Abstract
In budding yeast, an HO endonuclease-inducible double-strand break (DSB) is efficiently repaired by several homologous recombination (HR) pathways. In contrast to gene conversion (GC), where both ends of the DSB can recombine with the same template, break-induced replication (BIR) occurs when only the centromere-proximal end of the DSB can locate homologous sequences. Whereas GC results in a small patch of new DNA synthesis, BIR leads to a nonreciprocal translocation. The requirements for completing BIR are significantly different from those of GC, but both processes require 5′ to 3′ resection of DSB ends to create single-stranded DNA that leads to formation of a Rad51 filament required to initiate HR. Resection proceeds by two pathways dependent on Exo1 or the BLM homolog, Sgs1. We report that Exo1 and Sgs1 each inhibit BIR but have little effect on GC, while overexpression of either protein severely inhibits BIR. In contrast, overexpression of Rad51 markedly increases the efficiency of BIR, again with little effect on GC. In sgs1Δ exo1Δ strains, where there is little 5′ to 3′ resection, the level of BIR is not different from either single mutant; surprisingly, there is a two-fold increase in cell viability after HO induction whereby 40% of all cells survive by formation of a new telomere within a few kb of the site of DNA cleavage. De novo telomere addition is rare in wild-type, sgs1Δ, or exo1Δ cells. In sgs1Δ exo1Δ, repair by GC is severely inhibited, but cell viaiblity remains high because of new telomere formation. These data suggest that the extensive 5′ to 3′ resection that occurs before the initiation of new DNA synthesis in BIR may prevent efficient maintenance of a Rad51 filament near the DSB end. The severe constraint on 5′ to 3′ resection, which also abrogates activation of the Mec1-dependent DNA damage checkpoint, permits an unprecedented level of new telomere addition.
Author Summary
A chromosomal double-strand break (DSB) poses a severe threat to genome integrity, and budding yeast cells use several homologous recombination mechanisms to repair the break. In gene conversion (GC), both ends of the DSB share homology to an intact donor locus, and the break is repaired by copying the donor to create a small patch of new DNA synthesis. In break-induced replication (BIR), only one side of the DSB shares homology to a donor, and repair involves assembly of a recombination-dependent replication fork that copies sequences to the end of the template chromosome, yielding a nonreciprocal translocation. Both processes require that the DSB ends be resected by 5′ to 3′ exonucleases, involving several proteins or protein complexes, including Exo1 and Sgs1-Rmi1-Top3-Dna2. We report that ectopic BIR is inhibited independently by Sgs1 and Exo1 and that overexpression of Rad51 recombinase further improves BIR, while GC is largely unaffected. Surprisingly, when both Sgs1 and Exo1 are deleted, and resection is severely impaired, half of the cells acquire new telomeres rather than completing BIR or GC. New telomere addition appears to result from the lack of resection itself and from the fact that, without resection, the Mec1 (ATR) DNA damage checkpoint fails to inactivate the Pif1 helicase that discourages new telomere formation.
Introduction
DNA double-strand breaks (DSBs) are generated by normal cellular processes including DNA replication or by exposure to DNA damaging agents or ionizing radiation. To maintain cell viability and preserve genomic integrity, cells employ multiple pathways of homologous recombination (HR) to repair DSBs [1]–[4]. A key initial step in HR is 5′ to 3′ resection of DSB ends to create single-stranded DNA (ssDNA) that recruits formation of a Rad51 filament, which engages in a search for homologous sequences. The predominant HR pathway is gene conversion (GC), a conservative mechanism in which both ends of the DSB share homologous sequences on a sister chromatid, a homologous chromosome, or at an ectopic location. Rad51-mediated strand invasion of the 3′-ended ssDNA allows the initiation of new DNA synthesis to copy a short region of the template and patch up the DSB. When only one DSB end shares homology to a template elsewhere in the genome, a less-efficient HR mechanism, break-induced replication (BIR), can be used to repair the break [5], [6]. In BIR, recombination is used to establish an uni-directional replication fork that can copy the template DNA to the end of the chromosome. If homologous sequences are located ectopically, BIR will result in formation of a non-reciprocal translocation with loss of the distal part of the broken chromosome and may be a significant source of gross chromosomal rearrangements (GCRs) and genomic instability [7]. BIR requires the non-essential subunit of the Polδ polymerase, Pol32, and all of the essential replication machinery except those excluisvely required for formation of the pre-replicative complex [8], [9]. BIR can be used to restart stalled or collapsed replication forks during DNA replication [10] and elongate telomeres in the absence of telomerase [8]. An alternative way to repair the DSB is through de novo telomere addition through the action of telomerase [11]–[13], although this is a very inefficient process that is improved by elimination of the Pif1 helicase [14].
Genetic and in vivo molecular biological experiments indicate that the early steps of GC and BIR are shared [15]–[17]. Following the generation of a DSB, the Tel1/ATM kinase is loaded at sites of DSBs in an Mre11-Rad50-Xrs2 (MRX)-dependent manner [18], [19]. Tel1 in turn phosphorylates MRX [20], [21]. The Sae2 and MRX proteins mediate the initial resection [22], [23] which is continued via two alternate pathways, one using the Exo1 nuclease and the other employing the multifunctional RecQ family helicase Sgs1, in concert with Top3, Rmi1 and the essential helicase/nuclease Dna2 [22]–[24]. DNA resection is also essential to activate the Mec1-dependent DNA damage checkpoint kinase cascade that triggers a cell cycle arrest, allowing time for the cell to repair the beak prior to mitosis [25].
Following resection, Rad51-mediated strand invasion of the donor template occurs with similar kinetics, but the initiation of DNA synthesis at the 3′-end of the invading strand is greatly delayed in BIR as compared to GC [16], [17]. Recently, Jain et al [16] showed that a “Recombination Execution Checkpoint” (REC) delays the initiation of BIR synthesis if a second DSB end has not become engaged nearby on the same template. It is unclear if the delay in BIR synthesis is due to a restructuring of the strand invasion D-loop and/or the recruitment of BIR associated proteins. The efficiency of BIR is inhibited by Sgs1, as there is an increase in BIR in sgs1Δ cells [16]. Sgs1 also has been shown to disrupt HR intermediates [26], inhibit homeologous recombination [27]–[29], and to dissolve double Holiday Junctions (dHJ) to yield noncrossovers [30]–[32].
To better understand the role of Sgs1 in BIR, we examined mutations of non-essential genes that either cooperate or act redundantly with Sgs1 in many of its roles in DNA metabolism, including DNA resection. Here we show that deletion of SGS1 or EXO1 increases the efficiency of BIR whereas overabundance of Sgs1 or Exo1 strongly inhibits it. Overexpression of Exo1 also inhibits GC. Deletion of other non-essential factors responsible for DNA resection, TEL1 or SAE2, modestly increases the efficiency of BIR whereas deletion of MRX impairs BIR. Additionally, we find that overexpression of Rad51 markedly improves the efficiency of BIR but has little effect on GC. Finally, we show that Sgs1 and Exo1 redundantly prevent remarkably efficient de novo telomere addition at broken chromosome ends, a pathway dependent on both telomerase and Sae2.
Results
Assays to study break-induced replication and gene conversion in S. cerevisiae
To study BIR we used the haploid Saccharomyces cerevisiae strain JRL346. A galactose-inducible HO endonuclease is expressed to induce a DSB at a modified CAN1 locus approximately 30 kb from the telomere in the non-essential terminal region on Chromosome V (Ch V) (Figure 1A). The HO endonuclease cut site and an adjacent hygromycin-resistant marker, HPH-MX, was integrated into the CAN1 locus, deleting the 3′ portion of the gene but retaining the 5′ portion of the gene (denoted as CA). A 3′ portion of the gene (denoted as AN1) with 1,157 base pairs of shared homology to CA on Ch V was introduced in the same orientation into Ch XI, 30 kb from its telomere. Prior to HO induction, these cells are canavanine-resistant (CanR) because CAN1 is disrupted. Completion of BIR results in a non-reciprocal translocation that duplicates the donor sequences and the more distal part of the left arm of Ch XI, thus restoring an intact CAN1 gene. These cells become canavanine-sensitive (CanS) and hygromycin sensitive (HphS). About 20% of cells are viable with 99.85% of these cells repairing by BIR and a small fraction by nonhomologous end-joining (NHEJ). The efficiency of BIR repair allows us to physically monitor the kinetics of repair by PCR, Southern blot and pulse-field gel electrophoresis (PFGE), as described in Materials and Methods.
Figure 1. Experimental systems of break-induced replication (BIR) and gene conversion (GC).
(A) In the experimental system to study BIR, an HPHMX marked HO cut site (gray bar) is integrated into the CAN1 gene on Ch V, deleting the 3′ end portion of the gene, the remaining sequences are represented as CA. The AN1 donor sharing 1,157 bp homology with CAN1 is integrated into Ch XI. PCR with primers P1 and P2 monitors the initiation of new DNA synthesis while PCR with primers P1 and P4 detects synthesis past the AN1 sequences, specifc to the donor sequences on Ch XI. Southern blot analysis of AvaI-digested (marked by “A”) DNA probed with CAN1 sequences monitors extension of the BIR fork. Completion of BIR is monitored by Pulse-field gel electrophoresis (PFGE) followed by Southern blot analysis using the MCH2 sequences that are duplicated when the entire donor chromosome arm is copied. (B) In the experimental system to study ectopic GC. A galactose inducible HO endonuclease generates a DSB within the CAN1 locus (disrupted by URA3 creating a 376 bp gap) on Ch V. An additional 2,404 bp of homologous sequences to the gene conversion donor sequences found on Ch XI are distal to the cut site and are denoted as “1.” PCR with primers P1 and P2 monitors both the starting strain and repair into the CAN1 sequences. PCR with primers P1 and P3 monitors repair by GC in which the distal end of the break is retained.
To compare the effects of mutations on GC, we used the isogenic strain JRL475 (Figure 1B). The GC strain was modified from the BIR strain by introducing 2,404 bp of homology marked by URA3 to the other end of the break (denoted as 1, for the 3′-end of CAN1). The insertion of the URA3-1 sequences also deleted 376 bp in the middle of the CAN1 so there is a gap between the homology shared by the two DSB ends created by HO cleavage (CA-URA3-1) with the donor sequences on Ch XI (AN1). Repair by GC results in restoration of the CAN1 gene, rendering cells CanS, but, unlike BIR, the Ch V arm distal to the cut site is retained. When there is a second end of homology to a DSB break, the cell strongly favors GC over BIR [16], [17], [33], so that after induction of a DSB cell viability increases from 20% in the BIR strain to nearly 70% when there are two ends of homology and GC is used to repair the break (Figure 1B and Figure 2B).
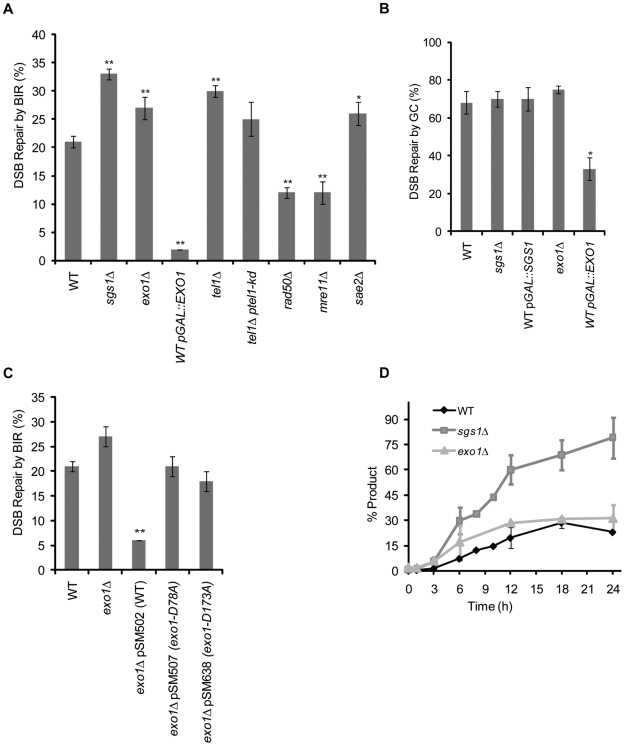
Figure 2. Sgs1 and Exo1 negatively regulate BIR.
(A) Efficiency of BIR in cells as measured by viability following a DSB. (B) Efficiency of GC in cells as measured by viability following a DSB. (C) Efficiency of BIR in wild type (WT), exo1Δ, overexpression of EXO1 and overexpression of EXO1 nuclease-dead alleles measured by viability following a DSB. For (A–C), data are the mean ±standard error of the mean. Values marked with asterics are statistically significant (*represents p<0.05, ** represents p<0.01 compared to wild type). (D) The kinetics of repair are shown for PCR of BIR induced in cycling WT, sgs1Δ and exo1Δ cells amplified with P1 and P2 primer set labeled as “CAN1” and the standard FLO9 locus of. Data are the mean ±standard deviation.
Deletion of SGS1 increases the efficiency of BIR
To better understand the role of Sgs1 in BIR, we first measured the viability of sgs1Δ cells after inducing a DSB (Figure 1A). As previously shown [16], sgs1Δ cells are 1.5 times more efficient in BIR compared to wild type cells (Figure 2A), repairing the break with 33% efficiency (p<0.001). To confirm that the increase in viability directly correlates with an increase in repair product, we monitored the kinetics of repair using the PCR assay that detects the first 242 bp of new DNA synthesis. The maximum amount of product detected by PCR (18% at 12 hours) in wild type cells (Figure 2D) is comparative to the viability of cells (21%) following induction of the DSB (Figure 2A). As expected, deletion of SGS1 increased the efficiency of product formation compared to wild type cells (Figure 2D). Using the previously described BIR system involving the LEU2 sequences [16] we also showed that a helicase-dead allele of Sgs1 [34] behaves like the complete deletion of Sgs1 (Figure S1). We have previously shown that deletion of sgs1Δ does not increase the efficiency of GC events in which there is perfect homology or when there is a small gap in homology of 1.2 kb or less [16], [35]. We confirmed that sgs1Δ does not affect the efficiency of GC in the ectopic assay used here (Figure 1B and Figure 2B).
The non-essential genes required for DNA resection affect the efficiency of BIR
To better understand the role of Sgs1 in BIR, we investigated a number genes that have previously been shown to interact genetically with Sgs1 [27], [29], [36]–[41]. Deletions of MSH6, MUS81, YEN1, RAD27, ESC2, DIA2, YBR094w, or RNH202 did not have a statistically significant effect on BIR when tested for viability after inducing a DSB that can only be repaired by BIR (Table S1). However, we found that the other non-essential genes required for 5′ to 3′ resection of DSB ends all affect the efficiency BIR. A deletion of SAE2 resulted in a slight, but statistically significant, increase in viability (p = 0.02). In contrast, deleting subunits of the MRX complex, mre11Δ or rad50 Δ, decreased viability nearly 2 fold (both p = 0.003) (Figure 2A). The effect of deleting mre11Δ or rad50Δ is consistent with results previously seen in a diploid BIR assay in which a DSB is induced at the MAT locus on Ch III [17], [42], but differs from a transformation-based BIR assay that saw no requirement for MRX in BIR [15].
Because Tel1 plays a role in suppressing gross chromosomal rearrangements and enhances Sae2 and MRX activity in DNA resection [43] we asked if deletion of TEL1 would affect BIR. Similar to sae2Δ, deletion of TEL1 resulted in a small but statistically significant increase in viability (p = 0.008) (Figure 2A). Complementation of a tel1Δ strain with the kinase-dead allele [20] partially restored viability to wild type levels (Figure 2A).
The Exo1 nuclease acts redundantly with Sgs1 in DNA resection after the initial trimming of the ends by Sae2 and MRX, although by itself exo1Δ has a minimal impact on 5′ to 3′ resection [22]–[24]. Similar to sgs1Δ, deletion of EXO1 (p = 0.001) increased viability nearly 1.5 times compared to wild type (Figure 2A). Also like sgs1Δ, deletion of EXO1 increased the efficency of BIR when measured by PCR (Figure 2D) and does not affect the efficiency of GC (Figure 2B).
Overexpression of both SGS1 and EXO1 inhibit BIR
Plamids overexpressing Sgs1 pYES2-SGS1 [44] or Exo1 (pSL44) [45] were expressed under the control of a galactose-inducible promoter on a high copy plasmid. These overexpression plasmids are denoted as pGAL::SGS1 and pGAL::EXO1, respectively. Expression is induced concomitantly with HO induction. In cells carrying pGAL::SGS1, the efficiency of BIR decreased 5 fold (p<0.001) whereas in pGAL::EXO1 the efficiency of BIR decreased 10 fold (p<0.001) (Figure 2A). Overexpression of these genes did not affect cell viability in cells that lacked an HO cleavage site (data not shown). Furthermore, we found that Exo1 overexpression inhibited BIR prior to inhibition of new DNA synthesis, by monitoring the kinetics of repair by PCR (Figure S2). The strong inhibition of BIR by overexpressing Exo1 depends on the nuclease activity of this protein, as there is no such inhibition when we overexpressed plasmids carrying exo1 mutations that are required for exonuclease activity (Figure 2C). As shown previously [8], increasing the homology in our BIR assay more than two fold to 2,977 bp increases the efficiency of BIR (Figure 3C). The increase in homology results in slightly higher viability but does not significantly suppress the effects of overexpressing SGS1 or EXO1 (Figure 3C). When tested in the GC assay, overexpressing Sgs1 had no effect on viability but overproduction of Exo1 decreased viability by half (Figure 2B).
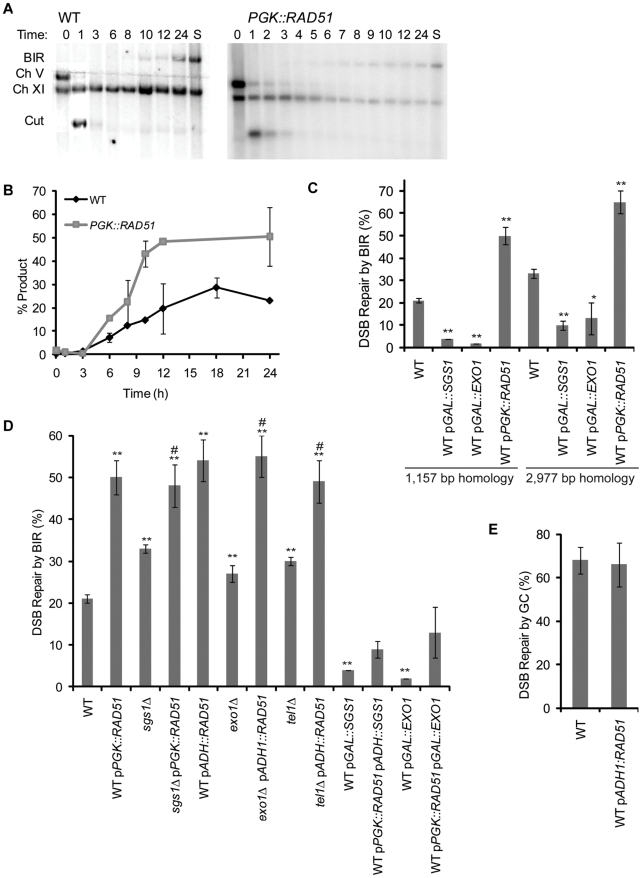
Figure 3. Overexpression of RAD51 increases the kinetics and efficiency of BIR.
(A) Southern blot analysis of the kinetics of repair product in wild type and pPGK::RAD51 cycling cells as indicated in Figure 1A. Lane S contains DNA from a colony where BIR occurred. (B) Kinetics of repair are shown for PCR of BIR induced in cycling wild type (WT) and pPGK::RAD51 cells. Data are the mean ±data range. (C) Efficiency of BIR in cells as measured by viability following a DSB in a BIR assay with increased homology (2,977 bp homology). Data from Figure 2A and Figure 3D (1,157 bp homology strain) are shown for comparison. Data are the mean ±s.e.m. Values marked with asterics are statistically significant (*represents p < 0.05, ** represents p < 0.01 compared to wild type). (D) Efficiency of BIR in strains graphed in Figure 2 also carrying either pPGK::RAD51 or pADH::RAD51 as measured by viability following a DSB. Data are the mean ±s.e.m. Values marked with asterics or number sign are statistically significant (*represents p < 0.05, ** represents p < 0.01 compared to wild type. # represents p < 0.05 to the corresponding single mutant). (E) Efficiency of GC in WT and pPGK::RAD51 as measured by viability following a DSB.
Overexpression of Rad51 increases the efficiency and kinetics of BIR
The initiation of BIR is delayed several hours after the ends of the DSB begin to be resected at a wild type rate of about 4 kb/hr [22], [46]. We have also previously shown that the abundance of Rad51 is sufficient to continuously coat only about 10 kb of ssDNA on either side of the break [47]; consequently it is possible that excess ssDNA would interfere with forming or maintaining a stable and efficient Rad51 filament that is needed to promote strand invasion and initiation of new DNA synthesis. Excess ssDNA has been previously shown to interfere with recombination in meiotic cells [48]. We therefore asked if overexpression of Rad51 would also increase the efficiency of BIR, using well-characterized high-copy plasmids in which RAD51 was expressed under the ADH1 promoter (pDBL(RAD51)) [49] or under the PGK promoter (pSJ5). Strikingly, overexpressing RAD51 in wild type cells caused a 2.5-fold increase in viability (p<0.001) when expressed under control of either promoter (Figure 3D). When we tested the same plasmids in the GC assay we found that there was a slight but not statistically significant decrease in viability (Figure 3E). These results clearly indicate that Rad51 overexpression preferentially stimulates BIR. Overexpression of RAD51 in the BIR assay with longer homology further increased the efficiency of BIR (Figure 3C). We also find that the efficiency of BIR is increased when we tested the kinetics of repair by Southern blot (Figure 3A) and PCR (Figure 3B). However, when normalized to the percent of final product the kinetics of repair are not different from wild type cells (data not shown).
An elevated level of Rad51 increased the viability of sgs1Δ, exo1Δ or tel1Δ cells to the level seen for overexpressed RAD51 alone (Figure 3D), so the effects of RAD51 expression and deleting SGS1 or EXO1 are not additive. However, overexpressing RAD51 in cells also overexpressing SGS1 or EXO1 did not significantly suppress the inhibition of BIR that is seen with overexpressing SGS1 or EXO1 alone (Figure 3D). These results could suggest that Sgs1 and Exo1 act prior to the rate-limiting step carried out by Rad51. In the case of Sgs1, it could be in dismantling transient strand invasion encounters; for Exo1, there is no evident mechanism at this point unless a modest increase in resection [45] would overwhelm excess Rad51.
Sgs1 and Exo1 redundantly inhibit new telomere addition at DSBs
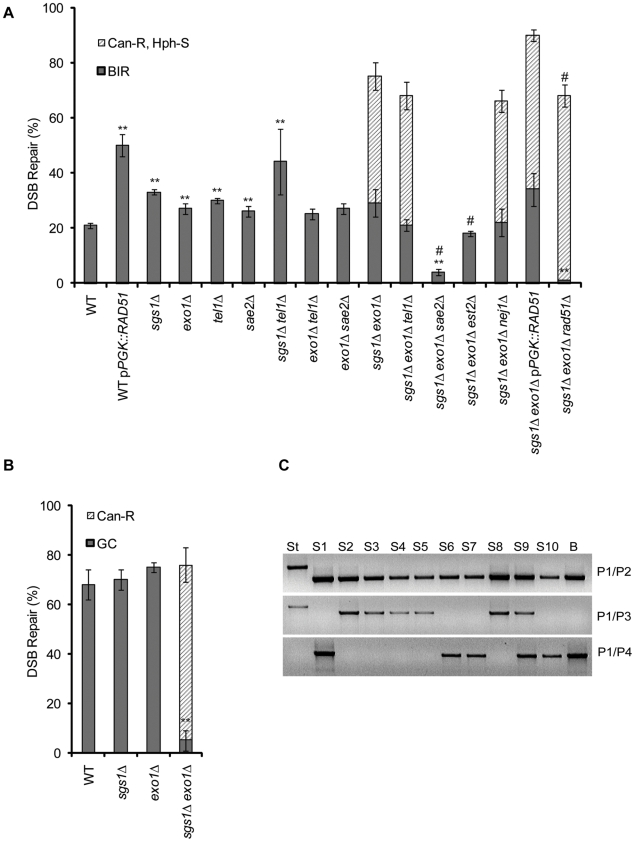
We examined a a dramatic 2-fold increase in viability in an sgs1D exo1D double mutant compared to sgs1Δ or exo1Δ alone when tested in the BIR assay (Figure 4A); however this increase is not in the level of BIR. Instead, it is due to a dramatic increase in new telomere addition, as described below. There is in fact no increase in BIR events compared to the single mutants and repair appears to be no better than wild type cells when repair was monitored by PCR (Figure S3). As has previoulsy been reported [22]–[24], we found that resection is severely impaired in sgs1Δ exo1Δ cells as evident by the persistence of the cut chromosome band seen by Southern blot (data not shown). Although TEL1 and SAE2 moderately inhibit BIR and are involved in DNA resection like SGS1 and EXO1 [50], deleting TEL1 did not cause new telomere additions at the DSB when ablated in combination with sgs1Δ or exo1Δ nor did deletion of SAE2 in combination with exo1Δ (Figure 4A).
Figure 4. The effect of sgs1Δ exo1Δ on the viability and repair product in BIR and GC.
(A) The viability and phenotypic characterization of wild type (WT), sgs1Δ, exo1Δ, tel1Δ, sgs1Δsgs1Δ exo1Δ, pPGK::RAD51 and indicated double and triple mutant combination cells following a DSB in the BIR assay. BIR colonies (CanS HphS) represent those that have repaired the DSB by BIR while CanR HphS colonies represent those that have a truncated chromosome. Data are the mean ±s.e.m. Values marked with asterics or number sign are statistically significant (*represents p < 0.05, ** represents p < 0.01 compared to wild type BIR. # represents p < 0.05 to the sgs1Δ exo1Δ CanR HphS colonies). (B) The viability and phenotypic characterization of cells following a DSB in the GC assay. HR colonies (CanS) represent those that have repaired by Homologus Recombination (either BIR or GC) while CanR colonies represent those that have a truncated chromosome. Data are the mean ±s.e.m. ** represents p < 0.01 compared to wild type. (C) Repair of CanS colonies in the GC assay as monitored by PCR. Included are the starting GC strain (ST), ten CanS colonies (S1–S10) and a colony that has repaired by BIR (B). PCR with primers P1 and P2 detects the starting band and shift to smaller size upon repair into the CAN1 sequences if repair occurs either by GC or BIR. PCR of primers P1 and P3 monitors retention of the distal end of the DSB and is indicative of repair by GC. PCR with primers P1 and P4 monitors repair specific to BIR (see Figure 1).
DSBS are frequently repaired by telomere addition in sgs1Δ exo1Δ cells
As mentioned above, when we analyzed the viablity of sgs1Δ exo1Δ cells, we found that half of the survivors did not have the CanS HphS phenotype indicative of repair by BIR (Figure 4A). Instead, the new survivors were HphS but CanR, suggesting that they might have lost the terminal non-essential portion of Ch V distal to the cut site but failed to restore a functional CAN1 locus.
Sgs1 has previously been shown to inhibit homeologous recombination [27], [29], specifically the formation of translocations between CAN1 and two highly diverged CAN1 homologs, LYP1 and ALP1, on Ch XIV [51]; these rearrangements might be further elevated by the absence of Exo1. Alternatively, given that sgs1Δ exo1Δ severely retards 5′ to 3′ resection, the chromosome end could be stabilized, allowing new telomere addition. To distinguish between these possibilities, we performed pulse field gel electrophoresis (PFGE) on 12 independent CanR HphS colonies, comparing them to the starting strain and a survivor that repaired by BIR (CanS HphS) (Figure 5). The ethidium bromide-stained agarose gel (Figure 5A) shows that the majority of the CanR HphS survivors (lanes 1–11) have a smaller chromosome than the starting (ST) strain or one repaired by BIR (B). (There is no size difference in Ch V size prior to DSB induction and after BIR because the 30 kb of non-essential region distal to the cut site on Ch V is replaced by a duplication of 30 kb from Ch XI.) We confirmed by Southern blot that the band remaining at the original position of Ch V is Ch VIII, which is approximately the same size as Ch V in this strain background (data not shown). One CanR HphS colony (lane 12) increased in size from the original strain. These data indicate the CanR colonies are not due to mutations in a restored CAN1 gene, and are therefore not repaired by BIR nor by NHEJ that could have deleted a small region including HPH. To confirm that none of the CanR HphS colonies were repaired by BIR, we probed with the MCH2 probe that hybridizes proximal to the telomere on Ch XI (Figure 5B). The MCH2 probe hybridized to sequences on Ch XI in every sample, but only to Ch V in the CanS HphS colony that repaired by BIR.
Figure 5. Characterization of CanR HPHS sgs1Δ exo1Δ colonies in the BIR strain by PFGE.
(A) Ethidium bromide-stained agarose gel PFGE gel of sgs1Δ exo1Δ colonies that have repaired the DSB. Included are the ladder (L), starting strain prior to DSB induction (ST), CanS HPHS colony that has repaired by BIR (B), and twelve CanR HPHS colonies (1–12). Arrows indicate additional uncharacterized chromosomal fragments. (B) Southern blot analysis of (5A) by hybridization with a probe for MCH2 that normally lies 6 kb from the telomere on Ch XI (See Figure 1). (C) The blot was stripped and Southern blot analysis was performed by hybridization with a probe for CAN1 that normally lies 33 kb from the telomere on Ch V and is 1 kb proximal to the HO cut site (See Figure 1). (D) The blot was stripped and Southern blot analysis was performed by hybridization with a probe for NPR2 that normally lies 36 kb from the telomere on Ch V and is 4 kb proximal to the HO cut site (See Figure 1). (E) Southern blot analysis was performed on (5D) by hybridization with a probe for PRB1 that normally lies 40 kb from the telomere on Ch V and is 8 kb proximal to the HO cut site (See Figure 1).
To determine what sequences of Ch V were retained in the CanR HphS colonies, we next probed the blot with a CAN1 probe that hybridizes to the donor sequences on Ch XI and just proximal (1 kb) to the cut site on Ch V (Figure 1A, Figure 5C). The CAN1 probe hybridized to sequences on Ch XI in all samples and to Ch V in the starting and BIR strains, but only to three CanR HphS colonies (1, 9 and 12). This result indicates that at least 1 kb of sequence was deleted in the 9 other CanR HphS survivors. To determine approximately how much sequence was deleted in the other CanR HphS colonies we probed the Southern blot with a NPR2 probe that specifically hybridizes to Ch V 4 kb proximal to the cut site (Figure 1A and Figure 5D). In this case, the NPR2 probe hybridized to all CanS samples except lanes 3, 5, 6, and 7. When we probed with PRB1 that hybridizes approximately 9 kb proximal to the cut site on Ch V, the probe hybridized to Ch V in all CanS survivors (Figure 1A and Figure 5E). We also probed the blot with the highly diverged ALP1 and LYP1 sequences on Ch XIV with which CAN1 forms translocations in sgs1Δ cells [51], but these sequences did not hybridize to the novel chromosome in lane 12 (data not shown). We have not explored further the structure of this translocation.
Based on our PFGE and Southern blot analysis we conclude that the great majority of the CanR HphS survivors result in a truncation of Ch V after limited resection. To show if the sequences at the terminus of the truncations are indeed new telomeres, we determined the breakpoint of five independent sgs1Δ exo1Δ CanR HphS repaired colonies by PCR, using a Ch V-specific primer and a telomere-specific primer as previously described [52], [53]. As shown in Figure 6, the presence of a new telomere is indicated by a laddered PCR product. We then sequenced the PCR product using the Ch V-specific primer. As shown in Table 1, all five sgs1Δ exo1Δ CanR HphS colonies have new telomere sequences directly added to the Ch V sequences. Consistent with the PFGE and Southern blot analysis, the breakpoints were not at a uniform location. Based on our results, we hypothesize that in the absence of both Sgs1 and Exo1, a DSB frequently results in a truncated chromosome with newly added telomeres and that these additions can occur at several different sites, often as far as between 1 and 4 kb away from the DSB end. To confirm that these events are telomerase-dependent, we deleted EST2, an essential components of telomerase. As shown in Figure 4A, deletion of EST2 does not affect repair by BIR but eliminates recovery of CanR colonies.
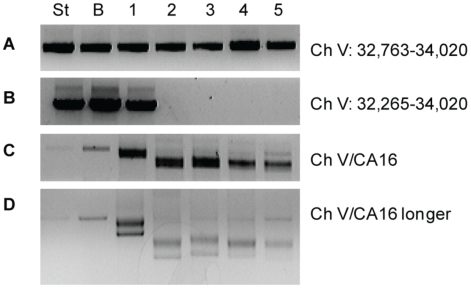
Figure 6. Marking of the breakpoint and detection of de novo telomere formation by PCR in sgs1Δ exo1Δ CanR HphS cells.
From the BIR assay. (A) PCR analysis of a starting strain prior to DSB induction (ST), CanS HphS colony that has repaired by BIR (B), and five CanR HPHS colonies (1–5) with primers that amplify sequences (Ch V 32,763–34,020) approximately 750 bp proximal to the break. (B) PCR with primers that amplify sequences (Ch V 32,265–34,020) approximately 250 bp proximal to the break. (C) PCR with a Ch V-specific primer that amplifies all colonies indicated and primer CA16, a telomere-specific primer. (D) PCR product from 6C ran longer an agarose gel to better display the laddered PCR product indicative of de novo telomere formation in samples 1–5.
Table 1. Sequenced breakpoints in sgs1Δ exo1Δ CANR HPHS repaired colonies.
| CANR Sample | Ch V Breakpoint | Sequence |
| 1 | 32209 | AAATTCCTGTCAAGGACCACCAAAGGTGTGTGTGGTGTGTGGGTG |
| 2 | 32657 | TTGGAGAAACCCAGGTGCCTGGGGTGTGTGGGTG |
| 3 | 32636 | TAAAAACGAAGGGAGGTTCTTAGGTGTGTGGGTGTGGGTGT |
| 4 | 32657 | TTGGAGAAACCCAGGTGCCTGGGGTGTGGGTGTGGTGTG |
| 5 | 32708 | GTTTTTGTATGGTTTGTGGTGCTGGGTGTGGGTG |
We next asked if NHEJ or HR pathways contributed to de novo telomere formation (Figure 4A). Telomere addition was not dependent on NEJ1, which is required for NHEJ. We next deleted RAD51, which is required for both BIR and GC. We confirmed that nearly all BIR is eliminated in sgs1Δ exo1Δ rad51Δ cells but also found a 20% increase in the number of cells with new telomeres. Although overexpression of RAD51 increased the efficiency of BIR it did not suppress new telomere addition (Figure 4A). We then tested if the MRX-associated exonuclease Sae2 plays a role in new telomere addition. Recently, Sae2 and Sgs1 have also been shown to act in parallel telomere processing pathways [54]. Interestingly, when resection is nearly eliminated by deletion of sae2Δ in combination with sgs1Δ exo1Δ, new telomere addition is eliminated and BIR is significantly reduced (Figure 4A). When TEL1 was deleted in combination with sgs1Δ exo1Δ there was no change in levels of BIR or de novo telomeres compared to sgs1Δ exo1Δ cells.
It has previously been seen that sgs1Δ exo1Δ cells are defective in GC when tested for the ability to successfully complete MAT switching [23]. When we tested the viability of sgs1Δ exo1Δ cells in our GC assay there was no discenrable effect on viability. However, when the phenotypes of the viable colonies were examined only 5% were CanS, which is indicative of repair by GC, while the remaining viabile colonies were CanR, consistent with a truncated chromosome (Figure 4B). The drastic decrease in GC is consistent with previously published defects seen in sgs1Δ exo1Δ cells. We analyzed 10 independent CanS colonies by PCR to ascertain if the break was repaired by GC (Figure 4C). In fact, only 5 of the 10 colonies analyzed (samples S2, S3, S4, S5, S8) repaired by GC whereas 4 of the colonies repaired the break by BIR (S1, S6, S7, S10). One colony (S9) had PCR products consistent with repair by both GC and PCR. The use of BIR to repair half of the sgs1Δ exo1Δ colonies is consistent with the failure of these cells to activate the DNA damage checkpoint and thus to enter mitosis in the absence of DSB repair.
To verify that that the DNA damage checkpoint was impaired by the lack of normal 5′ to 3′ resection of the DSB ends we microscopically monitored the length of the cell cycle of individual cells plated on YEP-Gal to induce HO endonuclease, from the time that an unbudded G1 cell formed a bud until the dumbbell-shaped mother-daughter pair formed the next bud [55]. Wild type cells in which the DSB cannot be repaired remain arrested prior to anaphase for approximately 6 cell division times relative to an isogenic strain lacking the HO cleavage site [55]. In contrast, cells of the BIR strain lacking SGS1, EXO1 and RAD51, so that they could not repair the DSB by homologous recombination, show a brief, but significant arrest. These cells extend the cell cycle 1.8 times the length of time of a derivative that lacks the cut site (6.2 h versus 3.5 h). Thus, there is still a brief activation of DSB-induced cell cycle arrest but much shorter than when extensive resection activates Mec1.
As was the case with CanR sgs1Δ exo1Δ colonies found in the BIR assay, the CanR colonies in the GC assay appear to be chromosome truncations with de novo telomere formation. PCR analysis showed that the broken chromosomes were truncated at different points proximal of the DSB (Figure S4). When representative isolates were tested by PCR as mentioned above we found that consistent with new telomere addition there was a laddered PCR product as seen in sgs1Δ exo1Δ cells in the BIR assay (Figure S4).
We conclude that eliminating both Sgs1 and Exo1, by markedly reducing 5′ to 3′ resection and most likely by preventing full activation of the Mec1-dependent DNA damage checkpoint (see Discussion), allows a dramatic increase in new telomere formation, rescuing almost half of all cells suffering a DSB.
Discussion
In this work we show that the RecQ family helicase, Sgs1, and the Exo1 exonuclease negatively regulate BIR to maintain genomic integrity. From the observation that the efficiency of BIR was no greater in sgs1Δ exo1Δ than in a single mutant one might conclude that the helicase/endonuclease (Sgs1-Rmi1-Top3/Dna2) and Exo1 act in the same pathway, but since the sgs1Δ exo1Δ double mutant has such distinctly different phenotypes from sgs1Δ or exo1Δ it is difficult to know precisely why the double mutant does not show an increase in BIR similar to that seen when Rad51 is overexpressed in sgs1Δ or exo1Δ alone. We note also that other proteins responsible for 5′ to 3′ DNA resection, Sae2 and MRX, do not inhibit BIR in the same fashion; but the behavior of sae2Δ or mre11Δ may be explained by their other important roles in other steps in HR [1], [3],[4].
Sgs1 and Exo1 likely do not act in precisely the same way in inhibiting BIR. Sgs1-mediated inhibition of BIR may involve unwinding of a nascent strand invasion D-loop, as demonstrated in vitro for the human Sgs1 homolog, BLM [56], [57]. In vivo it is clear that the Sgs1 helicase can dismantle strand annealings and strand invasions if the heteroduplex DNA contains mismatches [27]–[29]. In meiotic recombination, Sgs1 prevents independent strand invasions of alternative templates [58], [59]. If Sgs1 dismantles heteroduplex DNA, we might expect that increased homology between the DSB end and the donor template would lead to a more stable D-loop that would counteract Sgs1. Increasing the extent of homology from 1.1 kb to ∼3 kb did not significantly change the response of cells to overexpression of Sgs1. It is also possible that Sgs1 inhibits the recruitment of some of the BIR-associated proteins. We note that the effect of deleting Sgs1 or Exo1 is not apparent in a different BIR assay system in a diploid in which nearly all homologous sequences distal to the DSB are deleted [17], [60]; and where there are 100 kb of homologous sequences centromere-proximal to the DSB that can be used to initiate BIR. However, even in this case, many BIR events fail to retain a marker 3 kb proximal to the DSB, suggesting either that more extensive homology increases BIR or that some more proximal sequences are especially favored in initiating BIR [61].
Rather than acting on D-loop stability, Exo1 may act on the assembly of the BIR replication fork. In response to DNA damage or defective checkpoint activation, Exo1 has also been shown to process stalled replication forks and resect nascent strands [62], [63]. The mechanism by which Exo1 interferes with fork integrity is unclear; it may be possible that the intermediate steps at which the BIR replication fork is assembled are an Exo1 substrate. We have previously shown that overexpression of Exo1 increases the rate of resection [45]; this has not been tested for Sgs1 overexpression.
A unifying hypothesis would be that BIR is severely limited if resection of the DSB ends is too extensive. There is a limited amount of Rad51 in the cell (about 3,500 molecules), enough to cover continuously about 10 kb of ssDNA [47]. Although Rad51 will initially form a filament with sequences close to the DSB (including the relevant “CA” sequences that engage in BIR), as resection proceeds the continuous polymerization and depolymerization of Rad51 may leave patches of Rad51 along much of the ssDNA so that by the time BIR is seen, many DSBs will not have a continuous Rad51 filament near the 3′ end to promote the completion of recombination. Thus, even in wild type cells, overexpressing Rad51 would ensure that there would be a functional filament over the CA sequences and BIR would consequently be more efficient. Deletions of Sgs1 or Exo1 would partially suppress the problem by slowing down resection (hence BIR is increased 1.5 times wild type), although we again note that exo1Δ by itself has little visible effect on resection. Overexpression of Rad51 is apparently unable to suppress the consequences of overexpressing Exo1 or Sgs1. It is important to note that Exo1 overexpression is only effective if nuclease activity is preserved; at least some of Exo1's functions in meiosis are independent of nuclease activity (N. Hunter, personal communication; L. Symington, personal communication). Increasing homology in our assay does not suppress these effects but further increases in homology may do so, as noted above.
It is possible that overexpressing Rad51 could ensure that the 3′-ended single-stranded DNA was better protected against degradation over the long time required to enact BIR, as previously suggested [64]. However, we have previously shown that in single-strand annealing where one of the flanking 1-kb homologies is very close to the DSB and the other is exposed only after 6 hr of 5′ to 3′ resection, at least 85% of cells are able to accomplish SSA, which would be impossible if even 1 kb of the 3′-end were degraded in the 6-hr period. Moreover, SSA was equally possible with and without Rad51 [16], arguing that Rad51 did not provide end-protection to the 3′-ended single-strand.
Eliminating both Sgs1 and Exo1 had a marked defect in completing GC but did not impair BIR so severely. Because resection is severely impaired in the sgs1Δ exo1Δ double mutant, it is possible that the more severe defect in GC is attributable to the need to resect more than 1 kb of intervening sequence before the “1” end of homology would be single-stranded (see Figure 1B). However, it is also possible that the difference reflects still another defect in sgs1Δ exo1Δ strains, a failure to activate the DNA damage checkpoint because of a lack of sufficient ssDNA [25], [65]. If mitosis is not arrested, then cells that have an unrepaired DSB will proceed through mitosis. This may lead to the loss of the acentric fragment, as we have shown in other assays [66], so that only the centromere-proximal DSB end will be inherited. This situation is not fatal for BIR, which only uses homology on that side of the DSB; indeed previous studies [17], [67] have shown that BIR may actually increase in a checkpoint-deficient situation whereas GC will be defective. Thus, even when GC should be possible, half of the HR outcomes of the sgs1Δ exo1Δ GC assay proved to be BIR events.
Strikingly, Sgs1and Exo1 also redundantly inhibit new telomere formation. In a previous study [12], when an HO-induced DSB was generated in a rad52Δ strain that could not carry out recombination but had apparently normal 5′ to 3′ resection, only about 1% of cells created new telomeres, and this was only in a situation where a “seed” of T2G4 telomere sequences was located centromere-proximal to the DSB. In the absence of the T2G4 repeats, new telomeres arose less than 0.1% of the time. The remarkably high level of new telomere formation (up to 50% of all cells) must be attributable to the elimination of vigorous resection in the double mutant strain, but it is also likely that the failure to activate the Mec1 DNA damage checkpoint also plays a key role. Recently, Makovets and Blackburn [68] have shown that the Pif1 helicase, which antagonizes new telomere formation [69], is phosphorylated in a Mec1-dependent fashion; hence if sgs1Δ exo1Δ block resection and that prevents Mec1 activation, new telomeres should increase. However, in the assay used by Makovets and Blackburn [68] the level of new telomeres added near an HO endonuclease-induced DSB was only about 2%. Moreover, Chung et al [60] also find that new telomere addition is much less efficient in cells lacking MEC1 compared to sgs1Δ exo1Δ cells. Hence, it is likely that the 40–50% level of de novo telomere formation we find reflects both the failure to activate Pif1 when the checkpoint is not strongly activated and the severe block on resection itself.
Apparently de novo telomere formation does not require the recruitment of the MRX-Tel1 complex, as a tel1Δ mutant does not affect the formation of new telomeres in an sgs1Δ exo1Δ strain. When resection is blocked by deletion of SAE2 in sgs1Δ exo1Δ cells, new telomeres are absent. The fact that new telomeres were added as far as 4 kb from the DSB site indicates that there is a residual resection activity that–over a period of perhaps many hours–can chew away the chromosome end and expose sites suitable for new telomere addition. However, we show that the MRX-asociated endonuclease SAE2 is required for de novo telomere formation.
In this work we have expanded our understanding of the genetic relationships of factors that negatively regulate BIR. Furthermore, we have provided evidence for a novel repair pathway that is redundantly impaired by Sgs1 and Exo1. Understanding the interplay of these factors in response to DNA damage and uncovering the molecular details of signaling between them to maintain genomic integrity will be an area of much future research.
Materials and Methods
Strains and plasmids
The wild type JRL346 was derived from JRL092 [8] by first disrupting the LEU2 marker with a leu2::hisG construct from pNKY85 [70] to generate strain JRL187. The HMRa-stk gene was then knocked out with an hmr::ADE3 fragment generated by PCR with mixed oligos to generate JRL346. All strains used to study BIR are isogenic to JRL346 and were created by standard gene disruption methods and confirmed by PCR unless otherwise stated [71]. In order to generate an assay to study GC that is isogenic with JRL346, an HOcs-HPH cassette [8] was integrated into Ch V between nucleotides 31,644 and 32,020, resulting in a truncation of the CAN1 ORF at nucleotide 1,146 to create strain JRL017 (CL11-7 can1,1-1446::HOcs::HPH). JRL017 was then modified by transforming in a hphmx::URA3 “marker swap” cassette [72] to generate JRL472 (CL11-7 can1,1-1446::HOcs::URA3::AVT2). To introduce another 2,404 bp of homology to the donor, the can1,1-1446::HOcs::URA3::AVT2 region with Ch V sequences 29,146 to 32,976 was amplified from JRL472 and integrated distal to the HO cut site into Ch V in strain JRL346 to generate JRL475 (can1,1-1446::HOcs::URA3::AVT2 ykl215c::leu2::hisG::can1DEL1-289::AVT2). As a result, there are Ch V sequences 33,177–32,020 shared between the donor and sequences proximal to the break, Ch V sequences 31,644–29,240 shared between the donor and sequences distal to the break and a 376 bp gap of homology. All mutant strains were created by standard gene disruption methods and confirmed by PCR. Plasmid pSJ5 was constructed by subcloning a XhoI-NotI fragment containing the RAD51 ORF under the PGK promoter form pNSU256 [47] into pRS314 [73].
The wild type JRL346 was derived from JRL092 [8] by first disrupting the LEU2 marker with a leu2::hisG construct from pNKY85 [70] to generate strain JRL187. The HMRa-stk gene was then knocked out with an hmr::ADE3 fragment generated by PCR with mixed oligos to generate JRL346. All strains used to study BIR are isogenic to JRL346 and were created by standard gene disruption methods and confirmed by PCR unless otherwise stated [71]. In order to generate an assay to study GC that is isogenic with JRL346, an HOcs-HPH cassette [8] was integrated into Ch V between nucleotides 31,644 and 32,020, resulting in a truncation of the CAN1 ORF at nucleotide 1,146 to create strain JRL017 (CL11-7 can1,1-1446::HOcs::HPH). JRL017 was then modified by transforming in a hphmx::URA3 “marker swap” cassette [72] to generate JRL472 (CL11-7 can1,1-1446::HOcs::URA3::AVT2). To introduce another 2,404 bp of homology to the donor, the can1,1-1446::HOcs::URA3::AVT2 region with Ch V sequences 29,146 to 32,976 was amplified from JRL472 and integrated distal to the HO cut site into Ch V in strain JRL346 to generate JRL475 (can1,1-1446::HOcs::URA3::AVT2 ykl215c::leu2::hisG::can1DEL1-289::AVT2). As a result, there are Ch V sequences 33,177–32,020 shared between the donor and sequences proximal to the break, Ch V sequences 31,644–29,240 shared between the donor and sequences distal to the break and a 376 bp gap of homology. All mutant strains were created by standard gene disruption methods and confirmed by PCR. Plasmid pSJ5 was constructed by subcloning a XhoI-NotI fragment containing the RAD51 ORF under the PGK promoter form pNSU256 [47] into pRS314 [73].
Viability measurements
Logarithmically growing cells grown in YEP+2% Raffinose, or the appropriate drop-out media +2% Raffinose, were plated on either YEPD or YEP-Gal, and grown into colonies. Colonies were counted and were then replica plated onto plates containing either canavanine or hygromycin to confirm repair occurred by BIR. Experiments were performed at least 5 times for each strain unless otherwise indicated. To determine the statistical significance between strains the student's t-test was used (paired, two-tailed, n≥4 for all strains).
HO induction and measurement of kinetics of DSB repair
Strains were grown in YEP+2% Raffinose to a cell density of 3×10e6 to 1×10e7 cells/mL. A 50 mL aliquot of cells was removed for the zero time point. Freshly made galactose was added to final concentration of 2% to induce HO expression. Cell aliquots were taken at the indicated time points throughout the time course.
DNA analysis
PCR analysis of BIR was performed as previously described [8]. Briefly, we monitor the initiation of new BIR DNA synthesis using a PCR assay in which one primer is specific to Ch V and the other primer is specific to the donor sequence on Ch XI. Once a covalent molecule is formed, corresponding to the first 242 bp of new DNA synthesis, we see PCR product. At least three PCR reactions from three different experiments were performed for wild type, sgs1Δ and exo1Δ strains. For all other strains tested, at least three PCR reactions from two experiments were performed. The technical replicates from each biological experiment was first averaged and then the technical averages were averaged among the two experiments to obtain a biological average. Data were graphed as the biological averages normalized to the maximum product obtained by amplifying DNA from a strain that has repaired the DSB by BIR. Error bars represent the data range between the biological averages.
Repair is also measured by Southern blot that detects approximately the first 3 kb of new DNA synthesis was performed as previously described [8]. The analysis by Southern blot or pulse-field (CHEF) gel electrophoresis followed by Southern blot was performed as described [8] using the probes indicated in Figure 1. The breakpoints and sequences of sgs1Δ exo1Δ CanR HphS repaired colonies were performed as described [52], [53].
Supporting Information
The helicase-domain of Sgs1 is required to inhibit BIR. (A) In this assay to study BIR, an HO cut site is integrated into an ectopically located LEU2 gene on Chromosome V (Ch V) in which the 3′ end portion of the gene is deleted, the remaining sequences are represented as LE. The donor sequences are the endogenous LEU2 gene on Ch III. Repair of the DSB only occurs by BIR resulting in duplication of the LEU2 gene and the distal sequences on Ch III. (B) Efficiency of BIR as measured by viability following a DSB in wild type (WT), sgs1Δ, or sgs1Δ cells complemented with a plasmid expressing the sgs1-hd allele (psgs1-hd).
(0.21 MB TIF)
Overexpression of EXO1 inhibits BIR. Kinetics of repair are shown for PCR assays of BIR induced in cycling wild type (WT) and GAL::EXO1 cells. Data are the mean ±data range.
(0.13 MB TIF)
The efficiency of BIR is not increased in sgs1Δ exo1Δ cells. Kinetics of repair are shown for PCR assays of BIR induced in cycling wild type (WT) and sgs1Δ exo1Δ cells. Data are the mean ± data range for two experiments.
(0.16 MB TIF)
Marking of the breakpoint and detection of de novo telomere formation by PCR in sgs1Δ exo1Δ CANR survivors from the GC assay. (A) PCR analysis of a starting strain prior to DSB induction (ST), CanS colony that has repaired by HR (S), and ten CanR colonies (R1–R10) with primers that amplify sequences (Ch V 39,744–42,157) approximately 7.7 kb proximal to the break. (B) PCR with primers that amplify sequences (Ch V 34,271–37,985) approximately 2.2 kb proximal to the break. (C) PCR with primers that amplify sequences (Ch V 33,007–35,272) approximately 1 kb proximal to the break. (D) PCR with primers that amplify sequences (Ch V 32,265–34,020) approximately 250 bp proximal to the break. (E) PCR with a Ch V-specific primer that amplifies all colonies indicated and primer CA16, a telomere-specific primer.
(1.41 MB TIF)
The effect of varied mutants on the efficiency of BIR. The viability of cells that could repair a DSB by BIR as shown in Figure 1A was compared by plating cells on YEP-galactose to induce expression of HO endonuclease and on YEPD, as described in Materials and Methods.
(0.07 MB DOCX)
Acknowledgments
We are grateful to Ian Hickson, Loraine Symington, and David T. Weaver for the gift of plasmids and to Grzegorz Ira and Anna Malkova for sharing results prior to publication.
Footnotes
The authors have declared that no competing interests exist.
NIH grants GM20056 and GM76020. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.San Filippo J, Sung P, Klein H. Mechanism of eukaryotic homologous recombination. Annu Rev Biochem. 2008;77:229–257. doi: 10.1146/annurev.biochem.77.061306.125255. [DOI] [PubMed] [Google Scholar]
- 2.Agmon N, Pur S, Liefshitz B, Kupiec M. Analysis of repair mechanism choice during homologous recombination. Nucleic Acids Res. 2009;37:5081–5092. doi: 10.1093/nar/gkp495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krogh BO, Symington LS. Recombination proteins in yeast. Annu Rev Genet. 2004;38:233–271. doi: 10.1146/annurev.genet.38.072902.091500. [DOI] [PubMed] [Google Scholar]
- 4.Pâques F, Haber JE. Multiple pathways of recombination induced by double-strand breaks in Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 1999;63:349–404. doi: 10.1128/mmbr.63.2.349-404.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Llorente B, Smith CE, Symington LS. Break-induced replication: what is it and what is it for? Cell Cycle. 2008;7:859–864. doi: 10.4161/cc.7.7.5613. [DOI] [PubMed] [Google Scholar]
- 6.McEachern MJ, Haber JE. Break-induced replication and recombinational telomere elongation in yeast. Annu Rev Biochem. 2006;75:111–135. doi: 10.1146/annurev.biochem.74.082803.133234. [DOI] [PubMed] [Google Scholar]
- 7.Chen C, Kolodner RD. Gross chromosomal rearrangements in Saccharomyces cerevisiae replication and recombination defective mutants. Nat Genet. 1999;23:81–85. doi: 10.1038/12687. [DOI] [PubMed] [Google Scholar]
- 8.Lydeard JR, Jain S, Yamaguchi M, Haber JE. Break-induced replication and telomerase-independent telomere maintenance require Pol32. Nature. 2007;448:820–823. doi: 10.1038/nature06047. [DOI] [PubMed] [Google Scholar]
- 9.Lydeard JR, Lipkin-Moore Z, Sheu YJ, Stillman B, Burgers PM, et al. Break-induced replication requires all essential DNA replication factors except those specific for Pre-RC assembly. Genes Dev. in press doi: 10.1101/gad.1922610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saleh-Gohari N, Bryant HE, Schultz N, Parker KM, Cassel TN, et al. Spontaneous homologous recombination is induced by collapsed replication forks that are caused by endogenous DNA single-strand breaks. Mol Cell Biol. 2005;25:7158–7169. doi: 10.1128/MCB.25.16.7158-7169.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murray AW, Claus TE, Szostak JW. Characterization of two telomeric DNA processing reactions in Saccharomyces cerevisiae. Mol Cell Biol. 1988;8:4642–4650. doi: 10.1128/mcb.8.11.4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kramer KM, Haber JE. New telomeres in yeast are initiated with a highly selected subset of TG1-3 repeats. Genes Dev. 1993;7:2345–2356. doi: 10.1101/gad.7.12a.2345. [DOI] [PubMed] [Google Scholar]
- 13.Pennaneach V, Putnam CD, Kolodner RD. Chromosome healing by de novo telomere addition in Saccharomyces cerevisiae. Mol Microbiol. 2006;59:1357–1368. doi: 10.1111/j.1365-2958.2006.05026.x. [DOI] [PubMed] [Google Scholar]
- 14.Schulz VP, Zakian VA. The saccharomyces PIF1 DNA helicase inhibits telomere elongation and de novo telomere formation. Cell. 1994;76:145–155. doi: 10.1016/0092-8674(94)90179-1. [DOI] [PubMed] [Google Scholar]
- 15.Davis AP, Symington LS. RAD51-dependent break-induced replication in yeast. Mol Cell Biol. 2004;24:2344–2351. doi: 10.1128/MCB.24.6.2344-2351.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jain S, Sugawara N, Lydeard J, Vaze M, Tanguy Le Gac N, et al. A recombination execution checkpoint regulates the choice of homologous recombination pathway during DNA double-strand break repair. Genes Dev. 2009;23:291–303. doi: 10.1101/gad.1751209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malkova A, Naylor ML, Yamaguchi M, Ira G, Haber JE. RAD51-dependent break-induced replication differs in kinetics and checkpoint responses from RAD51-mediated gene conversion. Mol Cell Biol. 2005;25:933–944. doi: 10.1128/MCB.25.3.933-944.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Falck J, Coates J, Jackson SP. Conserved modes of recruitment of ATM, ATR and DNA-PKcs to sites of DNA damage. Nature. 2005;434:605–611. doi: 10.1038/nature03442. [DOI] [PubMed] [Google Scholar]
- 19.Nakada D, Matsumoto K, Sugimoto K. ATM-related Tel1 associates with double-strand breaks through an Xrs2-dependent mechanism. Genes Dev. 2003;17:1957–1962. doi: 10.1101/gad.1099003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Usui T, Ogawa H, Petrini JH. A DNA damage response pathway controlled by Tel1 and the Mre11 complex. Mol Cell. 2001;7:1255–1266. doi: 10.1016/s1097-2765(01)00270-2. [DOI] [PubMed] [Google Scholar]
- 21.D'Amours D, Jackson SP. The yeast Xrs2 complex functions in S phase checkpoint regulation. Genes Dev. 2001;15:2238–2249. doi: 10.1101/gad.208701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu Z, Chung WH, Shim EY, Lee SE, Ira G. Sgs1 helicase and two nucleases Dna2 and Exo1 resect DNA double-strand break ends. Cell. 2008;134:981–994. doi: 10.1016/j.cell.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mimitou EP, Symington LS. Sae2, Exo1 and Sgs1 collaborate in DNA double-strand break processing. Nature. 2008;455:770–774. doi: 10.1038/nature07312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gravel S, Chapman JR, Magill C, Jackson SP. DNA helicases Sgs1 and BLM promote DNA double-strand break resection. Genes Dev. 2008;22:2767–2772. doi: 10.1101/gad.503108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ira G, Pellicioli A, Balijja A, Wang X, Fiorani S, et al. DNA end resection, homologous recombination and DNA damage checkpoint activation require CDK1. Nature. 2004;431:1011–1017. doi: 10.1038/nature02964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cejka P, Kowalczykowski SC. The full-length Saccharomyces cerevisiae Sgs1 protein is a vigorous DNA helicase that preferentially unwinds holliday junctions. J Biol Chem. 2010;285:8290–8301. doi: 10.1074/jbc.M109.083196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Myung K, Datta A, Chen C, Kolodner RD. SGS1, the Saccharomyces cerevisiae homologue of BLM and WRN, suppresses genome instability and homeologous recombination. Nat Genet. 2001;27:113–116. doi: 10.1038/83673. [DOI] [PubMed] [Google Scholar]
- 28.Spell RM, Jinks-Robertson S. Examination of the roles of Sgs1 and Srs2 helicases in the enforcement of recombination fidelity in Saccharomyces cerevisiae. Genetics. 2004;168:1855–1865. doi: 10.1534/genetics.104.032771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sugawara N, Goldfarb T, Studamire B, Alani E, Haber JE. Heteroduplex rejection during single-strand annealing requires Sgs1 helicase and mismatch repair proteins Msh2 and Msh6 but not Pms1. Proc Natl Acad Sci U S A. 2004;101:9315–9320. doi: 10.1073/pnas.0305749101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu L, Hickson ID. The Bloom's syndrome helicase suppresses crossing over during homologous recombination. Nature. 2003;426:870–874. doi: 10.1038/nature02253. [DOI] [PubMed] [Google Scholar]
- 31.Ira G, Malkova A, Liberi G, Foiani M, Haber JE. Srs2 and Sgs1-Top3 suppress crossovers during double-strand break repair in yeast. Cell. 2003;115:401–411. doi: 10.1016/s0092-8674(03)00886-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lo YC, Paffett KS, Amit O, Clikeman JA, Sterk R, et al. Sgs1 regulates gene conversion tract lengths and crossovers independently of its helicase activity. Mol Cell Biol. 2006;26:4086–4094. doi: 10.1128/MCB.00136-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malkova A, Ivanov EL, Haber JE. Double-strand break repair in the absence of RAD51 in yeast: a possible role for break-induced replication. Proc Natl Acad Sci U S A. 1996;93:7131–7136. doi: 10.1073/pnas.93.14.7131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mullen JR, Kaliraman V, Brill SJ. Bipartite structure of the SGS1 DNA helicase in Saccharomyces cerevisiae. Genetics. 2000;154:1101–1114. doi: 10.1093/genetics/154.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ira G, Haber JE. Characterization of RAD51-independent break-induced replication that acts preferentially with short homologous sequences. Mol Cell Biol. 2002;22:6384–6392. doi: 10.1128/MCB.22.18.6384-6392.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stith CM, Sterling J, Resnick MA, Gordenin DA, Burgers PM. Flexibility of eukaryotic Okazaki fragment maturation through regulated strand displacement synthesis. J Biol Chem. 2008;283:34129–34140. doi: 10.1074/jbc.M806668200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mankouri HW, Ngo HP, Hickson ID. Esc2 and Sgs1 Act in Functionally Distinct Branches of the Homologous Recombination Repair Pathway in S. cerevisiae. Mol Biol Cell. 2009 doi: 10.1091/mbc.E08-08-0877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ip SC, Rass U, Blanco MG, Flynn HR, Skehel JM, et al. Identification of Holliday junction resolvases from humans and yeast. Nature. 2008;456:357–361. doi: 10.1038/nature07470. [DOI] [PubMed] [Google Scholar]
- 39.Collins SR, Miller KM, Maas NL, Roguev A, Fillingham J, et al. Functional dissection of protein complexes involved in yeast chromosome biology using a genetic interaction map. Nature. 2007;446:806–810. doi: 10.1038/nature05649. [DOI] [PubMed] [Google Scholar]
- 40.Ii M, Brill SJ. Roles of SGS1, MUS81, and RAD51 in the repair of lagging-strand replication defects in Saccharomyces cerevisiae. Curr Genet. 2005;48:213–225. doi: 10.1007/s00294-005-0014-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tong AH, Evangelista M, Parsons AB, Xu H, Bader GD, et al. Systematic genetic analysis with ordered arrays of yeast deletion mutants. Science. 2001;294:2364–2368. doi: 10.1126/science.1065810. [DOI] [PubMed] [Google Scholar]
- 42.Signon L, Malkova A, Naylor ML, Klein H, Haber JE. Genetic requirements for RAD51- and RAD54-independent break-induced replication repair of a chromosomal double-strand break. Mol Cell Biol. 2001;21:2048–2056. doi: 10.1128/MCB.21.6.2048-2056.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee K, Zhang Y, Lee SE. Saccharomyces cerevisiae ATM orthologue suppresses break-induced chromosome translocations. Nature. 2008;454:543–546. doi: 10.1038/nature07054. [DOI] [PubMed] [Google Scholar]
- 44.Mankouri HW, Craig TJ, Morgan A. SGS1 is a multicopy suppressor of srs2: functional overlap between DNA helicases. Nucleic Acids Res. 2002;30:1103–1113. doi: 10.1093/nar/30.5.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee SE, Bressan DA, Petrini JH, Haber JE. Complementation between N-terminal Saccharomyces cerevisiae mre11 alleles in DNA repair and telomere length maintenance. DNA Repair (Amst) 2002;1:27–40. doi: 10.1016/s1568-7864(01)00003-9. [DOI] [PubMed] [Google Scholar]
- 46.Fishman-Lobell J, Rudin N, Haber JE. Two alternative pathways of double-strand break repair that are kinetically separable and independently modulated. Mol Cell Biol. 1992;12:1292–1303. doi: 10.1128/mcb.12.3.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sugawara N, Wang X, Haber JE. In vivo roles of Rad52, Rad54, and Rad55 proteins in Rad51-mediated recombination. Mol Cell. 2003;12:209–219. doi: 10.1016/s1097-2765(03)00269-7. [DOI] [PubMed] [Google Scholar]
- 48.Johnson R, Borde V, Neale MJ, Bishop-Bailey A, North M, et al. Excess single-stranded DNA inhibits meiotic double-strand break repair. PLoS Genet. 2007;3:e223. doi: 10.1371/journal.pgen.0030223. doi: 10.1371/journal.pgen.0030223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Milne GT, Ho T, Weaver DT. Modulation of Saccharomyces cerevisiae DNA double-strand break repair by SRS2 and RAD51. Genetics. 1995;139:1189–1199. doi: 10.1093/genetics/139.3.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mantiero D, Clerici M, Lucchini G, Longhese MP. Dual role for Saccharomyces cerevisiae Tel1 in the checkpoint response to double-strand breaks. EMBO Rep. 2007;8:380–387. doi: 10.1038/sj.embor.7400911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schmidt KH, Kolodner RD. Suppression of spontaneous genome rearrangements in yeast DNA helicase mutants. Proc Natl Acad Sci U S A. 2006;103:18196–18201. doi: 10.1073/pnas.0608566103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Motegi A, Myung K. Measuring the rate of gross chromosomal rearrangements in Saccharomyces cerevisiae: A practical approach to study genomic rearrangements observed in cancer. Methods. 2007;41:168–176. doi: 10.1016/j.ymeth.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 53.Smith S, Hwang JY, Banerjee S, Majeed A, Gupta A, et al. Mutator genes for suppression of gross chromosomal rearrangements identified by a genome-wide screening in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 2004;101:9039–9044. doi: 10.1073/pnas.0403093101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bonetti D, Martina M, Clerici M, Lucchini G, Longhese MP. Multiple pathways regulate 3′ overhang generation at S. cerevisiae telomeres. Mol Cell. 2009;35:70–81. doi: 10.1016/j.molcel.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 55.Dotiwala F, Haase J, Arbel-Eden A, Bloom K, Haber JE. The yeast DNA damage checkpoint proteins control a cytoplasmic response to DNA damage. Proc Natl Acad Sci U S A. 2007;104:11358–11363. doi: 10.1073/pnas.0609636104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bachrati CZ, Borts RH, Hickson ID. Mobile D-loops are a preferred substrate for the Bloom's syndrome helicase. Nucleic Acids Res. 2006;34:2269–2279. doi: 10.1093/nar/gkl258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hu Y, Raynard S, Sehorn MG, Lu X, Bussen W, et al. RECQL5/Recql5 helicase regulates homologous recombination and suppresses tumor formation via disruption of Rad51 presynaptic filaments. Genes Dev. 2007;21:3073–3084. doi: 10.1101/gad.1609107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jessop L, Lichten M. Mus81/Mms4 endonuclease and Sgs1 helicase collaborate to ensure proper recombination intermediate metabolism during meiosis. Mol Cell. 2008;31:313–323. doi: 10.1016/j.molcel.2008.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oh SD, Lao JP, Hwang PY, Taylor AF, Smith GR, et al. BLM ortholog, Sgs1, prevents aberrant crossing-over by suppressing formation of multichromatid joint molecules. Cell. 2007;130:259–272. doi: 10.1016/j.cell.2007.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chung WH, Zhu Z, Papusha A, Malkova A, Ira G. Defective resection at DNA double-strand breaks leads to de novo telomere formation and enhances gene targeting. PLoS Genet. 2010;6:e948. doi: 10.1371/journal.pgen.1000948. doi: 10.1371/journal.pgen.1000948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Malkova A, Signon L, Schaefer CB, Naylor ML, Theis JF, et al. RAD51-independent break-induced replication to repair a broken chromosome depends on a distant enhancer site. Genes Dev. 2001;15:1055–1060. doi: 10.1101/gad.875901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cotta-Ramusino C, Fachinetti D, Lucca C, Doksani Y, Lopes M, et al. Exo1 processes stalled replication forks and counteracts fork reversal in checkpoint-defective cells. Mol Cell. 2005;17:153–159. doi: 10.1016/j.molcel.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 63.Segurado M, Diffley JF. Separate roles for the DNA damage checkpoint protein kinases in stabilizing DNA replication forks. Genes Dev. 2008;22:1816–1827. doi: 10.1101/gad.477208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zierhut C, Diffley JF. Break dosage, cell cycle stage and DNA replication influence DNA double strand break response. EMBO J. 2008 doi: 10.1038/emboj.2008.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aylon Y, Liefshitz B, Kupiec M. The CDK regulates repair of double-strand breaks by homologous recombination during the cell cycle. EMBO J. 2004;23:4868–4875. doi: 10.1038/sj.emboj.7600469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kaye JA, Melo JA, Cheung SK, Vaze MB, Haber JE, et al. DNA breaks promote genomic instability by impeding proper chromosome segregation. Curr Biol. 2004;14:2096–2106. doi: 10.1016/j.cub.2004.10.051. [DOI] [PubMed] [Google Scholar]
- 67.Galgoczy DJ, Toczyski DP. Checkpoint adaptation precedes spontaneous and damage-induced genomic instability in yeast. Mol Cell Biol. 2001;21:1710–1718. doi: 10.1128/MCB.21.5.1710-1718.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Makovets S, Blackburn EH. DNA damage signalling prevents deleterious telomere addition at DNA breaks. Nat Cell Biol. 2009;11:1383–1386. doi: 10.1038/ncb1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Myung K, Chen C, Kolodner RD. Multiple pathways cooperate in the suppression of genome instability in Saccharomyces cerevisiae. Nature. 2001;411:1073–1076. doi: 10.1038/35082608. [DOI] [PubMed] [Google Scholar]
- 70.Alani E, Cao L, Kleckner N. A method for gene disruption that allows repeated use of URA3 selection in the construction of multiply disrupted yeast strains. Genetics. 1987;116:541–545. doi: 10.1534/genetics.112.541.test. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eissenberg JC, Ayyagari R, Gomes XV, Burgers PM. Mutations in yeast proliferating cell nuclear antigen define distinct sites for interaction with DNA polymerase delta and DNA polymerase epsilon. Mol Cell Biol. 1997;17:6367–6378. doi: 10.1128/mcb.17.11.6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Voth WP, Jiang YW, Stillman DJ. New ‘marker swap’ plasmids for converting selectable markers on budding yeast gene disruptions and plasmids. Yeast. 2003;20:985–993. doi: 10.1002/yea.1018. [DOI] [PubMed] [Google Scholar]
- 73.Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The helicase-domain of Sgs1 is required to inhibit BIR. (A) In this assay to study BIR, an HO cut site is integrated into an ectopically located LEU2 gene on Chromosome V (Ch V) in which the 3′ end portion of the gene is deleted, the remaining sequences are represented as LE. The donor sequences are the endogenous LEU2 gene on Ch III. Repair of the DSB only occurs by BIR resulting in duplication of the LEU2 gene and the distal sequences on Ch III. (B) Efficiency of BIR as measured by viability following a DSB in wild type (WT), sgs1Δ, or sgs1Δ cells complemented with a plasmid expressing the sgs1-hd allele (psgs1-hd).
(0.21 MB TIF)
Overexpression of EXO1 inhibits BIR. Kinetics of repair are shown for PCR assays of BIR induced in cycling wild type (WT) and GAL::EXO1 cells. Data are the mean ±data range.
(0.13 MB TIF)
The efficiency of BIR is not increased in sgs1Δ exo1Δ cells. Kinetics of repair are shown for PCR assays of BIR induced in cycling wild type (WT) and sgs1Δ exo1Δ cells. Data are the mean ± data range for two experiments.
(0.16 MB TIF)
Marking of the breakpoint and detection of de novo telomere formation by PCR in sgs1Δ exo1Δ CANR survivors from the GC assay. (A) PCR analysis of a starting strain prior to DSB induction (ST), CanS colony that has repaired by HR (S), and ten CanR colonies (R1–R10) with primers that amplify sequences (Ch V 39,744–42,157) approximately 7.7 kb proximal to the break. (B) PCR with primers that amplify sequences (Ch V 34,271–37,985) approximately 2.2 kb proximal to the break. (C) PCR with primers that amplify sequences (Ch V 33,007–35,272) approximately 1 kb proximal to the break. (D) PCR with primers that amplify sequences (Ch V 32,265–34,020) approximately 250 bp proximal to the break. (E) PCR with a Ch V-specific primer that amplifies all colonies indicated and primer CA16, a telomere-specific primer.
(1.41 MB TIF)
The effect of varied mutants on the efficiency of BIR. The viability of cells that could repair a DSB by BIR as shown in Figure 1A was compared by plating cells on YEP-galactose to induce expression of HO endonuclease and on YEPD, as described in Materials and Methods.
(0.07 MB DOCX)