Abstract
Development of chemoresistance limits the clinical efficiency of platinum-based therapy. Although many resistance mechanisms have been demonstrated, genetic/molecular alterations responsible for drug resistance in the majority of clinical cases have not been identified. We analyzed three pairs of testicular germ cell tumor cell lines using Affymetrix expression microarrays and revealed a limited number of differentially expressed genes across the cell lines when comparing the parental and resistant cells. Among them, CCND1 was the most significantly differentially expressed gene. Analysis of testicular germ cell tumor clinical samples by quantitative reverse transcription PCR analysis revealed that overall expression of CCND1 was significantly higher in resistant cases compared with sensitive samples (P < 0.0001). We also found that CCND1 was dramatically overexpressed both in induced and intrinsically resistant samples of ovarian and prostate cancer. Finally combined CCND1 knockdown using small-interfering RNA and cisplatin treatment inhibited cell growth in vitro significantly more effectively than any of these single treatments. Therefore, deregulation of CCND1 may be a major cause of cisplatin resistance in testicular germ cell tumors and may also be implicated in ovarian and prostate cancers. CCND1 could be potentially used as a marker for treatment stratification and as a molecular target to improve the treatment of platinum-resistant tumors.
Cisplatin has dramatically improved the clinical outcome for testicular germ cell tumors (TGCTs) and remains the first line treatment of several other solid tumors such as, ovarian, breast, head and neck, and small cell lung cancers.1,2 However, in many cases, cancer cells develop a resistant phenotype, and the outcome for these patients is very poor.3,4 Many mechanisms associated with resistance to platinum drugs have been identified, such as alteration of drug transporters, drug uptake, and efflux in cells, abnormalities in DNA damage repair and apoptosis induction.1,4,5,6,7,8 However, the evidence for these mechanisms in a clinical setting has not been clearly established.4,5
TGCTs, which include seminomas and non-seminomas, provide an ideal model to study the molecular mechanisms of cisplatin resistance, due to their extreme sensitivity to cisplatin-based chemotherapy.4,8 However, although TGCT development and pathogenesis have been extensively studied, and some genetic aberrations have been described as characterization markers of adult TGCTs, such as the extra copies of the short arm of chromosome 12,9 our knowledge of the genetic mechanisms in TGCT chemoresistance is still limited. In TGCTs, mutations of TP53 account for a very small subset of refractory cases.4,10,11 Increased activity of DNA repair genes, such as ERCC1, XPA, XPD, and XPB, has also been reported to be associated with TGCT cisplatin resistance. However, their clinical significance has yet to be confirmed.4,5,8,12 The association between microsatellite instability/mismatch repair and resistance has been extensively investigated. Although the results from different studies were controversial,12,13,14,15,16 a recent study of a large series of resistant TGCT samples generally confirmed the involvement of microsatellite instability/mismatch repair in cisplatin resistance, and also revealed the association between BRAF mutation and cisplatin resistance.17 Seladin-1, a multifunctional protein, has also recently been identified as a putative player in cisplatin sensitivity of TGCTs.18 Since these resistance mechanisms only account for a limited proportion of the resistant cases, further studies are required. In this study, we performed a genome-wide gene expression analysis of three pairs of TGCT parental and resistant cell lines and identified CCND1 (Cyclin D1) overexpression in the three resistant lines. We confirmed the common involvement of CCND1 deregulation in cisplatin-resistant TGCT clinical cases, as well as in ovarian and prostate cancer samples.
Materials and Methods
Cell Lines and Clinical Samples
Three TGCT cell lines, 833K, Susa, and GCT27, nine ovarian cancer cell lines, A2780, CH1, 41M, OVCAR3, OVCAR4, OVCAR8, SKOV3, PXN94, and HX62, and four prostate cancer cell lines, PC3, DU-145, LNCaP, and 22RV1, were used for this study. The ovarian cancer lines OVCAR8, SKOV3, PXN94, and HX62 were cisplatin-resistant whereas A2780, CH1, 41M, and OVCAR3 were sensitive lines as previously reported.19,20,21 Cisplatin-resistant derivative sublines were available for 833K, Susa, GCT27, A2780, CH1, and 41M, namely 833KR, SusaR, GCT27R, A2780R, CH1R, and 41MR. All of the parental cell lines were made resistant to cisplatin by long-term exposure to the drug. The TGCT cell lines have previously been analyzed by single nucleotide polymorphism arrays22,23 and karyotyped by 24-color fluorescence in situ hybridization (unpublished data). The genomic alterations were similar to those detected in the original cell lines using low resolution genetic analyses soon after they were established, including the gain of 12p which is specific for TGCTs. All of the cell lines were maintained by standard cell culture as previously described.22,24
Fourteen fresh-frozen and 25 formalin-fixed, paraffin-embedded (FFPE) TGCTs (see Supplemental Table S1 at http://ajp.amjpathol.org), and 15 fresh frozen prostate cancer samples were collected from Barts and The London Hospital and the Royal Marsden Hospital with ethical approval. For one FFPE TGCT platinum-resistant case, samples from two separate blocks (P24 and P25) were analyzed. All of the TGCT clinical samples were selected from cases treated with platinum-based regimens, and those with progression or partial remission tumors were defined as resistant cases. Samples were reviewed by a consultant pathologist (D.M.B.) and cancer lesions were macrodissected so that all TGCT samples contained at least 80% tumor material and the prostate cancer contained more than 50% cancer cells.
Cisplatin Responsiveness Measured by ATP Cell Viability Assay
ATP cell viability assays were performed by plating cells at a density of 5 × 104 cells per well in 96-well plates and subsequently treated with one of six serial dilutions of cisplatin (Sigma, St. Louis, MO), including a negative control. Cell viability for each drug concentration was measured using the HS Vialight Assay kit (Lonza, Conshohocken, PA) on a FluoroStar automated plate reader (BMG Labtechnologies, Durham, UK). Each experiment was performed in triplicate.
Cell Cycle Distribution Assay
Cell cycle distribution was established using a FACScalibur flow cytometer (Becton Dickinson, Franklin Lakes, NJ). Cells were harvested 24 hours after replating and fixed in 70% ethanol. The cells were stained using a propidium iodide solution containing 50 μg/ml propidium iodide and 50 μg/ml RNase A (Sigma) before flow cytometric analysis. Five thousand cells were acquired and the proportion of cells in each of the cell cycle phases was quantified using the cell cycle analysis WinMDI v2.8 program.
Total RNA Extraction
RNA was extracted from cell lines and fresh frozen samples using TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s recommendations. RNA integrity was assessed using the Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA). For each FFPE sample, RNA was extracted from a 7-μm section using the Paradise Whole Transcript RT Reagent System (MDS Analytical Technologies, Mountain View, CA) following the manufacturer’s instructions.
Affymetrix Gene Expression Microarray Profiling and Data Analysis
The Affymetrix gene expression microarray profiling technology (Human Genome U133 plus 2.0 arrays) was applied using total RNA (5 μg) and a one-cycle eukaryotic target-labeling assay (Affymetrix, Santa Clara, CA) according to the manufacturer’s recommendations. Microarray data have been deposited in the Gene Expression Omnibus under Accession No. GSE14231. Unsupervised one-way cluster analysis with Spearman correlation was firstly applied to the data. Then data analysis was performed using GeneSpring software version 7.2 (Agilent Technologies). In vitro expression changes associated with cisplatin resistance in TGCTs were analyzed by comparing the cisplatin-sensitive and -resistant cell lines. Samples were processed using the Affymetrix Microarray Suite version 5.0 (MAS 5.0). Affymetrix default analysis settings and global scaling for normalization were used. Once imported onto GeneSpring software, data were further log-transformed, normalized per chip (50th percentile) and per gene (median). An additional “per gene to specific sample” normalization step was added. Absent calls were removed and statistical filters were applied. Different fold-change cut-off values were tested to identify differentially expressed genes/transcripts across the three pairs of cell lines when comparing resistant and parental cell lines. Finally, ontological analysis was undertaken using the Genecards database version 2.36 (http://www.genecards.org/) to determine the biological functions of candidate genes and their relevance to the mechanisms of chemoresistance.
Comparative Genomic Hybridization and Comparative Expressed Sequence Hybridization Analyses on Chromosomes
Comparative genomic hybridization (CGH) and comparative expressed sequence hybridization (CESH) analyses to profile genomic imbalances and differential expression patterns, respectively, on chromosomes, were performed as previously published.25,26 For CGH analysis, DNA from each cell line was hybridized against a sample from a normal healthy female. For paired parental and cisplatin-resistant lines, samples were directly co-hybridized onto slides. The 0.8 and 1.2 cut-off values were used to identify chromosome copy number changes. For CESH analysis, each RNA sample was hybridized against a pooled RNA control from several individual muscle tissues. The paired parental and cisplatin-resistant cell lines were co-hybridized. Similar threshold values to the ones used for CGH analysis were used to identify expression variations.
Quantitative Reverse-Transcription PCR
Total RNA (1 μg) from cell lines and fresh frozen tissues was reverse transcribed using random hexamers (50 μmol/L) (Sigma) and M-MLV RT RNase H Minus, point mutant enzyme (200 U/μL) (Promega, Madison, WI), and from FFPE tissues using the Paradise Whole Transcript RT reagent System (MDS Analytical Technologies), both following the manufacturer’s recommendations. The quality of reverse transcribed cDNA was checked by standard PCR using ß-actin primers (Forward sequence: 5′-GCGGGAAATCGTGCGTGCGTGACATT-3′; Reverse sequence: 5′-GATGGAGTTGAAGGTAGTTTCGTG-3′) (Sigma) following standard PCR technique with an annealing temperature of 62°C. PCR products were visualized by electrophoresis and only samples with positive ß-actin products were used for CCND1 quantitative reverse transcription (qRT)-PCR analysis.
Keeping the default settings for baselines and thresholds, qRT-PCR was performed using the ABI Prism 7700 Sequence Detector (Applied Biosystems, Foster City, CA). Pre-designed Taqman gene expression assays targeting CCND1 (product number: Hs99999004_m1, exon boundary 4 to 5) and the endogenously expressed GAPDH gene (product number: Hs99999905_m1) were purchased (Applied Biosystems). Each reaction was performed in triplicate.
Western Blot
CCND1 was detected using mouse monoclonal antibodies against CCND1 (sc-20044, Santa Cruz Biotechnology, Santa Cruz, CA) and the standard Western Blotting analysis, as previously described.27 Whole cell extracts of protein were obtained by lysing cells using PBS-1% Triton X-100 (T8532, Sigma) and 25 μg were loaded onto 12% acrylamide gels and were separated by SDS polyacrylamide gel electrophoresis, keeping the default settings for baselines and thresholds electrophoresis. Proteins were subsequently transferred to polyvinylidene difluoride membranes (Immobilon-P, Milllipore, Billerica, MA) and incubated with the monoclonal antibodies against CCND1 and β-Actin (A5441, Sigma). Bands were detected using horseradish peroxidase chemiluminescence-based detection kit (Millipore).
Immunohistochemistry Analysis
The standard avidin-biotin complex method (Vector ABC kit, Vector Laboratories Inc, Burlingame, CA) was used for immunostaining. Briefly, 4-μm tissue sections were dewaxed in xylene and rehydrated. Antigen retrieval was then performed by pressure cooking (10 minutes) in citrate buffer, pH 6.0 using Vector laboratories antigen unmasking solution. Endogenous peroxidase activity was blocked by hydrogen peroxide and nonspecific staining was prevented using diluted (1/50) normal horse serum. Sections were then incubated with diluted (1/50) CCND1 Clone SP4, VP-RMO3 (Vector Laboratories Inc) primary antibody for 40 minutes at room temperature. The bound antibody was detected with biotinylated universal secondary antibody for 30 minutes and avidin-biotin complex for 20 minutes, and 3, 3′-diaminobenzidine (BioGenex, San Ramon, CA) was used as the chromogen. Finally, slides were counterstained with hematoxylin. In control experiments, the primary antibody was replaced by bovine serum albumin phosphate buffer solution. The basal epithelial cells of the normal tonsil were used as positive controls. CCND1 expression was scored as negative, weakly positive, and strongly positive stain and the percentage of positively stained cells was counted for each sample.
Cell Proliferation and Viability after Combined Gene Knockdown and Cisplatin Treatment
Cells from SusaR and PC3 cell lines were transfected with 40 nmol/L and 133.3 nmol/L CCND1 ON-TARGET plus SMARTpool small-interfering RNA (siRNA) respectively using Oligofectamine transfection reagent (Invitrogen). Negative controls were added that included untreated and nontargeting siRNA-transfected cells. Following a 48-hour transfection, cells were treated with 0.5 μmol/L and 8.65 μmol/L cisplatin respectively. Both floating and attached cells were harvested for cell count analysis using a Beckman Coulter Vi-CELL XR cell counter (Beckman Coulter, Fullerton, CA). For SusaR cells, ATP assay was also used as described above to determine cell viability.
Statistical Analyses
For the gene expression microarray profiling study, a one-way parametric non-equal variance analysis of variance (between groups) test (P < 0.05) was applied to the normalized data (Welch analysis of variance test). Genes (probes) with statistically significant differences in mean expression value were identified. No further multiple testing correction was applied. The gene (probe) list generated after Welch analysis of variance test was then filtered on confidence with “t-test P value” as the measurement. The final statistically significant genes (probes) were retained for further clustering and ontology analysis. We applied two-tailed Student’s t-tests for the analysis of cell cycle distribution and qRT-PCR CCND1 expression; and one-tailed Student’s t-tests for the analysis of cell viability and other functional analyses.
Results
Characterization of the Three Pairs of TGCT Cell Lines
No apparent change in growth rate or morphology associated with the resistant phenotype was observed when comparing the parental and resistant TGCT cell lines. By comparing the EC50 of resistant and parental cells, a 1.78(0.27 vs. 0.48 μmol/L)-, 2.67(1.25 vs. 3.34 μmol/L)-, and 3.80 (0.51 vs. 1.94 μmol/L)-fold resistance to cisplatin treatment was observed in the 833K, GCT27, and Susa cell lines, respectively. The cell response curves to cisplatin from which the EC50s were derived have been published separately.28 Cell cycle distribution was monitored across the three pairs of TGCT cell lines (see Supplemental Table S1 at http://ajp.amjpathol.org). When comparing the parental lines to their corresponding resistant derivatives, a significantly higher number of 833KR and GCT27R cells were found to accumulate in G1/0 (P = 0.0356 and 0.006, respectively), as compared with the parental lines. Apoptosis was significantly reduced in SusaR (P = 0.012) and GCT27R (P = 0.002), but was at a similar level in 833K sensitive and resistant cells.
Identification of Differentially Expressed Genes Across the Three Paired TGCT Cell Lines Using Microarray Analysis
Unsupervised hierarchical clustering analysis of Affymetrix gene expression microarray data showed a clear grouping of the biological duplicates together, indicating good quality array data. Interestingly, sublines derived from the same cell line clustered together, rather segregating by sensitive and resistant cell lines into two distinct groups. This suggested fewer gene expression changes between parental and resistant sublines than between different cell lines. Using GeneSpring supervised analysis, we identified a number of differentially expressed genes/transcripts between individual cell line pairs when comparing the corresponding parental and resistant cell lines. In this study, more importance was given to genes/transcripts that were consistently differentially expressed across all of the three pairs of cell lines, representing genes that are more likely to be found in a large proportion of resistant samples. To achieve this, we determined the transcript probes with a fold-change between resistant and sensitive cells larger than certain cut-off value in each cell line pair. Applying a 1.5-fold cut-off value, only seven up-regulated transcript probes, including three well-characterized genes, and no down-regulated transcript probes, were identified in all three resistant cell lines (Table 1). The CCND1 probe (accession number 208712_at) showed the most dramatic changes across the three pairs of cell lines with a greater than sevenfold overexpression in the SusaR line compared with its parental counterpart. Two transcript probes of CCND1 are present on the U133 plus 2.0 chips (accession number 208712_at and 208711_s_at). We manually checked the expression profile for the second probe (208711_s_at) and found a 1.4-, 1.6-, and 3.9-fold change in 883K, GCT27, and Susa pair of cell lines, respectively. Therefore, although just missing the 1.5-fold change cut-off in 833K, the second probe confirmed that CCND1 is consistently differentially expressed in all of the cell line pairs. The differential expression of CCND1 between parental and resistant cells was confirmed using qRT-PCR in Susa and GCT27 cell lines, although not in the least resistant line 833K28 (Figure 1A). Differences in expression of CCND1 protein were less obvious than that at RNA level, possibly due to the lower dynamic range of the approach, such that only the Susa pair of cell lines showed apparent difference between sensitive and resistant sublines (2.5 fold) (see Supplemental Figure S1 at http://ajp.amjpathol.org.).
Table 1.
Consistently Up-regulated Transcripts (1.5-fold) in the Three Resistant TGCT Cell Lines, as Compared with Their Parental Sensitive Lines
| ID Number | Name | Location | Description | Hybridization value (normalized)
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| 833K | 833KR | GCT27 | GCT27R | Susa | SusaR | ||||
| 1560133_at | GIGYF2 | 2q37 | GRB10 interacting GYF protein 2 | 0.999 | 1.614 | 0.999 | 1.792 | 0.938 | 1.647 |
| 242593_at | — | Transcribed sequence with weak similarity to protein NP_112159.1 (Hs) | 0.977 | 1.503 | 1.000 | 1.715 | 0.709 | 1.888 | |
| 1562169_at | — | mRNA full length insert cDNA clone EUROIMAGE 131775 | 0.895 | 1.435 | 0.981 | 1.959 | 0.997 | 2.840 | |
| 212240_s_at | PIK3R1 | 5q13.1 | phosphoinositide-3-kinase, regulatory subunit 1 (alpha) | 0.995 | 1.564 | 0.998 | 2.168 | 1.000 | 2.048 |
| 235274_at | — | Transcribed sequence with weak similarity to protein NP_060312.1 (Hs) | 0.997 | 1.506 | 0.932 | 1.813 | 0.983 | 2.542 | |
| 212239_at | PIK3R1 | 5q13.1 | phosphoinositide-3-kinase, regulatory subunit 1 (alpha) | 1.314 | 2.096 | 0.998 | 2.916 | 0.999 | 1.577 |
| 208712_at | CCND1 | 11q13 | Cyclin D1 | 0.992 | 1.708 | 0.977 | 1.849 | 0.888 | 6.875 |
Figure 1.
CCND1 expression determined by qRT-PCR in TGCTs. A: CCND1 expression in the three pairs of parental and cisplatin-resistant cell lines. B: CCND1 expression in fresh frozen clinical samples. C: CCND1 expression in FFPE clinical samples. The CCND1 gene expression was normalized to the expression of the housekeeping gene GAPDH and calibrated to the GCT27 cell line.
We manually examined the expression microarray data for a panel of known drug resistance-associated candidate genes (ie, TP53, BCL2, MDM2, ABCB1, ABCC2, ABCG2, LRP, MDR, P21, ERCC1, XPA, and GST). No differential expression was noted between the parental and resistant cells. We also investigated the expression of TP53, P21, MDM2, BCL2, ERCC1, XPA, and GST protein levels by Western blotting analysis and did not find apparent difference between parental and resistant cells for any of these genes (28 and unpublished data).
TGCT Clinical Sample Analysis
The two fresh-frozen, resistant samples expressed CCND1 at a higher level than any of the chemosensitive clinical samples (Figure 1B). Since only a small proportion of TGCT patients are chemo-resistant, which limits the availability of fresh frozen cases, we performed qRT-PCR on 25 FFPE TGCT samples, aiming to maintain the balance between the number of sensitive (n = 13) and resistant (n = 12) cases. The overall RNA expression level of CCND1 was significantly higher in the resistant cases, as compared with the sensitive samples (P < 0.0001). In two-thirds (8/12) of the resistant cases, CCND1 RNA was expressed at a level higher than any of the sensitive tumors (Figure 1C). There was no difference in CCND1 expression between seminomas and non-seminomas at RNA level (P = 0.274). At protein level and as detected by immunohistochemistry, TGCTs generally expressed dramatically low levels of CCND1 that, even in chemoresistant cases, only a restricted proportion of cells were positively stained (see Supplemental Table S1 at http://ajp.amjpathol.org.). In contrast to RNA expression distribution, CCND1 protein was apparently expressed in more cells in non-senimomas than seminomas, although within each subtype, CCND1 was generally expressed in more cells in the resistant cases than the sensitive ones. Representative immunohistochemistry images are shown in Figure 2, A–D.
Figure 2.
Expression of CCND1 in TGCT clinical samples detected by immunohistochemistry. A: In the normal tonsil control sample, positive CCND1 signal was observed in some of the epithelial cells. B: Some strong and weak positive cells were detected in a chemoresistant embryonic carcinoma sample. C: In a chemosensitive embryonic carcinoma sample, no positive cells were detected. D: In a chemosensitive seminoma sample, no positive cells were detected.
CCND1 Expression in Other Cancers
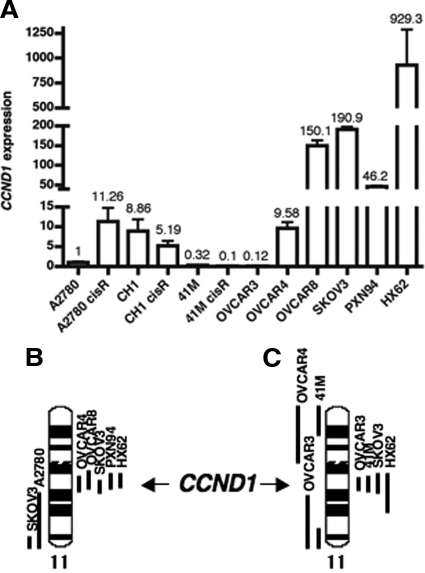
In the three pairs of parental and resistant ovarian cancer cell lines, we revealed an 11-fold overexpression of CCND1 in the A2780R, as compared with A2780, and reduced expression comparing the resistant to the sensitive cells in the CH1 and 41M pairs (Figure 3A). When comparing the intrinsically resistant to sensitive cell lines, we found that CCND1 expression was markedly increased in the cisplatin-resistant OVCAR8, SKOV3, PXN94, and HX62 cell lines compared with the sensitive lines A2780, CH1, 41M, and OVCAR3 (Figure 3A). The qRT-PCR data correlated with our CESH data that showed overexpression of genes on 11q13 region, where CCND1 is located, in SKOV3, HX62, PXN94, OVCAR4, and OVCAR8 cell lines (Figure 3, A and B). These results also correlated with genomic gain in SKOV3 and HX62 but not in 41M and OVCAR3 (Figure 3C).
Figure 3.
Genetic changes of CCND1 in ovarian cancer cell lines. A: Relative CCND1 expression detected by qRT-PCR normalized to the expression of the housekeeping gene GAPDH and calibrated to A2780 cell line. B and C: Gene expression and genomic copy number change profile, respectively, of the ovarian cancer cell lines on chromosome 11 determined by CESH (B) and CGH (C) analysis. The black bars on the right side of the chromosome represent the chromosomal regions with relative gene overexpression (CESH) or genomic gains (CGH) and the bars on the left indicate the chromosomal regions with relative underexpression of genes (CESH) or genomic losses (CGH).
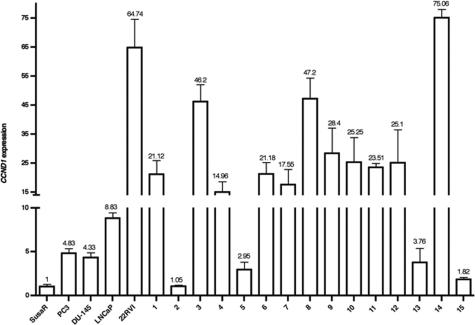
We analyzed four prostate cancer cell lines and 15 clinical samples using qRT-PCR analysis and found that CCND1 was expressed at a higher level in all of the samples except one clinical case, as compared with the SusaR cell line (Figure 4).
Figure 4.
CCND1 expression in prostate cancers detected by qRT-PCR. The relative CCND1 gene expression level was normalized to the housekeeping gene GAPDH and calibrated to the resistant TGCT cell line SusaR.
Re-Sensitization of the Resistant Cells by CCND1 Knockdown
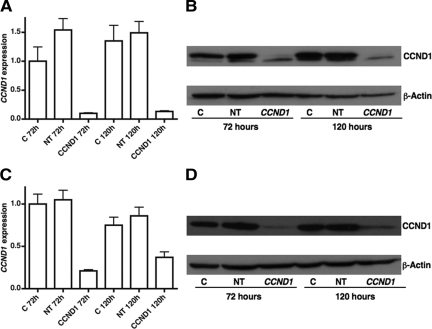
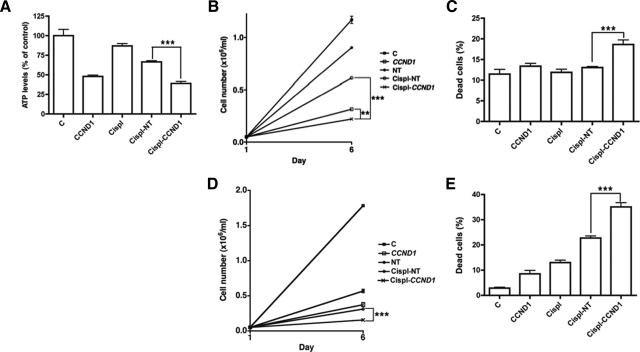
We determined the effect of altering CCND1 expression on the cell viability and proliferation of SusaR and PC3 cell lines in response to cisplatin treatment using siRNA-mediated transient transfection. We knocked down CCND1 both at the RNA and protein level (93.5% and 86% respectively at 72 hours; and 92.28% and 97% at 120 hours) in SusaR cells (Figure 5, A and B). The combined treatment with cisplatin inhibited cell growth and killed more efficiently than CCND1 knockdown and cisplatin (together with nontargeting control siRNA) alone analyzed using both ATP assay (P = 0.0192 and P = 0.0004 respectively) and the Beckman Coulter Vi-CELL XR cell counter (P = 0.0014 and P < 0.0001 respectively for the cell proliferation data and P = 0.0012 and P = 0.0008 respectively for the cell viability data; Figure 6, A–C). As the analysis using the Beckman Coulter Vi-CELL XR cell counter generated more information than the ATP assay, we discontinued using the ATP assay in the PC3 prostate cancer cell line. PC3 cells required a greater concentration of siRNA to knockdown CCND1 as they expressed this gene at a higher level than SusaR (Figure 4). We knocked CCND1 RNA and protein expression down to 21% and 15% respectively of its original level at 72 hours after transfection and 49.3% and 29% after 120 hours (Figure 5, C and D). Combining CCND1 knockdown with cisplatin we also showed that it inhibited cell growth and killed more efficiently than CCND1 knockdown and cisplatin (together with nontargeting control siRNA) alone in PC3 cells (P < 0.001 for all these comparisons; Figure 6, D–E).
Figure 5.
The effect of CCND1 knockdown using siRNA. A and B: show the knockdown effect at RNA and protein levels respectively in SusaR cells and (C) and (D) show the knockdown effect in PC3 cells. RNA expression was measured using qRT-PCR and protein expression was detected by standard Western blotting using a mouse monoclonal antibody against CCND1. C: control cells; NT: Cells transfected with nontargeting siRNA; CCND1: Cells with CCND1 knockdown.
Figure 6.
Cell count and viability after siRNA knockdown of CCND1 in cancer cells. A: Cell viability of SusaR cells with different treatment measured by ATP assay. B and C: Cell proliferation (by cell count) and viability (represented as percentage of dead cells) respectively of SusaR cells with different treatments analyzed using the Beckman Coulter Vi-CELL XR cell counter. D and E: Cell proliferation and viability respectively measured using the cell counter of PC3 cells treated in different way. C: control cells without any treatment, CCND1: cells with CCND1 knockdown, Cispl: cells treated with cisplatin only, Cispl-NT: cells transfected with nontargeting siRNA and treated with cisplatin, and Cispl-CCND1: cells transfected with CCND1 siRNA and treated with cisplatin. *P < 0.05; **P < 0.01; ***P < 0.001.
Discussion
Although many mechanisms of cisplatin resistance have been previously identified,4,5,6,7 they were mainly observed in cell culture models. In a large number of clinically resistant cases the resistance mechanisms are unknown.4,7 In this study, by analyzing gene expression in three pairs of TGCT parental and cisplatin-resistant sublines we identified CCND1 overexpression as the most apparent gene expression change in association with cisplatin resistance. As CCND1 has been previously reported in other cancers for its role in cisplatin resistance,29,30,31,32 we investigated further the association of CCND1 overexpression with platinum-resistance and revealed significantly higher levels of CCND1 expression in resistant TGCT clinical samples than in the sensitive ones. Identification of the overexpression of CCND1 in the majority of chemoresistant clinical samples treated with platinum-based regimen suggests that CCND1 overexpression may be one of the major mechanisms of cisplatin resistance in TGCTs. There may be other genes that act together with CCND1, such as PIK3R1, which was also overexpressed in our microarray analysis of the model cell lines (Table 1). Further studies are required to identify the cooperative genes.
In this study, although a clear correlation between CCND1 expression and cisplatin sensitivity was observed in the majority of TGCT samples, it could not explain the higher sensitivity of 833K than Susa parental cells, which were found to express the lowest level of CCND1 among the TGCT cell lines studied. CCND1 was also not highly overexpressed in 833K resistant cells and there was overlap of CCND1 expression levels between the sensitive and resistant clinical TGCT cases. Therefore, other genes or resistance mechanisms must contribute to platinum resistance in these cases with low-level CCND1 expression.
Although the association of CCND1 deregulation and cisplatin resistance has been previously reported in other cancers including head and neck cancer and pancreatic cancer,29,30,31,32 previous studies were mainly performed in cultured cells. Therefore, the importance of CCND1 overexpression with regard to cisplatin resistance has been largely overlooked.5,6 In this study, we revealed CCND1 overexpression in the majority of resistant TGCT clinical samples both at RNA and protein level. Our data, together with those previously published, suggest that CCND1 overexpression is a common mechanism of cisplatin resistance. CCND1 overexpression has been reported in many human cancers33,34,35,36 and is generally associated with a poor prognosis.34,35,36 Our findings should justify the expansion of clinical investigations targeting CCND1 expression.
Our preliminary data from cell lines also showed that CCND1 overexpression accounts for cisplatin resistance in a large proportion of ovarian cancer, another tumor type predominantly treated by platinum-based therapies.1,12 We showed that the intrinsically resistant cell lines expressed CCND1 at a dramatically higher level than sensitive lines and CCND1 was also overexpressed in A2780R, compared with A2780 cells (Figure 3A). Other mechanisms certainly also contribute to cisplatin resistance, as CCND1 was not overexpressed in the resistant cells of the other two of the three paired cell lines (Figure 3A). Most interestingly, compared with the TGCT cell lines, CCND1 was expressed at a much higher level in the prostate cancer samples, a tumor that is not clinically treated with platinum-based therapy due to its poor response. This, considered alongside our data from the ovarian cancer cell lines, suggests that in many tumors intrinsic resistance to cisplatin may be caused by high-level expression of CCND1, which broadens the potential clinical impact of targeting CCND1.
CCND1 may induce cisplatin resistance both through cell cycle control and inhibition of cellular apoptosis pathways, which have been previously observed37 and supported by our CCND1 knockdown study (Figure 5). The role of CCND1 in cell cycle control is well documented. CCND1 accumulates in cells at middle and late G1 phase and stimulate G1 progression to S phase. The proportion of parental cells in G1/0 correlated with the cisplatin sensitivity, with 833K cells having the highest G1/0 population cells and lowest EC50 value and GCT27 the lowest G1/0 population but highest EC50 score. This indicates that CCND1 expression may reduce cell sensitivity to cisplatin by accelerating G1/S progression. However, in the resistant and sensitive line pair-wise comparison, both 833KR and GCT27R have higher proportion of G1/0 cells than their parental lines. One possible explanation would be that the resistant lines contain more G0 cells, which are generally more resistant to DNA damaging agents. Certainly, other genes may also be involved in cisplatin resistance and cell cycle distribution, which makes the influence of CCND1 more complicated. The anti-apoptotic role of CCND1 has also been documented31,38,39 and correlates with our data, which shows that an increase in CCND1 expression paralleled the reduction in the proportion of apoptotic cells (Figure 1A and see Supplemental Table S2 at http://ajp.amjpathol.org). However, further studies are required to understand the precise mechanism of how CCND1 affects the cell cycle distribution and the sensitivity of cells to cisplatin.
The effect of CCND1 knockdown for sensitizing cancer cells to cisplatin has been demonstrated in several studies both in vitro and in vivo.31,36,40,41,42,43,44 We also demonstrated that combined CCND1 inhibition and cisplatin treatment is an effective way to kill cisplatin-resistant cancer cells, both in TGCT and prostate cancer cell lines (Figure 6). These data not only confirm that CCND1 overexpression results in cisplatin resistance, but also suggest that inhibition of CCND1 expression may be an effective approach in treating the large number of cisplatin-resistant cancers seen with CCND1 up-regulation. It has been demonstrated that inhibiting CCND1 has limited effects on the normal development of mice,45 making CCND1 a potential safe clinical therapeutic target.
In summary, we identified CCND1 overexpression in the majority of cisplatin resistant TGCTs. It is also involved in cisplatin-resistant ovarian and prostate cancer, which is consistent with previous observations in pancreatic cancer and head and neck cancer. This finding encourages the clinical investigation of CCND1 expression status as a biomarker for stratified cancer treatment. It also indicates the potential of using combined CCND1 inhibition and platinum agent chemotherapy to improve the treatment of many cancers with high-level CCND1 expression.
Acknowledgments
We thank Prof. John Masters, Dr. Robert F. Ozols, Dr. Jennifer Parrington, Dr. Martin Pera, and Dr. Anne P. Wilson for their kind donation of the relevant cancer cell lines; Ms. Naomi Tranter and Ms. Ela Stankiewicz for sample and clinical data collection, Dr. Yongwei Yu for reviewing immunostaining results, and the late Prof. Lloyd Kelland who initiated the ovarian cancer study.
Footnotes
Address reprint requests to Yong-Jie Lu, M.D., Ph.D., Molecular Oncology & Imaging Centre, Cancer Institute, Barts and London School of Medicine and Dentistry, Queen Mary, University of London, Charterhouse Square, London EC1M 6BQ, UK. E-mail: y.j.lu@qmul.ac.uk.
Supported by Orchid Cancer Appeal, Cancer Research UK and Barts and London Charitable Foundation (UK).
E.E.N. and M.Y.-V. contributed equally to this work.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer. 2007;7:573–584. doi: 10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]
- Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, Erfan J, Zabolotnyy D, Kienzer HR, Cupissol D, Peyrade F, Benasso M, Vynnychenko I, De Raucourt D, Bokemeyer C, Schueler A, Amellal N, Hitt R. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359:1116–1127. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- Kollmannsberger C, Nichols C, Bokemeyer C. Recent advances in management of patients with platinum-refractory testicular germ cell tumors. Cancer. 2006;106:1217–1226. doi: 10.1002/cncr.21742. [DOI] [PubMed] [Google Scholar]
- Mayer F, Honecker F, Looijenga LH, Bokemeyer C. Towards an understanding of the biological basis of response to cisplatin-based chemotherapy in germ-cell tumors. Ann Oncol. 2003;14:825–832. doi: 10.1093/annonc/mdg242. [DOI] [PubMed] [Google Scholar]
- Borst P, Rottenberg S, Jonkers J. How do real tumors become resistant to cisplatin? Cell Cycle. 2008;7:1353–1359. doi: 10.4161/cc.7.10.5930. [DOI] [PubMed] [Google Scholar]
- Houldsworth J, Korkola JE, Bosl GJ, Chaganti RS. Biology and genetics of adult male germ cell tumors. J Clin Oncol. 2006;24:5512–5518. doi: 10.1200/JCO.2006.08.4285. [DOI] [PubMed] [Google Scholar]
- Mayer F, Stoop H, Scheffer GL, Scheper R, Oosterhuis JW, Looijenga LH, Bokemeyer C. Molecular determinants of treatment response in human germ cell tumors. Clin Cancer Res. 2003;9:767–773. [PubMed] [Google Scholar]
- Masters JR, Koberle B. Curing metastatic cancer: lessons from testicular germ-cell tumours. Nat Rev Cancer. 2003;3:517–525. doi: 10.1038/nrc1120. [DOI] [PubMed] [Google Scholar]
- Looijenga LH, Zafarana G, Grygalewicz B, Summersgill B, Debiec-Rychter M, Veltman J, Schoenmakers EF, Rodriguez S, Jafer O, Clark J, van Kessel AG, Shipley J, van Gurp RJ, Gillis AJ, Oosterhuis JW. Role of gain of 12p in germ cell tumour development. Apmis. 2003;111:161–171. doi: 10.1034/j.1600-0463.2003.11101201.x. discussion 172–163. [DOI] [PubMed] [Google Scholar]
- Houldsworth J, Xiao H, Murty VV, Chen W, Ray B, Reuter VE, Bosl GJ, Chaganti RS. Human male germ cell tumor resistance to cisplatin is linked to TP53 gene mutation. Oncogene. 1998;16:2345–2349. doi: 10.1038/sj.onc.1201770. [DOI] [PubMed] [Google Scholar]
- Kersemaekers AM, Mayer F, Molier M, van Weeren PC, Oosterhuis JW, Bokemeyer C, Looijenga LH. Role of P53 and MDM2 in treatment response of human germ cell tumors. J Clin Oncol. 2002;20:1551–1561. doi: 10.1200/JCO.2002.20.6.1551. [DOI] [PubMed] [Google Scholar]
- Martin LP, Hamilton TC, Schilder RJ. Platinum resistance: the role of DNA repair pathways. Clin Cancer Res. 2008;14:1291–1295. doi: 10.1158/1078-0432.CCR-07-2238. [DOI] [PubMed] [Google Scholar]
- Mayer F, Gillis AJ, Dinjens W, Oosterhuis JW, Bokemeyer C, Looijenga LH. Microsatellite instability of germ cell tumors is associated with resistance to systemic treatment. Cancer Res. 2002;62:2758–2760. [PubMed] [Google Scholar]
- Velasco A, Corvalan A, Wistuba II, Riquelme E, Chuaqui R, Majerson A, Leach FS. Mismatch repair expression in testicular cancer predicts recurrence and survival. Int J Cancer. 2008;122:1774–1777. doi: 10.1002/ijc.23291. [DOI] [PubMed] [Google Scholar]
- Helleman J, van Staveren IL, Dinjens WN, van Kuijk PF, Ritstier K, Ewing PC, van der Burg ME, Stoter G, Berns EM. Mismatch repair and treatment resistance in ovarian cancer. BMC Cancer. 2006;6:201. doi: 10.1186/1471-2407-6-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olasz J, Mandoky L, Geczi L, Bodrogi I, Csuka O, Bak M. Influence of hMLH1 methylation, mismatch repair deficiency and microsatellite instability on chemoresistance of testicular germ-cell tumors. Anticancer Res. 2005;25:4319–4324. [PubMed] [Google Scholar]
- Honecker F, Wermann H, Mayer F, Gillis AJ, Stoop H, van Gurp RJ, Oechsle K, Steyerberg E, Hartmann JT, Dinjens WN, Oosterhuis JW, Bokemeyer C, Looijenga LH. Microsatellite instability, mismatch repair deficiency, and BRAF mutation in treatment-resistant germ cell tumors. J Clin Oncol. 2009;27:2129–2136. doi: 10.1200/JCO.2008.18.8623. [DOI] [PubMed] [Google Scholar]
- Nuti F, Luciani P, Marinari E, Erdei E, Bak M, Deledda C, Rosati F, Mazzinghi B, Danza G, Stoop H, Looijenga LH, Peri A, Serio M, Krausz C. Seladin-1 and testicular germ cell tumours: new insights into cisplatin responsiveness. J Pathol. 2009;219:491–500. doi: 10.1002/path.2622. [DOI] [PubMed] [Google Scholar]
- Hills CA, Kelland LR, Abel G, Siracky J, Wilson AP, Harrap KR. Biological properties of ten human ovarian carcinoma cell lines: calibration in vitro against four platinum complexes. Br J Cancer. 1989;59:527–534. doi: 10.1038/bjc.1989.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louie KG, Behrens BC, Kinsella TJ, Hamilton TC, Grotzinger KR, McKoy WM, Winker MA, Ozols RF. Radiation survival parameters of antineoplastic drug-sensitive and -resistant human ovarian cancer cell lines and their modification by buthionine sulfoximine. Cancer Res. 1985;45:2110–2115. [PubMed] [Google Scholar]
- Schilder RJ, Hall L, Monks A, Handel LM, Fornace AJ, Jr, Ozols RF, Fojo AT, Hamilton TC. Metallothionein gene expression and resistance to cisplatin in human ovarian cancer. Int J Cancer. 1990;45:416–422. doi: 10.1002/ijc.2910450306. [DOI] [PubMed] [Google Scholar]
- Noel EE, Perry J, Chaplin T, Mao X, Cazier JB, Joel SP, Oliver RT, Young BD, Lu YJ. Identification of genomic changes associated with cisplatin resistance in testicular germ cell tumor cell lines. Genes Chromosomes Cancer. 2008;47:604–613. doi: 10.1002/gcc.20564. [DOI] [PubMed] [Google Scholar]
- Lu YJ, Yang J, Noel E, Skoulakis S, Chaplin T, Raghavan M, Purkis T, McIntyre A, Kudahetti SC, Naase M, Berney D, Shipley J, Oliver RT, Young BD. Association between large-scale genomic homozygosity without chromosomal loss and nonseminomatous germ cell tumor development. Cancer Res. 2005;65:9137–9141. doi: 10.1158/0008-5472.CAN-05-1697. [DOI] [PubMed] [Google Scholar]
- Mao X, James SY, Yanez-Munoz RJ, Chaplin T, Molloy G, Oliver RT, Young BD, Lu YJ. Rapid high-resolution karyotyping with precise identification of chromosome breakpoints. Genes Chromosomes Cancer. 2007;46:675–683. doi: 10.1002/gcc.20452. [DOI] [PubMed] [Google Scholar]
- Lu YJ, Birdsall S, Osin P, Gusterson B, Shipley J. Phyllodes tumors of the breast analyzed by comparative genomic hybridization and association of increased 1q copy number with stromal overgrowth and recurrence. Genes Chromosomes Cancer. 1997;20:275–281. [PubMed] [Google Scholar]
- Lu YJ, Williamson D, Clark J, Wang R, Tiffin N, Skelton L, Gordon T, Williams R, Allan B, Jackman A, Cooper C, Pritchard-Jones K, Shipley J. Comparative expressed sequence hybridization to chromosomes for tumor classification and identification of genomic regions of differential gene expression. Proc Natl Acad Sci USA. 2001;98:9197–9202. doi: 10.1073/pnas.161272798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeste-Velasco M, Folch J, Casadesus G, Smith MA, Pallas M, Camins A. Neuroprotection by c-Jun NH2-terminal kinase inhibitor SP600125 against potassium deprivation-induced apoptosis involves the Akt pathway and inhibition of cell cycle reentry. Neuroscience. 2009;159:1135–1147. doi: 10.1016/j.neuroscience.2009.01.035. [DOI] [PubMed] [Google Scholar]
- Perry J, Powles T, Shamash J, Veerupillai A, McGrowder E, Noel E, Lu YJ, Oliver T, Joel S. The relative activity of cisplatin, oxaliplatin and satraplatin in testicular germ cell tumour sensitive and resistant cell lines. Cancer Chemother Pharmacol. 2009;64:925–933. doi: 10.1007/s00280-009-0944-6. [DOI] [PubMed] [Google Scholar]
- Zhang P, Zhang Z, Zhou X, Qiu W, Chen F, Chen W. Identification of genes associated with cisplatin resistance in human oral squamous cell carcinoma cell line. BMC Cancer. 2006;6:224. doi: 10.1186/1471-2407-6-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksson E, Baldetorp B, Borg A, Kjellen E, Akervall J, Wennerberg J, Wahlberg P. p53 mutation and cyclin D1 amplification correlate with cisplatin sensitivity in xenografted human squamous cell carcinomas from head and neck. Acta Oncol. 2006;45:300–305. doi: 10.1080/02841860600547380. [DOI] [PubMed] [Google Scholar]
- Biliran H, Jr, Wang Y, Banerjee S, Xu H, Heng H, Thakur A, Bollig A, Sarkar FH, Liao JD. Overexpression of cyclin D1 promotes tumor cell growth and confers resistance to cisplatin-mediated apoptosis in an elastase-myc transgene-expressing pancreatic tumor cell line. Clin Cancer Res. 2005;11:6075–6086. doi: 10.1158/1078-0432.CCR-04-2419. [DOI] [PubMed] [Google Scholar]
- Warenius HM, Seabra LA, Maw P. Sensitivity to cis-diamminedichloroplatinum in human cancer cells is related to expression of cyclin D1 but not c-raf-1 protein. Int J Cancer. 1996;67:224–231. doi: 10.1002/(SICI)1097-0215(19960717)67:2<224::AID-IJC13>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Alao JP. The regulation of cyclin D1 degradation: roles in cancer development and the potential for therapeutic invention. Mol Cancer. 2007;6:24. doi: 10.1186/1476-4598-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostner J, Ahnstrom Waltersson M, Fornander T, Skoog L, Nordenskjold B, Stal O. Amplification of CCND1 and PAK1 as predictors of recurrence and tamoxifen resistance in postmenopausal breast cancer. Oncogene. 2007;26:6997–7005. doi: 10.1038/sj.onc.1210506. [DOI] [PubMed] [Google Scholar]
- Myo K, Uzawa N, Miyamoto R, Sonoda I, Yuki Y, Amagasa T. Cyclin D1 gene numerical aberration is a predictive marker for occult cervical lymph node metastasis in TNM stage I and II squamous cell carcinoma of the oral cavity. Cancer. 2005;104:2709–2716. doi: 10.1002/cncr.21491. [DOI] [PubMed] [Google Scholar]
- Wang MB, Yip HT, Srivatsan ES. Antisense cyclin D1 enhances sensitivity of head and neck cancer cells to cisplatin. Laryngoscope. 2001;111:982–988. doi: 10.1097/00005537-200106000-00010. [DOI] [PubMed] [Google Scholar]
- Zhou X, Zhang Z, Yang X, Chen W, Zhang P. Inhibition of cyclin D1 expression by cyclin D1 shRNAs in human oral squamous cell carcinoma cells is associated with increased cisplatin chemosensitivity. Int J Cancer. 2009;124:483–489. doi: 10.1002/ijc.23964. [DOI] [PubMed] [Google Scholar]
- Sauter ER, Nesbit M, Litwin S, Klein-Szanto AJ, Cheffetz S, Herlyn M. Antisense cyclin D1 induces apoptosis and tumor shrinkage in human squamous carcinomas. Cancer Res. 1999;59:4876–4881. [PubMed] [Google Scholar]
- Ahmed KM, Fan M, Nantajit D, Cao N, Li JJ. Cyclin D1 in low-dose radiation-induced adaptive resistance. Oncogene. 2008;27:6738–6748. doi: 10.1038/onc.2008.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornmann M, Arber N, Korc M. Inhibition of basal and mitogen-stimulated pancreatic cancer cell growth by cyclin D1 antisense is associated with loss of tumorigenicity and potentiation of cytotoxicity to cisplatinum. J Clin Invest. 1998;101:344–352. doi: 10.1172/JCI1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima T, Clayman GL. Antisense inhibition of cyclin D1 in human head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2000;126:957–961. doi: 10.1001/archotol.126.8.957. [DOI] [PubMed] [Google Scholar]
- Oridate N, Kim HJ, Xu X, Lotan R. Growth inhibition of head and neck squamous carcinoma cells by small interfering RNAs targeting eIF4E or cyclin D1 alone or combined with cisplatin. Cancer Biol Ther. 2005;4:318–323. doi: 10.4161/cbt.4.3.1504. [DOI] [PubMed] [Google Scholar]
- Shuai XM, Han GX, Wang GB, Chen JH. Cyclin D1 antisense oligodexoyneucleotides inhibits growth and enhances chemosensitivity in gastric carcinoma cells. World J Gastroenterol. 2006;12:1766–1769. doi: 10.3748/wjg.v12.i11.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yde CW, Issinger OG. Enhancing cisplatin sensitivity in MCF-7 human breast cancer cells by down-regulation of Bcl-2 and cyclin D1. Int J Oncol. 2006;29:1397–1404. [PubMed] [Google Scholar]
- Lee YM, Sicinski P. Targeting cyclins and cyclin-dependent kinases in cancer: lessons from mice, hopes for therapeutic applications in human. Cell Cycle. 2006;5:2110–2114. doi: 10.4161/cc.5.18.3218. [DOI] [PubMed] [Google Scholar]