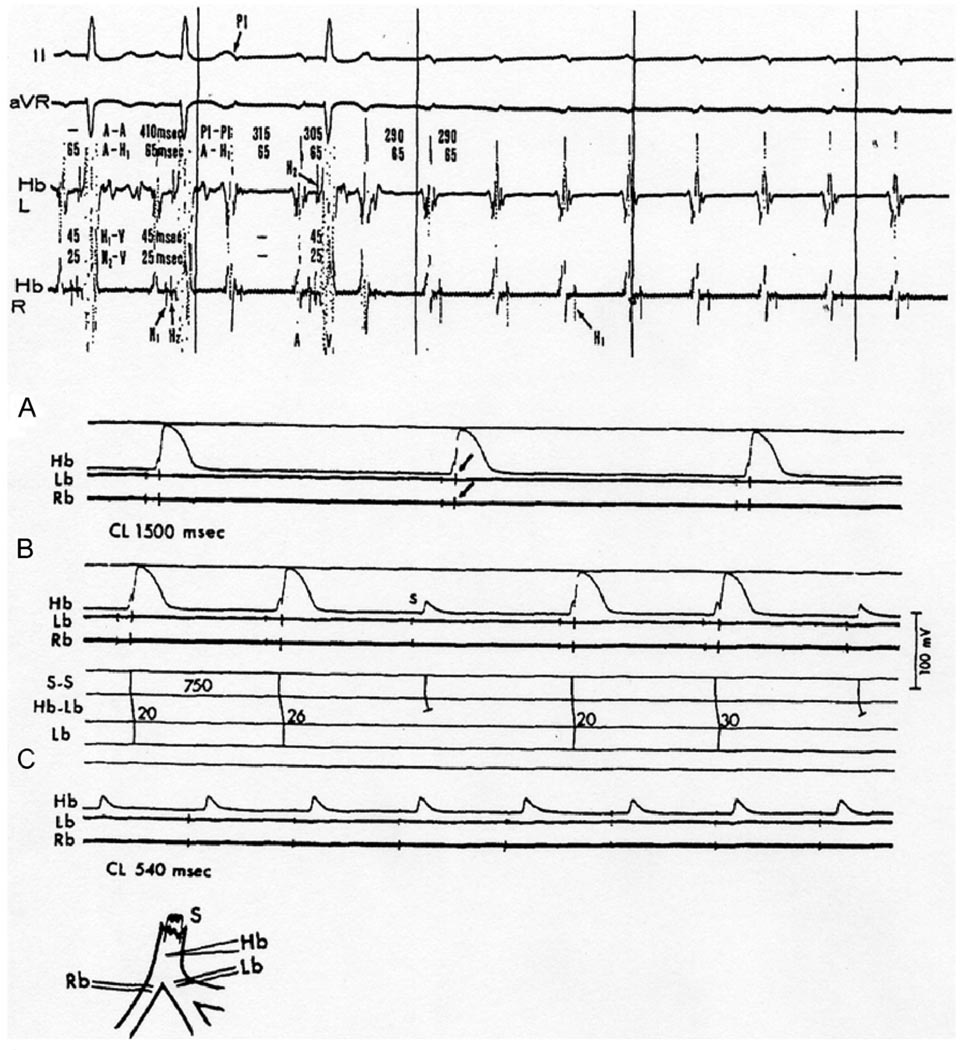
Figure 4.
Ischemic canine proximal His-Purkinje system after ligation of the anteroseptal artery shows Mobitz type II block and TD-PAVB. Top, in vivo experiment; intracardiac His bundle electrograms from both left and right sides of the ventricular septum (Hb-L and Hb-R) showing a split His potential (H1 and H2). After the first two sinus beats, incremental atrial pacing started (P1) resulting in 2:1 AVB followed by TD-PAVB in the His bundle. Bottom, in vitro recordings from an ischemic His-Purkinje preparation. Inset, arrangement of electrodes: stimuli applied to proximal His bundle (S); intracellular recording electrode in penetrating portion of His bundle (Hb). Note the slow approach to threshold preceding each action potential (foot potentials) as a result of slow conduction through the bundle; extracellular bipolar recordings from proximal right (Rb) and left (Lb) bundles. A: 1:1 conduction from His bundle to both bundle branches (arrows point at the extracellular recording spikes) at a slow driving rate (40/min). B: Increasing the rate to 75/min resulted in 3:2 response with slight (6 ms) increment of Hb-Lb conduction time before the blocked beat. The Hb recording showed only a local potential during block. C: Further increase of the rate to 111/min resulted in complete conduction failure; the Hb recording showed repetitive local potentials with failure of inscription of a regenerative action potential. Note that conduction failure occurred at cycle lengths (CLs) that far exceeded the action potential duration (i.e., postrepolarization refractoriness). The Rb and Lb electrograms have been retouched for clarity. (Reprinted with permission of the American College of Cardiology from El-Sherif et al, Am J Cardiol 1974;35:421– 434).
PD-PAVB
Although the majority of cases of PAVB appear to be tachycardia dependent,2 several examples of PAVB have been described where the onset of AVB is ushered by a pause.4–6 The latter could be a compensatory pause before an atrial or ventricular premature beat (Figure 5A), spontaneous slowing of the sinus rate, or overdrive suppression of sinus rhythm after a fast atrial rhythm. Several hypotheses have been proposed to explain the mechanism of the rare phenomenon of PD-PAVB, including concealed conduction, hypoxia, vagal effects, and supernormality of the affected bundle (reviewed in 25). In 1967, Singer, Lazzara, and Hoffman26 demonstrated that depolarization of depressed Purkinje fibers can lead to propagation impairment and produce slow conduction or even blockade. Their experiments suggested that phase 4 depolarization in potentially automatic cells could explain the conduction abnormalities associated with prolongation of the cycle length. Their postulate is illustrated in Figures 5B and 5C. Subsequently, Rosenbaum et al27 proposed a model in which entrance block and phase 4 depolarization (phase 4 block) in the diseased conducting system played a major role in the development of bradycardia-dependent BBB. Recently, Lee, Wellens, and Josephson28 emphasized the distinction between PD-PAVB and vagal AV block. Similar to Rosenbaum et al, they defined PD-PAVB as “. . . a sudden, pause dependent phase 4 AV block occurring in disease conduction system” and argued convincingly for the essentiality of understanding its mechanism and promptly recognizing its presentation to prevent asystole and sudden cardiac death.28
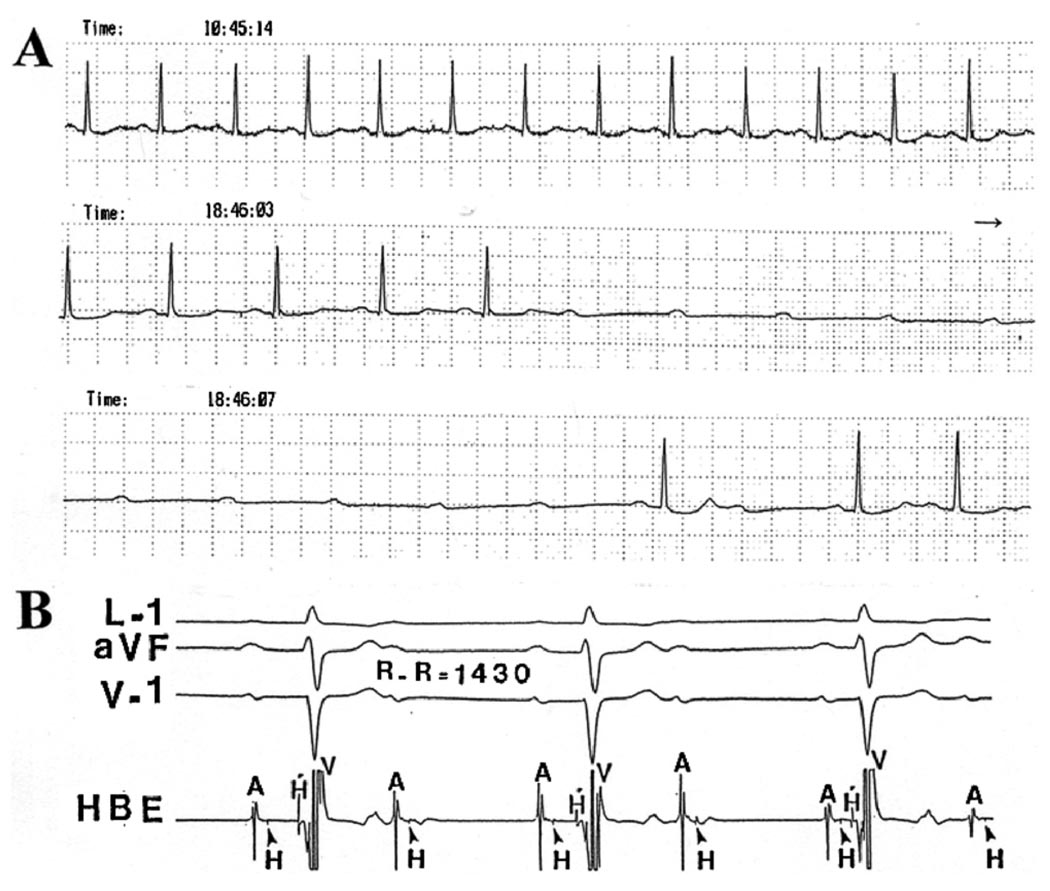
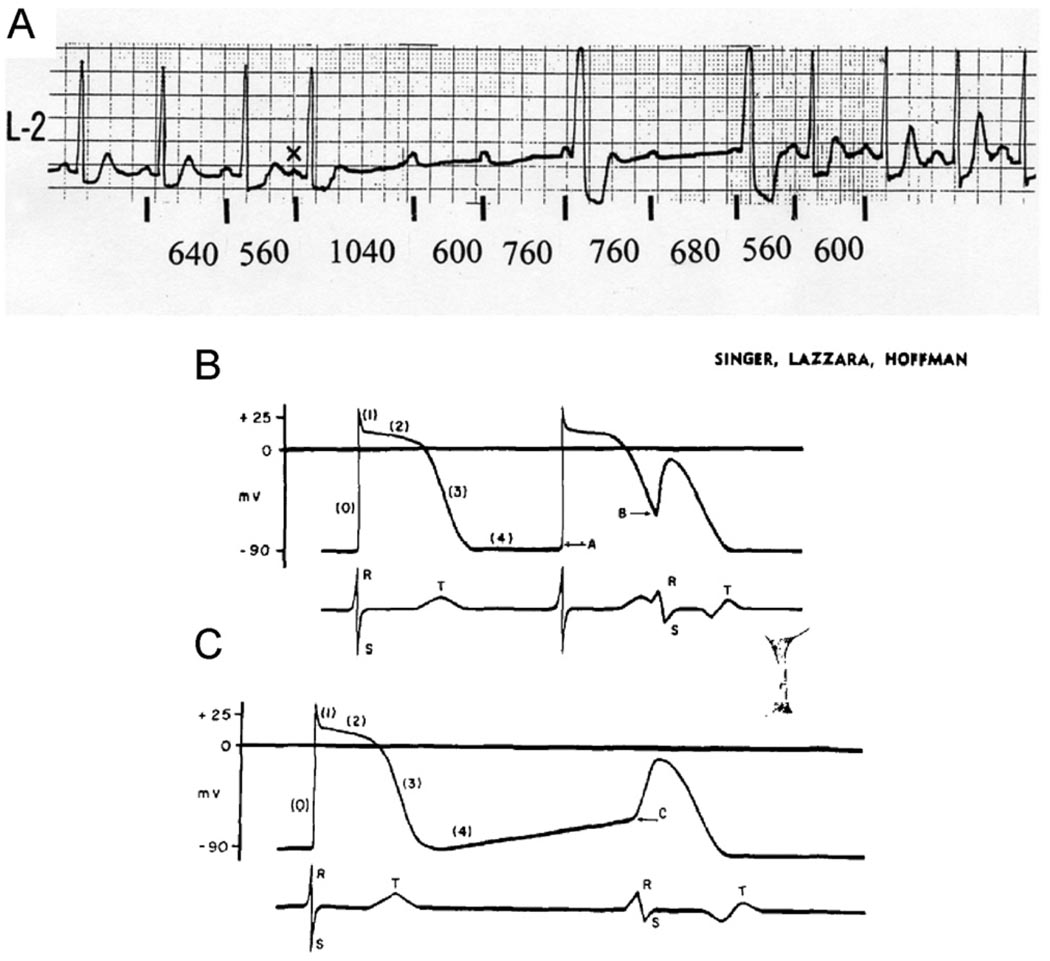
Figure 5.
A: Clinical case of pause-dependent paroxysmal AV block. A brief period of complete AV block with idioventricular escape rhythm was initiated by a long atrial cycle that followed the atrial premature beat marked X. On the right, 1:1 AV conduction resumed on slight acceleration of the sinus rate. Note the marked degree of sinus arrhythmia associated with AV block and resumption of normal conduction. B and C: Armchair model used by Singer, Lazzara, and Hoffman26 to illustrate their idea of phase 4 block. Panels represent transmembrane potentials from a Purkinje cell (top) and simultaneously recorded surface electrogram (bottom). B: Left, transmembrane action potential recorded during stimulation at a rate sufficiently fast to suppress phase 4 depolarization. Right, normal action potential initiated at −90 mV (response A) followed by a premature response initiated during repolarization at −60 mV (response B). The latter had decreased amplitude and upstroke velocity, as well as reduced rate of propagation as indicated by aberration of electrogram. C: Left, normal action potential and ECG complex. Right, the subsequent action potential is initiated in same fiber after development of phase 4 depolarization at the same level of membrane potential (C) as the premature response; its amplitude, upstroke velocity, and speed of propagation are expected to be reduced, and the ECG is expected to be aberrant (modified with permission of the American Heart Association, Inc., from Singer DS, et al, Circ Res 1967;21:537–558).
The idea that PD-PAVB or aberrancy involves a diseased His-Purkinje system undergoing phase 4 depolarization (see Figure 5C) is appealing. However, experiments in isolated Purkinje fibers conducted in the early 1980s25 demonstrated that phase 4 depolarization distal to an area of impaired conductivity may facilitate propagation. Figure 6 illustrates one such experiment. It shows that phase 4 depolarization in cells located downstream of an area of depressed excitability (trace B) can greatly facilitate conduction across a diseased bundle, despite a substantial decrease in the action potential upstroke velocity and thus sodium channel availability, compared with the control condition (trace A).25 Phase 4 hyperpolarization (trace C) takes the membrane away from the threshold potential. It therefore impairs conduction and leads to block. Other experiments demonstrated rate-dependent alterations of excitability in partially depressed tissues that do not undergo phase 4 depolarization. 20,25,29 These alterations have been related to changes in the amplitude of the slow-response action potential as a function of the basic cycle length (BCL; Figure 7).25 Hence, paroxysmal AV block at slow rates of stimulation need not be explained in terms of phase 4 depolarization. Even when diastolic depolarization shifts the takeoff potential to levels at which the sodium channels are completely inactivated, propagation can still be sustained through the slow inward current, and, under these conditions, time to excitation of the depolarized fiber remains practically unchanged, despite a decrease in the upstroke velocity. Thus, one may assume that if slow diastolic depolarization develops in the tissue distal to the zone of block, conduction would be accelerated. Accordingly, it is very unlikely that bradycardia-dependent conduction impairment in a specialized bundle can result from entrance block secondary to phase 4 depolarization in excitable cells within or distal to a depressed zone in the bundle.
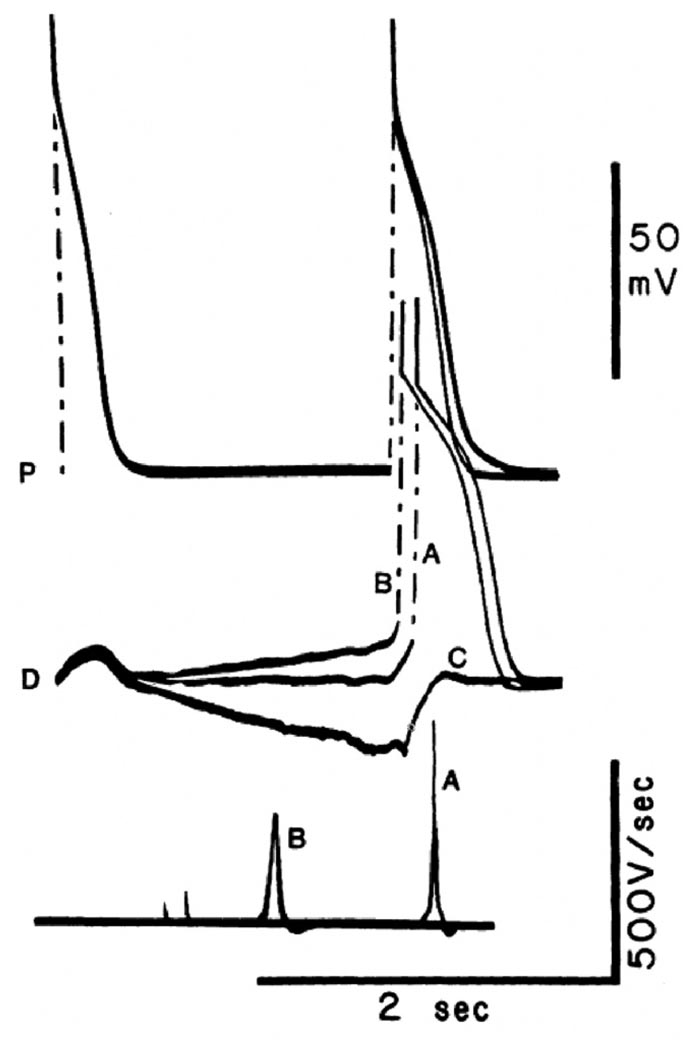
Figure 6.
Influence of diastolic depolarization versus hyperpolarization on conduction in a heterogeneous Purkinje fiber bundle placed in a three compartment tissue bath. Sucrose superfusion of the middle segment. The two outer fiber segments were superfused with normal Tyrode’s solution, stimulation was applied to the proximal segment (P), and activity was recorded from P and from the distal segment (D). Three superimposed recordings are shown. Top traces, P; middle traces, D; bottom traces dV/dt of D. In all sweeps, the initial action potential is the last of a series of 10 evoked in P at BCL = 500 ms. At this rate, complete PD block occurred, and only subthreshold depolarizations were apparent in the distal segment. A postmature action potential initiated in P after 1850 ms activated the distal fiber after 141 ms (discharge A). This long delay occurred despite the large amplitude (89 mV) and dV/dt max (475 V/s) of the distal action potential. In the next sweep, application of a slow depolarizing current ramp to D increased its slope of phase 4 depolarization. When, at the end of the ramp, the proximal segment was stimulated, propagation was again successful, but at a much reduced P-D conduction time (59 ms). Rapid propagation occurred even though the amplitude and dV/dt of phase 0 were significantly decreased in the distal segment, as a result of a reduction of the takeoff potential. In contrast, when the slope of phase 4 was inverted by the application of a hyperpolarizing ramp, complete block was induced (C), and only the electrotonic image of the proximal action potential was apparent in the distal trace. (Reproduced with permission of the American Heart Association from Jalife J, et al, Circulation 1983;67:912–922).
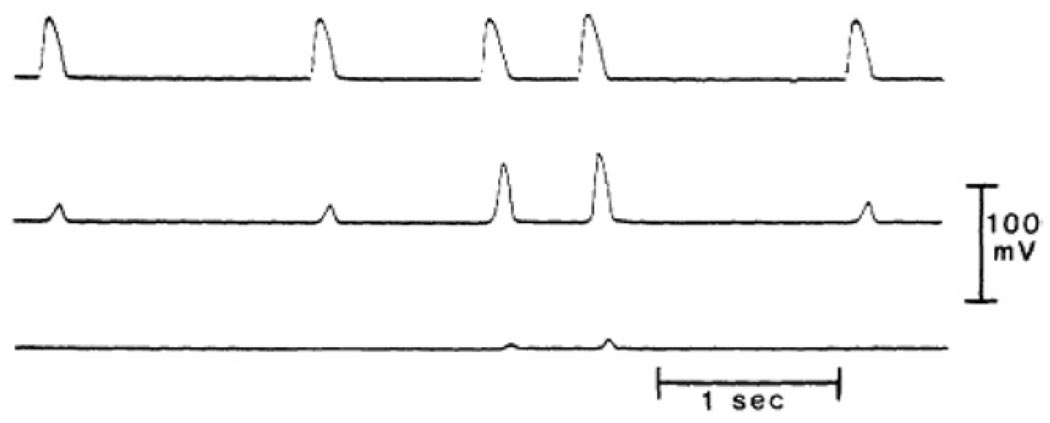
Figure 7.
PD conduction block in an isolated false tendon (dog) exposed to 20 mM KCL Tyrode’s solution. Top trace obtained from cell close to stimulation site; middle trace, from center of the bundle; and bottom trace, recorded more distally. At BCL = 1500 ms all impulses were blocked before they reached the middle segment. Successful activation of that segment was obtained when the stimulus interval was reduced to 500 ms. However, block persisted at the distal segment (reproduced with permission of the American Heart Association from Jalife J, et al, Circulation 1983;67:912–922).
Although phase 4 depolarization may contribute to a decrease in amplitude of proximal responses and may therefore facilitate the development of block, its presence is not absolutely necessary. Frequency-dependent changes in the magnitude of the slow inward current30 can alter the amplitude of action potentials in normally polarized Purkinje fibers proximal to a zone of block and can lead to changes in the efficacy of electrotonic inputs to activate tissue distal to the block. Therefore, conduction impairment or block may develop as a result of source-to-sink mismatch. However, these changes are independent of the maximal upstroke velocity, and they have been shown to occur in the complete absence of slow diastolic depolarization.20,25 Thus, some examples of bradycardia-dependent BBB31 can be explained on the basis of exit block associated with slow diastolic depolarization in conducting fibers proximal to an area of impaired conductivity. Yet a necessary condition for block to occur is the absence of phase 4 depolarization in cells distal to the area of impairment. In other words, while strictly speaking phase 4 block does occur during phase 4 of the cardiac electrical cycle, it may not require phase 4 depolarization.
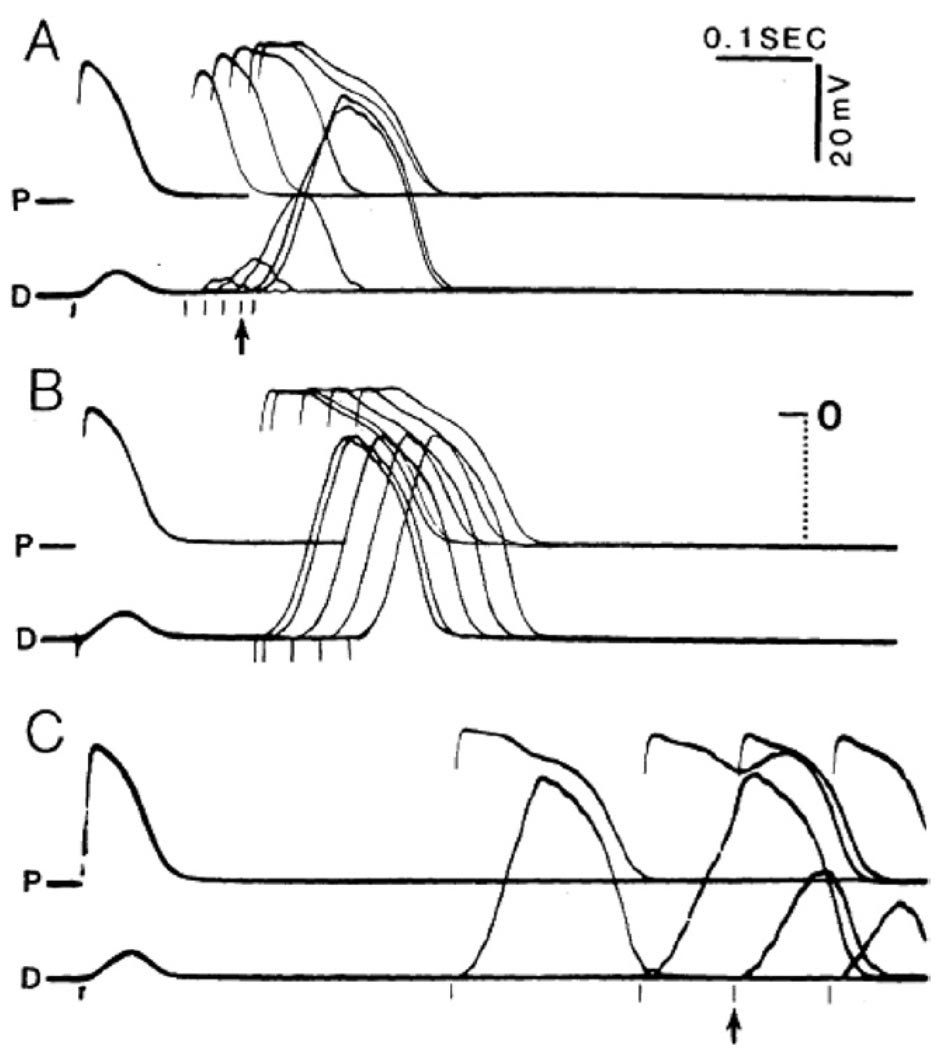
In a series of eight cases and a review of 10 similar cases from the literature, Rosenbaum et al1 showed that PAVB could occur both at rapid and slow rates, with intermediate normal conduction range. In other words, these were cases of combined bradycardia-dependent and TD BBB in which the excitability during diastole seemed to follow a biphasic time course, with relatively early recovery followed by subsequent loss at long diastolic intervals. In an effort to understand the possible mechanism underlying this interesting phenomenon, and to establish definitely the inapplicability of the terms phase 3 and phase 4 block to define it, Jalife et al25 conducted experiments in isolated dog Purkinje fibers exposed to 20 mM KCl, which ensured depressed excitability and complete lack of phase 4 depolarization. A representative experiment is reproduced in Figure 8. Two cells, each located at one extreme of the fiber, were impaled with microelectrodes. In all panels, several superimposed traces are shown. The upper traces were recorded from a site very close to the bipolar stimulating electrodes (P). The lower traces were taken from a site 12 mm distal to the bipolar electrode (D). The preparation was driven with trains of 10 stimuli at a BCL of 500 ms, and test stimuli were applied after every tenth basic beat to scan the long diastolic interval separating the trains. At the basic stimulation frequency, all slow responses were blocked before they reached the distal cell, as shown by the brief duration of the P action potentials and the subthreshold depolarizations in the D trace during the initial responses of all panels. However, when test stimuli were applied at progressively later intervals, progressively greater subthreshold depolarizations were induced in the D cell until, at stimulus intervals of 180 ms (arrow, panel A), the distal depolarization reached threshold and a propagated response ensued. The P-to-D (peak-to-peak) conduction time for this response was about 100 ms. In panel B, all slow responses initiated at intervals between 200 and 480 ms were conducted to the distal site. Yet at test intervals of 385 ms or longer (panel C), P-to-D conduction time increased progressively until at the stimulus interval of 680 ms (arrow, panel C) complete block reappeared. Only a subthreshold depolarization was again apparent in the distal impalement. The amplitude of this depolarization decreased progressively at the later intervals, and no distal responses were obtained thereafter. This biphasic time course of loss followed by recovery and then loss of excitability was highly reproducible in all experiments.25 As suggested by Gilmour, Salata, and Davis,32 the rate-related decline of action potential amplitude may be due in part to time-dependent recovery of the early outward current.
Figure 8.
Coexistence of TD and PD block of slow responses in a depressed dog cardiac Purkinje fiber exposed to 20 mM KCl and 9 mM CaCl2 and stimulated at a BCL of 2000 ms. Premature stimuli were applied every tenth beat with an increasing delay to scan the diastolic interval. The superimposed records in all three panels were recorded within 3 minutes. The upper record (labeled P) in each of them corresponds to a site proximal to the bipolar stimulating electrode; the lower record (labeled D), to a site 12 mm apart. A: Early premature slow responses are blocked before they reach the distal recording. B: Slow responses initiated at intermediate intervals propagate throughout the fiber. C: Slow responses initiated very late in diastole are unable to propagate through the fiber. See text for details.
Epilogue
Altogether, the data reviewed above provide definite demonstration that the terms “phase 3 block” and “phase 4 block” are misnomers. Therefore, their use in the context of TD- or PD-PAVB is inappropriate and should be abandoned.
References
- 1.Rosenbaum MB, Elizari MV, Levi RJ, Nau GJ. Paroxysmal atrioventricular block related to hypopolarization and spontaneous diastolic depolarization. Chest. 1973;63:678–688. doi: 10.1378/chest.63.5.678. [DOI] [PubMed] [Google Scholar]
- 2.Langendorf R. The role of spontaneous diastolic depolarization in second degree A-V block: the mechanism of “paroxysmal” A-V block and of a new form of pseudo-supernormal A-V conduction (editorial) Chest. 1973;63:652–655. doi: 10.1378/chest.63.5.652. [DOI] [PubMed] [Google Scholar]
- 3.El-Sherif N, Scherlag BJ, Lazzara R, Hope R, Williams DO, Samet P. The pathophysiology of tachycardia-dependent paroxysmal atrioventricular block after myocardial ischemia. Experimental and clinical observations. Circulation. 1974;50:515. doi: 10.1161/01.cir.50.3.515. [DOI] [PubMed] [Google Scholar]
- 4.Coumel P, Fabiato A, Waynberger M, Motte G, Slama R, Bouvrain Y. Bradycardia-dependent atrio-ventricular block. Report of two cases of A-V block elicited by premature beats. J Electrocardiol. 1971;4:168–177. doi: 10.1016/s0022-0736(71)80010-9. [DOI] [PubMed] [Google Scholar]
- 5.Shohat-Zxabarski R, Lakobishvilli Z, Kusniec J, Mazur A, Strasberg B. Paroxysmal atrioventricular block: clinical experience with 20 patients. Int J Cardiol. 2004;97:399–405. doi: 10.1016/j.ijcard.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 6.Bortone A, Albenque JP, Marijon E, Donzeau J-P. Complete atrioventricular block and asystole in a patient with an inferior acute myocardial infarction: what is the mechanism? Heart Rhythm. 2008;5:1077–1079. doi: 10.1016/j.hrthm.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Brignole M, Meozzi C, Moya A, et al. Mechanism of syncope in patients with bundle branch block and negative electrophysiological test. Circulation. 2002;104:2045–2050. doi: 10.1161/hc4201.097837. [DOI] [PubMed] [Google Scholar]
- 8.Mobitz W. Uber die unvollstandige Storung der Erregungsuberleitung zwischen Vorhof und Kammer des menschlichen Herzens. Zschr Exper Med. 1924;41:180–237. [Google Scholar]
- 9.Erlanger J. On the physiology of heart block in mammals with special reference to causation of Stokes-Adams disease. J Exp Med. 1906;8:8–58. doi: 10.1084/jem.8.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Langendorf R, Pick A. Concealed conduction. Further evaluation of a fundamental aspect of propagation of the cardiac impulse. Circulation. 1956;13:381–399. doi: 10.1161/01.cir.13.3.381. [DOI] [PubMed] [Google Scholar]
- 11.Langendorf R, Pick A. Concealed intraventricular conduction in the human heart. Adv Cardiol. 1975;14:40–50. doi: 10.1159/000397637. [DOI] [PubMed] [Google Scholar]
- 12.Ursell S, Habbab MA, El-Sherif N. Atrioventricular and intraventricular conduction disorders: Clinical aspects. In: El-Sherif N, Samet P, editors. Cardiac Pacing and Electrophysiology. Philadelphia: W.B. Saunders Co.; 1991. pp. 140–169. [Google Scholar]
- 13.Stock RJ, Macken DL. Observations on heart block during continuous electrocardiographic monitoring in myocardial infarction. Circulation. 1968;38:993–1005. doi: 10.1161/01.cir.38.5.993. [DOI] [PubMed] [Google Scholar]
- 14.McHenry PL, Knoebel SB. Acceleration of the sinoatrial rate leading to complete heart block, an unusual mechanism for the Adams-Stokes syndrome. Am Heart J. 1966;72:681–685. doi: 10.1016/0002-8703(66)90352-8. [DOI] [PubMed] [Google Scholar]
- 15.Donoso E, Adler LN, Friedberg CK. Unusual forms of second-degree atrioventricular block, including Mobitz type II block associated with the Morgagni-Adams-Stokes syndrome. Am Heart J. 1964;67:150–157. doi: 10.1016/0002-8703(64)90362-x. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz SP, Schwartz LS. Adams-Stokes syndrome during normal sinus rhythm and transient heart block. III. The paradoxical effects of carotid sinus digital pressure and deep breathing on patients with Adams-Stokes seizures during normal sinus rhythm and transient heart block. Am J Cardiol. 1961;7:204–210. doi: 10.1016/0002-9149(61)90275-2. [DOI] [PubMed] [Google Scholar]
- 17.Carmeliet E, Vereek J. Adrenaline and the plateau phase of the cardiac action potential. Plug Arch Pathol. 1969;313:300–315. doi: 10.1007/BF00593955. [DOI] [PubMed] [Google Scholar]
- 18.Narula OS, Rung M. Accommodation of A-V nodal conduction and fatigue phenomenon in the His-Purkinje system. In: Wellens HJJ, Lie KI, Janse MJ, editors. The Conduction System of the Heart. Philadelphia: Lea and Febiger; 1976. pp. 529–544. [Google Scholar]
- 19.Sasano T, Okishige K, Azegami K, Ohira H, Yamashita K, Satake S. Transient complete atrioventricular block provoked by ventricular pacing in a patient with nonsustained ventricular tachycardia. J Electrocardiol. 1999;32:185–190. [PubMed] [Google Scholar]
- 20.El-Sherif N, Gann D, Samet P. Pathophysiology of atrioventricular and bundle branch block in acute myocardial infarction. In: Samet P, El-Sherif N, editors. Cardiac Pacing. New York: Grune and Stratton; 1980. pp. 409–438. [Google Scholar]
- 21.Weidmann S. The effect of cardiac membrane potential on the availability of the sodium carrier system. J Physiol. 1955;127:213–224. doi: 10.1113/jphysiol.1955.sp005250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muñoz V, Grzeda KR, Desplantez T, et al. Adenoviral expression of IKs contributes to wavebreak and fibrillatory conduction in neonatal rat ventricular cardiomyocyte monolayers. Circ Res. 2007;101:475–483. doi: 10.1161/CIRCRESAHA.107.149617. [DOI] [PubMed] [Google Scholar]
- 23.Cranfield PF, Klein HO, Hoffman BF. Conduction of the cardiac impulse. I. Delay, block and one-way block in depressed Purkinje fibers. Circ Res. 1971;28:199–209. doi: 10.1161/01.res.28.2.199. [DOI] [PubMed] [Google Scholar]
- 24.Moe GK, Abildskov JA, Mendez C. An experimental study of concealed conduction. Am Heart J. 1964;67:338–356. doi: 10.1016/0002-8703(64)90008-0. [DOI] [PubMed] [Google Scholar]
- 25.Jalife J, Antzelevitch C, Lamanna V, Moe GK. Rate-dependent changes in excitability of depressed cardiac Purkinje fibers as a mechanism of intermittent bundle branch block. Circulation. 1983;67:912–922. doi: 10.1161/01.cir.67.4.912. [DOI] [PubMed] [Google Scholar]
- 26.Singer DS, Lazzara R, Hoffman BF. Interrelationships between automaticity and conduction in Purkinje fibers. Circ Res. 1967;21:537–558. doi: 10.1161/01.res.21.4.537. [DOI] [PubMed] [Google Scholar]
- 27.Rosenbaum MB, Elizari MV, Lazzari JO, Halpern MS, Nau GJ, Levi RJ. The mechanism of intermittent bundle branch block: relationship to prolonged recovery hypopolarization and spontaneous diastolic depolarization. Chest. 1973;63:666–677. doi: 10.1378/chest.63.5.666. [DOI] [PubMed] [Google Scholar]
- 28.Lee S, Wellens HJJ, Josephson ME. Paroxysmal atrioventricular block. Heart Rhythm. 6:1229–1234. doi: 10.1016/j.hrthm.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi N, Gilmour RF, Jr, Zipes DP. Overdrive suppression of conduction in the canine His Purkinje system following anterior septal artery occlusion. Circulation. 1984;70:495–505. doi: 10.1161/01.cir.70.3.495. [DOI] [PubMed] [Google Scholar]
- 30.Mary-Rabine L, Hoffman BF, Rosen MR. Participation of slow inward current in the Purkinje fiber action potential overshoot. Am J Physiol. 1979;237:204–212. doi: 10.1152/ajpheart.1979.237.2.H204. [DOI] [PubMed] [Google Scholar]
- 31.Halpern MS, Chiale PA, Nau GJ, Przybylski J, Lazzari JO, Rosenbaum MB. Effects of isoproterenol on abnormal intraventricular conduction. Circulation. 1980;62:1357–1364. doi: 10.1161/01.cir.62.6.1357. [DOI] [PubMed] [Google Scholar]
- 32.Gilmour RF, Salata JJ, Davis JR. Effects of 4-aminopyridine on rate-related depression of cardiac action potentials. Am J Physiol Heart Circ Physiol. 1986;251:H297–H306. doi: 10.1152/ajpheart.1986.251.2.H297. [DOI] [PubMed] [Google Scholar]