Abstract
Numerous studies show that pharmacological inhibition of p38 mitogen-activated protein kinases (p38s) before lethal ischemia prevents conditioning. However, these inhibitors have off-target effects and do not discriminate between the alpha and beta isoforms; the activation of which is thought to have diverse and perhaps opposing actions with p38α aggravating, and p38β reducing, myocardial injury. We adopted a chemical genetic approach using mice in which either the p38α (DRα) or p38β (DRβ) alleles were targeted to substitute the “gatekeeper” threonine residue for methionine, thereby preventing the binding of a pharmacological inhibitor, SB203580. Isolated, perfused wild-type (WT), DRα and DRβ mouse hearts underwent ischemic preconditioning with 4 cycles of 4 min ischemia/6 min reperfusion, with or without SB203580 (10 µM), followed by 30 min of global ischemia and 120 min of reperfusion. In WT and DRβ hearts, SB203580 completely abolished the reduction in myocardial infarction seen with preconditioning and also the phosphorylation of downstream substrates of p38. These effects of SB203580 were not seen in DRα hearts. Furthermore ischemic preconditioning occurred unaltered in p38β null hearts. Contrary to expectation the activation of p38α, and not p38β, is necessary for ischemic preconditioning. Since p38α is also the isoform that leads to lethal myocardial injury, it is unlikely that targeted therapeutic strategies to achieve isoform-selective inhibition will only prevent the harmful consequences of activation.
Abbreviations: βKO, p38β knock-out; I/R%, myocardial infarction as a percentage of myocardium at risk of infarction; DRα, drug-resistant p38α; DRβ, drug-resistant p38β; HSP27, heat stress protein of 27 kDa; MAPK, mitogen-activated protein kinase; MAPKK, mitogen-activated protein kinase kinase; MAPKKK, mitogen-activated protein kinase kinase kinase; P38, p38 mitogen-activated protein kinase; SAPK, stress activated protein kinase; TAB1, transforming growth factorβ-activated protein kinase 1-binding protein 1; T106M, substitution of threonine 106 for methionine; WT, wild type
Keywords: Myocardial infarction, SB203580, Ischemic preconditioning, p38 MAPK, Chemical genetics
1. Introduction
The heart is a dynamic organ that must respond and adapt to a variety of stresses throughout life. These adaptations are achieved through numerous signaling pathways of which the aptly named stress-activated protein kinases (SAPKs) are amongst the most evolutionarily conserved. The SAPKs consist of the Jun N-terminal kinases and the p38 mitogen-activated protein kinases (p38s). The p38s in turn comprise 4 family members, encoded by separate genes, p38α, p38β, p38γ and p38δ [1]. There are considerable data suggesting that inhibition of p38s during lethal ischemia is cardioprotective whilst under identical conditions the same inhibitors can block the protection seen with ischemic preconditioning [2]. The pharmacological inhibitors used in these studies, such as SB203580, only affect the p38α and p38β isoforms since their selectivity relies on their probing of a “hole” at the edge of the ATP-binding pocket guarded by a “gatekeeper” threonine residue (106 in murine and human p38α and p38β) [3,4]. In p38γ and p38δ this position is occupied by a more bulky methionine residue that prevents binding of the inhibitor and therefore its competition with ATP. By substituting threonine 106 for methionine (T106M), p38α and p38β can be rendered similarly resistant to pharmacological inhibition without altering kinase activity [4]. SB203580 is a frequently used cell-permeate pharmacological inhibitor with a rapid onset and offset of action but it is not selective and known to inhibit a number of other kinases [5]. Using the T106M knock-in mice, we are able to discriminate between the on and off-target effects of this inhibitor.
The reason remains uncertain why pharmacological inhibition of p38α and p38β should result in protection during lethal ischemia and the prevention of ischemic preconditioning before lethal ischemia. However, the favored explanation is that p38α is the dominant isoform activated during lethal ischemia, and its activation aggravates injury; whilst p38β is the dominant isoform activated during the sublethal ischemia/reperfusion of preconditioning, and its activation diminishes injury [6–8]. Defining the exact contribution of these isoforms in order to only selectively inhibit the detrimental form is important since it may enhance the magnitude of benefit in planned and ongoing (NCT00910962) clinical trials in myocardial infarction.
A number of studies that do not rely on pharmacological inhibitors suggest that p38α aggravates lethal injury [3,9,10]. Therefore, our hypothesis was that p38β is the isoform responsible for inhibitors blocking preconditioning.
2. Materials and methods
2.1. Mice
All experiments were performed in accordance with United Kingdom Home Office Guidance on the Operation of Animals (Scientific Procedures) Act 1986, published by Her Majesty's Stationary Office, London.
The knock-in mice harboring a T106M substitution in p38α (Drug-Resistant, DRα), or in p38β (DRβ), were on an isogenic C57BL/6 background shared with their corresponding wild type mice (B6) and have been described previously [11]. The knock-out mice for p38β (p38βKO) that were on a C57BL/6 background resulting from backcrossing for at least 10 generations have also been described previously [12] and were compared with their corresponding wild type mice (WT).
2.2. Perfusion of isolated murine hearts
Adult male mice were anesthetized with pentobarbital (300 mg/kg) and heparin (150 U) intraperitoneally. Hearts were rapidly isolated, mounted onto a Langendorff apparatus and retrogradely perfused at a constant pressure of 80 mm Hg with Krebs–Hensleit buffer (in mmol/L: 118.5 NaCl, NaHCO3, 4.75 KCl, 0.18 KH2PO4, 1.19 MgSO4, 11.0 d-glucose, and 1.41 CaCl2) equilibrated with 95% O2 and 5% CO2 at 37 °C. A fluid-filled balloon inserted into the left ventricular cavity monitored contractile function. The balloon was gradually inflated until the left ventricular end-diastolic pressure (LVEDP) was between 2 and 8 mm Hg. Atrial pacing was performed at 580 bpm and coronary flow (CF) was measured by timed collection of perfusate.
2.3. Infarction assessment in isolated murine hearts
Hearts were stabilized for 50 min after initiation of retrograde perfusion. For inclusion, all hearts had to fulfill the following criteria: CF between 1.5 and 4.5 mL/min, initial heart rate > 300 bpm (unpaced), left ventricular developed pressure (LVDP) > 55 mm Hg, time from thoracotomy to aortic cannulation < 3 min, and no persistent dysrhythmias during the stabilization period. All hearts underwent 30 min of global ischemia followed by 2 h of reperfusion. At the end of reperfusion hearts were perfused for 1 min with 5 mL of 1% triphenyl tetrazolium chloride (TTC) in PBS and then placed in an identical solution at 37 °C for 10 min. The atria were then removed, and the hearts were blotted dry, weighed, and stored at − 20 °C. Hearts were subsequently thawed, placed in 2.5% glutaraldehyde for 1 min and set in 5% agarose. The agarose heart blocks were then sectioned from apex to base in 0.75 mm slices using a vibratome (Agar Scientific). Sections were compressed between glass plates and scan-imaged (Epson model G850A). After magnification, planimetry was carried out using image analysis software (Adobe Photoshop 7.0). Risk and infarct volumes were calculated from surface area analysis of whole myocardium and TTC-negative myocardium, respectively, multiplied by tissue thickness. After summation of individual slices, infarct volume was expressed as a percentage of ventricular volume. Infarct analysis was performed in all cases by an investigator blinded to the group assignments.
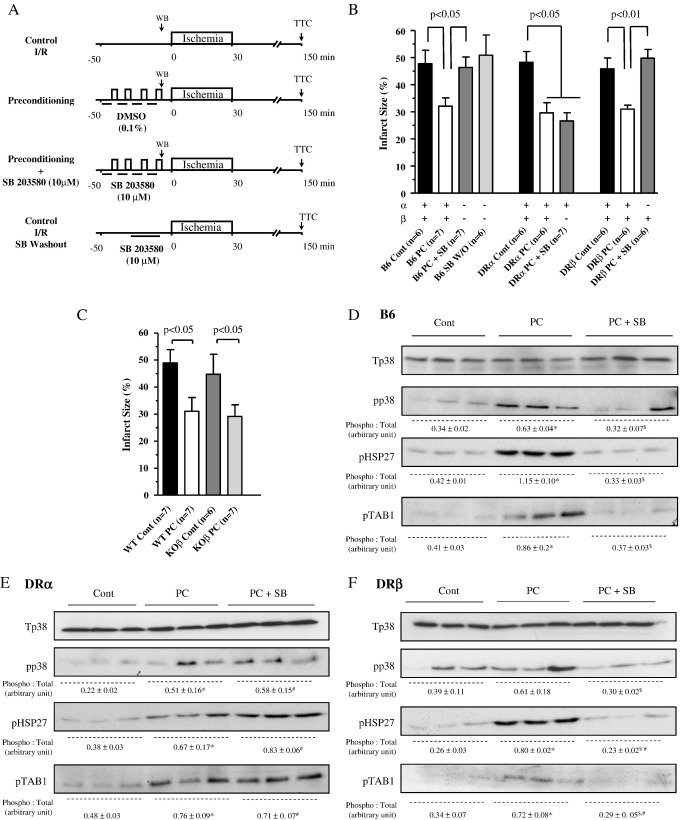
The experimental protocol appears as in Fig. 1(A).
Fig. 1.
Experimental protocol and infarct sizes. Panel (A) All hearts were subjected to 50 min of stabilization and 30 min of global ischemia. Preconditioning was by 4 cycles of 4 min global ischemia with 6 min of reperfusion with blinding to corresponding vehicle (DMSO 0.1%). SB203580 (10 μmol/L) started 6 min before, and during, preconditioning but was washed out during the last reperfusion. After 120 min of reperfusion the infarct size was determined by TTC staining. Hearts were harvested for immunoblotting on the last period of preconditioning ischemia. Panels (B) and (C) Infarct size is expressed as percent of the left ventricular volume with statistical comparisons as shown. The number of observations on which the data are based appears as the numeral inset in the bar labels. The “+” and “−” signs below each bar denote which p38 isoform(s) remains active under the set conditions. Panels (D), (E) and (F) Activation of p38 and phosphorylation of downstream substrates in the presence and absence of pharmacological inhibition with SB203580 in B6 (panel (D)), DR α (panel (E)) and DRβ (panel (F)) hearts. In the immunoblots below Tp38 is total p38, pp38 dual phospho-p38 (on Thr180 and Tyr182), pHSP27 phosphorylated 27 kDa heat shock protein (on Ser82) and pTAB1 phosphorylated TAB1 (on Ser423). Panels (D) and (F) demonstrate that SB present during preconditioning cycles of ischemia prevents the downstream phosphorylation of HSP27, a substrate of mitogen-activated protein kinase activated protein kinase-2 that is in turn activated by p38 and TAB1 a direct substrate of p38. The phosphorylation of these downstream substrates is still present in DRα hearts exposed to SB confirming the lack of p38 inhibition despite the presence of SB203580. Protein in each lane is from a different heart. The figures below each band represent quantification of band density from 3 separate experiments expressed as mean + SEM. ⁎P < 0.05 Cont vs. PC; $P < 0.05 PC vs. PC + SB; #P < 0.05 Cont vs. PC + SB.
2.4. Immunoblotting
Samples were thawed and homogenized in protein extraction buffer (50 mmol/L Tris–HCl; pH 7.5, 1 mmol/L EDTA, 1 mmol/L EGTA, 1% (w/v) Triton X-100, 0.1% β-mercaptoethanol, 1 mmol/L sodium orthovanadate, 50 mmol/L sodium fluoride, 5 mmol/L sodium pyrophosphate and protease inhibitor tablets (Complete®, Roche Applied Science), (1 Tab/50 mL of buffer)). One millilitre of extraction buffer was used per 100 mg of frozen tissue. Homogenates were centrifuged at 4 °C for 10 min at 13,000 rpm and the insoluble fraction discarded. Protein content was estimated using a Bio-Rad Protein Assay Kit. Twenty micrograms of protein was electrophoresed on a 10% polyacrylamide gel and then blotted onto a polyvinylidene difluoride membrane.
PVDF membranes which were blocked for 2 h with 5% non-fat milk + 1%BSA in TRIS-buffered saline (pH 7.4) containing 0.1% Triton (TBST) and probed overnight at 4 °C with the appropriate primary antibody recognizing total-p38 (#9212) (Tp38), diphospho-p38 (#9211, polyclonal) (pp38) or phospho-HSP27 (#2401) all from Cell Signaling, or to phospho-TAB1(S423) from Sir Philip Cohen (Dundee UK). After washing and exposure for 2 h at room temperature to HRP-conjugated secondary antibody, antibody–antigen complexes were visualized by enhanced chemiluminescence as previously described [13].
2.5. Statistics
Data sets were analyzed by two-way ANOVA and groups were compared using Tukey's test. A P value less than 0.05 was considered significant.
3. Results and discussion
In this study, we tested the hypothesis that p38β is the isoform responsible for the loss of preconditioning with pharmacological inhibitors that do not discriminate between isoforms (Fig. 1(A)). In order to examine this question, we used DRα and DRβ mice in which the substitution of threonine 106 for methionine renders p38α or p38β resistant to pharmacological inhibition without altering kinase activity.
Baseline coronary flow and contractile parameters were similar at the end of stabilization across mouse hearts of all genotypes (Table 1). In B6/WT hearts preconditioning resulted in significant improvements in post-ischemic hemodynamic recovery that reflected infarct size (Table 1 and Fig. 1(B)). Exposure to SB203580 during preconditioning cycles completely abolished its benefit in B6 and in DRβ mice (I/R% 32.1% vs. 46.4%, P < 0.05 and 31.0% vs. 49.8%, respectively, P < 0.01, Fig. 1(B)) in which p38α, but not p38β, was sensitive to inhibition. However, SB203580 failed to prevent preconditioning in the DRα mice (I/R% 29.6 vs. 26.6%, P > 0.05, Fig. 1(B)) in which p38β, but not p38α, was sensitive to inhibition. These differential effects of SB203580 by genotype were mirrored in post-ischemic contractile recovery (Table 1).
Table 1.
Baseline characteristics and hemodynamic recovery in drug resistant p38α (DRα) and p38β (DRβ) mice and their corresponding wild-type (B6) and in p38β null mice (p38β KO) and their corresponding wild-type (WT).
| Treatment | n | Body weight (g) | LVDP (mm Hg) |
LVEDP (mm Hg) |
CF (mL/min) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 60 min Rep | 120 min Rep | Baseline | 60 min Rep | 120 min Rep | Baseline | 60 min Rep | 120 min Rep | |||
| B6 | 6 | 28.6 ± 1.0 | 69.8 ± 5.7 | 6.5 ± 3.4 | 8.2 ± 4.7 | 6.2 ± 2.9 | 68.7 ± 10.6 | 62.4 ± 10.5 | 2.6 ± 0.3 | 1.5 ± 0.3 | 1.2 ± 0.5 |
| B6 PC | 7 | 27.9 ± 1.2 | 65.1 ± 6.3 | 15.2 ± 8.3⁎,† | 18.5 ± 5.6⁎,† | 5.3 ± 2.8 | 55.6 ± 10.9⁎ | 47.6 ± 10.5⁎ | 2.3 ± 0.3 | 1.7 ± 0.4 | 1.5 ± 0.5 |
| B6 PC + SB | 7 | 26.8 ± 1.7 | 62.3 ± 7.2 | 3.6 ± 3.2 | 5.5 ± 2.9 | 7.6 ± 2.7 | 63.3 ± 10.2 | 56.3 ± 8.6 | 2.8 ± 0.4 | 1.7 ± 0.4 | 1.4 ± 0.6 |
| B6 SB WO | 6 | 24.3 ± 2.4 | 63.6 ± 5.4 | 3.3 ± 3.6 | 4.8 ± 3.6 | 5.3 ± 3.7 | 61.8 ± 6.7 | 55.8 ± 6.7 | 2.9 ± 0.3 | 1.3 ± 0.2 | 1.2 ± 0.3 |
| DR α | 6 | 25.9 ± 1.5 | 67.5 ± 6.5 | 5.8 ± 4.7 | 6.0 ± 4.5 | 4.8 ± 2.2 | 65 ± 6.2 | 58.1 ± 9.8 | 2.4 ± 0.2 | 1.2 ± 0.4 | 1.0 ± 0.3 |
| DR α PC | 6 | 25.1 ± 1.3 | 62.2 ± 5.9 | 16.8 ± 7.1‡ | 17.6 ± 4.7 | 5.1 ± 2.0 | 54.3 ± 8.8 | 47.5 ± 5.8‡ | 2.3 ± 0.3 | 1.6 ± 0.4 | 1.5 ± 0.5 |
| DR α PC + SB | 7 | 25.1 ± 1.5 | 62.5 ± 3.8 | 14.2 ± 6.5§ | 15.1 ± 4.7 | 6.7 ± 3.3 | 57.5 ± 8.1 | 50.5 ± 8.2§ | 2.7 ± 0.5 | 1.7 ± 0.3 | 1.5 ± 0.6 |
| DR β | 6 | 27.6 ± 1.2 | 68.5 ± 5.7 | 4.1 ± 2.3 | 2.3 ± 3.5 | 5.6 ± 1.6 | 67.6 ± 12.7 | 59.3 ± 10.6 | 2.7 ± 0.4 | 1.4 ± 0.4 | 1.3 ± 0.6 |
| DR β PC | 6 | 24.9 ± 1.0 | 62.1 ± 5.8 | 12.7 ± 7.9||,# | 13.8 ± 7.8||,# | 4.5 ± 1.9 | 54.2 ± 14.5||,# | 46.7 ± 13.0 | 2.6 ± 0.4 | 1.8 ± 0.4 | 1.7 ± 0.5 |
| DR β PC + SB | 6 | 25.9 ± 1.2 | 65.1 ± 3.0 | 3.2 ± 3.1 | 3.3 ± 2.7 | 7.9 ± 1.8 | 65.8 ± 10.7 | 56.3 ± 10.5 | 3.0 ± 0.5 | 1.8 ± 0.6 | 1.5 ± 0.6 |
| WT | 7 | 29.9 ± 0.6 | 71.0 ± 11.1 | 15.5 ± 6.7 | 18.3 ± 6.8 | 7.8 ± 1.1 | 54.3 ± 13.1 | 47.3 ± 14.5 | 3.8 ± 0.9 | 2.0 ± 0.6 | 1.5 ± 0.5 |
| WT PC | 7 | 29.3 ± 0.5 | 69.9 ± 10.9 | 26.5 ± 13.1⁎⁎ | 36.7 ± 13.6⁎⁎ | 8.6 ± 2.8 | 33.6 ± 11.6 | 27.7 ± 6.6⁎⁎ | 3.2 ± 0.8 | 2.2 ± 0.7 | 2.3 ± 0.8⁎⁎ |
| p38 β KO | 6 | 30.5 ± 0.8 | 71.4 ± 8.6 | 21.7 ± 7.2 | 20.1 ± 9.6 | 6.8 ± 1.3 | 50.2 ± 17.7 | 45.1 ± 13.8 | 3.7 ± 0.6 | 2.2 ± 0.6 | 1.8 ± 0.8 |
| p38 β KO PC | 7 | 28.4 ± 0.6 | 75.9 ± 6.6 | 18.6 ± 10.5 | 31.3 ± 9.2†† | 6.7 ± 4.9 | 45.8 ± 22.1 | 34.3 ± 18.9 | 3.5 ± 0.4 | 2.5 ± 2.1 | 2.4 ± 1.3 |
Values are means ± SEM. ⁎P < 0.05 B6 PC vs. B6, †P < 0.05 B6 PC vs. B6 PC + SB, ‡P < 0.05 DR α PC vs. DR α, §P < 0.05 DR α PC + SB vs. DR α, ||P < 0.05 DRβ PC vs. DR β, #P < 0.05 DR β PC vs. DR β PC + SB; ⁎⁎P < 0.05 WT PC vs. WT; ††P < 0.05 p38 β KO PC vs. p38 β KO.
Left ventricular developed pressure (LVDP), left ventricular end-diastolic pressure (LVEDP) and coronary flow (CF) were measured after 30 min of stabilization (Baseline), and at 60 min (Rep 60 min) and 120 min (Rep 120 min) after the onset of reperfusion.
In order to confirm that ischemic preconditioning occurred independently of p38β we subjected βKO mouse hearts to an identical protocol. Baseline coronary flow and contractility were similar at the end of the stabilization period in all groups (Table 1). Preconditioning similarly improved hemodynamic recovery (Table 1) and reduced infarct size (Fig. 1(C)) in WT hearts with, and p38βKO hearts without, p38β. Taken together with the results in the drug-resistant mouse hearts, the findings indicate that ischemic preconditioning is dependent on the activation of p38α, but not of p38β.
We next examined the effects of SB203580 on downstream signaling (Figs. 1(D)–(F)) by investigating the intensity of the dual phosphorylation of p38 as well as the phosphorylation of the downstream substrates TAB1 and HSP27 on Ser423 and Ser82, respectively which likely reflects the transient activation of p38 in Langendorff perfused heart. The phosphoacceptor sites on TAB1 and HSP27 are both sensitive to SB203580 in WT and DRβ hearts (Figs. 1(D), (F)) but become resistant in DRα hearts, reflecting the dominance of this isoform during preconditioning and its resistance to inhibition by SB203580 (Fig. 1(E)).
Thus, the major finding in this study is that contrary to expectation the activation of p38α and not p38β is necessary for ischemic preconditioning. This conclusion is based both on a chemical-genetic approach using targeted mouse-lines with a single amino-acid substitution in p38α or p38β and is corroborated in p38β-null mice.
To our knowledge, this is the first report which causally links the activation of p38α with cardioprotection. Furthermore, it is also one of the first examples of in-vivo murine chemical-genetics applied to a cardiovascular phenotype and circumvents many of the disadvantages that cloud data interpretation in transgenic mouse models [14].
Our findings are surprising since detrimental consequences of p38α activation are supported by studies that do not simply rely on pharmacological inhibition or ectopic overexpression of a wild-type isoform. For example, the protective effect of SB203580 on isolated cardiomyocytes exposed to lethal simulated ischemia is lost with expression of p38α resistant to pharmacological inhibition [3]. Similarly, mice heterozygous for a p38α null allele, leading to reduced protein content [10], are resistant to infarction as are mice transgenic for a kinase dead form of p38α [9]. Thus there is strong direct evidence that the activation of p38α during lethal ischemia increases injury. However, recent studies in conditional cardiac-specific p38α null mice subjected to pressure overload similarly suggest that this isoform can play an unexpected protective role [15].
There are several reasons why under some circumstances the activation of a single isoform of p38 can lead to reduced, and under others increased, injury, including the timing of activation, differential localization and perhaps most importantly different modes of activation [1]. Instead of the classical MAPKKK–MAPKK–MAPK canonical activation cascade, p38α is also known to auto-activate through at least 2 mechanisms one dependent on the kinase ZAP-70, and the other the scaffold protein TAB-1 [1,16]. In the later case at least this seems relevant to ischemia and may antagonise the canonical pathway [1].
Although it would be interesting to replicate the protocol in an in-vivo model this is impractical since it is essential to washout the p38 inhibitor prior to lethal ischemia in order to ensure data interpretation is not clouded by the direct, but separate, cardioprotective effects of these compounds during sustained ischemia [1].
The findings of our study suggest that it will not be possible to avoid interfering with the beneficial effects of p38 activation by selectively inhibiting p38α. Instead attention needs to focus on the circumstance specific components of this pathway that lie upstream and downstream of this key isoform.
Acknowledgments
We thank Sir Philip Cohen (Dundee, UK) for providing anti-phospho-TAB1 (S423) antibody.
This work was supported by project grants from the Medical Research Council (G0802033) and British Heart Foundation (07/073/23432).
References
- 1.Clark J.E., Sarafraz N., Marber M.S. Potential of p38-MAPK inhibitors in the treatment of ischaemic heart disease. Pharmacol Ther. 2007;116:192–206. doi: 10.1016/j.pharmthera.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 2.Sanada S., Kitakaze M., Papst P.J., Hatanaka K., Asanuma H., Aki T. Role of phasic dynamism of p38 mitogen-activated protein kinase activation in ischemic preconditioning of the canine heart. Circ Res. 2001;88:175–180. doi: 10.1161/01.res.88.2.175. [DOI] [PubMed] [Google Scholar]
- 3.Martin J.L., Avkiran M., Quinlan R.A., Cohen P., Marber M.S. Antiischemic effects of SB203580 are mediated through the inhibition of p38alpha mitogen-activated protein kinase: evidence from ectopic expression of an inhibition-resistant kinase. Circ Res. 2001;89:750–752. doi: 10.1161/hh2101.099504. [DOI] [PubMed] [Google Scholar]
- 4.Eyers P.A., van d I., Quinlan R.A., Goedert M., Cohen P. Use of a drug-resistant mutant of stress-activated protein kinase 2a/p38 to validate the in vivo specificity of SB 203580. FEBS Lett. 1999;451:191–196. doi: 10.1016/s0014-5793(99)00552-9. [DOI] [PubMed] [Google Scholar]
- 5.Godl K., Wissing J., Kurtenbach A., Habenberger P., Blencke S., Gutbrod H. An efficient proteomics method to identify the cellular targets of protein kinase inhibitors. Proc Natl Acad Sci U S A. 2003;100:15434–15439. doi: 10.1073/pnas.2535024100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saurin A.T., Martin J.L., Heads R.J., Foley C., Mockridge J.W., Wright M.J. The role of differential activation of p38-mitogen-activated protein kinase in preconditioned ventricular myocytes. FASEB J. 2000;14:2237–2246. doi: 10.1096/fj.99-0671com. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y., Huang S., Sah V.P., Ross J., Jr., Brown J.H., Han J. Cardiac muscle cell hypertrophy and apoptosis induced by distinct members of the p38 mitogen-activated protein kinase family. J Biol Chem. 1998;273:2161–2168. doi: 10.1074/jbc.273.4.2161. [DOI] [PubMed] [Google Scholar]
- 8.Martindale J.J., Wall J.A., Martinez-Longoria D.M., Aryal P., Rockman H.A., Guo Y. Overexpression of mitogen-activated protein kinase kinase 6 in the heart improves functional recovery from ischemia in vitro and protects against myocardial infarction in vivo. J Biol Chem. 2005;280:669–676. doi: 10.1074/jbc.M406690200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaiser R.A., Bueno O.F., Lips D.J., Doevendans P.A., Jones F., Kimball T.F. Targeted inhibition of p38 mitogen-activated protein kinase antagonizes cardiac injury and cell death following ischemia–reperfusion in vivo. J Biol Chem. 2004;279:15524–15530. doi: 10.1074/jbc.M313717200. [DOI] [PubMed] [Google Scholar]
- 10.Otsu K., Yamashita N., Nishida K., Hirotani S., Yamaguchi O., Watanabe T. Disruption of a single copy of the p38alpha MAP kinase gene leads to cardioprotection against ischemia–reperfusion. Biochem Biophys Res Commun. 2003;302:56–60. doi: 10.1016/s0006-291x(03)00096-2. [DOI] [PubMed] [Google Scholar]
- 11.O'Keefe S.J., Mudgett J.S., Cupo S., Parsons J.N., Chartrain N.A., Fitzgerald C. Chemical genetics define the roles of p38alpha and p38beta in acute and chronic inflammation. J Biol Chem. 2007;282:34663–34671. doi: 10.1074/jbc.M704236200. [DOI] [PubMed] [Google Scholar]
- 12.Beardmore V.A., Hinton H.J., Eftychi C., Apostolaki M., Armaka M., Darragh J. Generation and characterization of p38beta (MAPK11) gene-targeted mice. Mol Cell Biol. 2005;25:10454–10464. doi: 10.1128/MCB.25.23.10454-10464.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumphune S., Bassi R., Jacquet S., Sicard P., Clark J.E., Verma S. A chemical genetic approach reveals that p38alpha MAPK activation by diphosphorylation aggravates myocardial infarction and is prevented by the direct binding of SB203580. J Biol Chem. 2010;285:2968–2975. doi: 10.1074/jbc.M109.079228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cook S.A., Clerk A., Sugden P.H. Are transgenic mice the ‘alkahest’ to understanding myocardial hypertrophy and failure? J Mol Cell Cardiol. 2009;46:118–129. doi: 10.1016/j.yjmcc.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 15.Nishida K., Yamaguchi O., Hirotani S., Hikoso S., Higuchi Y., Watanabe T. p38alpha mitogen-activated protein kinase plays a critical role in cardiomyocyte survival but not in cardiac hypertrophic growth in response to pressure overload. Mol Cell Biol. 2004;24:10611–10620. doi: 10.1128/MCB.24.24.10611-10620.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ashwell J.D. The many paths to p38 mitogen-activated protein kinase activation in the immune system. Nat Rev Immunol. 2006;6:532–540. doi: 10.1038/nri1865. [DOI] [PubMed] [Google Scholar]