Abstract
Background and Objectives
About 10-15% of Kawasaki disease (KD) is refractory to intravenous immunoglobulin (IVIG) therapy. This study was designed to investigate the predicting factors for refractory KD.
Subjects and Methods
We reviewed retrospectively the clinical records of 77 patients with typical KD admitted at Wonju Christian Hospital from January, 2005, to December, 2008. The variance of laboratory and demographic parameters between the IVIG-responsive group and IVIG-resistant group were analyzed. Thirteen patients with urinary tract infections were randomly collected as a febrile control group.
Results
Among 77 patients diagnosed with complete KD, 13 patients (16.9%) were IVIG-resistant. The febrile period and hospital days were significantly longer in the IVIG-resistant group than IVIG-responsive group (p<0.001, p=0.002). Serum levels of albumin and sodium were significantly lower in the IVIG-resistant group (p=0.025). The Kobayashi score could differentiate these two groups (p=0.015). Fewer lymphocytes was observed during the subacute phase in the IVIG-resistant group (p=0.032). Coronary arterial dilatations (CADs) were observed in 10.9% (7/64) of IVIG-responders and 38.5% (5/13) of IVIG-resistant patients (p=0.038).
Conclusion
The percentage of neutrophils and lymphocytes in patients with KD, in addition to known risk factors for refractory KD, may help predict IVIG-resistance in patients with KD.
Keywords: Mucocutaneous Lymph Node Syndrome, Risk factors, Coronary arteries
Introduction
Kawasaki disease (KD) is subcategory of systemic vasculitis that starts after infectious stimuli. Characteristic clinical features are prolonged fever, bilateral conjunctival injection, erythematous induration of palms and soles, mucocutaneous changes of oropharynx and lips, polymorphous skin rashes, and cervical lymphadenopathy.1) The incidence of KD has increased in Japan, reaching 174.0 per 100,000 in 2004, for children younger than 5 years of age.2) KD is a leading cause of acquired heart disease in children in the developed countries.3) Coronary arterial lesions develop in less than 5 percent of cases after high dose intravenous immunoglobulin (IVIG) treatment.4) However, 10-15% of KD patients show persistent fever despite high dose IVIG treatment (2 g/kg),5) a condition known as refractory KD. As the incidence of KD has increased, refractory KD also has also increased.5-7) A higher portion of band counts, lower serum levels of albumin, abnormal findings in initial echocardiography,8) hyponatremia, early treatment within 4 days of fever, elevated aspartate aminotransferase (AST), high proportion of neutrophils, C-reactive protein (CRP) ≥10 mg/dL, younger than 12 months old, thrombocytopenia,7) and recurrent KD9) are known risk factors for refractory KD. Most risk factors are related to severe inflammatory reactions and are not suppressed by IVIG alone. Furthermore, in addition to pro-inflammatory cytokines, growth factors such as vascular endothelial growth factors or platelet-derived growth factor and chemokines are also involved in the pathogenesis of KD.10) Kobayashi et al.7) suggested a useful scoring system that can predict refractory KD. This scoring system reflects clinical data as the consequences of increased inflammatory cascades. Other predictive factors are still needed.
Genetic defects in the apoptosis of immune cells may contribute to the poor responsiveness to IVIG during the acute phase of KD.11) The increased percentage of neutrophils may reflect apoptotic defects and the consequent restriction of effective clonal expansion of lymphocytes against infectious stimuli at the acute phase of KD.11) Therefore, we measured differences in the subpopulation of white blood cells as a potential risk factor for refractory KD.
Subjects and Methods
A total of 77 patients who fulfilled the criteria for complete KD at the Wonju Christian Hospital from January, 2005, to December, 2008, were included in this study. We retrospectively reviewed clinical records of these patients. The criteria for the diagnosis of complete KD followed the 5th revision of the diagnostic guideline for KD,12) and included fever (body temperature >38℃) for at least 5 days accompanied by the presence of at least 4 of these 5 findings: bilateral conjunctival injection, changes in the lips and oral cavity, nonpurulent cervical lymphadenopathy, polymorphous rashes, and changes in the extremities.
Among 77 patients, 13 patients (16.9%) were treated twice with IVIG. The age at disease onset ranged from 3 months to 8 years (median age 2.49 years). All patients were treated with 2 g/kg of IVIG along with aspirin (60 mg/kg/day) at the acute phase of KD.
Patients were classified into two groups: IVIG-resistant patients required a second dose of IVIG because of a reappearance of fever within 48 hours after the initial IVIG treatment. The IVIG-responsive group became afebrile after receiving a single dose of IVIG treatment and showed marked improvement of the initial inflammatory indices. Thirteen randomly selected age- and sex-matched febrile patients with urinary tract infections were adopted as the febrile control group.
Laboratory variables (complete blood count with differential count, AST, alanine aminotransferase, serum levels of albumin and sodium, erythrocyte sedimentation rate and CRP) were analyzed along with demographic variables (gender, age in months, hospital stay and days of illness at initial treatment). Laboratory data were obtained before IVIG treatment (acute phase) and 7 days after the initial IVIG treatment (subacute phase).
We used the scoring model established by Kobayashi et al.7) for predicting IVIG-resistance: high serum levels of AST, CRP, high percentage of neutrophils, young age at disease onset, low serum levels of sodium, low platelet counts and early administration of IVIG in the course of illness are independent risk factors. The points scored for each variable were as follows: sodium ≤133 mmol/L, 2 points; days of illness at initial treatment ≤4, 2 points; AST ≥100 IU/L, 2 points; % neutrophils ≥80%, 2 points; CRP ≥10 mg/dL, 1 point; age ≤12 months, 1 point; and platelet count ≤300×103/mm3, 1 point.7)
Two-dimensional echocardiography was performed to measure coronary artery (CA) size at the time of diagnosis. Follow up echocardiograms were performed at 7 days, 6 months, and 1 year after diagnosis to compare the incidence of coronary arterial lesions. CA lesions were defined as follows; 1) a coronary arterial luminal diameter exceeded 3 mm in a child <5 years old, or 4 mm in a child ≥5 years; 2) an internal segment diameter was at least 1.5 times as large as that of an adjacent segment; or 3) the lumen appeared irregular.13)
Statistical analysis
Variables including clinical manifestations, laboratory findings, and responses to treatment were analyzed with SAS version 9.1 (SAS Institute Inc., Cary, NC, USA) using the chi-square test and Student's t-test. For inclusion of the control group, we used the one-way analysis of variance test. P<0.05 was considered statistically significant.
Results
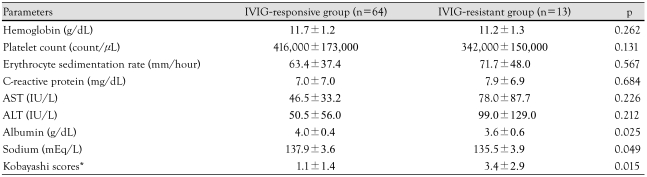
The duration of fever and hospital day were significantly longer in the IVIG resistant group than IVIG responsive group (p<0.001, p=0.002) (Table 1). The IVIG-resistant group showed lower serum albumin (p=0.025) and lower serum sodium (p=0.049). Kobayashi scores were significantly higher in the IVIG-resistant group than the IVIG-responsive group (p=0.015) (Table 2).
Table 1.
Demographic characteristics in IVIG-responsive group and IVIG-resistant group in Kawasaki disease
*Days with documented fever before initiation of IVIG treatment. M: male, F: female, IVIG: intravenous immunoglobulin
Table 2.
Laboratory values in IVIG-responsive group and IVIG-resistant group in Kawasaki disease
*Scores calculated by the methods by Kabayashi et al.7) AST: aspartate aminotransferase, ALT: alanine aminotransferase, IVIG: intravenous immunoglobulin
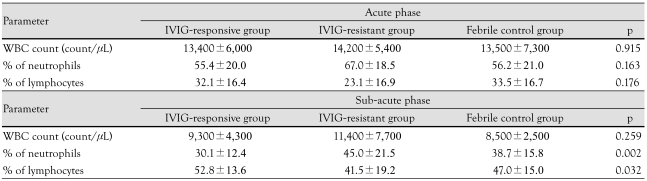
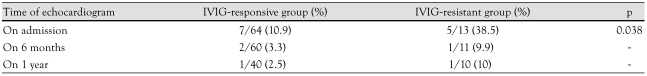
Both groups had a similar percentage of neutrophils and lymphocytes during the acute phase. However, a higher percentage of neutrophils and a lower percentage of lymphocytes were observed in the IVIG-resistant group than the IVIG-responsive group or controls during the subacute phase (p=0.002, 0.032) (Table 3). Coronary arterial dilatations (CADs) were observed in 10.9% (7/64) of the IVIG-responsive group and in 38.5% (5/13) of IVIG-resistant group during the acute phase (p=0.038) (Table 4). One patient in the IVIG-resistant group developed a coronary aneurysm that measured 0.54 cm.
Table 3.
Percentage of neutrophils and lymphocytes in IVIG-resistant group and IVIG-responsive group during acute phase and subacute phase of Kawasaki disease
IVIG: intravenous immunoglobulin, WBC: white blood cell
Table 4.
The serial incidence of coronary arterial dilatations in IVIG-responsive group and IVIG-resistant group in Kawasaki disease
IVIG: intravenous immunoglobulin
Discussion
Although the cause of KD remains unknown, epidemiologic features suggest that it is related to infectious agents, and aberrant immune mechanisms may play an important role in the pathogenesis of KD.14) The representative clinical parameters of IVIG-resistant KD are also associated with severe immune reactions after antigenic stimuli.8),15)
The scoring system suggested by Kobayashi et al.7) can help predict risk factors for IVIG-resistant KD and lead to timely management and better clinical outcomes.5) We also measured subpopulations of leukocytes in patients with KD.12),16-18) Fewer lymphocytes in peripheral blood may represent early tissue infiltration of effector T cells and B cells, potentially due to genetic defects in apoptosis of activated immune cells.11),19-23) Here, we tested the usefulness of the Kobayashi score in Korean patients with KD, but a further multicenter study would further establish useful predictive factors.
IVIG-resistant KD is characterized by prolonged fever and more severe coronary outcomes. Fever represents ongoing inflammatory reactions in vessels and may reflect defects in inhibition of immunologic stimuli.24) Therapies for IVIG-resistant KD could decrease inflammation and induce apoptosis of activated immune cells. Treatment with high dose corticosteroids improves fever and degradation of activated cells,22),25) but there are few well-controlled prospective cohort studies. A tumor necrosis factor-α blocker26) and low dose methotrexate14),26),27) may also have therapeutic potential. The significance of coronary outcomes emphasizes the importance of early aggressive management of IVIG-resistant KD. CAD occurs significantly more frequently in IVIG-resistant KD than in the IVIG-responsive group.28),29) This study also showed a higher incidence of CAD in the IVIG-resistant group. Furthermore the sequelae of KD could be related with subclinical atherosclerosis in adolescence.30)
In conclusion, this study showed a high Kobayashi score during the acute phase and a relatively high proportion of neutrophils and a low proportion of lymphocytes during the subacute phase may predict IVIG-resistant KD. Further, an expansive multicenter study is required for determining the predictive factors for IVIG-resistant KD.
References
- 1.Kawasaki T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children. Arerugi. 1967;16:178–222. [PubMed] [Google Scholar]
- 2.Nakamura Y, Yashiro M, Uehara R, Oki I, Kayaba K, Yanagawa H. Increasing incidence of Kawasaki disease in Japan: nationwide survey. Pediatr Int. 2008;50:287–290. doi: 10.1111/j.1442-200X.2008.02572.x. [DOI] [PubMed] [Google Scholar]
- 3.Burns JC, Glode MP. Kawasaki syndrome. Lancet. 2004;364:533–544. doi: 10.1016/S0140-6736(04)16814-1. [DOI] [PubMed] [Google Scholar]
- 4.Moon SY, Kim NS, Lee HB, Lee H. Comparative study about the therapeutic effect between single and five-day administration of gammaglobulin in Kawasaki disease. Korean Circ J. 1994;24:77–85. [Google Scholar]
- 5.Freeman AF, Shulman ST. Refractory Kawasaki disease. Pediatr Infect Dis J. 2004;23:463–464. doi: 10.1097/01.inf.0000125893.66941.e0. [DOI] [PubMed] [Google Scholar]
- 6.Dominguez SR, Friedman K, Seewald R, Anderson MS, Willis L, Glode MP. Kawasaki disease in a pediatric intensive care unit: a case-control study. Pediatrics. 2008;122:e786–e790. doi: 10.1542/peds.2008-1275. [DOI] [PubMed] [Google Scholar]
- 7.Kobayashi T, Inoue Y, Takeuchi K, et al. Prediction of intravenous immunoglobulin unresponsiveness in patients with Kawasaki disease. Circulation. 2006;113:2606–2612. doi: 10.1161/CIRCULATIONAHA.105.592865. [DOI] [PubMed] [Google Scholar]
- 8.Ashouri N, Takahashi M, Dorey F, Mason W. Risk factors for nonresponse to therapy in Kawasaki disease. J Pediatr. 2008;153:365–368. doi: 10.1016/j.jpeds.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 9.Uehara R, Belay ED, Maddox RA, et al. Analysis of potential risk factors associated with nonresponse to initial intravenous immunoglobulin treatment among Kawasaki disease patients in Japan. Pediatr Infect Dis J. 2008;27:155–160. doi: 10.1097/INF.0b013e31815922b5. [DOI] [PubMed] [Google Scholar]
- 10.Harada T, Ito S, Shiga K, et al. A report of two cases of Kawasaki disease treated with plasma exchange. Ther Apher Dial. 2008;12:176–179. doi: 10.1111/j.1744-9987.2008.00566.x. [DOI] [PubMed] [Google Scholar]
- 11.Popper SJ, Shimizu C, Shike H, et al. Gene-expression patterns reveal underlying biological processes in Kawasaki disease. Genome Biol. 2007;8:R261. doi: 10.1186/gb-2007-8-12-r261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ayusawa M, Sonobe T, Uemura S, et al. Revision of diagnostic guidelines for Kawasaki disease (the 5th revised edition) Pediatr Int. 2005;47:232–234. doi: 10.1111/j.1442-200x.2005.02033.x. [DOI] [PubMed] [Google Scholar]
- 13.Cha S, Yoon M, Ahn Y, Han M, Yoon KL. Risk factors for failure of initial intravenous immunoglobulin treatment in Kawasaki disease. J Korean Med Sci. 2008;23:718–722. doi: 10.3346/jkms.2008.23.4.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Newburger JW, Takahashi M, Gerber MA, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Pediatrics. 2004;114:1708–1733. doi: 10.1542/peds.2004-2182. [DOI] [PubMed] [Google Scholar]
- 15.Ahn SY, Kim DS. Treatment of intravenous immunoglobulin-resistant Kawasaki disease with methotrexate. Scand J Rheumatol. 2005;34:136–139. doi: 10.1080/03009740510026328. [DOI] [PubMed] [Google Scholar]
- 16.Yun SW. Diagnostic value of serum cardiac troponin T, troponin I and CK-MB in acute Kawasaki disease. Korean Circ J. 2004;34:582–592. [Google Scholar]
- 17.Hamamichi Y, Ichida F, Yu X, et al. Neutrophils and mononuclear cells express vascular endothelial growth factor in acute Kawasaki disease: its possible role in progression of coronary artery lesions. Pediatr Res. 2001;49:74–80. doi: 10.1203/00006450-200101000-00017. [DOI] [PubMed] [Google Scholar]
- 18.Inoue Y, Kato M, Kobayashi T, Shinohara M, Sone K, Morikawa A. Increased circulating granulocyte colony-stimulating factor in acute Kawasaki disease. Pediatr Int. 1999;41:330–333. doi: 10.1046/j.1442-200x.1999.01074.x. [DOI] [PubMed] [Google Scholar]
- 19.Tsujimoto H, Takeshita S, Nakatani K, Kawamura Y, Tokutomi T, Sekine I. Intravenous immunoglobulin therapy induces neutrophil apoptosis in Kawasaki disease. Clin Immunol. 2002;103:161–168. doi: 10.1006/clim.2002.5209. [DOI] [PubMed] [Google Scholar]
- 20.Yi QJ, Li CR, Yang XQ. Effect of intravenous immunoglobulin on inhibiting peripheral blood lymphocyte apoptosis in acute Kawasaki disease. Acta Paediatr. 2001;90:623–627. [PubMed] [Google Scholar]
- 21.Koga M, Hasegawa S, Furukawa S. No increase in soluble Fas and Fas ligand in Kawasaki disease: comment on the article by Nozawa et al. Arthritis Rheum. 1998;41:568–570. doi: 10.1002/1529-0131(199803)41:3<568::AID-ART32>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 22.Sundel RP, Baker AL, Fulton DR, Newburger JW. Corticosteroids in the initial treatment of Kawasaki disease: report of a randomized trial. J Pediatr. 2003;142:611–616. doi: 10.1067/mpd.2003.191. [DOI] [PubMed] [Google Scholar]
- 23.Chun JK, Kang DW, Yoo BW, Shin JS, Kim DS. Programmed death-1 (PD-1) gene polymorphisms lodged in the genetic predispositions of Kawasaki Disease. Eur J Pediatr. 2010;169:181–185. doi: 10.1007/s00431-009-1002-4. [DOI] [PubMed] [Google Scholar]
- 24.Matsubara T, Ichiyama T, Furukawa S. Immunological profile of peripheral blood lymphocytes and monocytes/macrophages in Kawasaki disease. Clin Exp Immunol. 2005;141:381–387. doi: 10.1111/j.1365-2249.2005.02821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lang BA, Yeung RS, Oen KG, et al. Corticosteroid treatment of refractory Kawasaki disease. J Rheumatol. 2006;33:803–809. [PubMed] [Google Scholar]
- 26.Oishi T, Fujieda M, Shiraishi T, et al. Infliximab treatment for refractory Kawasaki disease with coronary artery aneurysm. Circ J. 2008;72:850–852. doi: 10.1253/circj.72.850. [DOI] [PubMed] [Google Scholar]
- 27.Kim NY, Choi DY, Jung MJ, Jeon IS. A case of refractory Kawasaki disease complicated by peripheral ischemia. Pediatr Cardiol. 2008;29:1110–1114. doi: 10.1007/s00246-008-9221-4. [DOI] [PubMed] [Google Scholar]
- 28.Lee SY, Gwon HC, Park SW, et al. Acute myocardial infarction in young patient probably due to Kawasaki disease. Korean Circ J. 2001;31:119–124. [Google Scholar]
- 29.Egami K, Muta H, Ishii M, et al. Prediction of resistance to intravenous immunoglobulin treatment in patients with Kawasaki disease. J Pediatr. 2006;149:237–240. doi: 10.1016/j.jpeds.2006.03.050. [DOI] [PubMed] [Google Scholar]
- 30.Dalla Pozza R, Bechtold S, Urschel S, Kozlik-Feldmann R, Netz H. Subclinical atherosclerosis, but normal autonomic function after Kawasaki disease. J Pediatr. 2007;151:239–243. doi: 10.1016/j.jpeds.2007.03.057. [DOI] [PubMed] [Google Scholar]