Abstract
The salivary glands often are severely and permanently damaged by therapeutic irradiation for cancer of the head and neck. The markedly reduced quantity and quality of saliva results in greatly increased susceptibility to dental caries and infection of the oral mucosa and alveolar bone. Recently, subcapsular injection of cultured mouse salivary gland cells has achieved a significant degree of regeneration in a previously irradiated mouse salivary gland. However, the recovery was limited to one lobule. Here we describe a method of delivering donor rat salivary gland cells via the main duct that distributes several thousand cells throughout the recipient rat’s salivary gland. The donated cells exhibited the cytodifferentiation of the structures in which they lodged, i.e., acini, granular convoluted tubules and the several types of duct. This method may facilitate the simultaneous functional recovery of almost all of the lobules of irradiated rat salivary glands.
Keywords: differentiation, radiation, rat, regeneration, salivary glands, stem cells
Salivary glands in the path of therapeutic irradiation for head and neck cancer often are severely damaged despite recent modifications in radiation delivery, especially when the patient is unable to tolerate drugs that offer partial protection of normal tissues (reviewed by Redman 2008). This results in chronic oral mucositis and increased susceptibility to periodontitis and dental caries. Without meticulous home and professional dental care, rampant caries ensues, and the sequellae of periapical infections and extractions carry a high risk of precipitating osteonecrosis. It was suggested (Coppes et al. 2001, Nagler 2002, Redman 2008) that the reason for the poor functional recovery of salivary glands subjected to greater than 35 Gray of ionizing radiation is that the mature acinar and ductal cells and their progenitors are either destroyed or rendered incapable of proliferation. It follows that the most promising approach to restoring function in the damaged glands would be to promote regeneration via injection of donor salivary gland cells or the patient’s marrow stem cells. The stem cells have a propensity to migrate into badly damaged tissues and differentiate into functioning replacement cells. Thus, simply increasing the number of the otherwise uncommon stem cells in the circulation might boost the number migrating into irradiated salivary glands. This has been tried in mouse (Lombaert et al. 2006). Some of the stem cells mobilized by granulocyte-stimulating factor indeed did lodge in the irradiated glands and seemed to partially ameliorate the damage, but the result otherwise was discouraging. The stem cells remained in the stroma and did not differentiate into acinar or other epithelial cells. Subsequently, however, Lombaert and colleagues (2008) cultered dissociated male mouse salivary gland cells which after 3 to 5 days became enriched with ductal progenitor or stem cells, identified by immunohistochemistry (IHC) of markers such as c-Kit, Muhashi-1 and SCA-1. Further enrichment was achieved by flow cytometry. When cells dissocciated from such a cultue were injected into an irradiated female mouse salivary gland, they proliferated and differentiated to such an extent that an entire lobule underwent significant structural and functional restoration. In situ hybridization for the Y-chromosome revealed that most of the cells participating in the restoration were male, i.e,, donor cells. This is a landmark achievement, but as they noted, functional restoration of larger glands will require a much wider distribution of replacement cells than was achieved by injecting them though the capsule. We report here just such a wide distribution and retention of salivary gland cells from donor rats when these were infused up the ductal trees of normal salivary glands of recipient rats.
Materials and methods
The following studies were approved by the appropriate research review bodies of the Washington, DC, Department of Veterans Affairs Medical Center. The idea to infuse donor cells into salivary glands via the ductal system was based on an observation (by RSR) during experiments by Baccaglini et al. (2001) in which a cationic liposome vector was being used for gene insertion into rat salivary gland cells. The vector was infused up the ductal tree via a cannula inserted into the oral orifice of the main duct. When more fluid was infused than the amount that was required to distribute the vector to all cells bordering on lumens, scattered foci of inflammation indicated that some of the fluid had broken through the epithelial barrier into the stroma. This suggested that deliberately infusing too much fluid carrying dispersed cells might promote the retention of donor cells throughout the recipient gland.
Experimental animals
Sprague-Dawley rats (Rattus norvegicus albinus, certified pathogen free) were obtained from Harlan, Indianapolis, IN, and housed in the Animal Research Facility of the Washington, DC, Department of Veterans Affiars Medical Center. They were kept two each in plastic cages with shredded corncob bedding in a quiet room with controlled temperature and humidity, with lights on at 0700 and off at 1900 h and with a commercial pelleted diet and water available ad libitum. For each infusion experiment, three male rats age 40 to 45 days were the donors and three to four female rats age 55 to 65 days (195 to 210 g body weight) were the recipients. The strategy was to identify male salivary gland cells that lodged in the female salivary glands by in situ hybridization using a Y-chromosome probe.
Preparation of dispersed submandibular cells
Male rats were killed by inhalation of Halothane (2-bromo-2-chloro-1,1,1-trifluorethane; Sigma-Aldrich, St. Louis, MO) and, using aseptic technique, the submandibular glands were excised, separated from the sublingual glands which lie in the same capsule, and cleaned of adipose and connective tissues. A slurry of mostly acinar cells was prepared from the six glands as described by Quissell and Redman (1979). The dispersion medium was composed of a modified (no calcium or magnesium) balanced Hanks’ solution (with 15 mM Hepes), 50–75 units/mL chromatographically purified collagenase (CLSPA: Worthington Biochemical, Lakewood, NJ), 1.0 mg/mL hyaluronidase (Sigma-Aldrich), 1.0 mg/mL Fungizone (Amphoteracin B; Invitrogen, Grand Island, NY), and 100 μg/mL Gentamycin sulfate (Sigma-Aldrich). The pH was adjusted to 7.4 with Na3PO4. The 2.0% bovine serum albumin (BSA) used in the 1979 protocol for cell culture was omitted. The wash medium consisted of the dispersion medium without the collagenase and hyaluronidase and with 0.001 M CaCl2 added. All chemicals were ACS or reagent grade. Twenty mL of the dispersion medium in a siliconized (Sigmacote®, Sigma-Aldrich, St. Louis, MO) 125 mL Erlenmeyer flask and 100 mL of the wash medium in a non-siliconized 125 mL Erlenmeyer flask were placed in a shaking water bath (Precision Model 25, Fisher Scientific, Suwanee, GA) at 120 cpm and 37°C and gassed with 95% O2 and 5% CO2. The glands were minced in pairs from each rat in 2 mL of the dispersion medium and transferred to the siliconized flask. After 40 min incubation, dispersion was facilitated by pipetting 5 mL up and down x 10 in a 10 mL plastic pipette. After 1 h incubation, the cells were centrifuged (Adams Dynac 0101, Becton Dickinson, Sparks, MD) for 4 min at 1,100 × g in a 50 mL centrifuge tube. After four washes in wash medium, all but 3 mL of the last supernatant were drawn off and the pellet was resuspended, ready for infusion. Samples observed by light microscopy on glass slides were approximately 85% acinar cells and 90% viable, i.e., resisted penetration by trypan blue (TX1580, B430, Matheson, Coleman & Bell, Norwood, OH). It is worth noting that the acinar cells constituted a much greater proportion (85% vs. 60%) of these samples than they did in the original report on the dispersion procedure (Quissell and Redman, 1979).
Infusion procedure
As the preparation of dispersed cells approached completion, one submandibular duct was cannulated as described by Baccaglini et al. (2001). Female rats were anesthetized by intramuscular (i.m.) injection of a mixture of ketamine (Hospira, Lake Forest, IL) and xylazine (Lloyd Laboratories, Shenadoah, IA), 8–10 and 60–80 mg/kg body weight, respectively. When the rat was sufficiently anesthetized, 0.15 mg/kg of atropine was injected i.m. to prevent salivary secretion from interfering with the infusions. Each recipient rat then was placed in the dorsal position on a platform with the maxillary incisors on a wire bar and a rubber band stretched across the lower incisors to hold the mouth open vertically. A stainless steel wire frame then was inserted to hold the mouth open horizontally and a cotton puff was placed to push the tongue up into the palate (Fig. 1). A cannula made from polyethylene tubing (Intramedic ™ PE10, Becton Dickinson) was inserted into the left submandibular (Wharton’s) duct and fixed in place with a drop of cyanoacrylate adhesive (Krazy Glue®, Elmer’s Products, Columbus, OH). The volume of dispersed cells to be infused had been determined by preliminary trials as follows. India ink was injected via a 1.0 mL syringe with a 28 gauge needle inserted into the distal end of the cannula. The infused glands were fixed in 4.0 % formaldheyde prepared from paraformaldehyde (Electron Microscopy Sciences, Ft. Washington, PA) in sodium phosphate buffer, pH 7.4, and analyzed in routine paraffin sections stained with hematoxylin and eosin obtained as ready-to-use solutions from Surgipath Medical Industries, Richmond, IL (no lot or C.T. number listed). When 200 to 300 μL of ink were injected and held in place for 15 min, ink filled the lumens of all ducts and acini and numerous small depots of ink occurred in the stroma throughout the gland (not illustrated). No ink entered the sublingual glands.
Fig. 1.
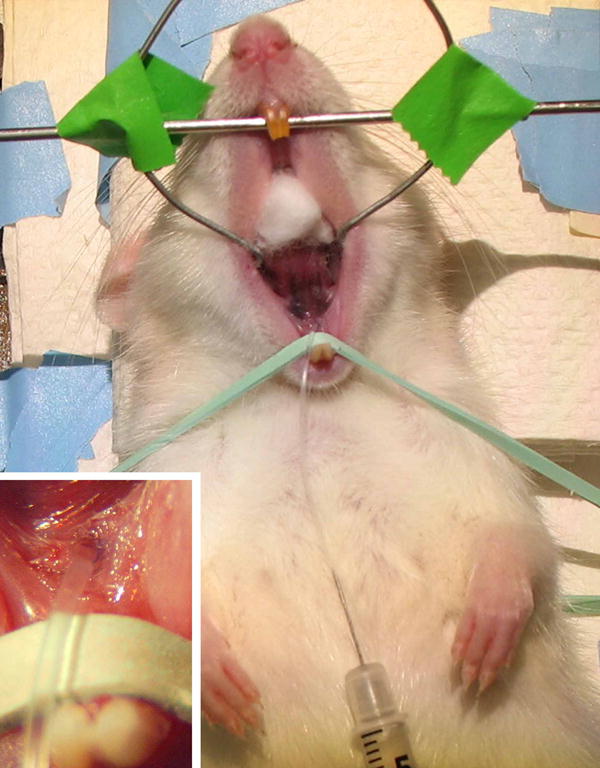
A cannula is inserted into the main (Wharton’s) excretory duct of the left submandibular gland of a female rat. A syringe carrying dispersed cells lies on the abdomen with the needle inserted into the distal end of the cannula. Insert: detail of the cannula inserted into Wharton’s duct.
Accordingly, the standard infusate was determined to be 250 to 300 μL of the dispersed cell slurry. To inhibit immune rejection of the infused cells, the recipient rats received Cyclosporin (Sandimmune® oral solution, Novartis, East Hanover, NJ) approximately 15 mg/kg/day via the drinking water. Recipient rats were killed by Halothane inhalation at 1 h and 1, 4, 8, 14 and 21 days after infusion. The infused glands were excised, cleaned, bisected in the long axis, fresh frozen in 4-methyl butane (isopentane; Sigma-Aldrich) suspended in liquid nitrogen, wrapped in aluminum foil, sealed in a plastic bag, and stored at −70° C. Several of the contralateral, non-infused glands and glands from male rats were used for Y-chromosome negative and positive controls, respectively.
Histology
Experiments with fluoresence in situ hybridization (FISH) showed that, although nuclei with the Y-chromosome were readily identified, distinguishing among the several types of parenchymal cells (acinar and intercalated, striated and excretory ductal) was uncertain. Therefore, another method, autoradiography with a radiolabeled probe, was tried. Attempts to preserve the mucins that are markers of acinar cells in rat submandibular glands included fixation with 0.5 % or 2.0 % phosphate-buffered formaldehyde prior to freezing and sectioning and fixing the air-dried, slide-mounted sections of fresh frozen glands with phosphate-buffered 4.0% formaldehyde and methacarn or Carnoy’s solution with or without 0.5% formaldehyde. Fixation prior to freezing inhibited the in situ hybridization procedure with the Y-chromosme probe, and in situ hybridization inhibited staining with hematoxylin, eosin and Alcian blue (Mowry 1963) and immunohistochemical reactions with an anti-human blood group A antibody (Sigma-Aldrich), which also reacts with rat submandibular gland acinar mucin (Moreira et al. 1989, 1991). However, all the types of parenchymal cells except myoepithelial cells were readily identified after staining the radiolabeled sections with either basic fuchsin/toluidine blue or Giemsa. Method details follow.
Sectioning
Frozen sections were cut at 8 μm, mounted on Superfrost®/plus glass slides (Fisher Scientific, Pittsburgh, PA), air-dried for 30 min on a slide-warmer at 37° C, sealed in aluminum foil, placed in plastic bags and stored at −80° C. Samples were taken as needed for FISH (Eglitis and Mezey 1997) or autoradiography with a 35S-labeled probe (Radiolabled ISH; Rogers 1979). In all of the following, the sections remained mounted on the slides.
Prehybridization
Sections were removed from −80°C and placed immediately into 4% formaldehyde in phosphate-buffered saline (PBS), pH 7.4, for 4 min. They were rinsed twice in PBS, followed by a 10 min. treatment in acetic anhydride with triethanolamine, and two washes in 2X Saline Sodium Citrate (2XSSC). Slides intended for FISH were removed from 2XSSC and incubated at 81°C for 10 min. in 50% formamide/2XSSC for denaturation. Slides intended for radiolabled ISH were removed from 2XSSC and run through increasing concentrations (70, 80, 95 and 100%) of ethanol for 1 minute each. They were then allowed to air dry until ready for hybridization.
Probe Preparation
A 1Kb length DNA probe containing five repeat units specific for rat chromosome Y (9.1ES8; Essers et al 1995) was a kind gift from Dr. Barbara Hoebee. For FISH, the probe was labeled with digoxigenin -11-UTP (uridine triphosphate) using a labeling kit (Roche Applied Science, Indianapolis, IN, Cat. # 1209256). The Y chromosome signal was visualized in fluorecence using an FITC-tyramide system (Eglitis and Mezey 1997) and the nuclei were demonstrated with 4′,6-diamidinno-2 phenyliindole dihydrochloride (DAPI); Invitrogen). For radiolabeled ISH, the probe was labeled with 35S by nick translation using an Ambion maxiscript transcription kit (Applied Biosystems/Ambion, Austin, TX, Cat. # 1324). After development of the latent images in the emulsion, the sections were stained either with basic fuchsin/toluidine blue (Ball and Redman 1984) or Giemsa (Luna 1968) and coverslipped with Cytoseal 60 (Stephens Scientific). Dyes were obtained as follows: basic fuchsin (760154) and toluidine blue O (766486), Fisher Scientific; Alcian blue 8Gx (C.T. 74240), Sigma-Aldrich; Giemsa, Fluka (Cat.# 48900).
Sections labeled with FISH were viewed and photographed with a Leica DMI6000B inverted fluorescent microscope equipped with a Hamamatsu-Orca camera. The radiolabeled sections were viewed and photographed in an Olympus BX41 light microscope equipped with an Olymous Q Color 5 Digital Camera.
Cell counts
A cell was considered to bear a Y-chromosome when there was a bright yellow-green spot in the nucleus with FISH (e.g., Figure 2A) or a radiating pattern of more than eight silver grains over or very near to a nucleus with the radiolabeled probe. Labels occurring in the cytoplasm, not over tissue, or over nuclei in the sublingual glands of female rats, were considered to be “false positives”. The radiolabeled nuclei were counted by cell type in five sections from each sample. To estimate the efficiency of the probe, labeled and unlabeled nuclei were counted to a combined total of 1,000 cells in each of the two male controls with radiolabeled Y-chromosomes. The average proportion of labeled nuclei was 75.75%. (765/1000 + 750/1000 = 1515/2000 = 0.7575).
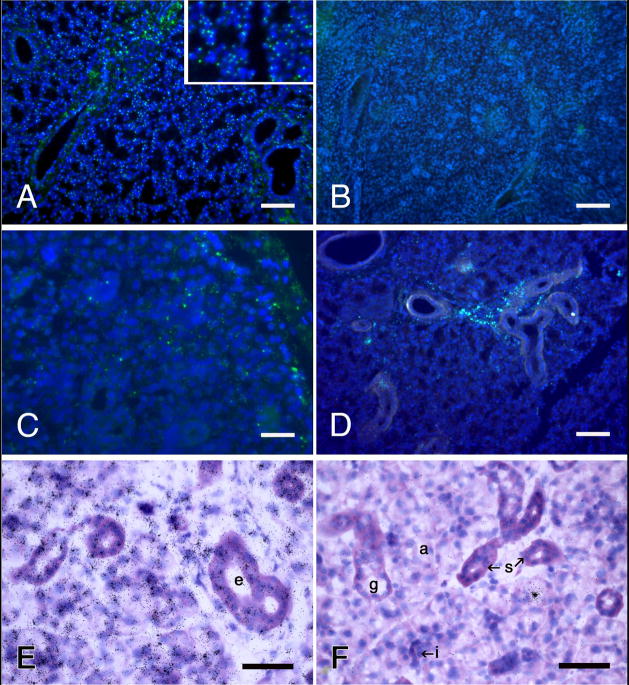
Fig. 2.
Identification of the Y-chromosome in rat submandibular glands by fluorescence in situ hybridization (A–D) and autoradiography of 35S-labeled antibodies to digoxygenin coupled to the probe (E, F). A, E, male controls; B, F, female controls; C, D, glands from female rats one hour and eight days, respectively, after being infused with dispersed cells from male rat submandibular glands. Y-chromosomes (small, bright yellow-green spots) are located in about 70% of the blue nuclei in A and none of the nuclei in B. Similarly, tight, radiating clusters of silver grains are atop the nuclei in E, and atop none in F. The lone cluster of grains in F is over the secretory granules of an acinar cell, illustrating a rare “false positive” artifact. E, F, basic fuchsin and toluidine blue stains. Scale bars = 100 μm (A, B and D), 25 μm (C), and 50 μm (E and F).
Results
Photomicrographs of sections subjected to FISH and the radiolabeled probes are displayed in Figures 2 and 3, and the number of radiolabeled nuclei by cell type are presented in Table 1. At one hour post-infusion, labeled cells had engorged the lumens of the larger ducts and many had lodged among the cells of the ducts and acini. The number of labeled cells per five sections of gland dropped precipitously in the next 24 hours but then remained relatively constant through 21 days post-infusion. Obviously, many of the infused cells either did not lodge successfully or were fatally damaged during the processes of dispersion and infusion. Two observations are of paramount importance. The donor cells exhibited the cytodifferention of the structures in which they lodged, i.e., acinar, granular convoluted tubule, or intercalated, striated or excretory duct, and they lodged here and there throughout the recipient gland, from in or near the excretory ducts to near the capsule. Labeled nuclei in the sublingual glands adjacent to infused submandibular glands and in non-infused female submandibular glands were rare, ranging from 0 to 2 per 5 sections.
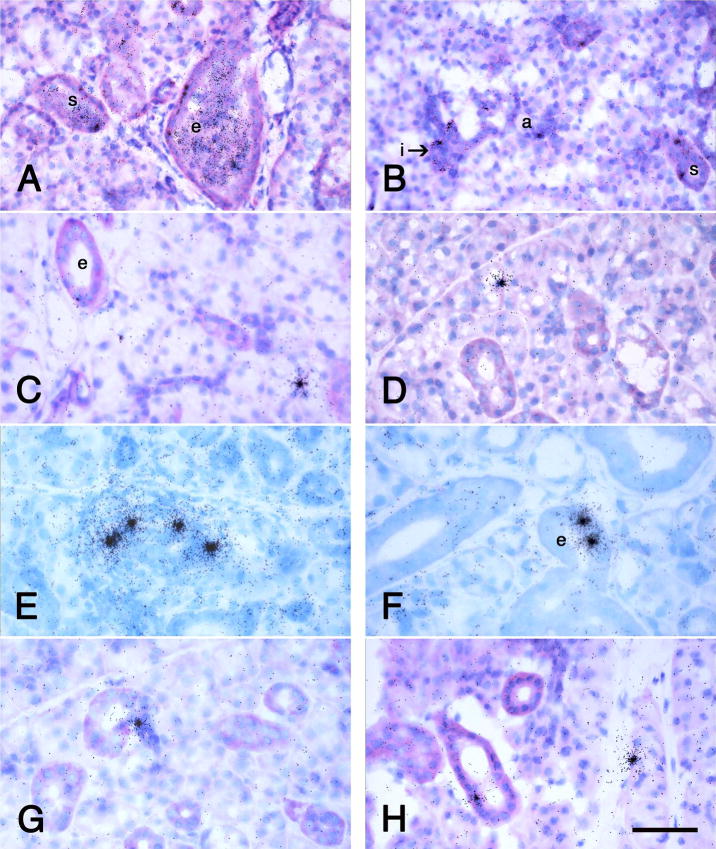
Fig. 3.
Autoradiographs of sections of female rat submandibular glands infusd with dispersed submandibular gland cells from male rats. The donor cells are identified by radiolabeled in situ hybridization by a Y-chromosome probe as in Figure 2E and F. The times post-infusion are 1 h (A, B), 1 day (C), 14 days (D, E, F) and 21 days (G, H). At 1 h, labeled cells pack the lumens of the excretory and striated ducts and many have lodged among the cells of both ducts and acini. Labeled cells have lodged in and have the cytodifferentiation of excretory ducts (e) in A, C, E, and H, striated ducts (s) in A, B, and E, intercalated ducts (i) in B, E, and G, and acini (a) in B, C, D, E, and H. A. B, C, D, G and H, basicfuchsin and toluidine blue stains; E, F, Giemsa stain. Scale bar = 70 μm for A and 50 μm for B through H.
Table 1.
Number of Y-Chromosome Cells by Cell Type and Time Post-Infusion of Male Rat Submandibular Gland Cells into Female Rat Submandibular Glands
| Time Post- Infusion (n) |
Cell Type |
|||||
|---|---|---|---|---|---|---|
| Acinar | I.D. | G.C.T. | S.D. | E.D. | Total | |
| 1 hour (1) | 37 | 35 | 10 | 50 | 18 | 150 |
| 1 day (2) | 27 | 8 | 4 | 6 | 1 | 46 |
| 4 days (2) | 17 | 9 | 2 | 6 | 1 | 35 |
| 8 days (2) | 17 | 8 | 8 | 5 | 1 | 39 |
| 14 days (3) | 16 | 8 | 5 | 4 | 3 | 36 |
| 21 days (2) | 18 | 8 | 4 | 4 | 1 | 35 |
Rat Y-chromosomes marked via autoradiography of 35S-labeled probes were counted in five 8 μm histologic sections per rat. G.C.T. = granular convoluted tubules; I.D., S.D. and E.D = intercalated, striated and excretory ducts, respectively.
The diameter of several submandibular glands of female rats age 60 days was measured in the direction of sectioning and all were about 6 mm, or 6,000 μm. The cells in the experimental (recipient) glands were counted in five 8 μm sections, or 40 μm of that diameter. Therefore, the number of cells lodged per gland can be estimated by the formula [n × 6,000 ÷ 40] ÷ 0.7575 (labeling efficiency) = n × 198 (rounded to 200 for simplicity). Thus, an average of 7,000 (35 × 200) or more donor cells remained lodged per gland at 4 days and later post-infusion.
Discussion
The accuracy of the method for estimating the number of donor cells that lodge in the gland is important in gauging the potential usefulness of this method for the functional restoration of damaged salivary glands. In this regard, two opposing factors that bear on this estimate need to be considered. The curvature of the gland surface would result in the first and last several sections of a completely sectioned gland being smaller than the other sections, with the total cells per gland being overestimated accordingly. However, the labeled cells were counted mostly in sections in which the sublingual gland was included as a built-in negative (non-infused) control. As sectioning proceeds in the long axis, the submandibular gland replaces the sublingual gland to the height of the sublingual/submandibular gland complex. Thus the submandibular gland area is much larger in the ca. 40% of sections made past the much smaller sublingual gland. This factor outweighs the other, so that the total labeled cells per gland actually were underestimated. Other considerations in this regard are the efficiency and specificity of the Y-chromosome probe. Short sequences of DNA identical to those on the Y-chromosome have been identified on other chromosomes in rats, suggesting a potential for the probe to bind to female cell nuclei (Essers et al, 1995; Hoebee and de Stoppelaar 1996). The results indicate that in the present study using stringent conditions in the in situ procedure, the probe used provided very high specificity and excellent efficiency.
Though seven to almost ten thousand donor cells were effectively implanted in the recipient glands, is this range sufficient to be useful in the functional restoration of damaged rat submandibular glands? Based on experiments with the atrophy and recovery of ligated and unligated rat salivary glands, the answer is yes. The glands recover with or without intervention (Tamarin 1971; Takahashi et al. 2004), but infusion of basic epidermal growth factor can accelerate the process (Okazaki et al. 2001). More to the point, T. Sugito and colleagues (2004) grew rat submandibular gland cells in primary culture, labeled them with PKH 26, a fluorescent linkage marker, and injected them (together with India ink, to mark the site of injection) into normal glands and glands previously rendered atrophic by main duct ligation. In the normal glands, the cells remained close to the site of injection, and quickly became scarce. In the glands recovering from the effects of duct ligation, many more cells were present after four weeks and some had migrated some distance away from the site of injection. However, almost all stayed in the stroma, and few, if any, differentiated into acinar cells. That and the previously cited work of Lombaert et al. (2008), and experience with stem cells in brain and other tissues (Mahmood et al. 2001), indicate that candidate progenitor cells added to normal tissues with low proliferative activity remain widely scattered for long periods of time. However, they tend to lodge in much greater numbers and proliferate in tissues that normally proliferate rapidly (Tran et al. 2003) or are recovering from injury. It is also evident that the seeded cells must be able to establish connections with the lumens of residual parenchymal structures, as widespread acinar cell atrophy and death usually occur when there is no outlet for secretion, e.g., with obstructed ducts (Tamarin 1971, Walker and Gobé 1987, Takahashi et al 2004). In this regard, the ductal infusion method presented here has the advantage of delivering the donor cells directly to the structures with lumenal borders.
Based on the above results it seems feasible to imagine that healthy salivary gland cells might be collected from patients before irradiation, possibly expanded in culture, and infused back through the excretory ducts into the patient’s salivary gland following radiation. Since there would not be immunological problems receiving their own cells, this approach might help to promote functional recovery after irradiation.
Acknowledgments
This research was supported in part by Grant DE 14995 (RSR) and the intramural program (EM) of The National Institute of Dental and Craniofacial Research, The National Institutes of Health, Bethesda, MD, the United States Department of Veterans Affairs (RSR), and the Institute for Clinical Research, Inc., Washington DC (RSR). We thank Dr. David O. Quissell and Ms. Katherine Barzen for teaching one of us (RSR) the fine points of preparing dispersed rat submandibular acinar cells, Ms. Tong-Hui Mixon and the late Mr. Edward Flores for assistance with the paraffin-embedded tissues, and Drs. Paul Denny and Simon D. Tran for encouragment during this research.
Footnotes
W.D. Ball has not read this manuscript, as contact with him was lost during its preparation.
References
- Baccaglini L, Shamsul Hoque ATM, Wellner RB, Goldsmith CM, Redman RS, Sankyar V, Kingman A, Barnhart KM, Wheeler CJ, Baum BJ. Cationic liposome-mediated gene transfer to rat salivary epithelial cells in vitro and in vivo. J Gene Med. 2001;3:82–90. doi: 10.1002/1521-2254(2000)9999:9999<::AID-JGM151>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Ball WD, Redman RS. Two independenetly regulated secretory sustems within the acini of the submandibular gland of the perinatal rat. Eur J cell Biol. 1984;33:112–122. [PubMed] [Google Scholar]
- Coppes RP, Zeilstra LJW, Kampinga HH, Konings AWT. Early to late sparing of radiation damage to the parotid gland by adrenergic and muscarinic receptor agonists. Brit J Cancer. 2001;85:1055–1063. doi: 10.1054/bjoc.2001.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eglitis MA, Mezey É. Hematopoietic stem cells differentiate into microglia and macroglia in the brains of adult mice. Proc Natl Acd Sci USA. 1997;94:4080–4085. doi: 10.1073/pnas.94.8.4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essers J, de Stoppelaar JM, Hoebee B. A new rat repetitive DNA family shows preferential localization on chromosomes 3, 12 and Y after fluorescence in situ hybridization and contains a subfamily which is Y-chromosome specific. Cytogenet Cell Genet. 1995;69:246–252. doi: 10.1159/000133974. [DOI] [PubMed] [Google Scholar]
- Hoebee B, de Stoppelaar JM. The isolation of rat chromosome probes and their application in cytogenetic tests. Mutat Res. 1996;372:205–210. doi: 10.1016/s0027-5107(96)00140-6. [DOI] [PubMed] [Google Scholar]
- Lombaert IMA, Brunsting JF, Wierenga PK, Faber H, Stokman MA, Kok T, Visser WH, Kampinga HH, de Haan G, Coppes RP. Rescue of salivary gland function after stem cell transplantation in irradiated glands. PLoS ONE. 2008;3:e2063. 1–16. doi: 10.1371/journal.pone.ooo2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombaert IM, Wierenga PK, Kok T, Kampinga HH, deHaan G, Coppes RP. Mobilization of bone marrow stem cells by granulocyte colony-stimulating factor ameliorates radiation-induced damage to salivary glands. Clin Cancer Res. 2006;12:1804–1812. doi: 10.1158/1078-0432.CCR-05-2381. [DOI] [PubMed] [Google Scholar]
- Luna LG. Manual of Histologic Staining Methods of the Armed Forces Institute of Pathology. 3. McGraw-Hill; New York, NY: 1968. pp. 127–128. [Google Scholar]
- Mahmood A, Lu D, Wang L, Li Y, Lu M, Chopp M. Treatment of traumatic brain injury in female rats with intravenous administration of bone marrow stromal cells. Neurosurgery. 2001;49:1196–1203. [PubMed] [Google Scholar]
- Moreira JE, Ball WD, Mirels L, Hand AR. Accumulation and localization of two adult acinar cell secretory proteins during development of the rat submandibular gland. Am J Anat. 1991;191:167–184. doi: 10.1002/aja.1001910204. [DOI] [PubMed] [Google Scholar]
- Moreira JE, Tabak LA, Bedi GS, Culp DJ, Hand AR. Light and electron microscopic immunolocalization of rat submandibular gland mucin-glycoprotein and glutamine/glutamic acid-rich proteins. J Histochem Cytochem. 1989;37:515–528. doi: 10.1177/37.4.2926128. [DOI] [PubMed] [Google Scholar]
- Mowry RW. The special value of methods that color both acidic vicinal hydroxyl groups in the histochemical study of mucins. With revised directions for the colloidal iron stain, the use of Alcian blue 8GX and their combinations with the periodic acid-Schiff reaction. Ann NY Acad Sci. 1963;106:402–423. [Google Scholar]
- Nagler RM. The enigmatic mechanism of irradition-induced damage to the major salivary glands. Oral Dis. 2002;8:141–146. doi: 10.1034/j.1601-0825.2002.02838.x. [DOI] [PubMed] [Google Scholar]
- Okazaki Y, Kagami H, Hattori T, Hishidi S, Shigetomi T, Ueda M. Acceleration of rat salivary gland tissue repair by basic fibroblast growth factor. Arch Oral Biol. 2001;45:911–919. doi: 10.1016/s0003-9969(00)00035-2. [DOI] [PubMed] [Google Scholar]
- Quissell DO, Redman RS. Functional characteristics of dispersed rat submandibualr cells. Proc Natl Acad Sci USA. 1979;76:2789–2793. doi: 10.1073/pnas.76.6.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman RS. On Approaches to the functional restoration of salivary glands damaged by therapeutic irradiation for head and neck cancer, with a review of related aspects of salivary gland morphology and development. Biotech Histochem. 2008;83:103–130. doi: 10.1080/10520290802374683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers AW. Techniques of Autoradiography. Elsevier; NY: 1979. pp. 1–429. [Google Scholar]
- Sugito T, Kagami H, Hata K, Nishiguchi H, Ueda M. Transplantation of cultured salivary cells into an atrophic salivary gland. Cell Transplant. 2004;13:691–699. doi: 10.3727/000000004783983567. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Shinzato K, Nakamura S, Domon T, Yamamato T, Wakita M. Cell death and cell proliferation in the regeneration of atrophied rat submandibular glands after duct ligation. J Oral Pathol Med. 2004;33:23–29. doi: 10.1111/j.1600-0714.2004.00191.x. [DOI] [PubMed] [Google Scholar]
- Tamarin A. Submaxillary gland recovery from obstruction. 1 Overall changes and electron microscopic alterations of granular duct cells 2 Electron microscopic alterations of acinar cells. J Ultrastruct Res. 1971;34:276–302. doi: 10.1016/s0022-5320(71)80072-2. [DOI] [PubMed] [Google Scholar]
- Tran SD, Pillemer SR, Dutra A, Barrett AJ, Brownstein MJ, Keys S, Pak E, Leakan RA, Kingman A, Yamada KM, Baum BJ, Mezey E. Differentiation of human bone marrow-derived cells into buccal epithelial cells in vivo: a molecular analytical study. Lancet. 2003;361:1084–1088. doi: 10.1016/S0140-6736(03)12894-2. [DOI] [PubMed] [Google Scholar]
- Walker NI, Gobé GC. Cell death and cell proliferation during atrophy of the rat parotid gland induced by duct obstruction. J Pathol. 1987;3:167–175. doi: 10.1002/path.1711530407. [DOI] [PubMed] [Google Scholar]