Abstract
Objectives To compare the safety, reactogenicity, and immunogenicity of an adjuvanted split virion H1N1 vaccine and a non-adjuvanted whole virion vaccine used in the pandemic immunisation programme in the United Kingdom.
Design Open label, randomised, parallel group, phase II study.
Setting Five UK centres (Oxford, Southampton, Bristol, Exeter, and London).
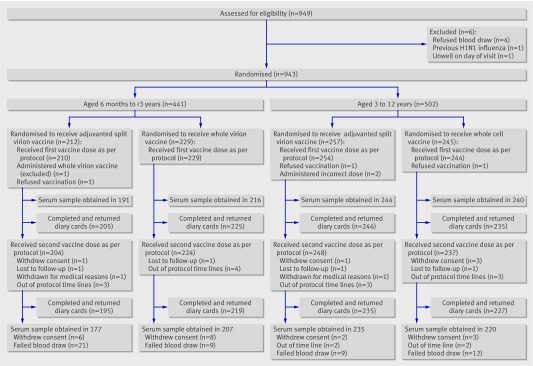
Participants Children aged 6 months to less than 13 years for whom a parent or guardian had provided written informed consent and who were able to comply with study procedures were eligible. Those with laboratory confirmed pandemic H1N1 influenza or clinically diagnosed disease meriting antiviral treatment, allergy to egg or any other vaccine components, or coagulation defects, or who were severely immunocompromised or had recently received blood products were excluded. Children were grouped by age: 6 months-<3 years (younger group) and 3-<13 years (older group). Recruitment was by media advertising and direct mailing. Recruitment visits were attended by 949 participants, of whom 943 were enrolled and 937 included in the per protocol analysis.
Interventions Participants were randomised 1:1 to receive AS03B (tocopherol based oil in water emulsion) adjuvanted split virion vaccine derived from egg culture or non-adjuvanted whole virion vaccine derived from cell culture. Both were given as two doses 21 days apart. Reactogenicity data were collected for one week after immunisation by diary card. Serum samples were collected at baseline and after the second dose.
Main outcome measures Primary reactogenicity end points were frequency and severity of fever, tenderness, swelling, and erythema after vaccination. Immunogenicity was measured by microneutralisation and haemagglutination inhibition assays. The primary immunogenicity objective was a comparison between vaccines of the percentage of participants showing seroconversion by the microneutralisation assay (fourfold rise to a titre of ≥1:40 from before vaccination to three weeks after the second dose).
Results Seroconversion rates were higher after the adjuvanted split virion vaccine than after the whole virion vaccine, most notably in the youngest children (163 of 166 participants with paired serum samples (98.2%, 95% confidence interval 94.8% to 99.6%) v 157 of 196 (80.1%, 73.8% to 85.5%), P<0.001) in children under 3 years and 226 of 228 (99.1%, 96.9% to 99.9%) v 95.9%, 92.4% to 98.1%, P=0.03) in those over 3 years). The adjuvanted split virion vaccine was more reactogenic than the whole virion vaccine, with more frequent systemic reactions and severe local reactions in children aged over 5 years after dose one (13 (7.2%, 3.9% to 12%) v 2 (1.1%, 0.1% to 3.9%), P<0.001) and dose two (15 (8.5%, 4.8% to 13.7%) v 2 (1.1%, 0.1% to 4.1%), P<0.002) and after dose two in those under 5 years (15 (5.9%, 3.3% to 9.6%) v 0 (0.0%, 0% to 1.4%), P<0.001). Dose two of the adjuvanted split virion vaccine was more reactogenic than dose one, especially for fever ≥38ºC in those aged under 5 (24 (8.9%, 5.8% to 12.9%) v 57 (22.4%, 17.5% to 28.1%), P<0.001).
Conclusions In this first direct comparison of an AS03B adjuvanted split virion versus whole virion non-adjuvanted H1N1 vaccine, the adjuvanted vaccine, while more reactogenic, was more immunogenic and, importantly, achieved high seroconversion rates in children aged less than 3 years. This indicates the potential for improved immunogenicity of influenza vaccines in this age group.
Trial registration Clinical trials.gov NCT00980850; ISRCTN89141709.
Introduction
During the 2009-10 influenza A (H1N1) pandemic, children experienced pandemic A(H1N1) infections at four times the rate of adults and were more commonly admitted to hospital.1 2 Although most cases in children were mild, severe disease and deaths occurred, mainly in those with comorbidities.2 3 This, along with the role that children play in virus transmission4 5 made children a priority group for vaccination against pandemic influenza in many countries.5 Some countries routinely vaccinate children against seasonal influenza,6 but this strategy is compromised by the limited immunogenicity and efficacy of inactivated seasonal influenza vaccines in children.7 8 In the United Kingdom, seasonal influenza vaccination with inactivated split virion or surface antigens vaccines is recommended only for children with comorbidities who are deemed at high risk, with children receiving two doses in their first year of vaccination and one thereafter. While there are substantial safety data regarding the use of trivalent seasonal split and subunit non-adjuvanted inactivated influenza vaccines in children, similar data on safety and efficacy for novel H1N1 vaccines were lacking9 10 11 12 and only limited data from H5N1 “mock-up” vaccines were available.5 Novel adjuvants had not been routinely used in early childhood before this pandemic but were believed to provide enhanced immunogenicity, particularly in infants in whom traditional influenza vaccines have limited efficacy,7 and potentially allow antigenic sparing and induction of cross clade immunity.13 14 15
While the H5N1 “mock-up” whole virion vaccines were well tolerated,16 whole virion influenza vaccines have previously been associated with unacceptable reactogenicity rates.7 Cell culture for manufacture of influenza vaccines should shorten production times by avoiding the bottleneck caused by the supply of hens’ eggs17 and avoid reactions in those allergic to eggs.18
The UK Department of Health purchased two H1N1 vaccines for the national immunisation programme, an AS03B adjuvanted split virion vaccine derived from egg culture and a non-adjuvanted whole virion vaccine derived from Vero cell culture.17 We evaluated the safety, tolerability, and immunogenicity of the two vaccines in children aged 6 months to 12 years to inform the scientific community, policy makers, and parents.
Methods
Vaccines
We compared two novel H1N1 vaccines: an AS03B adjuvanted split virion vaccine (GlaxoSmithKline Vaccines, Rixensart, Belgium) and a non-adjuvanted whole virion vaccine (Baxter Vaccines, Vienna). The adjuvanted split virion vaccine was constructed from the A/California/7/2009 (H1N1) v-like strain antigen (New York Medical College x-179A), generated by classical re-assortment in eggs, combining the HA, NA, and PB1 genes of A/California/7/2009 (H1N1)v with the PR8 strain backbone.12 19 Each dose (0.25 ml, half the adult dose) contained 1.875 μg of haemagglutinin antigen, the oil in water emulsion based adjuvant AS03B (containing squalene (5.345 mg), DL alpha tocopherol (5.93 mg), and polysorbate 80 (2.43 mg)) and thiomersal and was supplied as suspension and emulsion multidose vials.
The non-adjuvanted whole virion vaccine, derived from Vero cell culture, was supplied in multidose vials. Opened vials were used within three hours; each dose (0.5 ml) contained 7.5 μg of haemagglutinin from influenza A/California/07/2009 (H1N1).
Study design
Between 26 September and 11 December 2009, during the second wave of the influenza A (H1N1) pandemic in the UK, we conducted an open label, randomised, parallel group, phase II study at five UK sites (Oxford, Bristol, Southampton, Exeter, and London) in children aged 6 months to 12 years to compare the safety, reactogenicity, and immunogenicity of two novel H1N1 vaccines in a two dose regimen.
Recruitment by media advertising and direct mailing began on 26 September, before the start of the UK pandemic influenza immunisation programme. Enrolment continued during the initial phase of the national programme targeting immunisation of children in high risk groups. Extension of the national programme to all children under the age of 5 years occurred after the end of study enrolment. Parents or guardians gave written informed consent, and children aged 6 months to less than 13 years for whom a parent or guardian had provided written informed consent and who were able to comply with study procedures were eligible for inclusion. We also sought verbal assent from children aged 7 years and older. To target an immunologically naive population we excluded those with laboratory confirmed pandemic H1N1 influenza or with clinically diagnosed disease meriting antiviral treatment. For safety reasons, those with allergy to egg or any other vaccine components and coagulation defects were excluded. Other exclusions included those who were severely immunocompromised, were receiving immunosuppressive treatment, had recently received blood products, were going to be immunised with another H1N1 vaccine, or were taking part in another clinical trial. Participants were grouped into those aged 6 months to less than 3 years (younger group) and 3 years to less than 13 years (older group).
Randomisation and masking
Children were stratified for age group and randomly assigned in a 1:1 ratio to receive one of the two vaccines. Randomisation was in block sizes of 10, which were generated by a statistician who was not directly involved in enrolment. Assignment was by sequentially numbered, identical, opaque sealed envelopes.
Procedures
Vaccines were administered by intramuscular injection (deltoid or anterior-lateral thigh depending on age and muscle bulk) at enrolment and at day 21 (plus or minus 7 days). A minimum interval of 14 days between doses was allowed to reduce dropout rates, to facilitate the rapid data collection that was needed in this study setting, and to provide protection as early as possible in the context of the pandemic. Serum samples were collected immediately before vaccination (study day 0) and 21 days (−7 to 14) after second vaccination.
Up to the seventh day after vaccination parents or guardians used diary cards to record axillary temperature, reactions at the injection site, solicited and unsolicited systemic symptoms, and medications given (including antipyretics/analgesics). Primary reactogenicity end points were frequency and severity of fever, tenderness, swelling, and erythema after vaccination. Secondary end points were the frequency and severity of non-febrile solicited systemic reactions or use of analgesics/antipyretics. Solicited systemic reactions were different in those aged under and over 5 years to reflect the child’s ability to articulate symptoms. Erythema and swelling were graded by diameter as mild (1-24 mm), moderate (25-29 mm), or severe (≥50 mm). Other reactions were graded by effect on daily activity as none, mild (transient reaction, no limitation in activity), moderate (some limitations), or severe (unable to perform normal activities) or by frequency/duration into none, mild, moderate, and severe categories.
Medically important adverse events (ongoing solicited reactions or events necessitating a doctor’s visit or study withdrawal after day seven after vaccination) were recorded on a diary card. We also undertook monitoring of adverse events of special interest, as recommended by the European Medicines Agency,20 and a data monitoring committee monitored safety. Stopping procedures were in place but were not required. All data from case report forms and participant diary cards were double entered and verified on computer.
Antibody responses were detected by microneutralisation and haemagglutination inhibition with 0.5% turkey erythrocytes by using standard methods21 22 at the Centre for Infections, Health Protection Agency. Assays were performed with egg grown reverse genetics NIBRG-121 virus containing haemagglutinin and neuraminidase from A/California/7/2009 (H1N1v); the seed virus was supplied by the National Institute for Biological Standards and Controls (NIBSC). The negative control was pooled human sera, and the positive control was ferret antisera raised to A/California/7/2007 (NIBSC). For haemagglutination inhibition, serum samples were treated with receptor destroying enzyme (RDEII) and initial dilution was 1:8. Samples were titrated to determine absolute end point titres: for samples that showed titres ≥1024, a further dilution series was performed ending at a dilution of 1:16 384. For microneutralisation, the initial dilution of heat treated sera was 1:10. The final dilution was 1:320, unless further dilutions were necessary to determine fourfold rises from baseline. The neutralisation assay was performed by infection of a MDCK cell suspension, and final infectivity was detected by the use of monoclonal antibody directed against influenza nucleoprotein in an enzyme immunoassay detection format. Serum samples were tested in duplicate and the geometric mean value for each pair used.
The primary immunogenicity objective was a comparison between vaccines of the percentage of participants showing seroconversion by the microneutralisation assay, with seroconversion defined as a fourfold rise to a titre of ≥1:40 from before the vaccination to three weeks after the second dose. A secondary objective based on the microneutralisation assay was a comparison between vaccines of the percentage with titres ≥1:40 after the second dose. Further secondary objectives based on the haemagglutination inhibition assay were comparisons between vaccines in the percentage with fourfold rises to titres ≥1:32, the percentage with titres ≥1:32, geometric mean rises from baseline, and geometric mean titres, all after the second dose.
Statistical analysis
With 200 participants in each age and vaccine group the study had 80% power to detect differences of −14% to 12% around a 70% reactogenicity and seroconversion rate. Planned recruitment was up to 250 participants per group to allow for drop out and non-availability of serum samples.
For each age and vaccine group we calculated proportions with local or systemic reactions and with seroconversion or titres above given thresholds. Comparisons between vaccines were made with a two sided Fisher’s exact test. For reactions, we used the sign test for paired data for comparisons between doses.
Geometric mean haemagglutination inhibition titres and rises were calculated for each age and vaccine group along with 95% confidence intervals. Logged haemagglutination inhibition titres after vaccination were compared between vaccines by using normal errors regression in a univariable model and then in a multivariable model with adjustment for age, study site, sex, and interval from second vaccine dose to obtaining final serum sample. The interaction between age and vaccine was also investigated.
A planned interim analysis on the reactogenicity data from the first 500 participants was performed to provide rapid data to the UK Department of Health. The study site investigators remained blinded to the results of this analysis while visits were ongoing.
Data analysis was undertaken with Stata software, version 10. The level of significance was 5%. The data were analysed per protocol. As planned, we did not conduct intention to treat analyses because fewer than 10% of participants would have been classified differently in such an analysis.
Results
Recruitment visits were attended by 949 participants, of whom 943 were enrolled and 937 were included in the per protocol analysis (fig 1 and table 1) . Of these, 913 received the second vaccine dose per protocol at a mean interval of 20 days (range 14-28 days). Serum samples were obtained in 839 participants after the second vaccine dose as per protocol at a mean interval of 20 days (14-35). For one participant a second dose hamagglutination inhibition titre but not microneutralisation titre was available and for two participants a microneutralisation titre result, but no haemagglutination inhibition titre result, was available. Vials of the adjuvanted split virion vaccine were used within 12 hours of opening and vials of the whole virion vaccine within three hours of opening.
Fig 1 Enrolment and follow-up of study participants
Table 1.
Baseline characteristics of children in study by age and vaccine. Figures are numbers of children unless specified otherwise
| Age 6 months-<3 years | Age 3-12 years | ||||
|---|---|---|---|---|---|
| Adjuvanted split virion (n=210) | Whole virion (n=229) | Adjuvanted split virion (n=254) | Whole virion (n=244) | ||
| Race or ethnic group: | |||||
| White | 189 | 201 | 231 | 222 | |
| Indian | 0 | 1 | 0 | 0 | |
| Pakistani | 1 | 0 | 2 | 1 | |
| Asian other | 1 | 2 | 1 | 0 | |
| Mixed ethnic group | 14 | 19 | 9 | 10 | |
| Black African | 1 | 3 | 3 | 3 | |
| Black Caribbean | 2 | 0 | 3 | 1 | |
| Chinese | 0 | 0 | 2 | 2 | |
| Other | 2 | 3 | 3 | 5 | |
| Sex: | |||||
| Male | 116 | 123 | 131 | 121 | |
| Female | 94 | 106 | 123 | 123 | |
| Previous seasonal influenza vaccine | 5 | 5 | 22 | 28 | |
| Median (range) age (months) | 23 (6-35) | 23 (6-35) | 82 (36-151) | 84 (36-155) | |
| Site: | |||||
| Bristol | 44 | 46 | 41 | 42 | |
| Exeter | 16 | 23 | 24 | 19 | |
| Oxford | 70 | 79 | 66 | 59 | |
| Southampton | 67 | 58 | 72 | 80 | |
| St Georges | 13 | 23 | 51 | 44 | |
Safety and tolerability
Tables 2 and 3 provide data on solicited reactions . The adjuvanted split virion vaccine was associated with more frequent severe local reactions than the whole virion vaccine after either dose in those aged over 5 years (7.2% v 1.1%, P<0.001, for dose one; 8.5% v 1.1%, P=0.002, for dose two) and after dose two in those under 5 years (5.9% v 0.0%, P<0.001). There were also more systemic reactions among participants aged 6 months to less than 5 years, with more irritability after either dose (45.6% v 35.5% for dose one; 48% v 28.4% for dose two) and more decreased feeding (40.6% v 29.9%) and decreased activity (31.9% v 17.3%) after dose two. Participants aged over 5 years experienced more muscle pain after either dose (32.6% v 13.8% for dose one; 25% v 12.6% for dose two) and were more often generally unwell after dose two (26.1% v 14.9%).
Table 2.
Local and systemic reactions in children aged 6 months to <5 years by vaccine and dose. Figures are numbers (percentage, 95% confidence interval)
| Adjuvanted split virion | Whole virion vaccine | ||||
|---|---|---|---|---|---|
| Dose 1 | Dose 2 | Dose 1 | Dose 2 | ||
| Total vaccinated | 278 | 275 | 286 | 285 | |
| With diary card | 270 | 254 | 279 | 271 | |
| Pain: | |||||
| Mild | 77 (28.5, 23.2 to 34.3) | 79 (31.1, 25.5 to 37.2) | 48 (17.2, 13 to 22.2) | 46 (17, 12.7 to 22) | |
| Moderate | 6 (2.2, 0.8 to 4.8) | 19 (7.5, 4.6 to 11.4) | 3 (1.1, 0.2 to 3.1) | 1 (0.4, 0 to 2) | |
| Severe | 2 (0.7, 0.1 to 2.7) | 2 (0.8, 0.1 to 2.8) | 0 (0, 0 to 1.3) | 0 (0, 0 to 1.4) | |
| Any | 85 (31.5, 26 to 37.4)*† | 100 (39.4, 33.3 to 45.7)*† | 51 (18.3, 13.9 to 23.3)* | 47 (17.3, 13 to 22.4)* | |
| Redness (mm): | |||||
| 1-24 | 67 (24.8, 19.8 to 30.4) | 59 (23.2, 18.2 to 28.9) | 64 (22.9, 18.1 to 28.3) | 52 (19.2, 14.7 to 24.4) | |
| 25-49 | 9 (3.3, 1.5 to 6.2) | 8 (3.1, 1.4 to 6.1) | 0 (0, 0 to 1.3) | 0 (0, 0 to 1.4) | |
| ≥50 | 0 (0, 0 to 1.4) | 11 (4.3, 2.2 to 7.6) | 0 (0, 0 to 1.3) | 0 (0, 0 to 1.4) | |
| Any | 76 (28.1, 22.9 to 33.9) | 78 (30.7, 25.1 to 36.8)* | 64 (22.9, 18.1 to 28.3) | 52 (19.2, 14.7 to 24.4)* | |
| Swelling (mm): | |||||
| 1-24 | 42 (15.6, 11.4 to 20.4) | 37 (14.6, 10.5 to 19.5) | 26 (9.3, 6.2 to 13.4) | 17 (6.3, 3.7 to 9.9) | |
| 25-49 | 8 (3, 1.3 to 5.8) | 6 (2.4, 0.9 to 5.1) | 0 (0, 0 to 1.3) | 1 (0.4, 0 to 2) | |
| ≥50 | 2 (0.7, 0.1 to 2.7) | 7 (2.8, 1.1 to 5.6) | 0 (0, 0 to 1.3) | 0 (0, 0 to 1.4) | |
| Any | 52 (19.3, 14.7 to 24.5)* | 50 (19.7, 15 to 25.1)* | 26 (9.3, 6.2 to 13.4)* | 18 (6.6, 4 to 10.3)* | |
| Any local severe | 4 (1.5, 0.4 to 3.7)† | 15 (5.9, 3.3 to 9.6)*† | 0 (0, 0 to 1.3) | 0 (0, 0 to 1.4)* | |
| Decreased feeding: | |||||
| Mild | 67 (24.8, 19.8 to 30.4) | 70 (27.6, 22.2 to 33.5) | 75 (26.9, 21.8 to 32.5) | 59 (21.8, 17 to 27.2) | |
| Moderate | 17 (6.3, 3.7 to 9.9) | 27 (10.6, 7.1 to 15.1) | 17 (6.1, 3.6 to 9.6) | 14 (5.2, 2.9 to 8.5) | |
| Severe | 5 (1.9, 0.6 to 4.3) | 6 (2.4, 0.9 to 5.1) | 2 (0.7, 0.1 to 2.6) | 8 (3, 1.3 to 5.7) | |
| Any | 89 (33, 27.4 to 38.9) | 103 (40.6, 34.5 to 46.9)* | 94 (33.7, 28.2 to 39.6) | 81 (29.9, 24.5 to 35.7)* | |
| Decreased activity: | |||||
| Mild | 34 (12.6, 8.9 to 17.2) | 45 (17.7, 13.2 to 23) | 26 (9.3, 6.2 to 13.4) | 33 (12.2, 8.5 to 16.7) | |
| Moderate | 17 (6.3, 3.7 to 9.9) | 33 (13, 9.1 to 17.8) | 24 (8.6, 5.6 to 12.5) | 11 (4.1, 2 to 7.1) | |
| Severe | 4 (1.5, 0.4 to 3.7) | 3 (1.2, 0.2 to 3.4) | 2 (0.7, 0.1 to 2.6) | 3 (1.1, 0.2 to 3.2) | |
| Any | 55 (20.4, 15.7 to 25.7)† | 81 (31.9, 26.2 to 38)*† | 52 (18.6, 14.2 to 23.7) | 47 (17.3, 13 to 22.4)* | |
| Increased irritability: | |||||
| Mild | 89 (33, 27.4 to 38.9) | 84 (33.1, 27.3 to 39.2) | 64 (22.9, 18.1 to 28.3) | 45 (16.6, 12.4 to 21.6) | |
| Moderate | 28 (10.4, 7 to 14.6) | 34 (13.4, 9.5 to 18.2) | 28 (10, 6.8 to 14.2) | 26 (9.6, 6.4 to 13.7) | |
| Severe | 6 (2.2, 0.8 to 4.8) | 4 (1.6, 0.4 to 4) | 7 (2.5, 1 to 5.1) | 6 (2.2, 0.8 to 4.8) | |
| Any | 123 (45.6, 39.5 to 51.7)* | 122 (48, 41.7 to 54.4)* | 99 (35.5, 29.9 to 41.4)* | 77 (28.4, 23.1 to 34.2)* | |
| Persistent crying: | |||||
| Mild | 52 (19.3, 14.7 to 24.5) | 49 (19.3, 14.6 to 24.7) | 32 (11.5, 8 to 15.8) | 35 (12.9, 9.2 to 17.5) | |
| Moderate | 8 (3, 1.3 to 5.8) | 13 (5.1, 2.8 to 8.6) | 12 (4.3, 2.2 to 7.4) | 13 (4.8, 2.6 to 8.1) | |
| Severe | 1 (0.4, 0 to 2) | 1 (0.4, 0 to 2.2) | 2 (0.7, 0.1 to 2.6) | 1 (0.4, 0 to 2) | |
| Any | 61 (22.6, 17.7 to 28.1) | 63 (24.8, 19.6 to 30.6) | 46 (16.5, 12.3 to 21.4) | 49 (18.1, 13.7 to 23.2) | |
| Vomiting: | |||||
| Mild | 28 (10.4, 7 to 14.6) | 28 (11, 7.5 to 15.5) | 29 (10.4, 7.1 to 14.6) | 26 (9.6, 6.4 to 13.7) | |
| Moderate | 6 (2.2, 0.8 to 4.8) | 5 (2, 0.6 to 4.5) | 3 (1.1, 0.2 to 3.1) | 3 (1.1, 0.2 to 3.2) | |
| Severe | 0 (0, 0 to 1.4) | 0 (0, 0 to 1.4) | 0 (0, 0 to 1.3) | 0 (0, 0 to 1.4) | |
| Any | 34 (12.6, 8.9 to 17.2) | 33 (13, 9.1 to 17.8) | 32 (11.5, 8 to 15.8) | 29 (10.7, 7.3 to 15) | |
| Diarrhoea: | |||||
| Mild | 54 (20, 15.4 to 25.3) | 49 (19.3, 14.6 to 24.7) | 58 (20.8, 16.2 to 26) | 46 (17, 12.7 to 22) | |
| Moderate | 9 (3.3, 1.5 to 6.2) | 6 (2.4, 0.9 to 5.1) | 10 (3.6, 1.7 to 6.5) | 12 (4.4, 2.3 to 7.6) | |
| Severe | 3 (1.1, 0.2 to 3.2) | 3 (1.2, 0.2 to 3.4) | 3 (1.1, 0.2 to 3.1) | 4 (1.5, 0.4 to 3.7) | |
| Any | 66 (24.4, 19.4 to 30) | 58 (22.8, 17.8 to 28.5) | 71 (25.4, 20.4 to 31) | 62 (22.9, 18 to 28.3) | |
| Any severe symptoms | 14 (5.2, 2.9 to 8.5) | 19 (7.5, 4.6 to 11.4) | 12 (4.3, 2.2 to 7.4) | 14 (5.2, 2.9 to 8.5) | |
| Fever ≥38°C | 24 (8.9, 5.8 to 12.9)† | 57 (22.4, 17.5 to 28.1)*† | 26 (9.3, 6.2 to 13.4) | 34 (12.5, 8.8 to 17.1)* | |
| Any analgesic or antipyretic | 85 (31.5, 26 to 37.4)† | 111 (43.7, 37.5 to 50)*† | 77 (27.6, 22.4 to 33.2) | 64 (23.6, 18.7 to 29.1)* | |
*P<0.05 for comparison between vaccines.
†P<0.05 for comparison between doses.
Table 3.
Local and systemic reactions in participants aged 5-12 years by vaccine and dose. Figures are numbers (percentage, 95% confidence interval)
| Adjuvanted split virion | Whole virion | ||||
|---|---|---|---|---|---|
| Dose 1 | Dose 2 | Dose 1 | Dose 2 | ||
| Total vaccinated | 181 | 188 | 187 | 185 | |
| With diary card | 181 | 176 | 181 | 175 | |
| Pain: | |||||
| Mild | 89 (49.2, 41.7 to 56.7) | 78 (44.3, 36.8 to 52) | 68 (37.6, 30.5 to 45.1) | 65 (37.1, 30 to 44.8) | |
| Moderate | 44 (24.3, 18.3 to 31.2) | 43 (24.4, 18.3 to 31.5) | 4 (2.2, 0.6 to 5.6) | 8 (4.6, 2 to 8.8) | |
| Severe | 3 (1.7, 0.3 to 4.8) | 4 (2.3, 0.6 to 5.7) | 0 (0, 0 to 2) | 1 (0.6, 0 to 3.1) | |
| Any | 136 (75.1, 68.2 to 81.3)* | 125 (71, 63.7 to 77.6)* | 72 (39.8, 32.6 to 47.3)* | 74 (42.3, 34.9 to 50)* | |
| Redness (mm): | |||||
| 1-24 | 41 (22.7, 16.8 to 29.4) | 40 (22.7, 16.8 to 29.6) | 38 (21, 15.3 to 27.7) | 34 (19.4, 13.8 to 26.1) | |
| 25-49 | 8 (4.4, 1.9 to 8.5) | 8 (4.5, 2 to 8.8) | 3 (1.7, 0.3 to 4.8) | 4 (2.3, 0.6 to 5.7) | |
| ≥50 | 7 (3.9, 1.6 to 7.8) | 9 (5.1, 2.4 to 9.5) | 0 (0, 0 to 2) | 0 (0, 0 to 2.1) | |
| Any | 56 (30.9, 24.3 to 38.2) | 57 (32.4, 25.5 to 39.8)* | 41 (22.7, 16.8 to 29.4) | 38 (21.7, 15.8 to 28.6)* | |
| Swelling: | |||||
| 1-24 | 24 (13.3, 8.7 to 19.1) | 28 (15.9, 10.8 to 22.2) | 21 (11.6, 7.3 to 17.2) | 24 (13.7, 9 to 19.7) | |
| 25-49 | 9 (5, 2.3 to 9.2) | 6 (3.4, 1.3 to 7.3) | 2 (1.1, 0.1 to 3.9) | 1 (0.6, 0 to 3.1) | |
| ≥50 | 8 (4.4, 1.9 to 8.5) | 5 (2.8, 0.9 to 6.5) | 2 (1.1, 0.1 to 3.9) | 1 (0.6, 0 to 3.1) | |
| Any | 41 (22.7, 16.8 to 29.4)* | 39 (22.2, 16.3 to 29) | 25 (13.8, 9.1 to 19.7)* | 26 (14.9, 9.9 to 21) | |
| Any local severe | 13 (7.2, 3.9 to 12)* | 15 (8.5, 4.8 to 13.7)* | 2 (1.1, 0.1 to 3.9)* | 2 (1.1, 0.1 to 4.1)* | |
| Loss of appetite: | |||||
| Mild | 33 (18.2, 12.9 to 24.6) | 26 (14.8, 9.9 to 20.9) | 17 (9.4, 5.6 to 14.6) | 16 (9.1, 5.3 to 14.4) | |
| Moderate | 5 (2.8, 0.9 to 6.3) | 5 (2.8, 0.9 to 6.5) | 2 (1.1, 0.1 to 3.9) | 3 (1.7, 0.4 to 4.9) | |
| Severe | 4 (2.2, 0.6 to 5.6) | 2 (1.1, 0.1 to 4) | 2 (1.1, 0.1 to 3.9) | 1 (0.6, 0 to 3.1) | |
| Any | 42 (23.2, 17.3 to 30)* | 33 (18.8, 13.3 to 25.3) | 21 (11.6, 7.3 to 17.2)* | 20 (11.4, 7.1 to 17.1) | |
| Generally unwell: | |||||
| Mild | 39 (21.5, 15.8 to 28.3) | 31 (17.6, 12.3 to 24.1) | 27 (14.9, 10.1 to 21) | 14 (8, 4.4 to 13.1) | |
| Moderate | 20 (11, 6.9 to 16.5) | 13 (7.4, 4 to 12.3) | 16 (8.8, 5.1 to 14) | 12 (6.9, 3.6 to 11.7) | |
| Severe | 3 (1.7, 0.3 to 4.8) | 2 (1.1, 0.1 to 4) | 2 (1.1, 0.1 to 3.9) | 0 (0, 0 to 2.1) | |
| Any | 62 (34.3, 27.4 to 41.7) | 46 (26.1, 19.8 to 33.3)* | 45 (24.9, 18.7 to 31.8)† | 26 (14.9, 9.9 to 21)*† | |
| Headache: | |||||
| Mild | 51 (28.2, 21.8 to 35.3) | 38 (21.6, 15.8 to 28.4) | 50 (27.6, 21.3 to 34.7) | 36 (20.6, 14.8 to 27.3) | |
| Moderate | 25 (13.8, 9.1 to 19.7) | 21 (11.9, 7.5 to 17.7) | 10 (5.5, 2.7 to 9.9) | 10 (5.7, 2.8 to 10.3) | |
| Severe | 1 (0.6, 0 to 3) | 1 (0.6, 0 to 3.1) | 1 (0.6, 0 to 3) | 0 (0, 0 to 2.1) | |
| Any | 77 (42.5, 35.2 to 50.1) | 60 (34.1, 27.1 to 41.6) | 61 (33.7, 26.9 to 41.1) | 46 (26.3, 19.9 to 33.5) | |
| Nausea/vomiting: | |||||
| Mild | 30 (16.6, 11.5 to 22.8) | 4 (2.2, 0.6 to 5.6) | 0 (0, 0 to 2) | 34 (18.8, 13.4 to 25.2) | |
| Moderate | 25 (14.2, 9.4 to 20.3) | 1 (0.6, 0 to 3.1) | 1 (0.6, 0 to 3.1) | 27 (15.3, 10.4 to 21.5) | |
| Severe | 20 (11, 6.9 to 16.5) | 1 (0.6, 0 to 3) | 1 (0.6, 0 to 3) | 22 (12.2, 7.8 to 17.8) | |
| Any | 15 (8.6, 4.9 to 13.7) | 0 (0, 0 to 2.1) | 2 (1.1, 0.1 to 4.1) | 17 (9.7, 5.8 to 15.1) | |
| Diarrhoea: | |||||
| Mild | 24 (13.3, 8.7 to 19.1) | 11 (6.3, 3.2 to 10.9) | 25 (13.8, 9.1 to 19.7) | 17 (9.7, 5.8 to 15.1) | |
| Moderate | 4 (2.2, 0.6 to 5.6) | 2 (1.1, 0.1 to 4) | 2 (1.1, 0.1 to 3.9) | 3 (1.7, 0.4 to 4.9) | |
| Severe | 0 (0, 0 to 2) | 1 (0.6, 0 to 3.1) | 0 (0, 0 to 2) | 1 (0.6, 0 to 3.1) | |
| Any | 28 (15.5, 10.5 to 21.6)† | 14 (8, 4.4 to 13)^ | 27 (14.9, 10.1 to 21) | 21 (12, 7.6 to 17.8) | |
| Muscle pain: | |||||
| Mild | 40 (22.1, 16.3 to 28.9) | 29 (16.5, 11.3 to 22.8) | 22 (12.2, 7.8 to 17.8) | 17 (9.7, 5.8 to 15.1) | |
| Moderate | 19 (10.5, 6.4 to 15.9) | 13 (7.4, 4 to 12.3) | 3 (1.7, 0.3 to 4.8) | 5 (2.9, 0.9 to 6.5) | |
| Severe | 0 (0, 0 to 2) | 2 (1.1, 0.1 to 4) | 0 (0, 0 to 2) | 0 (0, 0 to 2.1) | |
| Any | 59 (32.6, 25.8 to 39.9)* | 44 (25, 18.8 to 32.1)* | 25 (13.8, 9.1 to 19.7)* | 22 (12.6, 8 to 18.4)* | |
| Joint pain: | |||||
| Mild | 17 (9.4, 5.6 to 14.6) | 15 (8.5, 4.8 to 13.7) | 19 (10.5, 6.4 to 15.9) | 13 (7.4, 4 to 12.4) | |
| Moderate | 3 (1.7, 0.3 to 4.8) | 3 (1.7, 0.4 to 4.9) | 4 (2.2, 0.6 to 5.6) | 2 (1.1, 0.1 to 4.1) | |
| Severe | 0 (0, 0 to 2) | 1 (0.6, 0 to 3.1) | 0 (0, 0 to 2) | 0 (0, 0 to 2.1) | |
| Any | 20 (11, 6.9 to 16.5) | 19 (10.8, 6.6 to 16.3) | 23 (12.7, 8.2 to 18.5) | 15 (8.6, 4.9 to 13.7) | |
| Any severe symptoms | 5 (2.8, 0.9 to 6.3) | 5 (2.8, 0.9 to 6.5) | 3 (1.7, 0.3 to 4.8) | 2 (1.1, 0.1 to 4.1) | |
| Fever ≥38°C | 14 (7.7, 4.3 to 12.6) | 11 (6.3, 3.2 to 10.9) | 6 (3.3, 1.2 to 7.1) | 5 (2.9, 0.9 to 6.5) | |
| Any analgesic/antipyretic | 66 (36.5, 29.5 to 43.9)* | 50 (28.4, 21.9 to 35.7)* | 40 (22.1, 16.3 to 28.9)* | 29 (16.6, 11.4 to 22.9)* | |
*P<0.05 for comparison between vaccines.
†P<0.05 for comparison between doses.
In younger children, dose two of the adjuvanted split virion vaccine was more reactogenic than dose one, with more fever ≥38°C (22.4% v 8.9%, P<0.001), local severe reactions (5.9% v 1.5%, P=0.02), and decreased activity (31.9% v 20.4%, P<0.001). The second dose of the whole virion vaccine was associated with decreased frequency of being generally unwell (14.9% v 24.9% after first dose).
In keeping with the increased reactogenicity of the adjuvanted split virion vaccine, more recipients of that vaccine received antipyretics/analgesics after either dose of vaccine in the older children (36.5% v 22.1% in the whole virion group for dose one; 28.4% v 16.6% for dose two) and after the second dose in younger children (43.7% v 23.6%, P<0.001) (table 3).
Four adverse events of special interest occurred, one in a child receiving the whole virion vaccine (focal seizure, considered unrelated to vaccination) and three in participants receiving the adjuvanted split virion vaccine (one reactive knee arthritis, possibly related to vaccination, and two generalised seizures considered unrelated to vaccination). The reactive knee arthritis occurred in a child aged 11 months in the leg in which vaccine had been administered two days previously. The child became febrile on the evening of vaccination and was failing to bear weight two days later. Other joints and the vaccination site were normal as were results of blood tests and x ray investigation of the knee and pelvis. Throat swab and blood cultures yielded sterile results. The child made a full recovery after 10 days.
Five other serious adverse events occurred, but they were not in the category of adverse events of special interest and were considered unrelated to vaccination.
Immunogenicity
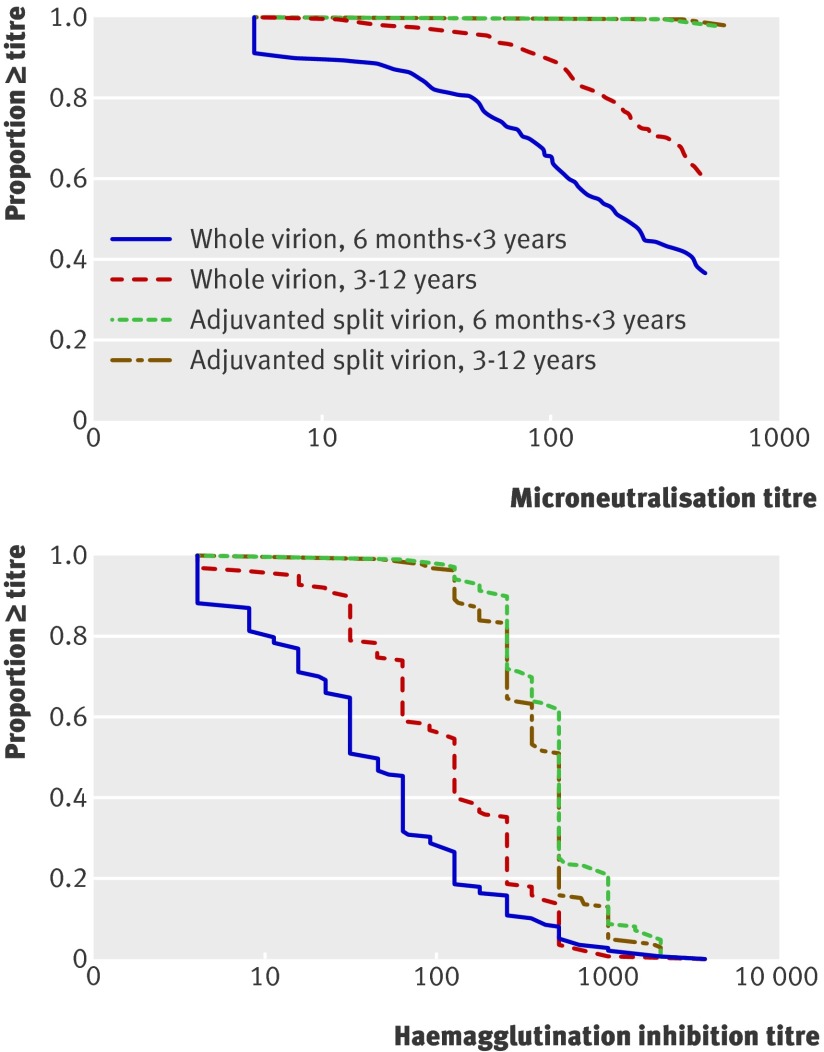
Before vaccination, 35 (4.0%) children (2.9% younger group, 5.0% older group) had microneutralisation titres ≥1:40, suggesting pre-existing immunity. Tables 4, 5, and 6 and figure 2 show antibody responses .
Table 4.
Seroconversion by microneutralisation titre (95% confidence interval)
| Vaccine and age (years) | Before vaccine | After second dose | Rise | |||||
|---|---|---|---|---|---|---|---|---|
| No | Titre % ≥1:40 | No | Titre %≥1:40 | No | Titre % ≥ fourfold to ≥1:40 | |||
| Whole virion | ||||||||
| <3 | 9/216 | 4.2 (1.9 to 7.8) | 166/206 | 80.6 (74.5 to 85.8) | 157/196 | 80.1 (73.8 to 85.5) | ||
| 3-12 | 11/240 | 4.6 (2.3 to 8.1) | 211/220 | 95.9 (92.4 to 98.1) | 208/217 | 95.9 (92.4 to 98.1) | ||
| All | 20/456 | 4.4 (2.7 to 6.7) | 377/426 | 88.5 (85.1 to 91.3) | 365/413 | 88.4 (84.9 to 91.3) | ||
| Adjuvanted split virion | ||||||||
| <3 | 3/191 | 1.6 (0.3 to 4.5) | 175/177 | 98.9 (96.0 to 99.9) | 163/166 | 98.2 (94.8 to 99.6) | ||
| 3-12 | 13/244 | 5.3 (2.9 to 8.9) | 234/235 | 99.6 (97.7 to 99.9) | 226/228 | 99.1 (96.9 to 99.9) | ||
| All | 16/435 | 3.7 (2.1 to 5.9) | 409/412 | 99.3 (97.9 to 99.8) | 389/394 | 98.7 (97.1 to 99.6) | ||
Table 5.
Seroconversion by haemagglutination inhibition titre
| Vaccine and age (years) | Before vaccine | After second dose | Rise | |||||
|---|---|---|---|---|---|---|---|---|
| No | Titre % ≥1:32 | No | Titre % ≥1:32 | No | Titre % ≥ fourfold to ≥1:32 | |||
| Whole virion | ||||||||
| <3 years | 8/216 | 3.7 (1.6 to 7.2) | 136/207 | 65.7 (58.8 to 72.1) | 126/197 | 64.0 (56.8 to 70.7) | ||
| 3-12 | 7/240 | 2.9 (1.2 to 5.9) | 198/220 | 90.0 (85.3 to 93.6) | 192/217 | 88.5 (83.5 to 92.4) | ||
| All | 15/456 | 3.3 (1.9 to 5.4) | 334/427 | 78.2 (74.0 to 82.0) | 318/414 | 76.8 (72.4 to 80.8) | ||
| Adjuvanted split virion | ||||||||
| <3 | 3/191 | 1.6 (0.3 to 4.5) | 174/175 | 99.4 (96.9 to 99.9) | 163/164 | 99.4 (96.6 to 99.9) | ||
| 3-12 | 13/244 | 5.3 (2.9 to 8.9) | 233/235 | 99.1 (97.0 to 99.9) | 225/228 | 98.7 (96.2 to 99.7) | ||
| All | 16/435 | 3.7 (2.1 to 5.9) | 407/410 | 99.3 (97.9 to 99.8) | 388/392 | 99.0 (97.4 to 99.7) | ||
Table 6.
Haemagglutination inhibition geometric mean titres
| Vaccine and age (years) | Before vaccine | After second dose | Rise | |||||
|---|---|---|---|---|---|---|---|---|
| No | Titre | No | Titre | No | Titre | |||
| Whole virion | ||||||||
| <3 | 216 | 4.6 (4.2 to 5.1) | 207 | 44.0 (35.6 to 54.3) | 197 | 9.5 (7.8 to 11.6) | ||
| 3-12 | 240 | 4.6 (4.2 to 4.9) | 220 | 106.3 (90.2 to 125.3) | 217 | 22.7 (19.3 to 26.8) | ||
| All | 456 | 4.6 (4.3 to 4.9) | 427 | 69.3 (60.3 to 79.6) | 414 | 15.0 (13.2 to 17.2) | ||
| Adjuvanted split virion | ||||||||
| <3 | 191 | 4.2 (4.0 to 4.5) | 175 | 461.0 (409.0 to 519.6) | 164 | 107.4 (93.9 to 122.9) | ||
| 3-12 | 244 | 4.8 (4.3 to 5.3) | 235 | 377.3 (339.2 to 419.7) | 228 | 78.5 (69.9 to 88.1) | ||
| All | 435 | 4.5 (4.3 to 4.8) | 410 | 411.0 (379.4 to 445.2) | 392 | 89.5 (81.9 to 97.8) | ||
Fig 2 Reverse cumulative distribution curves of antibody titres as measured by microneutralisation curves and haemagglutination inhibition assays by age group and vaccine
Seroconversion rates were higher with the adjuvanted split virion vaccine than with the whole virion unadjuvanted vaccine both by microneutralisation assay (younger group 98.2% v 80.1% (P<0.001), older group 99.1% v 95.9% (P=0.03), table 4) and haemagglutination inhibition assay (younger group 99.4% v 64.0%, older group 98.7% v 88.5%; P≤0.001 for both groups, table 5). Compared with the whole virion vaccine, the adjuvanted split virion vaccine was associated with a higher percentage of participants with microneutralisation titres ≥1:40 (99.3% v 88.5%; P<0.001), a higher percentage with haemagglutination inhibition titre ≥1:32 (99.3% v 78.2%; P<0.001), higher geometric mean haemagglutination inhibition titres (411.0 v 69.3), and greater geometric rise in haemagglutination inhibition titre from baseline (89.5 v 15.0) (P<0.001 for all comparisons).
The multivariable analysis on logged haemagglutination inhibition titres showed a significant interaction between age and vaccine (P<0.001), with 10.5-fold (95% confidence interval 8.1 to 13.5) titres induced by the adjuvanted split virion vaccine in the younger participants compared with 3.6-fold (3.0 to 4.3) titres in older children. We further evaluated this difference in the age effect by vaccine by including age as a continuous variable in the multivariable model. This showed a 3% decrease in titre per year of age (0.5% to 5%, P=0.02) for the split virion adjuvanted vaccine and a 16% increase per year (12% to 21%, P<0.001) for the whole virion vaccine.
Discussion
In this head to head study of an adjuvanted split virion H1N1 pandemic vaccine and a non-adjuvanted whole virion vaccine in children, both vaccines were well tolerated. The vaccine containing the novel adjuvant was more immunogenic than the whole virion vaccine, especially in young children, but was also more reactogenic.
In the UK, most influenza activity in 2009-10 has been caused by pandemic influenza A (H1N1). Serological evidence has shown low levels of immunity in children before the pandemic and correspondingly high attack rates.1 A UK vaccination programme, principally using the adjuvanted split virion vaccine,23 was announced in August 2009, initially targeting those with comorbidities, but was widened to all children aged 6 months to 5 years in December 2009 after a review of interim data from this study and other data.23
A recent serosurvey showed that the rates of H1N1 infection in English children after the first wave of the pandemic (as measured by haemagglutination inhibition titres ≥1:32) were higher than the 3.5% observed before immunisation in our study.1 This could reflect geographical differences in exposure risk1 and our exclusion of children with a history of confirmed H1N1 disease and those treated for suspected infection. Follow-up took place during the second wave of the UK pandemic, but any boosting effect of natural infection would be expected to be similar between vaccine groups.
Immunogenicity
The immunogenicity of both seasonal influenza vaccines7 and other, non-adjuvanted, H1N1 vaccines11 in young children is less than in older children and adults. New generation adjuvants (such as MF59 and AS03B) have been used to improve immunogenicity,13 14 24 though paediatric experience with these adjuvants is limited and AS03B has previously been used only in the H5N1 mock-up pandemic vaccine. In our study the adjuvanted split virion vaccine was highly immunogenic, even in young children, but was slightly less immunogenic in older children than in infants (3% per year with age), a pattern not previously described for inactivated vaccines. We also found a strongly age dependent response to the whole virion vaccine, with 15% higher immunogenicity per year with age. Other H1N1 vaccines, including both adjuvanted and non-adjuvanted vaccines, are immunogenic in children but contain considerably more antigen than the adjuvanted split virion vaccine used in this trial.10 25 Antigen sparing is important in a pandemic setting where requirements for vaccine exceed manufacturing capability.26 H5N1 vaccine trials before the pandemic showed the need for a two dose regimen in immunologically naive individuals,19 and two dose regimens of several H1N1 vaccines are more immunogenic than single dose regimens.10 11 25 Limited data, however, have suggested that the adjuvanted split virion vaccine used in our trial might be sufficient to meet licensing criteria,12 19 and the UK has recently recommended a single dose regimen in healthy children.23 Further studies evaluating the breadth and duration of the immune response to single and two dose regimens are needed.14
Even during periods between pandemics, children experience considerable morbidity and mortality from influenza infection, and their role in virus transmission results in a much wider burden.7 Unlike the UK, some countries recommend that all children are routinely immunised against seasonal influenza,6 although the available vaccines have low immunogenicity in young children.7 The favourable immunogenicity of the adjuvanted split virion vaccine in the youngest children in our study suggests that novel adjuvants could be used to improve the immunogenicity of seasonal influenza vaccines in this population.
Reactogenicity
Whole virion influenza vaccines have previously been associated with high reactogenicity rates.7 We have shown that a whole virion H1N1 vaccine in children is well tolerated. Increased reactogenicity was seen with an MF59 adjuvanted H1N1 vaccine in children27 as well as in adult trials of oil in water adjuvanted vaccines.12 13 14 15 24 The AS03B adjuvanted vaccine in this trial was similarly associated with more local reactions and some increase in systemic reactions compared with whole virion vaccine. Our observed local and systemic rates of reactogenicity were generally in keeping with data in the summary of product characteristics.12 19 Though we found the rate of fever to be slightly higher in infants after the second dose compared with the first, this was half the rate reported in the product characteristics (43% of 51 infants).19
Strengths and limitations of the study
Children with comorbidities are at increased risk of severe H1N1 disease, and for this reason we did not exclude children with pre-existing medical conditions (except immunodeficiency), making our findings particularly relevant to the general paediatric population.
The haemagglutination inhibition assay is used extensively in the serological assessment of immunity to influenza viruses and as a criterion for licensing.21 28 29 30 However, it measures antibody directed only to the receptor binding site, while the microneutralisation assay might be more sensitive as it detects antibody directed to this and other antigenic sites in the virus24 28 31 and was therefore chosen as the primary immunogenicity end point.
When we were designing this study, a two dose pandemic vaccine schedule was planned for children, and for this reason our pragmatic trial did not include a blood test after one dose to simplify the study in the face of the need for rapid recruitment. With the subsequent change to a single dose regimen in the UK, our results would have been strengthened by addition of assessment of immunogenicity after a single dose. Furthermore, a comparison with a non-adjuvanted split virion vaccine would be of interest but none was used in the UK during the 2009 H1N1 pandemic, and we limited the study to these two novel vaccines.
Conclusions and policy implications
In this direct comparison of two commercially available novel H1N1 vaccines, AS03B adjuvanted split virion vaccine was more immunogenic and induced high seroconversion rates in young children. These data provide important information to guide immunisation policy in an influenza pandemic and indicate the potential for improved immunogenicity of seasonal influenza vaccines in children.
What is already known on this topic
Children are a priority for pandemic influenza vaccination as they experience high rates of disease, are more often admitted to hospital than adults, and act as transmitters of the virus
Novel adjuvants such as AS03B were thought to enhance the immunogenicity of H1N1 vaccines but had not been routinely used in early childhood before the latest pandemic
Whole virion influenza vaccines were expected to shorten vaccine production times but have previously been associated with unacceptable reactogenicity rates, although H5N1 whole virion vaccines were well tolerated
What this study adds
AS03B adjuvanted split virion vaccine, while more reactogenic, was more immunogenic than the whole virion vaccine
This vaccine achieved high seroconversion rates in children aged less than 3 years, indicating the potential for improved immunogenicity of influenza vaccines in this age group
We thank the parents and children who participated in the trials and are grateful for the clinical and administrative assistance of the National Institute of Health Research (NIHR), National Research Ethics Service (NRES), Medicines and Healthcare Products Regulatory Agency (MHRA), National Health Service (NHS), Thames Valley, Hampshire and Isle of Wight, and South London NIHR CLRNs, NIHR Medicines for Children Research Network (MCRN), the Southampton University Hospitals NHS Trust Research and Development Office, Child Health Computer Departments of Primary Care Trusts in Oxford, Southampton, Bristol, Exeter, and London (Wandsworth), the NIHR Oxford Biomedical Research Centre (BRC), the Health Protection Agency Centre for Infections for both administrative assistance and laboratory support, the staff of the Clinical Trials Research Governance, University of Oxford, members of the trial steering committee (Stephen Evans (chair), Simon Bevan, Heather House, Simon Knoll, Stephen Welch, Elizabeth Miller, and Andrew Pollard) and the data monitoring committee (Philip Monk (chair), Delane Shingadia, Chris Verit, and Paula Williamson).
Contributors: AJP, MDS, EM, SNF, AF, PTH, ACC, NA, KH, and CSW designed the study with critical input from all authors. AJP was chief investigator. MDS, SNF, AF, PTH, and ACC were local principal investigators. NA was responsible for statistical analysis, KH for laboratory analysis and PW, PEE and ES for data management, all with oversight and guidance by EM (lead investigator). AR and RJA were project managers. CSW, WTW, CO, TJ, SW, MC, PEE, RA, IO, SL, ACC, PTH, AF, SNF, MDS, and AJP enrolled patients and/or contributed to data collection. CSW drafted this report, which was subsequently reviewed by all authors. All authors have seen the final submitted report and agree with its contents. All authors had full access to all the data (including statistical reports and tables) in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Funding: This study was funded by the NIHR Health Technology Assessment Programme and was supported by the NIHR Oxford Comprehensive Biomedical Research Centre programme (including salary support for AR, TMJ, and MDS), and the Thames Valley, Hampshire and Isle of Wight and Western (salary support for CDS) Comprehensive Local Research Networks and the Southampton Respiratory NIHR Biomedical Research Unit. None of the funders had a role in study design, data collection, data analysis, data interpretation, or writing of the report. This study was adopted by the NIHR Medicines for Children Research Network and supported by their South West and London-South East, North, Central, and East (SENCE) Local Research Networks. AJP is a Jenner Institute Investigator.
Competing interests: Vaccines were manufactured by GlaxoSmithKline vaccines and Baxter, both of whom donated the vaccine but had no role in study planning or conduct. All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare that they had (1) No Financial support for the submitted work. (2) AJP, AF, PTH, SNF, and ACC have received research grants and honoraria from vaccine manufacturers. All grants and honoraria are paid into accounts within the respective NHS trusts or universities, or to independent charities; AJP, AF, PTH, and SNF have participated in advisory boards for vaccine manufacturers but receive no personal payments for this work. MDS, PTH, and AF have received financial assistance from vaccine manufacturers to attend conferences. (3) No authors have spouses, partners, or children with relationships with commercial entities that might have an interest in the submitted work. (4) No authors have financial interests that may be relevant to the submitted work.
Ethical approval: This study was approved by the Oxfordshire Research Ethics Committee A (No 09/H0604/107), the UK Medicines and Healthcare Products Regulatory Agency (EUDRACT 2009-014719-11), and local NHS organisations by an expedited process.32 Parents or guardians gave written informed consent; children aged 7 and over gave verbal consent.
Data sharing: The authors will consider appropriate requests for data sharing that do not breach the consent obtained from the participants’ parents. Please contact claire.waddington@paediatrics.ox.ac.uk.
Cite this as: BMJ 2010;340:c2649
References
- 1.Miller E, Hoschler K, Hardelid P, Stanford E, Andrews N, Zambon M. Incidence of 2009 pandemic influenza A H1N1 infection in England: a cross-sectional serological study. Lancet 2010;375:1100-8. [DOI] [PubMed] [Google Scholar]
- 2.Pandemic (H1N1) 2009 in England: an overview of initial epidemiological findings and implications for the secondwave. Health Protection Agency, 2009.
- 3.Jain S, Kamimoto L, Bramley AM, Schmitz AM, Benoit SR, Louie J, et al. Hospitalized patients with 2009 H1N1 influenza in the United States, April-June 2009. N Engl J Med 2009;361:1935-44. [DOI] [PubMed] [Google Scholar]
- 4.Glezen WP, Couch RB. Interpandemic influenza in the Houston area, 1974-76. N Engl J Med 1978;298:587-92. [DOI] [PubMed] [Google Scholar]
- 5.Strategic Advisory Group of Experts on Immunization. Report of the extraordinary meeting on the influenza A (H1N1) 2009 pandemic, 7 July 2009. www.who.int/wer/2009/wer8430.pdf. [PubMed]
- 6.Fiore AE, Shay DK, Broder K, Iskander JK, Uyeki TM, Mootrey G, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep 2009;58(RR-8):1-52. [PubMed] [Google Scholar]
- 7.Belshe RB, Edwards KM, Vesikari T, Black SV, Walker RE, Hultquist M, et al. Live attenuated versus inactivated influenza vaccine in infants and young children. N Engl J Med 2007;356:685-96. [DOI] [PubMed] [Google Scholar]
- 8.Zangwill KM, Belshe RB. Safety and efficacy of trivalent inactivated influenza vaccine in young children: a summary for the new era of routine vaccination. Pediatr Infect Dis J 2004;23:189-97. [DOI] [PubMed] [Google Scholar]
- 9.Kelly H, Barr I. Large trials confirm immunogenicity of H1N1 vaccines. Lancet 2009;375:6-9. [DOI] [PubMed] [Google Scholar]
- 10.Liang XF, Wang HQ, Wang JZ, Fang HH, Wu J, Zhu FC, et al. Safety and immunogenicity of 2009 pandemic influenza A H1N1 vaccines in China: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet 2009;375:55-66. [DOI] [PubMed] [Google Scholar]
- 11.Plennevaux E, Sheldon E, Blatter M, Reeves-Hoche MK, Denis M. Immune response after a single vaccination against 2009 influenza A H1N1 in USA: a preliminary report of two randomised controlled phase 2 trials. Lancet 2009;375:41-8. [DOI] [PubMed] [Google Scholar]
- 12.Roman F, Vaman T, Gerlach B, Markendorf A, Gillard P, Devaster JM. Immunogenicity and safety in adults of one dose of influenza A H1N1v 2009 vaccine formulated with and without AS03(A)-adjuvant: preliminary report of an observer-blind, randomised trial. Vaccine 2010;28:1740-5. [DOI] [PubMed] [Google Scholar]
- 13.Chu DW, Hwang SJ, Lim FS, Oh HM, Thongcharoen P, Yang PC, et al. Immunogenicity and tolerability of an AS03(A)-adjuvanted prepandemic influenza vaccine: a phase III study in a large population of Asian adults. Vaccine 2009;27:7428-35. [DOI] [PubMed] [Google Scholar]
- 14.Leroux-Roels I, Roman F, Forgus S, Maes C, De Boever F, Drame M, et al. Priming with AS03(A)-adjuvanted H5N1 influenza vaccine improves the kinetics, magnitude and durability of the immune response after a heterologous booster vaccination: an open non-randomised extension of a double-blind randomised primary study. Vaccine 2009;28:849-57. [DOI] [PubMed] [Google Scholar]
- 15.Leroux-Roels I, Borkowski A, Vanwolleghem T, Drame M, Clement F, Hons E, et al. Antigen sparing and cross-reactive immunity with an adjuvanted rH5N1 prototype pandemic influenza vaccine: a randomised controlled trial. Lancet 2007;370:580-9. [DOI] [PubMed] [Google Scholar]
- 16.Ehrlich HJ, Muller M, Oh HM, Tambyah PA, Joukhadar C, Montomoli E, et al. A clinical trial of a whole-virus H5N1 vaccine derived from cell culture. N Engl J Med 2008;358:2573-84. [DOI] [PubMed] [Google Scholar]
- 17.Barrett PN, Mundt W, Kistner O, Howard MK. Vero cell platform in vaccine production: moving towards cell culture-based viral vaccines. Expert Rev Vaccines 2009;8:607-18. [DOI] [PubMed] [Google Scholar]
- 18.Erlewyn-Lajeunesse M, Brathwaite N, Lucas JS, Warner JO. Recommendations for the administration of influenza vaccine in children allergic to egg. BMJ 2009;339:b3680. [DOI] [PubMed] [Google Scholar]
- 19.European Medicines Agency. Pandemrix. Summary of product characteristics, approved by the European Commission on 22 December 2009. GlaxoSmithKlein, 2009.
- 20.CHMP recommendations for the core risk management plan for influenza vaccines prepared from viruses with the potential to cause a pandemic and intended for use outside of the core dossier context. European Medicines Agency, 2008.
- 21.Rowe T, Abernathy RA, Hu-Primmer J, Thompson WW, Lu X, Lim W, et al. Detection of antibody to avian influenza A (H5N1) virus in human serum by using a combination of serologic assays. J Clin Microbiol 1999;37:937-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ellis JS, Zambon MC. Molecular analysis of an outbreak of influenza in the United Kingdom. Eur J Epidemiol 1997;13:369-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.A (H1N1) swine flu influenza: phase two of the vaccination programme; children over 6 months and under 5 years: dosage schedule update. Department of Health, 2009.
- 24.Clark TW, Pareek M, Hoschler K, Dillon H, Nicholson KG, Groth N, et al. Trial of 2009 influenza A (H1N1) monovalent MF59-adjuvanted vaccine—preliminary report. N Engl J Med 2009;361:2424-35. [DOI] [PubMed] [Google Scholar]
- 25.Nolan T, McVernon J, Skeljo M, Richmond P, Wadia U, Lambert S, et al. Immunogenicity of a monovalent 2009 influenza A(H1N1) vaccine in infants and children: a randomized trial. JAMA 2010;303:37-46. [DOI] [PubMed] [Google Scholar]
- 26.Collin N, de Radigues X. Vaccine production capacity for seasonal and pandemic (H1N1) 2009 influenza. Vaccine 2009;27:5184-6. [DOI] [PubMed] [Google Scholar]
- 27.Arguedas A, Soley C, Lindert K. Responses to 2009 H1N1 vaccine in children 3 to 17 years of age. N Engl J Med 2010;362:370-2. [DOI] [PubMed] [Google Scholar]
- 28.Stephenson I, Heath A, Major D, Newman RW, Hoschler K, Junzi W, et al. Reproducibility of serologic assays for influenza virus A (H5N1). Emerg Infect Dis 2009;15:1252-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guidance for industry: clinical data needed to support the licensure of pandemic influenza vaccines. US Food and Drug Administration, 2007.
- 30.Guideline on influenza A vaccines prepared from viruses with the potential to cause a pandemic and intended for use outside of the core dossier content. European Medicines Agency, 2007.
- 31.Hancock K, Veguilla V, Lu X, Zhong W, Butler EN, Sun H, et al. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N Engl J Med 2009;361:1945-52. [DOI] [PubMed] [Google Scholar]
- 32.Pollard AJ, Reiner A, John T, Sheasby E, Snape M, Faust S, et al. Future of flu vaccines. Expediting clinical trials in a pandemic. BMJ 2009;339:b4652. [DOI] [PubMed] [Google Scholar]