Abstract
Reactive oxygen species (ROS) are produced during seed desiccation, germination, and ageing, leading to cellular damage and seed deterioration and, therefore, decreased seed longevity. The effects of simultaneous over-expression of two antioxidant enzymes on seed longevity and seed germination under stressful conditions were investigated. Transgenic tobacco simultaneously over-expressing the Cu/Zn-superoxide dismutase (CuZnSOD) and ascorbate peroxidase (APX) genes in plastids showed normal growth and seed development. Furthermore, the transgenic seeds displayed increased CuZnSOD and APX enzymatic activities during seed development and maintained antioxidant enzymatic activity after two years of dried storage at room temperature. The two-year stored non-transgenic seeds (aged NT seeds) had higher levels of ion leakage than the two-year stored transgenic seeds (aged CA seeds), indicating membrane damage caused by ROS was more severe in the aged NT seeds than the aged CA seeds. The aged CA seeds decreased germination rates as compared to newly harvested transgenic and non-transgenic seeds. The aged CA seeds, however, significantly increased germination rates under various abiotic stress conditions as compared to aged NT seeds. These data strongly suggest that simultaneous over-expression of the CuZnSOD and APX genes in plastids improves seed longevity and germination under various environmental stress conditions by attenuating the effects of oxidative stress produced by elongated storage conditions and harsh environmental stresses.
Keywords: Antioxidant enzymes, germination, seed longevity, transgenic plants
Introduction
Seeds suffer from extreme desiccation during seed development and storage (Priestley, 1986; Hendry, 1993; McDonald, 1999; Pammenter and Berjak, 1999). When plant tissues undergo drying, they have increased membrane permeability, lose membrane functions, and experience disturbances in metabolism (Bewley, 1986; Crowe et al., 1992; Vertucci and Farrant, 1995; Leprince et al., 1999). These metabolic disorders in drying seeds are accompanied by an accumulation of high amounts of reactive oxygen species (ROS). ROS include superoxide (O2−), hydrogen peroxide (H2O2), and hydroxyl radicals (· OH−). All living organisms produce ROS during normal metabolic processes as by-products or signal transducers. A slight alteration in the homeostatic state of intracellular ROS levels signals the cell to modulate its metabolism, gene expression, and post-translational modification of proteins (Finkel and Holbrook, 2000; Kannan and Jain, 2000; Halliwell and Guttrridge, 2002). Survival in the dried state involves retention of membrane functional integrity (Crowe et al., 1992; Hoekstra et al., 1997). Therefore, the ability of seeds to withstand desiccation can be positively related to their ability to scavenge ROS to avoid membrane lipid peroxidation and protein denaturation during desiccation (Hendry et al., 1992; Leprince et al., 1993; Vertucci and Farrant, 1995).
Seeds lose germinability and viability during prolonged storage periods (Smith and Berjak, 1995; McDonald, 1999). Reduced germination rates may be an indication of seed ageing. Aged seeds often have perturbed metabolic processes during seed germination, such as the establishment of respiration, ATP production, and protein synthesis (Bewley and Black, 1994). Aged seeds also have an increased leakage of ions, amino acids, and sugars during imbibition, indicating a loss of plasma membrane integrity (Bewley, 1986; Priestley et al., 1985). The increased loss of barrier properties of the plasma membranes gradually leads to an inability of cells to maintain turgor osmotically, eventually leading to death of the aged seeds (Parrish et al., 1982).
Many studies indicate that the loss of viability during seed ageing is mainly related to the loss of plasma membrane integrity, which is caused by the production of free radicals and ROS during storage (Roberts, 1972; Maguire, 1977; Senaratna et al., 1988; Bewley and Black, 1994; Bailly, 2004). Oxidative reactions during seed storage have been shown to be the major reactions responsible for the deterioration due to the production of free radicals and ROS (Spencer et al., 1973; Flood and Sinlair, 1981; Ohlrogge, 1982; Priestley and Leopold, 1983; Wilson and MacDonald, 1986). When ROS levels exceed the cellular antioxidant capacity, the disrupted cellular ROS homeostasis can result in oxidative injury. In a chain reaction, ROS can attack and damage membrane lipids (peroxidation of unsaturated fatty acids), proteins (denaturation of enzymes), carbohydrates (cleavage of polysaccharides), and nucleic acids (nicking, cross-linking, and scission of DNA strands) (Harrington, 1973; Gardner, 1979; Fujimoto et al., 1984; Priestley, 1986). This imbalance is either caused by excessive intracellular ROS production or by a deficiency in the antioxidant system that controls ROS levels (Lamb and Dixon, 1997).
Plants have evolved very efficient, but complicated, antioxidant systems to scavenge ROS to protect themselves from oxidative reactions (Allen, 1995; Halliwell and Gutteridge, 1999). The antioxidant system includes antioxidant enzymes, lipid-soluble membrane-associated antioxidants, such as α-tocopherol, β-carotene, and ubiquinone, and water-soluble antioxidants, such as glutathione and ascorbate (Monk et al., 1989; Halliwell and Gutteridge, 1999). Antioxidant enzymes are the most active, advanced, and efficient protective mechanism against ROS (Monk et al., 1989; Halliwell and Gutteridge, 2002). Important antioxidant enzymes are catalase, superoxide dismutases (SODs), peroxidases, and enzymes in the ascorbate–glutathione cycle. Among these antioxidant enzymes, SODs (EC 1.15.1.1) catalyse the dismutation of two superoxide (O2−) radicals and water into H2O2 and O2. Three SOD genes are distinguished by their locations and covalently-linked catalytic metal ions. Manganese SOD has Mn (III) at the active site and is localized to mitochondria (Zelko et al., 2002). Iron SOD has Fe (III) at the active site and is found in chloroplasts. CuZnSOD has Cu (II) plus Zn (II) at the active site and is found in the cytosol and plastids. Peroxidases oxidize organic substrates (R-H2) with H2O2 producing an oxidized substrate and water. The ascorbate–glutathione cycle, also known as the Halliwell–Asada or water–water cycle, utilizes serial enzymes, ascorbate peroxidase (APX), dehydroascorbate reductase, glutathione reductase, and monodehydroascorbate reductase to scavenge superoxide radicals and H2O2 in chloroplasts (Asada, 1999). CuZnSOD and APX are the first line of defence against oxidative stress in plastids.
There have been several reports describing synergistic effects of the simultaneous expression of ROS-scavenging enzymes on stress tolerance (Aono et al., 1995; Payton et al., 2001; Kwon et al., 2002). Previously, tobacco (Nicotiana tabacum cv. Xanthi) plants were transformed with both chloroplastic CuZnSOD and APX genes (CA plants) (Kwon et al., 2002). The CA plants displayed greater tolerance to oxidative stress induced by methyl viologen than did non-transgenic plants (NT plants) (Kwon et al., 2002). Using seeds from NT and CA plants, the effect on germination of simultaneous overexpression of CuZnSOD and APX in seeds during prolonged storage and adverse germination conditions was investigated further. These results presented here show that overexpression of both CuZnSOD and APX in plastids improved seed longevity and enhanced germination rates under unfavourable environmental conditions.
Materials and methods
Plant materials and germination assay
The CA plants were transgenic tobacco (Nicotiana tabacum cv. Xanthi) plants expressing both the CuZnSOD and APX genes under the control of a CaMV35S promoter, as previously reported (Kwon et al., 2002). CA plants showed higher resistance against MV-mediated oxidative stress than NT plants. CA and NT plants were grown in commercial mineral-mixed soil in a greenhouse maintained with a 16/8 h day/night and 30/22 °C day/night cycle. Aged NT and CA seeds were harvested at 90 DAA and were stored for up to 2 years under ambient dried conditions at room temperature. The newly harvested 90 DAA seeds and the 90 DAA seeds stored for two years are referred to as ‘new’ and ‘aged’, respectively.
For germination experiments, NT and CA seeds were surface-sterilized with 70% (v/v) ethanol for 10 s and 2% (v/v) Clorox for 15 min, washed thoroughly several times with distilled water, and then placed on Murashige–Skoog (MS) medium. The MS medium was added to 4% PEG 8000 for osmotic stress (abbreviated as O), to 100 mM NaCl for salt stress (abbreviated as OS), or incubated at 15 °C for low-temperature stress (abbreviated as OL). Seeds were exposed to individual (D, S, or L) or combined stresses, specifically osmotic stress and salt (OS), low-temperature and salt (LS), low-temperature and osmotic stress (LO), low-temperature, osmotic stress, and salt (LOS) during imbibition and germination. Rates of germination were scored by counting seeds with radicle protrusion of at least at 2 mm in length. All assays were replicated at least three times using 25 seeds per replicate.
ROS-release assay
Release of ROS from seeds during imbibition was determined as previously described by Schopfer et al. (2001) with some modifications. The ROS detection solution containing 50 μM dichlorofluorescein diacetate (DCFH-DA) (Serva, Heidelberg, Germany) in 20 mM K-phosphate buffer (pH 6.0) was incubated for 15 min at 25 °C with 0.1 g l−1 pig liver esterase (Boehringer Mannheim, Germany) for deacetylation to produce dichlorofluorescein (DCFH). Seeds (0.1 g) were imbibed in 1.5 ml freshly prepared ROS detection solution for 15 min at 25 °C and shaken at 150 rpm. One millilitre of the solution was used for measuring fluorescence (488 nm excitation and 525 nm emission) using a fluorescence spectrophotometer (LS-50B, Perkin-Elmer, Bucks, England). Blanks consisted of ROS detection solution without seeds, which were run in parallel to compensate for the spontaneous change of fluorescence. The fluorescence resulting from the oxidation of DCFH to fluorescent dichlorofluorescein by ROS was measured in the ROS detection solution containing seeds and in the blanks, and the difference was calculated.
Ion leakage analysis
The levels of ion leakage from CA and NT seeds were measured as previously described (Bowler et al., 1991) with some modifications. A total of 0.1 g CA or NT seeds soaked in 0.5 ml distilled water were maintained in an incubator at 25 °C for 5 d in the dark. The conductivities of the incubated solutions were measured using a conductivity meter (Model 455, Istek, Korea). All assays were replicated at least three times, and the averages and standard deviations were calculated.
SOD and APX enzymatic assays
CA and NT seeds in various maturation stages and seedlings were analysed to determine SOD and APX enzymatic activity. For protein extraction, seeds and seedlings were ground in liquid nitrogen and suspended in SOD or APX homogenization buffers. SOD and APX homogenization buffers were composed of 50 mM potassium phosphate (pH 7.0) with 0.1 mM EDTA, and 50 mM HEPES (pH 7.0) containing 1 mM ascorbate and 1% (v/v) Triton X-100, respectively. After centrifugation at 4 °C for 10 min at 12 000 g, supernatants were collected and used to determine activities of SOD and APX. The enzymatic activities of SOD isoenzymes were detected on a native gel as previously described (Beauchamp and Fridovich, 1971). The enzymatic activities of APX were identified using the method of Mittler and Zilinskas (1994). Total protein concentrations were measured using the Bradford method (Bradford, 1976) and the Bio-Rad Protein Assay Solution (Bio-Rad, Hercules, CA, USA).
Results
CuZnSOD and APX enzymatic activity during CA seed development
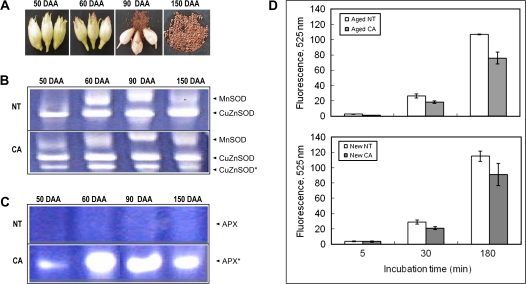
Antioxidant enzymatic activity during tobacco seed development was analysed using the native polyacrylamide gel method (Beauchamp and Fridovich, 1971). CA and NT seeds were collected at the early immature stage (50 d after anthesis, DAA), at the middle immature stage (60 DAA), at the harvesting stage (90 DAA), and at the dry stage (150 DAA) (Fig. 1A). In a previous report, actively growing leaves of CA plants had 1.2-fold and 7.6-fold higher specific SOD and APX enzymatic activities, respectively, than NT plants (Kwon et al., 2002). All maturation stages of CA and NT seeds had SOD and APX enzymatic activities (Fig. 1B, C). In all stages of the CA seeds, there were additional bands in the polyacrylamide gels corresponding to CuZnSOD and APX enzymatic activity that were not detected in NT seeds. These data indicate that the transgenes CuZnSOD and APX were successfully expressed during CA seed development (Fig. 1B, C). Enzymatic activities of CuZnSOD were low during the early immature stage, increased during the middle immature and harvesting stages, and then decreased during the dry storage period (Fig. 1B). The transgene APX enzymatic activity appeared at the early immature stage and dramatically increased after the early immature stage (Fig. 1C). The enzymatic activity of CuZnSOD and APX remained relatively high in dry seeds (Fig, 1B, C).
Fig. 1.
Enzymatic activities of SOD and APX in NT and CA plants during seed maturation. (A) Tobacco seed maturation at 50 d after anthesis (DAA), 60 DAA, 90 DAA, and 150 DAA. (B) SOD enzymatic activity in NT and CA seeds during seed maturation. (C) APX enzymatic activity in NT and CA seeds during seed maturation. (D) Emitted fluorescence at 30 min from 1.5 ml ROS detection solution containing 0.1 g NT or CA seeds. Data are presented as mean ±SD of three independent measurements. (This figure is available in colour at JXB online.)
To confirm the positive correlation between the increased enzymatic levels of CuZnSOD and APX and the scavenging capacities of ROS, the amount of ROS released from seeds was determined in the newly harvested seeds (90 DAA; new seeds) and in the dried seeds stored for two years at room temperature (aged seeds) (Fig. 1D). Production of ROS was detected in all new and old NT and CA seeds. New NT and CA seeds produced slightly higher levels of ROS than old NT and CA seeds without any statistical significance. However, ROS production from the new and aged NT seeds was significantly higher than that from the new and aged CA at 30 min of the reaction time (Fig. 1D).
Enhanced desiccation tolerance in aged CA seeds during prolonged storage
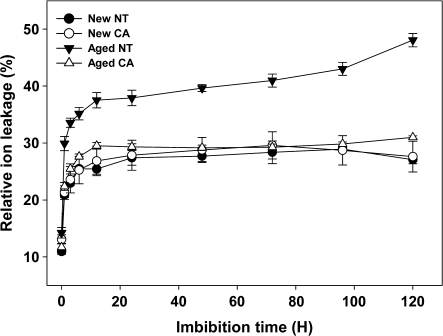
The level of ion leakage from imbibed NT and CA seeds was measured to evaluate membrane integrity (Fig. 2). There was no difference found in ion leakage between new NT and new CA seeds during the course of 5 d. However, aged NT seeds had higher amounts of ion leakage as compared with aged CA seeds following imbibition during all the 5 d of observation. After 5 d of imbibition (DAI), the aged NT seeds leaked 170% more ions than did the aged CA seeds.
Fig. 2.
Ion leakage assay to test membrane integrity of NT and CA seeds. The conductivity of distilled water containing new or aged NT or CA seeds was measured over 120 min. Data are presented as mean ±SD of three independent measurements.
Enhanced CA seed germination during various abiotic stress conditions
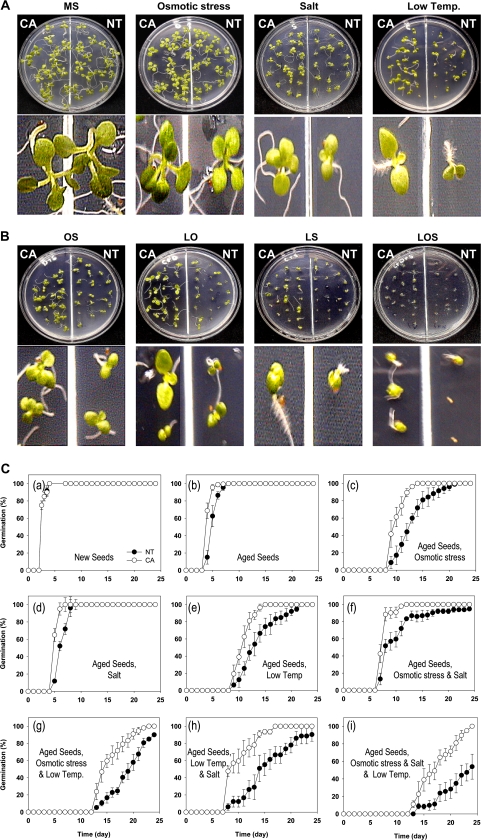
Osmotic stress, salt, and low temperatures adversely affect the germination of seeds. To evaluate the effect of the transgenes CuZnSOD and APX on the germination of aged NT and CA seeds, the germination rates of the aged NT and CA seeds were examined for 4 weeks. The germination behaviour of NT and CA seeds was measured during the various stress conditions. All conditions eventually led to the increased production of ROS by the seeds. The effect of the abiotic stresses on germination was measured by counting germinated seeds with root extrusions. The aged CA seeds showed enhanced germination and growth during osmotic stress, salt stress, and low temperature treatments (Fig. 3A). Germination of aged CA and NT seeds were also tested in combined stress conditions, including osmotic stress/salt (OS), low temperature/osmotic stress (LO), low temperature/salt (LS), and low temperature/osmotic stress/salt (LOS) (Fig. 3B).
Fig. 3.
Enhanced seed germination of aged CA plants under single or combined abiotic stress conditions. Twenty-five NT and CA seeds were germinated and the germination rates were recorded in Murashige-Skoog (MS) media with various environmental stress conditions for 4 weeks at 25 °C except low temperature (L) treatment at 15 °C. (A) Photographs of 1-week-old aged NT and CA seeds grown in MS medium (MS) or in MS medium treated with osmotic stress (O, MS media added with 4% PEG), salt (S, MS media added with 100 mM NaCl), or low temperature (L, grown at 15 °C). (B) Photographs of 1-week-old aged NT and CA seeds grown in MS medium under combined stress conditions, such as osmotic stress and salt (OS), low temperature and osmotic (LD), low temperature and salt (LS), and triple combined stress (LOS). (C) Germination rates of NT and CA seeds in MS media treated with environmental stressors. (a) Seed germination rates of new NT and CA seeds in an MS medium for 4 weeks. Seed germination rates were observed for 4 weeks for aged NT and CA seeds in an MS medium (b), and in an MS medium treated with osmotic stress (c), salt (d), low temperature (e), osmotic stress and salt (f), low temperature and osmotic stress (g), low temperature and salt (h), or low temperature, osmotic stress, and salt combined stresses (i). Data are presented as mean ±SD of three independent assays. (This figure is available in colour at JXB online.)
Aged CA seeds showed a significant increase in germination as compared to aged NT seeds under all abiotic stress conditions (Fig. 3C). New CA and NT seeds showed no difference in days needed for 90% germination (Fig. 3C, a). When aged CA and NT seeds were tested for germination, however, the aged CA seeds germinated 1 d earlier than aged NT seeds (Fig. 3C, b). Treatments of osmotic stress, salt or low temperatures significantly delayed the germination of aged NT seeds compared with that of aged CA seeds [Fig. 3C c, d, e), resulting in 89.2% germination of aged CA seeds at day 12 compared with 44.1% germination of aged NT seeds on the same day in the osmotic stress treatment, and in 88.0% germination of aged CA seeds at day 13 compared with 53.0% germination of aged NT seeds. Even during the combination of all three stress conditions, the germination of seeds was delayed at least 5 d longer than the non-stressed control seeds. The combination of osmotic stress and salt stresses delayed the germination of aged NT seeds for 5 d longer than aged CA seeds (Fig. 3C, f). When aged CA seeds were imbibed under the combined treatment of low temperature and osmotic stress, 90.9% germinated in 20 DAI, which was a delay of 2 weeks (Fig. 3C, g), but much faster than aged NT seeds, which only germinated 57.9% on the same day. The combined effect of low temperature and salt stress on aged CA and NT seeds was similar to the combined effect of low temperature and osmotic stress (Fig. 3C, h). When all three stresses were combined, the germination of aged seeds was significantly delayed (Fig. 3B, i). None of the aged CA and NT seeds germinated until 12 DAI. The germination of aged NT seeds was severely delayed, with only 53.9% of aged NT seeds germinating by day 24 of the treatment when 90% of aged CA seeds had germinated.
Changes of CuZnSOD and APX enzymatic activities in aged seeds during germination
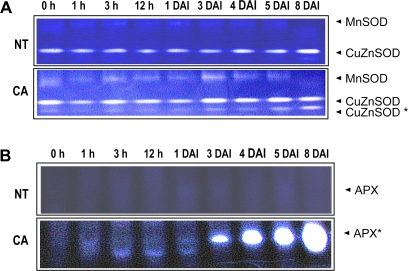
Enzymatic activities of CuZnSOD and APX in aged CA and NT seeds were measured during seed germination and seedling development (Fig. 4). The aged CA seeds had very small amounts of SOD enzymatic activity from the newly introduced CuZnSOD gene, even after 2 years of storage. The enzymatic activities of CuZnSOD increased gradually for 8 DAI in CA seeds. Aged NT plants did not display the enzymatic activities of the transgene CuZnSOD (Fig. 4A). The enzymatic activities for the transgene APX were detected at very high levels in aged CA seeds after 3 DAI and drastically increased over 8 DAI (Fig. 4B). Aged NT seeds did not have any APX enzymatic activities at the band location for the transgene APX product.
Fig. 4.
Enzymatic activity of SOD and APX in aged NT and CA seeds during germination. Aged NT and CA seeds were incubated in an MS medium for 8 d in an incubator maintained at 25 °C with a 16/8 h day/night cycle. The enzymatic activities of SOD (A) and APX (B) in aged NT and CA seeds were analysed by native polyacrylamide gel assays. Stars indicate the bands corresponding to enzymatic activity from the transgene products of CuZnSOD and APX. (This figure is available in colour at JXB online.)
Discussion
The dehydration state of stored-seed tissues is associated with an increased production of ROS, which can trigger numerous deleterious oxidative processes causing disruption and deteriorative changes (Leprince et al., 1993; Smirnoff, 1993). ROS accumulation in prolonged-storage and dried seeds may lead to oxidative stress and seed deterioration (Bailly, 2004). These oxidative processes are known to be common causes of decreased structural integrity and increased mortality of seeds (Priestley et al. 1985; Hendry et al., 1992; Smirnoff, 1993). Therefore, protection against oxidative processes may be a major determinant of longevity of stored seeds (Harrington, 1973; Spencer et al., 1973; Flood and Sinlair, 1981; Ohlrogge and Kernan, 1982; Priestley and Leopold, 1983; Wilson and McDonald, 1986). There have been several reports that enhanced antioxidative defence systems play important roles in the acquisition of seed desiccation tolerance (Smirnoff, 1993; Li and Sun, 1999; Bailly et al., 2001). In a previous study, the simultaneous expression of CuZnSOD and APX genes in tobacco chloroplasts increased tolerance to oxidative stresses (Kwon et al., 2002). Here, whether the increased antioxidant capacity of plastids is positively related to the germination rate of long-term stored dried seeds was investigated further.
The newly introduced CuZnSOD and APX gene products were actively expressed during seed maturation and maintained small amounts of enzymatic activity during prolonged seed storage over 2 years. The two genes were under the control of the 35S promoter actively expressed during maturation and germination. The CaMV 35S promoter, a constitutive promoter, is regulated developmentally and tissue-specifically (Sunilkumar et al., 2002). In the CA plants, the activities of antioxidant enzyme showed differences during seed maturation and germination (Figs 1, 4). These differences in enzyme activities during the developmental stages emphasize again that the CaMV 35S promoter is constitutive but is regulated developmentally. This high expression of the two transgenes resulted in reduced ROS production by aged CA seeds as compared with aged NT seeds (Figs 1D, 4). Among the many indicators of seed vigour and germination ability, the best indicator for low seedling vigour is the degree of leakage of various intracellular substances from imbibing seeds (Becwar et al., 1982; Kakefuda et al., 1982). Aged NT seeds had significantly higher levels of ion leakage during imbibition than the new NT seeds. However, overexpression of CuZnSOD and APX in plastids lowered the amount of ion leakage and maintained membrane integrity during imbibition by aged CA seeds (Fig. 2). Membrane lipids in seeds are the primary site for deterioration during the ageing process due to attack by ROS and free radicals during storage (Harrington, 1973; Spencer et al., 1973; Gardner, 1979; Flood and Sinlair, 1981; Priestley and Leopold, 1983; Wilson et al., 1986). The aged CA seeds maintained CuZnSOD and APX enzymatic activity during the 2 years of dried storage at room temperature, suggesting that dried seeds may sustain germination vigour as long as antioxidant enzymes maintain their enzymatic activities. There was no difference in ion leakage and germination rates in the new CA and NT seeds, indicating that newly harvested seeds would not have increased seed vigour, even with increased antioxidant capacities.
Osmotic stress, salt, and low-temperatures are common environmental stresses that adversely affect seed germination and plant growth (Bailly, 2004). In nature, plant seeds encounter a combination of these environmental stresses, which have much more harmful effects on germination than individual stresses. Each environmental stress reduces seed germination due to increased production of ROS (Bailly, 2004). Combinations of osmotic stress, salt, freezing, and low temperatures are accompanied by the formation of ROS, thereby, significantly decreasing seed germination (Holmberg and Bulow, 1998; Dat et al., 2000). All given environmental stresses, including combined stresses, reduced the germination rates more significantly for aged NT seeds than for aged CA seeds. Aged CA seeds achieved almost 100% germination under the combined treatment of osmotic stress, salt, and low temperature at 25 DAI, however, only 50% of aged NT seeds germinated. These results indicate that the germination vigour of aged NT seeds, decreased severely due to the triple stress combination, however, could be overcome by the enhanced antioxidative capacities in plastids.
ROS are the major molecules responsible for reducing seed longevity after prolonged storage, and can also be a factor inhibiting germination during stressful conditions (Bailly, 2004). Seed germination in nature can be affected by elongated storage times and unexpected serious environmental challenges, which cause increased ROS production in the seeds. The results in this report demonstrate that raised antioxidant enzymatic activities in plastids can increase seed longevity and germination rates under unfavorable conditions and may help seeds germinate more efficiently in unfavourable environments.
Acknowledgments
This study was supported by research grants (No. 20080401034045 to SY Kwon and No. 20070301034015 to SS Kwak) from the Biogreen21 program of the Rural Development Administration (RDA) and grant from the Crop Functional Genomics Center, Ministry of Education, Science, and Technology, Korea.
Glossary
Abbreviations
- APX
ascorbate peroxidase
- DCFH-DA
dichlorofluorescein diacetate
- ROS
reactive oxygen species
- SOD
superoxide anion dismutase
References
- Allen RD. Dissection of oxidative stress tolerance using transgenic plants. Plant Physiology. 1995;107:1049–1054. doi: 10.1104/pp.107.4.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aono M, Saji H, Sakamoto A, Tanaka K, Kondo N, Tanaka K. Paraquat tolerance of transgenic Nicotiana tabacum with enhanced activities of glutathione reductase and superoxide dismutase. Plant and Cell Physiology. 1995;36:1687–1691. [PubMed] [Google Scholar]
- Asada K. The water–water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annual Review of Plant Physiology and Plant Molecular Biology. 1999;50:601–639. doi: 10.1146/annurev.arplant.50.1.601. [DOI] [PubMed] [Google Scholar]
- Bailly C. Active oxygen species and antioxidant in seed biology. Seed Science Research. 2004;14:93–107. [Google Scholar]
- Bailly C, Audigier C, Ladonne F, Wagner MH, Coste F, Corbineau F, Come D. Changes in oligosaccharide content and antioxidant enzyme activities in developing bean seeds as related to acquisition of drying tolerance and seed quality. Journal of Experimental Botany. 2001;52:701–708. doi: 10.1093/jexbot/52.357.701. [DOI] [PubMed] [Google Scholar]
- Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Analytical Biochemistry. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Becwar MR, Stanwood PC, Roos EE. Dehydration effects on imbibitional leakage from desiccation sensitive seeds. Plant Physiology. 1982;69:1132–1135. doi: 10.1104/pp.69.5.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewley JD. Membrane changes in seeds as related to germination and the perturbation resulting from deterioration in storage. In: McDonald MB, Nelson CJ, editors. Physiology of seed deterioration. 1986. CSSA Special Publication No 11. Crop Science Society of America, Madison, WI: 27–45. [Google Scholar]
- Bewley JD, Black M. Seeds: physiology of development and germination. New York, NY: Plenum Press; 1994. [Google Scholar]
- Bowler C, Slooten L, Vandenbranden S, De Rycke R, Botterman J, Sybesma C, Van Montagu M, Inzé D. Manganese superoxide dismutase can reduce cellular damage mediated by oxygen radicals in transgenic plants. EMBO Journal. 1991;10:1723–1732. doi: 10.1002/j.1460-2075.1991.tb07696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Crowe JH, Hoekstra FA, Crowe LM. Anhydrobiosis. Annual Review of Physiology. 1992;54:570–599. doi: 10.1146/annurev.ph.54.030192.003051. [DOI] [PubMed] [Google Scholar]
- Dat J, Vandenabeele S, Vranová E, Van Montagu M, Inzé D, Van Breusegem F. Dual action of the active oxygen species during plant stress responses. Cellular and Molecular Life Sciences. 2000;57:779–795. doi: 10.1007/s000180050041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Flood RG, Sinlair A. Fatty acid analysis of aged permeable and impermeable of Trifolium subterraneum. Seed Science and Technology. 1981;9:475–477. [Google Scholar]
- Fujimoto K, Neff WE, Frankel EN. The reaction of DNA with lipid oxidation products, metals and reducing agents. Biochimica et Biophysica Acta. 1984;795:100–107. doi: 10.1016/0005-2760(84)90109-7. [DOI] [PubMed] [Google Scholar]
- Gardner HW. Lipid hydroperoxide reactivity with proteins and amino acids. a review. Journal of Agricultural and Food Chemistry. 1979;27:220–229. [Google Scholar]
- Halliwell B, Gutteridge JMC. Free radicals in biology and mMedicine. 3rd edn. New York, NY: Oxford University Press; 2002. [Google Scholar]
- Harrington JF. Biochemical basis of seed longevity. Seed Science Technology. 1973;1:453–461. [Google Scholar]
- Hendry GA. Oxygen, free radical processes and seed longevity. Seed Science Research. 1993;3:141–153. [Google Scholar]
- Hendry GA, Finch-Savage WE, Thorpe PC, Atherton NM, Buckland SH, Nilsson KA, Seel E. Free radical processes and loss of seed viability during desiccation in the recalcitrant species Quercus robur L. New Phytologist. 1992;122:273–279. doi: 10.1111/j.1469-8137.1992.tb04231.x. [DOI] [PubMed] [Google Scholar]
- Hoekstra FA, Wolkers WF, Buitink J, Golovina EA, Crowe JH, Crowe LM. Membrane stabilization in the dried state. Comparative Biochemistry and Physiology. 1997;117A:335–341. [Google Scholar]
- Holmberg N, Bülow L. Improving stress tolerance in plants by gene transfer. Trends in Plant Science. 1998;3:61–66. [Google Scholar]
- Kakefuda G, Schrader LE, Duke SH. Imbibitional leakage and seed death during germination in soybean cultivars with defective testae. Plant Physiology. 1982;69 S-1. [Google Scholar]
- Kannan K, Jain SK. Oxidative stress and apoptosis. Pathophysiology. 2000;7:153–163. doi: 10.1016/s0928-4680(00)00053-5. [DOI] [PubMed] [Google Scholar]
- Kwon SY, Jeong YJ, Lee HS, Kim JS, Cho KY, Allen RD, Kwak SS. Enhanced tolerances of transgenic tobacco plants expressing both superoxide dismutase and ascorbate peroxidase in chloroplasts against methyl viologen-mediated oxidative stress. Plant, Cell and Environment. 2002;25:873–882. doi: 10.1007/s00299-006-0199-1. [DOI] [PubMed] [Google Scholar]
- Lamb C, Dixon RA. The oxidative burst in plant disease resistance. Annual Review of Plant Physiology and Plant Molecular Biology. 1997;48:251–279. doi: 10.1146/annurev.arplant.48.1.251. [DOI] [PubMed] [Google Scholar]
- Leprince O, Buitink J, Hoekstra FA. Radicles and cotyledons of recalcitrant seeds of Castanea sativa Mill. exhibit contrasting responses of respiration to drying in relation to desiccation sensitivity. Journal Experimental Botany. 1999;338:1515–1524. [Google Scholar]
- Leprince O, Hendry GAF, Mckersie BD. The mechanism of desiccation tolerance in developing seeds. Seed Science Research. 1993;3:231–246. [Google Scholar]
- Li C, Sun WQ. Desiccation sensitivity and activities of free radical-scavenging enzymes in recalcitrant Theobroma cacaco seeds. Seed Science Research. 1999;9:209–217. [Google Scholar]
- Maguire JD. Seed quality and germination. In: Khan AA, editor. The physiology and biochemistry of seed dormancy and germination. 1977. Amsterdam: North Holland Publication Corporation, 219–235. [Google Scholar]
- McDonald MB. Seed deterioration: physiology, repair and assessment. Seed Science and Technology. 1999;27:177–237. [Google Scholar]
- Mittler R, Zilinskas BA. Regulation of pea cytosolic ascorbate peroxidase and other antioxidant enzymes during the progression of drought stress and following recovery from drought. The Plant Journal. 1994;5:397–405. doi: 10.1111/j.1365-313x.1994.00397.x. [DOI] [PubMed] [Google Scholar]
- Monk LS, Fagerstedt KV, Crawford RMM. Oxygen toxicity and superoxide dismutase as an antioxidant in physiological stress. Physiologia Plantarum. 1989;76:456–459. [Google Scholar]
- Ohlrogge JB. Oxygen dependent ageing of seeds. Plant Physiology. 1982;70:791–796. doi: 10.1104/pp.70.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlrogge JB, Kernan TP. Oxygen dependent ageing of seeds. Plant Physiology. 1982;70:791–796. doi: 10.1104/pp.70.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pammenter NW, Berjak P. A review of recalcitrant seed physiology in relation to desciccation-tolerance mechanisms. Seed Science Research. 1999;9:13–37. [Google Scholar]
- Parish DJ, Leopold AC, Hanna MA. Turgor changes with accelerated ageing of soybeans. Crop Science. 1982;22:666–669. [Google Scholar]
- Payton P, Webb R, Kornyeyev D, Allen R, Holaday AS. Protecting cotton photosynthesis during moderate chilling at high light intensity by increasing chloroplastic antioxidant enzyme activity. Journal of Experimental Botany. 2001;52:2345–2354. doi: 10.1093/jexbot/52.365.2345. [DOI] [PubMed] [Google Scholar]
- Priestley DA. Seed ageing: implications for seed storage and persistence in soil. Ithaca NY: Comstock Publication Association; 1986. [Google Scholar]
- Priestley DA, Leopold AC. Lipid changes during natural ageing of soybean seeds. Physiologia Plantarum. 1983;59:467–470. [Google Scholar]
- Priestley DA, Warner BG, Leopold AC, Mcbridge MB. Organic free radical levels in seeds and pollen: the effects of hydration and ageing. Physiologia Plantarum. 1985;70:88–94. [Google Scholar]
- Roberts EH. Cytological, genetical and metabolic changes associated with loss of viability. In: Roberts EH, editor. Viability of seeds. London: Chapman and Hall; 1972. pp. 253–306. [Google Scholar]
- Schopfer P, Plachy C, Frahry G. Release of reactive oxygen intermediates (superoxide radicals, hydrogen peroxide, and hydroxyl radicals) and peroxidase in germinating radish seeds controlled by light, gibberellin, and abscisic acid. Plant Physiolology. 2001;125:1591–1602. doi: 10.1104/pp.125.4.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senaratna T, Gusse JF, McKersie B. Age-induced changes in cellular membranes of imbibed soybean seed axes. Physiologia Plantarum. 1988;73:85–91. [Google Scholar]
- Smirnoff N. The role of active oxygen in the response of plants to water deficit and desiccation. New Phytologist. 1993;125:27–58. doi: 10.1111/j.1469-8137.1993.tb03863.x. [DOI] [PubMed] [Google Scholar]
- Smith MT, Berjak P. Deteriorative changes associated with the loss of viability of stored desiccation-tolerant and desiccation-sensitive seeds. In: Kigel J, Galili G, editors. Seed dDevelopment and gGermination. New York, NY: Marcel Dekker; 1995. pp. 701–746. [Google Scholar]
- Spencer GF, Earle FR, Wolff IA, Tallent WH. Oxygenation of unsaturated fatty acids in seeds during storage. Chemistry and Physics of Lipids. 1973;10:191–202. [Google Scholar]
- Sunilkumar G, Mohr L, Lopata-Finch E, Emani C, Rathore KS. Developmental and tissue-specific expression of CaMV 35S promoter in cotton as revealed by GFP. Plant Molecular Biology. 2002;50:463–474. doi: 10.1023/a:1019832123444. [DOI] [PubMed] [Google Scholar]
- Vertucci CW, Farrant JM. Acquisition and loss of desiccation tolerance. In: Kigel J, Galili G, editors. Seed development and germination. New York, NY: Marcel Dekker; 1995. pp. 237–271. [Google Scholar]
- Wilson DO, Jr., McDonald MB., Jr. The lipid peroxidation model of seed ageing. Seed Science and Technology. 1986;14:269–300. [Google Scholar]
- Zelko IN, Mariani TJ, Folz RJ. Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radical Biology and Medicine. 2002;33:337–349. doi: 10.1016/s0891-5849(02)00905-x. [DOI] [PubMed] [Google Scholar]