Abstract
There has been growing interest in the use of retinal imaging for tracking disease progression in multiple sclerosis. However, systematic and detailed pathological descriptions of retinal tissue in multiple sclerosis are lacking. Graded, histological evaluations on eyes from 82 patients with multiple sclerosis and 10 subjects with other neurological diseases, with immunohistochemistry on a subset, were performed and correlated with clinical and pathological findings. Multiple sclerosis cases demonstrated evidence of retinal atrophy and inflammation even in late-stage disease. Retinal ganglion cell loss was significant and remaining neurons appeared shrunken and were partially engulfed by human leukocyte antigen-DR positive cells with the phenotype of microglia in samples subjected to immunohistochemistry. Neurofilament staining revealed variable but prominent degrees of axonal loss and injury. Neuronal loss was noted in the inner nuclear layer with focal reduction in cell density. Foamy-appearing human leukocyte antigen-DR positive cells were evident near vessels and periphlebitis was found in a small but significant number of multiple sclerosis cases. Glial fibrillary acidic protein staining showed extensive astrocyte hypertrophy and proliferation with prominent gliosis in multiple sclerosis cases. Frequent but previously unreported abnormalities in the iris were documented in the majority of chronic multiple sclerosis cases. The injury to both iris and retina could be seen at all stages of disease. Severity of retinal atrophy was correlated with overall brain weight at time of autopsy (P = 0.04) and a trend for increased atrophy was seen with longer disease duration (P = 0.13). This study provides the first large-scale pathological description of retinas in multiple sclerosis, including patients with different subtypes of disease at all stages, and with variable clinical severity. Changes were seen not only in the retinal nerve fibre layer and ganglion cell layer, but also in the inner nuclear layer, suggesting that retinal injury is more widespread than previously appreciated. Furthermore, the human retina is devoid of myelin, but inflammation was demonstrated to be prominent in multiple sclerosis and to persist in the retina at late stages of disease. The prominent gliosis and inflammation surrounding vessels of the inner retina could potentially impact optical coherence tomography evaluations in multiple sclerosis—as standard techniques exploit presumed differences in tissue reflectivity and utilize automated edge detection algorithms to judge axon loss in the nerve fibre layer. Deciphering the relationships between the different types of retinal pathology may aid us in understanding the factors that drive both inflammation and tissue atrophy in multiple sclerosis.
Keywords: multiple sclerosis, eye, retinal periphlebitis, ocular pathology, inflammation
Introduction
The anterior visual pathway is a frequent site of injury during the course of multiple sclerosis. Damage to the anterior visual pathway, even in the absence of visual symptoms during life, has been recognized since the earliest pathological descriptions of multiple sclerosis (Oppenheim, 1887). As many as 20% of patients suffer injury to the anterior visual pathway as a sentinel event in multiple sclerosis with acute optic neuritis (Arnold, 2005; Balcer, 2006; Korsholm et al., 2007), and many more patients suffer acute optic neuritis at some point during the course of the disease. The presence of MRI abnormalities at the time of an acute optic neuritis attack is a strong predictor of the 15-year risk of multiple sclerosis (Optic Neuritis Study Group, 2008). Pathological assessments of the anterior visual pathway in patients with multiple sclerosis suggest that all patients with the disease suffer injury to the optic nerve and tracts at some stage in their disease (Lisch, 1933; Schlossman and Phillips, 1954; Lumsden, 1970; Toussaint et al., 1983; Ulrich and Groebke-Lorenz, 1983; Mogensen, 1990). Although, injury to the white matter tracts that comprise the anterior visual pathway is reasonably well characterized, the pathology of the retina is poorly understood.
Retinal periphlebitis, optic disc atrophy and localized disruptions in the retinal nerve fibre layer (slits) can be detected on routine ophthalmoscopy in multiple sclerosis (Rucker, 1972; Frisén and Hoyt, 1974). However, very little is known about the cellular and molecular changes underlying these clinical findings. Most pathological studies of the multiple sclerosis retina are from small case series, without detailed clinical information to help contextualize the findings. Moreover the technical advances in immunohistochemistry and the large range of antibodies available means that it is timely to define the molecular pathology of the retina more precisely in multiple sclerosis. High resolution quantitative retinal imaging technologies, e.g. Heidelberg retinal tomography, scanning laser polarimetry and optical coherence tomography (OCT) have increased our understanding of retinal injury in multiple sclerosis. They have firmly established the presence of retinal nerve fibre layer atrophy and reduction in macular thickness in multiple sclerosis (Trip et al., 2005; Fisher et al. 2006; Sepulcre et al., 2007; Henderson et al., 2008). Moreover, it has been shown that retinal nerve fibre layer imaging by OCT is qualitatively similar to histological measurement in the healthy retina (Blumenthal et al., 2009).
However, it is important to recognize that although OCT accurately reflects the anatomy of the healthy retina, interpretation of findings from retinal imaging must be viewed in context of the retinal histopathology of the disease under study. Current retinal imaging techniques do not possess the resolution to give cytological detail and therefore many of the retinal abnormalities in multiple sclerosis are imperfectly understood. For example, it is unknown if there is any relationship between retinal inflammation and retinal atrophy, or if both phenomena are a consequence of processes that are initiated in the optic nerve. Different experimental autoimmune encephalomyelitis models of optic neuritis have suggested both that neuronal/axonal loss occurs as a consequence of demyelination (Shindler et al., 2008; Dutt et al., 2009) and conversely that retinal ganglion cell (RGC) loss may precede histological evidence of retrobulbar inflammation (Hobom et al., 2004). Although retrobulbar demyelination and inflammation underlie the acute visual dysfunction seen in acute optic neuritis (Youl et al., 1991), patients with multiple sclerosis who do not have a history of clinically evident acute optic neuritis nonetheless have retinal nerve fibre layer and macular atrophy (Fisher et al., 2006). This potentially argues against the proposition that optic nerve inflammation underlies all the changes seen in the retina. In contrast it is important to note that there is a spectrum of retinal nerve fibre layer atrophy in patients with multiple sclerosis—with all eyes showing injury compared to disease free controls but more pronounced atrophy being evident in eyes after an acute optic neuritis (Trip et al., 2005; Costello et al., 2006). The pathological basis of this difference in the multiple sclerosis clinically-affected and non-affected eyes is not known. It is particularly unclear whether the retinal nerve fibre layer loss seen on OCT reflects loss of axons and neuronal elements, or atrophy of these structures. Furthermore, in spite of the fact that retinal vasculature sits embedded in the retinal nerve fibre layer, the impact of perivascular inflammation on inner retinal thickness measurements remains unexplored.
The prevailing model for multiple sclerosis highlights the distinction between an early inflammatory period of disease activity and a late neurodegenerative phase, dominated by axonal injury and grey matter pathology. However, the limits of this model are well known. MRI findings suggest early grey matter atrophy (De Stefano et al., 2007) and brain pathological studies document persistent inflammation in multiple sclerosis, revealed both on MRI and histopathology (Bruck et al., 1997; van Waesberghe et al., 1999). The pre-chiasmal anterior visual pathway is anatomically isolated and provides an attractive model pathway for understanding the relationships between inflammation, demyelination and neurodegeneration in multiple sclerosis. The inner retina is particularly amenable to study during life and provides clinically relevant tissue in which unmyelinated CNS axons can be assessed and quantified. Retinal periphlebitis has been previously described (ter Braak and van Herwaarden, 1933; Rucker, 1944; Sanders, 1987) and its presence suggests that myelin may not be necessary for establishing and/or maintaining inflammation in multiple sclerosis. Therefore, for many reasons, retinal pathology in multiple sclerosis deserves to be better understood. Here we report the results of a comprehensive analysis of ocular pathology in 82 patients with multiple sclerosis, and report a high prevalence of inflammation and neurodegeneration not present in patients with other neurological disease.
Materials and methods
Case selection, consent and ethical approval
All subjects (and/or their families) with a diagnosis of multiple sclerosis in Northern Ireland from 1967 to 1992 were invited to participate in this project and consent was obtained in advance of death, usually by several years, by a consultant neurologist. For those patients with other neurological disease, consent was obtained from the next of kin after death by a consultant neurologist. This study was approved by the NHS Research Ethics committee for Northern Ireland, and Research Governance approval was obtained from the Research Governance Office of the Royal Victoria Hospital, Belfast.
Multiple sclerosis cases
A total of 82 cases were selected in which one or both eyes had been taken at autopsy, irrespective of known retinal or optic nerve disease (Table 1 for clinical and pathological details).
Table 1.
Demographic and clinical information
| Diagnosis | Subjects | Eyes | Age (years) | Range | DAI | Brain weight (g) | Disease duration (years) | Sex (%F) |
|---|---|---|---|---|---|---|---|---|
| Acute multiple sclerosis | 6 | 9 | 35.5 ± 9.6 | 23–51 | 6.5 ± 4 | 1387 ± 102 | 4 mths ± 3 | 50 |
| Chronic multiple sclerosis | 76 | 121 | 57.6 ± 14.5 | 26–87 | 10 ± 6.5 | 1264 ± 150 | 23.6 ± 13 | 50 |
| Relapsing remitting multiple sclerosis | 8 | 15 | 45.1 ± 13.7 | 26–64 | 12 ± 4 | 1375 ± 190 | 13.8 ± 11 | 63 |
| Secondary progressive multiple sclerosis | 51 | 84 | 59.2 ± 15 | 33–87 | 10 ± 7 | 1229 ± 140 | 26.7 ± 14 | 59 |
| Primary progressive multiple sclerosis | 13 | 22 | 58.6 ± 11 | 40–75 | 10 ± 8 | 1324 ± 100 | 18.6 ± 9 | 39 |
| Unable to classify | 2 | 2 | 60.0 ± 2.8 | 58–62 | 8 ± 6 | 1550 ± 80 | NA | 50 |
| Other neurological diseasea | 10 | 16 | 53.5 ± 17 | 21–72 | 10.5 ± 4 | 1395 ± 120 | NA | 38 |
| Total | 92 | 148 | 55.5 ± 16 | 21–87 | 10 ± 6 | 1289 ± 150 | NA | 51 |
DAI = death-to-autopsy interval in hours; NA = not applicable.
Guillian–Barre n = 5; systemic lupus erythromatosis n =1; other demyelinating polyneuropathy n = 1; central pontine myelinolysis n = 1; inherited leukodystrophy n = 1; spinocerebellar degeneration n = 1.
Other neurological disease cases
Ten cases of other neurological disease were selected based on pathological diagnosis and availability of tissue.
Clinical records
Multiple sclerosis cases
Anonymized clinical records were independently reviewed at the time of this study to confirm diagnosis using the Poser criteria and to define clinical course and disease duration (Poser et al., 1983). Those who never had a clinical record of exacerbation with the accumulation of clinical disability were defined as primary progressive multiple sclerosis. Those who were still suffering from exacerbations but not progressive disability at the time of death were defined as relapsing remitting multiple sclerosis. Subjects who had previously suffered from exacerbations, but who at the time of death were suffering from progressive neurological disability without exacerbation, were defined as secondary progressive multiple sclerosis. Patients who died from their demyelinating condition within 1 year of diagnosis were defined as ‘acute’ multiple sclerosis (range 1–12 months). This category included four cases where the demyelinating illness was monophasic (Marburg-type) and two cases in which the subjects suffered relapses. Almost all multiple sclerosis subjects had received glucocorticosteroids or adrenocorticotropic hormone (ACTH) to treat exacerbations during the course of their disease. Three subjects (two acute multiple sclerosis, one relapsing remitting multiple sclerosis) were documented to be receiving glucocorticosteroids at the time of their death.
Other neurological disease cases
Cases were categorized from clinical and pathological records. In four cases diagnosed as polyneuropathy without identified brain pathology, e.g. Guillian-Barre, clinical records and peripheral nerve pathology were used to confirm diagnosis.
Autopsy
In all cases full autopsy was performed and CNS tissue was independently evaluated by a neuropathologist for confirmation of the diagnosis. Neuropathological material included brain, spinal cord and nerve roots, peripheral nerves, muscle and eyes.
Eye fixation, embedding, sectioning and staining
Globes were fixed in 10% neutral buffered formalin for 24–48 h at room temperature. Prior to paraffin embedding, inferior calottes (horizontal meridian) were removed from the fixed eyes. The surviving global ring included the central portion of the cornea, anterior chamber and its angle, lens, iris, ciliary body, a representative portion of the uveal tract and retina (including macula), optic disc and optic nerve. Eight micrometre microtome sections were cut from each eye, stained with haematoxylin and eosin and mounted on large slides containing the whole eye in cross section, including anterior compartments of the eye. Standard samples therefore included the optic nerve head and peripapillary retina including macula and peripheral retina.
Histology
Histological specimens were scored and evaluated by an ophthalmic pathologist (R.L.) and reviewed by a second ophthalmic pathologist (Professor William Lee) with an agreed protocol for each histological element of the eye and optic nerve. In each case the pathologist did not know the clinical and pathological diagnosis. In addition, the evaluating pathologist was unaware of the proportion of cases and controls in the sample. Each pathological feature was scored (present/absent) independently and sequentially in the following order: (i) iris, (ii) retina/macula, (iii) optic disc, (iv) optic nerve head and (v) optic nerve to reduce the risk of unblinding. Abnormalities were graded as severe or mild. This was a strictly qualitative distinction, but in the case of retinal atrophy, iris reaction, optic nerve head gliois and optic nerve head atrophy, a severe grade implied that the finding was widespread and prominent whereas a grade of mild was restricted in severity or location. In the case of periphlebitis a severe grade implied that the abnormality was found in multiple locations whereas a mild grade implied that it was found in only one or two locations. The primary ophthalmologic pathologist (R.L.) was responsible for all final pathologic determinations. Each case was then subsequently reviewed by the authors to highlight examples of particular pathological findings—but not to modify scoring.
Immunohistochemistry
On selected multiple sclerosis eye blocks (8), including both eyes from three cases, immunohistochemistry was performed on 7 μm formalin-fixed, paraffin-embedded sections using the following monoclonal antibodies and dilutions: astrocytes [glial fibrillary acidic protein (GFAP), clone number 1:100; Dako]; macrophages/microglia/B cells/activated T cells [human leukocyte antigen (HLA)-DR; CR3/43; 1:500; Dako]; axons (neurofilament 2F11, 1:100, Dako); and a rabbit polyclonal antibody to the tight junction associated protein ZO1 (1:200, Zymed). All sections were deparaffinized, and antigen retrieval was performed in 0.01 Km Tris–EDTA (Sigma-Aldrich; pH 9.0) in a pressure cooker at full steam pressure for 2 min. All antibodies were then incubated on sections overnight at 4°C and detected using either peroxidase-labelled Envision anti-mouse polymer (Dako) with diaminobenzidine (Dako) as chromogen or Alexa-488-conjugated goat anti-rabbit secondary antibody (1:500, Invitrogen) as described previously (Nihmaille et al., 2008).
Dual-labelling for astrocytes and tight junction proteins was achieved by incubation overnight at 4°C in a mix of anti-GFAP and anti-ZO1. After washes in Tris buffered saline, bound antibodies were detected by incubation in a mixture of goat anti-rabbit Alexa-568 and goat anti-mouse Alexa-488 (both 1:500; Invitrogen) for 2 h at 37°C. After final rinses in Tris–buffered saline, sections were mounted in 4-6-diamidino-2-phenylindole Vectashield (Vector Laboratories, Inc., Dublin, Ireland). All mounted immunofluoresence sections were analysed, and images were acquired using a Leica TCS/NT confocal microscope equipped with a krypton–argon laser as the source for the ion beam. Image stacks were collected through an optical plane of 8 μm. Negative controls were generated by omission of primary antibodies or by section pretreatment with proteinase K (Sigma-Aldrich).
Microscopic assessments were primarily done to describe variations in the pathology of multiple sclerosis eyes as compared to other neurological disease cases. Selected haematoxylin and eosin or immunohistochemistry-stained sections were digitally scanned using a Hamamatsu Nanozoomer with a ×40 objective. From these scans, selected images could be viewed or displayed at a range of magnifications.
Statistical analysis
Statistical analyses were performed to evaluate the likelihood that the differences observed between patients with multiple sclerosis and controls was a consequence of chance. Data were analysed using multivariate logistic and linear regression models controlling for age, sex and duration of disease when appropriate. In addition all statistical tests were adjusted for data clustering to control for subjects in whom two eyes were available for analysis. Analyses were performed using Intercooled Stata 9.0 software.
Results
Clinical records
The diagnosis of multiple sclerosis was confirmed in the 82 cases (100%) and disease subtype was established at time of death in 80 of the 82 cases (98%) (Table 1).
Multiple sclerosis cases
The majority of multiple sclerosis cases were subjects who suffered from secondary progressive disease at the time of death. Although, many of these individuals had a long clinical course, there was a broad range in disease duration. Age ranged from 26 to 87 years and duration of disease ranged from months to decades. Even among subjects with secondary progressive multiple sclerosis there was variation in duration of disease, ranging from 3 to 53 years (mean 26.7 ± 13.7). The primary progressive multiple sclerosis group showed the usual reversal of sex incidence with 39% female, while all other multiple sclerosis groups showed the usual female preponderance.
Other neurological disease cases
The control group showed a similar age range (mean 53.5 ± 17) to the multiple sclerosis group and had a similar sex ratio to the primary progressive multiple sclerosis group. Duration of disease in the other neurological disease control group ranged from 3 months to 15 years.
An overall summary of the pathological findings is shown in Table 2.
Table 2.
Summary of pathological findings
| Diagnosis | Subject | Eyes | Retina |
Optic nerve |
Iris | ||
|---|---|---|---|---|---|---|---|
| Periphlebitis | Cell loss | Gliosis | Atrophy | Reaction | |||
| Acute multiple sclerosis | 6 | 9 | 0% | 55% | 50% | 50% | 55% |
| Chronic multiple sclerosis | 76 | 121 | 13% (P = 0.125) | 80% (P < 0.001) | 72% (P < 0.001) | 71% (P < 0.001) | 72% (P = 0.001) |
| Relapsing remitting multiple sclerosis | 8 | 15 | 14% | 100% | 83% | 73% | 71% |
| Secondary progressive multiple sclerosis | 51 | 84 | 15% | 79% | 71% | 55% | 75% |
| Primary progressive multiple sclerosis | 13 | 22 | 5% | 67% | 67% | 70% | 58% |
| Unable to classify | 2 | 2 | 0% | 100% | 100% | 100% | 100% |
| Other neurological disease | 10 | 16 | 0% | 19% | 8% | 13% | 25% |
| Total | 92 | 148 | 11% | 73% | 65% | 65% | 67% |
All percentages are for numbers of eyes (not subjects). All P-values are for multivariable logistic regression comparing chronic multiple sclerosis to patients with other neurological disease controlling for age and gender.
Retina
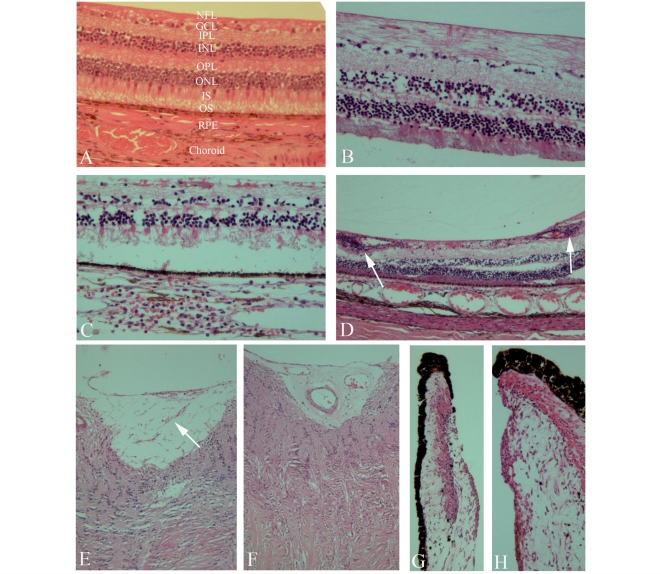
Inner retinal atrophy was prominent in multiple sclerosis eyes. Quantitative stereological estimates were not possible because of partial post-mortem detachment of retina in many of the eyes. However, there were obvious, substantive qualitative reductions in RGC populations in multiple sclerosis cases compared to the other neurological disease control eyes. Inner retinal atrophy (retinal nerve fibre layer and ganglion cell layer) was noted in 79% (48% severe) of multiple sclerosis eyes and was scored as present in 19% of other neurological disease control eyes (in all of which it was mild). In the six multiple sclerosis cases categorized as acute, RGC loss was noted in 55% of eyes (83% of cases). The odds of finding retinal atrophy in subjects with multiple sclerosis was more than 17 times greater than in non-multiple sclerosis subjects (odds ratio = 17.5, 95% confidence interval 4.9–61.7). Frequently, only one or two RGCs could be identified in the peripapillary retina per low power field in multiple sclerosis eyes. This compared with a continuous row of RGCs in the eyes from patients with other neurological disease (Fig. 1A, B and C).
Figure 1.
Haematoxylin and eosin and immunoperoxidase labelling of formalin-fixed, paraffin-embedded microtome sections from other neurological disease control or multiple sclerosis eye samples. Layers of retina are as follows: NFL = nerve fibre layer; GCL = ganglion cell layer; IPL = inner plexiform layer; INL = inner nuclear layer; OPL = outer plexiform layer; ONL = outer nuclear layer; IS = inner segments; OS = outer segments; RPE = retinal pigment epithelium; choroid. (A) Control sample: normal distribution of cells in the ganglion cell layer and inner nuclear layer. (B, C) Varying degrees of disruption in the ganglion cell layer and inner nuclear layer in patients with multiple sclerosis; B shows mild and C severe changes. (D) Perivascular cellular infiltration and periphlebitis in the retina (arrows). (E) Mild gliosis (arrow) in the optic nerve head. (F) Severe gliosis in the optic nerve head. (G, H) Inflammation involving the stroma of the iris.
In addition, prominent atrophy of the inner nuclear layer (predominantly constituted by horizontal and bipolar cells) was evident in a number of the multiple sclerosis eyes. This was noted in 40% of multiple sclerosis eyes and none of the control eyes. This finding was most pronounced in subjects who had longstanding and/or progressive disease and was not evident in any of the six cases categorized as acute multiple sclerosis. Furthermore, the severity of the inner nuclear atrophy appeared related to the severity of RGC atrophy—although this was not established quantitatively. Atrophy of the outer nuclear layer was not detected.
In addition, controlling for sex and age, atrophy of the retina was inversely associated with brain weight (i.e. patients with lower brain weight were more likely to have severe retinal atrophy) (P = 0.04). Using a similar multivariate model, there was a trend towards detecting an association between severe retinal atrophy and longer disease duration (P = 0.13).
Inflammatory changes in the retina
Localized inflammatory cellular infiltrates surrounding retinal veins in the connective tissue of the retinal nerve fibre layer and RGC layer were encountered in 29% of the relapsing remitting and secondary progressive multiple sclerosis eyes sampled but in only 5% of primary progressive multiple sclerosis eyes (Fig. 1D). Furthermore this finding was independent of disease duration. The majority of the perivascular inflammatory cells were mononuclear with compact cytoplasm and resembled lymphocytes, however a proportion had a foamy appearance suggesting that they were phagocytic. Similar mononuclear cells were seen scattered throughout the retinal nerve fibre layer and ganglion cell layer in 12% of cases (and never in controls). In a similar proportion of cases, elongated and foamy appearing cells were seen (some of which stained positive for HLA-DR—see below). These cells did not obey the boundaries of typical retinal layers and sometimes extended into the inner plexiform and inner nuclear layers.
Optic disc, optic nerve head and optic nerve
Pathological cupping of the optic disc was defined as an excavation or indenting, often with a fibrovascular membrane giving the appearance of retraction and atrophy of the disc. This abnormality was frequently encountered in multiple sclerosis eyes (Fig. 1E and F). Perivenular gliosis and thickening of small veins and venules were also observed in the optic disc. In longstanding multiple sclerosis cases, sections showed thinning of retinal nerve fibre layer adjacent to the optic nerve. This ranged from mild, with atrophy of individual bundles, to severe atrophy of the retinal nerve fibre layer. In addition, gliosis with a related glial-mesodermal reaction was seen in more than 70% of optic nerves from multiple sclerosis cases but was not present in control eye sections.
Anterior structures of the eye
Evaluation of the anterior eye revealed abnormalities involving the iris at a high frequency in multiple sclerosis cases. Inflammatory and fibrotic changes were seen in both the pigmented and stromal compartments of the iris. Degrees of rubeosis iridis (iris neovascularization), eversion of the pigmented layer at the pupillary margin and inflammation involving the iris stroma were seen in 72% of the multiple sclerosis cases (20% severe: Fig. 1G and H). Rarely these changes involved the sphincter pupillae. In some cases an inflammatory infiltrate could be seen in the anterior uvea. The presence of vascular changes in the iris was correlated with retinal atrophy (r = 0.4, P < 0.0001). Rubeosis iridis was found in only two cases (four eyes) of other neurological disease, and in both it was graded as mild. Eversion of the pupillary margin was not seen in these cases. A history of diabetes mellitus was not recorded in the multiple sclerosis or other neurological disease cases with anterior uveal abnormalities.
Immunohistochemistry
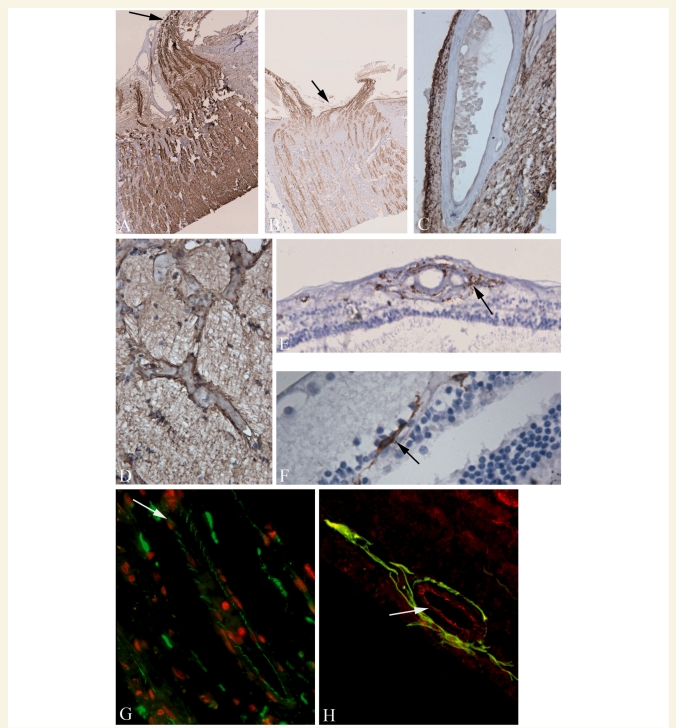
Immunohistochemistry was performed on selected eye and optic nerve sections of five multiple sclerosis cases, three of which had both eyes available for comparison (three secondary progressive, one relapsing remitting and one primary progressive multiple sclerosis). This sample supported the observations from the haematoxylin and eosin sections in the larger case sample. Immunohistochemistry for neurofilaments confirmed retinal ganglion cell loss, inner nuclear layer neuronal loss, retinal nerve fibre layer and optic nerve atrophy. However, immunohistochemistry appeared to be a more sensitive indicator of neuronal process loss than was demonstrated by haematoxylin and eosin staining. Within the optic nerve head, neurofilament immunohistochemistry demonstrated nerve bundle and axon abnormalities (Fig. 2A and B). GFAP staining was in general inversely proportional to neurofilament staining and highlighted perivenular astrocytic gliosis in the optic disc (Fig. 2C) and intense gliomesodermal reaction in the optic nerve (Fig. 2D).
Figure 2.
Haematoxylin and eosin, immunoperoxidase and dual immunofluoresence/confocal microscopy labelling of formalin-fixed, paraffin-embedded, microtome sections from multiple sclerosis eyes. (A, B) Contrast in distribution of neurofilaments in the optic nerve head (black arrows) in two patients with multiple sclerosis; A mild and B severe loss. GFAP immunohistochemistry highlights (C) perivenular gliosis in the optic disc and (D) intense glial-mesodermal reaction in the optic nerve. (E) Many of the inflammatory cells surrounding blood vessels expressed HLA-DR (arrow) and HLA-DR positive cells with ramified processes are also present in the inner nuclear layer in multiple sclerosis eye sections (F, arrow). (G) Tight junction expression (ZO1—Alexa-488, green; propidium iodide stained nuclei -red) in a retinal blood vessel. Most of the junction strands appear normal. However, there is evidence of ‘opening’ of tight junction strands between some endothelial cells (arrow). (H) Dual-imunofluoresence labelling for ZO1 (Alexa-568, red) and GFAP (Alexa-488, green), demonstrates loss of tight junction strands in a retinal blood vessel (arrowed) which is associated with a pronounced glial cell reaction.
Expression of HLA-DR positive cells in the anterior chamber in multiple sclerosis eyes was largely confined to the basal epithelial and sub-epithelial layers of the conjunctiva and cornea. Foci of HLA-DR positive cells were also seen in the iris. In the retinal nerve fibre layer, RGC layer and inner nuclear layer, optic disc and optic nerve, there was an increase in HLA-DR-expressing cells as compared with the published normal range (Yang et al., 2000) and positive cells were sometimes seen intertwined with degenerating neurons (primarily in the ganglion cell layer), and in nerve fibre tracts, in addition to their usual perivascular location (Fig. 2E and F). HLA-DR positive cells were also seen scattered in the inner nuclear and ganglion cell layer. In the one multiple sclerosis case in which immunohistochemistry could be compared in the retina and optic nerve of both eyes, one optic nerve showed severe neurofilament loss and gliosis with few HLA-DR positive cells in the retina; the other optic nerve was relatively normal but there was a marked increase in HLA-DR positive cells in the retina.
Immunohistochemistry with confocal microscopy for tight junction proteins ZO1 and occludin demonstrated that while most blood vessels in the retina in multiple sclerosis cases had a normal distribution of tight junction proteins, occasional blood vessels were present in both the retinal layers and optic nerve head that had an abnormal distribution of tight junctions. Tight junctional abnormalities included loss of staining and opening of tight junction strands (Fig. 2G). Dual-immunofluoresence staining for astrocytes and tight junction proteins revealed the presence of pronounced glial cell reaction which was associated with an abnormal distribution of tight junctions (Fig. 2H).
Influence of disease subtype
Patients with relapsing remitting, secondary progressive and primary progressive multiple sclerosis all demonstrated retinal atrophy and iris inflammatory and vascular changes. Vascular changes were seen with equal frequency in approximately 15% of relapsing remitting and secondary progressive multiple sclerosis, respectively, but were only seen in one case of primary progressive multiple sclerosis. Patients with progressive multiple sclerosis (secondary and primary) appeared to have a greater degree of retinal atrophy and were more likely to have atrophy of the inner nuclear layer. However, we could not detect an effect of progressive disease on the likelihood of having retinal atrophy—most probably because there were only eight patients with relapsing remitting disease and all 15 eyes available for study had some degree of retinal atrophy. There was a trend indicating a possible association between progressive disease and the presence of iris changes in patients with multiple sclerosis (P = 0.20).
Discussion
This is the largest retinal pathology series in multiple sclerosis to date—totalling nearly the full number of eyes that have previously been reported in the multiple sclerosis literature. Each of these cases was examined by several observers (blinded to the underlying diagnosis) in a uniform and consistent fashion to reduce bias. This is the only series to include subjects who were documented to suffer from relapsing remitting disease and to include an ‘other neurological disease’ control group. In addition, as almost all these cases were obtained prior to the advent of approved immunomodulatory treatment for multiple sclerosis, this series is likely to reflect the untreated natural pathology of the disease. Furthermore, in this series, immunohistochemistry was used, permitting more detailed evaluation of the abnormalities seen with haematoxylin and eosin. It is the first series to be assessed pathologically since the advent of OCT and was therefore analysed in the context of the growing interest in use of the inner retina to track disease in multiple sclerosis (Parisi et al., 1999; Trip et al., 2005; Fisher et al., 2006, Sepulcre et al., 2007; Henderson et al., 2008).
We have been able to confirm and reinforce results from previous smaller series. These include perivascular inflammation and fibrotic changes around vessels that did not have active inflammation. It also includes reduction in RGC density in the inner retina, demonstrating that inner retinal thinning in multiple sclerosis is not just a consequence of tissue atrophy but reflects neuronal and axonal loss. Given the size of our cohort, this analysis provides us with a more accurate point estimate of the real prevalence of these changes at end-of life in multiple sclerosis. Our careful clinical characterization of the cases allows us to document that these changes can be found throughout the course of disease and are not dependent on disease duration.
We have also documented a number of previously unreported pathological findings in this series, both in the retina and anterior chamber of multiple sclerosis eyes. For example, this series provides the first direct description of retinal neuronal loss beyond the RGC layer in multiple sclerosis. Despite the fact that the estimation of this injury was qualitative, it is prominent and frequent. It also comports with previously demonstrated but unexplained abnormalities in electroretinography performed in patients with multiple sclerosis (Coupland and Kirkham, 1982; Forooghian et al., 2006).
Cell loss in the deeper layers of the retina could potentially be the consequence of a number of different pathologic mechanisms. The first possibility is that both the RGC and inner nuclear layer suffer injury as a consequence of a direct immune-mediated process. However, the inflammation we have observed is more focal than the neurodegeneration observed. Furthermore, immune cells were not routinely detected in the inner nuclear layer of the samples that demonstrated atrophy—though the possibility of perineuronal diffusion of immune cell products cannot be excluded. The second possibility is that both RGCs and horizontal/bipolar cells share a susceptibility to a neurodegenerative process in multiple sclerosis. Lastly the loss of horizontal and/or bipolar cells could result from retrograde transneuronal degeneration. It is striking that in all cases RGC loss was more pronounced than that seen in the inner nuclear layer. Furthermore, both (i) the absence of inner nuclear atrophy in ‘acute’ multiple sclerosis cases; and (ii) an observed correlation between the severity of inner nuclear layer injury and RGC loss would support this retrograde trans-synaptic model. Anterograde trans-synaptic degeneration leading to loss of neurons in the lateral geniculate nucleus has been previously described in multiple sclerosis (Evangelou et al., 2001), in glaucoma (Gupta et al., 2007) and after chemical injury to the optic nerve (Madigan et al., 1996). Furthermore, retrograde trans-synaptic degeneration has been demonstrated using OCT, showing retinal nerve fibre layer thinning in patients who had suffered injury to the posterior visual pathway (Jindahra et al., 2009). However, this would be the first histological demonstration of trans-synaptic degeneration in a retrograde pattern.
The distinctive architecture of the retina lends itself to investigating the cascade of nerve injury in multiple sclerosis including transneuronal degeneration. RGCs lie directly anterior to the inner nuclear cells that synapse with them. Furthermore, as a sensory organ the retina has primary afferent function—therefore the direction of loss can be defined. Recent pathological data from glaucoma suggest that in areas of retina corresponding to scotoma, there is decline in RGC density and associated loss of deeper retinal neurons (Lei et al., 2008). This may contribute to the permanence of scotomata and could even contribute to the extension of a scotoma at its edges. If the findings described here represent retrograde trans-synaptic degeneration in multiple sclerosis, they may provide insights regarding the mechanisms underlying ‘diffuse’ axonal loss in multiple sclerosis (distal from sites of inflammation, whether contemporaneous or previous). This may also provide us an excellent model for investigating retrograde trans-synaptic degeneration in neurological disease.
The frequent presence of retraction of the optic nerve head is significant for a number of reasons. First a deep cup, when identified on ophthalmoscopy, is considered a hallmark of glaucoma or vascular-mediated optic neuropathies. In contrast, toxic, nutritional and demyelinating optic neuropathies are associated with temporal pallor of the optic disc. This observation highlights that, while injury to nerve fibre layer may be relatively selective and the pattern of injury may be dependent on the mechanism, the disease-associated differences are not absolute. In essence this supports the contention that with longstanding optic neuropathy of any aetiology the ‘final common finding’ is the globally-pale disc. The histological presence of cupping is likely to result from the loss of nerve fibres, retraction and the presence of a gliomesodermal reaction in the optic nerve head. The fact that this is seen so frequently in longstanding multiple sclerosis argues that ongoing degenerative injury to the anterior visual pathway occurs in multiple sclerosis even in the absence of episodes of clinically evident acute optic neuritis and loss of nerve fibres and gliomesodermal reaction are secondary changes that compound the injury. The presence of gliomesodermal reaction implies that deformation of disc architecture can be the consequence of various mechanical processes (i.e. retraction of the optic nerve head by contraction of cytoskeletal elements versus axon loss of the arcuate bundles). Admittedly, we do not have data on whether any of the subjects studied had concomitant glaucoma, although reasonably this could only explain a modest proportion of the cupping seen as ∼1–2% of patients in the UK over age 40 are thought to have open angle glaucoma (Kroese et al., 2002).
The findings reported here have important implications for OCT and other clinical retinal investigations in multiple sclerosis. OCT discerns retinal layers by using software detection algorithms based upon known pattern of backscatter off the healthy retina with incident low coherence infrared light (Blumenthal et al., 2009). It is unknown whether this backscatter pattern is modified by the pathological changes described here. The presence of perivascular inflammation and gliosis—especially given that the relevant vessels are situated in the inner retina—raises the possibility that retinal nerve fibre layer OCT thickness estimates may not be strictly measuring axons. Although this may undermine the reputed tissue specificity of OCT, newer, faster OCT methods (Fourier or spectral domain OCT) with improved image registration may permit us to identify the pathology described in this paper and modify our thickness estimating algorithms accordingly.
Descriptions of the pathologic features of multiple sclerosis in the retina have been based on a limited number of cases and predated a renewed interest in retinal injury in multiple sclerosis. Only Toussaint et al. (1983), who looked at the retinas of 15 patients with multiple sclerosis (and optic nerve in 17 additional patients), made any description of the clinical phenotype of disease. Unlike previous studies, the advent of agreed international criteria for subtype categorization of multiple sclerosis means that in this study it was possible to classify 80 of the 82 cases. However, some abnormalities described in this study confirmed those of earlier authors (ter Braak, 1933; Rucker, 1944; Gartner, 1953; Fog, 1965; Kerrison et al., 1994). A single case report described a patient with retinal periphlebitis identified at the time of diagnosis with detailed histopathology from the same location 14 years later at autopsy (Shaw et al., 1987). The retinal vascular changes observed in this patient closely resemble those seen in chronic plaques in the CNS in multiple sclerosis. In the present study, veins showing hyaline thickening of their walls but not inflammatory infiltrate were excluded in the assessment of retinal periphlebitis. Possibly, as in CNS plaques in multiple sclerosis, they were inflamed at some point in the disease. Furthermore, as a confirmation of the findings reported in this study, Gartner (1953) described (in 10 patients) extensive gliosis as well as optic nerve atrophy, and reduction of RGC density. In Gartner’s sample gliosis was especially prominent in the optic nerve head, sometimes severe enough to lead to a scar deforming the architecture of the disc and optic nerve.
As with all autopsy-based pathological studies in multiple sclerosis, this study is limited by an inability to sample tissue other than at the end of life. The frequency with which perivascular inflammation was observed at this late stage in the disease raises various questions. Understanding how a tissue devoid of myelin is capable of maintaining a robust inflammatory response is important for understanding the operative mechanisms in progressive multiple sclerosis. Interestingly, in a recent study using a transgenic mouse model of multiple sclerosis, myelin-specific T cells also recognized neuronal autoantigen (Krishnamoorthy et al., 2009). Using the retina as a model may even help to reconcile our conceptions of multiple sclerosis as both a neuroinflammatory disease and a neurodegenerative disease.
Of particular note were the findings in the anterior uvea. Given the correlation between the uveal and retinal changes, we suspect that these changes are a response to a distressed retina (as has been postulated to be the operative mechanism in the rubeosis iridis found in association with diabetic retinopathy). Understanding the mechanisms of this process may provide important insights into the molecular response to tissue injury in grey matter in multiple sclerosis. We consider these changes to possibly be a correlate of inflammation seen in the leptomeninges with cortical injury in multiple sclerosis (Kutzelnigg et al., 2005). In addition, from our work it is unclear when in the course of disease these changes become clinically manifest, and whether they are detectable during life. Efforts at clinical confirmation will help us ascertain whether these findings could serve a diagnostic or prognostic purpose.
There are number of reports arguing for an association between multiple sclerosis and clinically identifiable uveitis. However, these clinical series have been controversial because of potential sampling bias, have estimated concomitant uveitis to be an infrequent finding (1–15%) and have typically localized the predominant inflammation to be in the intermediate uveal compartment (pars plana) (Graham et al., 1989; Biousse et al., 1999; Le Scanff et al., 2008). With regards to our pathological findings, we cannot be certain that either the iris neovascularization or the changes at the pupillary margin are secondary to inflammation. Still our observation that these iris changes occur along with cellular infiltrate in some cases suggests that they could be related phenomena.
Given the observation that perivascular inflammation was never uniformly identified in all blood vessels from a single eye and considering the sampling method, it is possible that the frequency with which these changes occur has been underestimated. It has been reported that perivenous sheathing most frequently occurs in vessels beyond the mid-periphery of the retina—a region which in the present study is likely to be under-sampled (Rucker, 1972). However the results are in very close agreement with prior studies (Arnold et al., 1984; Birch et al., 1996; Schmidt et al., 2001).
A few significant additional limitations are worthy of mention. Although efforts were taken to insure the maintenance of blinding of the evaluating ophthalmic pathologist including (i) not disclosing the number or nature of control cases in the series; and (ii) having the evaluating pathologist review each pathological feature independently and in a specified order (optic disc and optic nerve last), we cannot rule out the possibility that the pathologist could have become partially unblinded by detecting pathology in a different location. Furthermore, our control group was primarily selected to account for the impact of (i) agonal changes; (ii) immune mediated injury; and (iii) treatment with glucocorticosteroids on retinal pathology (in order of importance). As a consequence, most of the control subjects suffered from peripheral nervous system demyelinating disorders. We therefore cannot rule out the possibility that similar pathological findings would be found in some other central nervous system disorders—especially those in which inflammation and involvement of the anterior visual pathway would be prominent (e.g. neurosarcoidosis). Lastly, although none of our patients had a concomitant significant ophthalmic condition documented in their available medical records, we cannot be certain that these records were exhaustive. Still, given the relatively low prevalence in the UK of the two most significant and common ophthalmic conditions, age related macular degeneration (3.5% > age 75; Evans et al., 2004) and glaucoma (1–2% > age 40; Kroese et al., 2002), it is unlikely that undiagnosed eye disease significantly confounds our results.
In conclusion, this is the largest retinal pathology series described in multiple sclerosis. It includes an embedded subset of control eyes essentially blinding the evaluating pathologist to the underlying diagnosis—demonstrating that the abnormalities seen are not an artefact of agonal processes or tissue preservation methods. This work confirms the common interpretation of OCT data that retinal atrophy in multiple sclerosis reflects loss of retinal ganglion cells and their axons. However, it also raises the question that other pathological processes involve the inner retina in multiple sclerosis and could confound interpretation of OCT measures. Gliosis and retinal inflammation (especially perivascular inflammation as inner retinal vessels lie in the retinal nerve fibre layer) could lead to increased thickness of retinal layers which is not due to expansion or swelling of neuronal/axonal elements. Furthermore, given OCT’s dependence on segmenting retinal layers based on presumed changes in tissue reflectivity at the interface of different retinal layers, OCT measures in multiple sclerosis should be interpreted with caution. We do not know how retinal inflammation or gliosis changes the backscatter of the low coherence infrared light source used in OCT. This series demonstrates that we cannot assume that retinal nerve fibre layer thickness measures in multiple sclerosis provide a pure estimate of axonal integrity in the anterior visual pathway. This does not diminish the capacity of OCT to serve as a meaningful measure of disease progression in multiple sclerosis, it only serves to provide greater context to prior observations and spur us forward in developing methods for distinguishing different pathological processes using OCT.
Ultimately, this work establishes that the retina is a site of important and underappreciated pathology in multiple sclerosis. We have demonstrated that retinal pathology in multiple sclerosis includes loss of RGCs and loss of neurons in the inner nuclear layer, further evidence that neuronal loss and atrophy in multiple sclerosis includes neuronal populations that are not myelinated. Finally we have documented a high rate of pathology in the anterior uvea, a phenomenon that may arise from either direct inflammation or as a consequence of the diffusion of factors from a distressed retina. Further investigation of these processes will provide valuable insights as we seek to uncover the mysteries of this destructive and capricious disease.
Funding
This work was performed with the generous support of the National Institutes of Health KL2 RR024130, National Multiple Sclerosis Society/American Academy of Neurology Foundation FN 1687A1, the Northern Ireland Branch of the Multiple Sclerosis Society, United Kingdom and the Nancy Davis Center Without Walls (Los Angeles).
Acknowledgements
The authors are indebted to Professor William Lee for his review and evaluation of the multiple sclerosis and control eye histopathology. We would like to thank Dr Gerry Mahon, vision sciences, QUB for expert technical assistance and Dr William F. Hoyt for his advice in preparation of these data.
Glossary
Abbreviations
- GFAP
glial fibrillary acidic protein
- HLA
human leukocyte antigen
- OCT
optical coherence tomography
- RGC
retinal ganglion cell
References
- Arnold C. Evolving management of optic neuritis and multiple sclerosis. J Ophthalmol. 2005;139:1101–108. doi: 10.1016/j.ajo.2005.01.031. [DOI] [PubMed] [Google Scholar]
- Arnold AC, Pepose JS, Hepler RS, Foos RY. Retinal periphlebitis and retinitis in multiple sclerosis. I. Pathologic characteristics. Ophthalmology. 1984;91:255–62. doi: 10.1016/s0161-6420(84)34296-8. [DOI] [PubMed] [Google Scholar]
- Balcer LJ. Optic Neuritis. N Engl J Med. 2006;354:1273–80. doi: 10.1056/NEJMcp053247. [DOI] [PubMed] [Google Scholar]
- Birch MK, Barbosa S, Blumhardt LD, O'Brien C, Harding SP. Retinal venous sheathing and the blood-retinal barrier in multiple sclerosis. Arch Ophthalmol. 1996;114:34–9. doi: 10.1001/archopht.1996.01100130032005. [DOI] [PubMed] [Google Scholar]
- Biousse V, Trichet C, Bloch-Michel E, Roullet E. Multiple sclerosis associated with uveitis in two large clinic-based series. Neurology. 1999;52:179–81. doi: 10.1212/wnl.52.1.179. [DOI] [PubMed] [Google Scholar]
- Blumenthal EZ, Parikh RS, Pe'er J, Naik M, Kaliner E, Cohen MJ, et al. Retinal nerve fibre layer imaging compared with histological measurements in a human eye. Eye. 2009;23:171–5. doi: 10.1038/sj.eye.6702942. [DOI] [PubMed] [Google Scholar]
- Brück W, Bitsch A, Kolenda H, Brück Y, Stiefel M, Lassmann H. Inflammatory central nervous system demyelination: correlation of magnetic resonance imaging findings with lesion pathology. Ann Neurol. 1997;42:783–93. doi: 10.1002/ana.410420515. [DOI] [PubMed] [Google Scholar]
- Costello F, Coupland S, Hodge W, Lorello GR, Koroluk J, Pan YI, et al. Quantifying axonal loss after optic neuritis with optical coherence tomography. Ann Neurol. 2006;59:963–9. doi: 10.1002/ana.20851. [DOI] [PubMed] [Google Scholar]
- Coupland SG, Kirkham TH. Flash electroretinogram abnormalities in patients with clinically definite multiple sclerosis. Can J Neurol Sci. 1982;9:325–30. doi: 10.1017/s0317167100044152. [DOI] [PubMed] [Google Scholar]
- De Stefano N, Battaglini M, Smith SM. Measuring brain atrophy in multiple sclerosis. J Neuroimaging. 2007;17(Suppl 1):10S–15S. doi: 10.1111/j.1552-6569.2007.00130.x. [DOI] [PubMed] [Google Scholar]
- Dutt M, Tabuena P, Ventura E, Rostami A, Shindler KS. Timing of corticosteroid therapy is critical to prevent retinal ganglion cell loss in experimental optic neuritis. Invest Ophthalmol Vis Sci. 2009 doi: 10.1167/iovs.09-4009. Nov 5. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evangelou N, Konz D, Esiri MM, Smith S, Palace J, Matthews PM. Size-selective neuronal changes in the anterior optic pathways suggest a differential susceptibility to injury in multiple sclerosis. Brain. 2001;124:1813–20. doi: 10.1093/brain/124.9.1813. [DOI] [PubMed] [Google Scholar]
- Evans JR, Fletcher AE, Wormald RP. Age-related macular degeneration causing visual impairment in people 75 years or older in Britain: an add-on study to the Medical Research Council Trial of Assessment and Management of Older People in the Community. Ophthalmology. 2004;111:513–7. doi: 10.1016/j.ophtha.2003.07.012. [DOI] [PubMed] [Google Scholar]
- Forooghian F, Sproule M, Westall C, Gordon L, Jirawuthiworavong G, Shimazaki K, et al. Electroretinographic abnormalities in multiple sclerosis: possible role for retinal autoantibodies. Doc Ophthalmol. 2006;113:123–32. doi: 10.1007/s10633-006-9022-0. [DOI] [PubMed] [Google Scholar]
- Fisher JB, Jacobs DA, Markowitz CE, Galetta SL, Volpe NJ, Nano-Schiavi ML, et al. Relation of visual function to retinal nerve fiber layer thickness in multiple sclerosis. Ophthalmology. 2006;113:324–32. doi: 10.1016/j.ophtha.2005.10.040. [DOI] [PubMed] [Google Scholar]
- Fog T. The topography of plaques in multiple sclerosis. Acta Neurol. 1965;41(suppl 15):154–56. [PubMed] [Google Scholar]
- Frisén L, Hoyt WF. Insidious atrophy of retinal nerve fibers in multiple sclerosis. Funduscopic identification in patients with and without visual complaints. Arch Ophthalmol. 1974;92:91–7. doi: 10.1001/archopht.1974.01010010097001. [DOI] [PubMed] [Google Scholar]
- Gartner S. Optic neuropathy in multiple sclerosis; optic neuritis. AMA Arch Ophthalmol. 1953;50:718–26. doi: 10.1001/archopht.1953.00920030729007. [DOI] [PubMed] [Google Scholar]
- Graham EM, Francis DA, Sanders MD, Rudge P. Ocular inflammatory changes in established multiple sclerosis. J Neurol Neurosurg Psychiatry. 1989;52:1360–3. doi: 10.1136/jnnp.52.12.1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N, Yucel YH. Glaucoma as a neurodegenerative disease. Curr Opin Ophthalmol. 2007;18:110–4. doi: 10.1097/ICU.0b013e3280895aea. [DOI] [PubMed] [Google Scholar]
- Henderson AP, Trip SA, Schlottmann PG, Altmann DR, Garway-Heath DF, Plant GT, et al. An investigation of the retinal nerve fibre layer in progressive multiple sclerosis using optical coherence tomography. Brain. 2008;13:277–87. doi: 10.1093/brain/awm285. [DOI] [PubMed] [Google Scholar]
- Hobom M, Storch MK, Weissert R, Maier K, Radhakrishnan A, Kramer B, et al. Mechanisms and time course of neuronal degeneration in experimental autoimmune encephalomyelitis. Brain Pathol. 2004;14:148–57. doi: 10.1111/j.1750-3639.2004.tb00047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jindahra P, Petrie A, Plant GT. Retrograde trans-synaptic retinal ganglion cell loss identified by optical coherence tomography. Brain. 2009;132:628–34. doi: 10.1093/brain/awp001. [DOI] [PubMed] [Google Scholar]
- Kerrison JB, Flynn T, Green WR. Retinal pathologic changes in multiple sclerosis. Retina. 1994;14:445–51. doi: 10.1097/00006982-199414050-00010. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy G, Saxena A, Mars LT, Domingues HS, Mentele R, Ben-Nun A, et al. Myelin-specific T cells also recognize neuronal autoantigen in a transgenic mouse model of multiple sclerosis. Nat Med. 2009;15:626–32. doi: 10.1038/nm.1975. [DOI] [PubMed] [Google Scholar]
- Korsholm K, Madsen KH, Frederiksen JL, Skimminge A, Lund TE. Recovery from optic neuritis: an ROI-based analysis of LGN and visual cortical areas. Brain. 2007;130:1244–53. doi: 10.1093/brain/awm045. [DOI] [PubMed] [Google Scholar]
- Kroese M, Burton H, Vardy S, Rimmer T, McCarter D. Prevalence of primary open angle glaucoma in general ophthalmic practice in the United Kingdom. Br J Ophthalmol. 2002;86:978–80. doi: 10.1136/bjo.86.9.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutzelnigg A, Lucchinetti CF, Stadelmann C, Bruck W, Rauschka H, Bergmann M, et al. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain. 2005;128:2705–12. doi: 10.1093/brain/awh641. [DOI] [PubMed] [Google Scholar]
- Le Scanff J, Sève P, Renoux C, Broussolle C, Confavreux C, Vukusic S. Uveitis associated with multiple sclerosis. Mult Scler. 2008;14:415–7. doi: 10.1177/1352458507083444. [DOI] [PubMed] [Google Scholar]
- Lei Y, Garrahan N, Hermann B, Becker DL, Hernandez MR, Boulton ME, et al. Quantification of retinal transneuronal degeneration in human glaucoma: a novel multiphoton-DAPI approach. Invest Ophthalmol Vis Sci. 2008;49:1940–5. doi: 10.1167/iovs.07-0735. [DOI] [PubMed] [Google Scholar]
- Lisch K. Die Veranderung der peripheren schbahn bei Mulipler Sklerose Arch f. Augehn. 1933;107:380. [Google Scholar]
- Lumsden CE. The Neuropathology of multiple sclerosis. In: Vinken PJ, Gruyn GW, editors. Handbook of Clinical Neurology. Chapter 8. Vol. 9. Amsterdam: North Holland; 1970. pp. 217–309. [Google Scholar]
- Madigan MC, Rao NS, Tenhula WN, Sadun AA. Preliminary morphometric study of tumor necrosis factor-alpha (TNF alpha)-induced rabbit optic neuropathy. Neurol Res. 1996;18:233–6. doi: 10.1080/01616412.1996.11740410. [DOI] [PubMed] [Google Scholar]
- Mogensen PH. Histopathology of anterior parts of the optic pathway in patients with multiplesclerose. Acta Ophthalmol. 1990;68:218–20. doi: 10.1111/j.1755-3768.1990.tb01908.x. [DOI] [PubMed] [Google Scholar]
- Nihmaille AN, McQuaid S, Windebank A, Cunnea P, McMahon J, Samali A, et al. Increased expression of endoplasmic reticulum stress-related signaling pathway molecules in multiple sclerosis lesions. J Neuropathol Exp Neurol. 2008;67:200–11. doi: 10.1097/NEN.0b013e318165b239. [DOI] [PubMed] [Google Scholar]
- Optic Neuritis Study Group. Multiple sclerosis risk after optic neuritis. Arch Neurol. 2008;65:727–32. doi: 10.1001/archneur.65.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim H. Zur pathologie der disseminirten sklerose. Berliner Klinische Wochenschrift. 1887;48:904–7. [Google Scholar]
- Parisi V, Manni G, Spadaro M, Colacino G, Restuccia R, Marchi S, et al. Correlation between morphological and functional retinal impairment in multiple sclerosis patients. Invest Ophthalmol Vis Sci. 1999;40:2520–7. [PubMed] [Google Scholar]
- Poser CM, Paty DW, Scheinberg L, McDonald WI, Davis FA, Ebers GC, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13:227–31. doi: 10.1002/ana.410130302. [DOI] [PubMed] [Google Scholar]
- Rucker CW. Sheathing of the retinal veins in multiple sclerosis. Mayo Clinic Proc. 1944;19:176–8. [PubMed] [Google Scholar]
- Rucker CW. Sheathing of the retinal veins in multiple sclerosis. Review of pertinent literature. Mayo Clin Proc. 1972;47:335–40. [PubMed] [Google Scholar]
- Sanders MD. Retinal arteritis, retinal vasculitis and autoimmune retinal vasculitis. Eye. 1987;1:441–65. doi: 10.1038/eye.1987.68. [DOI] [PubMed] [Google Scholar]
- Schlossman A, Phillips CC. Optic neuritis in relation to demyelinating diseases. Am J Ophthalomol. 1954;37:487–94. doi: 10.1016/0002-9394(54)92198-4. [DOI] [PubMed] [Google Scholar]
- Schmidt S, Wessels L, Augustin A, Klockgether T. Patients with Multiple Sclerosis and concomitant uveitis/periphlebitis retinae are not distinct from those without intraocular inflammation. J Neurol Sci. 2001;187:49–53. doi: 10.1016/s0022-510x(01)00520-2. [DOI] [PubMed] [Google Scholar]
- Sepulcre J, Murie-Fernandez M, Salinas-Alaman A, García-Layana A, Bejarano B, Villoslada P. Diagnostic accuracy of retinal abnormalities in predicting disease activity in MS. Neurology. 2007;68:1488–94. doi: 10.1212/01.wnl.0000260612.51849.ed. [DOI] [PubMed] [Google Scholar]
- Shaw PJ, Smith NM, Ince PG, Bates D. Chrnoic periphlebitis retinae in multiple sclerosis. A histopathological study. J Neurol Sci. 1987;77:147–52. doi: 10.1016/0022-510x(87)90117-1. [DOI] [PubMed] [Google Scholar]
- Shindler KS, Ventura E, Dutt M, Rostami A. Inflammatory demyelination induces axonal injury and retinal ganglion cell apoptosis in experimental optic neuritis. Exp Eye Res. 2008;87:208–13. doi: 10.1016/j.exer.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Braak JWG, van Herwaarden A. Ophthalmo-encephalomyelitis. Klin Monatsbl Augenheilkd. 1933;91:316–43. [Google Scholar]
- Toussaint D, Périer O, Verstappen A, Bervoets S. Clinicopathological study of the visual pathways, eyes, and cerebral hemispheres in 32 cases of disseminated sclerosis. J Clin Neuroophthalmol. 1983;3:211–20. [PubMed] [Google Scholar]
- Trip SA, Schlottmann PG, Jones SJ, Altmann DR, Garway-Heath DF, Thompson AJ, et al. Retinal nerve fiber layer axonal loss and visual dysfunction in optic neuritis. Ann Neurol. 2005;58:383–91. doi: 10.1002/ana.20575. [DOI] [PubMed] [Google Scholar]
- van Waesberghe JH, Kamphorst W, De Groot CJ, van Walderveen MA, Castelijns JA, Ravid R, et al. Axonal loss in multiple sclerosis lesions: magnetic resonance imaging insights into substrates of disability. Ann Neurol. 1999;46:747–54. doi: 10.1002/1531-8249(199911)46:5<747::aid-ana10>3.3.co;2-w. [DOI] [PubMed] [Google Scholar]
- Ulrich J, Groebke-Lorenz W. The optic nerve in multiple sclerosis: a morphological study with retrospective clinicopathological correlations. Neuro-Ophthalmology. 1983;3:149–59. [Google Scholar]
- Yang P, Das PK, Kijlstra A. Localization and characterization of immunocompetent cells in the human retina. Ocul Immunol Inflamm. 2000;8:149–57. [PubMed] [Google Scholar]
- Youl BD, Turano G, Miller DH, Towell AD, MacManus DG, Moore SG, et al. The pathophysiology of acute optic neuritis. An association of gadolinium leakage with clinical and electrophysiological deficits. Brain. 1991;114:2437–50. doi: 10.1093/brain/114.6.2437. [DOI] [PubMed] [Google Scholar]