Abstract
Members of the polo subfamily of protein kinases have emerged as important regulators in diverse aspects of the cell cycle and cell proliferation. A large body of evidence suggests that a highly conserved polo-box domain (PBD) present in the C-terminal non-catalytic region of polo kinases plays a pivotal role in the function of these enzymes. Recent advances in our comprehension of the mechanisms underlying mammalian polo-like kinase 1 (Plk1)-dependent protein–protein interactions revealed that the PBD serves as an essential molecular mediator that brings the kinase domain of Plk1 into proximity with its substrates, mainly through phospho-dependent interactions with its target proteins. In this review, current understanding of the structure and functions of PBD, mode of PBD-dependent interactions and substrate phosphorylation, and other phospho-independent functions of PBD are discussed.
Keywords: Polo kinase, Plk1, Polo-box domain, Mitosis, Cell proliferation
Introduction to polo-like kinases
Discoveries of Plks
Named after a defective spindle pole phenotype associated with Drosophila gene polo (which literally means “pole” in Spanish) mutations [1], the polo-like kinases (collectively, Plks) are a conserved subfamily of Ser/Thr protein kinases that play pivotal roles during the cell cycle and proliferation. In mammalian cells, four Plks that apparently exhibit differential functions and tissue distributions are expressed from distinct genetic loci (see reviews in [2–4]). They include three closely related members, Plk1 [5–8], Plk2 (also called Snk) [9, 10], and Plk3 (also called Prk or Fnk) [11, 12], and one distantly related member, Plk4 (also called Sak) [13]. Homologous to those in mammals, at least three related Plks, named Plx1/Plc1, Plx2/Plc2, and Plx3/Plc3, have been isolated from frogs and worms. On the other hand, organisms such as D. melanogaster, S. cerevisiae, and S. pombe appear to possess a single Plk (polo [1], Cdc5 [14], and Plo1 [15], respectively). Although it is not known whether the low eukaryotic organisms accomplish the function of all isoforms of Plk1–4 (collectively, Plks) using one homolog, it is likely that higher eukaryotic organisms have acquired functionally diversified Plks to cope with various physiological challenges for multicellular organisms. The function of Plks in various organisms has recently been extensively reviewed (see reviews in [2, 16, 17]).
One of the characteristic features of the Plks is the presence of the polo-box domain (PBD) in their respective C-terminal non-catalytic region. PBD is thought to play a critical role in targeting the catalytic activity of the enzymes to specific subcellular structures [18]. Therefore, understanding the PBD-dependent interactions and, furthermore, the spatiotemporal regulation of these events is central to better comprehend the physiological functions of Plks. In this review, we will primarily focus on discussing our current understanding of the structure and function of PBD and the underlying mechanisms of PBD-dependent biochemical and cellular events in mammalian Plks.
Differential functions of mammalian Plks
Among the four mammalian Plks, Plk1 has become the most extensively studied enzyme because of its tight association with oncogenesis. Plenty of evidence suggests that Plk1 regulates diverse cellular and biochemical events at multiple stages of M phase, including centrosome maturation, bipolar spindle formation, DNA damage adaptation, mitotic entry, activation of anaphase promoting complex, and cytokinesis (see reviews in [2, 3, 16, 17]). Consistent with the critical functions of Plk1 during M-phase progression, Plk1 is highly expressed in tumors of various origins. Consequently, Plk1 is thought to be an attractive anti-cancer drug target [19] that is selectively required for cancer cell viability [20, 21]. Paradoxically, however, downregulation of Plk1 also appears to promote tumorigenesis [22, 23], suggesting that the proper balance of Plk1 activity is important to ensure normal mitotic progression and cell proliferation.
Data accumulated from various studies suggest that mammalian Plks have both distinct and partially overlapped roles during the cell cycle and proliferation as evidenced by the differential expression patterns of these kinases [4]. During the cell cycle, Plk1 is highly expressed at the late G2 and M phases of the cell cycle [24, 25]. However, Plk2 has been shown to be transiently expressed in G1 [9, 26]. Plk2 appears to contribute to S phase entry [27] and plays a role in maintaining cell viability after spindle poisoning [28]. Plk3 is expressed at a constant level throughout the cell cycle [29]. Plk3 regulates mitotic entry and cytokinesis [30, 31], and appears to be activated in response to DNA damage and cellular stress [11, 32–36]. Both Plk2 and Plk3 are proposed to function as tumor suppressors [37, 38]. Interestingly, overexpression of either mammalian Plk1 or Plk3 rescues the lethality associated with the loss of the budding yeast polo kinase homolog Cdc5 [39, 40]. These observations suggest that both Plk1 and Plk3 can mediate some of the Cdc5 functions critically required for budding yeast viability, and that the critical functions of Plks from budding yeast to mammals are likely conserved throughout evolution. Distinct from the roles of Plk1–3 during the cell cycle, Plk4 appears to be a key regulator of centriole duplication [41–44]. Whether Plk1–3 directly regulates some of the processes important for centriole duplication is yet to be investigated.
Given the specific roles of these multiple Plks during the cell cycle and proliferation, it is imperative to understand how they interact specifically with their binding partners and physiological substrates and how these interactions are elaborately regulated without cross-recognition. Although temporal regulation of the expression of these kinases may serve as a mechanism for defining the function of each Plk at a distinct stage of the cell cycle, their expressions are still significantly overlapped with one another. On the other hand, since PBD plays a critical role in targeting the catalytic activity of each Plk to specific subcellular locations (see below), PBD-dependent spatial regulation of the activity of each kinase may be critical to delimit its functions. To better comprehend this spatial regulation, molecular mechanisms underlying the PBD-dependent protein–protein interactions and the physiological significance of these events are discussed below.
Polo-box domain: a multitalented mediator of protein–protein interactions
PBD-dependent subcellular localization of Plks
Plk1 has been shown to localize to the centrosomes and kinetochores in late interphase, and to remain at these locations until telophase. In anaphase, Plk1 localization to these sites weakens, as a fraction of Plk1 relocalizes to the spindle midzone (later it becomes midbody) [24, 25, 45, 46] (Fig. 1). These findings demonstrate that Plk1 binds to various targets at distinct subcellular locations in a temporally and spatially regulated manner. Interestingly, Plk2 also localizes to centrosomes [26, 47], while Plk3 localizes to centrosomes, mitotic spindle, midbody, nucleolus, and Golgi [48–50]. Overexpressed Plk3 was also found in midbody, cellular cortex, and perinuclear granules [31, 51]. Plk4 localizes to centrosomes and also to cleavage furrow when overexpressed [41, 52] (Table 1). Differential localization of Plks to multiple subcellular structures presages the functional complexity of these enzymes during the cell cycle.
Fig. 1.
Subcellular localization of Plk1 during the cell cycle. Plk1 localizes to the centrosomes as early as S phase. As Plk1 becomes more abundant during the late stages of the cell cycle, Plk1 localization to the centrosomes (arrows) and kinetochores (dotted red fluorescent signals) are manifest. In anaphase, a fraction of Plk1 relocalizes to the spindle midzone, which then condenses to a midbody in telophase
Table 1.
Subcellular localization of Plks
| Endogenous | Overexpressed | |
|---|---|---|
| Plk1 | Centrosome, kinetochore, midzone, midbody | Centrosome, kinetochore, mitotic spindle, midzone, midbody |
| Plk2 | Centrosome | Centrosome |
| Plk3 | Centrosome, mitotic spindle, midbody, around nuclear envelope, nucleous, Golgi | Centrosome, cellular cortex, midbody, nucleous, perinuclear granules, cytosolic granules |
| Plk4 | Centrosome | Centrosome, nucleous, around nuclear envelope, cleavage flow |
It is now widely appreciated that PBD plays a critical role in proper subcellular localization of Plks at specific subcellular structures. The initial clue for the importance of PBD in the subcellular localization of Plks originated from the analyses of various Plk1 PBD mutants in budding yeast. Without altering the kinase activity, a single point mutation at a conserved amino acid residue (W414F) within the polo-box 1 (PB1) of Plk1 (Fig. 2) was sufficient to delocalize Plk1 and disrupt its function [18]. Later studies confirmed that PBD is required for proper localization and functions of various other Plks in their native organisms [26, 53–56]. Furthermore, inhibition of the PBD function by a dominant-negative Plk1 PBD was sufficient to interfere with the PBD-dependent subcellular localization and mitotic functions of Plk1 [46, 57]. Thus, PBD-dependent protein–protein interactions are fundamentally required for proper functions of Plks from budding yeast to mammals.
Fig. 2.
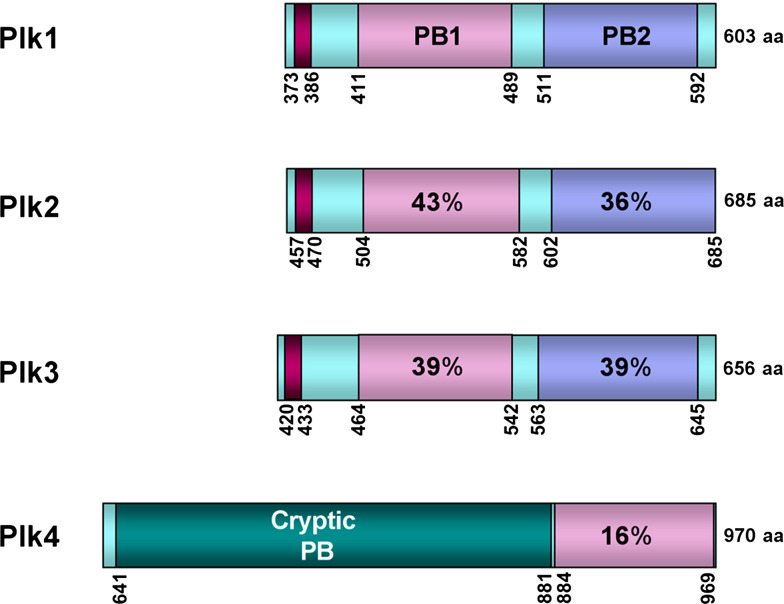
Schematic diagram of the PBDs from human Plk1–4. Sequence identities among PB1s (light pink) and PB2s (light blue) are given in percentages. A short sequence (burgundy) upstream of PB1 represent a short alpha-helix polo-cap denoted as αA in Fig. 3. The total lengths of Plk1–4 are indicated in amino acid residue numbers on the right. The numbers indicate the positions of the amino acid residues in a given gene. Cryptic PB indicates a region of Plk4 that is also sufficient for dimerization [2, 62]
Structures of the PBDs
Alignment of the C-terminal PBDs of mammalian Plk1 to Plk4 (for simplicity, we will call them PBD1 to PBD4, respectively, hereafter) revealed that PBD1–3 exhibit a high level of homology in two distinct polo-box motifs, PB1 (aa 411-aa 489 in Plk1) and PB2 (aa 511-aa 592 in Plk1) [5, 52] (Fig. 2). However, PBD4 present in one of the two Plk4 variants, Sak-a [13], contains a greatly divergent C-terminal sequence from the other three Plks and possesses only the PB1 motif (Fig. 2), hinting that the binding mode of PBD4 is distinct from that of PBD1–3.
A breakthrough in our understanding of the PBD-dependent protein–protein interaction came from the work of Yaffe and his colleagues who have been searching for cellular proteins binding to short phospho-Thr-Pro (pThr-Pro)-containing degenerate peptides. This study led to the identification of PBD1 as a specific pSer- or pThr-binding domain with a consensus binding motif of [Pro/Phe]-[Φ/Pro]-[Φ]-[Thr/Gln/His/Met]-Ser-[pThr/pSer]-[Pro/X] (Φ represents hydrophobic residues and X means any residues) [58, 59]. Additional peptide library screening for the PBDs from Homo sapiens Plk2 and Plk3, Xenopus lavis Plx1, and Sacchoromyces cerevisiae Cdc5 revealed that these PBDs also bind to a pSer/pThr peptide motif preceded by a Ser residue at the pThr-1 position (referred to hereafter as Ser-1 to indicate its relative position from the phosphorylated Thr residue) [59].
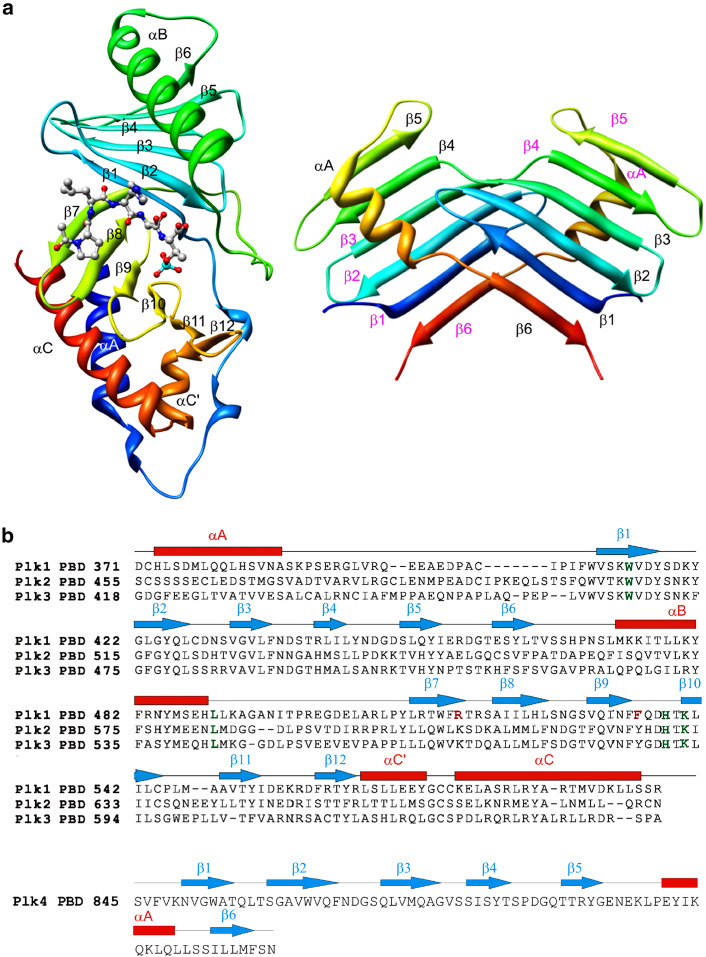
Analyses of the crystal structure of PBD1 in complex with its optimal phosphopeptide containing MQSpTPL revealed that PBD1 forms a single modular, pSer/pThr-binding, zipper-like structure with residues contributed alternatively from each PB (Fig. 3a) [59, 60]. The PB1 and PB2 contain identical folds of β6α (a six-stranded anti-parallel β-sheet and an α-helix) and form a hetero-dimeric phosphopeptide-binding module that bears a short alpha-helix polo-cap (αA) region (aa 373-aa 386 in Plk1) upstream of the PB1 (Fig. 3b). This cap region is thought to stabilize the fold [59, 60] (see Fig. 3a). Interestingly, the PBD1-binding phosphopeptide is located at one end of a shallow cleft between the two β-sheets from each PB. Consistent with the requirement of both PB1 and PB2 for the subcellular localization of Plk1 [46], the bound phospho-peptide interacts with key amino acid residues from both PBs. The Trp414 and Leu490 residues from PB1 are critical for the recognition of the phospho-peptide through non-polar interactions, while the His538 and Lys540 residues from PB2 are central for the interactions with the negatively charged phosphate of the pThr residue [59, 60]. In addition, a strict selectivity for the Ser-1 residue could be attributable to its engagement in two hydrogen bonding interactions and van der Waals interactions with the critical Trp414 residue [59]. As expected if the four residues—namely, Trp414, Lue490, His538, and Lys540—were crucial for the interactions with phosphopeptide, they are commonly found in the PBD1–3 (Fig. 3b).
Fig. 3.
Structures of the PBDs from human Plk1–4. a Comparison of the crystal structures of PBD1 and PBD4. The structure of PBD1 is shown in complex with PLHSpT drawn as a ball and stick representation [61]. The structure of PBD4 is adopted from Leung et al. [62]. Notations for α-helices and β-strands are given in order. b The sequence alignment of the PBDs from Plk1–3. Notations for α-helices and β-strands are done in the same manner as in (a). Four conserved residues (W414, L490, H538, and K540) from the PBD1 important for binding to the MQSpTPL-containing optimal peptide are highlighted in green. Two unique residues (R516 and F535) critical for accommodating the N-terminal Pro of PLHSpT are highlighted in red. PBD4 does not exhibit a significant level of homology with PBD1–3, and thus is shown separately
Comparative analyses of the crystal structures of PBD1 in complex with various phosphopeptide ligands or no peptide revealed that the binding of a phosphopeptide does not induce any detectable level of conformational change [61], suggesting that the interaction between PBD1 and its binding peptide follows a classical lock-and-key model. Although no structure is yet available to grasp the binding modes of PBD2 and PBD3, the observation that PBD2 and PBD3 also efficiently bind to Pro-directed phosphopeptides [59] suggests that phosphoepitope is universally required for the binding of all three PBDs. Determination of the crystal structures of PBD2 and PBD3 will be essential to provide new insights into the mechanism of how specific interactions between PBDs and their binding targets are achieved.
Although PBD4 appears to be crucially required for the function of Plk4, whether it mediates Plk4 function by binding to a phosphorylated target is not known. Analyses of the crystal structure of the single PB1 motif of PBD4 from murine Plk4/Sak-a revealed that, unlike the PB1 and PB2 motifs of PBD1, the PB1 motif of PBD4 generates an intermolecular dimer with another PB1 motif of the PBD4 molecule [62]. The dimerized PB1s of PBD4 contain two α-helices and two six-stranded anti-parallel β-sheets, and generates an interfacial, semi-enclosed, pocket (Fig. 3a), suggestive of a functionally important surface for binding. Each β-sheet is made of four strands from one molecule of PB1 and the other two strands from the other molecule of PB1, thus providing the molecular basis for intermolecular dimerization. Unexpectedly, however, a region N-terminal to the PB1 motif (from aa 641 to aa 881; also called a cryptic PB [2]) (Fig. 2) also appears to be sufficient for dimerization, suggesting the presence of more than one dimerization motif. Consistent with this observation, proper subcellular localization of Plk4/Sak-a requires both the PB1 and the cryptic PB motif [62].
A phospho-recognition domain with a myriad of interacting proteins
The dynamic nature of Plk1 localization to multiple subcellular structures suggests that PBD1 binds to a large number of proteins in a temporally and spatially regulated manner. Numerous PBD1-binding proteins have been isolated and characterized over the years (Table 2). To comprehensively identify additional PBD1-binding proteins, Lowery et al. [63] employed a combined biochemical and proteomic approach and identified more than 600 mitosis-specific proteins that bind to PBD1 in a phosphorylation-dependent manner. Given that some of the PBD1-binding proteins could be expressed under specific stages of the cell cycle or culture conditions, the number of PBD1-binding proteins may continue to increase. Interestingly, these binding targets include not only proteins involved in mitosis but also proteins required for proper translational control, RNA processing, and vesicle transport [63], suggesting that PBD1-dependent interactions are involved in a wide variety of cellular processes. The currently characterized physiological Plk1 substrates and both phospho-dependent and phospho-independent PBD1-binding proteins are listed in Table 2.
Table 2.
The substrates of Plks
| Protein | Plks phosphorylation site(s) | Plk bindinga | PBD binding | Determined PBD-binding site(s) | Cdk priming | References |
|---|---|---|---|---|---|---|
| Plk1 substrates | ||||||
| Bcl-xl | GYS23WSQ, several others | ND | ND | ND | ND | [87] |
| β-Catenin | YRS718FHS | Yes | ND | ND | ND | [88] |
| Bora | DMS497GYN, YNT501QNC | Yes | Yes* | ND | ND | [89] |
| Brca2 | DMS193WSS, DES239LKK | ND | ND | ND | ND | [90] |
| B23 | EDS4MDM | Yes | ND | ND | ND | [91] |
| Bub1 | ND | Yes | Yes | ST609P | Yes | [92] |
| BubR1 | EDS676REA | Yes | Yes | ST620P | Yes | [93] |
| Cdc25C | EFS198LKD | Yes | Yes | ST130P | Yes | [58, 94] |
| Cep55 | NES436LVE | Yes | ND | ND | ND | [95] |
| CEP170 | ND | Yes | ND | ND | ND | [96] |
| Cyclin B | ETS133GCA, AFS147DVI | Yes | ND | ND | ND | [97, 98] |
| Emil | EDS145GYS, YSS149FSL | ND | ND | ND | ND | [99] |
| FoxM1 | NDS715LSK, DIS724FPG | Yes | Yes | ST596P, ST678P | Yes | [100] |
| Grasp65 | ND | Yes | Yes | SS217P, several others | Yes | [101, 102] |
| HBO1 | DSS57PVR | Yes | Yes | PT85P, VT88P | Yes | [103] |
| HsCYK-4 | DES149GSI, DIS157FDK, DES164LDW, DSS170LVK, NES214IVA, DST260LNS | Yes | Yes | SS170L, ST260L, several others | No | [70, 71] |
| HSF1 | NDS216 GSA, LFS419PSV | Yes | ND | ND | ND | [104, 105] |
| IKKβ | DQS733FTA, DWS740WLQ, EHS750CLE | ND | ND | ND | ND | [106] |
| Kif2A | ND | Yes | ND | ND | ND | [107] |
| Kiz | DLT379ISI | Yes | Yes | ND | ND | [108] |
| MKLP1 | RRS904STV, RSS905TVA | Yes | Yes** | ND | ND | [109] |
| MKLP2 | EHS528LQV | Yes | Yes | HS528L | No | [65] |
| Myt1 | DSS426LSS, DDS435LGP, DLS469DIN, EDT495LDP | ND | ND | ND | ND | [110] |
| MyoGEF | EDT574DED | Yes | Yes** | ND | ND | [111] |
| Nedd1 | TDT382LSK, FSS397FDD, DES426IGK, RYS637VNE | Yes | Yes | ST550P | Yes | [112] |
| Nlp | EDS87S88SLE, ST161KEA, EKS686QEV | Yes | ND | ND | ND | [113] |
| NudC | ENS274KLS, DFS326KAK | Yes | Yes** | ND | ND | [114] |
| PBIP1 | HST78AIY | Yes | Yes | ST78A | No | [66] |
| PICH | ND | Yes | Yes | ST1063P | Yes | [115] |
| PIN1 | KHS65QSV | Yes | ND | ND | ND | [116] |
| PRC1 | AST578YSE, HST602NIQ | Yes | Yes | ST602N | No | [69] |
| Ran | AKS135IVF | Yes | ND | ND | ND | [117] |
| Rock2 | DAT967IAS, EES1099QIR, DSS1133SIG, NQS1374IRR | Yes | Yes | ND | ND | [63] |
| TAp73 | DST27YFD | Yes | ND | ND | ND | [118] |
| TCTP | DDS46LIG, TES64TVI | Yes | Yes** | ND | ND | [119] |
| Topors | YES718SYR | Yes | ND | ND | ND | [120] |
| TopoIIα | DFS1337DFD, EES1524DED | Yes | ND | ND | ND | [121] |
| TRF1 | ISS435DSE | Yes | Yes | GT344P, VT371P | Yes | [122] |
| Vimentin | QDS82VDF | Yes | Yes | SS55P | Yes | [123] |
| Wee1A | EDS53AFQ | Yes | Yes | SS123P | Yes | [124] |
| Plk2 substrates | ||||||
| α-Synuclein | MPS129EEG | ND | ND | ND | ND | [125] |
| SPAR | ND | Yes | Yes | SS1328P | Yes | [126] |
| Plk3 substrates | ||||||
| CDC25C | EIS191DEL, EFS198LK | Yes | ND | ND | ND | [127] |
| Chk2 | LSS62LET, LYS73IPE | ND | ND | ND | ND | [128] |
| p73 | ND | Yes | ND | ND | ND | [129] |
| TopoIIα | EKT1342DDE | Yes | ND | ND | ND | [130] |
| VRK1 | DLS342VVE | Yes | ND | ND | ND | [51] |
| Plk4 substrates | ||||||
| ND | ||||||
Yes Phospho-dependent PBD binding, Yes* phospho-independent PBD binding, Yes** phosphorylation-dependency remains unknown, ND not determined
aBased on co-immunoprecipitation and/or pull-down assays
Unlike a large number of PBD1-binding proteins, however, only a handful of potential PBD2- and PBD3-binding proteins have been isolated (Table 2). This is likely due to the limited number of studies on these two kinases compare to Plk1. Thus, isolation and characterization of PBD2- and PBD3-binding proteins are much needed to better comprehend both common and distinct characters of these PBDs in comparison to those of PBD1. Since the subcellular localizations of Plk1–3 appear to be significantly different (Table 1), the proteins that interact with the PBDs of these three enzymes are likely distinctive. In support of this view, in vitro pull-down assays revealed that PBD1 and PBD2 can bind to not only common but also distinct sets of proteins [64].
Then how is the specificity of PBD-dependent interaction achieved? Although largely unknown, a glimpse on attaining PBD1-binding specificity can be gained from the recently characterized interaction between the PBD1 and its specific phosphopeptide ligand derived from the T78 region of a kinetochores protein, PBIP1/MLF1IP/KLIP1/CENP-50/CENP-U (for simplicity, PBIP1 hereafter) [61]. Close examination of the binding mode of a minimal phospho-T78 (p-T78) peptide, PLHSpT, to the PBD1 revealed that the N-terminal residues are crucial for providing specificity to the interaction, while the C-terminal SpT dipeptide functions as a high-affinity anchor. The N-terminal Pro residue appears to play an important role in conferring the specificity by docking its side chain into a hydrophobic core surrounded by the Trp414, Phe535, and Arg516 residues, while concomitantly participating in polar contact (hydrogen bonding) between its carbonyl oxygen and the guanidinium moiety of Arg516 of the PBD1 [61]. The PBD2 and PBD3 possess Lys and Tyr residues at positions corresponding to the Plk1 Arg516 and Phe535 residues, respectively (Fig. 3b). As a result, they fail to generate as favorable an environment to interact with the N-terminal Pro residue, thus providing a molecular basis for the specificity of PBD1 binding.
Close analyses of the crystal structures of PBD1 in complex with various phosphopeptides revealed that many amino acid residues directly participate in the formation of the PBD1-binding cleft. Theoretically, any combination of these residues may potentially generate a binding pocket to stably interact with a given target. Thus, the mode of PBD1-dependent interactions may differ from one binding target to another. Further investigations on diverse PBD1-dependent protein–protein interactions will be necessary to gain additional insight into the mechanism of achieving PBD1-binding specificity.
Two distinct modes of PBD1 binding
Nonself-priming and binding
Screening of Ser-pThr-containing peptide libraries with PBD1 and systematic mutational analyses of an optimal PBD1-binding peptide unveiled that Pro is one of a few favored residues for the pThr + 1 position [58]. These observations suggest that PBD1-binding targets are commonly generated by Cdc2 or other Pro-directed kinases in vivo. Consistent with this notion, a large fraction of the currently characterized PBD1-dependent interactions involve Cdc2-dependent priming events at a Pro-directed site (Table 2). This finding is in good agreement with the observation that various mitotic processes frequently require a concerted action of Cdc2 and Plk1.
In the Pro-directed PBD1-binding model (referred to as “nonself-priming and binding” as opposed to “self-priming and binding”, described below), phosphorylation and generation of a PBD1-binding site by a priming kinase such as Cdc2 is a prerequisite step for subsequent PBD1 binding and PBD1-dependent Plk1 function (Fig. 4). Thus, the mechanism of nonself-priming imposes an additional layer of regulation in PBD1-dependent Plk1 functions. This mechanism also ensures that prior cell cycle events mediated by a priming kinase are completed before PBD1-dependent interaction allows Plk1 to carry out its own function. Currently, a large number of PBD1-binding proteins that appear to follow the nonself-priming and binding model have been isolated (Table 3), suggesting that this mechanism is commonly employed to mediate PBD1-dependent Plk1 function.
Fig. 4.
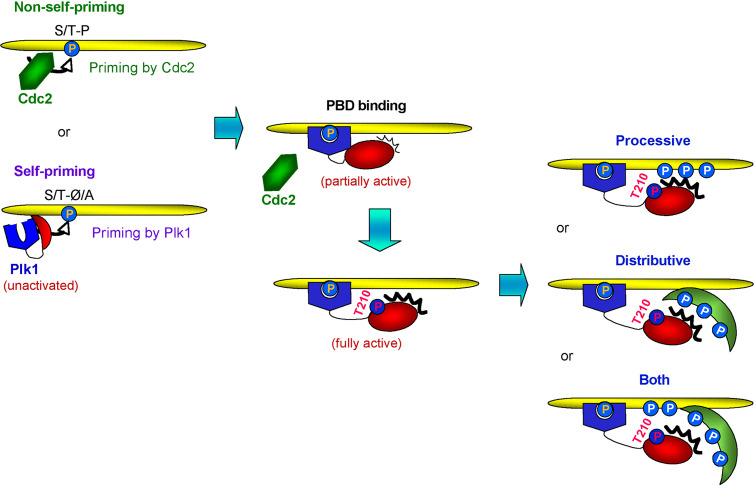
Schematic diagram for Plk1 activation, PBD1-dependent binding, and substrate phosphorylation. Generation of PBD1-binding targets is achieved either by a Pro-directed kinase such as Cdc2 (nonself-priming) or by Plk1 itself (self-priming). Upon binding to a phosphorylated target through PBD1, Plk1 becomes partially active via physical dissociation of its kinase domain from the PBD1. Phosphorylation of Plk1 at T210 by an upstream kinase such as Aurora A/Bora [80, 86] fully activates the enzyme prior to G2/M transition. Once Plk1 binds to a phosphorylated target, Plk1 phosphorylates either the same protein binding to its PBD1 (processive phosphorylation) or a third protein associating with the PBD1-binding target (distributive phosphorylation). These two phosphorylation models are not mutually exclusive and may operate in a concerted manner (both)
Table 3.
Self-priming or nonself-priming PBD1-binding targets
| Nonself-priming | Bub1, BubR1, Cdc25C, FoxM1, Grasp65, HBO1, Nedd1, PICH, TRF1, Vimentin, Wee1A |
| Self-priming | HsCYK-4, MKLP2, PBIP1, PRC1 |
Self-priming and binding
Besides the prevalent nonself-priming and binding mechanism described above, evidence accumulated in recent years suggests that Plk1 also generates PBD1-binding sites to promote its own localization to specific subcellular structures. The initial hint that led to the existence of this type of PBD1-binding mode came from the observation that Plk1 phosphorylates a mitotic kinesin-like protein Mklp2 at S528, and that this event is important for PBD1 binding in vitro [65]. The S528A mutant fails to recruit Plk1 to the central spindle [65], suggesting that Plk1-dependent S528 phosphorylation is likely required for proper Plk1 localization to the central spindle. Later, close analyses of the interaction between Plk1 and PBIP1 demonstrated that Plk1-dependent PBIP1 phosphorylation at T78 is critical for the Plk1–PBIP1 interaction both in vitro and in vivo [66]. Subsequent detailed investigations on the Plk1–PBIP1 interaction unequivocally demonstrated that Plk1 is the only enzyme that generates the p-T78 epitope and binds to it [67, 68]. Thus, to distinguish this alternative PBD1-binding mechanism from the above mechanism that involves another kinase, this new mechanism is termed “self-priming and binding” [67] (Fig. 4). Although not as frequently observed as the nonself-priming and binding, the number of PBD1-binding proteins that follow this alternative mechanism appears to grow. Recently, Neef et al. [69] showed that a microtubule-associating protein, PRC1, is an anaphase-specific PBD1-binding target that requires Plk1-dependent self-priming and binding. Similarly, Plk1-dependent phosphorylation of a subunit of the centralspindlin complex, HsCYK-4, promotes PBD1 binding in vitro, although the PBD1-docking site has not been determined [70, 71]. Since Cdc2 activity is low at the mitotic kinetochores or is already downregulated in anaphase, self-priming and binding of PBIP1, Mklp2, PRC1, and HsCYK-4 by Plk1 could be a means of ensuring proper regulation of these proteins during the intricate process of M-phase progression (Table 3).
Physiological significance
Whereas the nonself-priming and binding mechanism requires an action of another kinase to elicit PBD1 binding, the self-priming and binding mechanism necessitates an action of Plk1 itself. These differences seem to offer distinct physiological implications. Because of the requirement of another kinase for priming the PBD1-binding site, the nonself-priming and binding mechanism functions as a means of keeping an order of complexly organized cell cycle events. On the other hand, since the self-priming and binding mechanism is not contingent upon the completion of an earlier cell cycle event and is self-regulatory, it creates a condition that permits auto-amplification of Plk1-dependent events. For instance, newly recruited Plk1 molecules can, in turn, generate additional p-T78 epitopes for subsequent recruitment of other Plk1 molecules to the PBIP1-loaded kinetochores, thus inducing a rapid accumulation of a high level of active Plk1 population at this location. This mechanism would also guarantee a fast elimination of deactivating Plk1 population from a specific subcellular location, thus allowing an autonomous regulation of the level of Plk1 activity at this site. The self-priming and binding mechanism is functionally analogous to a positive amplification loop frequently observed in various signal transduction pathways.
It is noteworthy that, unlike Plk1-dependent priming of Mklp2, PRC1, and HsCYK-4, which happens when Plk1 is already activated, Plk1-dependent PBIP1 priming and binding occurs in early interphase sufficiently ahead of Plk1 activation in late G2. This raises a question of how Plk1 initially generates the p-T78 epitope on PBIP1 during early interphase. One plausible scenario is that unactivated Plk1 binds to PBIP1 in a low affinity as it is being expressed in S or early G2, and phosphorylates the T78 motif with its basal activity to generate a high affinity-binding site. Subsequent PBD1 binding to the p-T78 epitope partially activates Plk1, which then generates additional p-T78 motifs on other PBIP1 molecules more efficiently (Fig. 4). The early steps leading to a partial activation of Plk1 through the initial low-affinity PBD1 binding could be viewed as a prelude to the auto-amplification process observed in the self-priming and binding mechanism.
Mechanism of PBD1-dependent substrate phosphorylation; processive phosphorylation versus distributive phosphorylation
Although the interactions between PBD1 and its cognate binding targets are vitally important for proper Plk1 functions, a recent report clearly demonstrates that Plk1 binds to the p-S796 motif of a centrosomal protein hCenexin1, but does not phosphorylate the latter [72]. This finding led to the speculation that Plk1 not only phosphorylates the same protein bound to its PBD1 but also phosphorylates a distinct protein(s) that associates with its PBD1-binding target. To account for potentially diverse modes of PBD1-dependent Plk1 phosphorylation onto its substrates, Lowery et al. [73] originally proposed two mutually non-exclusive models (Fig. 4). In the first model, termed “processive phosphorylation”, the PBD1 binds to a primed site on a binding target and positions its catalytic activity to readily phosphorylate other sites on the same protein. A large number of PBD1-binding proteins have turned out to be physiological Plk1 substrates, thus following this mode of regulation (Table 4). A similar type of processive phosphorylation has been previously described for an SH2 domain-containing Tyr kinase Src [74–76] and a Ser/Thr kinase GSK-3 [77, 78].
Table 4.
Processive versus distributive phosphorylated target proteins
| Target proteins | |
|---|---|
| Processive | Bub1, BubR1, Cdc25C, FoxM1, Grasp65, HBO1, HsCYK-4, Kizuna, MKLP2, Nedd1, PBIP1, PICH, PRC1, Rock2, TRF1, Vimentin |
| Potential target proteins: MKLP1, MyoGEF, NudC, TCTP | |
| Distributive | ND |
| Binding only | hCenexin1 |
ND Not determined
In the second “distributive phosphorylation” model, a PBD1-dependent interaction with its binding target allows the catalytic activity of Plk1 to phosphorylate a third protein that is either directly interacting with the PBD1-binding protein or indirectly through another protein within a complex. At present, no solid example of a PBD1-binding target that links to subsequent Plk1-dependent phosphorylation onto another protein has been identified. However, at least one bona fide PBD1-binding protein, hCenexin1, does not appear to be phosphorylated by Plk1 [72] (Table 4), suggesting that the existence of a distributive phosphorylation mechanism is likely.
Other types of interactions
Phospho-independent or PBD1-independent interactions
It is now well appreciated that PBD1 is sufficient for proper subcellular localization of Plk1. In a further extension of this finding, Hanish et al. [57] have shown that a phosphopincer PBD1(H538A, K540M) mutant fails to localize to specific subcellular structures, suggesting that the phospho-binding module of the PBD1 is critically required for proper localization. However, additional evidence suggests that Plk1 may also localize to specific subcellular structures in a phospho-independent manner. The initial suggestion for this possibility arises from the work of Garcia-Alvarez et al. [79] which shows that the full-length Plk1 bearing the two phospho-pincer mutations can localize to the centrosomes efficiently. Close investigation of the localization pattern of EGFP-fused full-length Plk1 revealed that these mutations substantially cripple, but do not eliminate, the capacity of Plk1 to localize to the centrosomes, kinetochores, and midbody (Fig. 5). In line with these findings, the Drosophila polo localizes to interphase microtubules by interacting with a microtubule-associating protein, Map205, in a manner that requires both the PBD and the kinase domain, but not the priming phosphorylation [54]. It has also been shown that the interaction between Plk1 and Bora does not involve priming phosphorylation [80]. In this case, either the PBD1 or the kinase domain of Plk1 appears to be sufficient for the interaction [80]. These findings suggest that Plk1 can also interact with some of its binding targets through a phospho-independent or PBD1-independent binding mechanism, hinting that the modes of PBD1-dependent interactions are likely a lot more divergent than originally conjectured. Identification and characterization of additional PBD-binding proteins is necessary to better comprehend the various modes of interactions between Plks and their binding proteins.
Fig. 5.
Phospho-dependent interactions between PBD1 and its binding targets are critical but not essential for the subcellular localization of Plk1. HeLa cells infected with adenoviruses expressing either full-length EGFP-Plk1 (WT) or EGFP-Plk1 (H538A K540M) (AM) were fixed and imaged by confocal microscopy (a). The fluorescent intensities of Plk1 signals at the centrosomes, kinetochores, midzone, and midbody were analyzed (b)
Interfacial interactions between PBD1 and the kinase domain
In addition to the role of PBD1 in interacting with other cellular proteins, a large body of evidence suggests that PBD1 also regulates the function of its own kinase activity through interfacial interactions between the PBD1 and the kinase domain. Earlier studies report that the full-length Plk1 binds to a phosphorylated peptide significantly worse than the PBD1 alone [58], whereas the provision of PBD1 inhibits the Plk1 kinase activity [56]. In addition, deletion of the C-terminal PBD1 substantially increased the kinase activity of Plk1 [39, 81]. These observations suggest that the PBD1 and the kinase domain may interact with each other in a mutually inhibitory fashion. This interaction may induce conformational alterations for both the PBD1 and the kinase domain in such a way that reciprocally hinders their interactions with PBD1-binding targets and kinase substrates, respectively. A similar mechanism has been observed with the SHP family of phosphatases that show that the interaction between the phospho-binding SH2 domain and the C-terminal phosphatase domain not only occludes the catalytic cleft but also distorts the conformation of the SH2 domain [82]. Interestingly, provision of an optimal PBD1-binding phosphopeptide enhances the activity of the full-length Plk1 [59]. Further, activational phosphorylation of the T210 residue of Plk1 present within the kinase domain renders Plk1 insensitive to PBD1-dependent inhibition [56]. These observations led to the hypothesis that the PBD1-dependent interaction with its binding target and the T210 phosphorylation within the kinase activation loop [83] may cooperatively induce physical dissociation between the kinase domain and the PBD1, thus allowing Plk1 to adopt a fully activated conformation (see Fig. 4).
Currently, the molecular basis of how PBD1 specifically interferes with the function of its own kinase domain is not known. Since the kinase domain does not appear to possess any potential pseudo-PBD1-binding motif, the site of the inhibitory interaction between the PBD1 and the kinase domain is likely distinct from that of the interaction between the PBD1 and its binding target. Determination of the crystal structure of either an inactive full-length Plk1 or the kinase domain in complex with the PBD1 may shed new light on the nature of the interfacial interactions between the two domains.
Perspective
Since the characterization of PBD as a phospho-epitope-binding module, PBD has become a member of an expanding class of phosphopeptide-binding domains that play central roles in the assembly of diverse regulatory complexes [84, 85]. Over the years, a large number of PBD1-binding proteins have been identified and the physiological significance of these interactions has been characterized. In contrast, PBD2- and PBD3-binding proteins are only beginning to emerge. Thus, to determine the underlying mechanism of PBD-binding specificity and to better understand the physiological significance of these interactions in various cellular processes, isolation and characterization of additional proteins that specifically bind to each PBD will be necessary.
Clearly, the modes of PBD-mediated interactions are far more complicated than we initially thought. Given the diversity of Plks functions, different modes of PBD-dependent interactions undoubtedly provide additional finesse to various Plk-dependent events. Further investigation on the mechanism of how each Plk mediates various biochemical and cellular events will be imperative to better comprehend the distinct functions of Plks in regulating these processes. Future studies aimed at exploring the intricate nature of the spatial and temporal regulation of Plks may prove to be a worthwhile challenge for years to come.
Acknowledgments
We are grateful to the present and past members of our laboratory for their great work and stimulating discussions, and to many colleagues for generously sharing their views and insights. We apologize to all authors whose work could not be cited due to space limitations.
References
- 1.Sunkel CL, Glover DM. polo, a mitotic mutant of Drosophila displaying abnormal spindle poles. J Cell Sci. 1988;89:25–38. doi: 10.1242/jcs.89.1.25. [DOI] [PubMed] [Google Scholar]
- 2.Archambault V, Glover DM. Polo-like kinases: conservation and divergence in their functions and regulation. Nat Rev Mol Cell Biol. 2009;10:265–275. doi: 10.1038/nrm2653. [DOI] [PubMed] [Google Scholar]
- 3.Barr FA, Sillje HH, Nigg EA. Polo-like kinases and the orchestration of cell division. Nat Rev Mol Cell Biol. 2004;5:429–440. doi: 10.1038/nrm1401. [DOI] [PubMed] [Google Scholar]
- 4.Winkles JA, Alberts GF. Differential regulation of polo-like kinase 1, 2, 3, and 4 gene expression in mammalian cells and tissues. Oncogene. 2005;24:260–266. doi: 10.1038/sj.onc.1208219. [DOI] [PubMed] [Google Scholar]
- 5.Clay FJ, McEwen SJ, Bertoncello I, Wilks AF, Dunn AR. Identification and cloning of a protein kinase-encoding mouse gene, Plk, related to the polo gene of Drosophila . Proc Natl Acad Sci USA. 1993;90:4882–4886. doi: 10.1073/pnas.90.11.4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamanaka R, Maloid S, Smith MR, O’Connell CD, Longo DL, Ferris DK. Cloning and characterization of human and murine homologues of the Drosophila polo serine-threonine kinase. Cell Growth Differ. 1994;5:249–257. [PubMed] [Google Scholar]
- 7.Holtrich U, Wolf G, Bräuninger A, Karn T, Böhme B, Rübsamen-waigmann H, Strebhardt K. Induction and down-regulation of PLK, a human serine/threonine kinase expressed in proliferating cells and tumors. Proc Natl Acad Sci USA. 1994;91:1736–1740. doi: 10.1073/pnas.91.5.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lake RJ, Jelinek WR. Cell cycle- and terminal differentiation-associated regulation of the mouse mRNA encoding a conserved mitotic protein kinase. Mol Cell Biol. 1993;13:7793–7801. doi: 10.1128/mcb.13.12.7793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simmons DL, Neel BG, Stevens R, Evett G, Erikson RL. Identification of an early-growth-response gene encoding a novel putative protein kinase. Mol Cell Biol. 1992;12:4164–4169. doi: 10.1128/mcb.12.9.4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liby K, Wu H, Ouyang B, Wu S, Chen J, Dai W. Identification of the human homologue of the early-growth response gene Snk, encoding a serum-inducible kinase. DNA Seq. 2001;11:527–533. doi: 10.3109/10425170109041337. [DOI] [PubMed] [Google Scholar]
- 11.Donohue PJ, Alberts GF, Guo Y, Winkles JA. Identification by targeted differential display of an immediate early gene encoding a putative serine/threonine kinase. J Biol Chem. 1995;270:10351–10357. doi: 10.1074/jbc.270.17.10351. [DOI] [PubMed] [Google Scholar]
- 12.Li B, Ouyang B, Pan H, Reissmann PT, Slamon DJ, Arceci R, Lu L, Dai W. prk, a cytokine-inducible human protein serine/threonine kinase whose expression appears to be down-regulated in lung carcinomas. J Biol Chem. 1996;271:19402–19408. doi: 10.1074/jbc.271.32.19402. [DOI] [PubMed] [Google Scholar]
- 13.Fode C, Motro B, Yousefi S, Heffernan M, Dennis JW. Sak, a murine protein-serine/threonine kinase that is related to the Drosophila polo kinase and involved in cell proliferation. Proc Natl Acad Sci USA. 1994;91:6388–6392. doi: 10.1073/pnas.91.14.6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kitada K, Johnson AL, Johnston LH, Sugino A. A multicopy suppressor gene of the Saccharomyces cerevisiae G1 cell cycle mutant gene dbf4 encodes a protein kinase and is identified as CDC5 . Mol Cell Biol. 1993;13:4445–4457. doi: 10.1128/mcb.13.7.4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohkura H, Hagan IM, Glover DM. The conserved Schizosaccharomyces pombe kinase plo1, required to form a bipolar spindle, the actin ring, and septum, can drive septum formation in G1 and G2 cells. Genes Dev. 1995;9:1059–1073. doi: 10.1101/gad.9.9.1059. [DOI] [PubMed] [Google Scholar]
- 16.Petronczki M, Lénárt P, Peters JM. Polo on the rise-from mitotic entry to cytokinesis with Plk1. Dev Cell. 2008;14:646–659. doi: 10.1016/j.devcel.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 17.Takaki T, Trenz K, Costanzo V, Petronczki M. Polo-like kinase 1 reaches beyond mitosis–cytokinesis, DNA damage response, and development. Curr Opin Cell Biol. 2008;20:650–660. doi: 10.1016/j.ceb.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Lee KS, Grenfell TZ, Yarm FR, Erikson RL. Mutation of the polo-box disrupts localization and mitotic functions of the mammalian polo kinase Plk. Proc Natl Acad Sci USA. 1998;95:9301–9306. doi: 10.1073/pnas.95.16.9301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strebhardt K, Ullrich A. Targeting polo-like kinase 1 for cancer therapy. Nat Rev Cancer. 2006;6:321–330. doi: 10.1038/nrc1841. [DOI] [PubMed] [Google Scholar]
- 20.Luo J, Emanuele MJ, Li D, Creighton CJ, Schlabach MR, Westbrook TF, Wong KK, Elledge SJ. A genome-wide RNAi screen identifies multiple synthetic lethal interactions with the Ras oncogene. Cell. 2009;137:835–848. doi: 10.1016/j.cell.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sur S, Pagliarini R, Bunz F, Rago C, Diaz LAJ, Kinzler KW, Vogelstein B, Papadopoulos N. A panel of isogenic human cancer cells suggests a therapeutic approach for cancers with inactivated p53. Proc Natl Acad Sci USA. 2009;106:3964–3969. doi: 10.1073/pnas.0813333106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu LY, Wood JL, Minter-Dykhouse K, Ye L, Saunders TL, Yu X, Chen J. Polo-like kinase 1 is essential for early embryonic development and tumor suppression. Mol Cell Biol. 2008;28:6870–6876. doi: 10.1128/MCB.00392-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simizu S, Osada H. Mutations in the Plk gene lead to instability of Plk protein in human tumour cell lines. Nat Cell Biol. 2000;2:852–854. doi: 10.1038/35041102. [DOI] [PubMed] [Google Scholar]
- 24.Golsteyn RM, Mundt KE, Fry AM, Nigg EA. Cell cycle regulation of the activity and subcellular localization of Plk1, a human protein kinase implicated in mitotic spindle function. J. Cell Biol. 1995;129(6):1617–1628. doi: 10.1083/jcb.129.6.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee KS, Yuan Y-L, Kuriyama R, Erikson RL. Plk is an M-phase-specific protein kinase and interacts with a kinesin-like protein, CHO1/MKLP-1. Mol Cell Biol. 1995;15:7143–7151. doi: 10.1128/mcb.15.12.7143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma S, Liu MA, Yuan Y-L, Erikson RL. The serum-inducible protein kinase Snk is a G1 phase polo-like kinase that is inhibited by the calcium- and integrin-binding protein CIB. Mol Cancer Res. 2003;1:376–384. [PubMed] [Google Scholar]
- 27.Ma S, Charron J, Erikson RL. Role of Plk2 (Snk) in mouse development and cell proliferation. Mol Cell Biol. 2003;23:6936–6943. doi: 10.1128/MCB.23.19.6936-6943.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burns TF, Fei P, Scata KA, Dicker DT, El-Deiry WS. Silencing of the novel p53 target gene Snk/Plk2 leads to mitotic catastrophe in paclitaxel (taxol)-exposed cells. Mol Cell Biol. 2003;23:5556–5571. doi: 10.1128/MCB.23.16.5556-5571.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chase D, Feng Y, Hanshew B, Winkles JA, Longo DL, Ferris DK. Expression and phosphorylation of fibroblast-growth-factor-inducible kinase (Fnk) during cell-cycle progression. Biochem J. 1998;333:655–660. doi: 10.1042/bj3330655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ouyang B, Li W, Pan H, Meadows J, Hoffmann I, Dai W. The physical association and phosphorylation of Cdc25C protein phosphatase by Prk. Oncogene. 1999;18:6029–6036. doi: 10.1038/sj.onc.1202983. [DOI] [PubMed] [Google Scholar]
- 31.Conn CW, Hennigan RF, Dai W, Sanchez Y, Stambrook PJ. Incomplete cytokinesis and induction of apoptosis by overexpression of the mammalian polo-like kinase, Plk3. Cancer Res. 2000;60:6826–6831. [PubMed] [Google Scholar]
- 32.Bahassi EM, Conn CW, Myer DL, Hennigan RF, McGowan CH, Sanchez Y, Stambrook PJ. Mammalian Polo-like kinase 3 (Plk3) is a multifunctional protein involved in stress response pathways. Oncogene. 2002;21:6633–6640. doi: 10.1038/sj.onc.1205850. [DOI] [PubMed] [Google Scholar]
- 33.Xie S, Wu H, Wang Q, Kunicki J, Thomas RO, Hollingsworth RE, Cogswell J, Dai W. Genotoxic stress-induced activation of Plk3 is partly mediated by Chk2. Cell Cycle. 2002;1:424–429. doi: 10.4161/cc.1.6.271. [DOI] [PubMed] [Google Scholar]
- 34.Xie S, Wu H, Wang Q, Cogswell JP, Husain I, Conn C, Stambrook P, Jhanwar-Uniyal M, Dai W. Plk3 functionally links DNA damage to cell cycle arrest and apoptosis at least in part via the p53 pathway. J Biol Chem. 2001;276:43305–43312. doi: 10.1074/jbc.M106050200. [DOI] [PubMed] [Google Scholar]
- 35.Xie S, Wang Q, Wu H, Cogswell J, Lu L, Jhanwar-Uniyal M, Dai W. Reactive oxygen species-induced phosphorylation of p53 on serine 20 is mediated in part by polo-like kinase-3. J Biol Chem. 2001;276:36194–36199. doi: 10.1074/jbc.M104157200. [DOI] [PubMed] [Google Scholar]
- 36.Xie S, Xie B, Lee MY, Dai W. Regulation of cell cycle checkpoints by polo-like kinases. Oncogene. 2005;24:277–286. doi: 10.1038/sj.onc.1208218. [DOI] [PubMed] [Google Scholar]
- 37.Yang Y, Bai J, Shen R, Brown SA, Komissarova E, Huang Y, Jiang N, Alberts GF, Costa M, Lu L, Winkles JA, Dai W. Polo-like kinase 3 functions as a tumor suppressor and is a negative regulator of hypoxia-inducible factor-1 alpha under hypoxic conditions. Cancer Res. 2008;68:4077–4085. doi: 10.1158/0008-5472.CAN-07-6182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith P, Syed N, Crook T. Epigenetic inactivation implies a tumor suppressor function in hematologic malignancies for polo-like kinase 2 but not polo-like kinase 3. Cell Cycle. 2006;5:1262–1264. doi: 10.4161/cc.5.12.2813. [DOI] [PubMed] [Google Scholar]
- 39.Lee KS, Erikson RL. Plk is a functional homolog of Saccharomyces cerevisiae Cdc5, and elevated Plk activity induces multiple septation structures. Mol Cell Biol. 1997;17:3408–3417. doi: 10.1128/mcb.17.6.3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ouyang B, Pan H, Lu L, Li J, Stambrook P, Li B, Dai W. Human Prk is a conserved protein serine/threonine kinase involved in regulating M phase functions. J Biol Chem. 1997;272:28646–28651. doi: 10.1074/jbc.272.45.28646. [DOI] [PubMed] [Google Scholar]
- 41.Habedanck R, Stierhof YD, Wilkinson CJ, Nigg EA. The polo kinase Plk4 functions in centriole duplication. Nat Cell Biol. 2005;7:1140–1146. doi: 10.1038/ncb1320. [DOI] [PubMed] [Google Scholar]
- 42.Duensing A, Liu Y, Perdreau SA, Kleylein-Sohn J, Nigg EA, Duensing S. Centriole overduplication through the concurrent formation of multiple daughter centrioles at single maternal templates. Oncogene. 2007;26:6280–6288. doi: 10.1038/sj.onc.1210456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kleylein-Sohn J, Westendorf J, Le Clech M, Habedanck R, Stierhof YD, Nigg EA. Plk4-induced centriole biogenesis in human cells. Dev Cell. 2007;13:190–202. doi: 10.1016/j.devcel.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 44.Bettencourt-Dias M, Rodrigues-Martins A, Carpenter L, Riparbelli M, Lehmann L, Gatt MK, Carmo N, Balloux F, Callaini G, Glover DM. SAK/PLK4 is required for centriole duplication and flagella development. Curr Biol. 2005;15:2199–2207. doi: 10.1016/j.cub.2005.11.042. [DOI] [PubMed] [Google Scholar]
- 45.Arnaud L, Pines J, Nigg EA. GFP tagging reveals human polo-like kinase 1 at the kinetochore/centromere region of mitotic chromosomes. Chromosoma. 1998;107:424–429. doi: 10.1007/s004120050326. [DOI] [PubMed] [Google Scholar]
- 46.Seong YS, Kamijo K, Lee JS, Fernandez E, Kuriyama R, Miki T, Lee KS. A spindle checkpoint arrest and a cytokinesis failure by the dominant-negative polo-box domain of Plk1 in U-2 OS cells. J Biol Chem. 2002;277:32282–32293. doi: 10.1074/jbc.M202602200. [DOI] [PubMed] [Google Scholar]
- 47.Warnke S, Kemmler S, Hames RS, Tsai HL, Hoffmann-Rohrer U, Fry AM, Hoffmann I. Polo-like kinase-2 is required for centriole duplication in mammalian cells. Curr Biol. 2004;14:1200–1207. doi: 10.1016/j.cub.2004.06.059. [DOI] [PubMed] [Google Scholar]
- 48.Ruan Q, Wang Q, Xie S, Fang Y, Darzynkiewicz Z, Guan K, Jhanwar-Uniyal M, Dai W. Polo-like kinase 3 is Golgi localized and involved in regulating Golgi fragmentation during the cell cycle. Exp Cell Res. 2004;294:51–59. doi: 10.1016/j.yexcr.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 49.Wang Q, Xie S, Chen J, Fukasawa K, Naik U, Traganos F, Darzynkiewicz Z, Jhanwar-Uniyal M, Dai W. Cell cycle arrest and apoptosis induced by human Polo-like kinase 3 is mediated through perturbation of microtubule integrity. Mol Cell Biol. 2002;22:3450–3459. doi: 10.1128/MCB.22.10.3450-3459.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zimmerman WC, Erikson RL. Polo-like kinase 3 is required for entry into S phase. Proc Natl Acad Sci USA. 2007;104:1847–1852. doi: 10.1073/pnas.0610856104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.López-Sánchez I, Sanz-García M, Lazo PA. Plk3 interacts with and specifically phosphorylates VRK1 in Ser342, a downstream target in a pathway that induces Golgi fragmentation. Mol Cell Biol. 2009;29:1189–1201. doi: 10.1128/MCB.01341-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hudson JW, Kozarova A, Cheung P, Macmillan JC, Swallow CJ, Cross JC, Dennis JW. Late mitotic failure in mice lacking Sak, a polo-like kinase. Curr Biol. 2001;11:441–446. doi: 10.1016/S0960-9822(01)00117-8. [DOI] [PubMed] [Google Scholar]
- 53.Song S, Grenfell TZ, Garfield S, Erikson RL, Lee KS. Essential function of the polo box of Cdc5 in subcellular localization and induction of cytokinetic structures. Mol Cell Biol. 2000;20:286–298. doi: 10.1128/MCB.20.1.286-298.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Archambault V, D’Avino PP, Deery MJ, Lilley KS, Glover DM. Sequestration of polo kinase to microtubules by phosphopriming-independent binding to Map205 is relieved by phosphorylation at a CDK site in mitosis. Genes Dev. 2008;22:2707–2720. doi: 10.1101/gad.486808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jiang N, Wang X, Jhanwar-Uniyal M, Darzynkiewicz Z, Dai W. Polo box domain of Plk3 functions as a centrosome localization signal, overexpression of which causes mitotic arrest, cytokinesis defects, and apoptosis. J Biol Chem. 2006;281:10577–10582. doi: 10.1074/jbc.M513156200. [DOI] [PubMed] [Google Scholar]
- 56.Jang YJ, Lin CY, Ma S, Erikson RL. Functional studies on the role of the C-terminal domain of mammalian polo-like kinase. Proc Natl Acad Sci USA. 2002;99:1984–1989. doi: 10.1073/pnas.042689299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hanisch A, Wehner A, Nigg EA, Sillje HH. Different Plk1 functions show distinct dependencies on polo-box domain-mediated targeting. Mol Biol Cell. 2006;17:448–459. doi: 10.1091/mbc.E05-08-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Elia AE, Cantley LC, Yaffe MB. Proteomic screen finds pSer/pThr-binding domain localizing Plk1 to mitotic substrates. Science. 2003;299:1228–1231. doi: 10.1126/science.1079079. [DOI] [PubMed] [Google Scholar]
- 59.Elia AE, Rellos P, Haire LF, Chao JW, Ivins FJ, Hoepker K, Mohammad D, Cantley LC, Smerdon SJ, Yaffe MB. The molecular basis for phospho-dependent substrate targeting and regulation of Plks by the polo-box domain. Cell. 2003;115:83–95. doi: 10.1016/S0092-8674(03)00725-6. [DOI] [PubMed] [Google Scholar]
- 60.Cheng KY, Lowe ED, Sinclair J, Nigg EA, Johnson LN. The crystal structure of the human polo-like kinase-1 polo box domain and its phospho-peptide complex. EMBO J. 2003;22:5757–5768. doi: 10.1093/emboj/cdg558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yun SM, Moulaei T, Lim D, Bang JK, Park JE, Shenoy SR, Liu F, Kang YH, Liao C, Soung NK, Lee S, Yoon DY, Lim Y, Lee DH, Otaka A, Appella E, McMahon JB, Nicklaus MC, Burke TRJ, Yaffe MB, Wlodawer A, Lee KS. Structural and functional analyses of minimal phosphopeptides targeting the polo-box domain of polo-like kinase 1. Nat Struct Mol Biol. 2009;16:876–882. doi: 10.1038/nsmb.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Leung GC, Hudson JW, Kozarova A, Davidson A, Dennis JW, Sicheri F. The Sak polo-box comprises a structural domain sufficient for mitotic subcellular localization. Nat Struct Biol. 2002;9:719–724. doi: 10.1038/nsb848. [DOI] [PubMed] [Google Scholar]
- 63.Lowery DM, Clauser KR, Hjerrild M, Lim D, Alexander J, Kishi K, Ong SE, Gammeltoft S, Carr SA, Yaffe MB. Proteomic screen defines the polo-box domain interactome and identifies Rock2 as a Plk1 substrate. EMBO J. 2007;26:2262–2273. doi: 10.1038/sj.emboj.7601683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van de Weerdt BC, Littler DR, Klompmaker R, Huseinovic A, Fish A, Perrakis A, Medema RH. Polo-box domains confer target specificity to the polo-like kinase family. Biochim Biophys Acta. 2008;1783:1015–1022. doi: 10.1016/j.bbamcr.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 65.Neef R, Preisinger C, Sutcliffe J, Kopajtich R, Nigg EA, Mayer TU, Barr FA. Phosphorylation of mitotic kinesin-like protein 2 by polo-like kinase 1 is required for cytokinesis. J Cell Biol. 2003;162:863–875. doi: 10.1083/jcb.200306009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kang YH, Park J-E, Yu L-R, Soung N-K, Yun S-M, Bang JK, Seong YS, Yu H, Veenstra TD, Lee KS. Self-regulation of Plk1 recruitment to the kinetochores is critical for chromosome congression and spindle checkpoint signaling. Mol Cell. 2006;24:409–422. doi: 10.1016/j.molcel.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 67.Lee KS, Park JE, Kang YH, Zimmerman W, Soung NK, Seong YS, Kwak SJ, Erikson RL. Mechanisms of mammalian polo-like kinase 1 (Plk1) localization: self- versus non-self-priming. Cell Cycle. 2008;7:141–145. doi: 10.4161/cc.7.2.5272. [DOI] [PubMed] [Google Scholar]
- 68.Park J-E, Li L, Park J, Knecht R, Strebhardt K, Yuspa SH, Lee KS. Direct quantification of polo-like kinase 1 activity in cells and tissues using a highly sensitive and specific ELISA assay. Proc Natl Acad Sci USA. 2008;106:1725–1730. doi: 10.1073/pnas.0812135106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Neef R, Gruneberg U, Kopajtich R, Li X, Nigg EA, Sillje H, Barr FA. Choice of Plk1 docking partners during mitosis and cytokinesis is controlled by the activation state of Cdk1. Nat Cell Biol. 2007;9:436–444. doi: 10.1038/ncb1557. [DOI] [PubMed] [Google Scholar]
- 70.Wolfe BA, Takaki T, Petronczki M, Glotzer M. Polo-like kinase 1 directs assembly of the HsCyk-4 RhoGAP/Ect2 RhoGEF complex to initiate cleavage furrow formation. PLoS Biol. 2009;7:e1000110. doi: 10.1371/journal.pbio.1000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Burkard ME, Maciejowski J, Rodriguez-Bravo V, Repka M, Lowery DM, Clauser KR, Zhang C, Shokat KM, Carr SA, Yaffe MB, Jallepalli PV (2009) PLoS Biol 7:e1000111 [DOI] [PMC free article] [PubMed]
- 72.Soung NK, Park JE, Yu LR, Lee KH, Lee JM, Bang JK, Veenstra TD, Rhee K, Lee KS. Plk1-dependent and -independent roles of an ODF2 splice variant, hCenexin1, at the centrosome of somatic cells. Dev Cell. 2009;16:539–550. doi: 10.1016/j.devcel.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lowery DM, Mohammad DH, Elia AE, Yaffe MB. The polo-box domain: a molecular integrator of mitotic kinase cascades and Polo-like kinase function. Cell Cycle. 2004;3:128–131. [PubMed] [Google Scholar]
- 74.Mayer BJ, Hirai H, Sakai R. Evidence that SH2 domains promote processive phosphorylation by protein–tyrosine kinases. Curr Biol. 1995;5:296–305. doi: 10.1016/S0960-9822(95)00060-1. [DOI] [PubMed] [Google Scholar]
- 75.Pellicena P, Stowell KR, Miller WT. Enhanced phosphorylation of Src family kinase substrates containing SH2 domain binding sites. J Biol Chem. 1998;273:15325–15328. doi: 10.1074/jbc.273.25.15325. [DOI] [PubMed] [Google Scholar]
- 76.Scott MP, Miller WT. A peptide model system for processive phosphorylation by Src family kinases. Biochemistry. 2000;39:14531–14537. doi: 10.1021/bi001850u. [DOI] [PubMed] [Google Scholar]
- 77.Cohen P, Frame S. The renaissance of GSK3. Nat Rev Mol Cell Biol. 2001;2:769–776. doi: 10.1038/35096075. [DOI] [PubMed] [Google Scholar]
- 78.Frame S, Cohen P. GSK3 takes centre stage more than 20 years after its discovery. Biochem J. 2001;359:1–16. doi: 10.1042/0264-6021:3590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.García-Alvarez B, de Cárcer G, Ibañez S, Bragado-Nilsson E, Montoya G. Molecular and structural basis of polo-like kinase 1 substrate recognition: implications in centrosomal localization. Proc Natl Acad Sci USA. 2007;104:3107–3112. doi: 10.1073/pnas.0609131104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Seki A, Coppinger JA, Jang CY, Yates JR, Fang G. Bora and the kinase Aurora A cooperatively activate the kinase Plk1 and control mitotic entry. Science. 2008;320:1655–1658. doi: 10.1126/science.1157425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mundt KE, Golsteyn RM, Lane HA, Nigg EA. On the regulation and function of human polo-like kinase 1(PLK1): effects of overexpression on cell cycle progression. Biochem Biophys Res Comm. 1997;239:377–385. doi: 10.1006/bbrc.1997.7378. [DOI] [PubMed] [Google Scholar]
- 82.Hof P, Pluskey S, Dhe-Paganon S, Eck MJ, Shoelson SE. Crystal structure of the tyrosine phosphatase SHP-2. Cell. 1998;92:441–450. doi: 10.1016/S0092-8674(00)80938-1. [DOI] [PubMed] [Google Scholar]
- 83.Marshall CJ. Hot lips and phosphorylation of protein kinases. Nature. 1994;367:686. doi: 10.1038/367686a0. [DOI] [PubMed] [Google Scholar]
- 84.Pawson T, Nash P. Assembly of cell regulatory systems through protein interaction domains. Science. 2003;300:445–452. doi: 10.1126/science.1083653. [DOI] [PubMed] [Google Scholar]
- 85.Manke IA, Lowery DM, Nguyen A, Yaffe MB. BRCT repeats as phosphopeptide-binding modules involved in protein targeting. Science. 2003;302:636–639. doi: 10.1126/science.1088877. [DOI] [PubMed] [Google Scholar]
- 86.Macůrek L, Lindqvist A, Lim D, Lampson MA, Klompmaker R, Freire R, Clouin C, Taylor SS, Yaffe MB, Medema RH. Polo-like kinase-1 is activated by Aurora A to promote checkpoint recovery. Nature. 2008;455:119–123. doi: 10.1038/nature07185. [DOI] [PubMed] [Google Scholar]
- 87.Tamura Y, Simizu S, Muroi M, Takagi S, Kawatani M, Watanabe N, Osada H. Polo-like kinase 1 phosphorylates and regulates Bcl-x(L) during pironetin-induced apoptosis. Oncogene. 2009;28:107–116. doi: 10.1038/onc.2008.368. [DOI] [PubMed] [Google Scholar]
- 88.Arai T, Haze K, Iimura-Morita Y, Machida T, Iida M, Tanaka K, Komatani H. Identification of beta-catenin as a novel substrate of polo-like kinase 1. Cell Cycle. 2008;7:3556–3563. doi: 10.4161/cc.7.22.7072. [DOI] [PubMed] [Google Scholar]
- 89.Seki A, Coppinger JA, Du H, Jang CY, Yates JR, Fang G. Plk1- and beta-TrCP-dependent degradation of Bora controls mitotic progression. J Cell Biol. 2008;181:65–78. doi: 10.1083/jcb.200712027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lin HR, Ting NS, Qin J, Lee WH. M phase-specific phosphorylation of BRCA2 by polo-like kinase 1 correlates with the dissociation of the BRCA2-P/CAF complex. J Biol Chem. 2003;278:35979–35987. doi: 10.1074/jbc.M210659200. [DOI] [PubMed] [Google Scholar]
- 91.Zhang H, Shi X, Paddon H, Hampong M, Dai W, Pelech S. B23/nucleophosmin serine 4 phosphorylation mediates mitotic functions of polo-like kinase 1. J Biol Chem. 2004;279:35726–35734. doi: 10.1074/jbc.M403264200. [DOI] [PubMed] [Google Scholar]
- 92.Qi W, Tang Z, Yu H. Phosphorylation- and polo-box-dependent binding of Plk1 to Bub1 is required for the kinetochore localization of Plk1. Mol Biol Cell. 2006;17:3705–3716. doi: 10.1091/mbc.E06-03-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Elowe S, Hümmer S, Uldschmid A, Li X, Nigg EA. Tension-sensitive Plk1 phosphorylation on BubR1 regulates the stability of kinetochore microtubule interactions. Genes Dev. 2007;21:2205–2219. doi: 10.1101/gad.436007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Toyoshima-Morimoto F, Taniguchi E, Nishida E. Plk1 promotes nuclear translocation of human Cdc25C during prophase. EMBO Rep. 2002;3:341–348. doi: 10.1093/embo-reports/kvf069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fabbro M, Zhou BB, Takahashi M, Sarcevic B, Lal P, Graham ME, Gabrielli BG, Robinson PJ, Nigg EA, Ono Y, Khanna KK. Cdk1/Erk2- and Plk1-dependent phosphorylation of a centrosome protein, Cep55, is required for its recruitment to midbody and cytokinesis. Dev Cell. 2005;9:477–488. doi: 10.1016/j.devcel.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 96.Guarguaglini G, Duncan PI, Stierhof YD, Holmstrom T, Duensing S, Nigg EA. The forkhead-associated domain protein Cep170 interacts with polo-like kinase 1 and serves as a marker for mature centrioles. Mol Biol Cell. 2005;16:1095–1107. doi: 10.1091/mbc.E04-10-0939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Toyoshima-Morimoto F, Taniguchi E, Shinya N, Iwamatsu A, Nishida E. Polo-like kinase 1 phosphorylates cyclin B1 and targets it to the nucleus during prophase. Nature. 2001;410:215–220. doi: 10.1038/35065617. [DOI] [PubMed] [Google Scholar]
- 98.Jackman M, Lindon C, Nigg EA, Pines J. Active cyclin B1-Cdk1 first appears on centrosomes in prophase. Nat Cell Biol. 2003;5:143–148. doi: 10.1038/ncb918. [DOI] [PubMed] [Google Scholar]
- 99.Moshe Y, Boulaire J, Pagano M, Hershko A. Role of polo-like kinase in the degradation of early mitotic inhibitor 1, a regulator of the anaphase promoting complex/cyclosome. Proc Natl Acad Sci USA. 2004;101:7937–7942. doi: 10.1073/pnas.0402442101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fu Z, Malureanu L, Huang J, Wang W, Li H, van Deursen JM, Tindal DJ, Chen J. Plk1-dependent phosphorylation of FoxM1 regulates a transcriptional programme required for mitotic progression. Nat Cell Biol. 2008;10:1076–1082. doi: 10.1038/ncb1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lin CY, Madsen ML, Yarm FR, Jang YJ, Liu X, Erikson RL. Peripheral Golgi protein GRASP65 is a target of mitotic polo-like kinase (Plk) and Cdc2. Proc Natl Acad Sci USA. 2000;97:12589–12594. doi: 10.1073/pnas.220423497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Preisinger C, Korner R, Wind M, Lehmann WD, Kopajtich R, Barr FA. Plk1 docking to GRASP65 phosphorylated by Cdk1 suggests a mechanism for Golgi checkpoint signalling. EMBO J. 2005;24:753–765. doi: 10.1038/sj.emboj.7600569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wu ZQ, Liu X. Role for Plk1 phosphorylation of Hbo1 in regulation of replication licensing. Proc Natl Acad Sci USA. 2008;105:1919–1924. doi: 10.1073/pnas.0712063105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kim SA, Yoon JH, Lee SH, Ahn SG. Polo-like kinase 1 phosphorylates heat shock transcription factor 1 and mediates its nuclear translocation during heat stress. J Biol Chem. 2005;280:12653–12657. doi: 10.1074/jbc.M411908200. [DOI] [PubMed] [Google Scholar]
- 105.Lee YJ, Kim EH, Lee JS, Jeoung D, Bae S, Kwon SH, Lee YS. HSF1 as a mitotic regulator: phosphorylation of HSF1 by Plk1 is essential for mitotic progression. Cancer Res. 2008;68:7550–7560. doi: 10.1158/0008-5472.CAN-08-0129. [DOI] [PubMed] [Google Scholar]
- 106.Higashimoto T, Chan N, Lee YK, Zandi E. Regulation of I(kappa)B kinase complex by phosphorylation of (gamma)-binding domain of I(kappa)B kinase (beta) by polo-like kinase 1. J Biol Chem. 2008;283:35354–35367. doi: 10.1074/jbc.M806258200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jang CY, Coppinger JA, Seki A, Yates JR, Fang G. Plk1 and Aurora A regulate the depolymerase activity and the cellular localization of Kif2a. J Cell Sci. 2009;122:1334–1341. doi: 10.1242/jcs.044321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Oshimori N, Ohsugi M, Yamamoto T. The Plk1 target Kizuna stabilizes mitotic centrosomes to ensure spindle bipolarity. Nat Cell Biol. 2006;8:1095–1101. doi: 10.1038/ncb1474. [DOI] [PubMed] [Google Scholar]
- 109.Liu X, Zhou T, Kuriyama R, Erikson RL. Molecular interactions of polo-like-kinase 1 with the mitotic kinesin-like protein CHO1/MKLP-1. J Cell Sci. 2004;117:3233–3246. doi: 10.1242/jcs.01173. [DOI] [PubMed] [Google Scholar]
- 110.Nakajima H, Toyoshima-Morimoto F, Taniguchi E, Nishida E. Identification of a consensus motif for Plk (polo-like kinase) phosphorylation reveals Myt1 as a Plk1 substrate. J Biol Chem. 2003;278:25277–25280. doi: 10.1074/jbc.C300126200. [DOI] [PubMed] [Google Scholar]
- 111.Asiedu M, Wu D, Matsumura F, Wei Q. Phosphorylation of MyoGEF on Thr-574 by Plk1 promotes MyoGEF localization to the central spindle. J Biol Chem. 2008;283:28392–28400. doi: 10.1074/jbc.M801801200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang X, Chen Q, Feng J, Hou J, Yang F, Liu J, Jiang Q, Zhang C. Sequential phosphorylation of Nedd1 by Cdk1 and Plk1 is required for targeting of the gammaTuRC to the centrosome. J Cell Sci. 2009;122:2240–2251. doi: 10.1242/jcs.042747. [DOI] [PubMed] [Google Scholar]
- 113.Casenghi M, Meraldi P, Weinhart U, Duncan PI, Korner R, Nigg EA. Polo-like kinase 1 regulates Nlp, a centrosome protein involved in microtubule nucleation. Dev Cell. 2003;5:113–125. doi: 10.1016/S1534-5807(03)00193-X. [DOI] [PubMed] [Google Scholar]
- 114.Zhou T, Aumais JP, Liu X, Yu-Lee LY, Erikson RL. A role for Plk1 phosphorylation of NudC in cytokinesis. Dev Cell. 2003;5:127–138. doi: 10.1016/S1534-5807(03)00186-2. [DOI] [PubMed] [Google Scholar]
- 115.Baumann C, Körner R, Hofmann K, Nigg EA. PICH, a centromere-associated SNF2 family ATPase, is regulated by Plk1 and required for the spindle checkpoint. Cell. 2007;128:101–114. doi: 10.1016/j.cell.2006.11.041. [DOI] [PubMed] [Google Scholar]
- 116.Eckerdt F, Yuan J, Saxena K, Martin B, Kappel S, Lindenau C, Kramer A, Naumann S, Daum S, Fischer G, Dikic I, Kaufmann M, Strebhardt K. Polo-like kinase 1-mediated phosphorylation stabilizes Pin1 by inhibiting its ubiquitination in human cells. J Biol Chem. 2005;280:36575–36583. doi: 10.1074/jbc.M504548200. [DOI] [PubMed] [Google Scholar]
- 117.Feng Y, Yuan JH, Maloid SC, Fisher R, Copeland TD, Longo DL, Conrads TP, Veenstra TD, Ferris A, Hughes S, Dimitrov DS, Ferris DK. Polo-like kinase 1-mediated phosphorylation of the GTP-binding protein Ran is important for bipolar spindle formation. Biochem Biophys Res Commun. 2006;349:144–152. doi: 10.1016/j.bbrc.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 118.Soond SM, Barry SP, Melino G, Knight RA, Latchman DS, Stephanou A. p73-mediated transcriptional activity is negatively regulated by polo-like kinase 1. Cell Cycle. 2008;7:1214–1223. doi: 10.4161/cc.7.9.5777. [DOI] [PubMed] [Google Scholar]
- 119.Yarm FR. Plk phosphorylation regulates the microtubule-stabilizing protein TCTP. Mol Cell Biol. 2002;22:6209–6221. doi: 10.1128/MCB.22.17.6209-6221.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yang X, Li H, Zhou Z, Wang WH, Deng A, Andrisani O, Liu X. Plk1-mediated phosphorylation of Topors regulates p53 stability. J Biol Chem. 2009;284:18588–18592. doi: 10.1074/jbc.C109.001560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li H, Wang Y, Liu X. Plk1-dependent phosphorylation regulates functions of DNA topoisomerase IIalpha in cell cycle progression. J Biol Chem. 2008;283:6209–6221. doi: 10.1074/jbc.M709007200. [DOI] [PubMed] [Google Scholar]
- 122.Wu ZQ, Yang X, Weber G, Liu X. Plk1 phosphorylation of TRF1 is essential for its binding to telomeres. J Biol Chem. 2008;283:25503–25513. doi: 10.1074/jbc.M803304200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yamaguchi T, Goto H, Yokoyama T, Silljé H, Hanisch A, Uldschmid A, Takai Y, Oguri T, Nigg EA, Inagaki M. Phosphorylation by Cdk1 induces Plk1-mediated vimentin phosphorylation during mitosis. J Cell Biol. 2005;171:431–436. doi: 10.1083/jcb.200504091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Watanabe N, Arai H, Iwasaki J, Shiina M, Ogata K, Hunter T, Osada H. Cyclin-dependent kinase (CDK) phosphorylation destabilizes somatic Wee1 via multiple pathways. Proc Natl Acad Sci USA. 2005;102:11663–11668. doi: 10.1073/pnas.0500410102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Inglis KJ, Chereau D, Brigham EF, Chiou SS, Schöbel S, Frigon NL, Yu M, Caccavello RJ, Nelson S, Motter R, Wright S, Chian D, Santiago P, Soriano F, Ramos C, Powell K, Goldstein JM, Babcock M, Yednock T, Bard F, Basi GS, Sham H, Chilcote TJ, McConlogue L, Griswold-Prenner I, Anderson JP. Polo-like kinase 2 (PLK2) phosphorylates alpha-synuclein at serine 129 in central nervous system. J Biol Chem. 2009;284:2598–2602. doi: 10.1074/jbc.C800206200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Seeburg DP, Feliu-Mojer M, Gaiottino J, Pak DT, Sheng M. Critical role of CDK5 and polo-like kinase 2 in homeostatic synaptic plasticity during elevated activity. Neuron. 2008;58:571–583. doi: 10.1016/j.neuron.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bahassi EM, Hennigan RF, Myer DL, Stambrook PJ. Cdc25C phosphorylation on serine 191 by Plk3 promotes its nuclear translocation. Oncogene. 2004;23:2658–2663. doi: 10.1038/sj.onc.1207425. [DOI] [PubMed] [Google Scholar]
- 128.Bahassi EM, Myer DL, McKenney RJ, Hennigan RF, Stambrook PJ. Priming phosphorylation of Chk2 by polo-like kinase 3 (Plk3) mediates its full activation by ATM and a downstream checkpoint in response to DNA damage. Mutat Res. 2006;596:166–176. doi: 10.1016/j.mrfmmm.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 129.Sang M, Ando K, Okoshi R, Koida N, Li Y, Zhu Y, Shimozato O, Geng C, Shan B, Nakagawara A, Ozaki T. Plk3 inhibits pro-apoptotic activity of p73 through physical interaction and phosphorylation. Genes Cells. 2009;14:775–788. doi: 10.1111/j.1365-2443.2009.01309.x. [DOI] [PubMed] [Google Scholar]
- 130.Iida M, Matsuda M, Komatani H. Plk3 phosphorylates topoisomerase IIalpha at Thr(1342), a site that is not recognized by Plk1. Biochem J. 2008;411:27–32. doi: 10.1042/BJ20071394. [DOI] [PubMed] [Google Scholar]