Abstract
Mutations in the myocilin gene are associated with juvenile and adult-onset primary open-angle glaucoma. However, the pathogenic mechanisms of myocilin-induced glaucoma are still largely unknown. To investigate these mechanisms, we developed stably transfected HEK293 cell lines expressing wild-type or mutant (Y437H and I477N) myocilins under an inducible promoter. Expression of two mutant myocilins led to different levels of endoplasmic reticulum stress and increased apoptosis after treatment of cells with hydrogen peroxide. The Y437H mutant myocilin cell line showed the highest sensitivity to the oxidant treatment. Several antioxidant genes were down-regulated in the Y437H mutant myocilin cell line, but not in other cell lines. The Y437H mutant myocilin cell line also produced more reactive oxygen species than other cell lines examined. Consistent with the data obtained in cultured cells, the endoplasmic reticulum stress marker, 78 kDa glucose-regulated protein, was up-regulated, whereas antioxidant proteins, paraoxonase 2 and glutathione peroxidase 3, were down-regulated in the eye angle tissue of 18-month-old transgenic mice expressing Y437H myocilin mutant. In addition, a pro-apoptotic factor, CCAAT/enhancer-binding protein-homologous protein, was up-regulated in the aged transgenic mouse angle tissue. Our results suggest that expression of mutated myocilins may have a sensitization effect, which can lead to a severe phenotype in combination with oxidative stress. Mutant myocilins may confer different sensitivity to oxidative stress depending on the mutation.
Glaucoma is a group of chronic optic neuropathies characterized by the death of retinal ganglion cells, leading to a specific deformation of the optic nerve, known as glaucomatous cupping, and progressive loss of the visual field. Glaucoma is one of the leading causes of irreversible blindness in the world. It is estimated to affect more than 60 million and blind about 4.5 million people worldwide.1 Elevated intraocular pressure (IOP) is the most important risk factor for the onset and progression of glaucoma. Different forms of glaucoma are classified by the appearance of the iridocorneal angle, which is important in regulating the drainage of aqueous humor from the eye. In primary open-angle glaucoma (POAG), the most common form of glaucoma in populations of European and African ancestry, there are no clear abnormalities in the geometry and appearance of the iridocorneal angle. Several theories have been suggested to explain possible deficiencies in the eye drainage structures that may lead to POAG.2,3 Family history of POAG, old age, black race, myopia, and low diastolic perfusion pressure are well established risk factors for POAG.2,4
It is now well demonstrated that mutations in the MYOCILIN (MYOC) gene are associated with elevated IOP and with POAG.5,6 They are found in more than 10% of cases of juvenile POAG occurring between the ages of 3 and 40 years4 and 3% to 4% of cases of adult onset POAG occurring after 40 years of age.5,7,8 The MYOC gene is expressed in various ocular and nonocular tissues with the highest levels of expression in the trabecular meshwork (TM) and sclera.6,9,10,11 It encodes a secreted protein that contains a leucine zipper in the N-terminus and the olfactomedin domain in the C-terminus.12,13,14 The olfactomedin domain was originally identified in the protein isolated from frog olfactory neuroepithelim.15 This domain was subsequently found in several proteins in species ranging from Caenorhabditis elegans to Homo sapiens.16
The functions of myocilin protein are still not well understood. Biochemical data indicate that myocilin may interact with several intracellular and extracellular matrix proteins.10,17,18,19,20,21 Our recent data demonstrated that myocilin interacts with several Wnt receptors in the Frizzled family as well as with Wnt antagonists in the secreted Frizzled-related protein family and Wnt inhibitory factor-1.22
The absence of open-angle glaucoma in an elderly woman homozygous for the Arg46Stop mutation23 as well as the absence of glaucoma in people hemizygous for MYOC24 suggests that the loss of functional myocilin is not sufficient for the development of glaucoma or critical for normal eye function. We suggested that the absence of a glaucoma phenotype with myocilin null mutations in the eye may be explained by the compensatory action of Wnt proteins.22
A glaucoma phenotype appears to be dependent on expression of mutated myocilin protein in eye tissues. More than 70 glaucoma-causing mutations have been identified in the MYOC gene and greater than 90% of them are located in the region encoding the olfactomedin domain.7,25 Mutations that are associated with a severe glaucoma phenotype reduce the solubility of myocilin, lead to retention of the protein in the endoplasmic reticulum (ER), and prevent its secretion.26,27,28,29 Moreover, secretion of wild-type myocilin is impeded in the presence of mutated myocilin protein, making these mutations dominant.26,29,30,31 Accumulation of mutated myocilin in ER may be deleterious for cells and lead to cell death.32,33 However, the expression of mutated myocilin allele (Tyr423His) specifically in the mouse iridocorneal angle did not lead to detectable TM cell death.34,35
Environment may contribute to the effects of mutated myocilin. In particular, it is well established that oxidative stress may be a factor in the progression of many diseases, including glaucoma.36,37 Endothelial-like cells of the TM are in a direct contact with aqueous humor containing reactive oxygen species (ROS). Recent data demonstrate that the TM shows the highest sensitivity to the consequences of oxidative stress among the tissues of the eye anterior chamber.38 Expression of some myocilin mutants may induce ER stress in vitro,32,39 and ER stress in turn may cause oxidative stress.40 We hypothesized that expression of mutated myocilin can make cells more sensitive to oxidative stress both in vitro and in vivo.
In this article we demonstrate that expression of Y437H mutant myocilin in cultured cells and in transgenic mice in vivo induces the unfolded protein response (UPR) and suppresses expression of a number of proteins involved in the response to oxidative stress. Expression of myocilin mutants made cells more sensitive to oxidative stress, and different mutants provided different levels of sensitivity. Our data suggest that antioxidative agents should be assessed as therapeutic agents for myocilin-induced glaucoma.
Materials and Methods
Plasmids
The pTRE-Tight expression vector (Clontech Laboratories, Mountain View, CA) was used to produce HEK293 Tet-on stably transfected cell lines. Human cDNAs encoding wild-type myocilin or I447N mutant myocilin cloned in the pCS2-FLAG vector and Y437H mutant myocilin cloned in the pCS2 vector10 were digested with BamH1 and XbaI (New England BioLabs, Ipswich, MA). The restriction fragments were cloned into the pTRE-Tight vector digested with the same enzymes. The identity of the constructs was confirmed by sequencing. Wild-type myocilin cDNA and four myocilin mutants (P370L, Y437H, I477N, and N480K) were cloned into the pRcCMV vector30 and were kindly provided by Dr. V. Raymond (Molecular Endocrinology and Oncology Research Center, Laval University Hospital Research Center, Québec City, Québec, Canada).
Generation of Stable Cell Lines
HEK293 Tet-On cell line (Clontech Laboratories) that constitutively expressed the tetracycline activator was co-transfected with one of the four plasmids (pTRE, pTRE-MYOC-FLAG, pTRE-Y437H MYOC, or pTRE-I477N MYOC-FLAG) and the linearized plasmid encoding the hygromycin resistance gene for selection in 10:1 ratios by using lipofectamine 2000 (Stratagene, La Jolla, CA). Stably transfected clones were selected in the medium containing G418 (100 μg/ml; Clontech Laboratories) and hygromycin B (400 μg/ml; Clontech Laboratories). Individual clones were isolated and analyzed by Western blotting for the expression of the transgene after the treatment with 1 μg/ml of doxycycline (DOX; Clontech Laboratories) for 48 hours.
Cell Culture and Treatment
Tet-on stably transfected cells were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% Tet system-proved fetal bovine serum (Clontech Laboratories), penicillin (100 U/ml), streptomycin (100 μg/ml), hygromycin B (200 μg/ml), and G418 (100 μg/ml) at 37°C in a humidified atmosphere of 5% CO2. Oxidative stress was induced by the incubation with 100 to 500 μmol/L H2O2 (Sigma, St. Louis, MO) in the medium containing 2% Tet system-proved fetal bovine serum for 24 hours. To induce ER stress in control experiments, vector control cells were treated with 3 μmol/L thapsigargin (Sigma) in medium containing 2% Tet system-proved fetal bovine serum for 24 hours.
Terminal Deoxynucleotidyl Transferase-Mediated dUTP Nick-End Labeling Assay
Apoptotic cells were detected by a terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay by using a DeadEnd colorimetric TUNEL system (Promega, Madison, WI). Tet-on stably transfected cells that had been grown on CC2 treated four-well glass slides (Nalge Nunc International, Naperville, IL) were fixed in 4% paraformaldehyde and stained to detect apoptotic cells by following the manufacturer’s instructions.
Trypan Blue Exclusion Assay
Cells were incubated with H2O2 as described above, and then detached from the culture plates by using 0.05% trypsin and 0.5 mmol/L EDTA solution. Trypsin activity was quenched by using a soybean trypsin inhibitor (0.05 mg/ml; Sigma). Subsequently, an equal volume of 0.4% (w/v) trypan blue solution (Gibco-BRL, Grand Island, NY) was added to the cell suspension, and unstained (viable) and stained (nonviable) cells were counted by using a hemocytometer under an optical microscope. The ratio of dead cells was determined as a percentage of stained cells relatively total cells.
Western Blotting
The mouse iridocorneal angle tissues (the ciliary body, trabecular meshwork, and base of the iris and cornea) were dissected, homogenized in the lysis buffer (50 mmol/L Tris, pH 7.5, 5 mmol/L EDTA, 20 mmol/L dithiothreitol, 0.2% SDS, 1% Triton X-100, and 1% Tween 20), and centrifuged to remove insoluble material. Proteins of the soluble fraction (20 μg) were separated by NuPAGE 4–12% gradient Bis-Tris gel (Invitrogen, Carlsbad, CA) and transferred to a nitrocellulose membrane (Invitrogen). Membranes were pre-incubated in a blocking buffer (5% nonfat milk, 25 mmol/L Tris, 150 mmol/L NaCl, 0.05% Tween 20, pH 7.4) and then incubated with antibodies against myocilin (1:2000),41 anti-78 kDa glucose-regulated protein (GRP78; 1:500; Santa Cruz Biotechnology, Santa Cruz, CA), glutathione peroxidase 3 (GPx-3; 1:500; Abcam, Cambridge, MA), paraoxonase 2 (PON2; 1:750; Proteintech Group, Chicago, IL), or HSC70 (1:2000; Santa Cruz Biotechnology) in blocking buffer overnight at 4°C. Secondary antibodies (an anti-rabbit or anti-mouse horseradish peroxidase antibody; Amersham, Piscataway, NJ) were diluted 1:5000 in a blocking buffer and incubated for 2 hours at room temperature. Stably or transiently transfected cells were lysed in Mg2+ lysis buffer (Upstate, Temecula, CA) containing a protease inhibitor cocktail (Roche, Mannheim, Germany). The following antibodies and dilutions were used: anti-myocilin (1:1000; R&D Systems, Minneapolis, MN), GRP78 (1:1000; Cell Signaling Technology, Beverly, MA), anti-protein disulfide isomerase (PDI; 1:1000; BD Bioscience, San Diego, CA), anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH; 1:2000; Abcam), anti-cleaved poly (ADP-ribose) polymerase (PARP; 1:1000; Cell Signaling Technology), or anti-PON2 (1:1500; Proteintech Group). The immunoreactive bands were developed by using SuperSignal WestDura (Pierce, Rockford, IL). For the quantification, scanned images were analyzed by Image J software (NIH, Bethesda, MD).
Quantitative RT-PCR
Total RNA was extracted from Tet-On cells by using a Trizol reagent (Invitrogen). cDNA was obtained by reverse transcription of mRNA by using Oligo(dT) as a primer and a SuperScript III first-strand synthesis system (Invitrogen). Forward and reverse primers used are listed in Table 1. Primers for GAPDH were used as an internal control. Quantitative PCR was performed by using a SYBR Green PCR Master Mix (ABI, Foster City, CA) and a 7900HT Real Time Thermocycler (ABI).
Table 1.
Primers Used for Quantitative Real-Time PCR
| Target gene | Gene symbol | Sequence |
|---|---|---|
| Myocilin | MYOC | F: 5′-AGACACCGGATATGGAGAAC-3′ |
| R: 5′-CTCGCATCCACACACCATAC-3′ | ||
| Glyceraldehyde-3-phosphate dehydrogenase | GAPDH | F: 5′-GGAGTCCACTGGCGTCTTCAC-3′ |
| R: 5′-GAGGCATTGCTGATGTTGAGG-3′ | ||
| Glutathione peroxidase 3 | GPx-3 | F: 5′-ATGCTGGCAAATACGTCCTC-3′ |
| R: 5′-AGAATGACCAGACCGAATGG-3′ | ||
| Apolipoprotein E | APOE | F: 5′-GGTCGCTTTTGGGATTACCT-3′ |
| R: 5′-TCCAGTTCCGATTTGTAGGC-3′ | ||
| Paraoxonase 2 | PON2 | F: 5′-TGATTCAGCAAATGGGATCA-3′ |
| R: 5′-CCACCAGTGTATCCAGCTCA-3′ | ||
| Isocitrate dehydrogenase 1 | IDH1 | F: 5′-ACAGGAGACGTCCACCAATC-3′ |
| R: 5′-GCTTTGCTCTGTGGGCTAAC-3′ |
F, forward; R, reverse.
Detection of Intracellular ROS Accumulation
After treatment with doxycycline for 48 hours, cells were washed with warm PBS and then incubated in PBS containing 5 μmol/L 2′7′-dichlorodihydrofluorescein diacetate (Molecular Probes, Eugene, OR) at 37°C for 30 minutes. Subsequently, the cells were washed twice with PBS and observed by using an Axioplan-2 fluorescence microscope (Carl Zeiss, Jena, Germany) with excitation at 488 nm and emission at 530 nm. Fluorescent images were captured by using an Axioplan-2 microscope equipped with an Axiocam HRc digital camera. Pseudo colored images from the captured image were obtained by using Image J (NIH).
Eye Section and Immunohistochemistry
Mouse eyes were fixed in 10% neutral buffered formalin at 4°C for 24 hours before processing for paraffin embedding. Deparaffinized sections were examined under the Axioplan-2 fluorescence microscope (Carl Zeiss). The thickness of the region that included the TM, Schlemm’s canal, and surrounding tissues was measured by using Axiovision 4.7 software (Carl Zeiss). Immunohistochemistry was performed under the same conditions for wild-type and transgenic animals. Deparaffinized sections were incubated with antibodies against GRP78 (1:100; Santa Cruz Biotechnology), GPx-3 (1:100; Novus Biologicals, Littleton, CO), or CCAAT/enhancer-binding protein-homologous protein (CHOP; 1:50; Santa Cruz Biotechnology) in blocking buffer (2% normal goat serum, 0.2% Triton X-100 in PBS) for 3 hours at room temperature. The signals were visualized by staining with anti-rabbit antibody conjugated to Alexa 594 fluorophores (1:500; Molecular Probes) and 4′,6′-diamidino-2-phenylindole (DAPI; Molecular Probes). Images were collected by using an Axioplan-2 fluorescence microscope.
Microarray Analysis
RNA was extracted from control and myocilin expressing cells 48 hours after induction with selected concentrations of DOX by using a Trizol reagent (Invitrogene). Ten micrograms of total RNA was used for each microarray study. Gene expression profiling was performed as a service by Expression Analysis, Inc. (Durham, NC) by using the Human Affymetrix Gene Chip U133 Plus 2.0 (Affymetrix, Inc., Santa Clara, CA). The microarray gene expression was analyzed with the Affymetrix Microarray Suite software.
Results
Characterization of HEK293 Cell Lines Expressing Wild-Type and Mutated Myocilin
Prolonged expression of mutated myocilin may be deleterious to cells. Therefore, to study effects of wild-type and mutated myocilin on cell physiology and potential contribution of oxidative stress to these effects, we produced several HEK293 cell lines stably transfected with wild-type or mutated myocilin under the control of the tetracycline-inducible promoter. Two myocilin mutants associated with severe juvenile POAG, Y437H, and I477N42 were selected for cell line production. These two myocilin mutants produce a very similar glaucoma phenotype with maximum IOP of 40 to 44 mm Hg and a mean age at diagnosis of glaucoma of 20 to 21 years.8
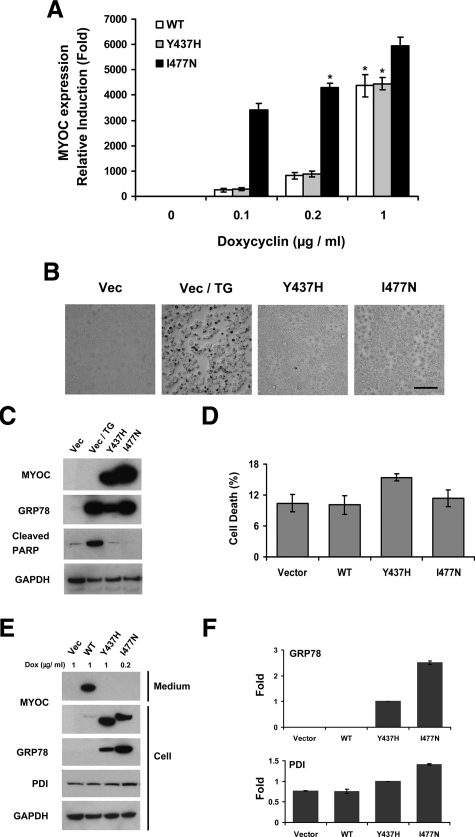
Several independent individual HEK293 cell lines were isolated after transfection with the vector, wild-type myocilin, Y437H, and I477N constructs. No myocilin expression was detected in any cell line without DOX induction. In the absence of DOX, all cell lines showed similar morphology, growth rate, and sensitivity to the treatment of thapsigargin or hydrogen peroxide (not shown). Individual myocilin-transfected cell lines demonstrated different levels of myocilin expression after stimulation with 1 μg/ml of DOX as estimated by Western blot experiments using antibodies against human myocilin (not shown). Lines demonstrating high inducibility were selected for subsequent experiments. Generally, expression levels of transgenes can be profoundly affected by position effect variation.43 It is possible that the I477N transgene may be inserted near an enhancer, which would explain higher inducibility of this mutant line compared with other lines. To find concentrations of DOX that produce similar levels of induction for different constructs, we used real-time PCR (Figure 1A). One microgram per milliliter of DOX for wild-type myocilin and the Y437H mutant and 0.2 μg/ml of DOX for the I477N mutant gave similar levels of MYOC mRNA induction. These DOX concentrations were selected for further experiments.
Figure 1.
Characterization of stably transfected HEK293 cell lines expressing wild-type and mutated myocilins. A: Established Tet-on HEK293 cells harboring a plasmid encoding wild-type, Y437H mutant, or I477N mutant myocilin were cultured in the presence of indicated concentration of DOX for 48 hours. Total RNA was isolated and the MYOC gene expression levels were quantified by quantitative PCR with a MYOC-specific primer set. Asterisks indicate the selected concentrations of DOX that were used in most subsequent experiments. Error bars represent ± SD of triplicate cultures. B: Tet-on HEK293 cells were cultured in media containing high concentration (5 μg/ml) of DOX for 48 hours. For the thapsigargin (TG) treatment, vector control cells were incubated with 3 μmol/L TG for 24 hours. Apoptotic cells were examined by the TUNEL assay. Dark spots represent apoptotic cells. Scale bar = 100 μm. C: Cell lysates from the HEK293 cells that were cultured as in B were immunoblotted with anti-myocilin, anit-GRP78, anti-cleaved PARP, and anti-GAPDH antibodies. D: The Tet-on HEK293 cells were cultured for 10 days in the medium containing 1 μg/ml DOX for vector, wild-type, and Y437H mutant myocilin cell lines or 0.2 μg/ml DOX for the I477N mutant myocilin cell line. The media were replaced every two days. The ratio of dead cells to total cells was determined by Trypan Blue staining. Error bars represent ± SD of triplicate cultures. E: The Tet-on HEK293 cells were cultured in the presence of indicated concentration of DOX for 48 hours. Cell lysates or conditioned media were immunoblotted with anti-myocilin, anti-GRP78, anti-PDI, and anti-GAPDH antibodies. Lower mobility of wild-type and the I477N myocilin mutant is explained by the presence of the FLAG tag at their C-terminus. F: Band densities for GRP78 and PDI in E were quantified by using Image J software. Comparisons of densitometry values were made relative to their value in Y437H mutant myocilin cell line. Error bars represent ± SD.
It has been previously reported that expression of myocilin mutants in TM cells in vitro may be cytotoxic32 and lead to cell death after long exposure.33 To test whether expression of myocilin mutants leads to cell death in our conditions, TUNEL assays were performed with cells expressing mutated myocilin 2 days after induction of myocilin expression with selected concentration of DOX or with 5 μg/ml of DOX producing the highest levels of myocilin induction. Even at a high DOX concentration, control and myocilin-expressing cells showed similar morphology and did not show significant apoptotic cell death as judged by the TUNEL assay, whereas treatment of cells with 3 μmol/L of thapsigargin, a strong inducer of the mammalian ER stress through the perturbance of calcium homeostasis, induced apoptotic cell death (Figure 1B). The absence of apoptotic cell death after expression of mutated myocilin for 2 days was confirmed by the absence of cleaved PARP-1. PARP-1 is proteolytically cleaved in the course of apoptosis producing two fragments.44 Thapsigargin treatment (3 μmol/L) induced the formation of a cleaved form of PARP-1, whereas expression of mutated myocilin did not produce higher levels of cleaved PARP-1 as compared with control samples (Figure 1C). Although expression of wild-type or mutated myocilins for 2 days did not induce detectable apoptotic cell death, their prolonged expression slightly increased the number of nonviable cells as compared with cells stably transfected with vector as judged by staining with Trypan Blue (Figure 1D).
As expected, wild-type myocilin was efficiently secreted from expressing cells and detected preferentially in conditioned medium, whereas both myocilin mutants were not secreted and located intracellularly (Figure 1, C and E). Inside the cells, mutated myocilins accumulated in ER. Accumulation of mutated myocilin in ER induced UPR, a major response to ER stress, as judged by the up-regulation of two end products of UPR, BiP/GRP78, and PDI (Figure 1, E and F). Expression of wild-type myocilin did not induce up-regulation of GRP78 and PDI when compared with control cells. I477N mutant induced higher levels of GRP78 and PDI when compared with Y437H mutant, and both mutants induced higher levels of GRP78 and PDI compared with wild-type myocilin (Figure 1, E and F).
Expression of Myocilin Mutants Induces Oxidative Stress in HEK293 Cells
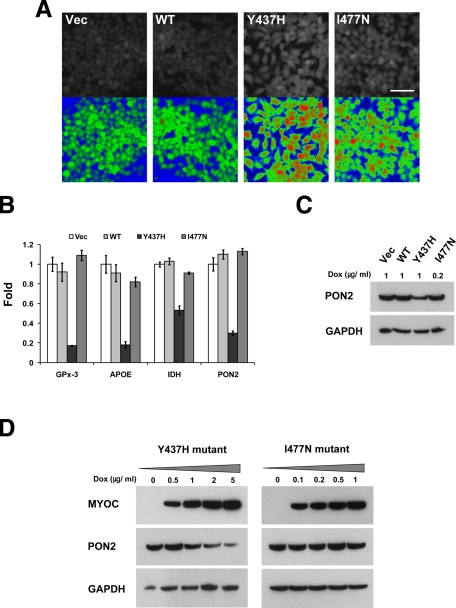
It has been reported that misfolding of new synthesized coagulation factor VIII leads to ER stress, causes oxidative stress, and induces apoptosis in vitro and in vivo.40 Recent data show that transfection of human TM cells with the P370L myocilin mutant also increases ROS levels.45 To test whether expression of wild-type and mutated myocilins may induce oxidative stress and lead to elevation of the ROS levels in HEK293 cells, intracellular ROS of individual cell lines were stained by using a fluorescent dye, 2′7′-dichlorodihydrofluorescein diacetate, 2 days after myocilin induction. Expression of both myocilin mutants in HEK293 cells increased the amount of ROS as compared with wild-type and control cells (Figure 2A). Although the I477N myocilin mutant induced higher expression levels of proteins involved in UPR, the Y437H mutant produced a higher level of ROS induction.
Figure 2.
Y437H mutant myocilin reduces the expression of several genes encoding antioxidant proteins. A: Tet-on HEK293 cells were cultured for 48 hours in the medium containing 1 μg/ml DOX for vector, wild-type, and the Y437H mutant myocilin cell line or 0.2 μg/ml DOX for the I477N mutant myocilin cell line. Intracellular ROS was monitored with a fluorescent indicator dye, 5 μmol/L Chloromethyl-2’7’-dichlorodihydrofluorescein. All images were taken at the same exposure setting. Images in the lower row were pseudo-colored according to fluorescence intensity. Red color represents high concentration of ROS and blue color represents low concentration of ROS. Scale bar = 50 μm. B: Tet-on HEK293 cells were cultured as in A. Total RNA was prepared, and quantitative PCR was performed with specific primer sets for each gene. All comparisons of the gene expression levels were made relative to their expression levels in control (vector) cells. Error bars represent ± SD of triplicate cultures. C: Cell lysates from the above cells were immunoblotted with anti-PON2 and anti-GAPDH antibodies. D: Y437H mutant (left) or I477N mutant (right) myocilin was induced by the adding of indicated concentration of DOX for 48 hours. Cell lysates were immunoblotted with anti-myocilin, anti-PON2, and anti-GAPDH antibodies.
To investigate possible mechanisms by which the Y437H myocilin mutant enables cells to produce a higher level of ROS than the I477N myocilin mutant, we compared genome-wide expression profiles of two mutant myocilin cell lines by using a microarray analysis. The gene expression profiles were analyzed in control and myocilin expressing HEK293 cells 2 days after myocilin induction (M.K.J. and S.I.T., unpublished). We paid special attention to genes involved in cell protection against oxidative stress. Some of them were specifically down-regulated in cells expressing the Y437H myocilin mutant as compared with cells expressing the I477N mutant (Table 2).
Table 2.
Antioxidant Genes That Were Down-Regulated in the Cell Line Expressing the Y437H Myocilin Mutant
| GenBank accession no. | Gene product | Gene symbol | Fold change (Y437H versus I477N) |
|---|---|---|---|
| NM_002084 | Glutathione peroxidase 3 | GPx-3 | −4.90 |
| NM_000041 | Apolipoprotein E | APOE | −3.68 |
| NM_000783 | Cytochrome P450, family 26, subfamily A, polypeptide 1 | CYP26A1 | −3.93 |
| NM_000305 | Paraoxonase 2 | PON2 | −2.49 |
| U94592 | Uncoupling protein 2 | UCP2 | −2.27 |
| BC012846 | Isocitrate dehydrogenase 1 | IDH1 | −2.31 |
To verify microarray data, we measured the expression levels of four genes identified by microarray hybridization by using real-time PCR. The results showed that expression levels of four selected genes, GPx-3, apolipoprotein E, isocitrate dehydrogenase, and PON2, were significantly reduced in cells expressing the Y437H mutant as compared with cells expressing wild-type myocilin or the I477N mutant (Figure 2B). For PON2, this result was confirmed by Western blotting (Figure 2C). Increasing levels of Y437H mutant myocilin led to further reduction of PON2 levels (Figure 2D, left panel). At the same time, increasing levels of the I477N myocilin mutant did not reduce PON2 levels (Figure 2D, right panel). Although the expression of the GPx-3 gene was well detected in our cell lines by quantitative PCR, we could not detect the corresponding protein by Western blotting (not shown). GPx-3 contains selenium at its active site in a form of selenocysteine.46 It has been reported that selenium in culture media is not efficiently used by cultured cells.47 Therefore, these cells may not efficiently produce selenoproteins in vitro.
Expression of Mutated Myocilins Makes HEK293 Cells More Sensitive to Oxidative Stress
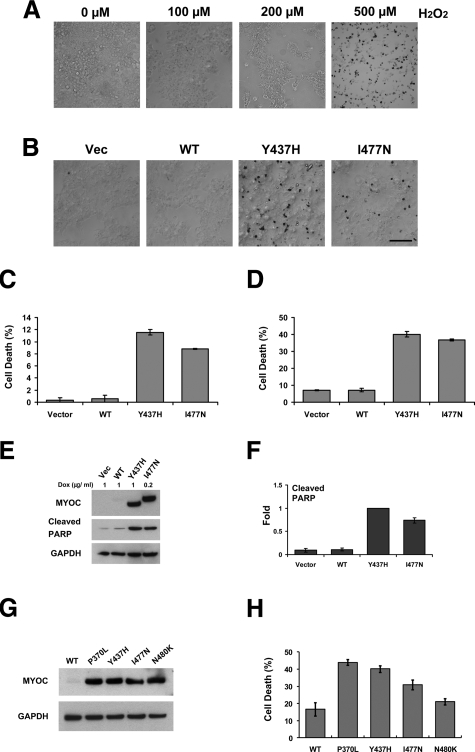
Because expression of mutated myocilins increased intracellular ROS levels, we hypothesized that cells expressing mutated myocilins might have different sensitivity to oxidative stress. To induce oxidative stress, we treated cells with H2O2. First, we demonstrated that treatment of control HEK293 cells with H2O2 does not induce apoptosis when concentration of H2O2 was below 200 μmol/L (Figure 3A). A 500 μmol/L concentration of H2O2 produced multiple apoptotic cells (Figure 3A). A 100 μmol/L concentration of H2O2, which did not produce visible damaging effect in control HEK293 cells, was selected for further experiments.
Figure 3.
Accumulation of mutant myocilins sensitizes cells to oxidative stress. A: Vector control cells were cultured in the media containing indicated concentration of H2O2 for 24 hours. Apoptotic cells (dark cells) were detected by the TUNEL assay. Only few apoptotic cells were detected when concentration of H2O2 was not more than 200 μmol/L. B: Tet-on HEK293 cells were pre-incubated for 24 hours with 1 μg/ml DOX for vector, wild-type, and the Y437H mutant myocilin cell line or 0.2 μg/ml DOX for the I477N mutant myocilin cell line, and then they were cultured in the media containing 100 μmol/L H2O2 and DOX for additional 24 hours. Apoptotic cells were identified by the TUNEL assay. Scale bar = 100 μm. C: TUNEL positive cells in B were counted. Error bars represent ± SD of triplicate cultures. D: Dead cells were counted by using a hemocytometer after Trypan Blue staining. Error bars represent ± SD of triplicate cultures. E: Cells were incubated as in B. Cell lysates were immunoblotted with anti-myocilin, anti-cleaved PARP, and anti-GAPDH antibodies. F: Band densities for cleaved PARP in E were quantified by using Image J software. Comparisons were made relative to the Y437H mutant myocilin cell line. Error bars represent ± SD. G: HEK293 cells were transiently transfected with constructs expressing wild-type or one of four different mutant myocilins. Cell lysates were prepared 48 hours after transfection and were immunoblotted with anti-myocilin and GAPDH antibodies. H: Transiently transfected HEK293 cells were cultured with 100 μmol/L H2O2 for 24 hours, and then the ratio of dead cells to total cells was determined by Trypan Blue staining. Error bars represent ± SD of triplicate cultures.
When cells expressing wild-type myocilin were treated with 100 μmol/L H2O2, no apoptotic cells were observed by TUNEL (Figure 3B). However, similar treatment of cells expressing mutated myocilins produced significant amounts of apoptotic cells. Cells expressing the Y437H mutant showed higher sensitivity to H2O2 than cells expressing the I477N mutant (Figure 3B). Of Y437H mutant myocilin cells and of I477N mutant myocilin cells, 11.6% ± 0.5% and 8.8% ± 0.1% were identified as TUNEL-positive, respectively (Figure 3C). After H2O2 treatment, more detached cells were observed in the two mutant myocilin cell lines as compared with control and wild-type myocilin cell lines. The number of dead cells was evaluated from the total cells, including the detached cells by Trypan Blue exclusion assay. Similar to the results shown in Figure 3C, both cell lines containing myocilin mutants showed increased cell death with Y437H mutant showing higher sensitivity to H2O2 than I477N mutant (Figure 3D). The number of Trypan Blue stained cells were higher than that of TUNEL positive cells, indicating that a large portion of the detached cells were dead cells. These results were confirmed in transient transfection experiments. HEK293 cells were transiently transfected with constructs encoding wild-type myocilin and four mutants, P370L, Y437H, I477N, and N480K. Four mutant constructs showed similar levels of expression as judged by Western blotting (Figure 3G). Cells expressing the P370L myocilin mutant were the most sensitive, and cells expressing the N480K mutants were the least sensitive to the H2O2 treatment (Figure 3H). Cells expressing the Y437H mutant were more sensitive to the treatment than cells expressing the I477N mutant, confirming that different sensitivities between mutant myocilin-containing cell lines is not due to artifact induced by the FLAG tag. A modification of cell membrane in the course of transient transfection generated additional sensitivity to the oxidative damage in these cells as compared with stably transfected cells (compare numbers for control cells in Figures 3, D and H).
Expression of mutated myocilins did not induce PARP cleavage by itself (Figure 1C), whereas the treatment of cells expressing mutant, but not wild-type, myocilins with H2O2 induced PARP cleavage. A slightly higher level of cleaved PARP was detected in the Y437H cell line as compared with the I477N cell line (Figure 3, E and F).
We concluded that expression of mutated myocilins makes cells more sensitive to oxidative stress as compared with cells expressing wild-type myocilin with the Y437H mutant providing higher sensitivity than I477N mutant.
Expression of the Y437H Mutant in Eye Angle Tissues of Transgenic Mice Induces UPR and Reduces the Levels of Oxidative Stress-Protective Proteins
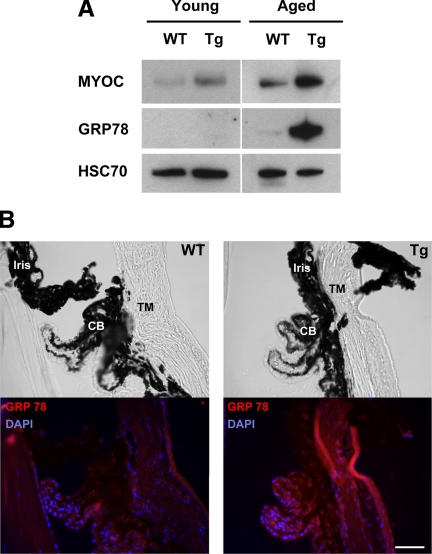
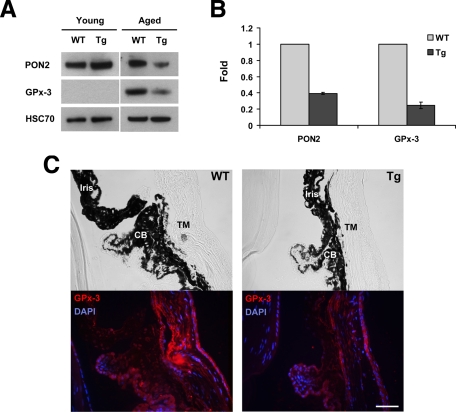
Previously, we have produced transgenic mice expressing the Y437H myocilin mutant in the eye angle tissues.48 These mice demonstrated moderate elevation of IOP and degeneration of retinal ganglion cells in the peripheral retina.48 Such changes were detected only in aged (15 to 18 months old) but not young (4 to 6 months old) transgenic mice.48 Young (4 months old) and aged (16 months old) transgenic mice showed elevated combined levels of mutated and wild-type myocilins as compared with wild-type littermates probably due to expression of mutated myocilin (Figure 4A). Aged animals showed higher combined levels of myocilin than young animals. To test whether some effects induced by the expression of mutated myocilin in HEK293 cells in vitro may be also observed in transgenic animals in vivo, we first checked the induction of UPR in the eye angle tissues of young and aged mice by using BiP/GPR78 as a marker. The expression of BiP/GRP78 was not detected in young transgenic animals when low levels of mutant myocilin were present in the eye angle tissues. However, accumulation of higher levels of mutated myocilin in aged animals induced a dramatic up-regulation of BiP/GRP78, indicating the induction of UPR (Figure 4A). Immunostaining of eye sections from aged mice with antibodies against GRP78 showed elevated levels of staining in the ciliary body, TM, and sclera (Figure 4B). Next, we checked the levels of two enzymes involved in the defense against oxidative stress, PON2, and GPx-3 in the angle tissues. We did not find any difference in the levels of these enzymes between young transgenic and wild-type mice. High levels of PON2 were detected in both transgenic and wild-type mice, whereas GPx-3 was not detected in wild-type and transgenic animals (Figure 5A). However, in aged mice, the levels of PON2 were significantly reduced in transgenic versus wild-type mice similar to in vitro results. We were also able to measure the levels of GPx-3, which were also dramatically reduced in the eyes of transgenic mice (Figure 5A). Quantitatively, PON2 and GPx-3 were reduced by 70% and 80%, respectively, in the eye angle tissues of transgenic as compared with their wild-type littermates (Figure 5B). Up-regulation of a protein involved in UPR (GRP78) and down-regulation of a protein participating in the defense against oxidative stress (GPx-3) were observed in an independent line of transgenic mice expressing the Y437H human myocilin mutant (not shown).
Figure 4.
GRP78 is up-regulated in the eye angle tissue of aged transgenic mice expressing Y437H mutant myocilin. A: Lysates from the dissected angle tissue of young (four months old) and aged (16 months old) wild-type or transgenic mice were immunoblotted with anti-myocilin, anti-GRP78, and anti-HSC70 antibodies. HSC70 was used for internal control. B: Eye sections of aged wild-type and transgenic mice were stained with anti-GRP78 antibody and DAPI (lower row). Upper row shows the bright field images. CB, ciliary body. Scale bar = 50 μm.
Figure 5.
Decrease in the levels of antioxidant proteins in the eye angle tissue of aged transgenic mice expressing Y437H mutant myocilin. A: Lysates from the dissected angle tissue of young and aged wild-type or transgenic mice were immunoblotted with anti-PON2, anti-GPx-3, and anti-HSC70 antibodies. Two pairs of mice for PON2 and three pairs of mice for GPx-3 were analyzed. HSC70 was used for internal control. B: Quantification of the results shown in A. Error bars represent ± SD. C: Eye sections of wild-type and transgenic mice were stained with anti-GPx-3 antibody and DAPI (lower row). Upper row shows the bright field images. CB, ciliary body. Scale bar = 50 μm.
Immunostaining of the eye sections from aged mice with antibodies against GPx-3 showed that the TM, the main site of myocilin expression, showed the most pronounced reduction in the levels of GPx-3 (Figure 5C).
We concluded that, similar to the cell culture experiments, high-level accumulation of the Y437H myocilin mutant in the eye angle tissues of aged transgenic animals induced UPR and reduced the levels of antioxidative enzymes.
Expression of the Y437H Mutant in the Eye Angle Tissues of Transgenic Mice Markedly Increases Levels of Pro-Apototic Protein, CHOP
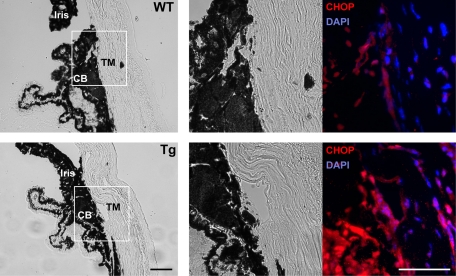
Analysis of eye sections produced from three pairs of aged wild-type and transgenic mice demonstrated that the region that included the TM, Schlemm’s canal, and surrounding tissues was thinner in transgenic than in wild-type mice, indicating that these tissues may be altered by the expression of the Y437H myocilin mutant. To investigate whether Y437H mutant myocilin-induced stress is capable of threatening survival of TM and surrounding tissues, we compared CHOP expression in aged transgenic and wild-type mice. CHOP is induced when ER stress is not alleviated and persists.49 CHOP is a key pro-apoptotic transcription factor, which can inhibit anti-apoptotic proteins such as Bcl-2 family members and promote apoptotic caspase activity.50 CHOP overexpression leads to cell cycle arrest and apoptosis, but the deletion of the CHOP gene protects cells and animals from cell death induced by ER stress.49,51 Therefore, induction of CHOP expression indicates that ER initiated apoptosis may be prompted. Immunostaining of the eye sections from aged mice with antibodies against CHOP showed that only a few cells in TM and its surrounding tissues of wild-type mice had weak fluorescence signals for CHOP, whereas most of DAPI positive cells in similar position of transgenic mice had stronger signals for CHOP (Figure 6). This result indicates that expression of Y437H myocilin mutant can provide fatal stress to TM and its surrounding tissues.
Figure 6.
CHOP is up-regulated in the eye angle tissue of aged transgenic mice expressing Y437H mutant myocilin. Eye sections of aged wild-type (upper row) and transgenic (lower row) mice were stained with anti-CHOP antibody and DAPI (right panel). Left panels show the bright field images and middle panels show high magnification of TM surrounding region from the white boxes in the left panels. CB, ciliary body. Scale bar = 50 μm.
Discussion
It is now well established that mutations in the MYOC gene may lead to juvenile and adult-onset glaucoma with elevated IOP in humans. Myocilin is highly expressed in the TM meshwork, the main component of the aqueous humor outflow system in the eye, and several reports suggest that expression of mutated myocilin is deleterious to cells and may lead to cell death.32,33,39,45 Most of these data were obtained in vitro in cell culture system by using not only TM cells but also other cell types. One possible mechanism of mutated myocilin-induced cell death involves a chronic induction of ER stress, although published data are somewhat controversial. It was reported that the level of BiP/GRP78 was up-regulated in TM cells after expression of the Q368X mutant,32 whereas the level of BiP/GRP78 was reduced in TM cells after transfection with the P370L myocilin mutant.52 Expression of mutated mouse myocilin Y423H corresponding to the Y437H human mutant in the mouse TM in vivo did not lead to the activation of UPR as judged by unchanged levels of several markers including GRP78.34
Here we used stably transfected HEK293 cells expressing wild-type and mutated myocilins under an inducible promoter to demonstrate that both mutations that we used, Y437H and I477N, induced UPR, with the I477N mutant producing higher activation of UPR markers (GRP78 and PDI) than the Y437H mutant. We do not believe that higher activation of UPR markers by the I477N mutant was due to the presence of the C-terminal FLAG tag, which was also present in wild-type myocilin. Expression of the Y437H myocilin mutant in the TM of transgenic mice also led to ER stress as judged by strong up-regulation of GRP78 in two independent lines of mice. This is the first demonstration in the literature that physiological concentrations of mutated myocilin induce ER stress in vivo. Existing discrepancies in the literature may be explained by different nature of constructs and mutants used, differing levels of mutated myocilin and exposure time, differences in the methods that were used for delivering mutated myocilin into cells, and different source of the cells.
Accumulating evidence suggests that protein misfolding and production of reactive oxygen species are closely related events.53 Accumulation of unfolded proteins in the ER lumens not only induces ER stress but is also able to cause oxidative stress.40 ROS by itself can cause protein misfolding in the ER and reduce protein secretion.40 Therefore, a combination of ER stress and oxidative stress may be more deleterious to cells than ER stress or oxidative stress alone. In this article we tested the hypothesis that expression of mutated myocilins can make cells more sensitive to oxidative stress. We demonstrated that expression of two myocilin mutants increased the levels of ROS in HEK293 cells with the Y437H mutant producing higher levels of ROS than I477N mutant. We expected that the I477N mutant may induce higher levels of ROS because this mutant appears to induce stronger UPR. However, further experiments demonstrated that expression of the Y437H mutant, unlike expression of the I477N mutant, inhibits expression of several enzymes involved in cell protection against oxidative stress. Cells expressing the I477N mutant showed similar levels of tested genes (GPx-3, apolipoprotein E, isocitrate dehydrogenase, and PON2) expression as cells expressing wild-type myocilin or control cells. At present, although we do not understand how the Y437H myocilin mutant inhibits the expression of antioxidative enzymes, we can suggest that down-regulation of some antioxidant genes in the cell line expressing Y437H mutant might be responsible for the increased sensitivity of the cell line to oxidative stress compared with the cell line expressing I477N mutant.
Remarkably, expression of the Y437H myocilin mutant inhibited antioxidative enzymes not only in cell culture but also in vivo in the TM of two independent lines of transgenic mice expressing mutated myocilin. This is the first demonstration in the literature that physiological levels of mutated myocilin inhibit antioxidative enzymes. PON2 and GPx-3 are well known antioxidant proteins. It has been reported that PON2 efficiently reduces oxidative stress and inhibits ER stress induced apoptosis.54 GPx-3 is one of major scavengers of ROS in extracellular matrix. Its reduced expression is associated with cardiovascular diseases and diabetes.55,56 Although its role in ocular tissues is yet unknown, high expression levels of this gene in the ciliary epithelium have been identified suggesting that this protein may have an important role in protection of the anterior segment of the eye from oxidative stress.57 Therefore, the reduced levels of these (and probably other) antioxidant proteins in the TM may lead to increased levels of ROS in the eye outflow system.
Expression of mutated myocilins makes cells in culture more sensitive to oxidative stress and stimulates apoptosis after application of oxidative stress. Cells expressing the Y437H mutant were more sensitive than cells expressing similar levels of the I477N mutant. Similar results were obtained with both stably transfected and transiently transfected cells. In transient transfected cells, we tested a broader collection of mutants and confirmed that mutant myocilins increase sensitivity of cells to oxidative stress and that different mutants may provide different sensitivity to the oxidative stress. Among the mutants tested, P370L mutant provided the highest sensitivity to oxidative stress. It is consistent with clinical data showing that the P370L myocilin mutant is more severe mutant than the Y437H or I477N mutants as judged by maximum IOP (45 mm Hg) and mean age at diagnosis (11 to 12 years old).8 It has been recently reported that expression of the P370L mutant in the culture of human TM cells also induced ROS generation and might impair mitochondrial functions.45 We suggest that expression of mutated Y437H myocilin in transgenic mice in vivo may make cells more sensitive not only to oxidative but also to other forms of stress and increase cell death. This could lead to a reduced thickness of the area containing the TM, Schlemm’s canal, and surrounding eye tissues of transgenic mice as compared with wild-type mice. Changes in the extracellular matrix due to inhibition of myocilin secretion may also contribute to a reduced thickness of this area in transgenic mice.
It is well documented that ROS can be produced as a result of exposure to multiple stresses including irradiation and environmental pollutants or as byproducts of mitochondrial respiration. People are continuously exposed to oxidative stress that can be brought by various exogenous and endogenous sources in daily life. According to our data, if a normal group and a group harboring mutations in the myocilin gene are exposed to similar levels of oxidative stress, the latter group can be more susceptible to the stress. Cigarette smoking is a well known inducer of ROS and oxidative stress.58 Although there is no clear association between smoking and development of POAG,59 this may be different for myocilin-induced glaucoma. People carrying myocilin mutations should be especially advised against smoking.
TM cells expressing the highest level of myocilin should be easily damaged by mutations in myocilin. The synergistic effect of mutant myocilin and oxidative stress may lead to the loss of TM cells, which is regarded as one of the potential mechanisms for glaucoma pathogenesis. It has been demonstrated that feeding mice with a lipid-soluble antioxidant, butylated hydroxyanisole, prevented ER-stress-induced oxidative damage, activation of UPR and apoptosis, and increased the secretion of the folding-defective coagulation factor VIII.40 A treatment with antioxidative agents may be considered as a possible treatment for some forms of myocilin-induced glaucoma.
Acknowledgments
We thank Dr. Vincent Raymond for the myocilin expression constructs, and Thomas V. Johnson for critically reading the article and helpful comments.
Footnotes
Address reprint requests to Stanislav I. Tomarev, Ph.D., Molecular Mechanisms of Glaucoma Section, Laboratory of Molecular and Developmental Biology, National Eye Institute, National Institutes of Health, 5635 Fishers Lane, Room 1124, Bethesda, MD 20892. E-mail: tomarevs@nei.nih.gov.
Supported by the Intramural Research Program of the National Institutes of Health, National Eye Institute.
This work was prepared as part of our official duties. Title 17 U.S.C. 105 provides that “Copyright protection under this title is not available for any work of the United States Government.” Title 17 U.S.C. 101 defines a U.S. Government work as a work prepared by a military service member or employee of the U.S. Government as part of that person’s official duties.
References
- Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004;363:1711–1720. doi: 10.1016/S0140-6736(04)16257-0. [DOI] [PubMed] [Google Scholar]
- Gabelt BT, Kaufman PL. Changes in aqueous humor dynamics with age and glaucoma. Prog Retin Eye Res. 2005;24:612–637. doi: 10.1016/j.preteyeres.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Kwon YH, Fingert JH, Kuehn MH, Alward WL. Primary open-angle glaucoma. N Engl J Med. 2009;360:1113–1124. doi: 10.1056/NEJMra0804630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone EM, Fingert JH, Alward WL, Nguyen TD, Polansky JR, Sunden SL, Nishimura D, Clark AF, Nystuen A, Nichols BE, Mackey DA, Ritch R, Kalenak JW, Craven ER, Sheffield VC. Identification of a gene that causes primary open angle glaucoma. Science. 1997;275:668–670. doi: 10.1126/science.275.5300.668. [DOI] [PubMed] [Google Scholar]
- Adam MF, Belmouden A, Binisti P, Brezin AP, Valtot F, Bechetoille A, Dascotte JC, Copin B, Gomez L, Chaventre A, Bach JF, Garchon HJ. Recurrent mutations in a single exon encoding the evolutionarily conserved olfactomedin-homology domain of TIGR in familial open-angle glaucoma. Hum Mol Genet. 1997;6:2091–2097. doi: 10.1093/hmg/6.12.2091. [DOI] [PubMed] [Google Scholar]
- Fingert JH, Heon E, Liebmann JM, Yamamoto T, Craig JE, Rait J, Kawase K, Hoh ST, Buys YM, Dickinson J, Hockey RR, Williams-Lyn D, Trope G, Kitazawa Y, Ritch R, Mackey DA, Alward WL, Sheffield VC, Stone EM. Analysis of myocilin mutations in 1703 glaucoma patients from five different populations. Hum Mol Genet. 1999;8:899–905. doi: 10.1093/hmg/8.5.899. [DOI] [PubMed] [Google Scholar]
- Fingert JH, Stone EM, Sheffield VC, Alward WL. Myocilin glaucoma. Surv Ophthalmol. 2002;47:547–561. doi: 10.1016/s0039-6257(02)00353-3. [DOI] [PubMed] [Google Scholar]
- Tamm ER. Myocilin and glaucoma: facts and ideas. Prog Retin Eye Res. 2002;21:395–428. doi: 10.1016/s1350-9462(02)00010-1. [DOI] [PubMed] [Google Scholar]
- Torrado M, Trivedi R, Zinovieva R, Karavanova I, Tomarev SI. Optimedin: a novel olfactomedin-related protein that interacts with myocilin. Hum Mol Genet. 2002;11:1291–1301. doi: 10.1093/hmg/11.11.1291. [DOI] [PubMed] [Google Scholar]
- Tomarev SI, Wistow G, Raymond V, Dubois S, Malyukova I. Gene expression profile of the human trabecular meshwork: nEIBank sequence tag analysis. Invest Ophthalmol Vis Sci. 2003;44:2588–2596. doi: 10.1167/iovs.02-1099. [DOI] [PubMed] [Google Scholar]
- Kubota R, Noda S, Wang Y, Minoshima S, Asakawa S, Kudoh J, Mashima Y, Oguchi Y, Shimizu N. A novel myosin-like protein (myocilin) expressed in the connecting cilium of the photoreceptor: molecular cloning, tissue expression, and chromosomal mapping. Genomics. 1997;41:360–369. doi: 10.1006/geno.1997.4682. [DOI] [PubMed] [Google Scholar]
- Nguyen TD, Chen P, Huang WD, Chen H, Johnson D, Polansky JR. Gene structure and properties of TIGR, an olfactomedin-related glycoprotein cloned from glucocorticoid-induced trabecular meshwork cells. J Biol Chem. 1998;273:6341–6350. doi: 10.1074/jbc.273.11.6341. [DOI] [PubMed] [Google Scholar]
- Ortego J, Escribano J, Coca-Prados M. Cloning and characterization of subtracted cDNAs from a human ciliary body library encoding TIGR, a protein involved in juvenile open angle glaucoma with homology to myosin and olfactomedin. FEBS Lett. 1997;413:349–353. doi: 10.1016/s0014-5793(97)00934-4. [DOI] [PubMed] [Google Scholar]
- Snyder DA, Rivers AM, Yokoe H, Menco BP, Anholt RR. Olfactomedin: purification, characterization, and localization of a novel olfactory glycoprotein. Biochemistry. 1991;30:9143–9153. doi: 10.1021/bi00102a004. [DOI] [PubMed] [Google Scholar]
- Tomarev SI, Nakaya N. Olfactomedin domain-containing proteins: Possible mechanisms of action and functions in normal development and pathology. Molec Neurobiol. 2009;40:122–138. doi: 10.1007/s12035-009-8076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fautsch MP, Vrabel AM, Johnson DH. The identification of myocilin-associated proteins in the human trabecular meshwork. Exp Eye Res. 2006;82:1046–1052. doi: 10.1016/j.exer.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Ueda J, Wentz-Hunter K, Yue BY. Distribution of myocilin and extracellular matrix components in the juxtacanalicular tissue of human eyes. Invest Ophthalmol Vis Sci. 2002;43:1068–1076. [PubMed] [Google Scholar]
- Joe MK, Sohn S, Choi YR, Park H, Kee C. Identification of flotillin-1 as a protein interacting with myocilin: implications for the pathogenesis of primary open-angle glaucoma. Biochem Biophys Res Commun. 2005;336:1201–1206. doi: 10.1016/j.bbrc.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Filla MS, Liu X, Nguyen TD, Polansky JR, Brandt CR, Kaufman PL, Peters DM. In vitro localization of TIGR/MYOC in trabecular meshwork extracellular matrix and binding to fibronectin. Invest Ophthalmol Vis Sci. 2002;43:151–161. [PubMed] [Google Scholar]
- Li Y, Aroca-Aguilar JD, Ghosh S, Sanchez-Sanchez F, Escribano J, Coca-Prados M. Interaction of myocilin with the C-terminal region of hevin. Biochem Biophys Res Commun. 2006;339:797–804. doi: 10.1016/j.bbrc.2005.11.082. [DOI] [PubMed] [Google Scholar]
- Kwon HS, Lee HS, Ji Y, Rubin JS, Tomarev SI. Myocilin is a modulator of Wnt signaling. Mol Cell Biol. 2009;29:2139–2154. doi: 10.1128/MCB.01274-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam DS, Leung YF, Chua JK, Baum L, Fan DS, Choy KW, Pang CP. Truncations in the TIGR gene in individuals with and without primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2000;41:1386–1391. [PubMed] [Google Scholar]
- Wiggs JL, Vollrath D. Molecular and clinical evaluation of a patient hemizygous for TIGR/MYOC. Arch Ophthalmol. 2001;119:1674–1678. doi: 10.1001/archopht.119.11.1674. [DOI] [PubMed] [Google Scholar]
- Gong G, Kosoko-Lasaki O, Haynatzki GR, Wilson MR. Genetic dissection of myocilin glaucoma. Hum Mol Genet. 2004;13 Spec No 1:R91–R102. doi: 10.1093/hmg/ddh074. [DOI] [PubMed] [Google Scholar]
- Jacobson N, Andrews M, Shepard AR, Nishimura D, Searby C, Fingert JH, Hageman G, Mullins R, Davidson BL, Kwon YH, Alward WL, Stone EM, Clark AF, Sheffield VC. Non-secretion of mutant proteins of the glaucoma gene myocilin in cultured trabecular meshwork cells and in aqueous humor. Hum Mol Genet. 2001;10:117–125. doi: 10.1093/hmg/10.2.117. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Vollrath D. A cellular assay distinguishes normal and mutant TIGR/myocilin protein. Hum Mol Genet. 1999;8:2221–2228. doi: 10.1093/hmg/8.12.2221. [DOI] [PubMed] [Google Scholar]
- Sohn S, Hur W, Joe MK, Kim JH, Lee ZW, Ha KS, Kee C. Expression of wild-type and truncated myocilins in trabecular meshwork cells: their subcellular localizations and cytotoxicities. Invest Ophthalmol Vis Sci. 2002;43:3680–3685. [PubMed] [Google Scholar]
- Malyukova I, Lee HS, Fariss RN, Tomarev SI. Mutated mouse and human myocilins have similar properties and do not block general secretory pathway. Invest Ophthalmol Vis Sci. 2006;47:206–212. doi: 10.1167/iovs.05-0220. [DOI] [PubMed] [Google Scholar]
- Gobeil S, Rodrigue MA, Moisan S, Nguyen TD, Polansky JR, Morissette J, Raymond V. Intracellular sequestration of hetero-oligomers formed by wild-type and glaucoma-causing myocilin mutants. Invest Ophthalmol Vis Sci. 2004;45:3560–3567. doi: 10.1167/iovs.04-0300. [DOI] [PubMed] [Google Scholar]
- Caballero M, Rowlette LL, Borras T. Altered secretion of a TIGR/MYOC mutant lacking the olfactomedin domain. Biochim Biophys Acta. 2000;1502:447–460. doi: 10.1016/s0925-4439(00)00068-5. [DOI] [PubMed] [Google Scholar]
- Joe MK, Sohn S, Hur W, Moon Y, Choi YR, Kee C. Accumulation of mutant myocilins in ER leads to ER stress and potential cytotoxicity in human trabecular meshwork cells. Biochem Biophys Res Commun. 2003;312:592–600. doi: 10.1016/j.bbrc.2003.10.162. [DOI] [PubMed] [Google Scholar]
- Liu Y, Vollrath D. Reversal of mutant myocilin non-secretion and cell killing: implications for glaucoma. Hum Mol Genet. 2004;13:1193–1204. doi: 10.1093/hmg/ddh128. [DOI] [PubMed] [Google Scholar]
- Gould DB, Reedy M, Wilson LA, Smith RS, Johnson RL, John SW. Mutant myocilin nonsecretion in vivo is not sufficient to cause glaucoma. Mol Cell Biol. 2006;26:8427–8436. doi: 10.1128/MCB.01127-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senatorov V, Malyukova I, Fariss R, Wawrousek EF, Swaminathan S, Sharan SK, Tomarev S. Expression of mutated mouse myocilin induces open-angle glaucoma in transgenic mice. J Neurosci. 2006;26:11903–11914. doi: 10.1523/JNEUROSCI.3020-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacca SC, Izzotti A. Oxidative stress and glaucoma: injury in the anterior segment of the eye. Prog Brain Res. 2008;173:385–407. doi: 10.1016/S0079-6123(08)01127-8. [DOI] [PubMed] [Google Scholar]
- Tezel G. Oxidative stress in glaucomatous neurodegeneration: mechanisms and consequences. Prog Retin Eye Res. 2006;25:490–513. doi: 10.1016/j.preteyeres.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzotti A, Sacca SC, Longobardi M, Cartiglia C. Sensitivity of ocular anterior chamber tissues to oxidative damage and its relevance to the pathogenesis of glaucoma. Invest Ophthalmol Vis Sci. 2009;50:5251–5258. doi: 10.1167/iovs.09-3871. [DOI] [PubMed] [Google Scholar]
- Yam GH, Gaplovska-Kysela K, Zuber C, Roth J. Aggregated myocilin induces russell bodies and causes apoptosis: implications for the pathogenesis of myocilin-caused primary open-angle glaucoma. Am J Pathol. 2007;170:100–109. doi: 10.2353/ajpath.2007.060806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra JD, Miao H, Zhang K, Wolfson A, Pennathur S, Pipe SW, Kaufman RJ. Antioxidants reduce endoplasmic reticulum stress and improve protein secretion. Proc Natl Acad Sci USA. 2008;105:18525–18530. doi: 10.1073/pnas.0809677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BS, Savinova OV, Reedy MV, Martin J, Lun Y, Gan L, Smith RS, Tomarev SI, John SW, Johnson RL. Targeted disruption of the myocilin gene (Myoc) suggests that human glaucoma-causing mutations are gain of function. Mol Cell Biol. 2001;21:7707–7713. doi: 10.1128/MCB.21.22.7707-7713.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alward WL, Fingert JH, Coote MA, Johnson AT, Lerner SF, Junqua D, Durcan FJ, McCartney PJ, Mackey DA, Sheffield VC, Stone EM. Clinical features associated with mutations in the chromosome 1 open-angle glaucoma gene (GLC1A). N Engl J Med. 1998;338:1022–1027. doi: 10.1056/NEJM199804093381503. [DOI] [PubMed] [Google Scholar]
- Wurm FM. Production of recombinant protein therapeutics in cultivated mammalian cells. Nature Biotechnol. 2004;22:1393–1398. doi: 10.1038/nbt1026. [DOI] [PubMed] [Google Scholar]
- Soldani C, Scovassi AI. Poly(ADP-ribose) polymerase-1 cleavage during apoptosis: an update. Apoptosis. 2002;7:321–328. doi: 10.1023/a:1016119328968. [DOI] [PubMed] [Google Scholar]
- He Y, Leung KW, Zhuo YH, Ge J. Pro370Leu mutant myocilin impairs mitochondrial functions in human trabecular meshwork cells. Mol Vis. 2009;15:815–825. [PMC free article] [PubMed] [Google Scholar]
- Kryukov GV, Castellano S, Novoselov SV, Lobanov AV, Zehtab O, Guigo R, Gladyshev VN. Characterization of mammalian selenoproteomes. Science. 2003;300:1439–1443. doi: 10.1126/science.1083516. [DOI] [PubMed] [Google Scholar]
- Brigelius-Flohe R, Lotzer K, Maurer S, Schultz M, Leist M. Utilization of selenium from different chemical entities for selenoprotein biosynthesis by mammalian cell lines. Biofactors. 1995;5:125–131. [PubMed] [Google Scholar]
- Zhou Y, Grinchuk O, Tomarev SI. Transgenic mice expressing the Tyr437His mutant of human myocilin protein develop glaucoma. Invest Ophthalmol Vis Sci. 2008;49:1932–1939. doi: 10.1167/iovs.07-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinszner H, Kuroda M, Wang X, Batchvarova N, Lightfoot RT, Remotti H, Stevens JL, Ron D. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 1998;12:982–995. doi: 10.1101/gad.12.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol. 2001;21:1249–1259. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman AD. GADD153/CHOP, a DNA damage-inducible protein, reduced CAAT/enhancer binding protein activities and increased apoptosis in 32D c13 myeloid cells. Cancer Res. 1996;56:3250–3256. [PubMed] [Google Scholar]
- Wang L, Zhuo Y, Liu B, Huang S, Hou F, Ge J. Pro370Leu mutant myocilin disturbs the endoplasm reticulum stress response and mitochondrial membrane potential in human trabecular meshwork cells. Mol Vis. 2007;13:618–625. [PMC free article] [PubMed] [Google Scholar]
- Malhotra JD, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal. 2007;9:2277–2293. doi: 10.1089/ars.2007.1782. [DOI] [PubMed] [Google Scholar]
- Horke S, Witte I, Wilgenbus P, Kruger M, Strand D, Forstermann U. Paraoxonase-2 reduces oxidative stress in vascular cells and decreases endoplasmic reticulum stress-induced caspase activation. Circulation. 2007;115:2055–2064. doi: 10.1161/CIRCULATIONAHA.106.681700. [DOI] [PubMed] [Google Scholar]
- Voetsch B, Jin RC, Bierl C, Benke KS, Kenet G, Simioni P, Ottaviano F, Damasceno BP, Annichino-Bizacchi JM, Handy DE, Loscalzo J. Promoter polymorphisms in the plasma glutathione peroxidase (GPx-3) gene: a novel risk factor for arterial ischemic stroke among young adults and children. Stroke. 2007;38:41–49. doi: 10.1161/01.STR.0000252027.53766.2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung SS, Kim M, Youn BS, Lee NS, Park JW, Lee IK, Lee YS, Kim JB, Cho YM, Lee HK, Park KS. Glutathione peroxidase 3 mediates the antioxidant effect of peroxisome proliferator-activated receptor gamma in human skeletal muscle cells. Mol Cell Biol. 2009;29:20–30. doi: 10.1128/MCB.00544-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Alonso JM, Ghosh S, Coca-Prados M. Cloning of the bovine plasma selenium-dependent glutathione peroxidase (GP) cDNA from the ocular ciliary epithelium: expression of the plasma and cellular forms within the mammalian eye. J Biochem. 1993;114:284–291. doi: 10.1093/oxfordjournals.jbchem.a124168. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Podlutsky A, Wolin MS, Losonczy G, Pacher P, Ungvari Z. Oxidative stress and accelerated vascular aging: implications for cigarette smoking. Front Biosci. 2009;14:3128–3144. doi: 10.2741/3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards R, Thornton J, Ajit R, Harrison RA, Kelly SP. Cigarette smoking and primary open angle glaucoma: a systematic review. J Glaucoma. 2008;17:558–566. doi: 10.1097/IJG.0b013e31815f530c. [DOI] [PubMed] [Google Scholar]