Abstract
Humoral immune responses depend on B cells encountering antigen, interacting with helper T cells, proliferating and differentiating into low-affinity plasma cells or, after organizing into a germinal center (GC), high-affinity plasma cells and memory B cells. Remarkably, each of these events occurs in association with distinct stromal cells in separate subcompartments of the lymphoid tissue. B cells must migrate from niche to niche in a rapid and highly regulated manner to successfully mount a response. The chemokine, CXCL13, plays a central role in guiding B cells to follicles whereas T-zone chemokines guide activated B cells to the T zone. Sphingosine-1-phosphate (S1P) promotes cell egress from the tissue, as well as marginal-zone B-cell positioning in the spleen. Recent studies have identified a role for the orphan receptor, EBV-induced molecule 2 (EBI2; GPR183), in guiding activated B cells to inter and outer follicular niche(s) and down-regulation of this receptor is essential for organizing cells into GCs. In this review, we discuss current understanding of the roles played by chemokines, S1P and EBI2 in the migration events that underlie humoral immune responses.
Keywords: antibody response, B-cell migration, chemokine, EBI2, lymphoid niche, S1P
Introduction
Secondary lymphoid organs, such as the spleen and lymph nodes, are specialized structures designed to filter the blood and lymph, respectively, and to promote appropriate immune responses against innumerous antigens belonging to a huge range of invading microbes. Strategic compartmentalization of B and T lymphocytes and highly regulated mechanisms that maximize the capture, processing and distribution of antigens to immune cells are components that evolved to maximize the probability that rare antigen-specific B and T cells encounter their cognate antigens and initiate appropriate immune responses. In this review, we summarize data documenting the migration of antigen-specific B and T lymphocytes during T-dependent antibody responses. The kinetics and synchrony of the events during a T-dependent response is variable and may depend on numerous factors. The model here described summarizes published data obtained in most cases with ‘ideal’ immunological conditions, including high frequency and affinity of antigen-specific lymphocytes and ‘optimal’ amounts of antigen and adjuvant. The understanding provided by these simplified systems provides an essential foundation for characterizing the changes that occur during more complex pathogen- and autoantigen-induced responses.
Traffic patterns of naive lymphocytes
Naive B lymphocytes, organized in primary follicles around the T zone (Fig. 1), move among a reticular network of stromal cell processes. Follicular stromal cells are heterogeneous (1) and include a central network of immune complex-trapping follicular dendritic cells (FDCs) (2, 3) and an outer population recently named marginal reticular cells (MRCs) (4, 5). The chemokine CXCL13 is made broadly by follicular stromal cells, including FDCs and MRCs (1, 2, 4), and B cells require the CXCL13 receptor, CXCR5, to access B-cell follicles (6). Expression of CXCL13 is dependent on the cytokine lymphotoxin (LT)-α1β2 and a positive feedback loop involving CXCR5-mediated induction of LTα1β2 expression by B cells contributes to maximal CXCL13 production and maturation of the FDC network (7). Lymphoid tissue-inducer cells are also likely involved in providing LTα1β2 and other inputs that promote CXCL13 production (8). B cells move within follicles in a ∼6 μm min−1 ‘random walk’ (9) and some work suggests that they move along stromal cell processes (10), though this is less well established than for T cells and is not evident in all studies (11) (Fig. 2).
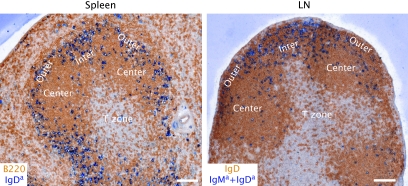
Fig. 1.
Positioning of activated antigen-specific B cells in inter and outer follicular niches of spleen and lymph node (LN) at days 2–3 of the response. Five to 10 million MD4 Ig-transgenic B cells that express IgMa and IgDa specific for hen egg lysozyme (HEL), and ovalbumin (OVA)-specific OTII TCR transgenic T cells were co-transferred into C57BL/6 mice immunized with 50 μg of HEL–OVA in Ribi adjuvant (Sigma) intraperitoneally for 2 days (spleen) or subcutaneously for 3 days (LN). Cryostat sections were stained with antibodies to B220 or IgD to detect all follicular B cells (brown) and to IgDa or IgMa + IgDa to detect the activated MD4 B cells (blue). Outer, Center and Inter refer to regions of the follicle. Scale bar is 100 μm.
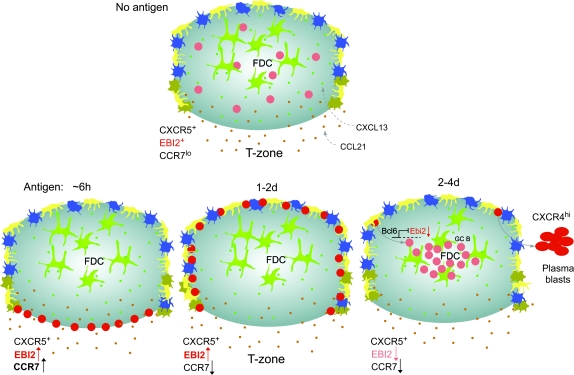
Fig. 2.
Model of EBI2 ligand distribution and B-cell migration in follicles during the early phase of T-cell-dependent antibody responses in spleen and lymph nodes. Naive B cells (pink circles) express CXCR5 and EBI2, reside in follicles and migrate over the processes of FDCs (green), MRCs (yellow), CD169+ macrophages (dark blue) and likely other stromal cells (not depicted). FDCs and MRCs produce CXCL13 (small green dots) and EBI2 ligand is suggested to be made by cells at the follicle perimeter, particularly in inter and outer follicular regions (blue–green gradient, where dark shading represents areas of highest ligand concentration). B-cell movement within the FDC-rich area is largely dependent on CXCR5 and CXCL13 and is critical for antigen scanning. Within 6 h after encountering cognate antigen, activated B cells (red circles) up-regulate CCR7 and EBI2. Activated B cells become more responsive to CCL21 (orange dots) and most likely to EBI2 ligand, while maintaining CXCR5 responsiveness. Balanced chemoattraction mediated by CXCR5 and CCR7, and possibly also responsiveness to local EBI2 ligand, promotes uniform distribution of activated B cells along the interface between follicles and T-cell areas (B/T zone), where cognate interactions with antigen-specific T cells are initiated. Between 1 and 2 days later, B cells down-regulate CCR7 while maintaining high levels of EBI2 (and CXCR5) and move to inter and outer follicular regions where EBI2 ligand concentration might be highest (dark shading). Inter and outer follicular niches are characterized by layers of CD169+ macrophages and MRCs and the interfollicular niche also contains dendritic cells (brown cells). B-cell proliferation and commitment to plasmablast or GC differentiation pathway ensues, perhaps influenced by signals received from local niche cells. Some activated B cells differentiate into plasmablasts and accumulate at extrafollicular sites in a CXCR4-dependent manner. Other activated B cells up-regulate Bcl6, which represses Ebi2, and take on a GC fate. GC-committed B cells move to the center follicle due to loss of EBI2 and perhaps attracted by CXCL13 or another FDC-derived cue and initiate the GC reaction that will produce high-affinity memory B cells and plasma cells.
Chemoattractant receptors activate the cell migration machinery by catalyzing the exchange of GTP for GDP on Gαi proteins (12). Visualization of Gαi2–/– B-cell behavior in follicles revealed a role for this subunit in promoting B-cell migration (13). It is likely that CXCR5, which couples to Gαi (14), contributes to B-cell migration in follicles, and such a requirement has been documented in germinal centers (GCs) (15). Downstream of the heterotrimeric G-protein, the rac-GEF DOCK2 is needed for B-cell migration within follicles (16). The small GTPase effector, Rap1, also promotes follicular B-cell motility (17). Although initially thought to function principally to activate integrins, integrin blocking has milder effects on lymphocyte motility than Rap1 deficiency (17, 18) suggesting additional roles for Rap1.
T cells utilize CCR7 to home and migrate within the T zone, a region enriched with fibroblastic reticular cells (FRCs), or T-zone reticular cells, that abundantly express CCR7 ligands CCL19 and CCL21. Deficiencies in CCR7, or in its ligands, disrupt the T-zone architecture, and T-cell homing and migration within the T zone are impaired (19–21). Naive B cells also express CCR7, though in lower amounts than on T cells, and they use this receptor along with CXCR4 and CXCR5, to enter lymphoid tissues from the blood (22, 23). CCL21, the more abundant of the T-zone chemokines, extends as a gradient into the B-cell follicle and access of naive B cells to the T-zone proximal half of B-cell follicles is more efficient when the cells express CCR7, suggesting that motility through this region is promoted by both CXCL13 and CCL21 (24, 25).
After migrating within a follicle for half a day to a day, possibly helping deliver opsonized antigens to FDCs (see below) but otherwise having an uneventful trip, non-cognate B cells leave the follicle and return to circulation in a manner dependent on sphingosine-1-phosphate receptor-1 (S1P1), which is a G-protein-coupled receptor (GPCR) for sphingosine-1-phosphate (S1P) (26). Recent studies have highlighted the role of lymphatic-related sinus structures, also known as cortical sinuses, as sites of S1P1-dependent lymphocyte egress in lymph nodes (27, 28). In situ staining for S1P1 showed that the receptor was down-modulated from the surface of B cells that had entered cortical sinuses, consistent with high ligand abundance (28). Through conditional ablation of sphingosine kinases, it was established that LyVE-1+ cells, likely the cells lining the sinuses, are a necessary S1P source promoting egress (29). The egress pathway from spleen is less well understood, but S1P1 and S1P again play a role (26).
Information regarding the location of S1P activity in the spleen has come from studies of marginal-zone (MZ) B cells that are positioned around follicles and are separated from them by the marginal sinus, a site of considerable blood flow (30). S1P1 and S1P are necessary for MZ B cells to position in the MZ, with this ligand–receptor system counteracting the attractive influence of CXCL13 (31). Despite their name, MZ B cells are also found within splenic follicles and a model has been proposed where MZ B cells shuttle continually between MZ and follicle as a result of cycles of S1P1-receptor desensitization and resensitization (32). We speculate that naive splenic B cells encounter the same S1P gradients as MZ B cells, but they lack the high integrin activity needed for retention in the MZ against the blood flow (33) and instead travel to venous sinuses in the red pulp, thereby returning to circulation.
Migration to follicles promotes B-cell antigen encounter
The logic behind follicular homing of B cells was implicit from the time B cells were defined, because it had earlier been discovered that intact antigens gained selective access to follicles and could be displayed in opsonized form for long periods on FDCs (34). Particulate antigens arriving via lymph to lymph nodes may be displayed transiently by subcapsular sinus (SCS)-lining CD169+ macrophages before reaching FDCs (35, 36). Cognate B cells can capture particulate antigens directly from SCS macrophages or from FDCs (11, 36). Non-cognate B cells are able to pick up opsonized antigens from SCS macrophages, or at other sites of exposure such as in the blood, via complement receptors (CR1/2) (36). As these antigen-loaded B cells perform their random walk through the follicle, they travel over the CR1/2hi processes of FDCs and discharge their cargo for retention and display. Antigen-loaded MZ B cells are thought to exhibit a similar behavior as they shuttle in and out of splenic follicles (32). Small antigens may gain direct access to follicles or travel via conduits, and current views on the importance of the various modes of B-cell antigen exposure have been summarized in recent reviews (35–37). B cells require CXCR5 to access antigens held on FDCs and the ability of FDCs to display opsonized antigens for many days allows late-arriving B cells that have traveled from distant sites a chance of antigen encounter (11). In the hours following cognate antigen encounter, B cells exhibit a reduction in migration velocity and move chemotactically toward the T zone (25).
Migration to the B/T border
Shortly after activation via the B-cell receptor (BCR), antigen-specific B cells increase their expression of CCR7 by ∼3 fold, while surface CXCR5 expression remains unaltered (38–40). The associated change in chemokine responsiveness causes activated B cells to migrate to the T zone where they become distributed along the B/T border (38) (Fig. 2). They also down-regulate S1P1, promoting their trapping in the lymphoid organ (31). The tissue concentration of CCL21 is higher than CCL19 (41), and activated B cells are proposed to position along the B/T border as a result of balanced chemoattraction to CCL21-expressing FRC and CXCL13-expressing follicular stroma (25, 42). In agreement with a central role for CCL21, positioning of antigen-engaged B cells occurred normally in Ccl19–/– mice (J. G. Cyster, unpublished observations). Positioning of activated B cells to the B/T border can last for a period of days and is thought to facilitate the interaction of cognate T and B cells and to accelerate development of T-dependent antibody responses (19).
The differentiation of T cells into T follicular helper (Tfh) cells that are specialized to help B-cell responses has been a recent focus of interest and reviews (43, 44). Follicular access of Tfh cells is dependent on up-regulation of CXCR5 (45–47), and this appears to occur in part due to Bcl6-mediated suppression of miRNAs that antagonize T-cell CXCR5 expression (48). The initial positioning of helper T cells at the B/T border and early interaction with activated B cells is associated with down-regulation of CCR7 function and may not require expression of CXCR5 (47). CXCR5 independence of this step would be consistent with evidence that strong induction of the Tfh phenotype depends on cognate interaction between T cells and B cells (43, 47). However, T-cell CXCR5 plays an important role in facilitating full development of the B-cell response as CXCR5 deficiency in T cells leads to defects in later stages of the plasma cell response and the GC response (45–47). These defects appear to reflect at least in part a reduced ability of the cells to localize in GCs but might also reflect roles for Tfh cells that distribute in other parts of the follicle.
EBI2 and the inter and outer follicular niches
EBI2 is involved as B cells move from the B/T border
The rapid movement of antigen-engaged B cells to the B/T border has been long appreciated and well studied, and it has fit with the satisfying logic that most antibody responses are T dependent and B cells need to seek T-cell help. Less appreciated, yet frequently observed, is the subsequent movement of activated B cells to inter follicular and outer follicle regions (25, 49–52) (Fig. 1). This behavior occurs under a variety of immunization conditions and across a period of several days and precedes the T-dependent plasma cell and GC response (Fig. 2). One recent study correlated movement of B cells to the outer follicle with a burst of proliferation prior to a suggested coalescence of the cells into GCs (49). The discovery that the orphan GPCR, EBI2, is required for activated B cells to move to inter and outer follicular regions and that EBI2-deficient mice mount reduced T-dependent antibody responses strengthens the notion that these niches have a specialized role in the B-cell response (39, 40).
Although up-regulated after activation, naive B cells already express considerable amounts of EBI2 (39, 40, 53). Remarkably, when naive B cells lack this receptor, they are defective in accessing inter and outer follicular regions in wild-type hosts and instead favor the follicle center (39, 40). In mice containing large numbers of Ebi2–/– B cells and small numbers of wild-type B cells, the wild-type cells distribute around the follicle perimeter and show a preference for inter and outer follicular regions (40), perhaps a consequence of increased ligand concentrations due to reduced receptor-mediated ligand depletion.
A hierarchy of EBI2 and other chemokines may regulate B-cell distribution
Despite the in vivo activity of EBI2 in naive B cells, in mice completely lacking EBI2, follicular organization appears normal (39, 40) suggesting that EBI2 function is obscured by the dominant follicular-organizing activity of CXCL13 and CXCR5 (7). Indeed, when mice lack CXCL13, EBI2 affects naive B-cell distribution. Although follicles are absent in the spleens of CXCL13-deficient mice, wild-type B cells continue to gain access to an ‘outer follicle-like’ region around the T zone, whereas EBI2-deficient B cells fail to reach this zone, and accumulate in the MZ instead (40). Future imaging studies will be needed to determine whether naive B-cell migration kinetics in inter and outer follicular regions of EBI2-deficient mice is normal.
EBI2 transcripts are up-regulated within hours of BCR engagement (39, 40, 54) and expression is further augmented by interaction with T cells or stimulation via CD40 (40). Initial up-regulation of EBI2 after BCR engagement may facilitate uniform distribution along the B/T border (39). In an experiment where BCR stimulation and T-cell help were provided separately, movement from the B/T border to the outer follicle by day 2 depended on receipt of T-cell help (40). Although expression of an EBI2-GFP reporter continued to increase between day 1 and 2 of the T-dependent response, it is not yet known if EBI2 protein expression increases across this interval; instead the key change between day 1 and 2 may be down-regulation of CCR7 (47, 55), allowing EBI2 to dominate and guide the cells to interfollicular regions and the outer follicle. This hierarchical receptor relationship in activated B cells is also suggested by the observation that CCR7-deficient B cells, instead of moving to the B/T boundary early after BCR engagement, move directly to the outer follicle (38).
EBI2-enabled migration to inter and outer follicular niches may promote B-cell responses
The reduced IgG response to T-dependent antigens in EBI2-deficient mice suggests that inter and/or outer follicular niches provide specialized signals that favor maturation of the plasmablast response. In some studies, activated B and T cells move deeper into lymph node interfollicular regions as a response progresses (25, 51) and it is possible that ongoing B–T interactions are favored by EBI2 directing the cells to this niche. However, there is less evidence for such interfollicular interactions occurring in the spleen and recent work questioned whether any T cells co-localized with activated B cells in the outer follicle (49) though the inability to track antigen-specific T cells may have caused some cells to be overlooked. Localization to the outer follicle might increase interactions with MRCs and molecules they express, such as TRANCE (4), or with macrophages displaying opsonized antigens or releasing inflammatory cytokines. One or more of these signals, and possibly EBI2 signaling itself, might promote proliferation and/or differentiation of the cells. EBI2 is also expressed by helper T cells and by various DC types (J. P. Pereira, L. M. Kelly, J. G. Cyster, unpublished observations) and future studies will be needed to determine whether expression in these cell types influences the antibody response.
Infection may affect EBI2-mediated lymphocyte migration directly and indirectly
As well as being sites enriched for newly arriving antigen, inter and outer follicular CD169+ macrophages become infected by various pathogen and activated CD8 T cells interact with the infected cells (56, 57). EBI2 is abundantly expressed in some CD8+ T cells (40) (J. P. Pereira, J. G. Cyster, unpublished observations) and may thus help guide CD8+ T cells to these areas, perhaps in combination with inflammatory chemokines. In humans, EBV-transformed B cells strongly up-regulate the expression of many genes including Ebi2 (53), and EBV-infected B cells have been found in inter and outer follicular areas (58–60), suggesting that in humans EBI2 may also regulate migration into these areas.
EBI2 ligand activity
Although one study suggested EBI2 is a constitutively active receptor (61), we have not found evidence for this and instead have identified in a tissue extract EBI2 ligand activity that is proteinase K resistant and hydrophobic in nature (J. P. Pereira, J. G. Cyster, unpublished observations). These observations and the tendency of the EBI2 sequence to be clustered with various lipid receptors (62) lead us to speculate that it has a lipid ligand. We suggest that the cells making this activity are enriched in inter and outer follicular regions, perhaps corresponding to MRCs or macrophages, whereas pathways for degradation of the activity may be enriched in the follicle center. The observation that LTα1β2 antagonism disrupted any segregation of wild-type and EBI2-deficient B cells within the spleen (40) suggests that LTβR signaling is needed for development and/or maintenance of EBI2 ligand concentration gradients (Fig. 2). MRCs and CD169+ macrophages, as well as FDCs, are all LTα1β2 dependent (4, 63, 64).
EBI2-enabled responses in inter and outer follicular niches may promote plasmablast differentiation
The connection between EBI2 and isotype switching is not yet clear. Although early IgM responses appeared intact in some experiments with EBI2-deficient mice, in an adoptive transfer study both IgM and IgG responses were diminished (39, 40). The sites where the first isotype switching events occur have not been well defined, though some evidence suggests the first IgG-switched cells appear within follicles (52, 55). Receipt of signals promoting isotype switching in the outer follicle might contribute to their early follicular appearance. B-cell differentiation into plasmablasts is synchronized with up-regulation of CXCR4 and down-regulation of CXCR5 (55) and migration of plasmablasts to splenic red pulp cords and lymph node medullary sinuses requires CXCR4 (65). Interestingly, Cxcr4–/– plasmablasts were found scattered around inter and outer follicular areas (65), perhaps a consequence of their EBI2 expression (39, 40) and an ability to respond to EBI2 ligand(s) when the dominant CXCR4 response is removed. The positioning of Cxcr4–/– plasmablasts in inter and outer follicular niches might also be a hint that commitment to plasmablast differentiation can occur at these sites.
EBI2 down-regulation and movement back to the center follicle
Within a few days after immunization, while some activated B cells differentiate into plasmablasts and migrate to extrafollicular sites, others differentiate into GC B cells and move to the FDC-rich follicle center (Fig. 2). As the GC matures, it develops a T-zone-proximal dark zone of rapidly dividing and somatically mutating GC B cells and a T-zone-distal light zone that contains GC B cells, antigen-bearing FDCs and Tfh cells and that is thought to support GC B-cell selection and differentiation events. The organization of GCs into dark and light zones is mediated by opposing gradients of CXCL12 and CXCL13, and GC B-cell positioning in the dark zone depends on high expression of the CXCL12 receptor, CXCR4 (66). How B cells become clustered in the center follicle during the GC reaction has been poorly understood.
EBI2 is markedly down-regulated during GC B-cell differentiation (39, 40, 54) and the recent studies on EBI2 function have now provided evidence that a critical step regulating migration to the follicle center is down-regulation of EBI2 (39, 40). As discussed previously, in lymphoid organs of Ebi2–/– and Ebi2+/+ mixed chimeric mice, the Ebi2–/– B cells favor the follicle center and intermingle with the FDC network, thus resembling the anatomy of GCs (40). Reciprocally, overexpression of EBI2 in GC B cells enforced migration to the outer follicle and exclusion from the center follicle and resulted in reduced GC responses (39, 40). GCs within EBI2-deficient mice exhibit a typical dark zone/light zone organization, consistent with GC B cells lacking expression of EBI2 (J. P. Pereira, L. M. Kelly, J. G. Cyster, unpublished observations). Bcl6 is a critical differentiation factor for GC B cells (54), and Ebi2 has been suggested to be a target of Bcl6-mediated transcriptional repression (54, 67). Thus, EBI2 down-regulation may be a necessary part of the BCL6 gene repression program that directs B cells to take on a GC fate. Additional cue(s), perhaps secreted by FDCs, might contribute to clustering of EBI2lo BCL6+ GC B cells in follicle centers. Given this sequence of events, it seems logical that commitment of activated B cells into the GC pathway already occurs in the periphery of follicular areas. However, the lack of differences in magnitude of GC B-cell responses in EBI2-deficient and sufficient mice suggests that positioning in inter and outer follicular regions is not critical for differentiation into GC B cells. Instead, the information available to date implicates EBI2-dependent movement of cells to inter and outer follicular niches as an event helping support the early plasma cell response.
Conclusions
During the course of T-dependent antibody responses, antigen-engaged B and T lymphocytes begin a ‘march’ that will take them initially to the B/T border, then to inter and outer follicular areas and then back to the center follicle where the GC reaction proceeds or to distal extrafollicular sites where plasmablasts proliferate and accumulate. While the mechanisms that regulate this pattern of lymphocyte trafficking are becoming increasingly elucidated, there are still many unanswered questions. For example, what are the EBI2 ligand(s), which cells produce them and how are their gradients maintained? It will also be interesting to determine whether EBI2 shares with lymphoid tissue chemokines the property of having roles in lymphoid tissue development and whether it functions in cell recruitment to sites of inflammation. Moreover, despite significant advances in the understanding of lymphocyte trafficking in secondary lymphoid organs, it is still unclear how trafficking through specific compartments shapes the immune response.
Inter and outer follicular niches contain various subsets of antigen-presenting cells and stromal cells. Activated lymphocytes are likely to interact with niche-specific cells and receive additional signals that alter the kinetics of antibody responses or influence commitment into specific differentiation pathways. Adding complexity to the microanatomy of immune responses is the finding that dramatic changes occur in stromal cell networks during infections (5). Future studies will need to define how pathogen-derived molecules alter the niches that support each step of the B-cell response. As well as contributing to our understanding of how we mount anti-pathogen responses, these studies may lead to improvements in vaccine design and to new methods for thwarting autoimmune responses.
Funding
NIH A140098 and A145073; Howard Hughes Medical Institute.
References
- 1.Cyster JG, Ansel KM, Reif K, et al. Follicular stromal cells and lymphocyte homing to follicles. Immunol. Rev. 2000;176:181. doi: 10.1034/j.1600-065x.2000.00618.x. [DOI] [PubMed] [Google Scholar]
- 2.Allen CD, Cyster JG. Follicular dendritic cell networks of primary follicles and germinal centers: phenotype and function. Semin. Immunol. 2008;20:14. doi: 10.1016/j.smim.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tew JG, Wu J, Qin D, Helm S, Burton GF, Szakal AK. Follicular dendritic cells and presentation of antigen and costimulatory signals to B cells. Immunol. Rev. 1997;156:39. doi: 10.1111/j.1600-065x.1997.tb00957.x. [DOI] [PubMed] [Google Scholar]
- 4.Katakai T, Suto H, Sugai M, et al. Organizer-like reticular stromal cell layer common to adult secondary lymphoid organs. J. Immunol. 2008;181:6189. doi: 10.4049/jimmunol.181.9.6189. [DOI] [PubMed] [Google Scholar]
- 5.Mueller SN, Germain RN. Stromal cell contributions to the homeostasis and functionality of the immune system. Nat. Rev. Immunol. 2009;9:618. doi: 10.1038/nri2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forster R, Mattis AE, Kremmer E, Wolf E, Brem G, Lipp M. A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell. 1996;87:1037. doi: 10.1016/s0092-8674(00)81798-5. [DOI] [PubMed] [Google Scholar]
- 7.Ansel KM, Ngo VN, Hyman PL, et al. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature. 2000;406:309. doi: 10.1038/35018581. [DOI] [PubMed] [Google Scholar]
- 8.Withers DR, Kim MY, Bekiaris V, et al. The role of lymphoid tissue inducer cells in splenic white pulp development. Eur J Immunol. 2007;37:3240. doi: 10.1002/eji.200737541. [DOI] [PubMed] [Google Scholar]
- 9.Miller MJ, Wei SH, Parker I, Cahalan MD. Two-photon imaging of lymphocyte motility and antigen response in intact lymph node. Science. 2002;296:1869. doi: 10.1126/science.1070051. [DOI] [PubMed] [Google Scholar]
- 10.Bajenoff M, Egen JG, Koo LY, et al. Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity. 2006;25:989. doi: 10.1016/j.immuni.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzuki K, Grigorova I, Phan TG, Kelly LM, Cyster JG. Visualizing B cell capture of cognate antigen from follicular dendritic cells. J. Exp. Med. 2009;206:1485. doi: 10.1084/jem.20090209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kehrl JH. Chemoattractant receptor signaling and the control of lymphocyte migration. Immunol. Res. 2006;34:211. doi: 10.1385/IR:34:3:211. [DOI] [PubMed] [Google Scholar]
- 13.Han SB, Moratz C, Huang NN, et al. Rgs1 and Gnai2 regulate the entrance of B lymphocytes into lymph nodes and B cell motility within lymph node follicles. Immunity. 2005;22:343. doi: 10.1016/j.immuni.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 14.Muller G, Lipp M. Signal transduction by the chemokine receptor CXCR5: structural requirements for G protein activation analyzed by chimeric CXCR1/CXCR5 molecules. Biol. Chem. 2001;382:1387. doi: 10.1515/BC.2001.171. [DOI] [PubMed] [Google Scholar]
- 15.Allen CD, Okada T, Tang HL, Cyster JG. Imaging of germinal center selection events during affinity maturation. Science. 2007;315:528. doi: 10.1126/science.1136736. [DOI] [PubMed] [Google Scholar]
- 16.Nombela-Arrieta C, Mempel TR, Soriano SF, et al. A central role for DOCK2 during interstitial lymphocyte motility and sphingosine-1-phosphate-mediated egress. J. Exp. Med. 2007;204:497. doi: 10.1084/jem.20061780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ebisuno Y, Katagiri K, Katakai T. Rap1 controls lymphocyte adhesion cascade and interstitial migration within lymph nodes in RAPL-dependent and -independent manners. Blood. 115 doi: 10.1182/blood-2009-03-211979. 804. [DOI] [PubMed] [Google Scholar]
- 18.Woolf E, Grigorova I, Sagiv A, et al. Lymph node chemokines promote sustained T lymphocyte motility without triggering stable integrin adhesiveness in the absence of shear forces. Nat. Immunol. 2007;8:1076. doi: 10.1038/ni1499. [DOI] [PubMed] [Google Scholar]
- 19.Forster R, Schubel A, Breitfeld D, et al. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- 20.Luther SA, Tang HL, Hyman PL, Farr AG, Cyster JG. Coexpression of the chemokines ELC and SLC by T zone stromal cells and deletion of the ELC gene in the plt/plt mouse. Proc. Natl Acad. Sci. USA. 2000;97:12694. doi: 10.1073/pnas.97.23.12694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okada T, Cyster JG. CC chemokine receptor 7 contributes to Gi-dependent T cell motility in the lymph node. J. Immunol. 2007;178:2973. doi: 10.4049/jimmunol.178.5.2973. [DOI] [PubMed] [Google Scholar]
- 22.Okada T, Ngo VN, Ekland EH, et al. Chemokine requirements for B cell entry to lymph nodes and Peyer's patches. J. Exp. Med. 2002;196:65. doi: 10.1084/jem.20020201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ebisuno Y, Tanaka T, Kanemitsu N, et al. Cutting edge: the B cell chemokine CXC chemokine ligand 13/B lymphocyte chemoattractant is expressed in the high endothelial venules of lymph nodes and Peyer's patches and affects B cell trafficking across high endothelial venules. J. Immunol. 2003;171:1642. doi: 10.4049/jimmunol.171.4.1642. [DOI] [PubMed] [Google Scholar]
- 24.Ekland EH, Forster R, Lipp M, Cyster JG. Requirements for follicular exclusion and competitive elimination of autoantigen-binding B cells. J. Immunol. 2004;172:4700. doi: 10.4049/jimmunol.172.8.4700. [DOI] [PubMed] [Google Scholar]
- 25.Okada T, Miller MJ, Parker I, et al. Antigen-engaged B cells undergo chemotaxis toward the T zone and form motile conjugates with helper T cells. PLoS Biol. 2005;3:e150. doi: 10.1371/journal.pbio.0030150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwab SR, Cyster JG. Finding a way out: lymphocyte egress from lymphoid organs. Nat. Immunol. 2007;8:1295. doi: 10.1038/ni1545. [DOI] [PubMed] [Google Scholar]
- 27.Grigorova IL, Schwab SR, Phan TG, Pham TH, Okada T, Cyster JG. Cortical sinus probing, S1P1-dependent entry and flow-based capture of egressing T cells. Nat. Immunol. 2009;10:58. doi: 10.1038/ni.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sinha RK, Park C, Hwang IY, Davis MD, Kehrl JH. B lymphocytes exit lymph nodes through cortical lymphatic sinusoids by a mechanism independent of sphingosine-1-phosphate-mediated chemotaxis. Immunity. 2009;30:434. doi: 10.1016/j.immuni.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pham TH, Baluk P, Xu Y, et al. Lymphatic endothelial cell sphingosine kinase activity is required for lymphocyte egress and lymphatic patterning. J. Exp. Med. doi: 10.1084/jem.20091619. 207:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kraal G. Cells in the marginal zone of the spleen. Int. Rev. Cytol. 1992;132:31. doi: 10.1016/s0074-7696(08)62453-5. [DOI] [PubMed] [Google Scholar]
- 31.Cinamon G, Matloubian M, Lesneski MJ, et al. Sphingosine 1-phosphate receptor 1 promotes B cell localization in the splenic marginal zone. Nat. Immunol. 2004;5:713. doi: 10.1038/ni1083. [DOI] [PubMed] [Google Scholar]
- 32.Cinamon G, Zachariah MA, Lam OM, Foss FW, Jr., Cyster JG. Follicular shuttling of marginal zone B cells facilitates antigen transport. Nat. Immunol. 2008;9:54. doi: 10.1038/ni1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu TT, Cyster JG. Integrin-mediated long-term B cell retention in the splenic marginal zone. Science. 2002;297:409. doi: 10.1126/science.1071632. [DOI] [PubMed] [Google Scholar]
- 34.Nossal GJ, Abbot A, Mitchell J, Lummus Z. Antigens in immunity. XV. Ultrastructural features of antigen capture in primary and secondary lymphoid follicles. J. Exp. Med. 1968;127:277. doi: 10.1084/jem.127.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Batista FD, Harwood NE. The who, how and where of antigen presentation to B cells. Nat. Rev. Immunol. 2009;9:15. doi: 10.1038/nri2454. [DOI] [PubMed] [Google Scholar]
- 36.Phan TG, Gray EE, Cyster JG. The microanatomy of B cell activation. Curr. Opin. Immunol. 2009;21:258. doi: 10.1016/j.coi.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gonzalez SF, Pitcher LA, Mempel T, Schuerpf F, Carroll MC. B cell acquisition of antigen in vivo. Curr. Opin. Immunol. 2009;21:251. doi: 10.1016/j.coi.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reif K, Ekland EH, Ohl L, et al. Balanced responsiveness to chemoattractants from adjacent zones determines B-cell position. Nature. 2002;416:94. doi: 10.1038/416094a. [DOI] [PubMed] [Google Scholar]
- 39.Gatto D, Paus D, Basten A, Mackay CR, Brink R. Guidance of B cells by the orphan G protein-coupled receptor EBI2 shapes humoral immune responses. Immunity. 2009;31:259. doi: 10.1016/j.immuni.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 40.Pereira JP, Kelly LM, Xu Y, Cyster JG. EBI2 mediates B cell segregation between the outer and centre follicle. Nature. 2009;460:1122. doi: 10.1038/nature08226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luther SA, Bidgol A, Hargreaves DC, et al. Differing activities of homeostatic chemokines CCL19, CCL21, and CXCL12 in lymphocyte and dendritic cell recruitment and lymphoid neogenesis. J. Immunol. 2002;169:424. doi: 10.4049/jimmunol.169.1.424. [DOI] [PubMed] [Google Scholar]
- 42.Okada T, Cyster JG. B cell migration and interactions in the early phase of antibody responses. Curr. Opin. Immunol. 2006;18:278. doi: 10.1016/j.coi.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 43.Crotty S, Johnston RJ, Schoenberger SP. Effectors and memories: Bcl-6 and Blimp-1 in T and B lymphocyte differentiation. Nat. Immunol. 2010;11:114. doi: 10.1038/ni.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vinuesa CG, Sanz I, Cook MC. Dysregulation of germinal centres in autoimmune disease. Nat. Rev. Immunol. 2009;9:845. doi: 10.1038/nri2637. [DOI] [PubMed] [Google Scholar]
- 45.Arnold CN, Campbell DJ, Lipp M, Butcher EC. The germinal center response is impaired in the absence of T cell-expressed CXCR5. Eur. J. Immunol. 2007;37:100. doi: 10.1002/eji.200636486. [DOI] [PubMed] [Google Scholar]
- 46.Hardtke S, Ohl L, Forster R. Balanced expression of CXCR5 and CCR7 on follicular T helper cells determines their transient positioning to lymph node follicles and is essential for efficient B-cell help. Blood. 2005;106:1924. doi: 10.1182/blood-2004-11-4494. [DOI] [PubMed] [Google Scholar]
- 47.Haynes NM, Allen CD, Lesley R, Ansel KM, Killeen N, Cyster JG. Role of CXCR5 and CCR7 in follicular Th cell positioning and appearance of a programmed cell death gene-1high germinal center-associated subpopulation. J. Immunol. 2007;179:5099. doi: 10.4049/jimmunol.179.8.5099. [DOI] [PubMed] [Google Scholar]
- 48.Yu D, Rao S, Tsai LM, et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity. 2009;31:457. doi: 10.1016/j.immuni.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 49.Coffey F, Alabyev B, Manser T. Initial clonal expansion of germinal center B cells takes place at the perimeter of follicles. Immunity. 2009;30:599. doi: 10.1016/j.immuni.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cyster JG, Goodnow CC. Antigen-induced exclusion from follicles and anergy are separate and complementary processes that influence peripheral B cell fate. Immunity. 1995;3:691. doi: 10.1016/1074-7613(95)90059-4. [DOI] [PubMed] [Google Scholar]
- 51.Garside P, Ingulli E, Merica RR, Johnson JG, Noelle RJ, Jenkins MK. Visualization of specific B and T lymphocyte interactions in the lymph node. Science. 1998;281:96. doi: 10.1126/science.281.5373.96. [DOI] [PubMed] [Google Scholar]
- 52.Pape KA, Kouskoff V, Nemazee D, et al. Visualization of the genesis and fate of isotype-switched B cells during a primary immune response. J. Exp. Med. 2003;197:1677. doi: 10.1084/jem.20012065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Birkenbach M, Josefsen K, Yalamanchili R, Lenoir G, Kieff E. Epstein-Barr virus-induced genes: first lymphocyte-specific G protein-coupled peptide receptors. J. Virol. 1993;67:2209. doi: 10.1128/jvi.67.4.2209-2220.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shaffer AL, Rosenwald A, Hurt EM, et al. Signatures of the immune response. Immunity. 2001;15:375. doi: 10.1016/s1074-7613(01)00194-7. [DOI] [PubMed] [Google Scholar]
- 55.Chan TD, Gatto D, Wood K, Camidge T, Basten A, Brink R. Antigen affinity controls rapid T-dependent antibody production by driving the expansion rather than the differentiation or extrafollicular migration of early plasmablasts. J. Immunol. 2009;183:3139. doi: 10.4049/jimmunol.0901690. [DOI] [PubMed] [Google Scholar]
- 56.Hickman HD, Takeda K, Skon CN, et al. Direct priming of antiviral CD8+ T cells in the peripheral interfollicular region of lymph nodes. Nat. Immunol. 2008;9:155. doi: 10.1038/ni1557. [DOI] [PubMed] [Google Scholar]
- 57.Chtanova T, Han SJ, Schaeffer M, et al. Dynamics of T cell, antigen-presenting cell, and pathogen interactions during recall responses in the lymph node. Immunity. 2009;31:342. doi: 10.1016/j.immuni.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Herbst H, Steinbrecher E, Niedobitek G, et al. Distribution and phenotype of Epstein-Barr virus-harboring cells in Hodgkin's disease. Blood. 1992;80:484. [PubMed] [Google Scholar]
- 59.Niedobitek G, Herbst H, Young LS, et al. Patterns of Epstein-Barr virus infection in non-neoplastic lymphoid tissue. Blood. 1992;79:2520. [PubMed] [Google Scholar]
- 60.Shaknovich R, Basso K, Bhagat G, et al. Identification of rare Epstein-Barr virus infected memory B cells and plasma cells in non-monomorphic post-transplant lymphoproliferative disorders and the signature of viral signaling. Haematologica. 2006;91:1313. [PubMed] [Google Scholar]
- 61.Rosenkilde MM, Benned-Jensen T, Andersen H, et al. Molecular pharmacological phenotyping of EBI2. An orphan seven-transmembrane receptor with constitutive activity. J. Biol. Chem. 2006;281:13199. doi: 10.1074/jbc.M602245200. [DOI] [PubMed] [Google Scholar]
- 62.Shimizu T. Lipid mediators in health and disease: enzymes and receptors as therapeutic targets for the regulation of immunity and inflammation. Annu. Rev. Pharmacol. Toxicol. 2009;49:123. doi: 10.1146/annurev.pharmtox.011008.145616. [DOI] [PubMed] [Google Scholar]
- 63.Browning JL. Inhibition of the lymphotoxin pathway as a therapy for autoimmune disease. Immunol. Rev. 2008;223:202. doi: 10.1111/j.1600-065X.2008.00633.x. [DOI] [PubMed] [Google Scholar]
- 64.Phan TG, Green JA, Gray EE, Xu Y, Cyster JG. Immune complex relay by subcapsular sinus macrophages and noncognate B cells drives antibody affinity maturation. Nat. Immunol. 2009;10:786. doi: 10.1038/ni.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cyster JG. Homing of antibody secreting cells. Immunol. Rev. 2003;194:48. doi: 10.1034/j.1600-065x.2003.00041.x. [DOI] [PubMed] [Google Scholar]
- 66.Allen CD, Ansel KM, Low C, et al. Germinal center dark and light zone organization is mediated by CXCR4 and CXCR5. Nat. Immunol. 2004;5:943. doi: 10.1038/ni1100. [DOI] [PubMed] [Google Scholar]
- 67.Basso K, Saito M, Sumazin P, et al. Integrated biochemical and computational approach identifies BCL6 direct target genes controlling multiple pathways in normal germinal center B cells. Blood. 2010 doi: 10.1182/blood-2009-06-227017. 115:975. [DOI] [PMC free article] [PubMed] [Google Scholar]