Abstract
Immunosuppressive treatments of systemic lupus (SLE) remain associated with significant toxicities; hence, compounds with better toxicity profiles are needed. Dihydroorotate dehydrogenase (DHODH) inhibition with leflunomide has proven to be effective in autoimmune diseases including SLE, but leflunomide can cause a variety of side effects. We hypothesized that 4SC-101, a novel DHODH inhibitor with a more favorable toxicity profile, would be as effective as high-dose cyclophosphamide (CYC) in controlling experimental SLE of female MRL(Fas)lpr mice. Daily oral gavage of 30, 100, and 300 mg/kg 4SC-101 from 12 to 22 weeks of age was compared with either vehicle or CYC treatment (30 mg/kg/week, i.p.) in terms of efficacy and toxicity. Three hundred milligrams per kilogram 4SC-101 was as effective as CYC in depleting spleen autoreactive T cells, B cells, and plasma cells as well as the respective DNA and RNA serum autoantibodies. This was associated with a comparable amelioration of the renal, dermal, and pulmonary SLE manifestations of MRL(Fas)lpr mice. However, even the highest dose of 4SC-101 had no effect on bone marrow neutrophil counts, which were significantly reduced in CYC-treated mice. Together, the novel DHODH inhibitor 4SC-101 is as effective as high dose CYC in controlling SLE without causing myelosuppression. Hence, DHODH inhibition with 4SC-101 might be suitable to treat active SLE with fewer side effects than CYC.
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease caused by multiple genetic polymorphisms and immunostimulatory environmental factors, which commonly affects young females.1,2 Mild disease manifestations such as fatigue, skin rashes, arthralgia, or fever can usually be controlled by low dose steroids and antimalarials.3 In many patients, however, autoimmune inflammation of solid organs holds the risk of progressive tissue remodeling and irreversible organ damage, which requires treatment with potent immunosuppressive drugs.3 For example, high dose cyclophosphamide (CYC) or mycophenolate mofetil has proven to be effective to control diffuse proliferative lupus nephritis in up to 60% to 80% of patients,4,5,6 and similar protocols have been applied in SLE patients with other types of severe immunopathologies.3 However, controlled trials revealed that immunosuppressive treatments are associated with significant morbidity and mortality in SLE. For example, in the Aspreva Lupus Management Study, mycophenolate mofetil caused serious adverse effects in 27.7% of patients and treatment-related death in 4.9% of patients; CYC caused serious adverse effects in 22.8% of patients and treatment-related death in 2.8% of patients.6 Most of the adverse effects and deaths were related to serious infections caused by the immunosuppressive and unspecific antiproliferative effects of CYC and mycophenolate mofetil, evident by myelosuppression and leukopenia. Novel drugs that can control autoimmune tissue inflammation more specifically, ie, without causing myelosuppression, may allow to further improve SLE treatment and to reduce the toxicity of current treatment protocols.3
The enzyme dihydroorotate dehydrogenase (DHODH) catalyzes the fourth step in the de novo biosynthesis of pyrimidine by converting dihydroorotate to orotate.7 This process is an important step in rapidly proliferating cells, hence, DHODH activity is necessary for the rapid expansion of autoreactive lymphocytes in autoimmune diseases.8 Because the α- and the β-barrel domains of DHODH form a tunnel to the active site of enzymatic activity, compounds interacting with the α- and the β-barrel domains can block DHODH activity. For example, leflunomide, 5-methyl-N-[4-(trifluoromethyl) phenyl]-isoxazole-4-carboxamide, inhibits DHODH by binding into this tunnel.9 Consistent with the role of DHODH in lymphocyte proliferation, leflunomide has proven effective and was approved for the treatment of rheumatoid arthritis and psoriasis arthritis.10,11 Leflunomide was shown to control lupus nephritis in experimental SLE,12 but this study lacked a detailed description of the autoimmune phenotype of experimental SLE, a comparison with CYC treatment efficacy or of toxicity aspects. Side effects of leflunomide treatment include diarrhea, arterial hypertension, liver dysfunction, myelosuppression, and interstitial lung disease.10 It remains unclear whether these effects relate to DHODH inhibition or to off-target effects of leflunomide.
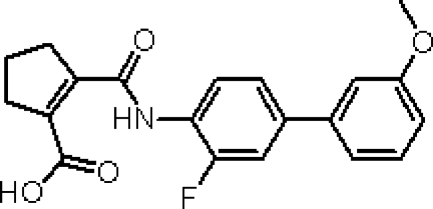
4SC-101, 2-(3-Fluoro-3′-methoxybiphenyl-4-carbamoyl)-cyclopent-1-enecarboxylic acid (formerly SC12267), is a novel small molecule DHODH inhibitor.13 The chemical structure of 4SC-101 (Figure 1) is considerably different from the active metabolite of leflunomide. 4SC-101 inhibits human DHODH with a half maximal inhibitory concentration (IC50) value of 134 nmol/L, and the proliferation of phytohemagglutinin-stimulated lymphocytes with an IC50 value of about 13 μmol/L.13 4SC-101 inhibits phytohemagglutinin-induced interleukin-17 secretion from human peripheral blood mononuclear cells (PBMCs) in a dose-related fashion with an estimated IC50 of 6 μmol/L and independently of lymphocyte proliferation.14 Because 4SC-101 is active in rodent models of arthritis and intestinal inflammation (own unpublished data), the compound is currently under clinical development for rheumatoid arthritis and inflammatory bowel disease. Can DHODH inhibition with 4SC-101 also control severe SLE? We hypothesized 4SC-101 to be effective in controlling experimental SLE, and we compared its effects with CYC in terms of efficacy and toxicity.
Figure 1.
Structure and biochemistry of 4SC-101. 4SC-101, 2-(3-fluoro-3′-methoxybiphenyl-4-carbamoyl)-cyclopent-1-enecarboxylic acid, is a novel inhibitor of human DHODH discovered by molecular design based on the X-ray structure of human DHODH. Different from leflunomide, this compound has virtually no effect on kinases and has therefore a much cleaner pharmacological profile.
Materials and Methods
Biochemistry and Pharmacological Profile of 4SC-101
In vitro DHODH inhibition assay mixture contained 50 μmol/L decycloubiquinone, 100 μmol/L dihydroorotate, and 60 μmol/L 2,6-dichloroindophenol. The amount of enzyme was adjusted such that an average slope of approximately 0.2 AU/min will be achieved in the assay for the positive control (eg, without inhibitor). Measurements were conducted in 50 mmol/L TrisHCl, 150 mmol/L KCl, 0.1% Triton X-100, and pH 8.0 at 30°C in a final volume of 1 ml. The components were mixed, and the reaction was started by adding dihydroorotate. The reaction was followed spectrophotometrically by measuring the decrease in absorption at 600 nm for 2 minutes. The assay was linear in time and enzyme concentration. Inhibitory studies were conducted in a standard assay with additional variable amounts of inhibitor. For the determination of the IC50 values (concentration of inhibitor required for 50% inhibition), eight different inhibitor concentrations were applied. Each data point was recorded in triplicates on a single measurement day.
Human PBMCs from healthy human donors were purified by centrifugation over Ficoll-Hypaque (Sigma-Aldrich, Taufkirchen, Germany). Purified PBMCs were then washed twice with phosphate-buffered saline and resuspended in RPMI1640 culture medium supplemented with 10% heat inactivated fetal calf serum, 1.5 mmol/L l-glutamine, 100 U penicillin/ml, and 100 mg streptomycin/ml. For stimulation studies, PBMCs were seeded at 1 × 105 cells/well, stimulated with 2 μg/ml phytohemagglutinin, and treated with compounds at the indicated concentrations for 48 hours. The vehicle for 4SC-101 (4SC AG, Planegg-Martinsried, Germany) was dimethyl sulfoxide (Acros Organics, Fischer Scientific GmbH, Niederrau, Germany). After incubation, proliferation was assessed by using the cell proliferation colorimetric BrdU-enzyme-linked immunosorbent assay kit from Roche (Mannheim, Germany) according to the manufacturer’s instructions.
Animals and Experimental Protocol
Eight-week-old female MRLlpr/lpr mice were obtained from Harlan Winkelmann (Borchen, Germany) and kept under normal housing conditions in a 12-hour light and dark cycle. Water and standard chow (Ssniff, Soest, Germany) were available ad libitum. At the age of 12 weeks, groups of 11 mice each started to receive either 30, 100, or 300 mg/kg/day 4SC-101 in 100 μl of suspension or placebo suspension only by daily oral gavage. Another group of MRLlpr/lpr mice received 30 mg/kg CYC once a week by intraperitoneal injection because this dose potently suppresses the autoimmune syndrome in MRLlpr/lpr mice.15 Twelve-week-old mice were selected because in our hands the presence of serum autoantibodies and albuminuria indicate significant systemic autoimmunity and early lupus nephritis at this age.16 4SC-101 plasma levels were determined at various time intervals after the first dose. All experiments were performed according to German animal protection laws and had been approved by the local government authorities.
Evaluation of Systemic Lupus
Urinary albumin/creatinine concentration ratios and serum immunoglobulin concentrations were determined by enzyme-linked immunosorbent assay as previously described.17 Two-micrometer paraffin sections for hematoxylin and eosin and periodic acid-Schiff stains were prepared from skin, lung, and kidney tissues samples following routine protocols.18 Skin lesions were scored semiquantitatively as described.19 In brief, hematoxylin and eosin skin sections were analyzed for the degree of acanthosis, mildly (1) to very markedly thickened epidermis (3); hyperkeratosis, mildly (1) or markedly increased thickness of the corneal layer (2); interface liquefaction, focal (1) or extensive damage to the basal cell layer (2); inflammation, sparse (1) or intense dermal lymphocytic cell infiltrates (2); fibrosis or mucin deposits, increased dermal cellularity with slight (1) or markedly thickened extracellular matrix or mucin deposits (2); vessels, focal (1) or diffuse (2) distribution of hemorrhagic vasodilation; and loss of epidermis, absence (0) or presence (1) of ulcer or erosion. The severity of the renal lesions was graded by using the indices for activity and chronicity as described for human lupus nephritis.20 The severity of the peribronchial inflammation was graded semiquantitatively from 0 to 3. Immunostaining was performed as previously described.21 The following primary antibodies were used: rat anti-mouse Mac2 (macrophages, Cederlane, ON, Canada, 1:50); anti-mouse CD3 (1:100, clone 500A2, BD Pharmingen, Heidelberg, Germany); and anti-mouse IgG1 (1:100, M32015, Caltag Laboratories, Burlingame, CA). Glomerular Ig deposits were scored from 0 to 3 on 15 glomerular sections where 0 = no glomerular IgG deposits, 1 = trace IgG deposits, 2 = moderate IgG deposits, and 3 = severe IgG deposits. Glomerular cells were counted in 15 cortical glomeruli per section. Interstitial cells were counted in 15 high power fields per section. All quantitative and semiquantitative analyses were performed by three blinded observers (Claudia Kanter, Miklos Sardy, Hans-J. Anders).
Glomerular Filtration Rate
Glomerular filtration rate (GFR) was determined by clearance kinetics of plasma fluorescein isothiocyanate (FITC)-inulin (Sigma-Aldrich) 5, 10, 15, 20, 35, 60, and 90 minutes after a single intravenous bolus injection.22 Fluorescence was determined with 485 nm excitation and read at 535 nm emission. GFR was calculated based on a two-compartment model of nonlinear regression by using a curve-fitting software (GraphPad Prism, GraphPad Software, Inc., San Diego, CA).
Flow Cytometry
Flow cytometry was performed by using a FACScalibur machine using the following primary antibodies: anti-mouse CD4-APC, CD8-PerCp, CD3-FITC, CD25-PerCp, CD45R-APC, CD21-FITC, CD23-PE, IgD-PE, IgM-FITC, κ light chain-PE, CD138-APC, 7/4-PE, Ly6G-FITC, CD11c-PE, and CD40-FITC were procured from BD Pharmingen. Analysis of bone marrow cells was performed as described.23
Statistical Analysis
Data were expressed as mean ± SEM. Comparisons among groups were performed by using univariate analysis of variance. Posthoc Bonferroni’s correction was used for multiple comparisons. A value of P < 0.05 indicated statistical significance. Due to the serial sacrifice design (each blood sample from an individual animal), no statistical evaluation of the pharmacokinetic data was performed.
Results
Pharmacodynamics of 4SC-101
DHODH inhibition was confirmed in an in vitro enzyme assay, which allows one to monitor the catalytic activity of the enzyme by indirect measurement of the reduced co-substrate ubiquinone. 4SC-101 caused a concentration-dependent inhibition of DHODH. The inhibition data for 4SC-101 in different DHODH species are given in Table 1. Human peripheral blood mononuclear cells isolated from healthy donors were stimulated with 2 μg/ml phytohemagglutinin. 4SC-101 caused a concentration dependent inhibition of phytohemagglutinin-stimulated PBMC proliferation. The IC50 values were determined by nonlinear regression of the inhibition curves by using a Hill equation curve fitting. The resulting data are given in Table 1. The inhibition of PBMC proliferation by 4SC-101 could be abrogated by addition of 50 to 500 μmol/L of uridine to the assay mixture, supporting the inhibition of pyrimidine de novo synthesis as the mode of action (data not shown).
Table 1.
Pharmacodynamics of 4SC-101
| Inhibition of | 4SC-101 (IC50) |
|---|---|
| Human DHODH | 0.048 ± 0.006 μg/ml (0.134 ± 0.016 μmol/L) |
| Rat DHODH | 0.46 ± 0.17 μg/ml (1.29 ± 0.47 μmol/L) |
| Mouse DHODH | 3.8 ± 0.3 μg/ml (10.6 ± 0.7 μmol/L) |
| Human PBMC proliferation | 4.6 ± 2.1 μg/ml (12.9 ± 5.9 μmol/L) |
Pharmacokinetics of Single Dose 4SC-101 in MRLlpr/lpr Mice
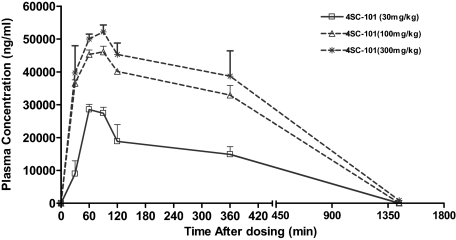
Pharmacokinetic studies were performed in 12-week-old female MRLlpr/lpr mice (n = 3 for each dose and time point). Pharmacokinetic parameters were calculated from geometric mean plasma levels per time point. Area under the curve was calculated by using the trapezoidal rule and extrapolated to infinity by adding Cz/λz. Key pharmacokinetic parameters are listed in Table 2. Plasma concentrations increased with increasing doses of 4SC-101, though, less than dose-proportionally (Figure 2). Maximum plasma levels were reached after 60 to 90 minutes, and the terminal half-life was 2.3 to 3 hours. Due to the lack of data points in the terminal phase, the values calculated for t1/2 were probably underestimated.
Table 2.
Pharmacokinetics of 4SC-101
| Dose, mg/kg | Cmax, μg/ml | tmax, h | AUC0-inf, μg/ml | t1/2, h |
|---|---|---|---|---|
| 30 | 28.4 | 1.0 | 229.0 | 2.3 |
| 100 | 46.0 | 1.5 | 499.7 | 2.3 |
| 300 | 52.1 | 1.5 | 583.3 | 3.0 |
Cmax, maximal concentration after single injection; tmax, time point of maximal concentration after single injection; AUC, area under the curve; t1/2, maximal concentration half time.
Figure 2.
Pharmacokinetics of 4SC-101 in mice. Twelve-week-old female MRLlpr/lpr mice received a single dose of either 30, 100, or 300 mg/kg 4SC-101 by oral gavage, and plasma samples were obtained at different intervals as indicated. The graph illustrates plasma 4SC-101 levels over time as geometric means of three data points per dose and sampling time.
Long-term 4SC-101 Treatment and B and T Cell Immunity in MRLlpr/lpr Mice
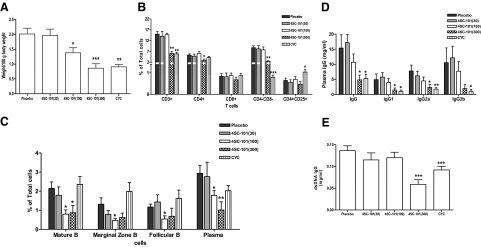
Next we assessed the effects of a long-term exposure with 4SC-101 on SLE in female MRLlpr/lpr mice. Mice were treated from 12 to 22 weeks of age with either 30, 100, or 300 mg/kg/day 4SC-101 by daily oral gavage. Additional groups of mice were treated either with placebo daily oral gavage or with CYC (30 mg/kg per week, i.p.) to serve as negative and positive controls, respectively. CYC and 300 mg/kg 4SC-101 were equipotent in reducing the weight of mesenterical lymph nodes at 22 weeks of age (Figure 3A). 4SC-101 at a dose of 100 mg/kg was less effective, and lymph node weight in the 30 mg/kg dose group was not significantly different from vehicle-treated MRLlpr/lpr mice (Figure 3A). Flow cytometry of spleen lymphocyte subsets revealed that 300 mg/kg 4SC-101 and CYC both significantly reduced the numbers of spleen CD3+ lymphocytes, mainly by reducing of CD4+ T helper cells and CD4/CD8 double negative “autoreactive” T cells (Figure 3B). By contrast, 300 mg/kg 4SC-101 did not significantly alter the CD8+ “cytotoxic” and CD4+/CD25+ “regulatory” T cell populations (Figure 3B). The lower doses of 4SC-101 had no significant effect on T cell populations in spleens of 22-week-old female MRLlpr/lpr mice. However, 100 and 300 mg/kg 4SC-101 both significantly reduced the B220+IgM+IgD+ “mature” B cells, the B220+CD21highCD23low “marginal zone” B cells, the B220+CD21lowCD23high “follicular” B cells, and the cytoplasmic κ+CD138+ plasma cells (Figure 3C). Interestingly, CYC did not significantly affect these B cell populations in MRLlpr/lpr mice, indicating that 4SC-101 has a predominant effect on B cells, which is different to the immunosuppressive effects of CYC. In addition, 300 mg/kg 4SC-101 and CYC significantly reduced serum total IgG and IgG isotype levels in 22-week-old female MRLlpr/lpr mice (Figure 3D). This effect did also apply to the production of lupus autoantibodies because anti-dsDNA IgG serum levels were only significantly reduced in the 300 mg/kg 4SC-101 and CYC groups (Figure 3E). Thus, reducing T cells is more important than reducing B cells to suppress hypergammaglobulinemia and autoantibody production in female MRLlpr/lpr mice, which was achieved by 300 mg/kg 4SC-101 and CYC.
Figure 3.
4SC-101 reduces systemic autoimmunity in MRLlpr/lpr mice. A: Mesenteric lymph node weight was determined at 22 weeks after ten weeks of vehicle, 4SC-101, or CYC treatment. Data are means ± SEM. *P < 0.05; ***P < 0.001 versus vehicle group. B and C: Spleen cell suspensions were prepared for flow cytometry by using specific antibodies that identify T cell (B) and B cell (C) subsets. Data are expressed as mean percentages of all splenocytes ± SEM. *P < 0.05; **P < 0.01 versus vehicle group. D and E: Serum samples were obtained at 22 weeks of age from mice of all groups, and total serum IgG and IgG isotype levels (D) and anti dsDNA IgG levels (E) were determined by enzyme-linked immunosorbent assay. Data are means ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001 versus vehicle group.
4SC-101 Improves Lupus Nephritis in MRLlpr/lpr Mice
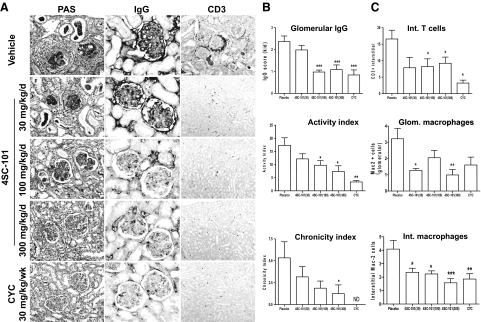
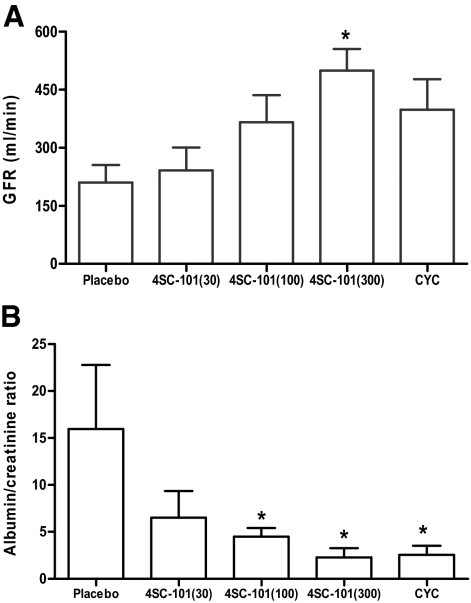
Female MRLlpr/lpr mice develop and subsequently die from proliferative immune complex glomerulonephritis with large similarities to diffuse proliferative lupus nephritis in humans.24 We treated female MRLlpr/lpr mice with 4SC-101, CYC, or vehicle from 12 to 22 weeks of age because at 12 weeks of age MRLlpr/lpr mice show first signs of proliferative immune complex glomerulonephritis and proteinuria.16 After 10 weeks of treatment, vehicle-treated MRLlpr/lpr mice showed diffuse proliferative immune complex glomerulonephritis characterized by glomerular IgG deposits, mesangioproliferative glomerulonephritis, considerable tubular atrophy, and interstitial fibrosis (Figure 4A). 4SC-101 reduced the glomerular IgG deposits and improved the lupus-like immune complex glomerulonephritis in female MRLlpr/lpr mice in a dose dependent manner (Figure 4A) as evidenced by semiquantitative morphometrical evaluation, eg, by assessing the activity and chronicity scores of lupus nephritis (Figure 4B). The activity score, a composite score of mostly glomerular abnormalities, and glomerular IgG deposits were significantly reduced by 100 as well as by 300 mg/kg 4SC-101 (Figure 4B). The chronicity score, a composite score of glomerular and interstitial scarring, was significant for the 300 mg/kg 4SC-101 group only. CYC was potent in reducing both parameters but not significantly different from 300 mg/kg 4SC-101. In 22-week-old vehicle-treated MRLlpr/lpr mice, lupus nephritis was associated with glomerular macrophage infiltration and a mixed periglomerular and interstitial inflammatory cell infiltrate consisting of glomerular and interstitial Mac2-positive macrophages and interstitial CD3-positive T cells (Figure 4A). CYC and 100 and 300 mg/kg 4SC-101 reduced renal macrophage and T cell infiltrates (Figure 4C). Next we determined the GFR as a functional marker of renal excretory function and urinary albumin/creatinine ratio as a marker of proteinuria, ie, glomerular filtration barrier dysfunction. 4SC-101 treatment was associated with an increase of GFR and a decrease of albuminuria in a dose-dependent manner (Figure 5, A and B). Doses of 100 or 300 mg/kg 4SC-101 were both as effective as CYC in improving both of these renal function parameters. We conclude that the dose-dependent impact of 4SC-101 on systemic autoimmunity translates into anti-inflammatory effects on lupus nephritis and improvement of renal dysfunction in MRLlpr/lpr mice.
Figure 4.
4SC-101 improves renal histopathology in MRLlpr/lpr mice. A: Renal sections of 22-week-old MRLlpr/lpr mice were stained with periodic acid Schiff and antibodies for IgG and CD3 (T cells) as indicated. Images are representative for seven to 12 mice in each group (original magnification, ×100 [PAS], ×400 [IgG], ×100 [CD3]). B: Morphometry was performed on IgG and PAS stained sections to quantify glomerular IgG deposits (score ranging from 0 to 3), the lupus nephritis disease activity index (score ranging from 0 to 24), and the lupus nephritis chronicity index (score ranging from 0 to 12). C: CD3+ T cells and Mac2+ interstitial cells were counted in 15 high power fields from renal sections of MRLlpr/lpr mice from all groups. Mac2+ cells were also counted in 15 glomeruli from mice of all groups of MRLlpr/lpr mice from all groups. Data represent means ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001 versus vehicle control group; ND, not detected.
Figure 5.
4SC-101 improves GFR and proteinuria in MRLlpr/lpr mice. At 22 weeks of age, GFR (A) and urinary albumin/creatinine ratios (B) were determined in MRLlpr/lpr mice from all groups as described in Materials and Methods. Note the dose-dependent increase in GFR and decline in albuminuria in 4SC-101-treated MRLlpr/lpr mice. Data represent means ± SEM. *P < 0.05 versus vehicle control group.
4SC-101 Reduces Cutaneous and Pulmonary SLE Manifestations of MRLlpr/lpr Mice
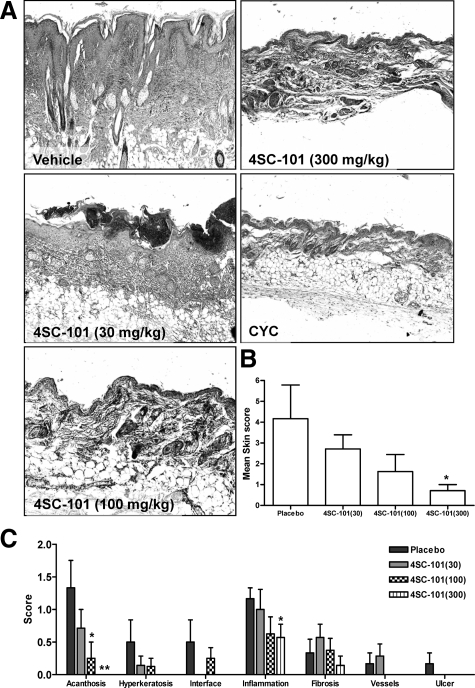
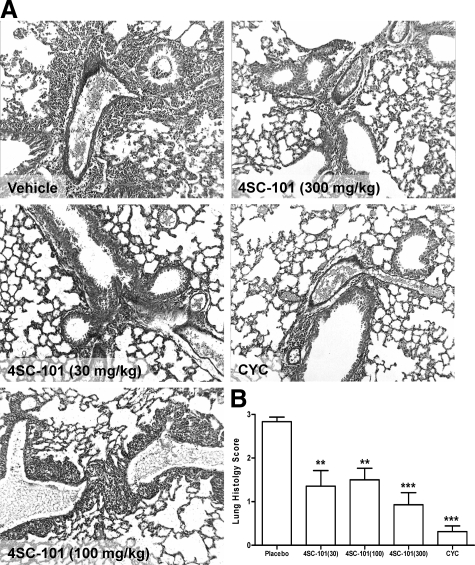
Skin and lungs are also commonly affected by autoimmune tissue injury in MRLlpr/lpr mice. In vehicle-treated mice, 6 of 11 mice suffered either from facial rash and/or discoid lesions in the neck area, few of them with neck ulcers. Histopathological evaluation of neck skin revealed various degrees of acanthosis, hyperkeratosis, widening of the interface, T cell infiltrates, dermal mucin and collagen deposits, and vasodilation (Figure 6A). The quantitative assessment of a composite score of all of these abnormalities revealed a dose-dependent improvement of skin disease in 4SC-101-treated female MRLlpr/lpr mice (Figure 6B). All of the aforementioned parameters were significantly improved by 300 mg/kg 4SC-101 except for dermal fibrosis (Figure 6C). None of the CYC-treated mice revealed any signs of skin disease. Autoimmune lung disease in MRLlpr/lpr mice was characterized by moderate to severe peribronchiolar and perivascular inflammatory cell infiltrates (Figure 7A). All dose regimens of 4SC-101 were effective in reducing lung pathology as evidenced by a semiquantitative lung injury score (Figure 7B). Obviously, higher doses of 4SC-101 are as potent as CYC to control kidney, skin, and lung pathology in autoimmune MRLlpr/lpr mice.
Figure 6.
4SC-101 and cutaneous lupus in MRLlpr/lpr mice. MRLlpr/lpr mice of all groups were regularly checked for skin manifestations, which typically occur in the facial or neck area. A: Representative images of hematoxylin and eosin stained skin biopsies from selected groups are shown as indicated (original magnification, ×100). A semiquantitative assessment of skin pathology allowed to calculate a composite skin injury score (B), which was derived from adding the scores of seven different criteria (C) as indicated. CYC-treated mice had no signs of skin disease. Data represent means ± SEM. *P < 0.05 versus vehicle control group, **P < 0.01 versus placebo control.
Figure 7.
4SC-101 improves autoimmune lung disease in MRLlpr/lpr mice. A: Lung sections of 22-week-old MRLlpr/lpr mice were stained with periodic acid Schiff. Images are representative for 7 to 12 mice in each group (original magnification, ×100). B: Morphometry was used to quantify the peribronchiolar and perivascular inflammation by using a score ranging from 0 to 3. Data represent mean scores ± SEM. **P < 0.01; ***P < 0.001 versus vehicle control group.
4SC-101 and Bone Marrow Cells in MRLlpr/lpr Mice
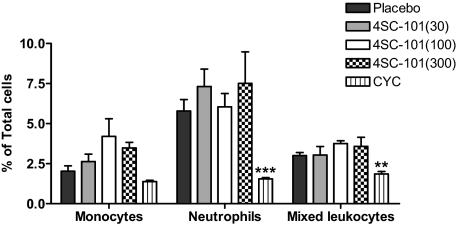
The therapeutic use of CYC is generally limited by myelosuppression, potentially causing fatal infectious complications, a side effect resulting from the unspecific antiproliferative mode of CYC action. We therefore performed flow cytometry on bone marrow cell suspensions to compare the effects of 4SC-101 and CYC on myelosuppression. CYC treatment significantly reduced the number of neutrophils in bone marrows to less than 30% and the mixed leukocyte population (monocytes, T cells and B cells) by 50% compared with vehicle-treated MRLlpr/lpr mice (Figure 8). By contrast, none of the 4SC-101 dose groups showed reduced bone marrow neutrophils (or macrophages) counts. We therefore conclude that a broad dose range of 4SC-101 does not cause myelosuppression in MRLlpr/lpr mice, which is one of the major risk factors for infectious complications during immunosuppressive therapy of SLE.
Figure 8.
4SC-101 and bone marrow cells in MRLlpr/lpr mice. Flow cytometry was used to quantify neutrophils and monocytes in bone marrows of 22-week-old female MRLlpr/lpr mice from all treatment groups. Monocytes were identified as 7/4bri Ly6G−. Neutrophils were identified as 7/4+Ly6G+. Data are shown as mean percentages ± SEM from at least five to six mice in each group. **P < 0.01, ***P < 0.001 versus vehicle control group.
Discussion
Immunosuppressants like CYC remain first-line treatment for severe SLE.3 However, many patients suffer from severe side effects like myelosuppression making patients susceptible to life threatening infections.2,3,4 Our data show that 4SC-101 is as effective as CYC in controlling SLE in MRLlpr/lpr mice but avoid these side effects.
The novel DHODH inhibitor 4SC-101 acts as a highly selective inhibitor of the de novo biosynthesis of pyrimidines, which inhibits the expansion of rapidly proliferating cells. DHODH inhibition was thought to be appropriate in SLE because the expansion of autoreactive lymphocytes demands large quantities of pyrimidine to support DNA synthesis, which renders autoreactive lymphocytes especially sensitive to the blockade of pyrimidine de novo synthesis.7 We found that 300 mg/kg of 4SC-101 can significantly reduce the numbers of CD4/CD8 double negative “autoreactive” T cells and plasma cells in spleen without affecting CD4/CD25+ “regulatory” T cells. This suppressive effect was sufficient to significantly reduce hypergammaglobulinemia and dsDNA autoantibodies as major hallmarks of polyclonal autoimmunity in SLE. Consistent with the concept of systemic autoimmunity driving tissue pathology,1,25 the reduction of autoreactive T and B cells was associated with almost complete prevention of cutaneous lupus lesions in all layers of the skin, improved renal pathology and function, and significant improvement of lung pathology. In terms of toxicity, CYC treatment caused significant myelosuppression as in our previous studies.15 Bone marrow 7/4+ monocytes and neutrophils were reduced by CYC treatment but not by 4SC-101, a finding not revealed by a study describing DHODH inhibition with leflunomide in murine SLE, basically because bone marrow was not analyzed.12
What is the clinical perspective of DHODH inhibition in human SLE? A double-blind, randomized, placebo-controlled study proved leflunomide to be effective in controlling diverse SLE manifestations in a small number of patients and during short-term follow-up.26 One patient withdrew from the study due to liver toxicity, and two patients discontinued treatment because of hypertension; both known side effects of leflunomide.7 The same group determined safety and efficacy of leflunomide in 17 patients with lupus nephritis with previous treatment-related toxicities, CYC contraindications or lack of response to CYC, azathioprine, or cyclosporin for at least 6 months.27 Leflunomide induced a partial remission in 13 of 17 patients and a complete remission in 5 of 17 patients, which serves as another proof-of-concept that DHODH inhibition can control severe SLE. However, leflunomide treatment was associated with hypertension in 6 of 17 patients and infections in 4 of 17 patients.27 Another trial compared efficacy and safety of leflunomide and CYC in 110 patients with biopsy-proven diffuse-proliferative lupus nephritis.28 Leflunomide was found to be equally effective as compared with CYC but caused the well known side effects.28 It is unknown whether these side effects are leflunomide-specific or a result of DHODH inhibition. The observation that leflunomide can induce cutaneous lupus may argue for drug-specific side effects rather than for DHODH inhibition.29,30,31 4SC-101 has been clinically tested in over 140 patients and healthy volunteers so far. In a randomized, double-blind, placebo-controlled sequential dose escalating phase I study, the safety, tolerability, and pharmacokinetics of ascending single oral doses of 4SC-101 (study number SC12267-1-2003) were evaluated in 42 healthy volunteers. In a corresponding phase I trial testing ascending multiple oral doses of 4SC-101 (study number SC12267-2-2004), additional 24 healthy volunteers have been treated with 4SC-101. In a randomized, double-blind, placebo-controlled phase IIa proof of concept study in rheumatoid arthritis (study number SC12267-3-2005), a total of 121 patients were randomized to three treatment groups (about 40 patients per group) to receive 20 mg tablets of 4SC-101, 35 mg tablets of 4SC-101, or matching placebo tablets once daily for a period of 12 weeks. In all completed trials, no liver toxicity, diarrhea, or arterial hypertension have been observed as treatment-related adverse events. 4SC-101 is currently being tested in two further ongoing phase II clinical trials: the “ENTRANCE” trial evaluating 4SC-101 in patients with inflammatory bowel diseases (http://www.clinicaltrials.gov, identifier: NCT00820365) and the “COMPONENT” trial assessing the efficacy of 4SC-101 in rheumatoid arthritis patients in combination with methotrexate (http://www.clinicaltrials.gov, identifier: NCT01010581).
In conclusion, so far 4SC-101 has been shown to be safe in human clinical trials without causing arterial hypertension, abnormal liver function tests, myelosuppression, or skin disease. Hence, 4SC-101 might be as effective as leflunomide or CYC in controlling severe SLE with fewer side effects.
Acknowledgments
The expert technical assistance of Dan Draganovic and Ewa Radomska is gratefully acknowledged.
Footnotes
Address reprint requests to Prof. Hans-Joachim Anders, Medizinische Poliklinik der LMU, Pettenkoferstr. 8a, 80336 Munich, Germany. E-mail: hjanders@med.uni-muenchen.de.
Supported by 4SC AG and Deutsche Forschungsgemeinschaft Graduiertenkolleg 1202 (M. Schnurr and H.-J.A.).
J.L., R.J., A.A., and R.D. are employees of 4SC AG, the manufacturer of 4SC-101.
4SC-101 is currently under clinical trial (http://www.clinicaltrials.gov); the “ENTRANCE” trial (identifier: NCT00820365) and the “COMPONENT” trial (identifier: NCT01010581).
References
- Kotzin BL. Systemic lupus erythematosus. Cell. 1996;85:303–306. doi: 10.1016/s0092-8674(00)81108-3. [DOI] [PubMed] [Google Scholar]
- D'Cruz DP, Khamashta MA, Hughes GR. Systemic lupus erythematosus. Lancet. 2007;369:587–596. doi: 10.1016/S0140-6736(07)60279-7. [DOI] [PubMed] [Google Scholar]
- Rahman A, Isenberg DA. Systemic lupus erythematosus. N Engl J Med. 2008;358:929–939. doi: 10.1056/NEJMra071297. [DOI] [PubMed] [Google Scholar]
- Austin HA, 3rd, Klippel JH, Balow JE, le Riche NG, Steinberg AD, Plotz PH, Decker JL. Therapy of lupus nephritis: controlled trial of prednisone and cytotoxic drugs. N Engl J Med. 1986;314:614–619. doi: 10.1056/NEJM198603063141004. [DOI] [PubMed] [Google Scholar]
- Boumpas DT, Austin HA, 3rd, Vaughn EM, Klippel JH, Steinberg AD, Yarboro CH, Balow JE. Controlled trial of pulse methylprednisolone versus two regimens of pulse cyclophosphamide in severe lupus nephritis. Lancet. 1992;340:741–745. doi: 10.1016/0140-6736(92)92292-n. [DOI] [PubMed] [Google Scholar]
- Appel GB, Contreras G, Dooley MA, Ginzler EM, Isenberg D, Jayne D, Li LS, Mysler E, Sanchez-Guerrero J, Solomons N, Wofsy D. Mycophenolate mofetil versus cyclophosphamide for induction treatment of lupus nephritis. J Am Soc Nephrol. 2009;20:1103–1112. doi: 10.1681/ASN.2008101028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffler M, Grein K, Knecht W, Klein A, Bergjohann U. Dihydroorotate dehydrogenase: profile of a novel target for antiproliferative and immunosuppressive drugs. Adv Exp Med Biol. 1998;431:507–513. [PubMed] [Google Scholar]
- Herrmann ML, Schleyerbach R, Kirschbaum BJ. Leflunomide: an immunomodulatory drug for the treatment of rheumatoid arthritis and other autoimmune diseases. Immunopharmacology. 2000;47:273–289. doi: 10.1016/s0162-3109(00)00191-0. [DOI] [PubMed] [Google Scholar]
- Liu S, Neidhardt EA, Grossman TH, Ocain T, Clardy J. Structures of human dihydroorotate dehydrogenase in complex with antiproliferative agents. Structure. 2000;8:25–33. doi: 10.1016/s0969-2126(00)00077-0. [DOI] [PubMed] [Google Scholar]
- Smolen JS, Kalden JR, Scott DL, Rozman B, Kvien TK, Larsen A, Loew-Friedrich I, Oed C, Rosenburg R. Efficacy and safety of leflunomide compared with placebo and sulphasalazine in active rheumatoid arthritis: a double-blind, randomised, multicentre trial. European Leflunomide Study Group. Lancet. 1999;353:259–266. doi: 10.1016/s0140-6736(98)09403-3. [DOI] [PubMed] [Google Scholar]
- Kaltwasser JP, Nash P, Gladman D, Rosen CF, Behrens F, Jones P, Wollenhaupt J, Falk FG, Mease P. Efficacy and safety of leflunomide in the treatment of psoriatic arthritis and psoriasis: a multinational, double-blind, randomized, placebo-controlled clinical trial. Arthritis Rheum. 2004;50:1939–1950. doi: 10.1002/art.20253. [DOI] [PubMed] [Google Scholar]
- Popovic S, Bartlett RR. Disease modifying activity of HWA 486 on the development of SLE in MRL/1-mice. Agents Actions. 1986;19:313–314. doi: 10.1007/BF01971235. [DOI] [PubMed] [Google Scholar]
- Leban J, Kralik M, Mies J, Gassen M, Tentschert K, Baumgartner R. SAR, species specificity, and cellular activity of cyclopentene dicarboxylic acid amides as DHODH inhibitors. Bioorg Med Chem Lett. 2005;15:4854–4857. doi: 10.1016/j.bmcl.2005.07.053. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick LR, Deml L, Hofmann C, Small JS, Groeppel M, Hamm S, Lemstra S, Leban J, Ammendola A: 4SC-101, a novel immunosuppressive drug, inhibits IL-17 and attenuates Colitis in two murine models of inflammatory bowel disease. Inflamm Bowel Dis. 2010 doi: 10.1002/ibd.21264 [DOI] [PubMed] [Google Scholar]
- Kulkarni O, Eulberg D, Selve N, Zollner S, Allam R, Pawar RD, Pfeiffer S, Segerer S, Klussmann S, Anders HJ. Anti-Ccl2 Spiegelmer permits 75% dose reduction of cyclophosphamide to control diffuse proliferative lupus nephritis and pneumonitis in MRL-Fas(lpr) mice. J Pharmacol Exp Ther. 2009;328:371–377. doi: 10.1124/jpet.108.142711. [DOI] [PubMed] [Google Scholar]
- Perez de Lema G, Maier H, Nieto E, Vielhauer V, Luckow B, Mampaso F, Schlondorff D. Chemokine expression precedes inflammatory cell infiltration and chemokine receptor and cytokine expression during the initiation of murine lupus nephritis. J Am Soc Nephrol. 2001;12:1369–1382. doi: 10.1681/ASN.V1271369. [DOI] [PubMed] [Google Scholar]
- Kulkarni O, Pawar RD, Purschke W, Eulberg D, Selve N, Buchner K, Ninichuk V, Segerer S, Vielhauer V, Klussmann S, Anders HJ. Spiegelmer inhibition of CCL2/MCP-1 ameliorates lupus nephritis in MRL-(Fas)lpr mice. J Am Soc Nephrol. 2007;18:2350–2358. doi: 10.1681/ASN.2006121348. [DOI] [PubMed] [Google Scholar]
- Patole PS, Schubert S, Hildinger K, Khandoga S, Khandoga A, Segerer S, Henger A, Kretzler M, Werner M, Krombach F, Schlondorff D, Anders HJ. Toll-like receptor-4: renal cells and bone marrow cells signal for neutrophil recruitment during pyelonephritis. Kidney Int. 2005;68:2582–2587. doi: 10.1111/j.1523-1755.2005.00729.x. [DOI] [PubMed] [Google Scholar]
- Chan OT, Paliwal V, McNiff JM, Park SH, Bendelac A, Shlomchik MJ. Deficiency in beta(2)-microglobulin, but not CD1, accelerates spontaneous lupus skin disease while inhibiting nephritis in MRL-Fas(lpr) nice: an example of disease regulation at the organ level. J Immunol. 2001;167:2985–2990. doi: 10.4049/jimmunol.167.5.2985. [DOI] [PubMed] [Google Scholar]
- Austin HA, 3rd, Muenz LR, Joyce KM, Antonovych TT, Balow JE. Diffuse proliferative lupus nephritis: identification of specific pathologic features affecting renal outcome. Kidney Int. 1984;25:689–695. doi: 10.1038/ki.1984.75. [DOI] [PubMed] [Google Scholar]
- Patole PS, Pawar RD, Lech M, Zecher D, Schmidt H, Segerer S, Ellwart A, Henger A, Kretzler M, Anders HJ. Expression and regulation of Toll-like receptors in lupus-like immune complex glomerulonephritis of MRL-Fas(lpr) mice. Nephrol Dial Transplant. 2006;21:3062–3073. doi: 10.1093/ndt/gfl336. [DOI] [PubMed] [Google Scholar]
- Qi Z, Whitt I, Mehta A, Jin J, Zhao M, Harris RC, Fogo AB, Breyer MD. Serial determination of glomerular filtration rate in conscious mice using FITC-inulin clearance. Am J Physiol Renal Physiol. 2004;286:F590–F596. doi: 10.1152/ajprenal.00324.2003. [DOI] [PubMed] [Google Scholar]
- Tsou CL, Peters W, Si Y, Slaymaker S, Aslanian AM, Weisberg SP, Mack M, Charo IF. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest. 2007;117:902–909. doi: 10.1172/JCI29919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen PL, Eisenberg RA. Lpr and gld: single gene models of systemic autoimmunity and lymphoproliferative disease. Annu Rev Immunol. 1991;9:243–269. doi: 10.1146/annurev.iy.09.040191.001331. [DOI] [PubMed] [Google Scholar]
- Goodnow CC. Multistep pathogenesis of autoimmune disease. Cell. 2007;130:25–35. doi: 10.1016/j.cell.2007.06.033. [DOI] [PubMed] [Google Scholar]
- Tam LS, Li EK, Wong CK, Lam CW, Szeto CC. Double-blind, randomized, placebo-controlled pilot study of leflunomide in systemic lupus erythematosus. Lupus. 2004;13:601–604. doi: 10.1191/0961203304lu1067sr. [DOI] [PubMed] [Google Scholar]
- Tam LS, Li EK, Wong CK, Lam CW, Li WC, Szeto CC. Safety and efficacy of leflunomide in the treatment of lupus nephritis refractory or intolerant to traditional immunosuppressive therapy: an open label trial. Ann Rheum Dis. 2006;65:417–418. doi: 10.1136/ard.2005.044248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HY, Cui TG, Hou FF, Ni ZH, Chen XM, Lu FM, Xu FF, Yu XQ, Zhang FS, Zhao XZ, Zhao MH, Wang GB, Qian JQ, Cai GY, Zhu TY, Wang YH, Jiang ZP, Li YN, Mei CL, Zou WZ. Induction treatment of proliferative lupus nephritis with leflunomide combined with prednisone: a prospective multi-centre observational study. Lupus. 2008;17:638–644. doi: 10.1177/0961203308089408. [DOI] [PubMed] [Google Scholar]
- Marzano AV, Ramoni S, Del Papa N, Barbareschi M, Alessi E. Leflunomide-induced subacute cutaneous lupus erythematosus with erythema multiforme-like lesions. Lupus. 2008;17:329–331. doi: 10.1177/0961203307087189. [DOI] [PubMed] [Google Scholar]
- Suess A, Sticherling M. Leflunomide in subacute cutaneous lupus erythematosus: two sides of a coin. Int J Dermatol. 2008;47:83–86. doi: 10.1111/j.1365-4632.2007.03333.x. [DOI] [PubMed] [Google Scholar]
- Chan SK, Hazleman BL, Burrows NP. Subacute cutaneous lupus erythematosus precipitated by leflunomide. Clin Exp Dermatol. 2005;30:724–725. doi: 10.1111/j.1365-2230.2005.01898.x. [DOI] [PubMed] [Google Scholar]