Abstract
Background and Objectives
Percutaneous occlusion of patent ductus arteriosus (PDA) has become increasingly attractive with the evolution of devices and techniques. We reviewed results for percutaneous occlusion of PDA using various devices in a single center.
Subjects and Methods
A retrospective review was done for 118 consecutive procedures performed in 111 patients with PDA between January 1996 and December 2007.
Results
The median age of the patients was 4.5 years (0.9 to 60.3 years); body weight was 16.9 kg (6.8 to 74.7 kg). The median PDA diameter at the pulmonic end was 3.8 mm (0.7 to 10 mm); mean pulmonary artery pressure was 21.0 mmHg (7 to 60 mmHg). Complete occlusion occurred in 76/111 (68.4%) immediately after implantation and in 100/111 (90.0%) at one year of follow-up. Second procedures for residual shunts were done in 7 patients. After the year 2001, the complete closure rate was 95.2% compared to 71.4% before 2001. Complications associated with the procedure were left pulmonary artery narrowing (all <20 mmHg) in 14, arrhythmia in 2, and death in 1.
Conclusion
Evolution of devices, cumulative experience, and health insurance covering the cost of devices have contributed to good outcomes in our center for percutaneous occlusion of PDA. Our results have improved over the years, particularly with the use of the Amplatzer duct occluder.
Keywords: Ductus arteriosus, patent
Introduction
Since Rao et al.1) first reported transcatheter occlusion (TCO) of a patent ductus arteriosus (PDA) with an Ivalon plug, several other devices have been developed including Rashkind double-umbrella (umbrella),2) Sideris buttoned device (SBD),3) Gianturco coil4) or Duct-occlud5) device (coil), and an Amplatzer Duct Occluder (ADO).6) We reviewed our experience with TCO of PDA using various devices to determine the efficacy and safety of TCO with regard to rates of success and complications.
Subjects and Methods
Subjects
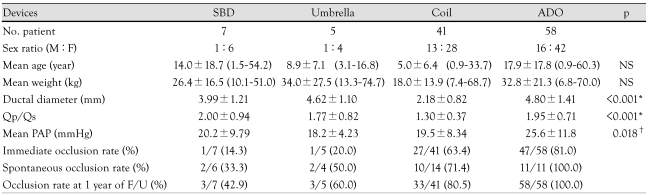
A retrospective review was done for 118 consecutive procedures performed in 111 patients (31 males, 80 females) for PDA during the 12 years from January 1996 to December 2007. Associated anomalies were ventricular septal defect (n=4), atrial septal defect (2), pulmonary valve stenosis (1), anomalous origin of the left coronary artery from the pulmonary artery (1), and Down syndrome (3). For TCO of PDA, we used a SBD (Custom Medical Devices, Athens, Greece; TX, USA), an umbrella (USCI, Billerica, MA, USA), coils such as a Gianturco coil (Cook, Inc., Bloomington, IN, USA), a Duct-occlud device (PFM, Cologne, Germany), and an ADO (AGA, MN, USA). Patients were divided into four groups based on the devices that were used: SBD, umbrella, coil, and ADO. In our center, SBD was used by the year 1997 and umbrella by 1999, both for somewhat large ductus with big shunts. After 1996, coils were favored in cases of a rather small ductus (ductal diameter ≤2.5 mm) with one or more coils. For secondary occlusion, we mostly used a coil. ADO has been used for ductus >2.5 mm (ductal diameter) since 1999 (Table 1).
Table 1.
Demographic and catheterization data for each device group
*Significant differences between coil and other device groups, †Significant difference between coil and ADO groups. Qp/Qs: determination of the pulmonary to systemic blood flow ratio, PAP: pulmonary arterial pressure, NS: no significant difference between groups, SBD: Sideris buttoned device, ADO: Amplatzer duct occluder, F/U: follow up
Procedures and follow up
The procedure for each device has been reported.2),3),5),7) The ductal diameter was measured at the pulmonic end in the lateral aortogram.8) The residual shunt was identified by aortogram, 10 to 15 minutes after the implantation of devices. The catheterization data and angiograms were reviewed by one examiner (NY Kim). Two-dimensional echocardiography and Doppler studies were performed on the next day, and 3, 6, and 12 months following the procedure. Attention was paid to the residual ductal flow and stenosis of the left pulmonary artery and the aortic arch.9),10)
Statistical analysis
Statistical Package for the Social Sciences (SPSS) for Windows (version 12.0, SPSS Inc., Chicago, IL, USA) was used for analysis. Comparisons among the four groups were done with the Kruskal-Wallis test. Comparisons between two groups were done using the Mann-Whitney U-method. A p<0.05 was considered significant.
Results
Comparisons between groups
SBD (n=7 cases), umbrella (n=5), coil (n=41), and ADO (n=58) were used. The patients' mean age was 12.5±15.4 years (0.9 to 60.3 years: SBD 14.0±18.7 years, umbrella 8.9±7.1 years, coil 5.1±6.5 years, ADO 17.9±17.8 years). Mean weight was 27.0±20.0 kg (6.8 to 74.7 kg: SBD 26.4±16 kg, umbrella 34.0±27.5 kg, coil 18.0±13.9 kg, ADO 32.8±21.3 kg). There was no difference between groups in age and weight. The mean ductal diameter was 3.81±1.72 mm (0.7 to 10 mm: SBD 3.99±1.21 mm, umbrella 4.62±1.10 mm, coil 2.18±0.82 mm, ADO 4.80±1.41 mm). The ADO group had the biggest ductus and the coil group the smallest (p<0.01). The ratio of pulmonary blood flow to systemic flow (Qp/Qs) in each group was SBD 2.00±0.94, umbrella 1.77±0.82, coil 1.30±0.37, and ADO 1.95±0.71. The mean pulmonary arterial pressure (m-PAP) was 22.6±10.6 mmHg (7 to 60 mmHg: SBD 20.2±9.79 mmHg, umbrella 18.2±4.23 mmHg, coil 19.5±8.34 mmHg, and ADO 25.6±11.8 mmHg). There was a significant difference in m-PAP only between coil and ADO (p<0.01). A total of 24 patients (21.6%) had pulmonary hypertension (m-PAP >25 mmHg) (Table 1).
Results of the procedures
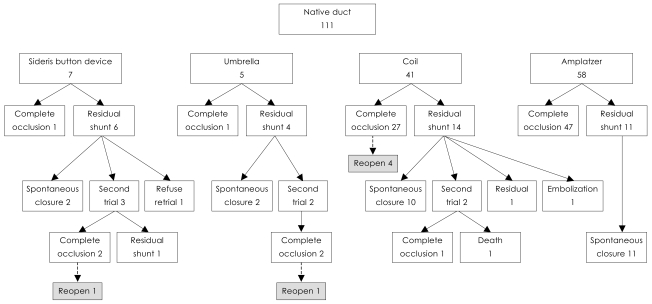
Complete occlusion occurred in 76 of 111 patients (68.4%) at the first trial. Of 35 patients with residual shunt, 25 (71.4%) showed spontaneous occlusion at 1 year of follow up. Reopening developed during follow up in 6 who had an immediate complete closure with a coil. Secondary procedures were performed in 7 using coils and one underwent an additional operation. The final occlusion rate at 1 year of follow up was 90.0% (100/111) (Fig. 1).
Fig. 1.
Outcomes of transcatheter occlusion of PDA cases with different devices. PDA: patent ductus arteriosus.
Sideris buttoned device
Only 1 of 7 SBDs was implanted successfully. Two showed spontaneous occlusion at follow up, 3 had successful second procedures with a coil but one of the three developed reopening. One was lost to follow up.
Rashkind double umbrella
Of 5 umbrellas, 1 was inserted uneventfully; 1 patient had spontaneous occlusion, 2 had a secondary procedure with success.
Coil
Coils were used in 41 patients, leading to immediate complete occlusion in 27 patients: 5 patients had two coils at the first trial; 10 of 14 who had residual shunt showed spontaneous occlusion at follow up, and two were lost to follow up.
Amplatzer duct occluder
ADO was implanted in 58 patients: 81% (47/58) had complete occlusion immediately after implantation and 11 with residual shunt showed spontaneous occlusion at follow up. Hence, complete occlusion was achieved in all cases.
Complications
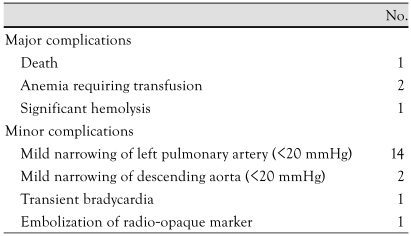
There was left pulmonary artery narrowing of less than 20 mmHg in 14 cases, arch obstruction in 2, transient bradycardia in 1, anemia requiring transfusion in 2, embolization of the device in 1, and death in 1 who had coil occlusion for ductus with a 2.5 mm of diameter but a residual shunt developed immediately after implantation and progressed to significant hemolytic anemia. A second procedure using ADO was planned but sudden uncontrollable arrhythmia developed during the procedure, and eventually led to the death of the patient (Table 2).
Table 2.
Procedural complications
The impact of health insurance
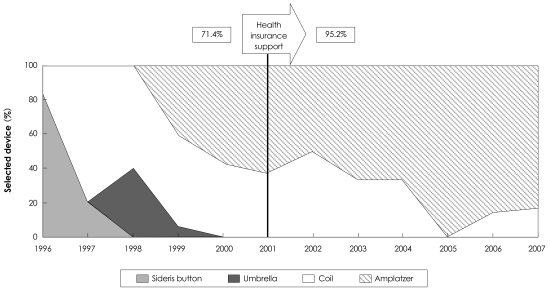
Health insurance has been applied to the use of ADO since 2001, which has resulted in the lessening of cost and a greater use of ADO. This has caused increase in the occlusion rate of PDA from 71.4% to 95.2% (Fig. 2).
Fig. 2.
Overall complete occlusion rate over several years.
Discussion
PDA is a common congenital heart disease accounting for 10% of all cases of congenital heart disease. Surgical closure of a PDA was a conventional treatment and has a negligible mortality rate. However, the morbidity of general anesthesia and thoracotomy such as phrenic nerve injury, scar formation, and bleeding, has driven the search for treatment options that are nonsurgical.10),13-16) In 1967, Porstmann and colleagues introduced a non-surgical closure of PDA with the use of a preshaped Ivalon plug, delivered through the femoral artery and requiring an 18 F sheath for implantation. In 1979, Rashkind and Cuaso developed a polyurethane foam disc umbrella requiring an 8 F or an 11 F sheath. But it is hardly applicable in infancy. Furthermore, the device induces a high residual leak and fracture of arm discs, and is not suitable for tubular shaped or short ductus or ductus >7 mm in diameter.9),11-13),17) In 1991, Sideris et al. developed an adjustable buttoned device that can be delivered via a 7 F sheath, but the device also shows a high residual leak and a significant failure rate, especially when the ductus has a long conical shape or is large.1),9),11),18-20) Thereafter, Lloyd and Moore and their coworkers reported coil occlusion of PDAs from the arterial side using a 5 F or a 6 F sheath.4),7),21) Single or multiple coils are useful for small or tubular shaped ductus. Several modifications such as retrievable, detachable coils, or use of a nitinol snare or forcep, has been tried to decrease the rate of embolization, a common complication.9),16),22),23) A Ductocclud device was designed to better match the shape and configuration of the PDA so that high stability on delivery and higher complete closure rates could be achieved with a single device.5),9) Multiple coils sometimes cause protrusion of the coil into the left pulmonary artery or aorta. In 1998, Masura et al.9) reported the use of ADO for TCO of moderate to large sized PDAs. The device has the benefit of less embolization, easier implantation, and a 98% to 100% occlusion rate.9),24) Our country also has spent considerable effort in developing a better device and the technology to increase the success rate.25) The results of our study show that the coil group had a low Qp/Qs ratio and worked for small ductus but the ADO was mostly used for large ductus with higher PAP. TCO with an umbrella double disc or a SBD produced a low occlusion rate and were discarded, which demonstrates that those devices were less effective (the reason might be that our center had little experience performing these procedures in the early stages of TCO). In the coil group, a well designed strategy for determining coil number according to ductal diameter would have given a better outcome. For instance, two or more coils for a ductus >2 mm or an immediate trial of a second coil in case of a residual shunt. The problems of reopening of a shunt and formation of a central residual shunt in coil TCO remain to be solved. Since public health insurance in Korea covers the cost of the ADO procedure, the performers have favored ADO for a ductus >2.5 mm and eventually the occlusion rate reached 100%, although the overall complete occlusion rate is 95.2%. Our study reveals that the success of TCO depends on the availability of safe, effective devices and an experienced interventionist. Health insurance support has also greatly affected outcomes.
Acknowledgments
This study was presented as a poster at the 2nd Asia-Pacific Congress of Pediatric Cardiology and Cardiac Surgery (PCCS 2008).
References
- 1.Rao PS, Sideris EB, Haddad J, et al. Transcatheter occlusion of patent ductus arteriosus with adjustable buttoned device: initial clinical experience. Circulation. 1993;88:1119–1126. doi: 10.1161/01.cir.88.3.1119. [DOI] [PubMed] [Google Scholar]
- 2.Rashkind WJ, Mullins CE, Hellenbrand WE, Tait MA. Nonsurgical closure of patent ductus arteriosus: clinical application of the Rashkind PDA Occluder System. Circulation. 1987;75:583–592. doi: 10.1161/01.cir.75.3.583. [DOI] [PubMed] [Google Scholar]
- 3.Rao PS, Wilson AD, Sideris EB, Chopra PS. Transcatheter closure of patent ductus arteriosus with buttoned device: first successful clinical application in a child. Am Heart J. 1991;121:1799–1802. doi: 10.1016/0002-8703(91)90029-h. [DOI] [PubMed] [Google Scholar]
- 4.Lloyd TR, Fedderly R, Mendelsohn AM, Sandhu SK, Beekman RH., 3rd Transcatheter occlusion of patent ductus arteriosus with Gianturco coils. Circulation. 1993;88:1412–1420. doi: 10.1161/01.cir.88.4.1412. [DOI] [PubMed] [Google Scholar]
- 5.Moore JW, Schneider DJ, Dimeglio D. The duct-occlud device: design, clinical results, and future directions. J Interv Cardiol. 2001;14:231–237. doi: 10.1111/j.1540-8183.2001.tb00741.x. [DOI] [PubMed] [Google Scholar]
- 6.Masura J, Walsh KP, Thanopoulous B, et al. Catheter closure of moderate- to large-sized patent ductus arteriosus using the new Amplatzer duct occluder: immediate and short-term results. J Am Coll Cardiol. 1998;31:878–882. doi: 10.1016/s0735-1097(98)00013-8. [DOI] [PubMed] [Google Scholar]
- 7.Zeevi B, Berant M, Bar-Mor G, Blieden L. Percutaneous closure of small patent ductus arteriosus: comparison of Rashkind double-umbrella device and occluding spring coils. Cathet Cardiovasc Diagn. 2006;36:44–48. doi: 10.1002/(SICI)1097-0304(199609)39:1<44::AID-CCD9>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 8.Krichenko A, Benson LN, Burrows P, Moes CA, McLaughlin P, Freedom RM. Angiographic classification of the isolated, persistently patent ductus arteriosus and implications for percutaneous catheter occlusion. Am J Cardiol. 1989;63:877–880. doi: 10.1016/0002-9149(89)90064-7. [DOI] [PubMed] [Google Scholar]
- 9.Jung MJ. Transcatheter closure of patent ductus arteriosus. J Korean Pediatr Cardiol Soc. 2003;7:185–190. [Google Scholar]
- 10.Hosking MC, Benson LN, Musewe N, Dyck JD, Freedom RM. Transcatheter occlusion of the persistently patent ductus arteriosus: forty-month follow-up and prevalence of residual shunting. Circulation. 1991;84:2313–2317. doi: 10.1161/01.cir.84.6.2313. [DOI] [PubMed] [Google Scholar]
- 11.Lee HD. Transcatheter closure of patent ductus arteriosus with the Rashkind PDA Occluder System. J Korean Pediatr Soc. 1997;40:63–68. [Google Scholar]
- 12.Kang MJ, Sohn S, Bae EJ, Park IS, Kim SH. Percutaneous closure of patent ductus arteriosus using coil embolization. J Korean Pediatr Soc. 1998;41:369–377. [Google Scholar]
- 13.Zhao HX, D'Agostino RS, Pitlick PT, Shumway NE, Miller DC. Phrenic nerve injury complicating closed cardiovascular surgical procedures for congenital heart disease. Ann Thorac Surg. 1985;39:445–449. doi: 10.1016/s0003-4975(10)61954-6. [DOI] [PubMed] [Google Scholar]
- 14.Sorensen KE, Kristensen B, Hansen OK. Frequency of occurrence of residual ductal flow after surgical ligation by color-flow mapping. Am J Cardiol. 1991;67:653–654. doi: 10.1016/0002-9149(91)90911-4. [DOI] [PubMed] [Google Scholar]
- 15.Mavroudis C, Backer CL, Gevitz M. Forty-six years of patient ductus arteriosus division at Children's Memorial Hospital of Chicago: standards for comparison. Ann Surg. 1994;220:402–409. doi: 10.1097/00000658-199409000-00016. discussion 409-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee YA, Hyun MC, Lee SB. Transcatheter closure of the patent ductus arteriosus. J Korean Pediatr Soc. 2000;43:1200–1206. [Google Scholar]
- 17.Wierny L, Plass R, Porstmann W. Transluminal closure of patent ductus arteriosus: long-term results of 208 cases treated without thoracotomy. Cardiovasc Intervent Radiol. 1986;9:279–285. doi: 10.1007/BF02577958. [DOI] [PubMed] [Google Scholar]
- 18.Kim SH, Park IS, Bae EJ, Shim WS, Sohn SJ. Modified method of transcatheter coil embolization of patent ductus arteriosus using Amplatz snare. Sejong Med J. 1994;11:113–117. [Google Scholar]
- 19.O'Donnell C, Neutze JM, Skinner JR, Wilson NJ. Transcatheter patent ductus arteriosus occlusion: evolution of techniques and results from the 1990s. J Paediatr Child Health. 2001;37:451–455. doi: 10.1046/j.1440-1754.2001.00689.x. [DOI] [PubMed] [Google Scholar]
- 20.Sideris EB, Rao PS, Zamora R. The Sideris buttoned devices for transcatheter closure of patent ductus arteriosus. J Interv Cardiol. 2001;14:239–246. doi: 10.1111/j.1540-8183.2001.tb00742.x. [DOI] [PubMed] [Google Scholar]
- 21.Yu JJ, Lee JY, Cheon EJ, et al. Clinical experience of transcatheter coil embolization in children. Korean Circ J. 1998;28:691–699. [Google Scholar]
- 22.Sommer RJ, Gutierrez A, Lai WW, Parness IA. Use of preformed nitinol snare to improve transcatheter coil delivery in occlusion of patent ductus arteriosus. Am J Cardiol. 1994;74:836–839. doi: 10.1016/0002-9149(94)90451-0. [DOI] [PubMed] [Google Scholar]
- 23.Brown S, Bruwer A, Al-Zaghal A, Claassens A. Effectiveness of single detachable COOK coils in closure of the patent ductus arteriosus. Cardiovasc J S Afr. 2004;15:76–80. [PubMed] [Google Scholar]
- 24.Thanopoulos BD, Hakim FA, Hiari A, Tsaousis GS, Paphitis C, Hijazi ZM. Patent ductus arteriosus equipment and technique: amplatzer duct occluder: intermediate-term follow-up and technical considerations. J Interv Cardiol. 2001;14:247–254. doi: 10.1111/j.1540-8183.2001.tb00743.x. [DOI] [PubMed] [Google Scholar]
- 25.Jang GJ, Lee SH, Jang Y, et al. Development of an occluder device for closure of patent ductus arteriosus. Korean Circ J. 1998;28:970–976. [Google Scholar]