Abstract
Proteolytic processing of the nuclear factor (NF)-κB2 precursor protein p100 generates the active NF-κB2 subunit p52, which in turn transcriptionally up-regulates p100 expression. p100 also functions as an IκB molecule capable of repressing p52 activity. The biological significance of this negative feedback control loop has yet to be demonstrated in vivo. Here we show that mice deficient in p100 but with constitutive expression of p52 in lymphocytes developed fatal lung inflammation characterized by diffuse alveolar damage with marked peribronchial fibrosis. In contrast, their littermates with only p100 deficiency or constitutive expression of p52 in lymphocytes developed mild lung inflammation with perivascular lymphocyte infiltration and had a normal life span. The fatal lung inflammation is associated with high-level induction of interferon-γ and its inducible inflammatory chemokines, suggesting the involvement of a T-helper-1 immune response. These findings demonstrate the physiological relevance of the NF-κB2 p100 precursor protein in limiting the potentially detrimental effects of constitutive NF-κB2 signaling in lymphocytes.
Nuclear factor (NF)-κB2 is a member of the NF-κB family of transcription factors that also include NF-κB1 (p105/p50), RelA (p65), RelB, and c-Rel. It is synthesized as a large precursor of 100-kDa protein (p100) and processed by the proteasome to generate p52, corresponding to the amino-terminal half of p100. The activation process is initiated by engagement of the receptors for B-cell-activating factor,1,2 lymphotoxin-β,3 CD40 ligand,4 and receptor activator of NF-κB ligand.5 The engagement activates a kinase cascade: NF-κB-inducing kinase activates inhibitor of NF-κB (IκB) kinase α, which in turn phosphorylates specific serine residues in the carboxyl-terminal region of p100, targeting it for ubiquitination and partial proteasomal degradation.6,7 p52 can form heterodimers with RelB or other Rel proteins. These dimers, once in the nucleus, bind a DNA sequence motif known as the κB site and regulate the expression of genes crucial to the development and functions of lymphocytes.8,9,10
The carboxyl-terminal region of p100 contains seven IκB-like ankyrin repeats with the capacity to retain NF-κB dimers and Rel proteins in the cytoplasm, including RelB, RelA, c-Rel, and p50,4,11,12 as well as p50-RelA and p50-RelB dimers.13,14 The physiological significance of p100 IκB activity is not well understood. In an early effort to address this question, Ishikawa et al15 generated the mutant mice NF-κB2Δc/Δc that lack p100 but still express p52. These mice display a marked increase in the nuclear κB-binding activity of p52-containing complexes, enhanced lymphocyte proliferation and cytokine production, enlarged lymph nodes, and severe gastric hyperplasia responsible for their early postnatal death. However, the constitutive production of p52 in the absence of p100 in these mice raises the question of whether the observed phenotype results from lack of p100, overproduction of p52, or both.8 In addition, it has been shown that the promoter region of NF-κB2 contains several κB-binding sites, which can be activated by the p52-RelA dimer as demonstrated in reporter assays,16,17 suggesting that p52 could up-regulate the expression of its precursor p100. The biological consequence of this autoregulatory loop has not been established.
To address these questions, we generated lymphocyte-specific p52 transgenic (p52-Tg) mice with or without the NF-κB2 p100 precursor protein. In contrast to their p100−/− or p52-Tg littermates, a majority of p52-Tg/p100−/− mice developed fatal lung inflammation characterized by diffuse alveolar damage and high-level induction of the T-helper-1 (TH1) signature cytokine interferon (IFN)-γ and IFN-γ-inducible inflammatory chemokines. These findings provide direct evidence for a physiological function of p100 serving as a surveillance mechanism against aberrant activation of its own signaling pathway.
Materials and Methods
Mice
p52-Tg mice (p52+/−, heterozygote) carry a human p52 transgene under the control of an H-2Kb promoter and an immunoglobulin μ chain enhancer (pHSE3′ expression vector),18 which direct the transgene expression specifically in T and B lymphocytes.18,19 The p52-Tg mice were originally generated on a mixed C57BL/6 × SJL genetic background18 and were subsequently backcrossed with C57BL/6 mice (The Jackson Laboratory, Bar Harbor, ME) for 10 generations. p100+/− mice20 were generated and maintained on the C57BL/6 genetic background. For this study, p100+/− and p52+/− mice (C57BL/6) were first crossed with SJL mice (The Jackson Laboratory) to generate p100+/− and p52+/− mice (F1) with the mixed C57BL/6 × SJL genetic background. The resulting F1 p100+/− and p52+/− mice were then interbred to obtain p52+/−/p100+/− and p100+/− mice (F2). Finally, F2 p52+/−/p100+/− and p100+/− mice were interbred to generate p52+/− (p52-Tg), p52-Tg/p100−/−, p100−/−, and wild-type mice (F3). Mice of the F3 generation were used in this study and were maintained under specific pathogen-free conditions at the animal facilities of the University of Toledo Health Science Campus and the Medical College of Georgia. Mice were euthanized when they became moribund and then were autopsied. All animal experiments were performed with age-matched littermates and were pre-approved by the Institutional Animal Care and Use Committees of both institutions.
Immunoblotting
Human fibrosarcoma HT1080 cells overexpressing NF-κB2 p100, p52, or green fluorescent protein (control) were generated by retroviral infection using pBabe-puro/p100, pBabe-puro/p52, or pBabe-GFP.21 The HT1080 cells and single-cell suspensions of splenocytes from 8-week-old p52-Tg and wild-type mice were directly suspended in SDS sample buffer. Whole-cell extracts were prepared as described previously.22 In brief, thymocytes from 4-week-old wild-type, p52-Tg, p52-Tg/p100−/−, and p100−/− mice were suspended in buffer C containing 20 mmol/L HEPES (pH 7.4), 25% glycerol, 420 mmol/L NaCl, 1.5 mmol/L MgCl2, 0.2 mmol/L EDTA, and 0.5 mmol/L phenylmethylsulfonyl fluoride. After three freeze-thaw cycles, insoluble materials were removed by a 10-minute spin in a microcentrifuge at 4°C, and the supernatants were collected for immunoblot analysis. Proteins (50 μg) were separated on 10% SDS-polyacrylamide gels, transferred to nitrocellulose membranes, probed with antibodies, and visualized by chemiluminescence. The following antibodies were used: rabbit anti-NF-κB2 (no. 4882, 1:500, Cell Signaling Technology, Danvers, MA), rabbit anti-NF-κB2 (no. 06-413, 1:1000, Upstate Biotechnology, Charlottesville, VA), rabbit anti-NF-κB1 p50 (sc-7178, 1:200), rabbit anti-RelA (sc-109x, 1:2000), rabbit anti-RelB (sc-226, 1:200), rabbit anti-c-Rel (sc-71x, 1:1000), rabbit anti-Sp1 (sc-59, 1:100), rabbit anti-β-actin (600-401-886, 1:2000, Rockland, Rockland, ME), and mouse anti-α-tubulin (B-5–1-2, 1:2000, Sigma-Aldrich, St. Louis, MO). Unless indicated, all antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Horseradish peroxidase-conjugated anti-mouse and anti-rabbit antibodies were used as secondary antibodies.
Electrophoretic Mobility Shift Assay
Nuclear extracts were prepared from 4-week-old mouse thymocytes using a NE-PER nuclear extraction kit (Pierce Chemical, Rockford, IL) and analyzed for κB-binding activity as described previously.21 For supershifting, 3 μg of extracts were incubated with 2 μl of either preimmune rabbit serum or rabbit antiserum against NF-κB2 (06-413; Upstate Biotechnology) for 30 minutes at 4°C before addition of the 32P-labeled κB probe 5′-CAGGGCTGGGGATTCCCCATCTCCACAGTTTCACTTC-3′.23
Flow Cytometry
Single-cell suspensions were prepared from mouse lymphoid organs according to standard procedures.24 Red blood cells were lysed in ACK buffer (150 mmol/L NH4Cl, 10 mmol/L KHCO3, and 0.1 mmol/L EDTA, pH 7.3), and dead cells were removed by passing through Lympholyte-M (Cedarlane, Burlington, NC). Lung-infiltrating cells were isolated as described previously.25 The cells were stained with fluorescein isothiocyanate-conjugated rat anti-mouse B220 (RA3-6B2), CD4 (GK1.5), and CD44 (IM7), hamster anti-mouse CD69 (H1.2F3), allophycocyanin-conjugated hamster anti-mouse CD3e (145-2C11), and phycoerythrin-conjugated rat anti-mouse CD4 (RM4-5), CD8a (53-6.7), IgM (R6-60.2), and F4/80 (12-4801, eBioscience, San Diego, CA). Unless indicated, all antibodies were purchased from BD Pharmingen (San Diego, CA). The cells were then sorted on an Epics Elite flow cytometer (Beckman Coulter, Fullerton, CA), and the data were analyzed with WinMDI 2.8 software.
Histopathology and Immunohistochemistry
Mouse tissues were fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned at 5 μm, and stained with H&E or Masson’s trichrome. For immunohistochemical staining, the sections were deparaffinized, rehydrated, and treated with 10 mmol/L citrate buffer (pH 6.0) or 1 mmol/L EDTA (pH 8.0) at 95°C for 25 minutes to retrieval antigens. After quenching of endogenous peroxidase activity with 3% H2O2 and blocking with normal serum, the sections were incubated with primary antibodies overnight at 4°C. The following antibodies were used: rat anti-mouse B220 (RA3-6B2, 5 μg/ml, BD Pharmingen), rat anti-mouse CCL2 (14-7996, 5 μg/ml, eBioscience), rat anti-mouse CCL5 (14-7993, 5 μg/ml, eBioscience), rat anti-human/mouse CD3 (MCA 1477, 10 μg/ml, Serotec, Oxford, UK), rat anti-mouse F4/80 (CI:A3-1, 10 μg/ml, Serotec), goat anti-mouse CXCL9 (AF-492-NA, 10 μg/ml, R&D Systems, Minneapolis, MN), rabbit anti-human/mouse S100A4 (A5114, 1.25 μg/ml, Dako, Carpinteria, CA), mouse anti-mouse αSMA (1E12 hybridoma supernatant, 1:1 dilution). After washing, a biotinylated secondary antibody (Vector Laboratories, Burlingame, CA) was applied for 30 minutes. The sections were then incubated for 30 minutes with an ABC Elite kit (Vector Laboratories), and immunostaining was visualized with 3,3-diaminobenzidine (Sigma-Aldrich). The sections were counterstained with hematoxylin.
Real-Time PCR
Total RNA was extracted from mouse lung tissues (n = 3 for each genotype, 4-month-old) or lung-infiltrating cells using TRIzol (Invitrogen, Carlsbad, CA), according to the manufacturer’s instruction. The quality of RNA samples was determined by 1% agarose gel electrophoresis analysis of 18S, 28S, and tRNA bands for lack of degradation. RNA samples were quantified by spectrophotometry. For real-time PCR analysis, 2.5 μg of RNA was reverse-transcribed using a RT First Strand Kit (SABiosciences, Frederick, MD). The generated cDNA samples were then used as the templates for real-time PCR quantification of mRNA expression of 84 mouse genes coding for common proinflammatory cytokines and receptors (RT Profiler PCR Array PAMM-011A, SABiosciences), and of the mouse T-bet gene (PPM03727, SABiosciences), using an RT2 SYBR Green/ROX PCR Master Mix (PA-012, SABiosciences) according to the manufacturer’s instruction. Data were analyzed with Bio-Rad iQ5 software.
Quantification and Statistical Analysis
p52 κB-binding activities in nuclear extracts, as revealed by electrophoretic mobility shifting assay (EMSA), were quantified using ImageJ (version 1.36b). Films were exposed for various times to ensure that all values obtained were within the linear range of the standard curve of optical density established with increasing amounts of the labeled probe. Masson’s trichrome staining was quantified by analysis of microscopic images using ImageJ (version 1.36b) as described previously.26 Results are expressed as collagen density (area and intensity of trichrome staining/micrometer length of basement membrane of bronchioles with 150- to 200-μm internal diameters). At least 10 images of bronchioles from three mice per genotype were quantified. For S100A4 and α-smooth muscle actin (αSMA) staining, positive cells were counted from four to five randomly selected ×400 fields per lung section per mouse, and sections from three mice per genotype were counted. All quantitative data were analyzed for statistical significance between two experimental groups (p52-Tg/p100−/− versus wild-type, p52-Tg, or p100−/−) by two-tailed Student’s t-test using Microsoft Excel software. P < 0.05 was considered significant in all comparisons.
Results
Negative Feedback Control of p52 Activity by p100
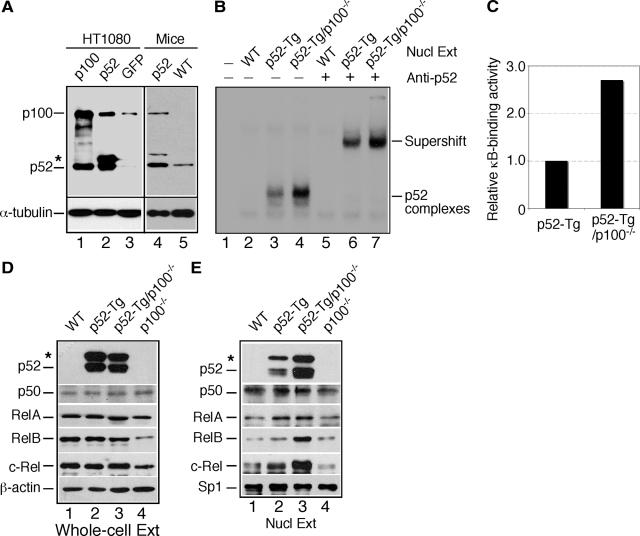
To confirm the ability of p52 to up-regulate p100 expression, we examined p100 levels in cells overexpressing p52. Compared with the control cells expressing green fluorescent protein, HT1080 cells overexpressing p52 had significantly higher levels of p100 (Figure 1A, lanes 1 to 3). Similar results were obtained with splenocytes isolated from 8-week-old p52-Tg and wild-type littermates (Figure 1A, lanes 4 and 5). Thus, in both cell- and animal-based systems, NF-κB2 expression is regulated in a positive autoregulatory manner.
Figure 1.
Negative feedback control of p52 activity by p100. A: Immunoblot analysis of NF-κB2 protein levels in HT1080 cells and splenocytes from 8-week-old wild-type (WT) and p52-Tg mice. Asterisk indicates unprocessed product of the p52 transgene. Levels of α-tubulin are shown as loading control. B: EMSA for κB-binding activity in nuclear extracts (Nucl Ext) of thymocytes from 4-week-old wild-type, p52-Tg, and p52-Tg/p100−/− mice. The κB-binding complexes containing p52 are indicated, based on anti-p52 antibody-mediated supershift. Preimmune rabbit IgG was used as control. C: The κB-binding activity was quantified by densitometry analysis of EMSA films using ImageJ. All values were transformed so that the κB-binding activity in p52-Tg extracts was equalized to 1.0. Data represent means of two independent experiments. D and E: Immunoblot analysis of the NF-κB family of proteins in thymocyte whole-cell (D) and nuclear (E) extracts from 4-week-old wild-type, p52-Tg, p52-Tg/p100−/−, and p100−/− mice. Asterisk indicates unprocessed product of the p52 transgene. Levels of β-actin and the basal transcription factor Sp1 are shown as loading control for whole-cell and nuclear extracts, respectively. WT, wild type.
Given the dual roles of p100 as the precursor for p52 and an IκB molecule capable of retaining NF-κB molecules in the cytoplasm, the up-regulation of p100 expression could lead to either an increase or a decrease in the nuclear κB-binding activity mediated by p52-containing complexes. We performed EMSA to investigate these possibilities. To focus on NF-κB2 activity, we used nuclear extracts prepared from unstimulated thymocytes of 4-week-old wild-type, p52-Tg, and p52-Tg/p100−/− mice. Compared with wild-type extracts (Figure 1B, lane 2), p52-Tg extracts contained significantly higher levels of constitutive κB-binding activity (Figure 1B, lane 3). The predominant κB-binding complexes in p52-Tg extracts could be supershifted by an antibody against human NF-κB2 (Figure 1B, lanes 6 and 7), indicating that they are p52-containing complexes. Importantly, p52-Tg/p100−/− extracts showed an approximately 2.7-fold increase in p52-mediated κB-binding activity relative to p52-Tg extracts (Figure 1, B and C), demonstrating that the up-regulation of p100 by p52 led to a decrease in nuclear p52 activity.
To better understand the regulation of nuclear p52 activity by p100, we examined the protein levels of various NF-κB family members in whole-cell and nuclear extracts prepared from unstimulated thymocytes of 4-week-old wild-type, p52-Tg, p52-Tg/p100−/−, and p100−/− mice. Immunoblot analysis of whole-cell extracts revealed that the presence or absence of p100 had no significant effect on the expression levels of the p52 transgene, NF-κB1 p50, and RelA (Figure 1D). In contrast, there was a substantial decrease in the levels of RelB and, to a lesser extent, c-Rel in p100−/− whole-cell extracts. It has been reported recently that p100 and p52 could stabilize RelB.27 Our finding suggests that p100 and p52 may have a similar role in stabilization of c-Rel protein. Importantly, in agreement with the finding of EMSA (Figure 1, B and C), p52-Tg/p100−/− nuclear extracts showed significantly higher levels of p52, RelB, and c-Rel in comparison with p52-Tg nuclear extracts (Figure 1E), indicating that the presence of p100 inhibited the nuclear translocation of p52, RelB, and c-Rel proteins. We obtained similar results with whole-cell and nuclear extracts of mouse splenocytes (data not shown). Taken together, these results suggest that NF-κB2 signaling is controlled by a negative feedback loop.
Development of Fatal Lung Inflammation in p52-Tg/p100−/− Mice
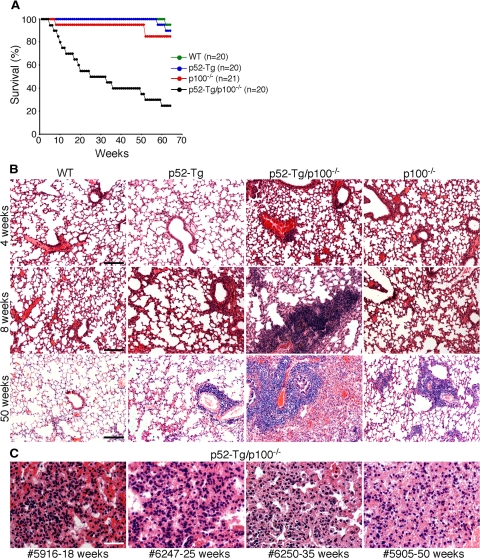
To assess the biological significance of this negative feedback control loop, we analyzed the phenotypes of p52-Tg mice with or without p100 expression. We have recently reported that p100−/− and p52-Tg mice are prone to the development of inflammatory autoimmune disease but have a life span similar to that of their wild-type littermates18,25 (Figure 2A). In addition, p52-Tg mice heterozygous for p100 (p52-Tg/p100+/−) had a mortality rate similar to that of p52-Tg mice (data not shown). In contrast, p52-Tg/p100−/− mice had a mortality rate of 75% by 64 weeks (Figure 2A).
Figure 2.
Development of fatal lung inflammation in p52-Tg/p100−/− mice. A: Survival curve of p52-Tg/p100−/− mice and their wild-type (WT), p52-Tg, and p100−/− littermates. Numbers of mice for each group are indicated. B: H&E staining of formalin-fixed lung sections from wild-type, p52-Tg, p52-Tg/p100−/−, and p100−/− littermates at the indicated ages. Scale bars = 400 μm. C: H&E staining of formalin-fixed lung sections from representative moribund p52-Tg/p100−/− mice at the indicated ages. Scale bar = 100 μm.
To determine the cause of the premature death of p52-Tg/p100−/− mice, we performed histological examination of major organs from p52-Tg/p100−/− mice at various ages, in comparison with their age-matched wild-type, p52-Tg, and p100−/− littermates. We observed no consistent histopathological changes in major organs of p52-Tg/p100−/− mice including the liver, kidney, bone marrow, and stomach (data not shown). However, p52-Tg/p100−/− mice developed severe lung inflammation in a time-dependent manner (Figure 2B). For mice sacrificed at the age of 4 weeks, there was no significant difference in the lung histological appearance between p52-Tg/p100−/− mice and their control littermates (Figure 2B, top panel). When sacrificed at 8 weeks, the lungs of p52-Tg/p100−/− mice displayed marked infiltration of predominantly lymphocytes, as judged by morphology (Figure 2B, middle panel). For all moribund p52-Tg/p100−/− mice, regardless of their ages, the primary pathological change seen in the lungs was diffuse alveolar damage characterized by pronounced pulmonary edema, type II pneumocyte hyperplasia, fibrin-like deposits in alveolar spaces, and interstitial thickening in the lung tissue, leading to collapse of alveoli and consolidation of the lung (Figure 2, B, bottom panel, and C). The diffuse alveolar damage was specific to p52-Tg/p100−/− mice. Although p52-Tg and p100−/− mice at old ages (≥50 weeks) are prone to the development of lung inflammation characterized by infiltration of activated lymphocytes, their lung architecture is typically preserved18,25 (Figure 2B).
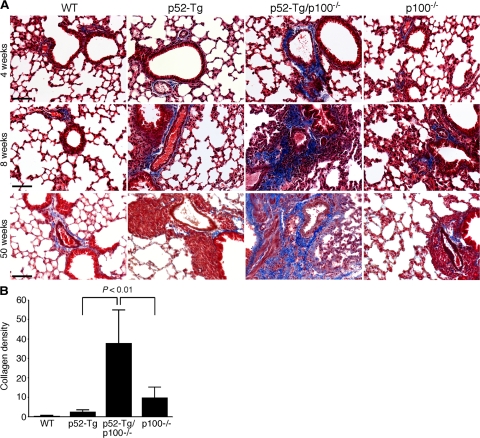
In addition, Masson’s trichrome staining of lung sections revealed the development of peribronchial fibrosis in p52-Tg/p100−/− mice in a similar time-dependent manner (Figure 3A). Quantification of microscopic images of lung sections stained with Masson’s trichrome using ImageJ software showed significantly higher levels of peribronchial collagen content in moribund p52-Tg/p100−/− mice relative to the levels in the age-matched control littermates (Figure 3B). The widespread diffuse alveolar damage in combination with lung fibrosis was probably a major cause of the premature death of p52-Tg/p100−/− mice. Thus, the presence of p100 significantly reduced the lung tissue damage caused by constitutive activation of p52 in lymphocytes.
Figure 3.
Development of progressive pulmonary fibrosis in p52-Tg/p100−/− mice. A: Masson’s trichrome staining of formalin-fixed lung sections from wild-type (WT), p52-Tg, p52-Tg/p100−/−, and p100−/− littermates at the indicated ages. Collagen-rich fibrotic lesions were stained blue. Scale bars = 200 μm. B: Quantification of the area and intensity of peribronchial trichrome staining by image analysis using ImageJ. Data represent means ± SD of at least 10 images of bronchioles from three moribund p52-Tg/p100−/− mice and their age-matched control littermates. Two-tailed Student’s t-test was used for statistical analyses, with P values indicated.
Lymphocyte Infiltration in the Lungs of p52-Tg/p100−/− Mice
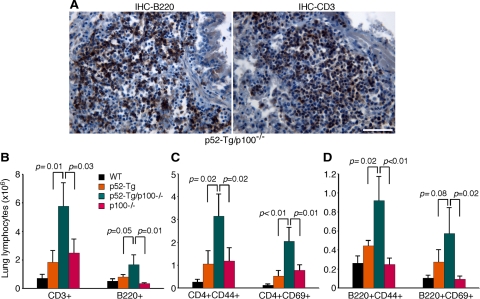
To shed light on the cellular basis of the fatal lung inflammation, we examined the infiltrating lymphocytes in the lungs of moribund p52-Tg/p100−/− mice. Immunohistochemical staining revealed that the infiltrating lymphocytes consisted of both B and T cells (Figure 4A), although T cells seemed to be predominant in most of the lung specimens. We also isolated infiltrating cells from the lungs of p52-Tg/p100−/− mice and their wild-type, p52-Tg, and p100−/− littermates at the age of 6 months (n = 3 per genotype) and quantified lymphocyte subpopulations by flow cytometry (Figure 4, B–D). The lungs of p52-Tg/p100−/− mice contained significantly more lymphocytes in comparison with those of their control littermates, and approximately 75% of them were CD3+ T cells (Figure 4B). Staining for the memory marker CD44 and the activation marker CD69 also revealed significant increases in the numbers of memory and activated CD4+ helper T cells and of memory B220+ B cells in the lungs of p52-Tg/p100−/− mice relative to those of their control littermates (Figure 4, C and D). These data suggest that the lymphocyte infiltration in the lungs of p52-Tg/p100−/− mice was the result of ongoing immune responses, which were much less severe in the presence of p100, indicating a critical role of p100 in limiting the lung immune response resulting from constitutive activation of p52 in lymphocytes.
Figure 4.
Lymphocyte infiltration in the lungs of p52-Tg/p100−/− mice. A: Immunohistochemical (IHC) staining of formalin-fixed lung sections of a moribund p52-Tg/p100−/− mouse (25 weeks). Most infiltrating cells stained positively either for CD3, a T-cell marker, or B220, a B-cell marker. Scale bar = 100 μm. B–D: Flow cytometry quantification of lung-infiltrating lymphocyte populations of 6-month-old p52-Tg/p100−/− mice and their age-matched wild-type, p52-Tg and p100−/− littermates. Data represent means ± SD from three mice per genotype. Two-tailed Student’s t-test was used for statistical analyses, with P values indicated.
Macrophage Infiltration in the Lungs of p52-Tg/p100−/− Mice
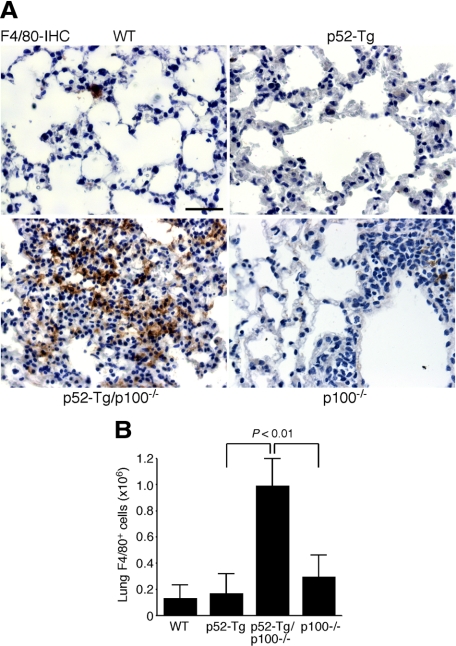
Alveolar macrophages play an important role in lung inflammatory responses.28 Therefore, we performed immunohistochemical staining of lung sections for F4/80, a marker for mouse mature macrophages.29 Compared with wild-type, p52-Tg, and p100−/− mice, there was a marked increase in the number of F4/80-expressing macrophages in the alveoli and interstitium of p52-Tg/p100−/− mice (Figure 5A). We also quantified the number of F4/80+ macrophages by flow cytometry analysis of lung-infiltrating cells isolated from 6-month-old wild-type, p52-Tg, p52-Tg/p100−/−, and p100−/− mice (n = 3 per genotype). Consistent with the result of immunohistochemical staining, the lungs of p52-Tg/p100−/− mice contained an average of 7.6-, 5.8-, and 3.3-fold more F4/80+ macrophages than their wild-type, p52-Tg, and p100−/− littermates, respectively (Figure 5B). Thus, in the absence of p100, constitutive activation of p52 in lymphocytes led to marked macrophage infiltration in the lung, which may play an important role in the development of diffuse alveolar damage in p52-Tg/p100−/− mice.
Figure 5.
Macrophage infiltration in the lungs of p52-Tg/p100−/− mice. A: Immunohistochemical (IHC) staining of formalin-fixed lung sections of a moribund p52-Tg/p100−/− mouse (25 weeks) and its age-matched wild-type (WT), p52-Tg, and p100−/− littermates for F4/80, a marker for alveolar macrophages. Scale bar = 100 μm. B: Flow cytometry quantification of lung F4/80+ macrophages of 6-month-old p52-Tg/p100−/− mice and their age-matched wild-type, p52-Tg and p100−/− littermates. Data represent means ± SD from three mice per genotype. Two-tailed Student’s t-test was used for statistical analyses, with P values indicated.
Accumulation of Fibroblasts and Myofibroblasts in the Lungs of p52-Tg/p100−/− Mice
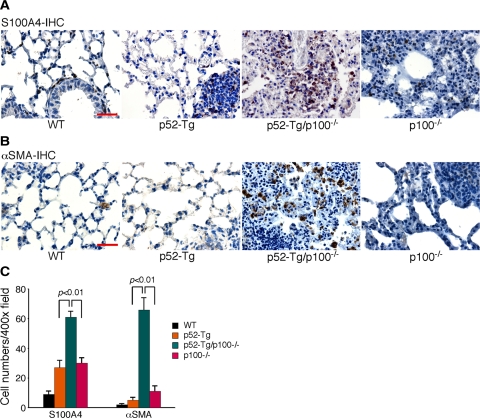
As described above, moribund p52-Tg/p100−/− mice showed prominent peribronchial fibrosis (Figure 3). This observation prompted us to examine the extents of fibroblasts and myofibroblasts in the lungs of moribund p52-Tg/p100−/− mice in comparison with their age-matched wild-type, p52-Tg, and p100−/− littermates. We first examined lung sections by immunohistochemical staining for S100A4, also known as fibroblast-specific protein 1,30 a marker for lung fibroblasts.31,32 In the wild-type lung, S100A4+ resident fibroblasts were found in the adventitia of vascular vessels, as well as in the lung interstitium and along alveolar walls (Figure 6A). A modest increase in the number of fibroblasts was observed in the lungs of p52-Tg and p100−/− mice, especially in the regions of heavy mononuclear cell infiltration (Figure 6A). In contrast, the lungs of p52-Tg/p100−/− mice showed a significant accumulation of S100A4+ fibroblasts, primarily in the perivascular and peribronchial regions (Figure 6A), where prominent fibrosis was also observed (Figure 3A). Quantitative analysis showed an approximately twofold increase in the number of S100A4+ fibroblasts in the lungs of p52-Tg/p100−/− mice, compared with p52-Tg and p100−/− littermates (Figure 6C).
Figure 6.
Accumulation of fibroblasts and myofibroblasts in the lungs of p52-Tg/p100−/− mice. Immunohistochemical (IHC) staining of formalin-fixed lung sections of a moribund p52-Tg/p100−/− mouse (50 weeks) and its age-matched wild-type (WT), p52-Tg, and p100−/− littermates for S100A4 (A), a marker for lung resident fibroblasts, and for α-SMA (B), a marker for myofibroblasts. Scale bar = 100 μm. C: Average numbers of S100A4+ and α-SMA+ cells per ×400 field. Data represent means ± SD of 12 to 15 fields from three mice per genotype. A Two-tailed Student’s t-test was used for statistical analyses, with P values indicated.
We next examined lung sections for the presence of myofibroblasts, given their important role in the pathogenesis of lung fibrosis.33 Myofibroblasts, also termed “activated fibroblasts,” are commonly identified by their expression of αSMA.34,35 We observed very few αSMA+ myofibroblasts in the lung sections of wild-type, p52-Tg, and p100−/− mice (Figure 6B). However, the lung sections of p52-Tg/p100−/− mice showed a marked increase in the number of αSMA+ myofibroblasts (Figure 6, B and C). Thus, in the absence of p100, constitutive activation of p52 signaling in lymphocytes promoted the accumulation of fibroblasts and myofibroblasts in the lung.
Induction of the TH1 Cytokine IFN-γ and Its Inducible Proinflammatory Chemokines in the Lungs of p52-Tg/p100−/− Mice
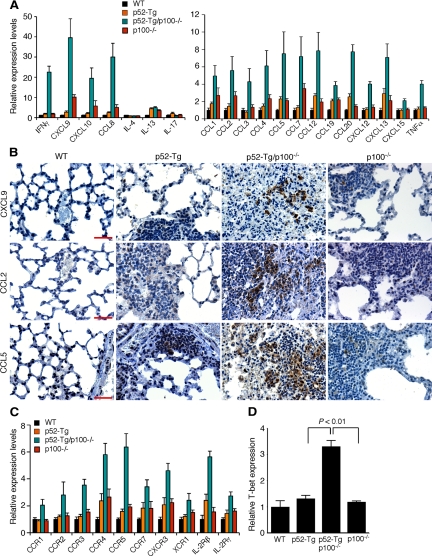
To understand the molecular mechanism underlying the fatal lung inflammation in p52-Tg/p100−/− mice, we examined mRNA expression levels of 84 proinflammatory cytokines and chemokines in lung tissues of 4-month old mice by quantitative real-time PCR. A large number of cytokines and chemokines and their receptors showed significant up-regulation in p52-Tg/p100−/− mice compared with their wild-type, p52-Tg, and p100−/− littermates (Figure 7, A–C).
Figure 7.
Induction of proinflammatory cytokines and chemokines in the lungs of p52-Tg/p100−/− mice. A: Quantitative real-time PCR analysis of mRNA levels of proinflammatory cytokines and chemokines in the lungs of p52-Tg/p100−/− mice and their wild-type (WT), p52-Tg, and p100−/− littermates at the age of 4 months (n = 3 per genotype). B: Immunohistochemical staining of formalin-fixed lung sections from 4-month-old p52-Tg/p100−/− mice and their age-matched wild-type, p52-Tg, and p100−/− littermates for CXCL9, CCL2, and CCL5. Scale bars = 100 μm. C: Quantitative real-time PCR analysis of mRNA levels of receptors for the proinflammatory cytokines and chemokines in the lungs of p52-Tg/p100−/− mice and their wild-type, p52-Tg, and littermates at the age of 4 months (n = 3 per genotype). D: Quantitative real-time PCR analysis of T-bet mRNA levels in lung-infiltrating cells from 6-month-old wild-type, p52-Tg, p52-Tg/p100−/−, and p100−/− littermates (n = 3 per genotype). All values were transformed so that the mRNA levels in the wild-type mice were equalized to 1.0. Data were analyzed for statistical significance by a two-tailed Student’s t-test. For all of the real-time PCR data presented, the differences in mRNA levels between p52-Tg/p100−/− mice and their control littermates are statistically significant (P < 0.05), except for interleukin (IL)-4, interleukin-13, and interleukin-17 (A). P values for data in D are indicated.
A prominent feature of the cytokine and chemokine response in the lungs of p52-Tg/p100−/− mice was the high-level induction of IFN-γ and its inducible chemokines CXCL9 and CXCL10 (Figure 7A). The expression of CXCR3, the receptor for both CXCL9 and CXCL10, was also up-regulated (Figure 7C). Other IFN-γ-inducible chemokines,36 including CCL2, CCL3, CCL4, and CCL5, and their receptors CCR1, CCR2, CCR3, and CCR5, were also significantly up-regulated in the lungs of p52-Tg/p100−/− mice (Figure 7, A and C). To confirm the result of real-time PCR assays, we examined the protein levels of CXCL9, CCL2, and CCL5 by immunohistochemical staining of lung sections. Age-matched wild-type, p52-Tg, and p100−/− mice showed no or few cells staining positively for these chemokines, whereas p52-Tg/p100−/− sections showed significant numbers of mononuclear cells expressing CXCL9, CCL2, or CCL5 (Figure 7B).
IFN-γ is a potent inflammatory cytokine produced primarily by TH1 cells.37 Its expression in TH1 cells is controlled by T-bet, a T box transcription factor with a critical role in the development and maintenance of TH1 cells.38 Real-time PCR analysis revealed an approximately 3-fold increase in T-bet mRNA levels in infiltrating cells isolated from the lungs of p52-Tg/p100−/− mice, compared with the cells from age-matched wild-type, p52-Tg, and p100−/− littermates (Figure 7D). We observed no significant difference between the mice in levels of TH2 cytokines, such as IL-4 and IL13 and of IL-17 (Figure 7A), a cytokine produced specifically by TH17 cells that have an important role in immune responses to infectious agents and in various autoimmune diseases.39 Taken together, these results indicate that the fatal lung inflammation in p52-Tg/p100−/− mice was associated with high-level induction of TH1 cytokines and chemokines.
Discussion
It is well documented that p100 can function as an IκB molecule,4,11,12,13,14 and p100 expression can be up-regulated by its processed product p52.16,17 In the present study, we confirm these observations in both in vitro and in vivo systems, showing that cells expressing higher levels of p52 also had higher levels of p100. Moreover, we show that abrogation of this positive autoregulation leads to a marked increase in the nuclear p52 κB-binding activity, suggesting that the autoregulation acts as a negative feedback loop for the control of NF-κB2 activity. Finally, we present genetic evidence that this negative feedback control mechanism has a critical role in limiting the inflammatory effect of sustained activation of NF-κB2 signaling. Mice deficient in NF-κB2 p100 but with constitutive expression of p52 in lymphocytes developed fatal lung inflammation, whereas their littermates with only p100 deficiency or p52 expression in lymphocytes developed mild lung inflammation at old ages and had a life span similar to that of their wild-type littermates.
The primary pathological change seen in the lungs of p52-Tg/p100−/− mice is diffuse alveolar damage with localized fibrosis, leading to alveolar collapse and lung consolidation. The lung-specific inflammation observed in p52-Tg/p100−/− mice remains a puzzle. We speculate that the constant exposure to antigenic stimuli, such as airborne microorganisms and particles, might make the lung more vulnerable to aberrant immune and inflammatory responses caused by constitutive activation of NF-κB2 signaling in lymphocytes. This notion is consistent with the observation that the infiltrating lymphocytes in the lungs of p52-Tg/p100−/− mice consisted predominantly of memory and activated CD4+ T helper cells, suggesting ongoing T cell-mediated immune responses.
Several lines of evidence suggest that TH1 cells play a major role in causing the lung inflammation in p52-Tg/p100−/− mice. The lung tissues of these mice showed consistently high-level induction of the TH1 cytokine IFN-γ and its inducible chemokines. In agreement with the finding, infiltrating cells isolated from the lungs of p52-Tg/p100−/− mice showed significantly higher mRNA levels of T-bet, a transcription factor specifically required for IFN-γ production in TH1 cells.38 We also observed significant up-regulation of CXCR3 and CCR5 in the lung tissues, which are preferentially expressed on polarized TH1 cells.40 Finally, the lung inflammation in p52-Tg/p100−/− mice is histologically similar to, although much severe than, the lung injury observed in mice transferred with alloreactive TH1 cells, which is characterized by widespread perivascular and interstitial infiltration of mononuclear cells and alveolitis.41,42 These findings also suggest that NF-κB2 signaling may have a key role in the development of TH1 cells.
Recruitment and activation of macrophages are important in inflammatory responses. IFN-γ-inducible chemokines, such as CXCL10, CCL2, CCL3, CCL4, and CCL5, play a prominent role in recruiting monocytes and macrophages.36 All of these chemokines were significantly up-regulated in the lungs of p52-Tg/p100−/− mice. In addition, IFN-γ is a major macrophage-activating cytokine.36,43 We also observed a significant increase in tumor necrosis factor-α (TNFα) expression in the lungs of p52-Tg/p100−/− mice (Figure 7A). TNFα is known to act synergistically with INF-γ in activation of macrophages.44 Activated macrophages can release cytotoxic mediators including oxygen radicals and nitric oxide and matrix metalloproteinases, resulting in cell death, matrix degradation, and tissue damage.45 Moreover, activated macrophages can produce proinflammatory chemokines including CXCL9, CXCL10, CCL2, CCL3, and CCL4,40,44 which in turn can amplify the inflammatory response by recruiting additional lymphocytes and monocytes into the site of inflammation,46,47,48,49 leading to a cycle of inflammatory processes.
The lungs of p52-Tg/p100−/− mice also displayed prominent peribronchial fibrosis, most likely as a consequence of the marked accumulation of fibroblasts and myofibroblasts, which are primarily responsible for the production and deposition of collagen. Several cytokines and chemokines with known regulatory functions in fibroblast proliferation and activation were up-regulated in the lungs of p52-Tg/p100−/− mice, including TNFα, CCR2 and its ligands, and CXCL12. TNFα can promote the proliferation of fibroblasts in vivo and in vitro.50,51 Moreover, transgenic mice with targeted expression of TNFα in alveolar type II epithelial cells develop progressive pulmonary fibrosis with fibroblast accumulation.52 The myofibroblasts could be derived from resident fibroblasts, mesenchymal progenitor cells, and circulating fibrocytes.33,53,54 CCR2 and its ligands have been shown to play an important role in recruiting fibrocytes into the lung, contributing to the pathogenesis of pulmonary fibrosis.55,56 Importantly, CCR2−/− mice are resistant to experimental pulmonary fibrosis induced by bleomycin or fluorescein isothiocyanate.57 CXCL12 is a chemoattractant for circulating fibrocytes.58 The TNFα, CCR2, and CXCL12 signaling pathways may act synergistically in inducing the accumulation of myofibroblasts and fibrotic lesions in the lungs of p52-Tg/p100−/− mice.
In summary, our analysis of the phenotypes of NF-κB2 p52-Tg mice with or without the NF-κB2 p100 precursor protein provides genetic evidence for a key role of p100 in the control of NF-κB2 signaling. It also illustrates an autoregulatory loop formed between a precursor protein and its processed product for a tight control of signaling output.
Acknowledgments
We thank Dr. William Gunning (University of Toledo Health Science Campus) for initial histopathology analysis.
Footnotes
Address reprint requests to Han-Fei Ding, Ph.D., Cancer Center and Department of Pathology, Medical College of Georgia, 1120 15th St., CN-4132, Augusta, GA 30912. E-mail: hding@mcg.edu.
See related Commentary on page 2595
Supported by the National Institutes of Health (grant CA106550 to H.-F.D.). H.-F.D. is a Georgia Cancer Coalition Distinguished Scholar.
Current address of H.C.: Department of Stem Cell Biology and Regenerative Medicine, Cleveland Clinic, Cleveland, Ohio; of Z.W.: Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, Massachusetts; of B.Z.: Immune Disease Institute, Harvard Medical School, Boston, Massachusetts.
References
- Claudio E, Brown K, Park S, Wang H, Siebenlist U. BAFF-induced NEMO-independent processing of NF-κB2 in maturing B cells. Nat Immunol. 2002;3:958–965. doi: 10.1038/ni842. [DOI] [PubMed] [Google Scholar]
- Kayagaki N, Yan M, Seshasayee D, Wang H, Lee W, French DM, Grewal IS, Cochran AG, Gordon NC, Yin J, Starovasnik MA, Dixit VM. BAFF/BLyS receptor 3 binds the B cell survival factor BAFF ligand through a discrete surface loop and promotes processing of NF-κB2. Immunity. 2002;17:515–524. doi: 10.1016/s1074-7613(02)00425-9. [DOI] [PubMed] [Google Scholar]
- Dejardin E, Droin NM, Delhase M, Haas E, Cao Y, Makris C, Li ZW, Karin M, Ware CF, Green DR. The lymphotoxin-β receptor induces different patterns of gene expression via two NF-κB pathways. Immunity. 2002;17:525–535. doi: 10.1016/s1074-7613(02)00423-5. [DOI] [PubMed] [Google Scholar]
- Coope HJ, Atkinson PG, Huhse B, Belich M, Janzen J, Holman MJ, Klaus GG, Johnston LH, Ley SC. CD40 regulates the processing of NF-κB2 p100 to p52. EMBO J. 2002;21:5375–5385. doi: 10.1093/emboj/cdf542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novack DV, Yin L, Hagen-Stapleton A, Schreiber RD, Goeddel DV, Ross FP, Teitelbaum SL. The IκB function of NF-κB2 p100 controls stimulated osteoclastogenesis. J Exp Med. 2003;198:771–781. doi: 10.1084/jem.20030116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao G, Harhaj EW, Sun SC. NF-κB-inducing kinase regulates the processing of NF-κB2 p100. Mol Cell. 2001;7:401–419. doi: 10.1016/s1097-2765(01)00187-3. [DOI] [PubMed] [Google Scholar]
- Senftleben U, Cao Y, Xiao G, Greten FR, Krahn G, Bonizzi G, Chen Y, Hu Y, Fong A, Sun SC, Karin M. Activation by IKKα of a second, evolutionary conserved. NF-κB signaling pathway. Science. 2001;293:1495–1499. doi: 10.1126/science.1062677. [DOI] [PubMed] [Google Scholar]
- Beinke S, Ley SC. Functions of NF-κB1 and NF-κB2 in immune cell biology. Biochem J. 2004;382:393–409. doi: 10.1042/BJ20040544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore TD. Introduction to NF-κB: players, pathways, perspectives. Oncogene. 2006;25:6680–6684. doi: 10.1038/sj.onc.1209954. [DOI] [PubMed] [Google Scholar]
- Weih F, Caamano J. Regulation of secondary lymphoid organ development by the nuclear factor-κB signal transduction pathway. Immunol Rev. 2003;195:91–105. doi: 10.1034/j.1600-065x.2003.00064.x. [DOI] [PubMed] [Google Scholar]
- Scheinman RI, Beg AA, Baldwin AS., Jr NF-κB p100 (Lyt-10) is a component of H2TF1 and can function as an IκB-like molecule. Mol Cell Biol. 1993;13:6089–6101. doi: 10.1128/mcb.13.10.6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solan NJ, Miyoshi H, Carmona EM, Bren GD, Paya CV. RelB cellular regulation and transcriptional activity are regulated by p100. J Biol Chem. 2002;277:1405–1418. doi: 10.1074/jbc.M109619200. [DOI] [PubMed] [Google Scholar]
- Derudder E, Dejardin E, Pritchard LL, Green DR, Korner M, Baud V. RelB/p50 dimers are differentially regulated by tumor necrosis factor-α and lymphotoxin-β receptor activation: critical roles for p100. J Biol Chem. 2003;278:23278–23284. doi: 10.1074/jbc.M300106200. [DOI] [PubMed] [Google Scholar]
- Basak S, Kim H, Kearns JD, Tergaonkar V, O'Dea E, Werner SL, Benedict CA, Ware CF, Ghosh G, Verma IM, Hoffmann A. A fourth IκB protein within the NF-κB signaling module. Cell. 2007;128:369–381. doi: 10.1016/j.cell.2006.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H, Carrasco D, Claudio E, Ryseck RP, Bravo R. Gastric hyperplasia and increased proliferative responses of lymphocytes in mice lacking the COOH-terminal ankyrin domain of NF-κB2. J Exp Med. 1997;186:999–1014. doi: 10.1084/jem.186.7.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liptay S, Schmid RM, Nabel EG, Nabel GJ. Transcriptional regulation of NF-κB2: evidence for κB-mediated positive and negative autoregulation. Mol Cell Biol. 1994;14:7695–7703. doi: 10.1128/mcb.14.12.7695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi L, Ciana P, Cappellini C, Trecca D, Guerrini L, Migliazza A, Maiolo AT, Neri A. Structural and functional characterization of the promoter regions of the NFKB2 gene. Nucleic Acids Res. 1995;23:2328–2336. doi: 10.1093/nar/23.12.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Zhang B, Yang L, Ding J, Ding HF. Constitutive production of NF-κB2 p52 is not tumorigenic but predisposes mice to inflammatory autoimmune disease by repressing Bim expression. J Biol Chem. 2008;283:10698–10706. doi: 10.1074/jbc.M800806200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pircher H, Mak TW, Lang R, Ballhausen W, Ruedi E, Hengartner H, Zinkernagel RM, Burki K. T cell tolerance to Mlsa encoded antigens in T cell receptor Vβ 8.1 chain transgenic mice. EMBO J. 1989;8:719–727. doi: 10.1002/j.1460-2075.1989.tb03431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caamaño JH, Rizzo CA, Durham SK, Barton DS, Raventos-Suarez C, Snapper CM, Bravo R. Nuclear factor (NF)-κB2 (p100/p52) is required for normal splenic microarchitecture and B cell-mediated immune responses. J Exp Med. 1998;187:185–196. doi: 10.1084/jem.187.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Cui H, Schroering A, Ding JL, Lane WS, McGill G, Fisher DE, Ding HF. NF-κB2 p100 is a pro-apoptotic protein with anti-oncogenic function. Nat Cell Biol. 2002;4:888–893. doi: 10.1038/ncb872. [DOI] [PubMed] [Google Scholar]
- Lernbecher T, Muller U, Wirth T. Distinct NF-κB/Rel transcription factors are responsible for tissue-specific and inducible gene activation. Nature. 1993;365:767–770. doi: 10.1038/365767a0. [DOI] [PubMed] [Google Scholar]
- Finco TS, Beg AA, Baldwin AS., Jr Inducible phosphorylation of IκBα is not sufficient for its dissociation from NF-κB and is inhibited by protease inhibitors. Proc Natl Acad Sci USA. 1994;91:11884–11888. doi: 10.1073/pnas.91.25.11884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelegrí C, Rodriguez-Palmero M, Morante MP, Comas J, Castell M, Franch A. Comparison of four lymphocyte isolation methods applied to rodent T cell subpopulations and B cells. J Immunol Methods. 1995;187:265–271. doi: 10.1016/0022-1759(95)00193-1. [DOI] [PubMed] [Google Scholar]
- Zhang B, Wang Z, Ding J, Peterson P, Gunning WT, Ding HF. NF-κB2 is required for the control of autoimmunity by regulating the development of medullary thymic epithelial cells. J Biol Chem. 2006;281:38617–38624. doi: 10.1074/jbc.M606705200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen ZJ, Esnault S, Rosenthal LA, Szakaly RJ, Sorkness RL, Westmark PR, Sandor M, Malter JS. Pin1 regulates TGF-β1 production by activated human and murine eosinophils and contributes to allergic lung fibrosis. J Clin Invest. 2008;118:479–490. doi: 10.1172/JCI32789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusco AJ, Savinova OV, Talwar R, Kearns JD, Hoffmann A, Ghosh G. Stabilization of RelB requires multidomain interactions with p100/p52. J Biol Chem. 2008;283:12324–12332. doi: 10.1074/jbc.M707898200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds HY. Lung inflammation and fibrosis: an alveolar macrophage-centered perspective from the 1970s to 1980s. Am J Respir Crit Care Med. 2005;171:98–102. doi: 10.1164/rccm.200406-788PP. [DOI] [PubMed] [Google Scholar]
- Leenen PJ, de Bruijn MF, Voerman JS, Campbell PA, van Ewijk W. Markers of mouse macrophage development detected by monoclonal antibodies. J Immunol Methods. 1994;174:5–19. doi: 10.1016/0022-1759(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- Lawson WE, Polosukhin VV, Zoia O, Stathopoulos GT, Han W, Plieth D, Loyd JE, Neilson EG, Blackwell TS. Characterization of fibroblast-specific protein 1 in pulmonary fibrosis. Am J Respir Crit Care Med. 2005;171:899–907. doi: 10.1164/rccm.200311-1535OC. [DOI] [PubMed] [Google Scholar]
- Tager AM, LaCamera P, Shea BS, Campanella GS, Selman M, Zhao Z, Polosukhin V, Wain J, Karimi-Shah BA, Kim ND, Hart WK, Pardo A, Blackwell TS, Xu Y, Chun J, Luster AD. The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat Med. 2008;14:45–54. doi: 10.1038/nm1685. [DOI] [PubMed] [Google Scholar]
- Phan SH. Biology of fibroblasts and myofibroblasts. Proc Am Thorac Soc. 2008;5:334–337. doi: 10.1513/pats.200708-146DR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332–337. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller MM, Fusenig NE. Friends or foes—bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004;4:839–849. doi: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-γ: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- Liew FY. TH1 and TH2 cells: a historical perspective. Nat Rev Immunol. 2002;2:55–60. doi: 10.1038/nri705. [DOI] [PubMed] [Google Scholar]
- Szabo SJ, Sullivan BM, Peng SL, Glimcher LH. Molecular mechanisms regulating Th1 immune responses. Annu Rev Immunol. 2003;21:713–758. doi: 10.1146/annurev.immunol.21.120601.140942. [DOI] [PubMed] [Google Scholar]
- Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat Rev Immunol. 2008;8:337–348. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Chen W, Chatta GS, Rubin WD, Clark JG, Hackman RC, Madtes DK, Ligitt DH, Kusunoki Y, Martin PJ, Cheever MA. T cells specific for a polymorphic segment of CD45 induce graft-versus-host disease with predominant pulmonary vasculitis. J Immunol. 1998;161:909–918. [PubMed] [Google Scholar]
- Clark JG, Madtes DK, Hackman RC, Chen W, Cheever MA, Martin PJ. Lung injury induced by alloreactive Th1 cells is characterized by host-derived mononuclear cell inflammation and activation of alveolar macrophages. J Immunol. 1998;161:1913–1920. [PubMed] [Google Scholar]
- Renauld JC. Class II cytokine receptors and their ligands: key antiviral and inflammatory modulators. Nat Rev Immunol. 2003;3:667–676. doi: 10.1038/nri1153. [DOI] [PubMed] [Google Scholar]
- Mosser DM. The many faces of macrophage activation. J Leukoc Biol. 2003;73:209–212. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- Duffield JS. The inflammatory macrophage: a story of Jekyll and Hyde. Clin Sci (Lond) 2003;104:27–38. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- Appay V, Rowland-Jones SL. RANTES: a versatile and controversial chemokine. Trends Immunol. 2001;22:83–87. doi: 10.1016/s1471-4906(00)01812-3. [DOI] [PubMed] [Google Scholar]
- Liao F, Rabin RL, Yannelli JR, Koniaris LG, Vanguri P, Farber JM. Human Mig chemokine: biochemical and functional characterization. J Exp Med. 1995;182:1301–1314. doi: 10.1084/jem.182.5.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins BJ, Yoshimura T, Leonard EJ, Pober JS. Cytokine-activated human endothelial cells synthesize and secrete a monocyte chemoattractant, MCP-1/JE. Am J Pathol. 1990;136:1229–1233. [PMC free article] [PubMed] [Google Scholar]
- Taub DD, Conlon K, Lloyd AR, Oppenheim JJ, Kelvin DJ. Preferential migration of activated CD4+ and CD8+ T cells in response to MIP-1α and MIP-1β. Science. 1993;260:355–358. doi: 10.1126/science.7682337. [DOI] [PubMed] [Google Scholar]
- Piguet PF, Grau GE, Vassalli P. Subcutaneous perfusion of tumor necrosis factor induces local proliferation of fibroblasts, capillaries, and epidermal cells, or massive tissue necrosis. Am J Pathol. 1990;136:103–110. [PMC free article] [PubMed] [Google Scholar]
- Sugarman BJ, Aggarwal BB, Hass PE, Figari IS, Palladino MA, Jr, Shepard HM. Recombinant human tumor necrosis factor-α: effects on proliferation of normal and transformed cells in vitro. Science. 1985;230:943–945. doi: 10.1126/science.3933111. [DOI] [PubMed] [Google Scholar]
- Miyazaki Y, Araki K, Vesin C, Garcia I, Kapanci Y, Whitsett JA, Piguet PF, Vassalli P. Expression of a tumor necrosis factor-α transgene in murine lung causes lymphocytic and fibrosing alveolitis. A mouse model of progressive pulmonary fibrosis. J Clin Invest. 1995;96:250–259. doi: 10.1172/JCI118029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol. 2007;170:1807–1816. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest. 2007;117:524–529. doi: 10.1172/JCI31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore BB, Kolodsick JE, Thannickal VJ, Cooke K, Moore TA, Hogaboam C, Wilke CA, Toews GB. CCR2-mediated recruitment of fibrocytes to the alveolar space after fibrotic injury. Am J Pathol. 2005;166:675–684. doi: 10.1016/S0002-9440(10)62289-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore BB, Murray L, Das A, Wilke CA, Herrygers AB, Toews GB. The role of CCL12 in the recruitment of fibrocytes and lung fibrosis. Am J Respir Cell Mol Biol. 2006;35:175–181. doi: 10.1165/rcmb.2005-0239OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharaee-Kermani M, McCullumsmith RE, Charo IF, Kunkel SL, Phan SH. CC-chemokine receptor 2 required for bleomycin-induced pulmonary fibrosis. Cytokine. 2003;24:266–276. doi: 10.1016/j.cyto.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Burdick MD, Hong K, Lutz MA, Murray LA, Xue YY, Belperio JA, Keane MP, Strieter RM. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest. 2004;114:438–446. doi: 10.1172/JCI20997. [DOI] [PMC free article] [PubMed] [Google Scholar]