Abstract
Protoplast cultures are remarkable examples of plant cell dedifferentiation. The state of dedifferentiation is evidenced by changes in cell morphology, genome organization, as well as by the capability of protoplasts to differentiate into multiple types of cells (depending on the type of the stimulus applied). The first change in the genome structure is connected with large-scale chromatin decondensation, affecting chromocentres involving various types of these repetitive sequences. This paper describes not only the de- and recondensation of satellite DNA type I and 5S rDNA repetitive sequences, but it also compares the recondensation level of chromatin with the levels of oxidative stress which were decreased by using an antioxidant, as well as the capabilities of the antioxidative systems within protoplasts, during the first 72 h of their culture. It is demonstrated that the treatment of protoplasts with ascorbic acid not only decreased the level of oxidative stress but also positively stimulated the expression of the ascorbate peroxidase and catalase. It also led to a greater recondensation of the chromatin (when compared to the untreated protoplasts); in addition, it supported cell proliferation. It is concluded that large-scale genome relaxation is more directly connected with oxidative stress than with large changes in the expression of genes; and further, that its recondensation is related to the start of (as well as the level of) protection by the antioxidative systems.
Keywords: Chromatin, nuclear organization, oxidative stress, protoplasts
Introduction
Plant protoplasts (plant cells devoid of a cell wall) represent a very spectacular example of cell dedifferentiation. After their isolation and subsequent culture they have the capability to dedifferentiate, re-enter the cell cycle, and then proliferate or regenerate into the various organs. They can also make new plants in the same way as zygotes (Grafi, 2004). This is one reason that protoplast cultures are such valuable tools for biotechnological applications such as somatic hybridization, increasing genetic variability by somaclonal variability, and genetic transformation (Debeaujon and Branchard 1992; Rakoczy-Trojanowska, 2002; Navrátilová et al., 2006). However, in many plant species, a high level of protoplast recalcitrance occurs (Papadakis and Roubelakis-Angelakis, 2002).
Protoplast dedifferentiation is characterized by a new balance between the less-dense portion of the genome and that which is condensed and with transcription repressed (heterochromatin). The heterochromatin decondensation of Arabidopsis protoplasts is accompanied by the structural relaxation of centromeric (180 bp), pericentromeric, and 5S rDNA repeats, as well as transposons localized at the chromocentres of leaves (Tessadori et al., 2007). Although the phenomenon of heterochromatin decondensation in protoplasts has recently been clearly described (Tessadori et al., 2007; Ondřej et al., 2009), the reasons for this relaxation (plus the process of the recondensation, together with its influence on protoplast differentiation) remain an open question.
This study is supportive of the hypothesis that there is a correlation between the recondensation of the chromatin in protoplast nuclei with the levels of oxidative stress in the protoplast cultures. As is generally known, oxidative stress, resulting from an imbalance between reactive oxygen species (ROS) generation and the antioxidant capacity of cells, has been proposed as contributing to the recalcitrance of plant protoplasts (reviewed in Papadakis and Roubelakis-Angelakis, 2002). The ROS superoxide anion radical (O2−·), hydrogen peroxide (H2O2), and hydroxyl radicals (·OH) are inevitably produced in higher plant cells during normal metabolism. Their production is enhanced during situations of stress such as drought, salinity, high and low temperatures, UV or ozone stress, as well as pathogen infections. ROS limit the spread of the pathogen by a strengthening of the plant cell walls and/or the killing of the pathogens directly; however, they also act as signalling molecules during biotic and abiotic stresses. In the same way as during a pathogen attack, ROS is generated during the enzymic maceration of the cell walls during protoplast isolation (reviewed in Papadakis and Roubelakis-Angelakis, 2002).
Materials and methods
Protoplast isolation and culture
Seeds of Cucumis sativus L. (cv. Marketer; SEMO Ltd., Smržice, Czech Republic) were sown, under sterile conditions, on half-strength MS medium (Duchefa). After germination, the seedlings were planted on MS medium (supplemented with 20 g l−1 sucrose, 0.8% agar, 0.049 μmol l−1 IBA, and 0.044 μmol/l BA) in plastic boxes. The plants were cultivated in a culture room with a 16 h day (light intensity 32–36 μmol m−2 s−1), and a temperature of 22±2 °C.
The leaves of the plantlets were chopped and digested in a maceration enzyme mixture containing 1% (w/v) Cellulase Onozuka R-10 (Duchefa) and 0.25% (w/v) Macerozyme R-10 (Duchefa), and then dissolved in PGly washing solution (Debeaujon and Branchard, 1992). The pieces of leaves were incubated in the enzyme mixture for 16–17 h in the dark at 27 °C. The protoplasts were isolated according to Gajdová et al. (2007), and then resuspended (2×105 protoplasts ml−1) in LCM1 culture medium (Debeaujon and Branchard, 1992) supplemented with 0, 100, and 200 mg l−1 of ascorbic acid in order to get the experimental series.
Fixation, fluorescence in situ hybridization (FISH), image acquisition
Freshly isolated protoplasts and cells from protoplast cultures at 24, 48, and 72 h after protoplasts isolation were fixed in ethanol:acetic acid (3:1 v/v) (EAA), and prepared for FISH. Cells from the cultures were treated with a solution of 1% Pectinase and 2% Cellulase Onozuka R-10 (Duchefa) for 15 min at 37 °C, to remove newly synthesized cell walls. Young leaves were also fixed in EAA, and then macerated in a solution of 1% Pectinase from Aspergillus niger (Serva) and 2% Cellulase for 30 min at 37 °C. They were then gently squashed in 45% acetic acid, next washed in 96% ethanol, and then dried and prepared for FISH.
FISH was carried-out on the interphase nuclei of the leaves, protoplasts, and protoplast-derived cells, with the slight modifications of the hybridization protocol used by Ondřej et al. (2008). The probe for telomere-associated regions was prepared by PCR amplification of the satellite DNA type I repeats (Ganal et al., 1986) from genomic DNA using primers: forward 5′-CTGGGTGGCCTCATTTTG-3′ and reverse 5′-GACCTTTGGCACCGTTGT-3′. The PCR products were identified by electrophoresis on 2% agarose gel, then purified (Gene Elute PCR Clean-Up Kit, Sigma), and finally labelled with a Biotin-nick translation kit (Roche) according to the manufacturer's protocol. The probe for 5S rDNA was prepared by DIG-labelled PCR using primers: forward 5′-GATCCCATCAGAACTCC-3′ and reverse 5′-GGTGCTTTAGTGCTGGTAT-3′. The hybridized probes were detected using mouse anti-digoxin rhodamine red-X-conjugated antibody and extravidin conjugated with FITC (Jackson Immunoresearch Laboratories, Inc.). Nuclei were counterstained using DAPI, diluted in Vectashield (Vector Laboratories, Burlingame, CA), in order to reduce photobleaching.
Image acquisition was carried out with a fluorescent microscope (Olympus BX 60) fitted with a CCD camera (Cool Snap, Photometrics). The images were analysed with ImageJ freeware (http://rsb.info.nih.gov/ij/index.html). The relative signal area was calculated as a percentage of the signal area (yellow or red signals of satellite DNA type I and 5S rDNA) in relation to the total area of DAPI stained nuclei. The data were presented as the mean plus errors and treated by one-way ANOVA analyses (Daniel's XL Toolbox version 2.57; http://xltoolbox.sourceforge.net).
ROS measurement
The concentration of H2O2 in the cell suspension of protoplasts was determined fluorimetrically using the fluorescent probe 2,7-dichlorodihydrofluorescein diacetate (H2DCF DA). H2DCF DA can permeate through the cell membrane into the cell, where it can there be metabolized by cell esterase into H2DCF. Then, H2DCF is oxidized by reactive oxygen species into fluorescent DCF (Gomes et al., 2006). The H2O2 assessments were performed on a Synergy microplate reader (Biotek, USA). Into 100 μl of cell suspension, 5 μl of 0.2 mM fluorescent probe H2DCF DA was added. The fluorescent signal (λexc–485 nm and λem–515 nm) was measured immediately after the probe's addition, as well as after 1 h of cell suspension incubation at 26 °C in the dark. For each variant, three measurements, for each of the above-mentioned three independent sets of samples, were conducted (i.e. nine values were obtained). The data were presented as a mean+standard deviation.
Localization of ROS production
The sedimented protoplasts were resuspended in PBS (pH 7.0) and then incubated in 1 mM Hoechst 33342 (Sigma-Aldrich, USA) for 20 min to stain the nuclei and in 20 μM solution of H2DCF DA (Axxora, USA) for 10 min to visualize H2O2 inside the cells. Samples were subjected to confocal laser scanning microscopy (Olympus Fluorview 1000 attached to inverted microscope IX81). Excitation was provided by the 405 nm diode laser (for DNA staining) and the 488 nm line of an argon ion laser (for H2O2 detection). Dye emissions were recorded using 430–470 nm and 505–525 nm band-pass filters, respectively. To adjust the correct intensity of the lasers, control samples without fluorescent probes were examined at the beginning of experiment.
Real-time PCR of ascorbate peroxidase and catalase
Total RNA was isolated from the young leaves of plantlets cultivated under sterile conditions and from freshly isolated protoplasts derived from protoplasts cultured using the Spectrum Total RNA isolation Kit (Sigma), and subsequently treated by RNAse free DNAse (Promega). RNA was reversely transcribed into cDNA by oligo dT(18) primers and a Transcriptor High Fidelity cDNA Synthesis Sample Kit (Roche). In order to describe the expression level of the cytosolic ascorbate peroxidase (accession number D88649) and catalase (accession number D63385) at each time point of the media series, a LightCycler 2.0 instrument (Roche) was used. The quantified cDNA was used as a template in order to perform the series of amplification analyses using a SYBR Green I containing kit and specific primers: APX, forward 5′-CTCTGGTGGTCACACATTGG-3′ and reverse 5′-CCTCATCGGCAGCATATTTT-3′; CAT, forward 5′-TTGACGATATTGGCATCC-3′ and reverse 5′-TAGCATCTTCCTCCAGCA-3′. The LightCycler Software 4.1 was used to get Cp, amplification, and the melting curves. The relative quantification was performed, in which the measured Cp for the leaves was taken as the standard.
Results
Oxidative stress in protoplasts
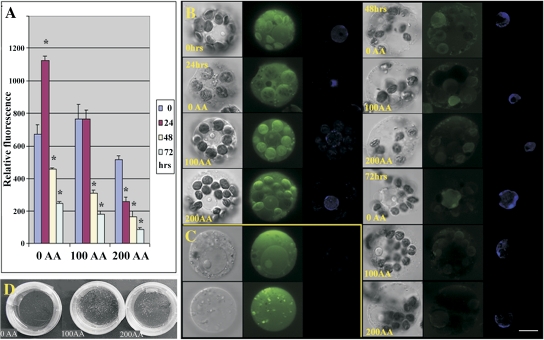
The protoplasts of cucumber were isolated from the mesophyll tissues originating from the young leaves of plantlets cultured in vitro. The viability of the isolated protoplasts was about 80% (estimated by FDA staining). Protoplasts were obtained in normal yields (2–5×106 cells g−1 of donor tissue) and were of normal size and shape (spherical). Immediately after isolation of the protoplasts, the ascorbate acid antioxidant was added to the culture media, in order to obtain the concentration series of 0, 100, and 200 mg l−1 of media. The detection of ROS was performed for the whole concentration series at four time points 0, 24, 48, and 72 h after isolation (AI). The results (summarized in Fig. 1A) demonstrate a decreasing tendency of the ROS level in the protoplasts in relation to the AA concentration and the time of culture. Only for the protoplast cultures without AA, were the highest levels of ROS measured 24 h AI. Protoplasts cultivated for 24 h in the medium with 100 mg l−1 of AA showed the same level of ROS as measured in freshly isolated protoplasts. Histochemical detection of ROS using confocal microscopy (Fig. 1B) not only showed ROS decreasing during protoplast cultivation, as described by fluorimetric ROS detection, but it was possible to localize ROS within the cells. The ROS were mostly found in the cytosol, chloroplasts, as well as in the nuclei.
Fig. 1.
The ROS measurement and cellular localization. The subset (A) shows measurements of ROS during protoplast culture (72 h) in relation to AA treatments (0, 100, and 200 mg l−1). The values marked with an asterisk were significantly different from values obtained in freshly isolated protoplasts (P <0.05). The subset (B) shows not only the level of ROS in the cells, in relation to the time of culture and AA concentration, but also demonstrates the localization of ROS within cells. The highest intensity signals of the ROS (green) are visible in freshly isolated protoplasts and protoplasts at 24 h AI localized mostly in chloroplasts, cytoplasm, peroxisomes, and also in nuclei that were stained by Hoechst (blue). The subset (C) demonstrates a part of the protoplast populations in cultures without AA and 100 mg l−1 AA occurring between 24–48 h AI. These types of protoplasts are characterized by high ROS content and enlarged weakly stained nuclei or without nuclei. The subset (D) displays, separately, a proliferation for 2 week cultures of protoplast-derived cells, compared to the AA treatment. Application of AA led to the increasing amounts of microcalli. The scale bar=10 μm.
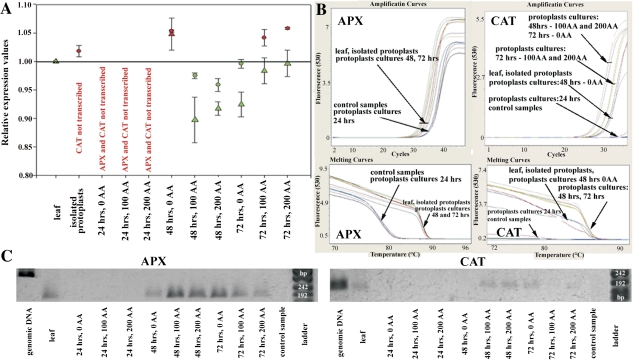
Thus, real-time PCR for the quantification of the cytosolic ascorbate peroxidase (APX) and catalase (CAT) transcripts was performed. The results show that the transcript level of APX in freshly isolated protoplasts decreased in relation to the leaves (Fig. 2A). Moreover, the expression of ascorbate peroxidase was not detected at 24 h AI, throughout all of the concentration series. The expression of CAT was not detected in freshly isolated protoplasts or in protoplasts 24 h AI in all of the concentration series (Fig. 2A–C). These facts correlate with the ROS measurement where protoplasts in the medium without AA display the highest level of ROS; indicating that the stress defence apparatus is not switched on at that time. At the following two time points, protoplast cultures with the addition of AA differed from cultures without AA in both their expression level and tendency. Protoplasts not treated with AA started slowly to switch on the expression of APX and CAT at 48 h AI, and reached the expression level of leaves at 72 h AI. By contrast, protoplasts treated with AA rise above the APX and CAT expression level of leaves at 48 h AI, and the low level of ROS led to the down-regulation of APX and CAT expression at 72 h AI (Fig. 2A–C).
Fig. 2.
APX and CAT expression profile in protoplast cultures, treated and untreated with AA. The subset (A) demonstrates the relative quantification of the APX (circles) and CAT (triangles) transcription by RT-PCR for protoplasts, during their culture and AA application. The transcription level was related to the expression measured in the leaves. The circles and triangles (marked in red) demonstrate down-regulation of AP expression; the green ones up-regulation. The subset (B) shows amplification and melting curves from RT-PCR experiments and (C) shows the size of RT-PCR products of APX and CAT for leaves (L) and protoplasts at 72 h AI (P). The PCR products of APX and CAT using genomic DNA as a APX template displaying intron are also shown.
The level of the AA also influenced the subsequent proliferation of the cultivated protoplasts. As demonstrated in Fig. 1D, the highest proliferation capacity was observed for protoplasts cultivated in medium supplemented by 100 mg l−1 of AA. Lower concentrations decreased the level of proliferation.
Chromatin de- and recondensation
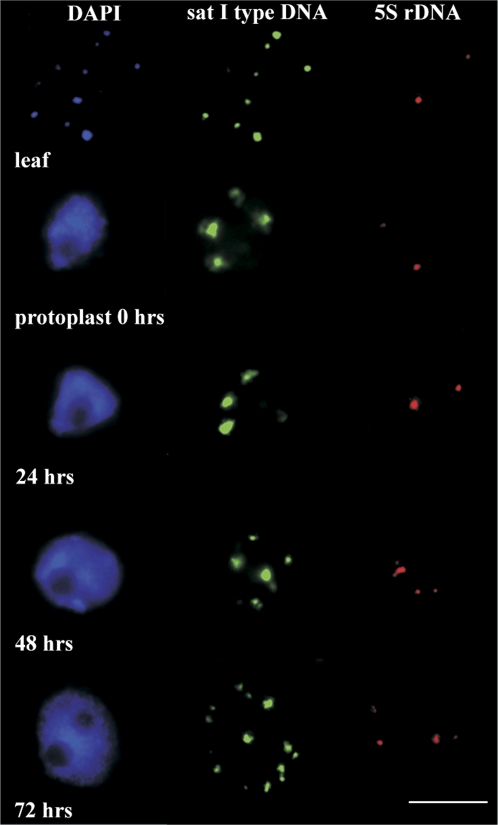
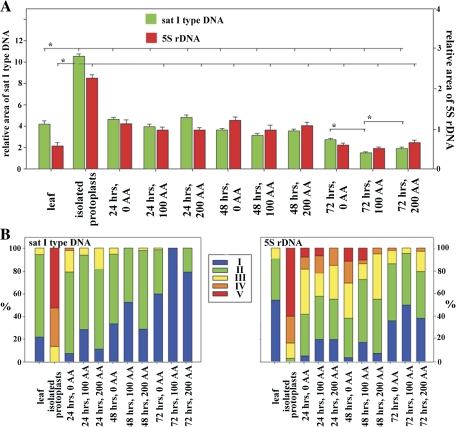
To study the level of the changes of chromatin condensation, both the satellite type I and 5S rDNA repetitive sequences were selected. Figures 3 and 4 demonstrate and summarize the relative level of de- and recondensation of the studied repeats in leaves, protoplasts at the same time-points, as well as the concentration series when the levels of ROS and antioxidative enzymes expression were measured. Freshly isolated protoplasts and protoplasts up to 24 h after their isolation showed as significant the highest levels of 5S rDNA and satellite type I DNA decondensation, compared with leaf nuclei and satellite type I DNA and also in relation to other time points of protoplast cultivation (Figs 3, 4). Satellite type I DNA also displayed four huge signals at the nuclear periphery (characteristic for this period of protoplast culture). This signal configuration became condensed into smaller ones during subsequent culturing (Fig. 3). Increases in the relative amounts of 5S rDNA signals in the protoplast nuclei at 48 h after isolation is significantly connected with replication during the S phase, through which the protoplasts had passed. 5S rDNA showing double dots at this time (Fig. 3) are replicated earlier then satellite DNA type I (of heterochromatin origin) at a time when the nuclear volume (in this case area) had not fully increased (as the result of whole genome replication). This could misrepresent the relative area of 5S rDNA at this time point. The strongest recondensation levels for both types of DNA were achieved at 72 h AI, at the time when the S phase had finished (double dots signals, two nucleolei) (Figs 3, 4). The changes in chromatin de- and recondensation levels at most time points were statistically significant (Fig. 4A).
Fig. 3.
The de- and recondensation of satellite DNA type I and 5S rDNA sequences in protoplast nuclei during the first 72 h of the culture and leaf nucleus. The figure shows nuclei (blue), satellite DNA type I (green), and 5S rDNA (red) condensation levels. Satellite DNA type I (green) display the highest decondensation after protoplast isolation, and during the first 24 h of the culture. The massive signals were located at four locations within the cell nucleus. Subsequently, these signals started to disintegrate and to form a greater number of smaller signals. The relaxation of 5S rDNA (red) was not as extensive as that observed for satellite DNA type I; the largest relaxation occurred at 24 h and 48 h into the culture. The double-dot signals indicate that the S-phase has passed and were observed at 48 h and 72 h AI. The scale bar=10 μm.
Fig. 4.
The subset (A) shows the calculated differences in the condensation level (mean of relative area of the signals+standard errors) of the studied sequences in relation to the time of culture and AA treatments (0, 100, and 200 mg l−1). For statistical analysis, cells from multiple experiments were pooled together for each experimental group and a one-way ANOVA test was performed. The statistically significant differences of condensation levels (marked by bracket with an asterisk =P <0.05) were found for most of the time points, freshly isolated protoplasts and leaves. The condensation levels in relation the concentration of AA significantly differs just for 72 h and for satellite DNA type I repeats. The subset (B) shows protoplast population variability during the time of cultivation and AA treatment. The protoplasts were divided into five categories according to the condensation level (relative area of the signals) of satellite DNA type I (categories: I, 0>3.00; II, 3.01–6.00; III, 6.01–8.00; IV, 8.01–10.00; V, 10.01<) and 5S rDNA (categories: I, 0>0.50; II, 0.51–1.00; III, 1.01–1.50; IV, 1.51–2.00; V, 2.01<).
The AA treatment supported recondensation of both types of repetitive sequences (statistically significant for the 72 h time point); however, the nuclei of protoplasts treated with 200 mg l−1 of AA displayed higher levels of chromatin decondensation than that measured for the 100 mg l−1 concentration. The highest level of repetitive DNA decondensation was observed during the period from protoplast isolation, until 24 h AI. This period corresponds to the time of the highest level of ROS, and the lowest expression of APX and CAT; revealing the connection between chromatin decondensation and oxidative stress in protoplasts. The period between 24 h and 48 h AI is characterized by a high variability of chromatin recondensation within the cell population (Fig. 4B). The cell population could be divided into two groups: the less condensed (6% with more of the relative area for satellite type I repeats, and 1% and greater for 5S rDNA) and more condensed ones (less than 6% of the relative area for satellite type I repeats and less than 1% for 5S rDNA). The highest number of the cells with more condensed chromatin was found for protoplast cultures treated with 100 mg l−1 of A and the lowest for cultures without AA (Fig. 4B). ROS localization by the confocal microscopy also revealed protoplasts with high levels of ROS across the whole cell and with totally decondensed, enlarged, and weakly stained nuclei (or without them); indicating the degradation of nuclei including chromatin in protoplasts undergoing high oxidative stress (Fig. 1C).
Discussion
The results presented here demonstrate that chromatin de- and recondensation correlate with the level of oxidative stress as well as the reactivation of the antioxidative systems. A common feature of in vitro cultures, including protoplasts, is the increased rate of ROS production, and also the accumulation of ROS. The extent of damaging effects of ROS depends upon the effectiveness of the antioxidative systems, which include low molecular mass antioxidants (ascorbate, glutathione, carotenoids), as well as several antioxidative enzymes (ascorbate peroxidase, glutathione reductase, catalase, superoxide dismutase) (reviewed in Bat'ková et al., 2008). In agreement with the results reviewed by de Marco and Roubelakis-Angelakis (1999), the application of ascorbate reduced the ROS level in protoplasts during the cultivation period; the reactivation of APX and catalase followed. However, a decrease of cytosolic APX expression in AA-treated protoplasts 72 h AI was determined; this could be connected with the ascorbate cycle in the cells. Protoplasts treated with AA displayed reduced ROS levels. This could lead to the depletion of the reduced ascorbate, on which the APX activity depends.
Moreover, it has been proposed that a reduced form of ascorbate, in contrast to an oxidized one, is required for cell division and to promote cell cycle progression in competent cells (Potters et al., 2000). Thus, protoplast cultures without AA treatment lack ascorbate to protect themselves from oxidative stress. They also did not proliferate in the experiments as much as was observed for AA treatments. However, the expression of APX at 72 h AI was reduced. The preservation of the ROS and levels of the reduced form of ascorbate were sufficient for the rapid cell proliferation of the AA-treated protoplasts.
Oxidative stress has been proposed as the main factor responsible for the recalcitrance of plant protoplast differentiation. It has been demonstrated that oxidative stress could cause cell death of protoplasts in a manner similar to the hypersensitivity responses during plant pathogen attacks (Maccarone et al., 2000; Papadakis and Roubelakis-Angelakis, 2002). Cell death is clearly characterized by DNA fragmentation and ladder formation during DNA electrophoresis (Maccarone et al., 2000). In this work, the chromatin changes under oxidative stress were studied directly in the cell nucleus. It was noted that heterochromatin organized in the chromocentres had undergone intense decondensation during protoplast isolation, and this had been observed for protoplasts of several species (Zhao et al., 2001; Tessadori et al., 2007; Ondřej et al., 2009). As shown here, this decondensation persists at an extreme level for the first 2 d of the cultivation, timed with maximum ROS occurrence; thereafter, the chromocentres started to reassemble, when the S-phase had passed. Subsequently, the protoplast-derived cells re-enter into the cell cycle and began to divide (Ondřej et al., 2009).
Several theories have been proposed to explain such massive chromatin decondensation. The decondensation is often connected with cell reprogramming and dedifferentiation at this stage of protoplast regeneration (Exner and Hennig, 2008). However, it has been documented that decondensation of the chromatin did not dramatically change the expression profile of protoplasts, and the up-regulation of the expression is connected with genes involving cell wall reconstruction and the cell cycle. Moreover, the chromocentres that were mainly affected by decondensation represent the constitutive heterochromatin—gene pure and the silent parts of the genome (Avramova, 2002; van Driel and Fransz, 2004). Further, the decondensed chromatin still retains its characteristics, with unchanged epigenetic markers (Tessadori et al., 2007). It has been demonstrated here that two repetitive sequences (5S rDNA satellite DNA type I located in chromocentres) are significantly less decondensed in relation to AA treatment, ROS level, and the reactivation of the antioxidative systems. It has also been shown that oxidative stress is connected with DNA damage, not only in the chloroplasts (Kim et al., 2000), but also in cell nuclei (Mancini et al., 2006) and in reaction to pathogen attacks (Sedlářová et al., 2007). Thus, large-scale decondensation of the chromatin could promote DNA damage as a response to cell wall degradation; or as a response to substances occurring in enzyme solution for cell wall degradation, which could behave as pathogen elicitors.
Overall, the results of this work have shown that large-scale decondensation of the chromatin within protoplast nuclei and the subsequent reassembly is connected with the levels of oxidative stress and the antioxidative systems. Reassembly of chromocentres (Ondřej et al., 2009) and an adequate dose of antioxidants could promote the re-entry into the cell cycle and the subsequent proliferation rate. It is hypothesized that the limitation of large-scale chromatin decondensation can also reduce the induction of changes in the genome, leading to the somaclonal variation known from plant biotechnologies. These changes are often associated with cytogenetic abnormalities, including changes in the ploidy level, chromosomal rearrangement, activation of transposons and retrotransposons, leading to mutations when transposed into genic regions (Grafi, 2009).
Acknowledgments
This work was supported by the Ministry of Education of the Czech Republic (MSM 6198959215 and 2E08018).
Glossary
Abbreviations
- AI
after isolation
- ROS
reactive oxygen species
- APX
ascorbate peroxidase
- CAT
catalase
- H2DCF DA
2,7-dichlorodihydrofluorescein diacetate
References
- Avramova ZJ. Heterochromatin in animals and plants. Similarities and differencies. Plant Physiology. 2002;129:40–49. doi: 10.1104/pp.010981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bat'ková P, Pospíšilová J, Synkvá H. Production of reactive oxygen species and development of antioxidative systems during in vitro growth and ex vitro transfer. Biologia Plantarum. 2008;52:413–422. [Google Scholar]
- Debeaujon I, Branchard M. Induction of somatic embryogenesis and callogenesis from cotyledons and leaf protoplasts-derived colonies of melon (Cucumis melo L.) Plant Cell Reports. 1992;12:37–40. doi: 10.1007/BF00232420. [DOI] [PubMed] [Google Scholar]
- de Marco A, Roubelakis-Angelakis KA. Specific features of the ascorbate/glutathione cycle in cultured protoplasts. Plant Cell Reporter. 1999;18:406–411. [Google Scholar]
- Exner V, Hennig L. Chromatin rearrangements in development. Current Opinion in Plant Biology. 2008;11:64–69. doi: 10.1016/j.pbi.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Gajdová J, Navrátilová B, Smolná J, Lebeda A. Factors affecting protoplast isolation and cultivation of Cucumis spp. Journal of Applied Botany and Food Quality. 2007;81:1–6. [Google Scholar]
- Ganal M, Riede I, Hemleben V. Organization and sequence analysis of two related satellite DNAs in cucumber (Cucumis satiuvus L.) Journal of Molecular Evolution. 1986;23:23–30. [Google Scholar]
- Gomes A, Femandes E, Lima JFC. Use of fluorescence probes for detection of reactive nitrogen species: a review. Journal of Fluorescence. 2006;16:119–139. doi: 10.1007/s10895-005-0030-3. [DOI] [PubMed] [Google Scholar]
- Grafi G. How cells dedifferentiate: a lesson from plants. Developmental Biology. 2004;268:1–6. doi: 10.1016/j.ydbio.2003.12.027. [DOI] [PubMed] [Google Scholar]
- Grafi G. The complexity of cellular dedifferentiation: implications for regenerative medicine. Trends in Biotechnology. 2009;27:329–332. doi: 10.1016/j.tibtech.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Kim JH, Shin SH, Cho SH, Lee WS. DNA endonuclease- and active oxygen-associated degradation of chloroplast DNA in response to paraquat-induced oxidative stress. Journal of Plant Biology. 2000;43:226–231. [Google Scholar]
- Maccarrone M, Van Zadelhoff G, Veldink GA, Vliegenthart JFG, Finazzi-Agró A. Early activation of lipoxygenase in lentil (Lens culinaris) root protoplasts by oxidative stress induces programmed cell death. European Journal of Biochemistry. 2000;267:5078–5084. doi: 10.1046/j.1432-1327.2000.01564.x. [DOI] [PubMed] [Google Scholar]
- Mancini A, Buschini A, Restivo FM, Rossi C, Poli P. Oxidative stress as DNA damage in different transgenic tobacco plants. Plant Science. 2006;170:845–852. [Google Scholar]
- Navrátilová B, Greplová M, Vyvadilová M, Klíma M, Gajdová J, Skálová D. Electrofusion of protoplasts in selected vegetables of Brassica, Cucumis, and Solanum genera. Acta Horticulturae. 2006;725:801–805. [Google Scholar]
- Ondřej V, Lukášová E, Krejčí J, Matula P, Kozubek S. Lamin A/C and polymeric actin in genome organization. Molecules and Cells. 2008;26:356–361. [PubMed] [Google Scholar]
- Ondřej V, Kitner M, Doležalová I, Nádvorník P, Navrátilová B, Lebeda A. Chromatin structural rearrangement during dedifferentiation of protoplasts of Cucumis sativus L. Molecules and Cells. 2009;27:443–447. doi: 10.1007/s10059-009-0057-4. [DOI] [PubMed] [Google Scholar]
- Papadakis AK, Roubelakis-Angelakis KA. Oxidative stress could be responsible for the recalcitrance of plant protoplasts. Plant Physiology and Biochemistry. 2002;40:549–559. [Google Scholar]
- Potters G, Horemans N, Caubergs RJ, Asard H. Ascorbate and dehydroascorbate influence cell cycle progression in a tobacco cell suspension. Plant Physiology and Biochemistry. 2000;38:531–540. doi: 10.1104/pp.124.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakoczy-Trojanowska M. Alternative methods of plant transformation: a short review. Cellular and Molecular Biology Letters. 2002;7:849–858. [PubMed] [Google Scholar]
- Sedlářová M, Luhová L, Petřivalský M, Lebeda A. Localization and metabolism of reactive oxygen species during Bremia lactucae pathogenesis in Lactuca sativa and wild Lactuca spp. Plant Physiology and Biochemistry. 2007;45:607–616. doi: 10.1016/j.plaphy.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Tessadori F, Chupeau MCh, Chupeau Y, Knip M, Germann S, van Driel R, Fransz P, Gaudin V. Large-scale dissociation and sequential reassembly of pericentric heterochromatin in dedifferentiated Arabidopsis cells. Journal of Cell Science. 2007;120:1200–1208. doi: 10.1242/jcs.000026. [DOI] [PubMed] [Google Scholar]
- van Driel R, Fransz P. Nuclear architecture and genome functioning in plants and animals: what can we learn from both? Experimental Cell Research. 2004;296:86–90. doi: 10.1016/j.yexcr.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Zhao J, Morozova N, Williams L, Libs L, Avivi Y, Grafi G. Two phases of chromatin decondensation of plant cells. Journal of Biological Chemistry. 2001;276:22772–22778. doi: 10.1074/jbc.M101756200. [DOI] [PubMed] [Google Scholar]