Abstract
The current study identifies within the Th1 subtype two distinct CD4+ populations: those capable of transferring inflammatory autoimmunity and others that regulate its development by suppressing Th17 in an interferon (IFN)-γ-dependent manner. These CD4+IFN-γhighIL-4lowIL-10lowTGF-βlowFOXp3− cells in fact function as antigen-specific regulatory cells that restrain the development of autoimmunity by increasing the threshold of Th17 activation. We show that development of autoimmune conditions within the central nervous system is dependent on the Fas ligand-mediated apoptosis of these regulatory cells at early stages of disease. We also show that not only is the function of these cells IFN-γ dependent but also that stable over expression of IFN-γ in encephalitogenic CD4+ T cells redirects their biological function to become antigen-specific regulatory cells. This may also explain, in part, the pleiotropic role of IFN-γ in the regulation of autoimmunity, as previously observed by others.
Experimental autoimmune encephalomyelitis (EAE) is a T cell-mediated autoimmune disease of the central nervous system (CNS) that serves as an experimental model for multiple sclerosis. It is believed that antigen-specific effector CD4+ T cells that produce interleukin (IL)-17 (ie, Th17) initiate the inflammatory process, which is later propagated by IFN-γhighIL-4low Th1 cells.1,2,3,4,5 IFN-γ is a key cytokine that suppresses the selection and activity of Th17 cells.6 It is therefore thought that the dynamics of the autoimmune process includes the initiation of disease by Th17 and, later on, the propagation of the inflammatory process by Th1, which, via IFN-γ, suppresses and replaces Th17,7 thus making the Th17/Th1 ratio an important factor in the dynamics of disease.1
Thus far, much attention has been devoted to identifying regulatory T cells that restrain effector T cell functions. These include CD25+Foxp3+CD4+ T cells, which are likely to suppress effector cell functions nonspecifically,8 antigen-specific regulatory T cells that produce IL-10 (ie, Tr1),9,10,11 and those producing transforming growth factor (TGF)-β (ie, Th3).12 Antigen-specific regulatory T cells that selectively restrain Th17 have not been so far identified.
We have previously shown that Fas ligand (FasL) plays a dual role in regulating EAE. Targeted neutralization of this tumor necrosis factor (TNF) family member at early stages of disease prevented the development of disease, whereas its neutralization at later stages, when the activity of Th1 drives the pathogenesis of disease,1 aggravated its manifestation.13 The mechanistic basis of exacerbating disease most likely includes inhibition of apoptosis of Th1 cells at the autoimmune site.13 Nevertheless, understanding why early neutralization of FasL suppresses EAE remains elusive.
The affinity of TCR to major histocompatibility complex II-peptide complex during CD4+ T cell selection affects their biological properties. Whereas higher affinity of biding enhances the selection of very high IFN-γ-producing Th1 cells, low affinity binding would results in lower IFN-γ-producing Th1 cells.14 Those CD4+ T cells that produce higher levels of IFN-γ were found to be more susceptible to FasL-mediated apoptosis, in an IFN-γ-dependent manner, probably as a part of the natural regulation of T cell homeostasis.15 The current study shows that these antigen-specific T cells emerge during early stages of an autoimmune condition and suppress Th17 cells in attempting to block the development of disease. Once they undergo FasL-induced apoptosis the autoimmune condition develops. Therefore, rescuing these cells by anti-FasL antibodies (Abs) at early stages of the disease suppresses its development.
Materials and Methods
Mice
Six-week-old female C57BL/6 mice were purchased from Harlan (Jerusalem, Israel) and maintained under specific pathogen-free conditions in our animal facility. Breeders of IFN-γ−/− C57BL/6 and of Fas-deficient (lpr) and Fas ligand-deficient (gld) mice on the same background were purchased from The Jackson Laboratory (Bar Harbor, ME), from which our colonies were established under pathogen-free conditions. All animal studies were conducted according our approved protocol reviewed by the Technion (Technion, Haifa Israel) ethics committee for experiments in animals, according the National Institutes of Health guideline.
Peptides
Myelin oligodendrocyte glycoprotein (MOG)p35-55 was constructed by the Protein and Nucleic Acid (PAN) facility of the Beckman Center at Stanford University (Stanford, CA). After purification by high-performance liquid chromatography, the sequence was confirmed by amino acid analysis, and the correct mass was checked by mass spectroscopy. Purification of the peptide that was used in the current study was >95%.
Development of MOGp35-55-Specific CD4+ T Cell Lines
Development of MOGp35-55-specific T cell lines was performed by repeated antigen-specific selections according to protocol described in Ref. 16. Briefly, 6-week-old female C57BL/6 mice were immunized s.c. with 200 μl of an emulsion containing 800 μg of Mycobacterium tuberculosis H37Ra and 200 μg of MOGp35-55. On days 9 to 11, draining lymph nodes were harvested, and draining lymph node cells were cultured for 72 hours in stimulation medium containing Dulbecco’s modified Eagle’s medium, 5% FBS, 2 mmol/L mercaptoethanol, sodium pyruvate, minimal essential medium nonessential amino acids, and penicillin-streptomycin and supplemented with 50 μg/ml MOGp35-55 and 20 ng/ml recombinant murine IL-2 (R&D Systems, Minneapolis, MN) in a humidified 7.5% CO2 atmosphere at 37°C. After 72 hours, cells were washed, counted, and resuspended (1 × 106 T cell blasts/ml, ∼10 × 106 draining lymph node cells/ml) in full medium containing Dulbecco’s modified Eagle’s medium, 10% FBS, 2 mmol/L mercaptoethanol, sodium pyruvate, minimal essential medium nonessential amino acids, and penicillin-streptomycin, supplemented with 20 ng/ml recombinant murine IL-2 (R&D Systems). After 4 days, cells were collected, washed, and co-cultured with irradiated (3500 rad) splenocytes (as Ag-presenting cells) in the presence of 50 μg/ml MOGp35-55 for another 72 hours, followed by another cycle of rest and stimulation.
Induction of Active and Adoptively Transferred Disease
EAE was induced by immunizing mice with MOGp35-55/complete Freund’s adjuvant, as described by Tompkins et al,16 as follows: i) active EAE: mice were immunized s.c. with 200 μl of an emulsion containing 800 μg of M. tuberculosis H37Ra (Difco, Detroit, MI) and 200 μg of MOGp35–55 distributed over three spots on the flank. Each mouse additionally received 200 ng of pertussis toxin (List Biological Laboratories, Campbell, CA) in 200 μl of PBS i.p. on days 0 and 2 post immunization. ii) Transferred EAE: Female donor mice (6 to 10 weeks old) were subjected to active EAE induction as described above. Draining lymph nodes were harvested from donor mice after 7 to 11 days for in vitro stimulation, as described above. After 72 hours of incubation, cells were counted, washed, and resuspended (2.5 × 107 T cell blasts/ml; 10 to 15 × 107 lymph node cells/ml) in buffered salt solution. T cell blasts were differentiated from other lymph node cells by size under microscopic observation. Recipient mice received 200 ng of pertussis toxin in 200 μl of PBS i.p. on days 0 and 2.
EAE was scored as follows: 0, clinically normal; 1, flaccid tail; 2, hind limb paralysis; 3, total hind limb paralysis, accompanied by an apparent front limb paralysis; 4, total hind limb and front limb paralysis; and 5, death.
Cytokine Enzyme-Linked Immunosorbent Assay
IL-2, IL-4, IL-10, IL-12, IL-17, TNF-α, and IFN-γ enzyme-linked immunosorbent assay kits were purchased from BioLegend (San Diego, CA). The TGF-β enzyme-linked immunosorbent assay kit was purchased from BD Biosciences (Franklin Lakes, NJ).
Cell Separation
Spinal cords were digested for 4 hours with 300 U/ml type IV collagenase (Worthington Biochemical, Lakewood, NJ) in Dulbecco’s modified Eagle’s medium. Cells were then separated by Ficoll, washed, and stained.
Monoclonal Abs
Anti-FasL monoclonal Ab (mAb) used for both in vitro and in vivo neutralization was purchased from R&D Systems (clone 101626). Anti-IFN-γ mAb used for in vivo neutralization was also purchased from R&D Systems (clone H22).
Flow Cytometry
Flow cytometry analysis was conducted according to the protocol described in detail elsewhere.17 The AnnexinV/propidium iodide kit was purchased from Bender Medsystems (Vienna, Austria). For intracellular staining, the Cytofix/Cytoperm kit and anti-mouse IL-4, IL-10, IL-17 and IFN-γ Abs, as well as anti-CD4 mAb (clone L3T4) for gating on CD4+ cells, were all purchased from BD Biosciences. The FOXp3/CD4/CD25 flow cytometry kit was purchased from BioLegend.
Histopathology and Immunostaining
The lumbar spinal cord was dissected, fixed in 4% paraformaldehyde, dehydrated, and embedded in paraffin. Next, 5-μm-thick sections were stained with H&E. Each section was evaluated for tissue damage and mononuclear infiltration using the following scale: 0, no mononuclear cell infiltration; 1, 1 to 5 perivascular lesions per section with minimal parenchymal infiltration; 2, 5 to 10 perivascular lesions per section with parenchymal infiltration; and 3, >10 perivascular lesions per section with extensive parenchymal infiltration. The mean histological score ± SE was calculated for each treatment group. To analyze and compare demyelination and axonal degeneration, we have subjected the sections to a Luxol fast blue staining. To analyze and compare gliosis, we determined the proliferation of astrocytes in the damaged area of the CNS using an anti-glial fibrillary acidic protein (Serotec, Raleigh, NC). Immunostaining of T cells and macrophages were conducted using anti-CD3 (Serotec) and F4/80 (Serotec) mAb.
Terminal Deoxynucleotidyl Transferase-Mediated dUTP Nick-End Labeling Apoptosis Assay
Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling apoptosis assay was conducted on spinal cord sections using a commercially available kit (MBL International, Woburn, MA), according to the manufacturer’s protocol.
Construction of the Retroviral Vectors
A 1337-bp EcoRI-HpaI fragment of the IRES-EGFP region from pIRES2-EGFP vector (BD Clontech, Palo Alto, CA) was introduced to the Moloney murine leukemia virus-derived vector pLXSN (BD Clontech) to construct the pLXSN-IRES-EGFP vector. Full-length murine IFN-γ cDNA was purified from concanavalin A-activated splenocytes. The cDNA was then subcloned into the pLXSN-IRES-EGFP vector to construct the pLXSN-IFN-γ-IRES-EGFP vector. The retroviral vectors were transfected into the 293/E3 packaging cells with FuGENE6 transfection reagent (Roche Diagnostic Systems, Somerville, NJ), according to the manufacturer’s protocol.
Retroviral Gene Transfer to T Cells
Retroviral gene transfer to T cells was done using the method developed by Flugel et al,18,19 with minor modifications.17 Briefly, primary T lymphocytes from mice that were injected 9 days earlier with 200 μg of MOGp35-55/complete Freund’s adjuvant were cocultivated with 293/E3 packaging cells producing replication incompetent retroviruses. After 72 hours, the lymphocytes were separated from the packaging cells, and selection with 0.4 mg/ml G418 was started and maintained for the 2-week culture period.
Statistical Analysis
Statistical analysis was conducted accordingly.20,21,22,23 A two-tailed Student’s t-test was applied for statistical comparison of two groups, or where appropriate, the two-way analysis of variance, followed by Bonferroni’s posthoc test for multiple comparisons and a Mann-Whitney test for nonparametric data (EAE scoring). A value of P ≤ 0.05 was considered significant. In all figures significance appears as follows: *P < 0.05, **P < 0.01, and ***P < 0.005 versus control.
Results
Targeted Neutralization of FasL, at Early Stages of EAE, Suppresses the Disease by an IFN-γ-Dependent Mechanism
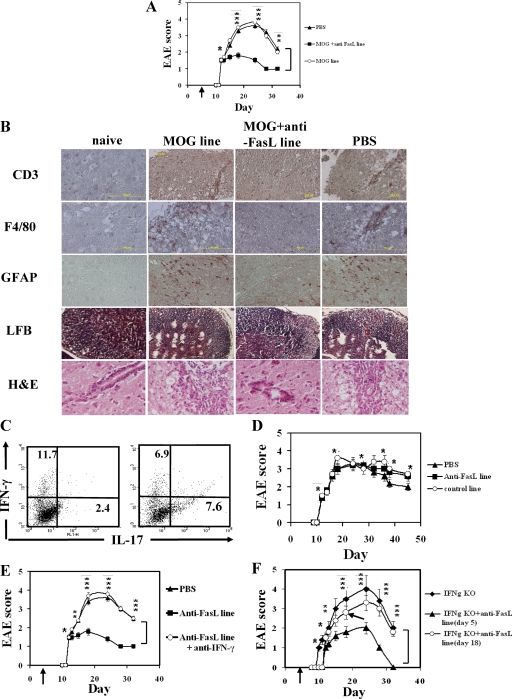
C57BL/6 mice were subjected to active induction of EAE and then were administered with anti-FasL neutralizing Abs (5, 7, and 9 days after active induction of disease, 300 μg/mouse) and monitored for development and progression of the disease (Figure 1A). In accordance with our previous observations in Lewis rats,13 these mice developed a significantly less severe form of disease (mean maximal score of 1.66 ± 0.3 compared with 4 ± 0.3 and 3.66 ± 0.5 in control groups; P < 0.001). We show here that targeted neutralization of FasL at early stages of disease also significantly reduced the relative number of apoptotic CD4+ T cells at the CNS, as determined by flow cytometry analysis for Annexin V+ cells (day 11, 23.7% in control mice administered with isotype-matched control IgG, compared with 8.2% in anti-FasL-treated mice; Figure 1B, left panel), and verified by terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling apoptosis assay on lumbar spinal cord sections of these mice (Figure 1C). We did not observe any significant changes in Annexin V+ CD8 or CD11b+ cells within the cells isolated from the CNS at that time (Figure 1D).
Figure 1.
Suppression of EAE following targeted neutralization of FasL is associated with altering the Th1/Th17 balance toward Th1. A: C57BL/6 mice were subjected to active induction of EAE and, on days five, seven, and nine, after the induction of EAE, were administered 300 μg/mouse anti-FasL neutralizing Abs (closed squares), isotype-matched control IgG (open circle), or PBS (closed triangles). Mice were monitored for the development and progression of the disease by an observer blind to the experimental protocol. The data represent one of three experiments with similar results. Results are shown as the mean maximal score of six mice ± SE Arrow indicates the first day of treatment. *P < 0.05, **P < 0.01, and ***P < 0.005 versus control. On day 11, CD4+ T cells (gated using L3T4 mAb; BD Biosciences) from the CNS, and spleens of treated mice were analyzed by flow cytometry for Annexin V/PI. B: Results were verified by terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling assay. C shows representative sections (1 of 18) per group. We have also analyzed the effect of this Ab administration on Annexin V expression on CD8+ T cells and CD11b+ cells isolated from the CNS (D), showing no significant difference. The above CD4+ cells (see B) were also subjected to intracellular staining of IL-17 versus IFN-γ. E: Results are shown after calibration with control nonspecific-labeled Abs.
Further analysis of the T cell subsets accumulating in the CNS revealed that inhibition of CD4+ T cell apoptosis led to a selective accumulation of IFN-γhighIL-17low over IFN-γlowIL-17highCD4+ T cells in the CNS (Figure 1E, left panel, in control mice 3.8% IFN-γhighIL-17low and 10.4% IFN-γlowIL-17highCD4+ T cells, compared with 10.2% IFN-γhighIL-17low and 3.5% IFN-γlowIL-17highCD4+ T cells in anti-FasL-treated mice). A similar pattern of results was obtained when the same parameters were tested on CD4+ T cells isolated from the periphery. Thus, splenic CD4+ T cells, isolated from these mice, also displayed a significant reduction in Annexin V+CD4+ T cells (Figure 1B, right panel) associated with a shift in T cell polarization that included an increase in the relative number of IFN-γhighIL17low cells and a decrease in IFNlowIL-17highCD4+ T cells that correlated with the reciprocal shift within the CNS (Figure 1E, right panel).
One possible interpretation of these observations is that IFN-γ-producing T cells, selected under these conditions, suppress Th17 and thereby EAE. We have taken several approaches to evaluate this hypothesis. The first included evaluating the ability of anti-FasL Abs to affect the course of disease in IFN-γ−/− mice, in comparison with wild-type mice. Before subjecting IFN-γ−/− EAE mice to anti-FasL therapy, we have monitored the kinetics of IL-17highCD4+ T cell accumulation in the CNS in these mice, compared with wild-type C57BL/6 mice. Figure 2A shows a significantly higher accumulation of IL-17highCD4+ T cells in the CNS of IFN-γ−/− mice as early as 3 days after the induction of disease (5.2% compared with 3.7%), peaking 4 days later at day 7(22.2% compared with 10.7%). Before the onset of disease (day 9) and later on (day 15), the relative numbers of these cells were markedly decreased in wild-type mice but not in their IFN-γ−/− counterparts. These mice continued to display a higher relative number of IL-17highCD4+ T cells at the CNS (12%, compared with 4.6 and 7.2%, compared with 4.7%, respectively). This selective accumulation of Th17 cells within the CNS may explain in part the aggravation of disease in these mice (mean maximal score of 4.16 ± 0.18, compared with 3.3 ± 0.23; P < 0.011; Figure 2B, upper left panel). These groups were administered with anti-FasL Abs (100 μg/mouse), isotype-matched Ab, or PBS, according to the protocol described in the legend to Figure 1, and followed for its development and progression by an observer blind to the experimental protocol. Figure 1B shows the effect of anti-FasL Abs on the development of disease in IFN-γ−/− mice, whereas Figure 2C shows the results obtained in wild-type mice. Although anti-FasL Abs administered before the onset of disease (days 5, 7, and 9) led to the development of an aggravated form of disease in wild-type mice (mean maximal score of 3.3 ± 0.23 in control mice and 2 ± 0.28 in mice treated with anti-FasL Abs; P < 0.025), they had no effect on the severity of disease in IFN-γ−/− mice (Figure 1B), implying that suppression of EAE by targeting FasL, at early stages of disease, is IFN-γ dependent. In an independent experiment, we have administered anti-IFN-γ Abs (PeproTech, Rocky Hill, NJ) or isotype-matched control Abs (100 μg/injection each) to wild-type EAE mice, either before the onset of disease (Figure 1D) or at its peak (Figure 1E). Our results show that although administration of these Abs before the onset of EAE led to the development of an aggravated form of disease (mean maximal score of 4.5 ± 0.26 compared with 3.5 ± 0.33; P < 0.001), their administration later on had no beneficial effect.
Figure 2.
Anti-FasL neutralizing Ab fails to suppress EAE in IFN-γ−/− mice. A: The kinetics of IL-17high CD4+ T cell (gated using L3T4 mAb; BD Biosciences) accumulation at the CNS of IFN-γ−/− mice and control wild-type mice at different time points throughout the development of active EAE. Analyses were conducted after calibration with control nonspecific-labeled Abs. B: EAE was induced in IFN-γ−/− and wild-type C57BL/6 mice. Upper left panel shows the manifestation of disease in each group. These groups were administered with anti-FasL Abs, isotype-matched Ab, or PBS, according the protocol described in the legend to Figure 1 and followed for its development and progression by an observer blind to the experimental protocol. The upper middle panel shows the effect of anti-FasL Abs on the development of disease in IFN-γ−/− mice, whereas the upper right panel shows the results obtained in wild-type mice. Bottom panels show the effect of anti-IFN-γ Abs on the kinetics of diseases (wild-type mice) if being administered before the onset of disease (left panel) or when it reaches its maximal severity (right panel). Each panel represents one of three experiments with similar results. Results are shown as the mean maximal score of six mice ± SE (*P < 0.05, **P < 0.01, and ***P < 0.005 versus control). C: Eight days after induction of EAE, CD4+ T cells from the CNS of IFN-γ−/− and wild-type mice that were treated with anti-FasL Abs or PBS, according the protocol described in Figure 2B, were subjected to intracellular staining for the relative number of IL-17-producing CD4+ cells (gated using L3T4 mAb; BD Biosciences). Analyses were conducted after calibration with control nonspecific-labeled Abs.
In a complementary experiment 8 days after induction of EAE, CD4+ T cells from the CNS of IFN-γ−/− and wild-type that were treated with anti-FasL Abs or PBS, as described above (Figure 2B, upper right panel), were subjected to intracellular staining for the relative number of IL-17-producing CD4+ cells (gated using L3T4 mAb; BD Biosciences). Comparative analysis of the accumulation of IL-17highCD4+ T cells in the CNS of these mice showed that, indeed, mice deficient of IFN-γ, which were or were not subjected to anti-FasL administration, displayed a comparable number of Th17 cells, whereas in wild-type mice, the relative number of IL-17highCD4+ T cells decreased from 10.3 to 4.3% (Figure 2C).
In Vitro Selection of Antigen-Specific T Cells in the Presence of Anti-FasL Abs Selects High IFN-γ-Producing T Cells that Suppress EAE
In attempt to isolate the potential antigen-specific T cells that suppress Th17 response during EAE, we selected MOGp35-55-specific T cell lines in the presence of anti-FasL Abs. Figure 3 summarizes data obtained from one of three different experiments with very similar data. In each experiment, we selected, independently, five different MOGp35-55-specific T cell lines from cultures supplemented with 20 μg/ml neutralizing anti-FasL Abs, and five control lines selected from cultures supplemented with the target antigen alone or isotype-matched control Abs. These lines were first tested for their ability to induce transferred EAE at different doses. All five lines, developed in the presence of anti-FasL Abs, were nonpathogenic at all doses, whereas control lines could all transfer EAE in a dose-dependent manner. Figure 3A summarizes the data of one of these experiments, showing that whereas the native line was encephalitogenic at all doses ranging from 3 × 106 to 107 cells per mouse (incidence of 3/6 at 3 × 106 cells/mouse, 6/6 at 5 × 106, and 6/6 at 107 cells/mouse), a line selected in the presence of anti-FasL Abs was entirely nonpathogenic, even at a dose of 107 cells per mouse. Further analysis of the cytokine profile of each of these lines (Figure 3B) showed that both pathogenic and nonpathogenic lines produce comparable levels of IL-2 and TNF-α, as well as very low levels of known regulatory/anti-inflammatory cytokines, including IL-10, IL-4, and TGF-β. Notably, the nonpathogenic line produced almost threefold more IFN-γ than the pathogenic line (1930 ± 170, compared with 720 ± 60 and 740 ± 50 pg/ml in control lines selected in the presence of MOGp35-55 alone or in cultures also supplemented with control IgG; P < 0.0001; Figure 3B) and markedly reduced levels of IL-17 (190 ± 25 compared with 410 ± 30 and 390 ± 40 pg/ml in control lines selected in the presence of MOGp35-55 alone or in cultures also supplemented with control IgG; P < 0.0001; Figure 3B). Intracellular flow cytometry analysis of CD4+ subsets showed a preferential selection for IFN-γhighIL-4low-producing T cells and a markedly reduced selection for IL-17high Th17 cells within the cells selected in the presence of anti-FasL Abs (Figure 3C). It should be noted that there isn’t any change in the proliferate response of the anti-MOGp33–55 lines that were developed in the presence or absence of anti-FasL (Figure 3B). Our results show that those selected in the presence of anti-FasL did not lead to a decreased proliferating rate in response to their target antigen (P < 0.005). This excludes the possibility that reduced pathogenic competence of this line is due to a reduction in its proliferative rate.
Figure 3.
Targeted neutralization of FasL selects IFN-γhighIL-17lowCD25−Foxp3−CD4+ T cells. A: Comparative analysis of the ability of MOGp35-55-specific CD4+ T cells that were selected in the absence (cont) or presence of anti-FasL neutralizing mAb (+ anti-FasL) to transfer EAE in a dose-dependent manner. B: Comparative analysis of cytokine production by MOGp35-55-specific control lines selected in cultures that were supplemented with PBS, isotype-matched control Ab, or anti-FasL mAb: IFN-γ, TGF-β, TNF-α, IL-2, IL-4, IL-17, IL-10, and proliferative response thymidine uptake. Results are shown as the mean cytokine concentration of triplicates ± SE and represent one of three experiments with similar results (*P < 0.05, **P < 0.01, and ***P < 0.005 versus control). C: Intracellular flow cytometry analysis of differing cytokine production by the control MOGp35-55-specific line and the line selected in the presence of anti-FasL mAb. Analyses were conducted after calibration with control nonspecific-labeled Abs. D: Flow cytometry analysis for the expression of FoxP3 versus CD25 by CD4+ (gated using L3T4 mAb; BD Biosciences) spleen cells from naive C57BL/6 mice and by the MOGp35-55-specific line selected in the absence (positive control) or presence of anti-FasL mAb. Analyses were conducted after calibration with control nonspecific-labeled Abs. E: Flow cytometry analysis for the expression of FoxP3 versus CD25 by CD4+ (gated using L3T4 mAb; BD Biosciences) in primary T cells from mice treated with anti-FasL Abs, isotype-matched IgG, or PBS.
Further analysis of these cells showed that they were FOXp3−CD25−CD4+ T cells. Splenic T cells from naive mice, as well as from those developing MOG-induced EAE (gated on CD4+), were used as a positive control in this experiment (Figure 3D). Thus, antigen-specific T cells, selected in the presence of anti-FasL Abs (three stimulation cycles), are CD4+CD25−Foxp3−IFN-γhigh-TGF-βlowIL-4lowIL-10low T cells, and a vast majority of them display a Th1-like phenotype (IFN-γhighIL-17low), whereas those selected under the same conditions without anti-FasL Abs display a mixed Th1/Th17 phenotype (Figure 3C).
In a complementary experiment, we have analyzed if the repeated administration of protective anti-FasL Abs, before the onset of disease, might affect the level of CD25+FOXp3+ in these mice. Thus, three groups of mice (six each) were subjected to the administration of either anti-FasL or isotype-matched control IgG (days 5, 7, and 9), and on day 11, the spleen T cells were analyzed (gated on CD4+) for the expression of CD25+ versus Foxp3+. Figure 3E shows that in accordance with its affect on cultured T cells (Figure 3D) targeted neutralization of FasL leads to a decreased level of CD4+CD25+FOXp3+ cells. Yet, these mice display a markedly reduced level of disease (see Figure 1A).
We have then explored the ability of these cells to affect the clinical manifestation of disease when administered 3 days before its onset (day 7), when the number of Th17 cells reaches its maximal relative number at the CNS (see Figure 2A). As shown in Figure 4A, administering these cells led to the development of a markedly reduced form of disease (mean maximal EAE score in control EAE group 3.3 ± 0.23 compared with 1.33 ± 0.23 and of those administered with the antigen-specific T cell line selected in the presence of anti-FasL Abs; P < 0.001) and to a decreased accumulation of mononuclear cells around high endothelium venules at the lumbar spinal cords, as determined histologically (day 18; Figure 4B). Our results clearly show a markedly reduced number of CD3+ cells in anti-FasL-treated mice compared with controls and also of F4/80+ macrophages. To analyze and compare demyelination and axonal degeneration, we have subjected the sections to a Luxol fast blue staining. Figure 4B clearly shows reduced white area, indicating damage to the myelin sheath, in anti-FasL-treated mice. To analyze and compare gliosis, we determined the proliferation of actrocytes in the damaged area of the CNS. Figure 4B shows reduced proliferative rate of astrocytes in anti-FasL-treated mice. Taken together, these results indicate a reduced severity in the development of disease in anti-FasL-treated mice. Flow cytometry analysis revealed that successful therapy was associated with a significant reduction in the relative number of CD4+ Th17 cells at the CNS (7.6% compared with 2.4%; Figure 4C). We then repeated the experimental protocol described in Figure 4A. However, in this experiment, cells were transferred only at the peak of disease (day 18), when the relative number of Th17 cells at the CNS was low. As shown in Figure 4D, this had no beneficial effect on the manifestation of disease. Finally, to determine whether this suppression was indeed IFN-γ dependent, we repeated the experiment described above, and mice that received the protective line were also subjected to a subsequent administration of anti-IFN-γ-neutralizing Abs (R&D Systems; 100 μg/mouse administered on day 7 together with the line and 3 days later) or to an isotype-matched control Ab. Figure 4E shows that the administration of neutralizing Abs to IFN-γ entirely reversed the therapeutic effect of our protective line. These results further suggest that the mechanistic basis, by which targeted neutralization of FasL participates in the regulation of EAE, is performed by selecting IFN-γhigh-producing CD4+ T cells that suppress Th17 and thereby the development of autoimmunity. Moreover, these results also imply that the function of these cells is limited to the phase of disease directed by Th17.
Figure 4.
MOGp35-55-specific T cells selected in the presence of neutralizing anti-FasL suppress EAE. A: C57BL/6 mice were subjected to active induction of EAE and after 5 days were administered with 20 × 106 CD4+ T cells from our encephalitogenic control line, the MOGp35-55-specific line selected in the presence of anti-FasL mAb or PBS. Mice were then monitored daily for the development and progression of the disease by an observer blind to the experimental protocol. The data represent one of three experiments with similar results. Results are shown as the mean maximal score of six mice ± SE The arrow indicates the first day of treatment (*P < 0.05, **P < 0.01, and ***P < 0.005 versus control). B: H&E, anti-CD3, F/40, glial fibrillary acidic protein and Luxol fast blue staining of the lumbar spinal cord of representative sections from a naive mouse, EAE mice injected with T cells from our encephalitogenic control line, EAE control mice, and EAE mice injected with the MOGp35-55-specific line selected in the presence of anti-FasL mAb. Each panel shows a representative section of 18 different sections analyzed from each mouse. C: Flow cytometry analysis for IL-17 versus IFN-γ production in CD4+ T cells (gated using L3T4 mAb; BD Biosciences) isolated from mice treated with either the MOGp35-55-specific line (left panel) or MOGp35-55-specific line selected in the presence of anti-FasL mAb (right panel). D: C57BL/6 mice were subjected to active induction of EAE and at the peak of disease (day 18) were administered with 20 × 106 CD4+ T cells of our encephalitogenic MOGp35-55-specific control line, 20 × 106 CD4+ T cells of MOGp35-55-specific line selected in the presence of anti-FasL mAb or PBS. Mice were then monitored daily for the development and progression of the disease by an observer blind to the experimental protocol. The data represent one of three experiments with similar results. Results are shown as the mean maximal score of six mice ± SE The arrow indicates the first day of treatment (*P < 0.05, **P < 0.01, and ***P < 0.005 versus control). E: C57BL/6 mice were subjected to active induction of EAE and 5 days later were administered with 20 × 106 CD4+ T cells of the MOGp35-55-specific line selected in the presence of anti-FasL mAb, PBS, or the MOGp35-55-specific line selected in the presence of anti-FasL mAb and anti-IFN-γ mAb (300 μg/mouse, days 7 and 10). Mice were then monitored daily for the development and progression of the disease by an observer blind to the experimental protocol. The data represent one of three experiments with similar results. Results are shown as the mean maximal score of six mice ± SE The arrow indicates the first day of treatment *P < 0.05, **P < 0.01, and ***P < 0.005 versus control). An additional group of mice was administered the MOGp35-55-specific line selected in the presence of anti-FasL mAb and then an isotype-matched control Ab show a very similar disease manifestation to the one administered with cells alone (data not shown). F: IFN-γ−/− mice were subjected to the administration of 20 × 106 cells of our IFN-γhighCD4+T cells either on day five (six days before the onset of disease) or just before the peak of disease (day 18). Results are shown as the mean maximal score of six mice ± SE The arrow indicates the first day of treatment (*P < 0.05, **P < 0.01, and ***P < 0.005 versus control).
IFN-γ−/− mice were subjected to the administration of 20 × 106 cells of our IFN-γhighCD4+ T cells either on day 5 (6 days before the onset of disease) or just before the peak of disease (day 18). Figure 4F shows that only the administration of this line at the early stage suppressed the development of disease (mean maximal score of 2 ± 0.23 compared with 4 ± 0.33; P < 0.005).
Stable Transduction of IFN-γ in an Effector Encephalitogenic T Cell Line Redirects Its Functional Properties
The accumulating data presented so far strongly suggest that IFN-γhigh-producing CD4+ T cells, which are noninflammatory, participate in the regulation of autoimmunity by suppressing Th17 in an IFN-γ-dependent mechanism and that the development of the autoimmune process depends on their apoptosis, which enables the acceleration of Th17 cells, driving the inflammatory process at its initial stages. These cells differ from inflammatory Th1 cells by the level of IFN-γ they produce (Figure 3B) and perhaps by other parameters yet to be identified. Would targeted overexpression of IFN-γ in inflammatory Th1 cells convert them into noninflammatory T cells that regulate Th17? We have explored this possibility by overexpressing IFN-γ (together with green fluorescent protein (GFP)) or GFP alone in our pathogenic MOGp35-55-specific line (stable transduction) and explored its ability to transfer disease and later to suppress an established disease. Overexpression of IFN-γ, but not GFP alone, led to increased production of IFN-γ (2041 ± 151 pg/ml, compared with 512 ± 16 and 531 ± 17 in control lines; P < 0.0001; Figure 5A). Overexpression of IFN-γ in these cells did not affect their production of proinflammatory cytokines, such as TNF-α, and also did not lead to increased production of known regulatory/anti-inflammatory cytokines, including IL-4, IL-10, and TGF-β (Figure 5A). It did, however, redirect their polarization from a mixed Th1/Th17 phenotype (Figure 3C) to IFN-γhighIL-17low-producing cells (Figure 5C). These cells were nonencephalitogenic, even if administered at a dose of 107 per mouse (0/12, compared with 12/12 in both control groups; Figure 5B). We then explored the ability of each line to affect the dynamics of disease when administered either 5 days after its induction (5 to 6 days before its onset) or just before the peak of disease (day 18). Apparently, only the early administration of IFN-γhigh-producing T cells could suppress the disease (mean maximal score of 2.66 ± 0.5 and 3.5 ± 0.66 in control untreated mice and mice administered GFP cells, compared with 1.83 ± 0.66 in those treated with IFN-γhigh- producing T cells; P < 0.001; Figure 5D). Administering these cells around the peak of the disease, when the relative number of Th17 cells at the CNS was low, had no beneficial effect on the manifestation of the disease (Figure 5E).
Figure 5.
Stable transduction of IFN-γ in an effector encephalitogenic T cell line redirects its functional properties. A: IFN-γ production by the MOGp35-55-specific line that have been subjected to retroviral infection with IRES-GFP (MOG (GFP)) or with IFN-γ-IRES-GFP (MOG (IFN-γ-GFP)), compared with the native line (MOG). Various cytokines were detected (enzyme-linked immunosorbent assay) following antigen-specific stimulation. Results are shown as the mean triplicates ± SE (*P < 0.05, **P < 0.01, and ***P < 0.005 versus control). B: Comparative analysis of the ability of the native MOGp35-55-specific line (MOG), the retroviral infected line with IRES-GFP (MOG (GFP)), or the IFN-γ-IRES-GFP-infected line (MOG (IFN-γ-GFP)) to adoptively transfer EAE. Results of one of three experiments with similar data are shown as the mean maximal score ± SE. C: Intracellular analysis of IFN-γ versus IL-17 production in the above IFN-γhigh-transduced line. C57BL/6 mice were subjected to active induction of EAE and on day 5 (D) or day 18 (E) were administered with 2 × 107 of IRES-GFP-infected cells, IFN-γ-IRES-GFP-infected cells, or PBS and were monitored daily for the development and progression of the disease by an observer blind to the experimental protocol. The data represent one of three experiments with similar results. Results are shown as the mean maximal score of six mice ± SE (*P < 0.05, **P < 0.01, and ***P < 0.005 versus control). The arrow indicates the first day of treatment. F: Left panel, lpr and wild-type C57BL/6 mice were subjected to active induction of EAE and monitored daily for the development and progression of the disease by an observer blind to the genetic differences between the groups. Results are shown as the mean maximal score of six mice ± SE (*P < 0.05, **P < 0.01, and ***P < 0.005 versus control). Right panel, Flow cytometry analysis for IL-17 versus IFN-γ production in CD4+ T cells (gated using L3T4 mAb; BD Biosciences) isolated from spleen (9 days after active disease induction) of these mice. G: Left panel, gld and wild-type C57BL/6 mice were subjected to active induction of EAE and monitored daily for the development and progression of the disease by an observer blind to the genetic differences between the groups. Results are shown as the mean maximal score of six mice ± SE (*P < 0.05, **P < 0.01, and ***P < 0.005 versus control). Middle panel, Flow cytometry analysis for IL-17 versus IFN-γ production in CD4+ T cells (gated using L3T4 mAb; BD Biosciences) isolated from spleen (9 days after active disease induction) of these mice. Right panel, These cells were tested for their ability to transfer EAE (2 × 107 cells/mouse). H: Following a subsequent selection in response to MOGp33–55 CD4+ T cells were analyzed, again, for IFN-γ versus IL-17 expression by flow cytometry (left panel) and then for their ability to affect the development of ongoing EAE in wild-type mice when administered (2 × 107) 5 days after the induction of disease (right panel). The data represent one of three experiments with similar results. Results are shown as the mean maximal score of six mice ± SE (*P < 0.05, **P < 0.01, and ***P < 0.005 versus control).
Previous EAE experiments with lpr mice lacking Fas, as well as with gld mice lacking FasL, provided controversial results. Whereas Kuchroo and colleagues24 found that these mice display an attenuated form of disease and, in accordance with these observations, Russell and his group25 showed that FasL-deficient lymphocytes transfer attenuated disease to wild-type, Miller and colleagues26 showed that lpr mice develop an exacerbated form of disease. None of these studies included Th17 follow-up, because these cells were not identified at that time. Because of the discrepancy between these studies and because of its high relevance to the current study, we have reconducted these experiments in C57BL/6 mice with either an lpr or gld background. Notably, gld mice are more relevant to our experimental system because both lack FasL activity, either due deficiency or Ab-based targeted neutralization. Each experiment included two groups of nine mice each, one of deficient mice and the other wild-type that were subjected to active induction of disease. On day 9, three mice of each group were sacrificed for cell analysis and the other six followed for development and progression of disease. Figure 5F, left panel, shows that Fas-deficient mice (lpr) developed a significantly lower form of disease (mean maximal score of 3.5 ± 0.24 compared with 1.83 ± 0.18; P < 0.001). Figure 5F, right panel, presents intracellular analysis of IFN-γ versus IL-17 in spleen cells of each group, showing a marked reduction in Th17 selection (1.9% compared with 5.9%) in these mice, which could explain, in part, their attenuated disease. Figure 5G presents the reciprocal set of experiments done with gld mice lacking FasL. We show that these mice also display an attenuated form of disease (mean maximal score of 1.66 ± 0.3 compared with 3.66 ± 0.3; P < 0.001; Figure 5G, left panel). Similarly to lpr mice, intracellular analysis of IFN-γ versus IL-17 in spleen cells of these mice also showed a marked reduction in Th17 selection (1.8% compared with 4.8%; Figure 5G, middle panel), which also could explain, in part, their attenuated disease (Figure 5G, left panel). These cells failed in transferring adoptive EAE to naive mice (0/6 compared with 6/6; Figure 5G, right panel) and were then selected for a second selection cycle (without addition of anti-FasL Abs), in comparison with wild-type. Figure 5H, left panel, shows that this line was selected into IFN-γhigh cells (42.3% compared with 22.6% in line of control wild-type mice) that could also suppress ongoing EAE when administered 5 days before the onset of disease (mean maximal score of 1.83 ± 0.3 compared with 3.5 ± 0.3; P < 0.001; Figure 5H, right panel). These results are comparable with those obtained using Ab-based neutralization of FasL (Figure 4).
Discussion
In the current article, we show that IFN-γhighCD4+ T cells, which are noninflammatory, participate in the regulation of autoimmunity by suppressing Th17 in an IFN-γ-dependent mechanism. Targeted neutralization of FasL at early stages of EAE, at a time when the contribution of Th17 to the development of disease is critical,1 prevented CD4+ T cell apoptosis, leading to increased accumulation of IFN-γhighIL-17lowCD4+ T cells, reduced accumulation of IL-17-producing CD4+ T cells in the CNS and periphery, and suppressed the development of EAE. IFN-γ is a major regulator of Th17.6 We show here that: i) the acceleration in producing IFN-γ is reciprocal to the decrease in Th17 at the CNS (Figure 1). ii) IFN-γ−/− mice display a significantly more severe form of disease in accordance with an elevated relative number of Th17 cells (Figure 2). In these mice, targeted neutralization of FasL had no effect either on disease manifestation or on the relative number of Th17 cells (Figure 2). iii) T cells selected in the presence of neutralizing Abs to FasL are IFN-γhigh nonpathogenic CD4+ T cells that suppress Th17 function and thereby EAE (Figure 3), an effect that could be reversed by neutralizing Abs to IFN-γ (Figure 3). iv) Targeted neutralization of IFN-γ before the onset of disease, when the relative number of Th17 CD4+ cells is very high (Figure 2A), aggravates EAE (Figure 2B, bottom left panel), whereas at the peak of disease it has no significant effect (Figure 2B, bottom right panel). v) The ability of these cells to suppress EAE depends on the persistence of a significant relative number of Th17 cells at the CNS (Figure 4D). vi) Antigen-specific CD4+ T cells transformed to overexpress IFN-γ exhibit properties very similar to those selected in the presence of anti-FasL Abs (Figure 5). Taken together, these results indicate the existence of noninflammatory IFN-γhigh-producing T cells that participate in regulating disease.
Why does targeted neutralization of FasL favor the selection of IFN-γhigh noninflammatory T cells? It has been shown that the affinity of TCR to major histocompatibility complex II-peptide complex during CD4+ T cell selection affects their biological properties. Higher affinity of biding enhances the selection of very high IFN-γ-producing Th1 cells, whereas low affinity binding results in low IFN-γ-producing Th1 cells.14 These cells that produce high levels of IFN-γ were found to be more susceptible to FasL-mediated apoptosis, in an IFN-γ-dependent manner, probably as a part of the natural regulation of T cell homeostasis.15 The biological significance of these findings remained elusive. The current study shows that these antigen-specific T cells emerge during early stages of an autoimmune condition and suppress Th17 cells in attempting to block the development of disease. Once they undergo FasL-induced apoptosis, the autoimmune condition develops. We suggest that rescuing them by anti-FasL Abs at early stages of disease suppresses its development.
The inflammatory activity of Th17 cells differs from Th1,27 as has recently been shown in experimental autoimmune uveitis28 and EAE.2 These cells are also likely to play an important role in human autoimmunity.29 Their aggressiveness against self is a drawback. They are essential for combating infectious disease, particularly fungal infections,30,31,32 as well as to restrain IgE hyperreactivates, via IL-21 signal transducer and activator of transcription-3-induced signaling.33 Although these activities are essential and beneficial for the host, a low threshold in their activation may result in increased susceptibility to autoimmunity. It is therefore likely that the presence of noninflammatory regulatory T cells that would restrain Th17 response, and allow its controlled development, owing to self apoptosis, may provide an appropriate balance between the beneficial and harmful effects of these cells. It also likely that, similarly to CD25+ regulatory T cells, the main function of these regulatory T cells has been to restrict infectious inflammatory response34; and their role in autoimmunity is secondary yet important.
Why, following a single selection cycle in the presence of anti-FasL Abs, do these cells continue to grow in the absence of Abs to FasL? It has been shown that IFN-γ produced by these cells control their selection in an autocrine manner by increasing their susceptibility to FasL-mediated apoptosis.15 One possibility is that targeted neutralization of FasL not only rescues these cells but also directs reselection of cells that are less sensitive to FasL-mediated apoptosis, thus continue to produce high levels of IFN-γ.
Do these cells differ from encephalitogenic CD4+ T cells only by the level of IFN-γ they produce? Under our working conditions (see Materials and Methods), development of a MOG-specific CD4+ lines (three stimulation cycles) from primary cultured cells that are obtained before the onset of disease (day 9) results in the selection of a mixed Th1/Th17 population with a typical balance as shown in Figure 3C. These cells adoptively transfer EAE (Figure 4). After additional two to three stimulation cycles, they are reselected into a more unique Th1-like (IFN-γhighIL-17low) cell line, but then lose their ability to transfer disease (which could potentially be independent of the loss of IL-17-producing cells). Subsequently, development of a preline from EAE mice later stages of disease do result in a rapid selection of a IFN-γhighIL-17low line, but once again, its ability transfer disease is very limited (G. Wildbaum and N. Karin, unpublished observations). Overexpression of IFN-γ in primary cells (during first stimulation), isolated on day 9 after EAE induction, led to a rapid selection of IFNγhighIL-17low CD4+ T cells (>90%; Figure 5C), which not only are incapable of transferring EAE but also suppress an established disease, if being administered at its early stages (Figure 5D). These results are in accordance with our other results showing that targeting FasL rescues IFN-γhigh-producing cells that suppress Th17 selection (in vitro and in vivo) and the progression of EAE (Figures 1 and 2). It has recently shown that both Th1 and Th17 are capable of transferring adoptive EAE and independently induce distinct types of EAE based on histological examination.2 Our IFN-γhigh-transduced T cell line, as the one selected in the presence of anti-FasL Abs, not only fail to adoptively transfer disease but also suppress it without producing any know regulatory cytokine (Figure 5A). These results together further imply that the mechanistic basis of their action is likely to include Th17 suppression via IFN-γ.
Administration of these cells at late stages of disease, when Th17 cells are suppressed, had no beneficial effect and even aggravated the disease (Figure 5E). IFN-γ is a pleiotropic cytokine that also direct inflammation, partly by up-regulating major histocompatibility complex II expression and inflammatory chemokine production.35 It is therefore likely that although in the presence of Th17, IFN-γ-producing Th1 suppresses inflammatory autoimmunity, by inhibiting Th17, but later in their absence it would generate an opposing effect. A very recent article by O'Connor et al36 indeed showed that Th1 preparations devoid of Th17 cells are highly pathogenic. Intriguingly, this article suggests that among the pleiotropic functions of Th1 they may also facilitate Th17 entry to the site of inflammation.
Ten years ago Tarrant et al37 showed that administration of IL-12 during early stages of experimental autoimmune uveitis protects from the disease, in part by eliciting IFN-γ production.37 At that time Th17 have not yet been discovered. As a suggested mechanism, the authors showed that high IFN-γ triggered inducible nitric oxide synthase and nitric oxide synthase production, which in turn caused apoptosis of cells in the draining lymph nodes and inhibited development of disease-associated immunity.37 The current article focuses on the role of IFN-γ on Th17 polarization. Both articles are therefore complementary. More recently, the same group showed that Th17 are essential for the induction of active experimental autoimmune uveitis, thus in the absence of IL-23, and thereby Th17 these mice showed a high state against active induction of disease. Th17 effector cells induced experimental autoimmune uveitis in the absence of IFN-γ. In contrast, IFN-γhighIL-17low could effectively transfer adoptive disease dispensable of Th17.28 Along this line, Kroenke et al2 showed that IL-12- and IL-23-modulated T cells induce distinct types of EAE based on histology, CNS chemokine profile, and response to cytokine inhibition. These results together may well explain why targeting Th17 at early stages of actively induced inflammatory autoimmune disease suppresses its development. In our study, we showed that Th17 were suppressed by IFN-γhigh-producing cells.
We do not exclude the possibility that more than one mechanism contributed to the interplay between IFN-γ and Th17 in which this cytokine suppresses Th17. This may include direct suppression of RORγT transcription (reviewed in Ref. 38), suppression of the proliferative response of polarized Th17, or induction their direct apoptosis.
Finally, recent data from humans suggest that Th17 cells play an important role in the pathogenesis of a diverse group of immune-mediated diseases, including psoriasis, rheumatoid arthritis, multiple sclerosis, and inflammatory bowel disease, and that in these diseases Th17 cells are likely to be the driver cells even at advanced stages.29 This makes the challenge of understanding the regulatory mechanisms underlying Th17 function highly applicative, even at advanced stages of these diseases.
Footnotes
Address reprint requests to Nathan Karin, Ph.D., Department of Immunology, Bruce Rappaport Faculty of Medicine, Haifa, Israel. E-mail: nkarin@tx.technion.ac.il.
Supported by grants from the Israel Science Foundation, the Israel Ministry of Health Chief Scientist, and the L. Aronberg Research Fund in Neurology.
CME Disclosure: None of the authors disclosed any relevant financial relationships.
References
- Stromnes IM, Cerretti LM, Liggitt D, Harris RA, Goverman JM. Differential regulation of central nervous system autoimmunity by Th1 and Th17 cells. Nat Med. 2008;14:337–342. doi: 10.1038/nm1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke MA, Carlson TJ, Andjelkovic AV, Segal BM. IL-12- and IL-23-modulated T cells induce distinct types of EAE based on histology, CNS chemokine profile, and response to cytokine inhibition. J Exp Med. 2008;205:1535–1541. doi: 10.1084/jem.20080159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, Zurawski S, Wiekowski M, Lira SA, Gorman D, Kastelein RA, Sedgwick JD. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatino JJ, Jr, Shires J, Altman JD, Ford ML, Evavold BD. Loss of IFN-γ enables the expansion of autoreactive CD4+ T cells to induce experimental autoimmune encephalomyelitis by a nonencephalitogenic myelin variant antigen. J Immunol. 2008;180:4451–4457. doi: 10.4049/jimmunol.180.7.4451. [DOI] [PubMed] [Google Scholar]
- Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1069–1070. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- Steinman L. A brief history of Th17, the first major revision in the Th1/Th2 hypothesis of T cell-mediated tissue damage. Nat Med. 2007;13:139–145. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- Kohm AP, Carpentier PA, Anger HA, Miller SD. Cutting edge: CD4+CD25+ regulatory T cells suppress antigen-specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. J Immunol. 2002;169:4712–4716. doi: 10.4049/jimmunol.169.9.4712. [DOI] [PubMed] [Google Scholar]
- Meiron M, Zohar Y, Anunu R, Wildbaum G, Karin N. CXCL12 (SDF-1α) suppresses ongoing experimental autoimmune encephalomyelitis by selecting antigen-specific regulatory T cells. J Exp Med. 2008;205:2643–2655. doi: 10.1084/jem.20080730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groux H, O'Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+ T cell subset inhibits antigen-specific T cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- Wildbaum G, Netzer N, Karin N. Tr1 cell-dependent active tolerance blunts the pathogenic effects of determinant spreading. J Clin Invest. 2002;110:701–710. doi: 10.1172/JCI15176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Kuchroo VK, Inobe J, Hafler D, Weiner HL. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994;265:1237–1240. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- Wildbaum G, Westermann J, Maor G, Karin N. A targeted DNA vaccine encoding Fas ligand defines its dual role in the regulation of experimental autoimmune encephalomyelitis. J Clin Invest. 2000;106:671–679. doi: 10.1172/JCI8759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer C, Stein J, Southwood S, Ketelaar H, Sette A, Bottomly K. Altered peptide ligands can control CD4 T lymphocyte differentiation in vivo. J Exp Med. 1995;181:1569–1574. doi: 10.1084/jem.181.4.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refaeli Y, Van Parijs L, Alexander SI, Abbas AK. Interferon γ is required for activation-induced death of T lymphocytes. J Exp Med. 2002;196:999–1005. doi: 10.1084/jem.20020666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompkins SM, Padilla J, Dal Canto MC, Ting JP, Van Kaer L, Miller SD. De novo central nervous system processing of myelin antigen is required for the initiation of experimental autoimmune encephalomyelitis. J Immunol. 2002;168:4173–4183. doi: 10.4049/jimmunol.168.8.4173. [DOI] [PubMed] [Google Scholar]
- Schif-Zuck S, Westermann J, Netzer N, Zohar Y, Meiron M, Wildbaum G, Karin N. Targeted overexpression of IL-18 binding protein at the central nervous system overrides flexibility in functional polarization of antigen-specific Th2 cells. J Immunol. 2005;174:4307–4315. doi: 10.4049/jimmunol.174.7.4307. [DOI] [PubMed] [Google Scholar]
- Flugel A, Berkowicz T, Ritter T, Labeur M, Jenne DE, Li Z, Ellwart JW, Willem M, Lassmann H, Wekerle H. Migratory activity and functional changes of green fluorescent effector cells before and during experimental autoimmune encephalomyelitis. Immunity. 2001;14:547–560. doi: 10.1016/s1074-7613(01)00143-1. [DOI] [PubMed] [Google Scholar]
- Flugel A, Willem M, Berkowicz T, Wekerle H. Gene transfer into CD4+ T lymphocytes: green fluorescent protein-engineered, encephalitogenic T cells illuminate brain autoimmune responses. Nat Med. 1999;5:843–847. doi: 10.1038/10567. [DOI] [PubMed] [Google Scholar]
- Fujita M, Otsuka T, Mizuno M, Tomi C, Yamamura T, Miyake S. Carcinoembryonic antigen-related cell adhesion molecule 1 modulates experimental autoimmune encephalomyelitis via an iNKT cell-dependent mechanism. Am J Pathol. 2009;175:1116–1123. doi: 10.2353/ajpath.2009.090265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashold FE, Spach KM, Spanier JA, Hayes CE. Estrogen controls vitamin D3-mediated resistance to experimental autoimmune encephalomyelitis by controlling vitamin D3 metabolism and receptor expression. J Immunol. 2009;183:3672–3681. doi: 10.4049/jimmunol.0901351. [DOI] [PubMed] [Google Scholar]
- Yang J, Jiang Z, Fitzgerald DC, Ma C, Yu S, Li H, Zhao Z, Li Y, Ciric B, Curtis M, Rostami A, Zhang GX. Adult neural stem cells expressing IL-10 confer potent immunomodulation and remyelination in experimental autoimmune encephalitis. J Clin Invest. 2009;119:3678–3691. doi: 10.1172/JCI37914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du C, Liu C, Kang J, Zhao G, Ye Z, Huang S, Li Z, Wu Z, Pei G. MicroRNA miR-326 regulates TH-17 differentiation and is associated with the pathogenesis of multiple sclerosis. Nat Immunol. 2009;10:1252–1259. doi: 10.1038/ni.1798. [DOI] [PubMed] [Google Scholar]
- Waldner H, Sobel RA, Howard E, Kuchroo VK. Fas- and FasL-deficient mice are resistant to induction of autoimmune encephalomyelitis. J Immunol. 1997;159:3100–3103. [PubMed] [Google Scholar]
- Sabelko-Downes KA, Cross AH, Russell JH. Dual role for Fas ligand in the initiation of and recovery from experimental allergic encephalomyelitis. J Exp Med. 1999;189:1195–1205. doi: 10.1084/jem.189.8.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suvannavejh GC, Dal Canto MC, Matis LA, Miller SD. Fas-mediated apoptosis in clinical remissions of relapsing experimental autoimmune encephalomyelitis. J Clin Invest. 2000;105:223–231. doi: 10.1172/JCI8561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman L. A rush to judgment on Th17. J Exp Med. 2008;205:1517–1522. doi: 10.1084/jem.20072066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luger D, Silver PB, Tang J, Cua D, Chen Z, Iwakura Y, Bowman EP, Sgambellone NM, Chan CC, Caspi RR. Either a Th17 or a Th1 effector response can drive autoimmunity: conditions of disease induction affect dominant effector category. J Exp Med. 2008;205:799–810. doi: 10.1084/jem.20071258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesmer LA, Lundy SK, Sarkar S, Fox DA. Th17 cells in human disease. Immunol Rev. 2008;223:87–113. doi: 10.1111/j.1600-065X.2008.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JJ, Ruddy MJ, Conti HR, Boonanantanasarn K, Gaffen SL. The interleukin-17 receptor plays a gender-dependent role in host protection against Porphyromonas gingivalis-induced periodontal bone loss. Infect Immun. 2008;76:4206–4213. doi: 10.1128/IAI.01209-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki G, Umemura M. Interleukin-17 as an effector molecule of innate and acquired immunity against infections. Microbiol Immunol. 2007;51:1139–1147. doi: 10.1111/j.1348-0421.2007.tb04008.x. [DOI] [PubMed] [Google Scholar]
- Carbonetti NH. Immunomodulation in the pathogenesis of Bordetella pertussis infection and disease. Curr Opin Pharmacol. 2007;7:272–278. doi: 10.1016/j.coph.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM, Dong C. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- Shevach EM. CD4+CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- Lukacs NW, Strieter RM, Elner VM, Evanoff HL, Burdick M, Kunkel SL. Intercellular adhesion molecule-1 mediates the expression of monocyte-derived MIP-1α during monocyte-endothelial cell interactions. Blood. 1994;83:1174–1178. [PubMed] [Google Scholar]
- O'Connor RA, Prendergast CT, Sabatos CA, Lau CW, Leech MD, Wraith DC, Anderton SM. Cutting edge: Th1 cells facilitate the entry of Th17 cells to the central nervous system during experimental autoimmune encephalomyelitis. J Immunol. 2008;181:3750–3754. doi: 10.4049/jimmunol.181.6.3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarrant TK, Silver PB, Wahlsten JL, Rizzo LV, Chan CC, Wiggert B, Caspi RR. Interleukin 12 protects from a T helper type 1-mediated autoimmune disease, experimental autoimmune uveitis, through a mechanism involving interferon γ, nitric oxide, and apoptosis. J Exp Med. 1999;189:219–230. doi: 10.1084/jem.189.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov II, Zhou L, Littman DR. Transcriptional regulation of Th17 cell differentiation. Semin Immunol. 2007;19:409–417. doi: 10.1016/j.smim.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]