Abstract
Although estrogens have long been known to accelerate healing in females, their roles in males remain to be established. To address this, we have investigated the influence of 17β-estradiol on acute wound repair in castrated male mice. We report that sustained exposure to estrogen markedly delays wound re-epithelialization. Our use of hairless mice revealed this response to be largely independent of hair follicle cycling, whereas other studies demonstrated that estrogen minimally influences wound inflammation in males. Additionally, we report reduced collagen accumulation and increased gelatinase activities in the wounds of estrogen-treated mice. Increased wound matrix metalloproteinase (MMP)-2 activity in these animals may i) contribute to their inability to heal skin wounds optimally and ii) stem, at least in part, from effects on the overall levels and spatial distribution of membrane-type 1-MMP and tissue inhibitor of MMP (TIMP)-3, which respectively facilitate and prevent MMP-2 activation. Using mice rendered null for either the α or β isoform of the estrogen receptor, we identified estrogen receptor-α as the likely effector of estrogen’s inhibitory effects on healing.
Although anecdotal evidence has long suggested that differences exist in the abilities of females and males (particularly the elderly) to heal acute wounds, only recently have they been substantiated by published research. Indeed, it was observed sex differences in key parameters such as restoration of the basement membrane1 and elastin regeneration2 that previously encouraged us to make detailed comparisons of healing in males and females. We discovered that, although repair is broadly similar in intact (young) male and female mice, castrated males heal acute skin wounds far better than do their ovariectomized female counterparts.3 Furthermore, males and females differed in their responsiveness to macrophage migration inhibitory factor (MIF): a potent inhibitor of repair in females, in males it has minimal influence.
These studies encouraged us to conclude that sex differences in the responses to cutaneous injury do exist but that they are masked in young individuals by the combined actions of gonadal sex steroids. In males, testosterone and its more potent metabolite 5α-dihydrotestosterone inhibit repair1,4; in females, estrogens such as 17β-estradiol accelerate healing.5,6
Although the effects of estrogens on female cutaneous physiology are well characterized, their roles in males are poorly understood. A handful of studies have sought to address this. In a group of aged males, locally administered 17β-estradiol was shown to reduce macroscopically determined day 7 wound areas in an excisional wounding model.6 It was recently shown that an overwhelming majority of genes displaying different wound expression between young and elderly human males are subject to estrogenic control.7 In a separate study, thrice-weekly application of 17β-estradiol to sun-protected skin in aged males induced the synthesis of collagen I; increased dermal collagen bundle thickness and density; and stimulated keratinocyte proliferation.8 Although these and other studies have provided useful insights, little is yet known about the healing properties of i) systemic and ii) prolonged estrogen treatment.
Having previously reported preliminary evidence that systemic 17β-estradiol treatment may impair cutaneous wound healing in castrated male mice,3 we aimed with the present study to fully characterize the effects of 17β-estradiol on the healing of acute wounds in males and to delineate the mechanisms underpinning any identified responses. Because estrogens are well-known to influence the cycling of hair follicles,9 which themselves were recently shown to be beneficial to repair,10 the contribution of hair to estrogen-impaired healing provided our initial focus. We report that estrogen treatment of castrated mice significantly retards wound re-epithelialization in both hairless (hr/hr) mice and strain-matched controls, confirming that systemic estrogen treatment does indeed inhibit repair and suggesting that the presence of cycling hair follicles is not critical to this response. Subsequent studies identified estrogen receptor (ER)-α as the likely effector of estrogenic inhibition and highlighted the potential involvement of increased matrix metalloproteinase (MMP)-2 activity in the reduced wound accumulation of collagen that we observed in estrogen-treated mice.
Materials and Methods
Wound Healing Experiments
All animal studies were approved by both the University of Manchester Institutional Animal Use Committee and the U.K. Government Home Office and all procedures performed in accordance with the Home Office regulations relating to animal care. Our wounding/estrogen treatment protocols are summarized in Table 1.
Table 1.
In Vivo Estrogen Treatment Protocols
| Sex | Type | Gonads | Day
|
|||
|---|---|---|---|---|---|---|
| −140+3+10 | ||||||
| M | MF1 | CSX | ←————————————————→ | |||
| M | MF1 | CSX | ←———————————→←————————————→ | |||
| M | MF1 | Intact | ←————————————————→ | |||
| M | hr/hr | CSX | ←————————————————→ | |||
| F | BALB/c | OVX | ←————————————————→ | |||
| F | BALB/c | OVX | ←———→ | |||
| M | WT* | CSX | ←————————————————→ | |||
| M | ER-α−/− | CSX | ←————————————————→ | |||
| M | ER-β−/− | CSX | ←————————————————→ | |||
| M | WT* | CSX | ←———→ | |||
| M | ER-α−/− | CSX | ←———→ | |||
| M | ER-β−/− | CSX | ←———→ | |||
Arrows indicate duration of treatment with 17β-estradiol (21-day slow-release 0.05 mg pellet; s.c.) Chronic treatment, commencing on day −14/acute, on day 0.
Castration/ovariectomy performed on day −14.
Mice wounded on day 0.
Wounds excised on days +3/+10 as indicated.
Mixed-strain littermates of ER-α−/− and ER-β−/− mice.
Six-week-old male MF1 mice were castrated. A subset additionally received 0.05 mg 17β-estradiol pellets (Innovative Research of America, Sarasota, FL), s.c. implanted using a trocar at the time of castration. Two weeks subsequently, the mice were anesthetized with isoflurane, and their dorsa shaved and cleansed with ethanol. Full-thickness 1-cm incisions were made 1 cm either side of the midline. (In a subset of mice-used as a source of day 10 wounds-estrogen pellets implanted at the time of castration were replaced with fresh 17β-estradiol pellets.) All mice received immediate analgesia in the form of buprenorphine (0.1 mg/kg) and were housed individually. Three or 10 days postwounding, the wounds were excised and bisected.
Eight-week-old intact (noncastrated) male MF1 mice were wounded (as described above), and their wounds were excised and bisected 3 days postwounding.
Six-week-old male hr/hr mice (on an MF1 background) were castrated as above. A subset received 0.05 mg 17β-estradiol pellets at the time of castration (see above). Two weeks later, the mice were wounded (see above). Wounds were excised and bisected 3 days postwounding.
Six-week-old female BALB/c mice were ovariectomized. A subset received 0.05 mg 17β-estradiol pellets at the time of ovariectomy (see above). Two weeks subsequently, the mice were wounded (see above). A further subset of ovariectomized mice not previously treated with estrogen was implanted with 0.05 mg 17β-estradiol pellets at the time of wounding. Wounds were excised and bisected 3 days postwounding.
Six-week-old male mixed-strain ER-α null (αKO)11 and ER-β null (βKO)11 mice and wild-type littermates were castrated as above. Subsets of αKO, βKO, and wild-type animals received 0.05 mg 17β-estradiol pellets at the time of castration (see above). Two weeks subsequently, the mice were wounded (see above). Further subsets of castrated αKO, βKO, and wild-type animals not previously treated with estrogen were implanted with 0.05 mg 17β-estradiol pellets at the time of wounding. Wounds were excised and bisected 3 days postwounding.
One-half of each wound was processed in 8% formaldehyde-based fixative; the other snap-frozen in liquid nitrogen and stored at −80°C. Unwounded dorsal skin was similarly processed in fixative. Sera were isolated from blood obtained by cardiac puncture.
Histology, Immunohistochemistry, and Image Analysis
Fixed tissue was embedded in paraffin. Five-micrometer-thick histological sections prepared from the center of each wound were stained with hematoxylin and eosin (H&E) and subjected to immunohistochemistry using polyclonal IgGs raised against MMP-2, ER-α, arginase I (Insight, Wembley, U.K.), ER-β, tissue inhibitor of MMP (TIMP)-3 (Abcam, Cambridge, U.K.), MIF and tumor necrosis factor (TNF)-α (R&D Systems, Abingdon, U.K.), and monoclonal IgGs raised against Ly6G, Mac3, and CD74 (BD Biosciences, Oxford, U.K.), and membrane-type (MT)1-MMP (Abcam). Bound IgG was subsequently detected using a colorimetric Vectastain avidin-biotin complex peroxidase kit (Vector Laboratories, Peterborough, U.K.) in conjunction with the enzyme substrate Novared (Vector Laboratories). Tissue sections treated with PBS in place of primary IgG (negative controls) yielded no signal. Wound areas, extent of re-epithelialization, cell numbers/unit area, the thicknesses of individual skin layers, and immunostained areas were lastly quantified using Image-Pro Plus software (Media Cybernetics, Marlow, U.K.).5
Picrosirius Red Staining
Paraffin-embedded tissue sections were immersed in 0.1% Sirius red (in picric acid) for 1 hour and then washed in acidified water. When examined under plane-polarized light, larger collagen fibers appear red, orange, or yellow and thinner ones green. This birefringence is highly specific to collagen.12
Quantification of Epidermal Protein Expression
Epidermal MMP-2 and MT1-MMP staining intensities were compared by four-grade semiquantitative scoring: 0, no staining; 1, weak; 2, moderate; and 3, strong. Data are presented as box-and-whisker plots.
Gelatin Zymography
Total protein was extracted from unwounded skin and wound tissue using denaturing, nonreducing buffer. Fifty micrograms of individual and pooled protein samples (n = 5 per treatment group) was tested for gelatinase activities as described previously.13 Briefly, an acrylamide gel containing 0.5 mg/ml gelatin was prepared. Test samples were separated by SDS-PAGE under nonreducing conditions alongside human MMPs 2 and 9, purified from stably transfected mouse myeloma cells.14 Following separation, gels were washed in 2.5% Triton X-100 (Sigma-Aldrich, Poole, U.K.) and, briefly, distilled H2O, before being incubated for 16 hours at 37°C in assay buffer (100 mmol/L Tris, 30 mmol/L CaCl2, 0.02% (w/v) sodium azide, 0.05% (v/v) Brij 35, pH 7.9). Finally, the gels were stained with Coomassie Brilliant Blue G for 20 minutes and then destained in 1% acetic acid and 30% methanol. Band intensities were quantified using Image-Pro Plus software. Samples separated by reducing SDS-PAGE were stained with Coomassie to serve as a loading control.
Enzyme Immunoassay
Levels of 17β-estradiol in serum samples isolated from MF1 and hr/hr mice were measured using an enzyme immunoassay kit (MP Biomedicals, Cambridge, U.K.), according to the manufacturer’s guidelines.
Immunoblotting
Total protein was extracted from unwounded skin and wound tissue using an SDS-based detergent buffer. One microgram of individual and pooled protein samples (n = 5 per treatment group) was tested as described previously.15 Briefly, samples separated by denaturing, reducing SDS-PAGE, were blotted to 0.2-μm nitrocellulose membranes (Bio-Rad, Hemel Hempstead, UK). Membranes were blocked for 16 hours in Tris-buffered saline (containing 0.1% (v/v) Tween-20 and 5% (w/v) nonfat dry milk). Following sequential 1-hour washes in primary and peroxidase-linked secondary antibodies, antigen binding was probed using ECL Plus detection reagent (GE Healthcare, Little Chalfont, U.K.). Polyclonal IgGs raised against type I collagen (Millipore, Watford, U.K.), ER-α (Insight), ER-β, MT1-MMP, and TIMP-3 (Abcam), and MMP-2 (Merck, Nottingham, UK), and a monoclonal IgG raised against β-actin (used as a loading control) (Sigma-Aldrich) were used in conjunction with sheep anti-mouse and donkey anti-rabbit secondary IgGs (both GE Healthcare). Signal intensities were determined densitometrically.
Quantitative Real-Time PCR
Total RNA was extracted from frozen day 3 wound tissue using TRIzol (Invitrogen, Paisley, U.K.) in conjunction with a PureLink RNA Mini Kit (Invitrogen). cDNA was synthesized from RNA (1 μg) and analyzed by quantitative real-time PCR (qPCR). Each test sample was serially diluted over 3 orders of magnitude and PCR performed using the primers listed in Table 2 and the combination of a SYBR Green I core kit (Eurogentec, Romsey, U.K.) and an Opticon qPCR thermal cycler (Genetic Research Instrumentation, Braintree, U.K.). The specificity of product amplification was assessed through melting curve analysis.
Table 2.
Primer Sequences and Product Sizes for qPCR-Amplified Genes
| Target gene | Forward primer sequence | Reverse primer sequence | Product size (bp) |
|---|---|---|---|
| Cd74 | 5′-ATGACCCAGGACCATGTGAT-3′ | 5′-ATCTTCCAGTTCACGCCATC-3′ | 128 |
| Col1a1* | 5′-GAGCGGAGAGTACTGGATCG-3′ | 5′-GTTCGGGCTGATGTACCAGT-3′ | 142 |
| Col1a2† | 5′-GTGTTCAAGGTGGCAAAGGT-3′ | 5′-GAGACCGAATTCACCAGGAA-3′ | 131 |
| Krt1 | 5′-CGTGGTGAGAAAGCACTCAA-3′ | 5′-TGCACTCTCCAGACATCCTG-3′ | 199 |
| Krt14 | 5′-CCTCTGGCTCTCAGTCATCC-3′ | 5′-TGAGCAGCATGTAGCAGCTT-3′ | 144 |
| Krt16 | 5′-GTGGTTTTGGTGCTGGTCTT-3′ | 5′-GTACCAGTCCCGGATCTTCA-3′ | 179 |
| Mif | 5′-AGGCCACACAGCAGCTTACT-3′ | 5′-AGCTCATGACTTTTAGCGGC-3′ | 112 |
| Mmp1a | 5′-GTGCTCTCCTTCCACAGAGG-3′ | 5′-GGTCCACGTCTCATCAAGGT-3′ | 135 |
| Mmp1b | 5′-CCTTCCTTTGCTGTTGCTTC-3′ | 5′-ATCACCTCCTTGCCATTCAC-3′ | 162 |
| Mmp2 | 5′-CTTCGCTCGTTTCCTTCAAC-3′ | 5′-ATGTCAGACAACCCGAGTCC-3′ | 78 |
| Mmp13 | 5′-AGTTGACAGGCTCCGAGAAA-3′ | 5′-GGCACTCCACATCTTGGTTT-3′ | 105 |
| Timp1 | 5′-TCCCCAGAAATCAACGAGAC-3′ | 5′-CATTTCCCACAGCCTTGAAT-3′ | 88 |
| Timp2 | 5′-CACAGACTTCAGCGAATGGA-3′ | 5′-CCAGCATGAGACCTCACAGA-3′ | 124 |
| Timp3 | 5′-TTATCCCATTGGGGCATTTA-3′ | 5′-TTGCTGCCTTTGACTGATTG-3′ | 161 |
| Tnfa | 5′-TCTCAGCCTCTTCTCATTCCTGCT-3′ | 5′-AGAACTGATGAGAGGGAGGCCATT-3′ | 124 |
| Rn18s‡ | 5′-AGTCCCTGCCTTTGTACACA-3′ | 5′-GATCCGAGGGCCTCACTAAC-3′ | 69 |
| Gapdh§ | 5′-TGCACCACCAACTGCTTAGC-3′ | 5′-GGCATGGACTGTGGTCATGAG-3′ | 87 |
| Hprt¶ | 5′-TGCTCGAGATGTCATGAAGG-3′ | 5′-AATCCAGCAGGTCAGCAAAG-3′ | 95 |
| Ywhaz∥ | 5′-TTCTTGATCCCCAATGCTTC-3′ | 5′-TTCTTGTCATCACCAGCAGC-3′ | 107 |
Encodes the α1 chain of type I collagen.
Encodes the α2 chain of type I collagen.
Encodes the 18S ribosomal RNA.
Encodes glyceraldehyde-3-phosphate dehydrogenase.
Encodes hypoxanthine guanine phosphoribosyl transferase.
Encodes tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, ζ.
Macrophage Isolation and Culture
Peritoneal macrophages were isolated from 8-week-old male mice by i.p. lavage with sterile ice-cold PBS and subsequently purified using Ficoll-Paque Premium (GE Healthcare). Cells were pooled and suspended at a concentration of 2 × 106 cells/ml in Phenol-Red-free Dulbecco’s modified Eagle’s medium (supplemented with 5% charcoal-stripped fetal calf serum), before being treated with bacterial lipopolysaccharide (LPS) (1 μg/ml) for 2 hours. Thus-activated cells were then treated with 100 nmol/L 17β-estradiol (Sigma-Aldrich), 1 μmol/L PPT (Tocris Bioscience, Bristol, U.K.), or 1 μmol/L diarylpropionitrile (Tocris Bioscience), or left untreated, for a further 3 hours. Total cellular RNA and protein samples were subsequently isolated and analyzed by qPCR and immunoblotting/zymography.
Keratinocyte Isolation and Culture
Male and female mouse neonates were euthanized by decapitation and their trunk skin removed and rinsed in 70% ethanol.16 The epidermis was loosened from the dermis through overnight incubation in 5 mg/ml dispase I (Sigma-Aldrich) at 4°C. Primary keratinocytes were isolated from the epidermis according to the protocol provided by CELLnTEC. Briefly, skin was washed in CnT-57 medium (CELLnTEC, Bern, Switzerland) to remove excess dispase and the epidermis separated from the dermis with forceps. The epidermal sheet was placed on a drop of TrypLE Select (Invitrogen) in a petri dish with the basal layer facing downward and incubated at room temperature for 30 minutes. The epidermis was gently rubbed on the bottom of the dish to disturb basal cells. The resulting cell suspension was centrifuged, and the thus-pelleted cells resuspended in CnT-57 medium before being seeded to cell culture flasks coated with collagen IV (BD Biosciences).
Pelage hairs were removed from dorsal skin of sacrificed adult male mice by wax-based depilation (using Veet (Reckitt Benckiser, Slough, U.K.)). The skin was excised, and the epidermis and dermis separated following overnight incubation in 0.25% trypsin/EDTA (Invitrogen) at 4°C. Isolation and culture of primary keratinocytes were performed as described previously.17
Keratinocyte Migration Assay
The migration of scratch-wounded mouse epidermal keratinocytes on type I collagen was assessed as described previously.18 The influence of 17β-estradiol at three concentrations (1, 100, and 1000 nmol/L) on cell migration was evaluated.
Cell Proliferation Assay
The proliferation of mouse epidermal keratinocytes was assessed using the colorimetric MTS-based CellTiter 96 AQueous One Solution cell proliferation assay (Promega, Southampton, U.K.), according to the manufacturer’s guidelines and as described previously.18 The effects on cell proliferation of 17β-estradiol (1, 100, and 1000 nmol/L) were evaluated.
Keratinocyte Expression Studies
Epidermal keratinocytes were seeded to type I collagen-coated 6-well plates and cultured to ∼ 90% confluence, before being treated with 17β-estradiol (1 or 1000 nmol/L). Total cellular RNA and protein samples were isolated 24 hours subsequently and were respectively analyzed by qPCR and immunoblotting.
Statistical Analysis
Simfit (version 6.7.15) (William Bardsley, University of Manchester, Manchester, U.K.) was used to test for statistical significance by one-way and two-way analysis of variance, unpaired Student’s t-tests and, for nonparametric data, systematic, pairwise Mann-Whitney U-tests with Bonferroni correction. Posthoc testing was performed using Bonferroni-corrected unpaired Student’s t-tests.
Results
Healing in Male Mice Is Impaired by Systemic Estrogen Treatment
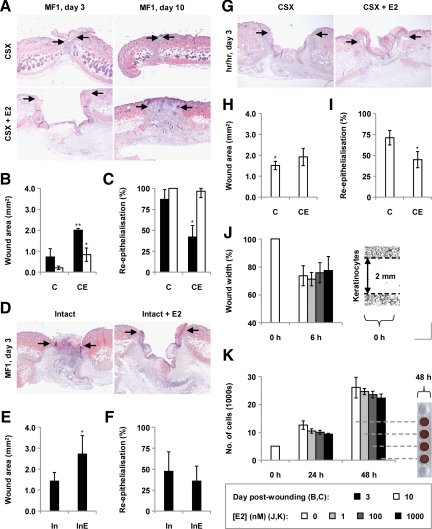
To investigate the roles of estrogen in the repair of acute skin wounds in males, we initially treated castrated male mice systemically with 17β-estradiol. Corroborating our previous findings,3 17β-estradiol markedly impaired healing (assessed in terms of day 3 and 10 wound areas) (Figure 1, A and B). Moreover, it greatly retarded wound re-epithelialization (Figure 1C).
Figure 1.
Effects of chronic 17β-estradiol on wound healing in male mice. A: H&E-stained day three and ten wounds from castrated (CSX, CX) MF1 mice, some treated with 17β-estradiol (E2) from days −14 to +3/+10 (CSX + E2, CXE). B: Day three and ten wound areas (statistical significance tested by two-way analysis of variance (P < 0.001, influence of time; P = 0.0047, influence of E2), followed by Bonferroni-corrected Student’s t-tests: *P < 0.05; **P < 0.01 (versus CX). C: Re-epithelialization of day three and ten wounds (Bonferroni-corrected pairwise Mann-Whitney U-tests: *P < 0.05 (versus CX)). D: H&E-stained day three wounds from intact (In) male MF1 mice, some treated chronically with E2 (InE). E: Day three wound areas (Mann-Whitney U-test: *P < 0.05). F: Re-epithelialization of day three wounds. G: H&E-stained day three wounds from CSX hr/hr mice, some treated chronically with E2. H: Day three wound areas (Bonferroni-corrected Student’s t-tests: *P < 0.05 (versus MF1 CX)). I: Re-epithelialization of day three wounds (Mann-Whitney U-test: *P < 0.05). J: Effect of 1, 10, and 100 nmol/L 17β-estradiol on the migration of epidermal keratinocytes derived from adult male mice in scratch wound assays. K: Effect of 17β-estradiol on keratinocyte proliferation (MTS-based assay). n = 5 per treatment group. Data are presented as mean ± SD. Arrows (A, D, and G) define the wound margins. Original magnification: ×40 (A, D, and G); ×100 (J).
To determine whether exogenous estrogen could influence healing against a background of normal circulating steroid levels, we similarly treated intact male mice with 17β-estradiol. We found that 17β-estradiol increased day 3 wound areas in intact animals (Figure 1, D and E) without significantly affecting the extent of re-epithelialization at that time point (Figure 1F).
To probe the potential involvement of hair follicles in this apparent inhibition of repair by estrogens, we likewise treated hr/hr mice (strain-matched) with 17β-estradiol. Intriguingly, estrogen delayed re-epithelialization in these animals without obviously influencing day 3 wound areas (Figure 1, G–I). This suggests that hair follicles do not contribute significantly to estrogenic inhibition of re-epithelialization but may play roles in additional repair processes that estrogen controls.
As others have previously shown,10 healing was significantly delayed in hr/hr mice relative to controls (Figure 1, B and H).
We subsequently found that three concentrations of 17β-estradiol did not influence the migration of scratch-wounded epidermal keratinocytes cultured from male mice (Figure 1J), or the proliferation of such cells (Figure 1K), suggesting that the inhibitory effect of estrogen in vivo is realized indirectly or via an alternative mechanism.
The effectiveness of our in vivo estrogen treatment protocol was determined by enzyme immunoassay of isolated sera (Table 3).
Table 3.
Serum 17β-Estradiol Levels following Systemic Replacement
| MF1
|
hr/hr
|
|||
|---|---|---|---|---|
| CSX | CSX + E2 | CSX | CSX + E2 | |
| 17β-Estradiol (ng/ml) | 18.15 (±6.10) | 63.21 (±9.48)* | 19.27 (±4.89) | 118.8 (±50.6)† |
Data are presented as mean (±SD), measured 3 days post-wounding. Statistical significance was determined by unpaired Student’s t-tests:
P < 0.01;
P < 0.05 (versus CSX). n = 5 per treatment group.
Estrogen Has Minimal Influence on Key Wound Inflammatory Parameters in Male Mice
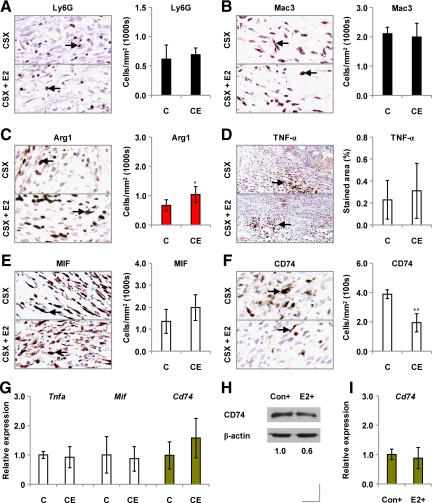
In an attempt to identify the mechanisms by which systemically administered estrogen impairs healing in castrated male mice, we examined its effects on wound inflammation. We found day 3 wound numbers of Ly6G-positive neutrophils to be comparable in estrogen-treated and untreated mice (Figure 2A). Similarly, day 3 wound Mac3-positive macrophage population sizes (Figure 2B) were unaffected by estrogen, although greater numbers of arginase 1-positive (alternatively activated (AA)) macrophages infiltrated the wounds of estrogen-treated animals than those of untreated controls (Figure 2C).
Figure 2.
Effects of chronic 17β-estradiol on wound inflammation in castrated mice. A: Immunostaining for the neutrophil marker Ly6G, and overall neutrophil numbers, in day three wounds from castrated (CSX, CX) MF1 mice, some treated with 17β-estradiol (E2) from days −14 to +3 (CSX +E2, CXE). B: Immunostaining for the macrophage marker Mac3, and numbers of macrophages, in day three wounds from E2-treated CSX MF1 mice. C: Immunostaining for arginase 1, a marker of AA macrophages, and day three wound numbers of arginase 1-positive cells, in E2-treated CSX MF1 mice. Immunostaining for TNF-α (D), MIF (E), and CD74 (F) in day three wounds from E2-treated CSX mice. G: Day three wound expression of the Tnfa, Mif, and Cd74 genes, determined by qPCR. H: Cellular levels of CD74 protein in peritoneal macrophages activated with LPS (+) for 2 hours (Con) and then treated with 100 nmol/L E2 or left untreated (Con) for a further three hours (immunoblotting). I: Expression of the Cd74 gene in control and 100 nmol/L E2-treated macrophages (qPCR). Statistical significance (C and F) was determined by unpaired Student’s t-tests: *P < 0.05; **P < 0.01. n = 5 per treatment group. Data are presented as mean ± SD Arrows (A–F) identify cell immunostaining. Original magnification: ×200 (A–C, E and F); ×100 (D).
Local levels of the proinflammatory cytokine TNF-α did not differ significantly between estrogen-treated mice and controls (Figure 2D).
Because estrogen exerts much of its influence on healing in female mice through its regulation of MIF expression,19 we proceeded to study its effects on MIF and CD74, a putative MIF receptor.20 Although treatment with 17β-estradiol did not influence day 3 wound MIF levels (Figure 2E), it did elicit a reduction in wound numbers of CD74-positive cells (Figure 2F), suggesting that estrogens may dampen the response to MIF in males. Having previously shown that healing in castrated male MIF null mice is unresponsive to exogenous MIF,3 our present findings reinforce our conclusion that MIF, so detrimental to repair in females, is of minimal influence in males.
In further studies, exogenous 17β-estradiol did not influence wound expression of the Tnfa, Mif, or Cd74 genes (Figure 2G). And, although it tended to decrease levels of CD74 protein in peritoneal macrophages (Figure 2H), 17β-estradiol (at a concentration of 100 nmol/L) did not modulate macrophage Cd74 gene expression (Figure 2I).
Collectively, these data suggest that it is highly unlikely that, in males, estrogens act to increase wound-associated inflammation (the observed increase in AA macrophage numbers would, if anything, be expected to reduce the magnitude of the inflammatory response), but instead retard healing through some alternative mechanism.
Skin Architecture in Estrogen-Treated Mice
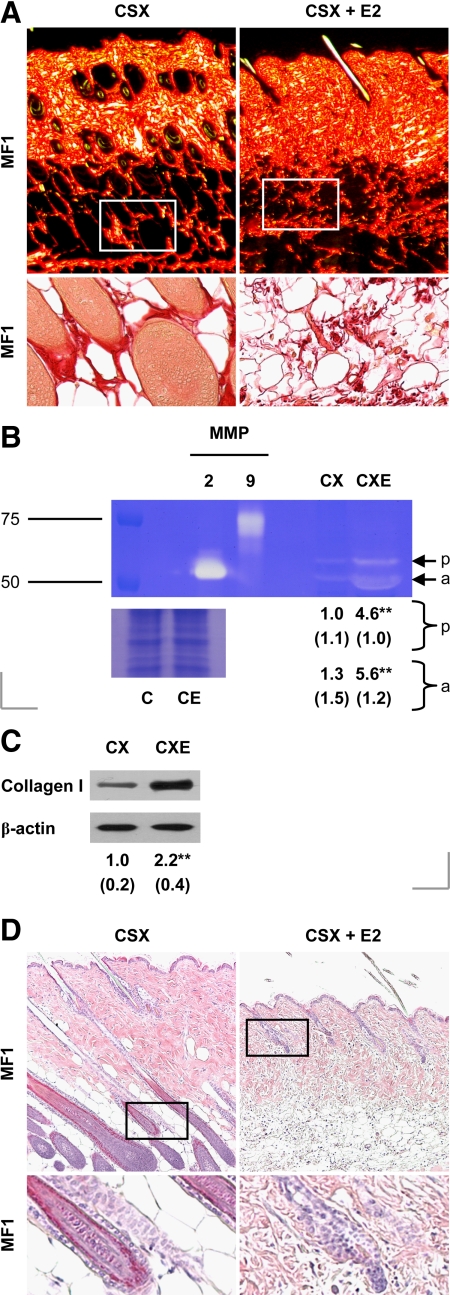
Previous studies have demonstrated the profound influence of estrogens on skin structure.21 We investigated whether and how this contributes to delayed repair in estrogen-treated mice. Chronic estrogen treatment did not greatly influence epidermal thickness in the MF1 mice used in this study (Table 4). By contrast, it significantly reduced the thicknesses of both the hypodermis and dermis (Table 4). Moreover, estrogen profoundly altered the appearance of the dermis: the collagen of the lower dermis was highly disorganized in estrogen-treated animals (Figure 3A).
Table 4.
Thicknesses of Individual Skin Layers in Estrogen-Treated MF1 Mice
| Thickness (μm)
|
||
|---|---|---|
| CSX | CSX + E2 | |
| Epidermis | 21 (±4) | 22 (±7) |
| Dermis | 538 (±32) | 450 (±19)* |
| Hypodermis | 643 (±112) | 396 (±200)† |
Data are presented as mean (±SD). Statistical significance was determined by unpaired Student’s t-tests:
P < 0.01;
P < 0.05 (versus CSX). n = 5 per treatment group.
Figure 3.
Changes in unwounded skin following chronic estrogen treatment. A: Picrosirius red-stained skin from castrated (CSX, CX) MF1 mice, some treated with 17β-estradiol (E2) from days −14 to +3 (CSX + E2, CXE) [upper panels, images taken under plane-polarized white light; lower panels, unpolarized light]. B: Gelatin zymogram of total skin protein isolated from CSX MF1 mice, some treated chronically with E2 [scale: RMM in kDa; p, pro-MMP-2; a, active MMP-2] (loading control: Coomassie-stained total skin protein). C: Skin type I collagen levels, determined by immunoblotting. D: H&E-stained skin from CSX MF1 mice, some treated chronically with E2, indicating hair cycle phase (longitudinal sections). Lower panels (A and D): magnifications of the indicated region of the corresponding upper panel. Statistical significance (B and C) was determined by unpaired Student’s t-tests: **P < 0.01. n = 5 per treatment group. Data are presented as mean ± SD. Original magnification: ×80 (A, upper panel); ×200 (A, lower panel); ×100 (D).
In our search for potential explanations, we noted greater skin MMP-2 activity in estrogen-treated mice relative to controls (Figure 3B; also Supplemental Figure 1, see http://ajp.amjpathol.org). However, we found overall cutaneous levels of type I collagen to be significantly increased as a result of estrogen treatment (Figure 3C).
It should be noted also that the hair cycles in untreated and estrogen-treated animals were not synchronized: on day 3 postwounding, the follicles in untreated mice were in anagen phase; those in estrogen-treated animals in telogen (Figure 3D). Because we and others have implicated hair follicles in the response to wounding, this last finding may partly account for estrogen’s effects on healing.
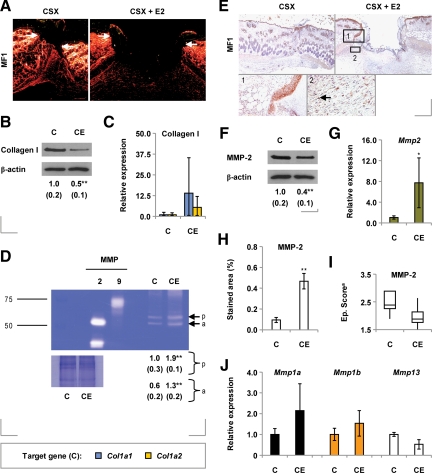
Effects of Estrogen on Wound Collagen Levels
After defining estrogen-invoked changes in the patterns of collagen distribution in unwounded skin, we sought to determine whether wound collagen profiles were likewise altered in estrogen-treated mice. We found that day 3 wound collagen I levels were reduced in estrogen-treated animals relative to controls (Figure 4, A and B; also Supplemental Figure 2A, see http://ajp.amjpathol.org). This response apparently did not stem from a prior effect on collagen gene expression: there was a trend toward increased wound levels of the mRNA species encoding the α1 and α2 chains of collagen I in estrogen-treated mice (Figure 4C). It was instead accompanied by increased activity of MMP-2 and several unidentified gelatinases of greater molecular weight (Figure 4D; also Supplemental Figure S2B, see http://ajp.amjpathol.org).
Figure 4.
Wound collagen deposition and proteolysis in mice treated chronically with estrogen. A: Picrosirius red-stained day three wounds from castrated (CSX, CX) MF1 mice, some treated with 17β-estradiol (E2) from days −14 to +3 (CSX + E2, CXE) [images taken under plane-polarized white light]. B: Day three wound type I collagen levels, determined by immunoblotting. C: Expression of the genes encoding the α1 (Col1a1) and α2 (Col1a2) chains of type I collagen in day three wounds (qPCR). D: Gelatin zymogram of total day three wound protein isolated from CSX MF1 mice, some treated chronically with E2 [scale: RMM in kDa; p, pro-MMP-2; a, active MMP-2]. E: MMP-2 immunoreactivity in day three wounds from CSX mice, some treated chronically with E2 [1: the neoepidermis; 2: inflammatory cells]. F: Overall day three wound MMP-2 levels (immunoblotting). G: Expression of the Mmp2 gene in day three wounds (qPCR). H: Granulation tissue MMP-2 immunostaining in day three wounds from CSX mice treated chronically with E2. I: Neoepidermal MMP-2 levels in CSX mice treated chronically with E2 [data are presented as a box-and-whisker plot (aCSX group: Q1 = 2.25, median = 2.38, Q3 = 2.88; CSX + E2: Q1 = 1.75, median = 1.88, Q3 = 2.13). J: Day three wound expression of the mRNA species encoding MMP-1 (mmp1a, mmp1b) and MMP-13. Statistical significance (B, D, F, G, and H) was determined by unpaired Student’s t-tests: *P < 0.05; **P < 0.01. n = 5 per treatment group. Data are presented as mean ± SD Arrows define the wound margins (A) and identify cell immunostaining (E). Original magnification: ×40 (A, E, upper panels); ×200 (E, lower panels).
In day 3 wounds, MMP-2 protein was highly expressed by neoepidermal keratinocytes and granulation tissue cells bearing a macrophage-like morphology (Figure 4E). Surprisingly, overall wound MMP-2 protein levels were lower in estrogen-treated mice than in controls (Figure 4F; also Supplemental Figure 2C, see http://ajp.amjpathol.org), despite estrogenic induction of wound MMP-2 mRNA levels (Figure 4G). Further analysis showed that, although granulation tissue (inflammatory cell) MMP-2 protein levels were increased in estrogen-treated animals (Figure 4H), neoepidermal MMP-2 levels tended to decrease (Figure 4I).
Because collagenase enzymes perform the initial digestion of native collagen, we examined the effects of estrogen on wound expression of the collagenase-encoding genes Mmp1a, Mmp1b and Mmp13. None was significantly influenced by estrogen (Figure 4J).
Collectively, these data suggest that reduced wound collagen accumulation in estrogen-treated mice may result from increased wound MMP-2 activity, which itself primarily stems from an increase in granulation tissue protein levels.
Estrogens May Alter the Balance between Wound Activators and Inhibitors of MMP-2
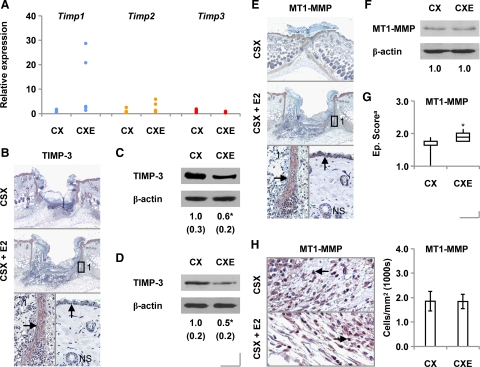
Having identified MMP-2 as a candidate effector of delayed healing in estrogen-treated male mice, we next attempted to evaluate the potential involvement of MMP activators and inhibitors in the response to estrogen. We found no change in overall wound expression of the genes encoding TIMPs 1, 2, or 3 in estrogen-treated mice (Figure 5A), although differences were observed when individual TIMP signals were normalized to those for epidermal markers (Supplemental Figure 3, see http://ajp.amjpathol.org), which suggests that estrogens may alter keratinocyte TIMP expression.
Figure 5.
Estrogen’s effects on wound levels of MMP activators and inhibitors. A: Expression of the genes encoding TIMP-1, TIMP-2, and TIMP-3 in day three wounds from castrated (CSX, CX) MF1 mice, some treated with 17β-estradiol (E2) from days −14 to +3 (CSX, CXE), determined by qPCR. B: TIMP-3 immunostaining in day three wounds and normal skin (NS) [1: the neoepidermis]. Normal skin (C) and day three wound (D) TIMP-3 protein levels (immunoblotting) (statistical significance tested by unpaired Student’s t-test: *P < 0.05). E: MT1-MMP immunostaining in day three wounds and normal skin [1: the neoepidermis]. F: Day three wound MT1-MMP levels (immunoblotting). G: Neoepidermal levels of MT1-MMP in CSX mice treated chronically with E2 [data are presented as a box-and-whisker plot (aCSX group: Q1 = 1.63, median = 1.63, Q3 = 1.75; CSX + E2: Q1 = 1.75, median = 1.88, Q3 = 2.00) (Mann-Whitney U-test: *P < 0.05). H: Granulation tissue MT1-MMP immunostaining and numbers of MT1-MMP-expressing cells, in CSX mice treated chronically with E2. n = 5 per treatment group. Data are presented as mean ± SD. Arrows (B, E, and H) identify cell immunostaining. Original magnification: ×40 (B and E, upper and middle panels); ×200 (B and E, lower panels, H).
Nonetheless, we decided to explore TIMP-3 further. We specifically immunolocalized TIMP-3 protein to the wound neoepidermis (Figure 5B), as others have done in acute human wounds.22 We subsequently determined that overall levels of TIMP-3 protein were considerably reduced in normal skin (Figure 5C) and day 3 wounds (Figure 5D) in estrogen-treated animals.
MMP-2 activation may be increased through either loss of TIMP-323 or elevated expression of MT1-MMP,24 which, unlike other MT-MMP subfamily members (MT2-MMP and MT3-MMP), is highly expressed in rodent wounds.25 We found MT1-MMP also to be strongly expressed by the neoepidermis in day 3 wounds (Figure 5E). Subsequent immunoblot analysis revealed overall wound MT1-MMP levels to be comparable between estrogen-treated and control mice (Figure 5F), although neoepidermal MT1-MMP levels were significantly increased as a consequence of estrogen treatment (Figure 5G).
Unlike TIMP-3, MT1-MMP was additionally immunolocalized to infiltrating inflammatory cells in the granulation tissue of day 3 wounds (Figure 5H). Site-specific measurements revealed overall wound numbers of MT1-MMP-expressing inflammatory cells to be comparable in control and estrogen-treated mice (Figure 5H).
Effects of Estrogen on the Protease Inhibitor Balance in Vitro
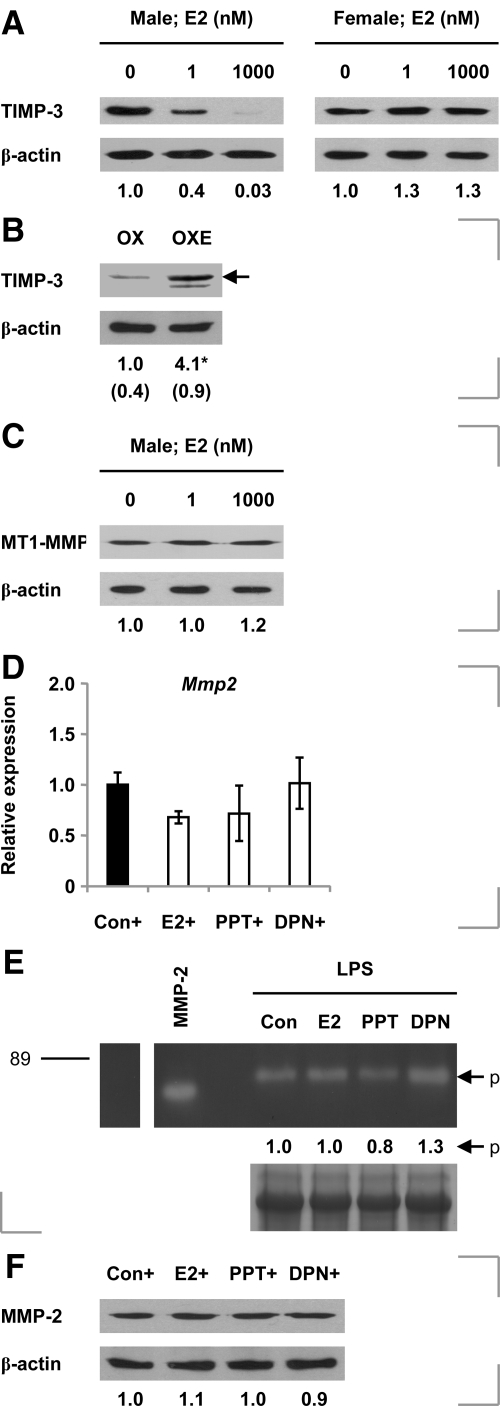
In an attempt to delineate the mechanisms by which estrogen apparently alters MMP-2 activity and TIMP-3 levels in vivo, we tested the effects of 17β-estradiol and receptor-selective agonists on isolated cells. We found that 17β-estradiol dose-dependently reduced TIMP-3 levels in keratinocytes derived from male but not female mice (Figure 6A). Interestingly, exogenous estrogen was subsequently shown to increase overall day 3 wound levels of TIMP-3 protein in ovariectomized female mice (Figure 6B).
Figure 6.
In vitro effects of estrogens on keratinocyte and macrophage protease/protease inhibitor levels. A: TIMP-3 levels in keratinocytes (from male and female neonates) treated with 1 or 1000 nmol/L E2 or left untreated, for 24 hours (determined by immunoblotting). B: Levels of TIMP-3 in day three wounds from ovariectomized (OX) mice, some treated with E2 from days −14 to +3 (OXE) (immunoblotting) (statistical significance tested by unpaired Student’s t-test: *P < 0.05). C: Levels of MT1-MMP protein in E2-treated male keratinocytes (immunoblotting). D: Expression of the Mmp2 gene in peritoneal macrophages activated with LPS (+) for 2 hours and then treated with 100 nmol/L 17β-estradiol (E2), 1 μmol/L PPT, or 1 μmol/L diarylpropionitrile (DPN), or left untreated (Con), for a further three hours (qPCR). E: Gelatin zymogram of total protein isolated from LPS-activated macrophages [scale: RMM in kDa; p, pro-MMP-2]. F: Macrophage levels of MMP-2 (immunoblotting); n = 5 per treatment group. Data are presented as mean ± SD.
17β-Estradiol failed to influence levels of MT1-MMP in keratinocytes obtained from male mice (Figure 6C) or MMP-2 levels in male or female cells (Supplemental Figure 4A, see http://ajp.amjpathol.org).
The mechanism underpinning estrogen’s induction of TIMP3 in male keratinocytes is seemingly nontranscriptional (Supplemental Figure 4B, see http://ajp.amjpathol.org). Expression of neither Timp1 nor Timp2 mRNA was estrogen-responsive in male cells (Supplemental Figure 4C, see http://ajp.amjpathol.org).
In LPS-activated peritoneal macrophages, neither 17β-estradiol nor the ER-α agonist PPT nor the ER-β agonist diarylpropionitrile influenced expression of the Mmp2 gene (Figure 6D), MMP-2 activity (Figure 6E), or cellular MMP-2 protein levels (Figure 6F). This suggests that estrogenic induction of inflammatory cell MMP-2 immunoreactivity observed in vivo occurs indirectly.
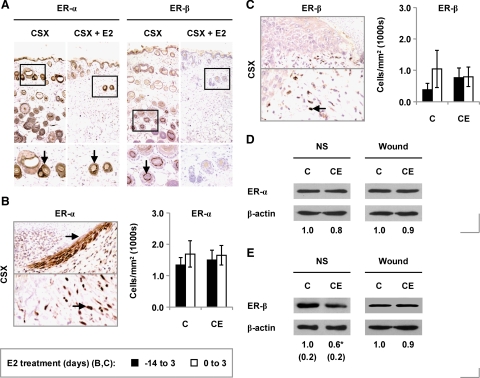
ER Expression in Skin and Wound Tissue in Castrated Male Mice
To determine whether altered receptor expression might contribute to the observed responses to estrogen, we next examined the effects of 17β-estradiol on cutaneous expression of the ERs. In unwounded skin, ER-α was strongly expressed in hair follicles and sebaceous glands (Figure 7A). The epidermis displayed weaker expression. ER-β expression was restricted to specific cells in the hair follicles (Figure 7A) in castrated mice; in estrogen-treated animals, ER-β expression was not detected immunohistochemically.
Figure 7.
ER expression in male mice treated chronically with estrogen. A: Immunostaining for ER-α and ER-β in unwounded skin from castrated (CSX, CX) MF1 mice, some treated with 17β-estradiol (E2) from days −14 to +3 (CSX +E2, CXE). B: Immunostaining for ER-α (upper panel, wound edge epidermis; lower panel, infiltrating inflammatory cells), and day three wound numbers of ER-α-expressing cells, in CSX MF1 mice treated chronically with E2. C: Immunostaining for ER-β (upper panel, epidermis; lower panel, inflammatory cells), and day three wound numbers of ER-β-expressing cells, in E2-treated CSX MF1 mice. D: Overall ER-α protein levels in normal skin (NS) and day three wounds (immunoblotting). E: Overall ER-β protein levels in normal skin and day three wounds (immunoblotting) (statistical significance tested by unpaired Student’s t-test: *P < 0.05). Statistical significance (B and C) was tested by two-way analysis of variance. n = 5–9 per treatment group. Data are presented as mean ± SD. Arrows (B and C) identify cell immunostaining. Original magnification: ×100 (A, upper panels); ×400 (A, lower panels); ×200 (B and C).
In day 3 wounds, ER-α was expressed by the proliferating epidermis and infiltrating inflammatory cells (Figure 7B) and ER-β exclusively by inflammatory cells (Figure 7C). Overall day 3 wound numbers of ER-α- and ER-β-expressing inflammatory cells were not affected by chronic or acute administration of 17β-estradiol (Figure 7, B and C). However, while overall levels of ER-α protein were comparable between estrogen-treated mice and controls in intact and wounded skin (Figure 7D), estrogen reduced overall ER-β levels in unwounded skin (Figure 7E).
Collectively, these findings suggest that altered ER expression may contribute to estrogen’s effects on normal skin but not those on healing acute wounds.
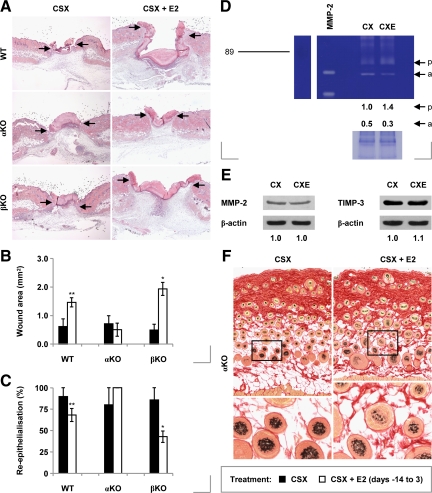
Estrogen Primarily Inhibits Repair by Signaling via ER-α
In an attempt to determine the relative influence of individual ER isoforms on healing, we administered 17β-estradiol to castrated ER-α and ER-β null mice. We found that, although estrogen inhibits re-epithelialization and delays repair in ER-β null mice, it has no measurable effect on these same parameters in ER-α null mice (Figure 8, A–C). This strongly suggests that estrogen’s inhibitory signals are transduced by ER-α homodimers.
Figure 8.
Healing in ER null mice treated chronically with 17β-estradiol. A: H&E-stained day three wounds from castrated (CSX, CX) wild-type (WT), ER-α knockout (αKO), and ER-β knockout (βKO) mice, some treated with 17β-estradiol (E2) from days −14 to +3 (CSX + E2, CXE). B: Day three wound areas in wild-type and ER null mice (statistical significance tested by two-way analysis of variance (P < 0.001, influence of genetic background; P < 0.001, influence of E2), followed by Bonferroni-corrected Student’s t-tests: *P < 0.05; **P < 0.01 (versus CSX)). C: Re-epithelialization of day three wounds in wild-type and ER null mice (Bonferroni-corrected pairwise Mann-Whitney U-tests: *P < 0.05; **P < 0.01 (versus CSX)). D: Gelatin zymogram of total day three wound protein isolated from CSX αKO mice, some treated chronically with E2 [scale: RMM in kDa; p, pro-MMP-2; a, active MMP-2]. E: Day three wound levels of MMP-2 and TIMP-3 in untreated and E2-treated CSX αKO mice (immunoblotting). (F) Picrosirius red-stained skin from CSX αKO mice, some treated chronically with E2 [images taken under unpolarized white light; lower panels: magnifications of the indicated region of the corresponding upper panel]. n = 5–8 per treatment group. Data are presented as mean ± SD. Arrows (A) define the wound margins. Original magnification: ×40 (A); ×100 (F, upper panels); ×200 (F, lower panels).
This conclusion was substantiated by our subsequent observations that several of the estrogenic responses we observed in wild-type mice do not occur in ER-α null animals. These include induction of MMP-2 activity in normal skin (Supplemental Figure 5A, see http://ajp.amjpathol.org) and day 3 wounds (Figure 8D), repression of overall day 3 wound MMP-2 and TIMP-3 protein levels (Figure 8E), and effects on hair follicle cycling (Figure 8F). Moreover, induction of neoepidermal MT1-MMP protein is prevented (Supplemental Figure 5, B and C, see http://ajp.amjpathol.org); the increase in the wound AA macrophage population reversed (Supplemental Figure 5D, see http://ajp.amjpathol.org); and repression of wound CD74 levels blocked (Supplemental Figure 5E, see http://ajp.amjpathol.org).
These data all relate to “chronic” (prolonged) estrogen treatment. Intriguingly, when administered at the time of wounding (“acute” treatment), 17β-estradiol did not influence healing in wild-type, ER-α null or ER-β null mice (Supplemental Figure 6, see http://ajp.amjpathol.org).
These results suggest that the timing and duration of estrogen treatment are critical to the nature and scale of the resultant healing response. Interestingly, the same is true in ovariectomized female mice (Supplemental Figure 7, see http://ajp.amjpathol.org). Day 3 wounds were smaller, re-epithelialization further advanced, and local macrophage populations reduced in mice treated with 17β-estradiol from the time of ovariectomy than in animals treated with estrogen from the time of wounding (Supplemental Figure 7, A–D, see http://ajp.amjpathol.org). These differences were not, intriguingly, accompanied by a significant difference in wound levels of MIF (Supplemental Figure 7E, see http://ajp.amjpathol.org), the estrogen-controlled effector of impaired wound healing in ovariectomized mice.19,26
Discussion
Historical reports of sex differences in skin wound healing, especially in the elderly, prompted us to conclude that endogenous sex steroids may perform vital roles in repair processes. Our preliminary studies identified estrogens and androgens as being, respectively, stimulators and inhibitors of repair in females and males.1,5 We subsequently discovered that sex steroids mask fundamental underlying differences in the ways that males and females heal acute wounds.3 These differences include marked dimorphism in the responses to MIF (which inhibits repair in females only) and testosterone (an inhibitor of repair in males but not females).
We here present a detailed analysis of the effects of estrogens on healing in male mice (Figure 9, A and B). We report that systemic 17β-estradiol treatment delays both wound re-epithelialization and the progressive reduction in incisional wound areas in castrated males, in which estrogens and androgens circulate at negligible levels. Estrogen similarly impeded healing in intact animals. These findings were surprising for several reasons. First, similar estrogen treatment in female mice accelerated re-epithelialization.27 Moreover, elderly males responded positively to estrogen applied to the edges of excisional wounds.6
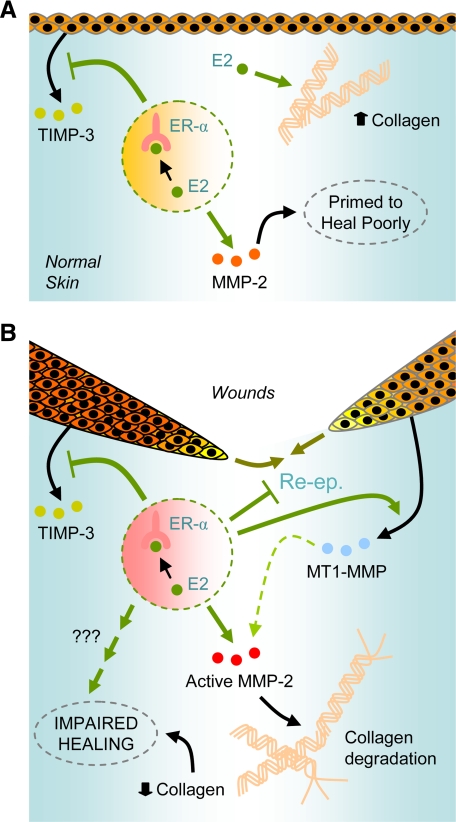
Figure 9.
Proposed mechanisms for the impairment of wound healing by estrogens. A: Although it increases overall cutaneous collagen levels, 17β-estradiol (E2), acting via ER-α, respectively, represses and induces TIMP-3 and MMP-2 in unwounded skin, which may predispose it to heal poorly following subsequent injury. B: In acute wounds, E2 signals via ER-α to delay wound re-epithelialization (Re-ep.) and to respectively repress and induce keratinocyte expression of TIMP-3 and MT1-MMP. These responses may underscore the increased local activation of MMP-2, which in turn would be expected to contribute to reduced local accumulation of neomatrix proteins including collagens.
It appears that the timing/duration and method of delivery are critical to the nature of the response to estrogen treatment. In male mice, estrogen treatment from 2 weeks before wounding greatly worsened healing; treatment from the day of wounding had little effect. In ovariectomized females, chronic estrogen treatment improved healing to a greater extent than did acute treatment. These data suggest that the success of any future estrogen-based healing therapies will depend on the careful optimization of dosing and delivery.
The sex difference in the effect of systemic estrogen treatment on wound re-epithelialization may partly be explained by a fundamental difference in the response of keratinocytes to estrogens: cells cultured from female mice migrate more rapidly following treatment with 17β-estradiol28; those from male donors were unresponsive to estrogen in the same assay.
In an effort to explain the inhibitory effects of estrogens on wound re-epithelialization in males, we probed the potential involvement of hair follicles, which were recently reported to benefit healing,10 and whose function is strongly influenced by estrogens.9 Although we showed that healing is impaired in untreated hr/hr mice relative to wild-type mice, we also found re-epithelialization to be comparably impaired in estrogen-treated hr/hr and wild-type animals. This suggests that cycling hair follicles are not critical to the inhibition of re-epithelialization by estrogens. Nonetheless, our observations that 17β-estradiol 1) failed to increase day 3 wound areas in hr/hr mice (as it did in wild-type animals) but 2) reversed castration’s arrest of the hair cycle in the anagen phase in wild-type animals suggest that hair follicles may play some role in the response to estrogens.
In female mice, estrogen is believed to facilitate repair by constraining local expression of MIF and by limiting the influx of macrophages and neutrophils.6,26 We have here shown that 17β-estradiol has little influence over wound inflammatory responses in male mice. Overall wound macrophage and neutrophil numbers and MIF and TNF-α levels were similar in estrogen-treated mice and controls. Intriguingly, 17β-estradiol significantly increased wound numbers of AA macrophages, as it was previously shown to do in females.29 This would not, however, be expected to inhibit healing because AA macrophages up-regulate proteins such as arginase I, the ultimate products of whose actions stimulate repair-promoting functions including cell proliferation and the biosynthesis of extracellular matrix proteins.30
Our failure to identify a profound effect on acute inflammation led us to hypothesize that prolonged exposure to exogenous estrogens may, in some way, prime the skin to repair itself poorly following subsequent insult. We found that dermal architecture was influenced by chronic estrogen treatment. Notably, the collagen of the lower dermis was highly disorganized in estrogen-treated mice. And although we did, like others before us,8 find that estrogen increases overall skin collagen content, it also increased overall MMP-2 activity in unwounded skin.
MMP-2 is implicated in the pathogenesis of chronic leg31 and diabetic foot32 ulceration and in the impairment of healing by aging15 and androgens,33 while limiting the activity of MMP-2 (and MMP-9) has been proposed as a mechanism by which healing might be improved.34 We thus inferred that estrogens might create a degradative environment in the dermis that may contribute to delayed repair following subsequent injury, and indeed, we found that the similar induction of MMP-2 activity in day 3 wounds in estrogen-treated animals was accompanied by reduced accumulation of collagen.
Estrogen has been shown to induce expression of MMP-2 in both mesangial35 and vascular smooth muscle cells.36 The authors of the latter study identified three putative half-palindromic estrogen response elements in the human MMP2 gene and others have identified additional estrogen response elements (some functional) (summarized in Ref. 37).
Although the parallel induction of MMP-2 mRNA in the wounds of estrogen-treated mice suggested that increased MMP-2 activity might have a transcriptional basis, the surprising reduction in overall wound MMP-2 protein levels implies otherwise. In an attempt to explain this apparent paradox, we highlighted an apparent shift in the spatial pattern of MMP-2 protein expression in estrogen-treated animals, with neodermal (ie, inflammatory cell) levels being increased (relative to controls) and neoepidermal production tending to fall. Our finding that 17β-estradiol did not influence the expression of MMP-2 in isolated macrophages suggests that effects on MMP-2 levels in vivo are realized indirectly.
We subsequently uncovered evidence that estrogen may increase wound MMP-2 activity by altering the balance between inhibitors and activators of MMPs. 17β-estradiol decreased expression of TIMP-3, a key inhibitor of MMP-2 activation,23 in unwounded skin and day 3 wounds and additionally suppressed keratinocyte TIMP-3 production in vitro.
Interestingly, this last response was observed only in cells derived from male mice and not females. Moreover, 17β-estradiol increased wound levels of TIMP-3 protein in ovariectomized female mice, in which healing is enhanced by exogenous estrogen.19,27,28 This suggests that sex-specific effects on TIMP3 expression may contribute to the noted sex dimorphism in the effects of estrogens on wound healing. That TIMP-3 itself is important for efficient repair is implied by its absence from poorly healing venous leg ulcers.38
Estrogen treatment also increased neoepidermal expression of MT1-MMP, a key activator of MMP-2.24 This, along with the down-regulation of TIMP3, may combine to increase the local activation of MMP-2 in males, thereby increasing wound proteolysis and interfering with the normal accumulation of neomatrix proteins including type I collagen.
That the TIMP-3 and MT1-MMP responses were specific to the neoepidermis is intriguing in light of the observed slowing of re-epithelialization by estrogens in vivo. It appears that effects on the epidermis contribute significantly to impaired healing in male rodents treated with estrogens. In the case of the TIMP3 response, this appears, from our in vitro studies, to be a direct effect, although it may also involve interactions with stromal cells, as has been reported previously.39
It is worth adding that the selective genetic ablation of ER-α in keratin 14-expressing cells of the epidermis markedly improved wound healing in a group of castrated male mice (our unpublished observation).
Our identification of ER-α as the putative primary effector of estrogen’s inhibitory effects on skin healing in males—responsible for delayed re-epithelialization and increased MMP-2 activity—is highly intriguing since we have separately identified ER-β as the likely effector of enhanced repair in estrogen-treated females (our unpublished observation). A comparative study in humans found patterns of ER expression to be indistinguishable in males and females.40 We ourselves found wound ER expression to be unmodulated by exogenous estrogen. Collectively, these observations suggest that accessory factors expressed by wound cells may determine the precise nature of the response to estrogen in males and females. It should also be noted that aromatase is strongly expressed by hair follicles in unwounded male skin,41 which suggests that the inhibitory effects of androgens on healing in males may, in part, stem from their local conversion to estrogens.
Failure of 17β-estradiol to influence hair follicle cycling in ER-α null mice is in agreement with the findings of a previous study that identified ER-α as the specific mediator of estrogen’s effects in this context.42
In summary, we have shown that estrogen has a strong negative effect on wound healing in male mice. It greatly retards re-epithelialization and inhibits the accumulation of collagen in the neodermis, seemingly by inducing skin gelatinolytic activity. This in turn may reflect a sex-specific effect on wound TIMP-3 levels. It is likely that the inhibitory effect on re-epithelialization is signaled via ER-α because 1) it does not occur in ER-α null mice and 2) wound edge keratinocytes do not express ER-β.
Our previous findings raised a key question: given that circulating levels of androgens (apparently detrimental to healing) gradually decline in human males with increasing age,43,44 which factors underscore the concurrent deterioration in the quality of repair? Although it remains unclear whether or not systemic estrogen levels too change with age in males,43,44 it is worth noting that a recent study identified estrogen, and not aging per se, as the primary mediator of delayed healing in elderly males.7
Acknowledgments
We thank Helen Thomason and Nichola Cooper for their assistance with keratinocyte isolation and culture.
Footnotes
Address reprint requests to Matthew J. Hardman, Ph.D., Faculty of Life Sciences, University of Manchester, AV Hill Building, Oxford Road, Manchester M13 9PT, U.K. E-mail: matthew.j.hardman@manchester.ac.uk.
Supported by Wellcome Trust Senior Fellowship in Clinical Science grant GR064256MA.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Ashcroft GS, Mills SJ. Androgen receptor-mediated inhibition of cutaneous wound healing. J Clin Invest. 2002;110:615–624. doi: 10.1172/JCI15704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft GS, Kielty CM, Horan MA, Ferguson MW. Age-related changes in the temporal and spatial distributions of fibrillin and elastin mRNAs and proteins in acute cutaneous wounds of healthy humans. J Pathol. 1997;183:80–89. doi: 10.1002/(SICI)1096-9896(199709)183:1<80::AID-PATH1104>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Gilliver SC, Ruckshanthi JP, Hardman MJ, Nakayama T, Ashcroft GS. Sex dimorphism in wound healing: the roles of sex steroids and macrophage migration inhibitory factor. Endocrinology. 2008;149:5747–5757. doi: 10.1210/en.2008-0355. [DOI] [PubMed] [Google Scholar]
- Gilliver SC, Ashworth JJ, Mills SJ, Hardman MJ, Ashcroft GS. Androgens modulate the inflammatory response during acute wound healing. J Cell Sci. 2006;119:722–732. doi: 10.1242/jcs.02786. [DOI] [PubMed] [Google Scholar]
- Ashcroft GS, Dodsworth J, van Boxtel E, Tarnuzzer RW, Horan MA, Schultz GS, Ferguson MW. Estrogen accelerates cutaneous wound healing associated with an increase in TGF-β1 levels. Nat Med. 1997;3:1209–1215. doi: 10.1038/nm1197-1209. [DOI] [PubMed] [Google Scholar]
- Ashcroft GS, Greenwell-Wild T, Horan MA, Wahl SM, Ferguson MW. Topical estrogen accelerates cutaneous wound healing in aged humans associated with an altered inflammatory response. Am J Pathol. 1999;155:1137–1146. doi: 10.1016/S0002-9440(10)65217-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardman MJ, Ashcroft GS. Estrogen, not intrinsic aging, is the major regulator of delayed human wound healing in the elderly. Genome Biol. 2008;9:R80. doi: 10.1186/gb-2008-9-5-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son ED, Lee JY, Lee S, Kim MS, Lee BG, Chang IS, Chung JH. Topical application of 17β-estradiol increases extracellular matrix protein synthesis by stimulating TGF-α signaling in aged human skin in vivo. J Invest Dermatol. 2005;124:1149–1161. doi: 10.1111/j.0022-202X.2005.23736.x. [DOI] [PubMed] [Google Scholar]
- Ohnemus U, Uenalan M, Inzunza J, Gustafsson JA, Paus R. The hair follicle as an estrogen target and source. Endocr Rev. 2006;27:677–706. doi: 10.1210/er.2006-0020. [DOI] [PubMed] [Google Scholar]
- Langton AK, Herrick SE, Headon DJ. An extended epidermal response heals cutaneous wounds in the absence of a hair follicle stem cell contribution. J Invest Dermatol. 2008;128:1311–1318. doi: 10.1038/sj.jid.5701178. [DOI] [PubMed] [Google Scholar]
- Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. Effect of single and compound knockouts of estrogen receptors α (ERα) and β (ERβ) on mouse reproductive phenotypes. Development. 2000;127:4277–4291. doi: 10.1242/dev.127.19.4277. [DOI] [PubMed] [Google Scholar]
- Junqueira LCU, Bignolas G, Brentani RR. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J. 1979;11:447–455. doi: 10.1007/BF01002772. [DOI] [PubMed] [Google Scholar]
- Heussen C, Dowdle EB. Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal Biochem. 1980;102:196–202. doi: 10.1016/0003-2697(80)90338-3. [DOI] [PubMed] [Google Scholar]
- Murphy G, Willenbrock F, Ward RV, Cockett MI, Eaton D, Docherty AJ. The C-terminal domain of 72 kDa gelatinase A is not required for catalysis, but is essential for membrane activation and modulates interactions with tissue inhibitors of metalloproteinases. Biochem J. 1992;283:637–641. doi: 10.1042/bj2830637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft GS, Horan MA, Herrick SE, Tarnuzzer RW, Schultz GS, Ferguson MW. Age-related differences in the temporal and spatial regulation of matrix metalloproteinases (MMPs) in normal skin and acute cutaneous wounds of healthy humans. Cell Tissue Res. 1997;290:581–591. doi: 10.1007/s004410050963. [DOI] [PubMed] [Google Scholar]
- Yuspa SH, Harris CC. Altered differentiation of mouse epidermal cells treated with retinyl acetate in vitro. Exp Cell Res. 1974;86:95–105. doi: 10.1016/0014-4827(74)90653-3. [DOI] [PubMed] [Google Scholar]
- Hager B, Bickenbach JR, Fleckman P. Long-term culture of murine epidermal keratinocytes. J Invest Dermatol. 1999;112:971–976. doi: 10.1046/j.1523-1747.1999.00605.x. [DOI] [PubMed] [Google Scholar]
- Gilliver SC, Ruckshanthi JP, Hardman MJ, Zeef LA, Ashcroft GS. 5α-Dihydrotestosterone (DHT) retards wound closure by inhibiting re-epithelialization. J Pathol. 2009;217:73–82. doi: 10.1002/path.2444. [DOI] [PubMed] [Google Scholar]
- Hardman MJ, Waite A, Zeef L, Burow M, Nakayama T, Ashcroft GS. Macrophage migration inhibitory factor: a central regulator of wound healing. Am J Pathol. 2005;167:1561–1574. doi: 10.1016/S0002-9440(10)61241-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng L, Metz CN, Fang Y, Xu J, Donnelly S, Baugh J, Delohery T, Chen Y, Mitchell RA, Bucala R. MIF signal transduction initiated by binding to CD74. J Exp Med. 2003;197:1467–1476. doi: 10.1084/jem.20030286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall G, Phillips TJ. Estrogen and skin: the effects of estrogen, menopause, and hormone replacement therapy on the skin. J Am Acad Dermatol. 2005;53:555–568. doi: 10.1016/j.jaad.2004.08.039. [DOI] [PubMed] [Google Scholar]
- Vaalamo M, Leivo T, Saarialho-Kere U. Differential expression of tissue inhibitors of metalloproteinases (TIMP-1, -2, -3, and -4) in normal and aberrant wound healing. Hum Pathol. 1999;30:795–802. doi: 10.1016/s0046-8177(99)90140-5. [DOI] [PubMed] [Google Scholar]
- English JL, Kassiri Z, Koskivirta I, Atkinson SJ, Di Grappa M, Soloway PD, Nagase H, Vuorio E, Murphy G, Khokha R. Individual Timp deficiencies differentially impact pro-MMP- 2 activation. J Biol Chem. 2006;281:10337–10346. doi: 10.1074/jbc.M512009200. [DOI] [PubMed] [Google Scholar]
- Sato H, Takino T, Kinoshita T, Imai K, Okada Y, Stetler Stevenson WG, Seiki M. Cell surface binding and activation of gelatinase A induced by expression of membrane-type-1-matrix metalloproteinase (MT1-MMP). FEBS Lett. 1996;385:238–240. doi: 10.1016/0014-5793(96)00389-4. [DOI] [PubMed] [Google Scholar]
- Okada A, Tomasetto C, Lutz Y, Bellocq JP, Rio MC, Basset P. Expression of matrix metalloproteinases during rat skin wound healing: evidence that membrane type-1 matrix metalloproteinase is a stromal activator of pro-gelatinase A. J Cell Biol. 1997;137:67–77. doi: 10.1083/jcb.137.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft GS, Mills SJ, Lei K, Gibbons L, Jeong MJ, Taniguchi M, Burow M, Horan MA, Wahl SM, Nakayama T. Estrogen modulates cutaneous wound healing by downregulating macrophage migration inhibitory factor. J Clin Invest. 2003;111:1309–1318. doi: 10.1172/JCI16288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardman MJ, Emmerson E, Campbell L, Ashcroft GS. Selective estrogen receptor modulators accelerate cutaneous wound healing in ovariectomized female mice. Endocrinology. 2008;149:551–557. doi: 10.1210/en.2007-1042. [DOI] [PubMed] [Google Scholar]
- Emmerson E, Campbell L, Ashcroft GS, Hardman MJ. Unique and synergistic roles for 17β-estradiol and macrophage migration inhibitory factor during cutaneous wound closure are cell type specific. Endocrinology. 2009;150:2749–2757. doi: 10.1210/en.2008-1569. [DOI] [PubMed] [Google Scholar]
- Routley CE, Ashcroft GS. Effect of estrogen and progesterone on macrophage activation during wound healing. Wound Repair Regen. 2009;17:42–50. doi: 10.1111/j.1524-475X.2008.00440.x. [DOI] [PubMed] [Google Scholar]
- Hesse M, Modolell M, La Flamme AC, Schito M, Fuentes JM, Cheever AW, Pearce EJ, Wynn TA. Differential regulation of nitric oxide synthase-2 and arginase-1 by type 1/type 2 cytokines in vivo: granulomatous pathology is shaped by the pattern of l-arginine metabolism. J Immunol. 2001;167:6533–6544. doi: 10.4049/jimmunol.167.11.6533. [DOI] [PubMed] [Google Scholar]
- Wysocki AB, Staiano-Coico L, Grinnell F. Wound fluid from chronic leg ulcers contains elevated levels of metalloproteinases MMP-2 and MMP-9. J Invest Dermatol. 1993;101:64–68. doi: 10.1111/1523-1747.ep12359590. [DOI] [PubMed] [Google Scholar]
- Neely AN, Clendening CE, Gardner J, Greenhalgh DG. Gelatinase activities in wounds of healing-impaired mice versus wounds of non-healing-impaired mice. J Burn Care Rehabil. 2000;21:395–402. doi: 10.1097/00004630-200021050-00001. [DOI] [PubMed] [Google Scholar]
- Gilliver SC, Ruckshanthi JP, Atkinson SJ, Ashcroft GS. Androgens influence expression of matrix proteins and proteolytic factors during cutaneous wound healing. Lab Invest. 2007;87:871–881. doi: 10.1038/labinvest.3700627. [DOI] [PubMed] [Google Scholar]
- Santos MC, Souza AP, Gerlach RF, Tabchoury CM, Line SR. Inhibition of human gelatinases (matrix metalloproteinase-2 and matrix metalloproteinase-9) activity by zinc oxide: a possible mechanism to enhance wound healing. Br J Dermatol. 2001;145:854–855. doi: 10.1046/j.1365-2133.2001.04502.x. [DOI] [PubMed] [Google Scholar]
- Guccione M, Silbiger S, Lei J, Neugarten J. Estradiol upregulates mesangial cell MMP-2 activity via the transcription factor AP-2. Am J Physiol Renal Physiol. 2002;282:F164–169. doi: 10.1152/ajprenal.0318.2000. [DOI] [PubMed] [Google Scholar]
- Wingrove CS, Garr E, Godsland IF, Stevenson JC. 17β-Oestradiol enhances release of matrix metalloproteinase-2 from human vascular smooth muscle cells. Biochim Biophys Acta. 1998;1406:169–174. doi: 10.1016/s0925-4439(97)00097-5. [DOI] [PubMed] [Google Scholar]
- Bourdeau V, Deschênes J, Métivier R, Nagai Y, Nguyen D, Bretschneider N, Gannon F, White JH, Mader S. Genome-wide identification of high-affinity estrogen response elements in human and mouse. Mol Endocrinol. 2004;18:1411–1427. doi: 10.1210/me.2003-0441. [DOI] [PubMed] [Google Scholar]
- Agren MS, Eaglstein WH, Ferguson MW, Harding KG, Moore K, Saarialho-Kere UK, Schultz GS. Causes and effects of the chronic inflammation in venous leg ulcers. Acta Derm Venereol Suppl (Stockh) 2000;210:3–17. [PubMed] [Google Scholar]
- Sawicki G, Marcoux Y, Sarkhosh K, Tredget EE, Ghahary A. Interaction of keratinocytes and fibroblasts modulates the expression of matrix metalloproteinases-2 and -9 and their inhibitors. Mol Cell Biochem. 2005;269:209–216. doi: 10.1007/s11010-005-3178-x. [DOI] [PubMed] [Google Scholar]
- Thornton MJ, Taylor AH, Mulligan K, Al-Azzawi F, Lyon CC, O'Driscoll J, Messenger AG. The distribution of estrogen receptor β is distinct to that of estrogen receptor α and the androgen receptor in human skin and the pilosebaceous unit. J Investig Dermatol Symp Proc. 2003;8:100–103. doi: 10.1046/j.1523-1747.2003.12181.x. [DOI] [PubMed] [Google Scholar]
- Sawaya ME, Penneys NS. Immunohistochemical distribution of aromatase and 3B- hydroxysteroid dehydrogenase in human hair follicle and sebaceous gland. J Cutan Pathol. 1992;19:309–314. doi: 10.1111/j.1600-0560.1992.tb01367.x. [DOI] [PubMed] [Google Scholar]
- Movérare S, Lindberg MK, Faergemann J, Gustafsson JA, Ohlsson C. Estrogen receptor α, but not estrogen receptor β, is involved in the regulation of the hair follicle cycling as well as the thickness of epidermis in male mice. J Invest Dermatol. 2002;119:1053–1058. doi: 10.1046/j.1523-1747.2002.00637.x. [DOI] [PubMed] [Google Scholar]
- Gray A, Feldman HA, McKinlay JB, Longcope C. Age, disease, and changing sex hormone levels in middle-aged men: results of the Massachusetts Male Aging Study. J Clin Endocrinol Metab. 1991;73:1016–1025. doi: 10.1210/jcem-73-5-1016. [DOI] [PubMed] [Google Scholar]
- Feldman HA, Longcope C, Derby CA, Johannes CB, Araujo AB, Coviello AD, Bremner WJ, McKinlay JB. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002;87:589–598. doi: 10.1210/jcem.87.2.8201. [DOI] [PubMed] [Google Scholar]