Abstract
The control of ripening of the non-climacteric grapevine fruit is still a matter of debate, but several lines of evidence point to an important role for the hormone abscisic acid (ABA). The effects of ABA treatments on Cabernet Sauvignon berries before and at véraison were studied using a 2-DE proteomic approach. Proteins from whole deseeded berries (before véraison) and berry flesh and skin (at véraison) treated with 0.76 mM ABA and collected 24 h after treatment were separated and analysed. A total of 60 protein spots showed significant variations between treated and control berries, and 40 proteins, mainly related to general metabolism and cell defence, were identified by LC MS/MS. Our results show that ABA acts mainly through the regulation of mostly the same proteins which are involved in the ripening process, and that several of these changes share common elements with the ABA-induced responses in vegetative tissues.
Keywords: 2D electrophoresis, abscisic acid, grape berry, proteomics, ripening, Vitis vinifera
Introduction
Grapevine is a major fruit crop worldwide since it is cultivated in all continents on an area of more than eight million hectares. Its fruits are the basis of one of the most economically important branches of the food industry. Beside its use in oenology, grape is becoming a significant source of secondary metabolites to be used for the production of pharmaceuticals and cosmetics. The ripening of the grape berry is a complex process, involving changes in texture, in anatomical structures, and in the composition of the vacuolar sap. The main changes which occur in the berries during their ripening are the accumulation of soluble sugars, flavonoids, and aromatic compounds, and the metabolism of organic acids (Ribereau-Gayon, 2000).
The molecular and physiological mechanisms underlying grape berry ripening are, however, still poorly understood. Recently, the availability of genomic information based on EST collections (da Silva et al., 2005; Deluc et al., 2007) and on genome sequencing (Jaillon et al., 2007) have boosted studies on molecular changes induced by ripening, which have been analysed at the transcriptomic (Terrier et al., 2005; Deluc et al., 2007; Pilati et al., 2007) and proteomic levels (Deytieux et al., 2007; Giribaldi et al., 2007; Negri et al., 2008; Zhang et al., 2008). These studies have opened several new perspectives in understanding the factors which control the ripening process at the molecular level.
The grape berry is a non-climacteric fruit, and although ethylene favours some aspects of ripening (Chervin et al., 2004), it is not a general trigger of ripening in grape. Auxin and brassinosteroids have been proposed as regulators of berry ripening, respectively negative and positive (Davies et al., 1997; Symons et al., 2006). Abscisic acid (ABA) contributes to the regulation of ripening in climacteric (Galpaz et al., 2008) and non-climacteric fruits, such as grape, cherry, strawberry, and orange (Coombe and Hale, 1973; Kondo and Inoue, 1997; Jiang and Joyce, 2003; Rodrigo et al., 2003). While in climacteric fruits ABA is thought to control ripening through activation of ethylene biosynthesis (Zhang et al., 2009), its role in non-climacteric fruits is much less clear.
Berry ABA concentrations increase just before véraison (the beginning of colour change) (Coombe and Hale, 1973; Scienza et al., 1978; Davies et al., 1997; Antolin et al., 2003). Transcripts and proteins linked to ABA biosynthesis have been found in ripening berries (Deluc et al., 2007; Giribaldi et al., 2007), although some evidence exists that ABA may also be synthesized in the leaves, and transported to the clusters via the phloem vessels (Shiozaki et al., 1999; Wheeler et al., 2009). The hypothesis that ABA may trigger ripening in the grape berry has been substantiated by the demonstration that exogenous ABA treatments at véraison enhance several processes involved in berry ripening, such as the accumulation of soluble solids, the decrease in the concentration of organic acids, and anthocyanin accumulation (Coombe and Hale, 1973; Pirie and Mullins, 1976; Palejwala et al., 1985; Wheeler et al., 2009). However, in most cases, feeding ABA to the berry resulted in the activation of some but not all the components of the ripening process. Thus the question is still open whether ABA is a main trigger of ripening, or whether it merely contributes to the activation of specific metabolic pathways.
The molecular effects of exogenous ABA have been intensively studied at the gene-specific level, detailing the effects of this phytohormone in the control of seed development and dormancy, and in the plant responses to environmental stress (Rock, 2000; Finkelstein et al., 2002). Genome-wide surveys of ABA-induced gene expression in vegetative tissues of Arabidopsis thaliana by applying deep sequencing and microarray analysis (Hoth et al., 2002; Seki et al., 2002; Suzuki et al., 2003; Sanchez et al., 2004) have confirmed that ABA induces a complex reprogramming of plant metabolism, in particular affecting genes involved in responses against stress, and genes involved in stress-dependent signalling networks. Such studies, however, have never been applied to fruit tissues, and in particular to fruits whose development seems to be controlled by ABA, such as grape. Gene-specific studies on ABA molecular effects in the grapevine berry have shown an activation of anthocyanin biosynthetic genes and of the anthocyanin-synthesis related VvmybA1 transcription factor (Ban et al., 2003; Jeong et al., 2004), and a delayed expression of condensed tannins biosynthetic genes (VvANR and VvLAR2) (Lacampagne et al., 2009). Supply of ABA to grape cell cultures induces an ABA/stress-related protein thought to stimulate sugar accumulation (Çakir et al., 2003). Genome-wide investigations on these fruits would supply valuable information on the mode of action of ABA, and could contribute to underpin its role in ripening. As a first contribution to this subject, the results of a proteomic analysis of the effects of exogenous ABA supply on grape berries at two different ripening stages are reported here, and it is shown that ABA affects the concentrations of several proteins with putative major roles in the ripening process.
Materials and methods
Biological material and treatments, ABA and maturity analyses
In order to work on homogeneous material, Vitis vinifera cv. Cabernet Sauvignon clusters were obtained from fruiting cuttings cultivated in a greenhouse at Bordeaux University (Bordeaux, France). Fruiting cuttings were produced following the procedure described by Ollat et al. (1998). Briefly, dormant cuttings were obtained from one-year-old, cane-pruned Cabernet Sauvignon shoots, collected in a vineyard at Bordeaux (France). The cuttings were propagated by a technique which ensured that the formation of adventitious roots preceeded bud burst (heating the base of the cuttings at 26 °C in a cold room at 4 °C). After 4 weeks, the rooted cuttings were planted in pots (10×10 cm) containing a perlite/sand mixture, and were transferred to a greenhouse under controlled conditions (45° north latitude, 27 °C day and 22 °C night, 70% relative humidity, natural photoperiod 14–16 h). A hydroponic solution was provided by drip irrigation (150 ml d−1 pot−1). Following budburst, the plants underwent normal development throughout flowering, setting, véraison, and ripening.
Clusters were treated before véraison (green berry stage) and at véraison (50% coloured berries) by spraying either an aqueous solution of 0.76 mM synthetic abscisic acid (±-cis,trans-ABA, Sigma) for the treated plants or water for the control plants. It was decided to use the racemic mixture of ABA instead of the natural form (–)-ABA, as Huang et al. (2007) demonstrated that the number of Arabidopsis genes whose expression was affected by (–)-ABA relative to (+)-ABA was small, and their expression ratios were low. Each cluster was sprayed with 10 ml solution (ABA or water) containing 0.05% (v/v) Tween 20 as a wetting agent. All spraying was carried out at dusk (sunset) to minimize ABA photodestruction.
For each treatment date, nine clusters from nine different cuttings were sprayed. Three clusters per treatment (control and ABA-treated) were collected after 24 h and 48 h, respectively, and 5 d after both treatment dates. Berries from the three clusters of each treatment at each sampling date were pooled and two subsamples of 30 berries and one of 20 berries were selected for each treatment. Berries were frozen at –80 °C until analysis.
On the first 30-berry subsample, the concentration of soluble solids was assessed using a refractometer (Atago), and the concentrations of anthocyanins and flavonoids were determined on grape skins after extraction in methanol containing 1% (v/v) hydrochloricric acid with a Lambda 25 (Perkin Elmer) spectrophotometer scan drive reading between 230 nm and 700 nm.
On the 20-berry subsample, ABA content was determined according to the method described by Antolin et al. (2003). Briefly, deseeded berries were extracted with 60 ml of 80% (v/v) methanol, then purified using polyvinyl-polypyrrolidone (PVPP), and finally extracted with diethyl-ether. ABA was quantified by HPLC analysis with UV spectrophotometry at 254 nm. Quantification was performed by regularly including an ABA standard solution in each HPLC sequence and endogenous ABA was quantified by the comparison of peak areas with those obtained from the respective ABA standard calibration curves. The assays were validated independently by mass spectrometry comparison with purified hormone extracts.
Protein extraction, 2-D electrophoresis, and statistical analyses
Green berries (treated before véraison) were analysed without separation of their components, except for deseeding, while deseeded coloured berries (treated at véraison) were divided into skin and flesh, and the two tissues were analysed separately. Frozen material (second 30-berry subsample) was ground in liquid nitrogen with a mortar and pestle with 10% (w/w) PVPP and 10% (w/w) sterile sand. The extraction protocol was essentially as described by Giribaldi et al. (2007). Extraction buffer contained 0.1 M TRIS-HCl (pH 7.5), 7 M urea, 2 M thiourea, 2% (v/v) Triton X-100, and 65 mM DTT. The frozen powders from green berries (3 g), from coloured berry skins (2 g), and from coloured berry flesh (3 g) were vortexed respectively in 10 ml, 6 ml, and 9 ml of extraction buffer. The suspensions were left for 30 min on ice, and then centrifuged for 30 min at 5000 g at 4 °C. Supernatants were then precipitated with 15% (w/v) TCA, vortexed, left for 10 min at 4 °C and centrifuged 15 min at 14 000 g at 4 °C. Protein pellets were washed twice in cold acetone (–20 °C), then incubated for 10 min in acetone at –20 °C, and centrifuged for 15 min at 14 000 g at 4 °C. Final pellets were then resuspended in IEF rehydration solution (7 M urea, 2 M thiourea, 2% (v/v) Triton X-100, 65 mM DTT, and 0.5% (v/v) IPG buffer 3-10 Non Linear). Total protein concentration was assessed using Plus One 2DQuant kit (GE Bioscience), using BSA as a standard.
IEF was carried out with 600 μg protein per strip using 24 cm long Immobiline Dry-strips pH 3-10 Non Linear (GE Bioscience). Running conditions for the first dimension were: passive rehydration for 8 h at 20 °C, active rehydration (50 V) for 9 h, then ramping up to 300 V in 1 min, 300 V for 30 min, then ramping up to 8000 V in 3 h, 8000 V for 11 h, then down again to 300 V in 3 h, and 300 V until about 110 kVh were reached. Strips were then equilibrated upon gentle agitation on a stirrer twice for 15 min in equilibration buffer (0.1 M TRIS-HCl pH 8.8, 6 M urea, 30% (v/v) glycerol, and 2% (w/v) SDS), containing the first time 65 mM DTT, and the second time 2.5% (w/v) iodoacetamide.
SDS-PAGE was performed in 24×24 cm, 11% (v/v) polyacrylamide gels using an Ettan Dalt six multiple apparatus (GE Biosciences) at 15 °C, according to the Laemmli protocol (Laemmli, 1970). Running conditions were 15 mA gel−1 for 1 h, then 25 mA gel−1 until the dye front reach the end of gel. Gels were stained with colloidal Coomassie Blue G-250 (Candiano et al., 2004), using at least 300 ml of staining solution per gel. Gels were then scanned with a GelDoc device (Bio-Rad), and analysed with PDQuest version 7.2 software (Bio-Rad).
Three replicate gels were run for each pooled sample. Spots showing significant variation between control and ABA-treated samples were selected according to the results of paired Student t tests (P ≤0.05). Only spots whose presence was detectable in at least two out of three replicates, whose rate of variation was ±0.5, and whose mean % volume was ≥0.03% were considered for further analysis.
In-gel digestion
Spots were excised from the gel and washed until destaining in 50 mM NH4HCO3/ACN (acetonitrile) (1:1 v/v). The washing solution was then removed and replaced with 100% (v/v) ACN. When the gel pieces had shrunk, ACN was removed and the gel pieces were dried in a vacuum centrifuge. The gel pieces were rehydrated with trypsin solution (10 ng μl−1 in 50 mM NH4HCO3), at 4 °C for 10 min, and finally incubated in trypsin solution overnight at 37 °C. The gel pieces were then incubated in 50 mM NH4HCO3 at room temperature with rotary shaking for 15 min. The supernatant was collected and a H2O/ACN/HCOOH (47.5:47.5:5 by vol.) extraction solution was added to the gel pieces for 15 min. This step was repeated and both supernatants were pooled and concentrated in a vacuum centrifuge to a final volume of 25 μl. Digests were finally acidified by adding 1.2 μl of 5% (v/v) acetic acid and stored at –20 °C.
On-line capillary HPLC nanospray ion trap MS/MS analyses and protein identification by database search
The peptide mixture was analysed by on-line capillary HPLC (LC Packings), coupled to a nanospray LCQ ion trap mass spectrometer. Ten microlitres of peptide digests were loaded onto a 300 μm inner diameter 35 mm C18 PepMap™ trap column (LC Packings) at a flow rate of 30 μl min−1. The peptides were eluted from the trap column onto an analytical 75 μm inner diameter×15 cm C18 PepMap™ column (LC Packings) with a 5–40% (v/v) linear gradient of solvent B in 35 min (solvent A was 0.1% (v/v) formic acid in 5% (v/v) ACN, and solvent B was 0.1% (v/v) formic acid in 80% (v/v) ACN). The separation flow rate was set at 200 nl min−1. The mass spectrometer operated in positive ion mode at a 2 kV needle voltage and a 3 V capillary voltage. Data were acquired in a data-dependent mode alternating a MS scan survey over the range m/z 150–200, a zoom scan on the most intense ion and its MS/MS spectrum using a 2 m/z units ion isolation window and 35% relative collision energy.
Data were searched by SEQUEST through Bioworks 3.3.1 interface (ThermoFinnigan) against a subset of the NCBI database restricted to Vitis vinifera proteins (55849 entries) for the first time, then against a subset of the Uniprot/SwissProt database restricted to plants. Data files were generated for MS/MS spectra that reached both a minimal intensity (5×104) and a sufficient number of ions (35). The data generation allowed the averaging of several MS/MS spectra corresponding to the same precursor ion with a tolerance of 1.4 Da. Spectra from the precursor ion higher than 3500 Da or lower than 500 Da were rejected. The search parameters were as follows: mass accuracy of the peptide precursor and peptide fragments was set to 1.5 Da and 0.5 Da, respectively; only b- and y-ions were considered for mass calculation; oxidation of methionine (+16) and carbamidomethylation of cysteine (+57) were considered as differential modifications; two missed trypsin cleavages were allowed.
Only peptides whose Xcorr was over 1.9 (single charge), 2.2 (double charge), and 3.75 (triple charge) were retained. In all cases, ΔCn was above 0.1. All protein identifications were based on a minimum of two peptide assignments, except where indicated.
After identification, theoretical molecular weight and pI of proteins were calculated by processing sequence entries at http://www.expasy.org/tools/pi_tool.html, and proteins were assigned to a functional categories (FunCat) by the Munich Information Center for Protein Sequences (MIPS) (http://mips.gsf.de/projects/funcat) according to their role described in the literature.
Results
Ripening parameters and ABA quantification
Berry soluble solids content was 3.5° Brix at the date of treatment before véraison, and 9° Brix at the date of treatment at véraison. Anthocyanins were absent before véraison, while at véraison anthocyanin content was 3.0 mg g−1 of berry skin. The concentrations of soluble solids and of anthocyanins did not significantly change within the five days following treatment, although a small and transient increase was noticed.
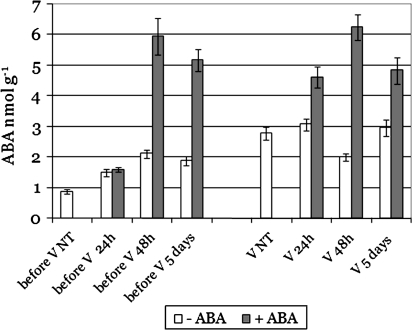
As expected, ABA concentration increased in the berries following treatment. The concentration of ABA was not significantly affected by ABA application 24 h after treatment before véraison, while it was significantly higher 48 h and 5 d after treatment before véraison. After treatment at véraison stage, ABA concentration was higher both 24 h and 48 h, and 5 d after treatment (Fig. 1).
Fig. 1.
ABA concentration in V. vinifera cv. Cabernet Sauvignon berries before treatment and 24 h and 48 h and 5 d after treatment with 0.76 mM ABA. Before V, treated before véraison; V, treated at véraison. Error bars represent standard errors (n=2).
2D gel analysis
Protein yield was higher for tissues treated at véraison (Table 1). The number of spots observed (average of three gel replicates) for each sample ranged between 679 and 379. A total of 64 spots showed significant variation between control and ABA-treated samples, mostly observed in whole berries treated before véraison (Table 1). Among these, most spots were up-regulated following ABA treatment, in particular, before véraison. The correlation of spot intensities between non-treated and ABA-treated samples was, however higher, in the case of treatment before véraison (Table 1).
Table 1.
Treatment time, tissue analysed, protein yield, total spot number, number of spots showing statistically significant difference (P ≤0.05; trend ≥±0.5), number of spots, respectively, up-regulated or down-regulated by ABA treatment, regression coefficient R2 of spot intensities between control and ABA-treated Cabernet Sauvignon samples
| Treatment time | Tissue | Protein yield (mg g−1) | Total spots | Differentially expressed spots | ABA up-regulated | ABA down-regulated | R2 |
| Before véraison | Whole berry | 0.46 | 679 | 34 | 25 | 9 | 0.90 |
| Véraison | Skin | 1.98 | 407 | 20 | 15 | 5 | 0.75 |
| Véraison | Flesh | 1.04 | 379 | 10 | 4 | 6 | 0.80 |
Spots showing significant differences were analysed by LC MS/MS, and a total of 40 yielded successful identifications (15 from whole berries treated before véraison, nine from berry flesh, and 16 from berry skins of berries treated at véraison) (Fig. 2). Almost all of the proteins identified belonged to members of the genus Vitis. Eight spots contained multiple proteins, for a total of 20 proteins, so the total number of proteins identified was 52 (Table 2).
Fig. 2.
Protein spots identified from Cabernet Sauvignon berries after ABA treatment. (A) Control green berries; (B) treated green berries; (C) control véraison flesh; (D) treated véraison flesh; (E) control véraison skin; F: treated véraison skin. For spot names see Table 2.
Table 2.
Spots identified by LC MS/MS
| Spot ID | Accession | Identified protein | % Cov | Exp/Hyp mass (kDa) | Vol% maxa | Exp/Hyp pI | Trendb |
| Whole berries before véraison | |||||||
| 7502 | A7QC85 | Elongation factor 1 gamma chain (EF1) | 19 | 47.15/47.72 | 0.05 | 7.11/6.43 | –1.00 |
| 9504 | A3FA69 | Aquaporin PIP2;4 (PIP2) | 10 | 51.79/30.23 | 0.23 | 8.85/8.28 | –0.78 |
| 5807 | A7NZW5 | Lipoxygenase (Lox) | 8 | 93.98/101.68 | 0.07 | 6.11/6.13 | –0.68 |
| 217 | Q7XAU6 | Class IV chitinase (C) | 18 | 28.94/27.53 | 0.94 | 4.83/5.38 | +0.50 |
| 2103 | A5ARN9 | (Early dehydratation induced) Glutathione S transferase (GST) | 39 | 25.41/25.55 | 0.07 | 5.36/5.57 | +0.55 |
| 1707 | A5AER7 | Cloroplast membrane-bound ATP dependent protease (Prot) | 14 | 67.67/74.36 | 0.05 | 5.18/5.8 | +0.72 |
| 3209 | Q1AFF4 | Ascorbate peroxidase (APX) | 37 | 27.56/27.62 | 0.07 | 5.71/5.58 | +0.77 |
| 2417 | A5AFH5 | Cysteine synthase (CS) | 49 | 37.00/34.37 | 0.25 | 5.35/5.39 | +0.85 |
| 2109 | Q8W3L8 | Xyloglucan endo-transglycosylase (XET) | 17 | 23.46/32.76 | 0.45 | 5.48/5.55 | +1.00 |
| 117 | Q9S944 | Vacuolar invertase 1 (GIN1) | 10 | 26.42/71.55 | 0.16 | 4.05/4.60 | +1.00 |
| 706 | P51117 | Chalcone isomerase (CHI) | 27 | 70.64/25.14 | 0.04 | 4.78/5.26 | +1.00 |
| 1311 | A5BHF8 | Spermidine synthase (SS) | 38 | 33.62/34.26 | 0.05 | 5.03/5.06 | +1.00 |
| 1513 | A5C0I8 | Alcohol dehydrogenase 2 (Adh) | 38 | 46.37/41.12 | 0.03 | 5.17/5.97 | +1.00 |
| 5317 | A5BGY1+P51110+Q7PCC4 | UDP-galactose-4-epimerase+Dihydroflavonol-4-reductase+Anthocyanidin reductase (Mix1) | 48 | 35.93/38.03+37.76+36.74 | 0.03 | 6.08/6.02+6.17+5.77 | +1.00 |
| 6109 | Q38JC9+A2T400 | Temperature-induced lipocalin+Ascorbate peroxidase (Mix2) | 34 | 21.95/21.54+19.66 | 0.04 | 6.37/6.63+5.51 | +1.00 |
| Berry flesh at véraison | |||||||
| 6706 | A5BDU8+A7R155 | Dihydrolipoyl dehydrogenase+Polyphenol oxidase, chloroplast precursor (Mix3) | 6 | 57.50/49.57+67.36 | 0.07 | 6.58/7.18+6.06 | –1.00 |
| 8303 | A7QIF2 | Protein phosphatase type 2C (Pho2C) | 15 | 28.16/26.18 | 0.13 | 7.59/5.52 | –1.00 |
| 5902 | A5BQI3 | Putative alpha-glucosidase (α-glu) | 3 | 86.71/100.95 | 0.08 | 5.58/5.78 | –0.93 |
| 7905 | A7QRI6 | Cobalamine-independent methonine synthase (metS) | 12 | 82.95/84.99 | 0.1 | 7.22/6.09 | –0.86 |
| 9004 | A5AKD8 | Peptidyl-prolyl cis-trans isomerase (iso) | 12 | 19.14/17.94 | 0.14 | 9.40/8.93 | –0.86 |
| 5709 | A7QI60 | Delta-1-pyrroline-5-carboxylate dehydrogenase (P5CDh) | 7 | 61.15/57.34 | 0.05 | 5.60/6.23 | –0.82 |
| 8805 | A7PEH8 | Malic enzyme (ME) | 24 | 66.66/54.84 | 0.21 | 7.38/8.27 | +0.79 |
| 9701 | P43311 | Polyphenol oxidase, chloroplast precursor (PPO) | 5 | 54.99/67.36 | 0.37 | 7.96/6.06 | +0.84 |
| 3806 | A7QS95+A7Q4B5 | HSP70+Vacuolar ATP synthase catalytic subunit A (Mix4) | 16 | 76.92/66.9+68.72 | 0.08 | 5.38/5.21+5.24 | +0.96 |
| Berry skin at véraison | |||||||
| 1914 | A5CAF6 | Phosphoglycerate kinase (Pgk) | 20 | 103.98/42.42 | 0.13 | 4.92/6.29 | –0.66 |
| 211 | A7P4N9+A7P5N4 | Ran-binding protein+Unknown function (Mix8) | 28 | 35.40/24.58+40.33 | 0.05 | 4.80/4.72+5.77+5.24 | –0.62 |
| 1707 | A5BVL7 | Protein disulphide isomerase-like protein (Disulph) | 5 | 68.25/64.99 | 0.08 | 4.95/4.98 | –0.62 |
| 1806 | A7PNA3 | HSP90 (HSP90) | 6 | 91.05/90.46 | 0.11 | 4.92/4.93 | –0.62 |
| 5203 | A7NZC2 | Cytosolic ascorbate peroxidase (APX) | 11 | 30.88/27.15 | 0.15 | 4.92/5.86 | –0.61 |
| 2002 | A5CAF6 | Phosphoglycerate kinase (Pgk2) | 17 | 20.17/42.42 | 0.71 | 5.13/6.29 | +0.50 |
| 5402 | A7NT93+A7PRU0 | Endo-1,4-beta-mannanase+Protein disulphide isomerase-like protein (Mix7) | 8 | 42.55/48.97+39.27 | 0.06 | 5.56/9.19+5.57 | +0.51 |
| 3601 | Q0ZJ35 | ATP synthase CF1 alpha subunit (cATPase) | 10 | 60.31/68.72 | 0.08 | 5.24/5.24 | +0.55 |
| 3803 | A7R8V8 | Eukaryotic initiation factor 3 subunit (EiF) | 8 | 81.24/38.79 | 0.04 | 5.26/5.31 | +0.60 |
| 4210 | A9UFX7 | Cytosolic ascorbate peroxidase (APX2) | 28 | 32.16/28.00 | 0.13 | 5.48/5.43 | +0.69 |
| 7206 | A5C6V1+A7PQR5 | Mov34 protein+Xyloglucan endo-transglycosylase (Mix6) | 12 | 36.41/34.51+32.7 | 0.11 | 6.41/6.31+5.74 | +0.79 |
| 4402 | A7PTT3 | Protease C56, putative (pC56) | 23 | 45.77/41.38 | 0.29 | 5.48/5.45 | +0.81 |
| 9102 | A7P5N4 | Unknown function (Unk) | 14 | 28.95/40.33 | 0.20 | 8.97/5.77 | +0.87 |
| 8109 | A7NZG0 | Beta proteasome subunit (B prot) | 20 | 28.72/27.58 | 0.07 | 8.28 | /6.44+0.88 |
| 8506 | A5APN1+A7QR94+A7Q2Y3+P93622 | Chorismate synthase+Citrate synthase+Acetyl-CoA acetyltransferase+Polyphenol oxidase (Mix5) | 9 | 52.05/47.13+52.39+38.86+67.39 | 0.06 | 7.89/7.64+7.66+5.64+6.39 | +0.95 |
| 5807 | A7NZW5 | Lipoxygenase (Lox) | 3 | 90.09/101.68 | 0.05 | 5.56/6.13 | +1.00 |
Spot ID: spot code. Accession: SwissProt code of the identified protein. Identified protein and spot name in Fig. 2. % Cov: the percentage of sequence covered by the identified peptides. Exp/Hyp mass (kDa): experimental/predicted molecular weight. Vol% max: maximum per cent volume of the spot. Exp/Hyp pI: experimental/predicted isoelectric point. Control/ABA trend.
Vol% max is calculated as the mean of the three replicates after normalization on total quantity in valid spots.
Trend is calculated as 1–(ABA treated/control). Positive values=up-regulated following ABA treatment, negative values=down-regulated following ABA treatment.
In the case of berries treated before véraison, three of the identified spots were down-regulated, while the majority of spots were up-regulated. Spot 117, identified as vacuolar invertase, showed a Mr three times lower than expected, so it probably is a degradation fragment of the native protein. In berries treated at véraison, 11 of the identified spots were down-regulated (six in flesh and five in skin) and also in this case the majority (14 spots) were up-regulated.
Discussion
ABA treatment affects ripening-related proteins
Throughout ripening of the grapevine berry, expression changes of genes and proteins related to sugar transport and metabolism, organic acid metabolism, biosynthesis of secondary metabolites, and cell wall softening have been well documented using gene specific and genome-wide investigation tools (Davies and Robinson, 2000; Terrier et al., 2005; Deluc et al., 2007; Deytieux et al., 2007; Giribaldi et al., 2007; Negri et al., 2008). The expression of many of these proteins was affected by ABA in our study. Vacuolar invertase (GIN1: Davies and Robinson, 1996) is thought to play a fundamental role in hexose accumulation in the berry. The appearance of a small spot, identified as GIN1, was noticed after ABA treatment before véraison. Although transcript level of GIN1 was reported to increase until véraison and to decrease thereafter (Davies and Robinson, 1996), the amount of corresponding protein was recorded as stable (Famiani et al., 2000; Giribaldi et al., 2007) during grape berry ripening. Based on the low Mr, the GIN1 spot observed probably represents a cleavage product, and thus ABA treatment seems to induce a faster degradation of vacuolar invertase. This agrees with the observation of a shift in the localization of sucrose hydrolysis from the vacuole to the apoplast during ripening, associated with a decreased expression of vacuolar invertases and a concomitant up-regulation of apoplastic invertases (Waters et al., 2005; Zhang et al., 2006).
A cytosolic NADP-dependent malic enzyme (ME) was found to increase after treatment at véraison in berry flesh. Malate is accumulated until véraison in the berry, while it is catabolized from the onset of ripening: malate breakdown is probably not controlled by malic enzyme, as its activity is high already before véraison (Taureilles-Saurel et al., 1995). However, NADP-ME could serve other roles in the developing berry cytosol, such as the provision of NADPH for biosynthetic purposes. NADPH could be used in the biosynthesis of phenolics, as hypothesized for bean seeds, based on the sensibility to stress of this enzyme (Pinto et al., 1999), or in the biosynthesis of lipids for epicuticular wax production. Contradictory data have been reported on NADP-ME protein abundance during berry ripening, including both a decrease after véraison (Giribaldi et al., 2007), a steady level (Famiani et al., 2000), and an increase (Negri et al., 2008). These contradictions are probably due to the relatively high number of isoforms of this protein (Jaillon et al., 2007).
Activation of anaerobic metabolism has been demonstrated during the ripening process, probably to cope with the decreased availability of oxygen within the berry (Sauvage et al., 1991; Tesnière and Verriès, 2000). Grape alcohol dehydrogenase (Adh) genes are differentially expressed and exhibit different biochemical properties, probably playing distinct metabolic roles. VvAdh1 expression is mainly detected in the green stage, as is VvAdh3. VvAdh2, on the contrary, shows very low expression after flowering, and transcript accumulation starts at véraison, in accordance with the observed trend for total Adh activity (Tesnière and Verriès, 2000). A VvAdh2 spot which was up-regulated upon ABA treatment before véraison was observed.
Ripening is linked to tissue softening in most fruits, and the grape berry is no exception, although the relevant molecular processes are still poorly known. Xyloglucan endotransglycosylase (XET), an enzyme involved in cell-wall restructuring, was found in berry skin by Deytieux et al. (2007) and by Negri et al. (2008), who both recorded an increase in its abundance at véraison. A similar conclusion was reached in transcriptomic studies made with extracts from deseeded berries (Glissant et al., 2008). XET transcripts increased in grape berries during ripening (Nunan et al., 2001; Ishimaru and Kobayashi, 2002). In our experiment XET protein expression was significantly induced by ABA treatment, both before and at véraison.
Grape berries accumulate high concentrations of secondary metabolites, including flavonoids, terpenes, and other aromatic compounds. Three proteins involved in flavonoid biosynthesis, chalcone isomerase, dihydroflavonol-4-reductase and anthocyanidin reductase (the last two included in a mixed spot with UDP-galactose-4 epimerase) were up-regulated following ABA treatment before véraison. The first two have been reported to be expressed early in berry development and again after véraison, when colour development occurs (Boss et al., 1996). Anthocyanidin reductase catalyses the formation of epicatechins and it has been shown to be highly expressed in flowers and young berries well before véraison (Bogs et al., 2005; Gagné et al., 2009). Our results confirm that ABA affects anthocyanin biosynthesis (Jeong et al., 2004) and suggest that it may also influence the proanthocyanidin pathway. This hypothesis is strengthened by the recent finding that ABA treatment on young berries may increase and delay both VvLAR2 and VvANR expression (Lacampagne et al., 2009). Also glutathione-S-transferase, whose expression is up-regulated by ABA, could be linked to secondary metabolism, as it is implicated in the accumulation of anthocyanidins in vacuoles of maize, Petunia, and Arabidopsis (Kitamura et al., 2004).
ABA treatment induces the expression of stress-related proteins in the ripening berry
ABA has been long known as a stress hormone and its biosynthesis, signalling, and molecular effects are activated under water-, salt-, and cold-stress. During ripening, the grape berry undergoes water stress because of its high solute potential (Matthews et al., 2009), while increased expression of abiotic and biotic stress defence genes has been reported in proteomic and transcriptional profiling studies of berry ripening (Davies and Robinson, 2000; Giribaldi et al., 2007; Deluc et al., 2007). Our results show that ABA treatment also induces proteins which are or may be linked to stress response.
Based on the activation of some genes linked to oxidative stress, it has been proposed that an oxidative burst can take place at ripening (Pilati et al., 2007), although this claim found only limited confirmation in transcriptomic and proteomic analyses of grape ripening (Terrier et al., 2005; Giribaldi et al., 2007; Negri et al., 2008). ABA treatment before véraison induced expression of proteins potentially involved in the oxidative stress response, such as two ascorbate peroxidases (a key enzyme for the elimination of the peroxide radical), and a temperature-induced lipocalin. Also, treatment at véraison affected expression of two ascorbate peroxidases, although in this case one was up-regulated and one down-regulated, but this is consistent with the shift among ascorbate peroxidase isoforms reported by Giribaldi et al. (2007) in ripening Nebbiolo berries.
Chitinase activity increases markedly at the onset of ripening, and class IV chitinases are up-regulated at véraison, even in the absence of pathogen challenge (Robinson et al., 1997). A marked expression activation of a class IV chitinase following ABA treatment before véraison was observed. Accordingly, in vegetative and seed tissue of model plants, chitinase activity can be induced by a number of chemical signals, including ethylene, jasmonic and salicylic acid, auxin, cytokinin, and ABA (Graham and Sticklen, 1994).
Two spots identified as lipoxygenase were affected by ABA treatment. The behaviour of these proteins was different in berries treated before and at véraison, as they are probably isoforms involved in different metabolic pathways. Lipoxygenase is involved in the octadecanoid pathway leading to jasmonate biosynthesis. It is also involved in the formation of compounds that generate the so-called ‘green odour’ in grape berries. Expression of five different lipoxygenases was found to be modified in water-stressed berries by Deluc et al. (2009), two being down-regulated in response to water deficit. Antagonistic interactions were found in Arabidopsis between components of the responses to ABA and to jasmonate (Anderson et al., 2004).
Proline is a key metabolite in plants, which may serve a protection role against osmotic and oxidative stress. Free proline is accumulated at high concentration in grape berries during ripening (Stines et al., 2000). The concentration of proline in plant tissues is controlled by the balance between biosynthetic enzymes, in particular Δ1-pyrroline-5-carboxylate synthase (P5CS), and degrading enzymes, namely proline dehydrogenase and Δ1-pyrroline-5-carboxylate dehydrogenase (P5CDH) (Deuschle et al., 2004). A decrease was observed in the abundance of P5CDH protein after ABA treatment before véraison. It is interesting to note that Stines et al. (1999) were not able to measure changes in gene or protein expression of P5CS or proline dehydrogenase in Cabernet Sauvignon berries after véraison. Our results suggest the alternative hypothesis that proline accumulation in ripening grape berries may be under ABA control through the inactivation of the degrading enzyme P5CDH.
Amino acid and polyamine metabolism were affected by ABA treatment: spermidine synthase and cysteine synthase increased following treatment before véraison. Concentration of polyamines changes during grape berry development. Early stages of berry development are associated with higher levels of polyamines (Gény et al., 1997), including free putrescine, spermidine, and spermine, followed by a decrease up to véraison, the concentration remaining stable afterwards (Shiozaki et al., 2000) or increasing (Gény et al., 1997). Polyamines, in particular spermidine and spermine, are also known to accumulate under several abiotic stresses, including salt and drought (Capell et al., 2004). Many plants also accumulate specific amino acids or their derivatives in response to environmental stresses (Bohnert and Jensen, 1996). The concentrations of most of the major free amino acids in grape berry are higher at véraison (Lamikanra and Kassa, 1999).
ABA signal transduction
Protein phosphorylation is a key factor for ABA signal transduction: a grapevine protein phosphatase 2C (PP2C; A7QIF2) was identified, which shares extensive similarity with AtABI1 and the related negative regulators of ABA signalling in Arabidopsis, which have been involved in many effects of ABA such as stomatal closure, drought-induced resistance, seed germination, and the inhibition of vegetative growth (Gosti et al., 1999; Hirayama and Shinozaki, 2007). This spot completely disappears in berry flesh soon after ABA treatment at véraison. The ABI1 protein has been shown to be negatively regulated in Arabidopsis (Suzuki et al., 2003; Sanchez et al., 2004; Xin et al., 2005) by ABA treatment or by secondary messengers of the ABA signal. It could therefore be a key protein for the regulation of the ABA signal in the ripening grape berry as well, and its down-regulation by ABA could represent a mechanism allowing a fine-tuning of ABA sensitivity.
ABA induces a general rearrangement of metabolism
The ‘classical’ effects of ABA on ripening and stress protection cannot be reached without a profound reorganization of metabolism in the berry. It is therefore not surprising that the present proteomic data highlight the effects of ABA on many diverse branches of metabolism and on protein modifications.
Two spots, identified as phosphoglycerate kinase (PGK), display molecular masses very different from the intact protein (about 42 kDa). Euglena gracilis chloroplast phosphoglycerate kinase was found to be encoded by a polyprotein precursor, whose Mr was about 100 kDa, formed by four mature subunits, separated by conserved tetrapeptide sequences (Nowitzki et al., 2004). The mature proteins are processed after import into the plastids. The similarity between database sequences of Vitis vinifera and Euglena PGKs rise up to 50%. One PGK spot (no. 1914) displays a mass very similar to Euglena PGK polyprotein precursor, and decreases after ABA treatment at véraison. At the same time, the other PGK spot (no. 2002) displays a mass consistent with that of the N terminal domain of the enzyme, and seems to increase after ABA treatment at véraison. Our hypothesis is that PGK is also synthesized as a polyprotein precursor in the grape berry and is then processed. To our knowledge, this is the first time that this phenomenon has been hypothesized in plants. In a previous proteomic report (Deytieux et al., 2007), plastidic PGK was repressed in Cabernet Sauvignon skins at the beginning and at the end of véraison. This suggests that ABA treatment at véraison may cause a halt in the synthesis of PGK polyprotein precursor, and a contemporary increase in its degradation products.
Cobalamine-independent methionine synthase catalyses the final step in L-methionine synthesis. This protein is already known to decrease from véraison onwards in whole Nebbiolo grape berries (Giribaldi et al., 2007), although it was found to have an opposite trend in the proteome of Barbera grape skins (Negri et al., 2008). In our study, ABA treatment before véraison negatively affected the concentration of this protein, and it can be hypothesized that a flesh- and not skin-localized isoform is under ABA control.
Given the important reorganization of metabolism induced by ABA, it is not surprising that protein fate is one of the MIPS classes most represented among proteins affected by ABA treatment, in particular at véraison. A similar effect on controlled proteolytic degradation was observed in Arabidopsis vegetative tissues by Hoth et al. (2002). In flesh, ABA treatment increased one heat shock protein 70, while one HSP90 decreased in berry skin. In the same class, peptidyl-propyl cis-trans isomerase, which accelerates protein folding by catalysing isomerization of proline imidic peptide bonds, increased after ABA treatment. An isomerase (spot no. 1707), acting on disulphide bonds between cysteine residues within proteins, displayed a decreasing trend after ABA treatment in skins, while a similar protein was part of a spot (no. 5402) induced by ABA in skins. A disulphide isomerase spot almost identical to our spot no. 1707 was found to be overexpressed in fully coloured skins of Cabernet Sauvignon by Deytieux et al. (2007). The increase in the skin of beta proteasome subunit after ABA treatment at véraison is predictable as subunit alpha has the same trend during Nebbiolo ripening (Giribaldi et al., 2007).
ABA treatment induced a decrease in the expression of a PIP2-type aquaporin in green berries. Aquaporins are water channels which facilitate the transport of water and other non-polar solutes through membranes. Pilati et al. (2007) identified two PIP2, sharing a 99% homology with our spot, displaying a decreasing trend after véraison. In Arabidopsis, several aquaporins are known to be down-regulated by water stress and by ABA (Jang et al., 2004). The higher molecular weight of the spot, compared to the calculated one, is due to the fact that this spot represents a dimer, as already reported on 2D gels by Santoni et al. (2003).
Conclusions
Our study consists of a proteomic approach to the effects of ABA on a fruit tissue and, as such, it allows answers to two unresolved questions to be proposed.
A first issue is whether, and by which mechanisms, ABA can induce or enhance ripening in the grape berry. The enhancement of ripening by ABA in grapevine has been observed at the compositional (Coombe and Hale, 1973; Pirie and Mullins, 1976; Wheeler et al., 2009) and, in some cases, at the molecular level (Ban et al., 2003; Jeong et al., 2004). Our results represent the first proteomic confirmation of these effects of ABA treatments. They show that ABA acts through the over- or underexpression of, mostly, the same proteins which are involved in the ripening process, in the same direction as observed during ripening: vacuolar invertase is down-regulated, Adh2, XET, enzymes of anthocyanin biosynthesis are up-regulated. It is also remarkable that these effects were mostly observed when berries were treated before véraison, thus confirming the results of Giribaldi et al. (2009), which showed that ABA was most effective in enhancing ripening when it was supplied before véraison and not at later stages, probably due to the fact that at these stages the endogenous ABA content was already high.
A second question is whether, in fruit tissues, the effects of ABA are similar or different from those observed in vegetative tissues. The effects of ABA have been largely studied in vegetative tissues of Arabidopsis thaliana using transcript profiling and high-throughput sequencing (Seki et al., 2002; Hoth et al., 2002). Although reproductive tissues of grape have a very distinct structural and metabolic organization than Arabidopsis vegetative tissues, it is a striking observation that most of the effects recorded by us at the protein level in grape berries are similar to those observed in Arabidopsis plants. Seki et al. (2002) observed up-regulation of Adh, XET, anthocyanin biosynthetic genes, glutathione-S-transferase, and membrane intrinsic proteins. Changes in proline metabolism were observed, although based on modifications of expression of the P5CS gene. They also observed down-regulation of PP2C. Both Seki et al. (2002) and Hoth et al. (2002) report on the activation of proteases and of genes involved in protein restructuring and degradation. Changes in expression of genes linked to amino acid and ascorbate metabolism were reported by Xin et al. (2005). This suggests that the pattern of gene regulation induced by ABA shares important common elements between vegetative and reproductive tissues, although it seems to have adapted to the requirements of the specific tissues.
Acknowledgments
MG and AS acknowledge financial support from Regione Piemonte, project CIPE 2004 ‘Genomica funzionale della vite’.
References
- Anderson JP, Badruzsaufari E, Schenk PM, Manners JM, Desmond OJ, Ehlert C, Maclean DJ, Ebert PR, Kazana K. Antagonistic interaction between abscisic acid and jasmonate-ethylene signaling pathways modulates defence gene expression and disease resistance in Arabidopsis. The Plant Cell. 2004;16:3460–3479. doi: 10.1105/tpc.104.025833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antolin MC, Baigorri H, De Luis I, Aguirrezabal F, Geny L, Broquedis M, Sànchez-Diaz M. ABA during reproductive development in non-irrigated grapevines (Vitis vinifera cv. Tempranillo. Australian Journal of Grape and Wine Research. 2003;9:169–176. [Google Scholar]
- Ban T, Ishimaru M, Kobayashi S, Goto-Yamamoto SN, Horiuchi S. Abscisic acid and 2,4-dichlorophenoxyacetic acid affect the expression of anthocyanin biosynthetic pathway genes in ‘Kyoho’ grape berries. Journal of Horticoltural Science and Biotechnology. 2003;78:586–589. [Google Scholar]
- Bogs J, Downey MO, Harvey JS, Ashton AR, Tanner GJ, Robinson SP. Proanthocyanidin synthesis and expression of genes encoding leucoanthocyanidin reductase and anthocyanidin reductase in developing grape berries and grapevine leaves. Plant Physiology. 2005;139:652–663. doi: 10.1104/pp.105.064238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnert HJ, Jensen RG. Strategies for engineering water-stress tolerance in plants. Trends in Biotechnology. 1996;14:89–97. [Google Scholar]
- Boss PK, Davies C, Robinson SP. Analysis of the expression of anthocyanin pathway genes in developing Vitis vinifera L. cv. Shiraz grape berries and the implications for pathway regulation. Plant Physiology. 1996;111:1059–1066. doi: 10.1104/pp.111.4.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Çakir B, Agasse A, Gaillard C, Saumonneau A, Delrot S, Atanassova R. A grape ASR protein involved in sugar and abscisic acid signalling. The Plant Cell. 2003;15:2165–2180. doi: 10.1105/tpc.013854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candiano G, Bruschi M, Musante L, Santucci L, Ghiggeri GM, Carnemolla B, Orecchia P, Zardi L, Righetti PG. Blue silver: a very sensitive colloidal Coomassie G-250 staining for proteome analysis. Electrophoresis. 2004;25:1327–1333. doi: 10.1002/elps.200305844. [DOI] [PubMed] [Google Scholar]
- Capell T, Bassie L, Christou P. Modulation of the polyamine biosynthetic pathway in transgenic rice confers tolerance to drought stress. Proceedings of the National Academy of Sciences, USA. 2004;101:9909–9914. doi: 10.1073/pnas.0306974101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chervin C, El-Kereamy A, Roustan JP, Latché A, Lamon J, Bouzayen M. Ethylene seems required for the berry development and ripening in grape, a non-climacteric fruit. Plant Science. 2004;167:1301–1305. [Google Scholar]
- Coombe BG, Hale CR. The hormone content of ripening grape berries and the effects of growth substance treatments. Plant Physiology. 1973;51:629–634. doi: 10.1104/pp.51.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva F, Iandolino A, Al-Kayal F, et al. Characterizing the grape transcritome. Analysis of expressed sequence tags from multiple Vitis species and development of a compendium of gene expression during berry development. Plant Physiology. 2005;139:574–597. doi: 10.1104/pp.105.065748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies C, Boss PK, Robinson SP. Treatment of grape berries, a nonclimacteric fruit with a synthetic auxin, retards ripening and alters the expression of developmentally regulated genes. Plant Physiology. 1997;115:1155–1161. doi: 10.1104/pp.115.3.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies C, Robinson SP. Sugar accumulation in grape berries. Cloning of two putative vacuolar invertase cDNAs and their expression in grapevine tissues. Plant Physiology. 1996;111:275–283. doi: 10.1104/pp.111.1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies C, Robinson SP. Differential screening indicates a dramatic change in mRNA profiles during grape berry ripening. Cloning and characterization of cDNAs encoding putative cell wall and stress responsive proteins. Plant Physiology. 2000;122:803–812. doi: 10.1104/pp.122.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deluc LD, Grimplet J, Wheatley MD, Tillett RL, Quilici DR, Osborne C, Schooley DA, Schlauch KA, Cushman JC, Cramer GR. Transcriptomic and metabolite analyses of Cabernet Sauvignon grape berry development. BMC Genomics. 2007;8:429. doi: 10.1186/1471-2164-8-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deluc LD, Quilici DR, Decendit A, Grimplet J, Wheatley MD, Schlauch KA, Mérillon JM, Cushman JC, Cramer GR. Water deficit alters differentially metabolic pathways affecting important flavour and quality traits in grape berries of Cabernet Sauvignon and Chardonnay. BMC Genomics. 2009;10:212. doi: 10.1186/1471-2164-10-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuschle K, Funk D, Forlani G, Stranski H, Biehl A, Leister D, van der Graaff E, Kunze R, Frommer WB. The role of Δ1-pyrroline-5-carboxylate dehydrogenase in proline degradation. The Plant Cell. 2004;16:3413–3425. doi: 10.1105/tpc.104.023622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deytieux C, Gény L, Lapaillerie D, Claverol S, Bonneu M, Donèche B. Proteome analysis of grape skins during ripening. Journal of Experimental Botany. 2007;58:1851–1862. doi: 10.1093/jxb/erm049. [DOI] [PubMed] [Google Scholar]
- Famiani F, Walker RP, Técsi L, Z–H Chen, Proietti P, Leegood RC. An immunohistochemical study of the compartmentation of metabolism during the development of grape (Vitis vinifera L.) berries. Journal of Experimental Botany. 2000;51:675–683. [PubMed] [Google Scholar]
- Finkelstein RR, Gampala SS, Rock CD. Abscisic acid signaling in seeds and seedlings. The Plant Cell. 2002;14:15–45. doi: 10.1105/tpc.010441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagné S, Lacampagne S, Claisse O, Gény L. Leucoanthocyanidin reductase and anthocyanidin reductase gene expression and activity in flowers, young berries and skin of Vitis vinifera L. cv. Cabernet Sauvignon during development. Plant Physiology and Biochemistry. 2009;47:282–290. doi: 10.1016/j.plaphy.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Galpaz N, Wang Q, Maenda N, Zamir D, Hirschberg J. Abscisic acid deficiency in the tomato mutant high-pigment 3 leading to increased plastid number and higher fruit lycopene content. The Plant Journal. 2008;53:717–730. doi: 10.1111/j.1365-313X.2007.03362.x. [DOI] [PubMed] [Google Scholar]
- Gény L, Deytieux C, Darrieumerlou A, Doneche B. Hormonal status in grape berry during ripening: importance of calcium to polyamine and abscisic acid synthesis. Acta Horticulturae. 1997;689:234–250. [Google Scholar]
- Giribaldi M, Hartung W, Schubert A. 2009. The effects of abscisic acid on grape berry ripening are affected by the timing of treatment. International Journal of Vine and Wine Science. in press. [Google Scholar]
- Giribaldi M, Perugini I, Sauvage FX, Schubert A. Analysis of protein changes during grape berry ripening by 2-DE and MALDI-TOF. Proteomics. 2007;7:3154–3170. doi: 10.1002/pmic.200600974. [DOI] [PubMed] [Google Scholar]
- Glissant D, Dédaldéchamp F, Delrot S. Transcriptomic analysis of grape berry softening during ripening. Journal International des Sciences de la Vigne et du Vin. 2008;42:1–13. [Google Scholar]
- Gosti F, Beaudoin N, Serizet C, Webb AA, Vartanian N, Giraudat J. ABI1 protein phosphatase 2C is a negative regulator of abscisic acid signaling. The Plant Cell. 1999;11:1897–1910. doi: 10.1105/tpc.11.10.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham LS, Sticklen MB. Plant chitinases. Canadian Journal of Botany. 1994;72:1057–1083. [Google Scholar]
- Hirayama T, Shinozaki K. Perception and transduction of abscisic acid signals: keys to the function of the versatile plant hormone ABA. Trends in Plant Science. 2007;12:343–351. doi: 10.1016/j.tplants.2007.06.013. [DOI] [PubMed] [Google Scholar]
- Hoth S, Morgante M, Sanchez JP, Hanafey MK, Tingey NV, Chua NH. Genome-wide gene expression profiling in Arabidopsis thaliana reveals new targets of abscisic acid and largely impaired gene regulation in the abi1-1 mutant. Journal of Cell Science. 2002;115:4891–4900. doi: 10.1242/jcs.00175. [DOI] [PubMed] [Google Scholar]
- Huang D, Jaradat MR, Wu W, Ambrose SJ, Ross AR, Abrams SR, Cutler AJ. Structural analogs of ABA reveal novel features of ABA perception and signaling in Arabidopsis. The Plant Journal. 2007;50:414–428. doi: 10.1111/j.1365-313X.2007.03056.x. [DOI] [PubMed] [Google Scholar]
- Ishimaru M, Kobayashi S. Expression of a xyloglucan endo-transglycosylase gene is closely related to grape berry softening. Plant Science. 2002;162:621–628. [Google Scholar]
- Jaillon O, Aury JM, Noel B, et al. The grapevine genome sequence suggests ancestral hexaploidization in major angiosperm phyla. Nature. 2007;449:463–467. doi: 10.1038/nature06148. [DOI] [PubMed] [Google Scholar]
- Jang JY, Kim DG, Kim YO, Kim JS, Kang H. An expression analysis of a gene family encoding plasma membrane aquaporins in response to abiotic stresses in Arabidopsis thaliana. Plant Molecular Biology. 2004;54:713–725. doi: 10.1023/B:PLAN.0000040900.61345.a6. [DOI] [PubMed] [Google Scholar]
- Jeong ST, Goto-Yamamoto N, Kobayashi S, Esaka M. Effects of plant hormones and shading on the accumulation of anthocyanins and the expression of anthocyanin biosynthetic genes in grape berry skins. Plant Science. 2004;167:247–252. [Google Scholar]
- Jiang Y, Joyce DC. ABA effects on ethylene production, PAL activity, anthocyanin and phenolic contents of strawberry fruit. Plant Growth Regulation. 2003;39:171–174. [Google Scholar]
- Kitamura N, Shikazono S, Tanaka A. TRANSPARENT TESTA19 is involved in the accumulation of both anthocyanins and proanthocyanidins in Arabidopsis. The Plant Journal. 2004;37:104–114. doi: 10.1046/j.1365-313x.2003.01943.x. [DOI] [PubMed] [Google Scholar]
- Kondo S, Inoue K. Abscisic acid and 1-aminocyclopropane-1-carboxylic acid (ACC) content during growth of ‘Satohnishiki’ cherry fruit, and the effect of ABA and ethephon application on fruit quality. Journal of Horticultural Science and Biotechnology. 1997;72:221–227. [Google Scholar]
- Lacampagne S, Gagné S, Gény L. Involvement of abscisic acid in controlling the proanthocyanidin biosynthesis pathway in grape skin: new elements regarding the regulation of tannin composition and leucoanthocyanidin reductase (LAR) and anthocyanidin reductase (ANR) activities and expression. Journal of Plant Growth Regulation. 2009 DOI 10.1007/s00344-009-9115-6. [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamikanra O, Kassa AK. Changes in the free amino acid composition with maturity of the Noble cultivar of Vitis rotundifolia Michx. grape. Journal of Agricultural and Food Chemistry. 1999;47:4837–4841. doi: 10.1021/jf981085e. [DOI] [PubMed] [Google Scholar]
- Matthews MA, Thomas TR, Shackel KA. Fruit ripening in Vitis vinifera L.: possible relation of veraison to turgor and berry softening. Australian Journal of Grape and Wine Research. 2009;15:278–283. [Google Scholar]
- Negri AS, Prinsi B, Rossoni M, Failla O, Scienza A, Cocucci M, Espen L. Proteome changes in the skin of the grape cultivar Barbera among different stages of ripening. BMC Genomics. 2008;8:378. doi: 10.1186/1471-2164-9-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowitzki U, Gelius-Dietrich G, Schwieger M, Henze K, Martin W. Chloroplast phosphoglycerate kinase from Euglena gracilis: endosymbiotic gene replacement going against the tide. European Journal of Biochemistry. 2004;271:4123–4131. doi: 10.1111/j.1432-1033.2004.04350.x. [DOI] [PubMed] [Google Scholar]
- Nunan KJ, Davies C, Robinson SP, Fincher GB. Expression patterns of cell wall-modifying enzymes during grape berry development. Planta. 2001;214:257–264. doi: 10.1007/s004250100609. [DOI] [PubMed] [Google Scholar]
- Ollat N, Gény L, Soyer JP. Grapevine fruit cuttings: validation of an experimental system to study grapevine physiology. I. Main vegetative characteristics. Journal International des Sciences de la Vigne et du Vin. 1998;32:1–9. [Google Scholar]
- Palejwala VA, Parikh HR, Modi VV. The role of abscisic acid in grape berry ripening. Physiologia Plantarum. 1985;65:498–502. [Google Scholar]
- Pilati S, Perazzolli M, Malossini A, Cestaro A, Demattè L, Fontana P, Dal Ri A, Viola R, Velasco R, Moser C. Genome-wide transcriptional analysis of grapevine berry ripening reveals a set of genes similarly modulated during three seasons and the occurrence of an oxidative burst at vèraison. BMC Genomics. 2007;8:428. doi: 10.1186/1471-2164-8-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto ME, Casati P, Su TP, Ku MSP, Edwards GE. Effects of UV-B radiation on growth, photosynthesis, UV-B absorbing compounds and NADP-malic enzyme in bean (Phaseolus vulgaris L.) grown under different nitrogen conditions. Journal of Photochemistry and Photobiology B. 1999;48:200–207. doi: 10.1016/s1011-1344(99)00031-7. [DOI] [PubMed] [Google Scholar]
- Pirie A, Mullins MG. Changes in anthocyanin and phenolics content of grapevine leaf and fruit tissues treated with sucrose, nitrate, and abscisic acid. Plant Physiology. 1976;58:468–472. doi: 10.1104/pp.58.4.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribéreau-Gayon P, Dubourdieu D, Donèche B, Lonvaud A. The grape and its maturation. In: Handbook of enology. Vol. I. John Wiley and Sons, Ltd. 2000:219–255. [Google Scholar]
- Robinson SP, Jacobs AK, Dry IB. A class IV chitinase is highly expressed in grape berries during ripening. Plant Physiology. 1997;114:771–778. doi: 10.1104/pp.114.3.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock CD. Pathways to abscisic acid-regulated gene expression. New Phytologist. 2000;148:357–396. doi: 10.1046/j.1469-8137.2000.00769.x. [DOI] [PubMed] [Google Scholar]
- Rodrigo MJ, Marcos JF, Alférez F, Mallent MD, Zacarias L. Characterization of Pinalate, a novel Citrus sinensis mutant with a fruit-specific alteration that results in yellow pigmentation and decreased ABA content. Journal of Experimental Botany. 2003;54:727–738. doi: 10.1093/jxb/erg083. [DOI] [PubMed] [Google Scholar]
- Sanchez JP, Duque P, Chua NH. ABA activates ADPR cyclase and cADPR induces a subset of ABA-responsive genes in Arabidopsis. The Plant Journal. 2004;38:381–395. doi: 10.1111/j.1365-313X.2004.02055.x. [DOI] [PubMed] [Google Scholar]
- Santoni V, Vinh J, Pflieger D, Sommerer N, Maurel C. A proteomic study reveals novel insights into the diversity of aquaporin forms expressed in the plasma membrane of plant roots. Biochemical Journal. 2003;373:289–296. doi: 10.1042/BJ20030159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauvage FX, Romieu CG, Flanzy C, Robin JP. Aminotransferases in grapes. Isolation and characterization of aspartate aminotransferase. American Journal of Enology and Viticulture. 1991;42:209–218. [Google Scholar]
- Scienza A, Miravalle R, Visai C, Fregoni M. Relationships between seed number, gibberellin and abscisic acid levels and ripening in Cabernet Sauvignon grape berries. Vitis. 1978;17:361–368. [Google Scholar]
- Seki M, Ishida J, Narusaka M, et al. Monitoring the expression pattern of around 7000 Arabidopsis genes under ABA treatments using a full-length cDNA microarray. Functional and Integrative Genomics. 2002;2:282–291. doi: 10.1007/s10142-002-0070-6. [DOI] [PubMed] [Google Scholar]
- Shiozaki S, Kamata Y, Ogata T, Horiuchi S, Kawase K. Localization of abscisic acid in grape berry by immunohistochemical techniques. Journal of the Japanese Society for Horticultural Science. 1999;68:1–9. [Google Scholar]
- Shiozaki S, Ogata T, Horiuchi S. Endogenous polyamines in the pericarp and seed of the grape berry during development and ripening. Scientia Horticulturae. 2000;83:33–41. [Google Scholar]
- Stines AP, Grubb J, Gockowiak H, Henschke PA, Høj PB, van Heeswijck R. Proline and arginine accumulation in developing berries of Vitis vinifera L. in Australian vineyards: influence of vine cultivar, berry maturity and tissue type. Australian Journal of Grape and Wine Research. 2000;6:150–158. [Google Scholar]
- Stines AP, Naylor DJ, Høj PB, van Heeswijck R. Proline accumulation in developing grapevine fruit occurs independently of changes in the levels of 1-pyrroline-5-carboxylate synthetase mRNA or protein. Plant Physiology. 1999;120:923–931. doi: 10.1104/pp.120.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Ketterling MG, Li QB, McCarty DR. Viviparous1 alters global gene expression patterns through regulation of abscisic acid signaling. Plant Physiology. 2003;132:1664–1677. doi: 10.1104/pp.103.022475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons GM, Davies C, Shavrukov Y, Dry IB, Reid JB, Mark RT. Grapes on steroids. Brassinosteroids are involved in grape berry ripening. Plant Physiology. 2006;140:150–158. doi: 10.1104/pp.105.070706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taureilles-Saurel C, Romieu C, Robin J-P, Flanzy C. Grape (Vitis vinifera L.) malate dehydrogenase. I. Intracellular compartmentation of the isoforms. II. Characterization of the major mithocondrial and cytosolic isoforms and their role in ripening. American Journal of Enology and Viticulture. 1995;46:22–36. [Google Scholar]
- Terrier N, Glissant D, Grimplet J, et al. Isogene specific oligo arrays reveal multifaceted changes in gene expression during grape berry (Vitis vinifera L.) development. Planta. 2005;222:820–831. doi: 10.1007/s00425-005-0017-y. [DOI] [PubMed] [Google Scholar]
- Tesnière C, Verriès C. Molecular cloning and expression of c-DNAs encoding alcohol dehydrogenases from Vitis vinifera L. during berry development. Plant Science. 2000;157:77–88. doi: 10.1016/s0168-9452(00)00274-0. [DOI] [PubMed] [Google Scholar]
- Waters DLE, Holton TA, Ablett EM, Lee LS, Henry RJ. cDNA microarray analysis of the developing grape (Vitis vinifera cv. Shiraz) berry skin. Functional and Integrative Genomics. 2005;5:40–58. doi: 10.1007/s10142-004-0124-z. [DOI] [PubMed] [Google Scholar]
- Wheeler S, Loveys B, Ford C, Davies C. The relationship between the expression of abscisic acid biosynthesis genes, accumulation of abscisic acid and the promotion of Vitis vinifera L. berry ripening by abscisic acid. Australian Journal of Grape and Wine Research. 2009;15:195–204. [Google Scholar]
- Xin Z, Zhao Y, Zheng ZL. Transcriptome analysis reveals specific modulation of abscisic acid signaling by ROP10 small GTPase in Arabidopsis. Plant Physiology. 2005;139:1350–1365. doi: 10.1104/pp.105.068064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Ma H, Feng J, Zeng L, Wang Z, Chen S. Grape berry plasma membrane proteome analysis and its differential expression during ripening. Journal of Experimental Botany. 2008;59:2979–2990. doi: 10.1093/jxb/ern156. [DOI] [PubMed] [Google Scholar]
- Zhang M, Yuan B, Leng P. The role of ABA in triggering ethylene biosynthesis and ripening in tomato fruit. Journal of Experimental Botany. 2009;60:1579–1588. doi: 10.1093/jxb/erp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XY, Wang XL, Wang XF, Xia GH, Pan QH, Fan RC, Wu FQ, Yu XC, Zhang DP. A shift of phloem unloading from symplastic to apoplastic pathway is involved in developmental onset of ripening in grape berry. Plant Physiology. 2006;142:220–232. doi: 10.1104/pp.106.081430. [DOI] [PMC free article] [PubMed] [Google Scholar]