Abstract
Epileptic cortex is characterized by paroxysmal electrical discharges. Analysis of these interictal discharges typically manifests as spike–wave complexes on electroencephalography, and plays a critical role in diagnosing and treating epilepsy. Despite their fundamental importance, little is known about the neurophysiological mechanisms generating these events in human focal epilepsy. Using three different systems of microelectrodes, we recorded local field potentials and single-unit action potentials during interictal discharges in patients with medically intractable focal epilepsy undergoing diagnostic workup for localization of seizure foci. We studied 336 single units in 20 patients. Ten different cortical areas and the hippocampus, including regions both inside and outside the seizure focus, were sampled. In three of these patients, high density microelectrode arrays simultaneously recorded between 43 and 166 single units from a small (4 mm × 4 mm) patch of cortex. We examined how the firing rates of individual neurons changed during interictal discharges by determining whether the firing rate during the event was the same, above or below a median baseline firing rate estimated from interictal discharge-free periods (Kruskal–Wallis one-way analysis, P<0.05). Only 48% of the recorded units showed such a modulation in firing rate within 500 ms of the discharge. Units modulated during the discharge exhibited significantly higher baseline firing and bursting rates than unmodulated units. As expected, many units (27% of the modulated population) showed an increase in firing rate during the fast segment of the discharge (±35 ms from the peak of the discharge), while 50% showed a decrease during the slow wave. Notably, in direct contrast to predictions based on models of a pure paroxysmal depolarizing shift, 7.7% of modulated units recorded in or near the seizure focus showed a decrease in activity well ahead (0–300 ms) of the discharge onset, while 12.2% of units increased in activity in this period. No such pre-discharge changes were seen in regions well outside the seizure focus. In many recordings there was also a decrease in broadband field potential activity during this same pre-discharge period. The different patterns of interictal discharge-modulated firing were classified into more than 15 different categories. This heterogeneity in single unit activity was present within small cortical regions as well as inside and outside the seizure onset zone, suggesting that interictal epileptiform activity in patients with epilepsy is not a simple paroxysm of hypersynchronous excitatory activity, but rather represents an interplay of multiple distinct neuronal types within complex neuronal networks.
Keywords: microelectrodes, focal epilepsy, spike–wave, single unit, microphysiology
Introduction
Importance of the interictal discharge
Epileptogenic cortex is characterized by paroxysmal bursts of activity (Gibbs et al., 1935) that occur independently from the synchronous neural activity that comprises a seizure. These interictal discharges, referred to as a spike, spike and wave or sharp wave on the electroencephalogram (EEG), are thought to represent pathologic alterations in normal cellular excitability and synchronization (Kooi, 1966; Chatrian et al., 1974; Gotman, 1980; Walczac and Jayakar, 1997). Many authors, particularly those trained as electroencephalographers, refer to interictal spikes. Instead, we have chosen the term interictal discharge in order to encompass all components of the event, including the slow wave, and to avoid confusion with single-unit action potentials that are also referred to as spikes, primarily by cellular physiologists (Niedermeyer et al., 1982). While the relationship between interictal abnormalities in the EEG and ictal events remains somewhat unclear, interictal discharges are important indicators of epileptogenicity, are routinely used in the diagnosis of epilepsy and play a critical role in the localization of seizure foci (Penfield and Jasper, 1954; Pedley, 1984; Baumgartner et al., 1995; Holmes et al., 2000; Blume et al., 2001a and b; de Curtis and Avanzini, 2001). A deeper and more comprehensive understanding of the neurophysiological mechanisms underlying the interictal discharge is crucial to developing more complete models of epileptic activity and more principled and effective methods for controlling seizures.
Prior studies in animal models suggest that interictal discharges reflect synchronous and excessive discharges from a large population of neurons. In these models, the interictal discharge results from a burst of action potentials at 200–500 Hz superimposed on a slow depolarizing potential, the paroxysmal depolarizing shift (Goldensohn and Purpura, 1963; Matsumoto and Ajmone Marsan, 1964). This has been observed in many models of focal epilepsy (see de Curtis and Avanzini, 2001 for a review). Whether this same mechanism underlies interictal events in human patients with idiopathic focal epilepsy is uncertain. In particular, does the interictal discharge arise from endogenous membrane instability leading to a paroxysmal depolarizing shift or is it the consequence of exogenous, perhaps network wide, activities? Examination of the relationship between single unit activity and the interictal discharge appears more complex than model systems would suggest. For example, only a subset of neurons seem to increase firing during the fast component of the interictal discharge (Wyler et al., 1982; Altafullah et al., 1986; Isokawa et al., 1989; Ulbert et al., 2004) and most human studies have not found a predictable correlation between neuronal firing or bursting of single units and interictal spikes (Ward and Thomas, 1955; Rayport and Waller, 1967; Babb, 1973; Wyler et al., 1982; Schwartzkroin et al., 1983; Williamson and Spencer, 1994). There is more consistency, however, with regard to the slow component of the discharge. During the slow wave there is a diminished rate of neuronal activity corresponding to a period of relative inhibition. In patients with focal epilepsy, for example, a decrease in multi-unit firing during the slow-wave (Altafullah et al., 1986) is accompanied by large current sources in middle cortical layers (Ulbert et al., 2004). Taken together, these studies point to greater complexity in the generation of the interictal discharge than the paroxysmal depolarizing shift model accounts for and raise the possibility that neuronal interactions and network relationships drive the paroxysm.
To understand the physiological basis of the interictal discharge in human epilepsy more completely, particularly the relationship between single unit activity and interictal discharges, we simultaneously recorded single unit activity and local field potentials in patients with intractable focal epilepsy. Consistent with prior studies, we found that many single units show an increase in neuronal firing at the discharge as well as a decrease during the following slow-wave. In contrast with those studies, however, for many units in or near the seizure focus there is either a decrease or an increase in their firing preceding the interictal discharge. There was also a wide variety of different unit firing patterns seen during the fast component of the discharge and the following slow wave that have not been described previously. This diversity in types of modulation was observed even within small cortical regions and was true in regions inside and outside the seizure focus. Together these patterns suggest that the interictal discharge does not result from a physiologic event limited to a single population of neurons but, instead, reflects a dynamic and complex network phenomenon, emerging from a heterogeneous population.
Materials and methods
Subjects
Twenty patients (ages 10–58 years, nine females) with medically intractable focal epilepsy who were already scheduled for clinically-indicated intracranial cortical recordings for epilepsy monitoring (Delgado-Escueta and Walsh, 1983; Engel et al., 1983) were enrolled in the study after informed consent was obtained. These procedures were monitored by local Institutional Review Boards in accordance with the ethical standards of the Declaration of Helsinki. Electrode targets and the duration of implantation were determined solely on clinical grounds.
Clinical electrodes and recordings
Patients were implanted with intracranial subdural grids, strips and/or depth electrodes (Adtech Medical Instrument Corporation, Racine, WI) for 5–10 days. They were monitored in a specialized hospital setting until sufficient data were collected to identify the seizure focus, at which time the electrodes were removed and, if appropriate, the seizure focus was resected. Continuous intracranial EEG was recorded with standard recording systems (XLTEK, Ontario Canada; BMSI, Viasys NeuroCare, Conshohocken, PA; CEEGRAPH, Bio-logic Systems, Mundelein, IL; and BIDMC/Apropos Medical, Boston, MA) with sampling rates between 200 and 500 Hz. All steps of the analysis of intracranial EEG data were performed using Neuroscan Edit 4.3 software (Compumedics, El Paso, TX) and custom designed MATLAB (MathWorks, Natick, MA) software.
Microelectrodes
Three types of microelectrodes were implanted in addition to the clinical macroelectrodes. The first type of electrode was the NeuroPort array (n = 3 patients, Cyberkinetics Inc/Blackrock Microsystems, Salt Lake City, UT), which has been used in several previous studies (Hochberg et al., 2006; Schevon et al., 2008; Truccolo et al., 2008; Waziri et al., 2009). The 4 mm × 4 mm microelectrode array is composed of 100 platinum-tipped silicon probes that are inserted 1.0 mm into the cortex. Recordings were made from 96 active electrodes and data were sampled at 30 kHz per electrode (0.3–7 kHz bandwidth).
The second type was a system of microelectrodes implanted perpendicularly to the cortical surface to sample the width of the cortex (n = 13 patients). This laminar microelectrode array has been described previously (Ulbert et al., 2001a) and also used in a number of studies (Ulbert et al., 2001b, 2004; Wang et al., 2005; Fabó et al., 2008; Cash et al., 2009; Keller et al., 2009). Each array was comprised of 24 electrodes in a single row with diameters of 40 µm spaced at 150 µm centre-to-centre. The entire array had a length of 3.6 mm. Differential recordings were made from each pair of successive contacts to establish a potential gradient across the cortical lamina. After wideband filtering (DC-10 kHz) and preamplification (gain 10×, CMRR 90 db, input impedance 1012 ohms), the signal was split into a low frequency field potential band (filtered at 0.2–500 Hz, gain 1000×, digitized at 2 kHz, 16 bit) and a high frequency multi- and single unit activity band (zero-phase digital high pass filtering above 300 Hz, 48 dB/oct, gain 1000×, digitized at 20 kHz, 12 bit) and stored continuously with stimulus markers.
The third type of microelectrode used in this study (n = 4 patients) was a microwire bundle (Adtech Medical Instrument Corporation, Racine, WI) that has, in similar form, also been used in several previous studies (Cameron et al., 2001; Staba et al., 2002; Ekstrom et al., 2003; Worrell et al., 2008). Patients were implanted with hybrid depth electrodes consisting of the microwire bundle located inside the clinical depth electrodes. The microwires protruded ∼3 mm beyond the macroelectrode tip and were used to record from mesial temporal structures (primarily hippocampus). Recordings were made from seven active electrodes and data were acquired at 30 kHz (0.3–7 kHz bandwidth) using the NeuroPort recording amplifiers.
Selection of interictal discharges
Interictal discharges were selected based on morphological characteristics typical for sharp waves, spikes and spike–wave discharges, as detected on subdural grids or strips in clinical practice. Events showing a biphasic or triphasic morphology with an initial fast phase of 200 ms or less which may or may not have been followed by a prolonged, slower phase were chosen through visual inspection. Time zero was defined as the peak of the fast component of the discharge. Events were selected from the microelectrode field potentials and verified by the adjacent macroelectrode EEG record to minimize potential variability due to the distance between the two types of electrodes. Discharges were selected from epochs in which the patient was awake as determined by inspection of the macroelectrode EEG recordings and video monitoring. Although interictal spikes may be present during sleep, we did not include such recordings since the background rhythmicity of slow wave sleep would confound our analysis regarding grouping and inter-relationships between single units (Staba et al., 2002). Only discharges separated by more than one hour from any ictal event were included in this analysis. The median firing rate of interictal discharges across all subjects was 3.46 discharges/min (minimum was 0.23, maximum 13.06). This high variability in rate resulted in a high variability of total number of discharges being examined for each patient (31–608 discharges).
Localization of electrodes
To co-register the electrodes to anatomical structures, we used Freesurfer software to compute the reconstruction of the cortical surface (Dale et al., 1999). A combination of in-house MATLAB software and Freesurfer was then used to co-register the preoperative MRI and post-operative CT or MRI scan and later align the electrodes on the cortical surface and to deeper structures. Such localization was checked against intraoperative notes and photographs of the placement of the microelectrode arrays. Determination of the seizure onset zone was performed by clinical neurophysiologists. For the purposes of this study, the seizure onset region was demarcated by electrodes that were involved in the initiation of the seizure as recorded with the intracranial electrodes. This determination was made without any knowledge of the research.
Data and statistical analysis
Time-frequency analysis of single trial interictal discharges was used to determine peri-event spectral changes and compare these changes to modulated unit firing rates. A sinusoidal wavelet method (short-time DFT) that returns the mean event-related spectral perturbation was employed using the EEGLAB toolbox for MATLAB (Delorme and Makeig, 2004). We used 100 linear-spaced frequencies up to 50 Hz for this analysis. To compute statistical power, we used a bootstrap method of 200 surrogate data trials.
Although single units were isolated slightly differently for each recording system, they were sorted similarly. For the laminar microelectrode, continuous microphysiology data were high-pass filtered (200 Hz–20 kHz, zero-phase shift, 48 dB/oct) and amplitude thresholded offline with Offline Sorter (Plexon, Dallas, TX). For the microwire bundle and NeuroPort array, the full analogue signal was automatically amplitude thresholded and reduced to waveform snapshots using Cerebus Online Classification software (Blackrock, Inc. Salt Lake City, UT) and then sorted with Plexon’s Offline Sorter.
Great care was taken to ensure that single, stable neurons were used for this analysis. Units sorted with the method described above were treated as single units based on criteria including waveform morphology and autocorrelation functions (Lewicki, 1994; Gale et al., 2009). Only neurons with an absence of spikes in a refractory period (2 ms) were considered single units; putative units with spikes during the refractory period were considered to represent multi-unit activity and were omitted.
To visualize the discharge-related activity of single neurons, peri-stimulus raster plots and timing histograms were constructed for a period 1 s before and after each event. After visual inspection of the local field potential of the event for all patients, we defined five distinct time periods around an interictal discharge. These were a pre-interictal discharge baseline period (−500 to −200 ms), a pre-interictal discharge period (−200 to −35 ms), the interictal discharge (−35 to 35 ms), the slow-wave (35–200 ms) and a post-interictal discharge period (200–500 ms).
Interictal discharges can be rhythmic and can occur at high frequencies, therefore activity directly before the interictal discharge cannot be taken as a distinct baseline. For this reason, baseline periods were created by randomly selecting epochs during interictal discharge-free interictal recording segments. A Kruskal–Wallis one-way analysis of variance tested the equality (P < 0.05) of medians for the firing rate of baseline periods and each period of interest around the interictal discharge (Gibbons, 1985; Hollander and Wolfe, 1999). This test accounts for the non-parametric distribution of spike trains. We used a Bonferroni correction to account for the multiple number of time periods being compared.
To study the effect of neuronal attributes on different populations, we dually characterized each unit. The ‘average firing rate’ was calculated by dividing the total number of spikes by the recording length of the segment. The ‘average bursting rate’ of a neuron was calculated by the algorithm described in Staba et al. (2002), in which a burst is defined by groups of three action potentials occurring within 20 ms such that none are observed 20 ms on either side of the first and last action potential in the cluster.
To study the effects of firing and bursting rate, a Kolmogorov–Smirnov test was used to compare the data set with a normal distribution (Massey, 1951; Marsaglia et al., 2003). In each case, the data were determined to be non-parametric (P < 0.05) and the Kruskal–Wallis test was utilized. Additionally, the peak:trough ratio (the maximum peak amplitude divided by the minimum trough amplitude) and spike half width (a spike’s duration at half-spike amplitude) were calculated in order to characterize action potential morphology and attempt to discriminate between cortical pyramidal cells and interneurons as described previously (Swadlow, 2003; Merchant et al., 2008; Cardin et al., 2009). We observed low variability in these calculations for a given recording system; however, we observed a high variability between modalities. This was presumably due to filtering differences in the different recording systems as well as distinct electrode properties.
Results
Clinical characteristics of patients
We recorded interictal discharges from 20 patients (11 males, 9 females) with intractable epilepsy, from five collaborating institutions (Beth Israel Deaconess Medical Centre, Boston; Brigham and Women’s Hospital, Boston; The Children’s Hospital, Boston; Massachusetts General Hospital, Boston; and New York University Medical Centre, New York City). The mean age was 30.0 ± 13.7 years (SD). Different aetiologies accounted for patient’s epilepsy including cortical dysplasias and hetereotopias, perinatal ischaemia, mesial temporal sclerosis, tumours (oligoastrocytoma and ganglioglioma), arteriovenous malformation and post-traumatic injury. In six patients the aetiology was not definitively established. In three cases this was because a resection was not performed, because the seizure onset zone involved eloquent cortex (n = 1) or was not fully defined (n = 2). In the remaining three cases, the pathology obtained was unremarkable. Similarly, different brain regions were affected, although the temporal lobe was most commonly involved. Microelectrodes were implanted into both the lateral neocortex (13 patients, 10 with laminar microelectrodes, three with NeuroPort) and mesial cortical structures (seven patients, three with laminar microelectrodes, four with microwires). Microelectrode arrays were placed within as well as outside the seizure focus (as determined by a team of clinical encephalographers). These data are summarized in Table 1.
Table 1.
Patient characteristics
| ID | Age | Gender | Electrode type | Anatomical structure | Proximity to seizure focus | Number of units | Seizure focus | Aetiology |
|---|---|---|---|---|---|---|---|---|
| S1 | 27 | F | Laminar | Cingulate gyrus | Far | 8 | Temporal | Cryptogenic (1) |
| S2 | 31 | M | Microwire | Hippocampus | Near | 3 | Temporal | Cryptogenic (2) |
| S3 | 58 | F | Microwire | Hippocampus | Far | 2 | Temporal | Cryptogenic (1) |
| S4 | 45 | F | Microwire | Hippocampus | Near | 10 | Parietal-occipital | Cryptogenic (3) |
| S5 | 28 | M | Microwire | Hippocampus | Within | 4 | Temporal | Post-traumatic |
| S6 | 17 | M | Laminar | Hippocampus | Within | 8 | Temporal | Cortical dysplasia |
| S7 | 47 | M | Laminar | Hippocampus | Far | 7 | Temporal | Hippocamal sclerosis |
| S8 | 19 | M | Laminar | Superior temporal gyrus | Within | 3 | Temporal | Cortical gliosis/hippocampal sclerosis |
| S9 | 21 | M | NeuroPort | Middle frontal gyrus | Near | 43 | Temporal | Cortical dysplasia |
| S10 | 52 | F | NeuroPort | Middle temporal gyrus | Near | 166 | Temporal | Cortical dysplasia |
| S11 | 43 | M | Laminar | Pre-central gyrus | Within | 2 | Temporal | Oligoastrocytoma |
| S12 | 29 | F | Laminar | Lateral occipital cortex | Near | 3 | Parietal | Heterotopia |
| S13 | 24 | M | Laminar(s) | Pre-central and middle temporal gyri | Far | 2 | Frontal | Cortical dysplasia |
| S14 | 12 | M | Laminar | Pre-central gyrus | Near | 9 | Parietal-occipital | Perinatal ischaemic injury |
| S15 | 10 | F | Laminar | Inferior temporal gyrus | Within | 8 | Temporal | Ganglioglioma with adjacent dysplasia |
| S16 | 14 | M | Laminar(s) | Inferior and middle temporal gyri | Near | 3 | Temporal | Hippocampal sclerosis |
| S17 | 34 | F | Laminar | Occipital lateral gyrus | Within | 2 | Occipital | AVM |
| S18 | 27 | F | Laminar | Inferior frontal gyrus | Far | 1 | Frontal | Cryptogenic (2) |
| S19 | 40 | M | Laminar | Middle temporal gyrus | Far | 2 | Frontal | Cryptogenic (2) |
| S20 | 22 | F | NeuroPort | Middle temporal gyrus | Within | 50 | Temporal | Cortical dysplasia |
Anatomical structure from which the microelectrode recording was made was determined by comparison of intra-operative notes and photographs and co-registration of pre- and post-operative imaging (MRI and/or CT). Proximity to seizure focus was established by comparison to clinically determined seizure onset regions. If the electrode was within the determined area it was considered within, near if within 3 cm and far if outside of 3 cm. Aetiology was determined based on pathology studies in the context of the other clinical data. Aetiology notes: multifocal or site of onset not defined so no resection was performed (1), pathology was unremarkable (2), eloquent cortex involved therefore no resection was performed (3). AVM = arteriovenous malformation.
Some, but not all, neurons change their firing rate during or near the interictal discharge
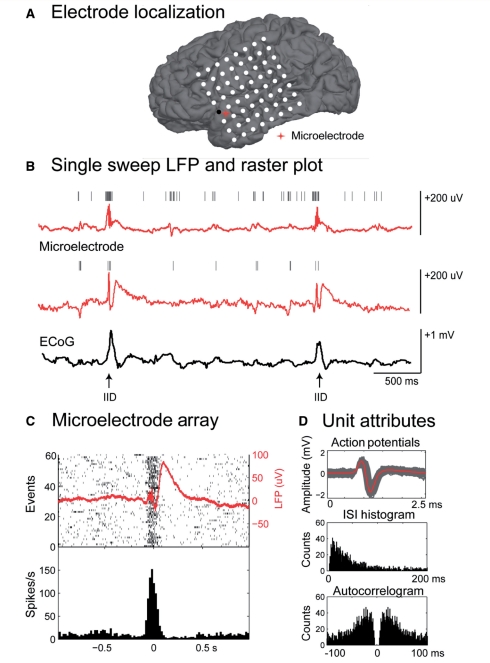
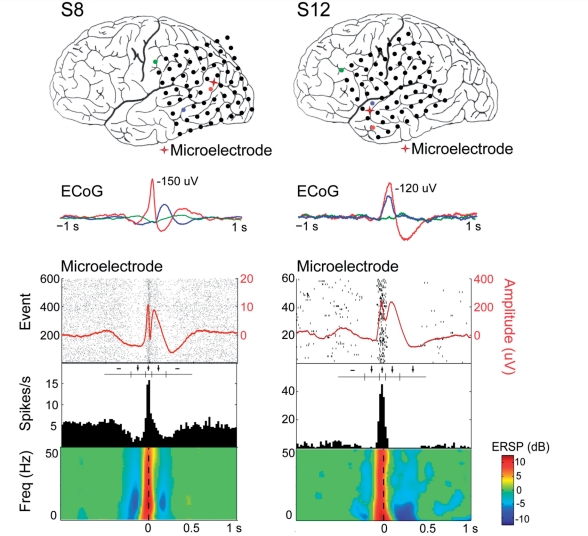
For each patient, the microelectrode array and macroelectrode contacts were localized with respect to cortical and subcortical structures. A common trigger system was used to ensure alignment of the corticography data and the microelectrode signals. An example of electrode localization, macro and microelectrode recordings and single unit analysis is shown in Fig. 1. Additionally, an example of the laminar recordings and unit bursting can be found in the online supplementary material (Supplementary Fig. 1).
Figure 1.
Relationship between macroelectrodes, microelectrodes and single unit activity during an interictal discharge. (A) Co-registration of pre-operative MRI and post-operative CT or MRI allows identification of the electrodes and anatomical structures. (B) Single sweep of microelectrode local field potential (LFP; red) and corticography (ECoG; black) from an adjacent electrode. Hash marks indicate discriminated action potentials. Selected interictal discharges (IID) are indicated with arrows. Note the increase in action potentials from both units during the fast component of the interictal discharge. (C) Raster plot and peri-spike timing histogram from one of the microelectrode channels shown above during the interictal discharge (n = 60). The averaged local field potential is overlaid on the raster plot in red. Histogram bin width is 5 ms. (D) Unit attributes: action potentials (each in grey, average in red), neuronal inter-spike time interval (ISI) histogram (1 ms bin width) and autocorrelogram (−100 to 100 ms; 1 ms bin width) from the unit isolated in (C).
We examined 336 isolated single units across the 20 patients. We defined a unit to be ‘modulated’ if the firing rate, in at least one of five defined time periods around the interictal discharge, was significantly different from a baseline firing rate (Kruskal–Wallis one-way analysis of variance with Bonferroni correction for multiple comparison). Based on this categorization, we found that 48% (158/336) of the units were modulated (P < 0.05) during the interictal discharge.
To determine if all the modulated units were participating in a given event or if only a subset contribute, we calculated the firing probabilities on a per unit basis. On average, a given modulated unit only had a 39.6% (±2.0% standard error) probability of firing during the five time periods previously defined during any single interictal discharge. Additionally, during any given interictal discharge period only 28.2% (±5.95% standard error) of the modulated units fired. Thus, for any given event only a small, and changing, population of neurons participated.
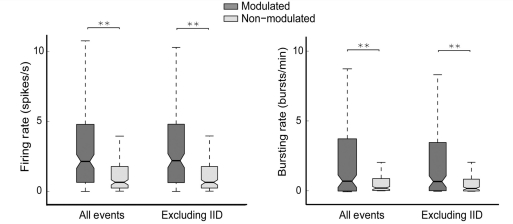
To determine if the modulated and non-modulated neurons had distinct characteristics we compared the firing and bursting rate of these populations. Median baseline frequencies in the modulated and non-modulated groups were 2.55 and 0.66 spikes/s and 1.20 and 0.20 bursts/min, respectively (Kruskal–Wallis, n1 = 158, n2 = 178, P < 0.01 for both measures, two-tailed). We recalculated these parameters excluding the interictal discharge to ensure that the difference was not solely due to the interictal discharge itself. The modulated population still had significantly higher firing and bursting rates than the non-modulated population (Fig. 2; Medmodulated = 2.56 spikes/s and 0.65 bursts/min; Mednon-modulated = 1.02 spikes/s and 0.20 bursts/min; for both measures P < 0.01).
Figure 2.
Firing and bursting rate for neurons modulated or not during the interictal discharge. Boxplots show the median (Med, ×5), lower (Q1, ×25) and upper quartile (Q3, ×75) in the shaded regions and the largest non-outlier observations (whiskers shown with dotted lines). The modulated population had significantly higher firing and bursting rates compared to the non-modulated population both including and excluding the interictal event (Med modulated = 2.56 spikes/s and 0.65 bursts/min; Med non-modulated = 1.02 spikes/s and 0.20 bursts/min; **P < 0.01, Kruskal–Wallis test, n1 = 158, n2 = 178). IID = interictal discharge.
We additionally calculated each unit’s spike half-width (a spike’s duration at half-spike amplitude) and peak:trough ratio (peak amplitude divided by the trough amplitude) as a possible measure of different neuronal types (Cardin et al., 2009). There were no noticeable effects of modulation on neuronal properties within a given recording modality (Supplementary Fig. 2). There were, however, significant changes in these values between modalities. Spike half-width values were significantly lower for units recorded by the laminar system when compared to the others (P < 0.01). Additionally, those units recorded by the NeuroPort system had significantly lower peak:trough ratios when compared to microwire-recorded units (Supplementary Fig. 2, P < 0.05).
We also examined if there were differences in these characteristics based on mesial temporal or cortical location. Overall, there was no statistically significant difference between the bursting or firing rates in mesial temporal versus cortical neurons. The spike half-width of mesial temporal neurons was slightly faster than cortical neurons. There were no significant differences in the peak:trough ratio (Supplementary Fig. 3).
Characterization of the modulated neuronal population
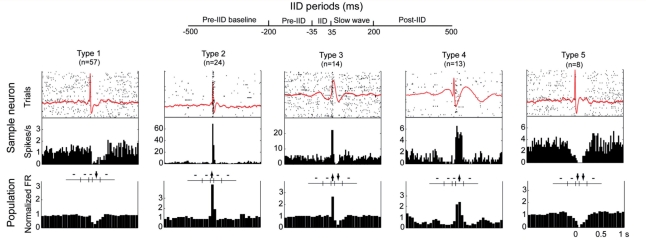
To categorize these different patterns of modulation further, we grouped the neurons based on changes in each of five time periods. Of the possible permutations (five time periods, each with either a significant increase, decrease or no change results in 243 possible outcomes), 16 different firing patterns occurred in more than one unit (Table 2). Five main groups constituted about 75% of the modulated neurons and were observed both inside and outside the seizure focus. In addition, modulated units were observed in both cortical (154 of 304 units) and mesial temporal locations (4 of 32). Figure 3 demonstrates a representative neuron and population averages for the five most common firing patterns. The average local field potential of the same microelectrode channel is overlaid on the raster plot with the peri-spike timing histogram of the representative neuron.
Table 2.
Classification of neuronal responses during interictal discharge
| Class | Number of units | Modulated neurons (%) | Patients represented (%) | Pre-IID (−500 to −200 ms) | Pre-IID (−200 to −35 ms) | IID (−35 to 35 ms) | Slow wave (35 to 200 ms) | Post-slow wave (200 to 500 ms) |
|---|---|---|---|---|---|---|---|---|
| 1 | 57 | 36.1 | 60 | – | – | – | ↓ | – |
| 2 | 24 | 15.2 | 50 | – | – | ↑ | – | – |
| 3 | 14 | 8.9 | 35 | – | – | ↑ | ↓ | – |
| 4 | 13 | 8.2 | 5 | – | – | – | ↑ | – |
| 5 | 8 | 5.1 | 20 | – | – | ↓ | ↓ | – |
| 6 | 5 | 3.2 | 20 | – | ↓ | ↑ | ↓ | – |
| 7 | 4 | 2.5 | 15 | – | ↑ | – | ↓ | – |
| 8 | 4 | 2.5 | 10 | – | ↓ | – | ↓ | – |
| 9 | 3 | 1.9 | 10 | ↑ | – | – | – | – |
| 10 | 3 | 1.9 | 5 | ↑ | – | – | ↓ | – |
| 11 | 3 | 1.9 | 5 | – | – | – | ↓ | ↓ |
| 12 | 2 | 1.3 | 10 | – | ↑ | – | ↑ | – |
| 13 | 2 | 1.3 | 5 | – | – | – | – | ↑ |
| 14 | 2 | 1.3 | 10 | – | – | ↑ | ↓ | ↓ |
| 15 | 2 | 1.3 | 10 | – | ↓ | ↑ | ↓ | ↓ |
| 16 | 2 | 1.3 | 10 | – | – | ↑ | ↑ | – |
Arrows indicate a significant change in firing during the selected time period as defined in the ‘Materials and methods’ section. IID = interictal discharge.
Figure 3.
Classification of neuronal responses during the interictal discharge (IID). Five defined interictal discharge periods are shown in the schematic at top. The averaged local field potential, raster plot and peri-event time histogram of a sample neuron and the population average peri-event time histogram of the top five modulated firing patterns around the interictal event are shown in each column. FR = firing rate. Dashes indicate no significant change and arrows up or down denote an increase or decrease in significance in the middle diagrams (P < 0.05, Kruskal–Wallis test, Bonferroni corrected).
Firing of Type 1 units significantly decreased during the slow-wave but was unchanged during the interictal discharge. They represented 36.1% of the modulated population and occurred in 60% of patients. Type 2 units had an increase in firing rate only during the fast component of the interictal discharge (±35 ms from the peak, 15.2% of the modulated units) and were found in 50% of the patients. In contrast, for Type 3, units firing increased during the interictal discharge but decreased during the slow-wave. Type 4 neurons were not modulated during the spike itself but increased in activity during the slow-wave and were only found in one patient. Action potentials of Type 5 units decreased during both the spike and slow-wave.
In an attempt to understand which classes of neurons may constitute any given type of firing pattern, we examined the baseline firing and action potential characteristics of each of the 10 most frequent types of patterns. Most patterns showed the same characteristics in terms of firing rate and bursting rate with the exception of Type 5 units. These showed a significantly higher firing and bursting rate than other types. This type is the only one of the most common types to have a decrease in firing during the fast component of the discharge. Types 3 and 4 appeared to have a lower firing and bursting rate than other types. Spike half-width and peak:trough values were not significantly different between different firing patterns (Supplementary Fig. 3).
Changes in neuronal firing preceding interictal discharges are found within or near the seizure focus
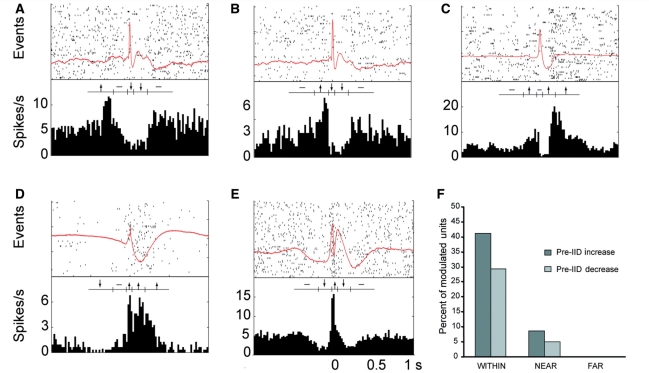
In addition to the five main types of single unit activity described above, many units showed a significant change in firing preceding the interictal discharge. Twelve single units (7.6% of the modulated population) significantly decreased their firing rate and 19 units (12.0%) significantly increased in one of the two time periods preceding the interictal discharge. Two of the 12 units (1.3% of the modulated population) decreased far before the interictal discharge (−500 to −200 ms) while 11 of the 19 units (7.0%) increased far before. We also found three units that increased long after the interictal discharge (200–500 ms; Fig. 4). These types of patterns were found only within or near the seizure focus. In fact, 70% of identified units within the seizure focus showed a pre-discharge change in activity. No such changes were seen in units well outside the seizure onset zone.
Figure 4.
Neuronal units whose firing changes preceded the interictal discharge (IID). Examples of units that (A) increase long before, (B) increase just before, (C) increase after, (D) decrease long before and (E) decrease just before the interictal discharge. (F) Histogram of percent of modulated units which show an increase or decrease before the interictal discharge in regions which were within, near or far from the seizure onset zone.
Comparison of firing rates, bursting rates and action potential characteristics amongst these different groups revealed few differences. There were no statistically significant differences between units which increased, decreased or had no change in their firing rate in terms of baseline firing rate, bursting rate or peak:trough ratio. Units with a decrease in firing before the event had a significantly shorter time at half peak (Supplementary Fig. 3).
Broadband (local field potential) and neuronal firing decrement preceding interictal discharge observed across subjects
Given the plurality of neurons demonstrating a decrement in single unit firing preceding the interictal discharge, we were interested in increasing our understanding of the relationship between this decrement and local field potentials during the pre-spike period. We identified units across subjects that exhibited similar attributes—a decrement before the interictal discharge (−200 to −35 ms), an increase during the interictal discharge (−35 to 35 ms) and a decrement after the fast component (−35 to 200 ms or 200–500 ms; Fig. 5). In four subjects we observed this neuronal firing pattern. In addition, we quantified the local field potential frequency components by averaging the single trial event-related spectral power around the interictal discharge. All of the subjects demonstrated a significant (P < 0.01) broadband decrement in spectral power preceding the event, a significant increase in spectral power during the fast component and a significant decrement again during the slow wave. These changes in spectral power correlate closely with the changes in neuronal firing and together suggest a cortical inhibitory period preceding the fast component, followed by an excitatory period during the fast component of the interictal discharge and an inhibitory period during the slow wave.
Figure 5.
Transient decrease in both local field potential spectral power and neuronal firing rates precede interictal discharges. Upper panels show the average LFP (red) overlaid on raster plots of neuronal firing. Below is the peri-event time histogram and then time-frequency plots of the LFP (non-significant values are plotted in green. Red indicates a significant increase and blue indicates a decrease (P < 0.01). The two columns are from two different patients.
Firing patterns are heterogenous even within a small cortical region and such variation is present inside and outside the seizure focus
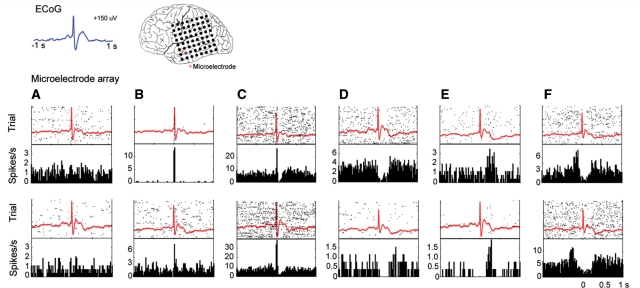
In each subject we found that the several different firing patterns of individual neuronal units could be observed even within a small area of cortex. Units with entirely different firing characteristics could be recorded from a single microelectrode or from microelectrodes within a few 100 µm of each other. These results are demonstrated in Fig. 6, in which the variability in firing patterns of a population of neurons in a restricted cortical region from a single patient is displayed. In this example, the recordings were made within a radius of ∼2.5 mm in layers III–IV of the neocortex, as determined from post-resection histology. Note that the local field potentials of all recordings are extremely similar in morphology despite the large variation in firing patterns of the single units. Unit attributes for this example (action potentials, interictal spike histograms, and autocorrelograms) can be found in the online supplementary material (Supplementary Fig. 4). In addition, not only were many different firing patterns found within a small region but many of the different patterns were observed in recordings made both inside and outside of the seizure focus.
Figure 6.
Neuronal responses are variable in a small cortical region. All 12 units in this analysis were found within 2.5 mm at the same depth in cortex and therefore, the same cortical layer. In each plot, the top panel shows the raster plot (60 events) overlaid with the average local field potential. The lower panel shows the peri-event time histogram. Each column shows examples of similar firing patterns in two different units. Neuronal responses to the interictal discharge were seen that (A) did not change, (B) increased during the fast component, (C) increased during the fast component and decreased during the slow wave, (D) did not change during the fast component but decreased during the wave, (E) increased after the IID peak and wave, and (F) increased before the fast component of the interictal discharge.
Discussion
A heterogeneous sub-population of neurons are involved in interictal discharge generation
We studied hundreds of neurons in ten different cortical and sub-cortical structures, both inside and outside the seizure focus, in 20 patients with intractable focal epilepsy. This is perhaps the most extensive survey of single unit activity during human interictal activity reported. In contrast to previous studies, our datasets included large numbers of simultaneously recorded single units—up to 166 units from a small patch of cortex—and covered a larger number of different regions and aetiologies. In this systematic characterization of cortical neuronal activities surrounding interictal discharge events we found a remarkable variety of distinct firing patterns, which we classified into more than 15 categories. Only about half of the recorded units showed some change in firing in or around the interictal discharge. This percentage of modulated neurons agrees with that described by Wyler et al. (1982), who found that 44% of recorded neurons showed primarily an increase in firing rate near the interictal discharge peak. Surprisingly, a considerable subset of units showed either an increase or decrease in firing rates well ahead of the interictal discharge event, in direct contrast to simple models of a paroxysmal depolarizing shift. These results have several implications for the mechanisms underlying epileptiform activity.
First, our results suggest that epileptiform activity, rather than requiring a large mass of neurons, can occur with relatively sparse single neuron participation. That is, for any given event, only a fraction of available neurons participate or are necessary for the generation of the epileptic discharge. In addition, it appears that a different subset of modulated neurons participated in any given event since any given interictal discharge only involved ∼30% of the total pool of recorded modulated units. This finding suggests that at the microphysiological level epileptiform activity can involve multiple neuronal groups or pathways. Certainly, our recording techniques are biased toward sampling the activities of neurons with large cell bodies. Smaller cells and neurons with low amplitude events may not be recorded and therefore their behaviour during the interictal discharge may not be revealed. As a result, it is possible that the total percentage of neurons involved in any given discharge is larger. Nonetheless, it is clear that (i) a substantial number of neurons are not necessary for, or perhaps are not able to, participate in the epileptic activity; and (ii) the same set of neurons are not always involved. Advances in recording techniques and comparisons with intracellular studies done in model systems should help to complete this picture in more detail.
Second, neurons that were modulated during the interictal discharge had significantly higher bursting and firing rates than those that did not. It is possible that the increase in bursting and firing rates found during the interictal state may be due solely to the interictal discharge itself. To exclude this possibility we analysed recording periods without interictal discharge and found that the modulated population still demonstrated higher firing and bursting propensities. These data lead to two possible conclusions. On the one hand, these findings support the notion, widely expressed in the literature, that neurons crucial for epileptic activity have distinct, if not abnormal, intrinsic activities. On the other hand, it is possible that these increased burst and firing rates reflect purely network phenomena; they are an essential reflection of the network properties in which the neuron is embedded. In either case, the distinct characteristics of these neurons do not explain how an interictal discharge is generated. Examination of the diversity of firing patterns seen before and during the interictal discharge suggests that a particular cascade of neuronal interactions is crucial for formation of the paroxysmal event.
Modulated firing patterns include changes preceding the interictal discharge
Of the subset of units whose activity was modulated during the interictal discharge, we observed multiple firing patterns both within and across patients. Some of these patterns are expected—an increase in firing during the fast component, a decrease during the slow wave, or both an increase during and a decrease after have all been reported previously (Wyler et al., 1982; Altafullah et al., 1986; Ulbert et al., 2004) and together constitute 60% of the units in our study. These patterns are consistent with the paroxysmal depolarizing shift mechanism—a burst of action potentials followed by a period of relative inhibition. The other activity patterns observed, however, depart from predictions of the simple paroxysmal depolarizing shift model. Most significantly, almost 20% of the units significantly increased or decreased their firing well before the interictal discharge. Such changes were only seen in or near the seizure onset zone. This is consistent with the common notion that autonomously generated epileptiform activity would have a different origin than similar activity which is propagated and may point toward methods for further delineation of the seizure onset-zone.
These pre-discharge changes were not an artefact of the recording process. While the morphology of the interictal discharge was highly variable across patients, we selected events such that time zero would be aligned to the peak of the fast component of the interictal discharge. Additionally, these local field potentials were derived from the local microelectrode channel; therefore, this temporal shift in different neuronal firing patterns could not result from a shift between the macroelectrode intracranial EEG recording and the microelectrode local field potential recording.
Concomitant with the decrease in firing rate for certain neurons, at least in some patients and locations, is a decrease in broadband local field potential activity. In addition, a different population of neurons displays an increase in firing rates during this same period. These results support the hypothesis that neuronal inhibition may be responsible, in part, for synchronizing cortical activity and generating the interictal discharge. Unfortunately, our analysis of the action potential characteristics associated with units which changed before the discharge showed only modest differentiation between classes. Thus, it is difficult to point conclusively to one type as excitatory and the other as inhibitory. This is, in part, due to our use of different recording systems but may also reflect the heterogeneity of aetiologies, brain regions and many other factors. Nevertheless, such pre-event changes have also been seen in advance of seizures in an animal model of temporal lobe epilepsy (Bower and Buckmaster, 2008) and around seizure onset in human temporal lobe epilepsy (Verzeano et al., 1971; Babb and Crandall, 1976; Babb et al., 1987). In addition, this period prior to the fast component of the interictal discharge appears similar to the cortical downstate in animal models of sleep (Amzica and Steriade, 1998; Steriade and Amzica, 1998) and in humans during slow wave sleep and evoked during K-complexes of sleep (Cash et al., 2009).
Previous evidence for this type of mechanism was confined mainly to animal studies of generalized epilepsy. Decreased neuronal firing in reticular thalamic cells during a depth positive EEG wave preceding the interictal discharge was recorded from cat cortex during generalized epilepsy (Steriade et al., 1995). Additionally, a correlation in firing was found between interneurons and high frequency oscillations preceded the interictal discharge in rat hippocampus (Ylinen et al., 1995). Based on these studies, it has been proposed that synchronously firing cortical interneurons that decrease firing directly before the interictal discharge permit the bursting of excitatory neurons that make up the fast component of the interictal discharge (de Curtis and Avanzini, 2001). This mechanism may certainly account for our data in which we observed some neurons with decreased firing immediately preceding the interictal discharge. The timescale of this event, however, may be fairly prolonged as there were clear changes for certain neurons hundreds of milliseconds before the event.
In this data set, there was remarkably little that distinguished units of one pattern from another. The action potential or firing characteristics of the neurons at baseline were not very different between different classes of units (with the exception of the Type 5 unit). It is possible that other measures of classification might distinguish these different neuronal types. It is also possible that both the numbers of units recorded here and the recording technologies themselves, being variants on extracellular systems, are not powerful or consistent enough to allow us to reliably distinguish between the neuron types. Further work with larger numbers of examples may eventually allow us to separate more precisely the physiological features corresponding to each neuronal firing pattern and within different brain regions.
An overall model of the interictal discharge
While the results presented here do not establish causal links between observed neuronal firing patterns and the recorded paroxysm, the existence of these different patterns suggests that activity in multiple different neuronal types, linked in a local but remarkable heterogeneous network, gives rise to the interictal event. One possible sequence of events is that chronic excitation in and around the seizure focus leads to a compensatory, chronic up-regulation of both excitatory and inhibitory activity. As a result, the bursting and firing rates of the neurons are generally increased. A subset of inhibitory neurons firing together may initially decrease the activity of other neurons in the network. Subsequently, these inhibitory interneurons cease to fire resulting in a hyper-synchronous, excitatory rebound. The post-synaptic effects of this rebound are manifest in the EEG recording of the interictal discharge. During the slow wave there is primarily a decrease in firing—though a small number of neurons increase during this period—suggesting, like the pre-ictal period, that this is an active event representative of network interactions and not just intrinsic currents. Of course, this sequence of events begs the question of what initiates the inhibitory wave of activity to start with. In some situations, sleep for example, ongoing slow oscillations may provide the underlying drive. In other states it may be a purely stochastic process. Further study will be necessary to provide further details of this sequence but the overall scheme highlights the possible importance of inhibition in sculpting epileptic events which have largely been considered purely excitatory in nature.
Conclusion
In this study we have used microelectrode recordings to quantify and characterize cortical neuronal firing patterns during interictal discharges in patients with focal seizures, finding a tremendous diversity of response including changes in firing that precede the defining interictal discharge itself. Such a variety of neuronal activities supports the hypothesis that interictal discharges are an emergent manifestation of a complex set of network interactions and highlights the potential importance of inhibitory activity in seemingly excitatory paroxysmal events.
Funding
This work was supported by CIMIT under U.S. Army Medical Research Acquisition Activity Cooperative Agreement W81XWH-09-2-0001, an EFA-Grass-Morison Fellowship, Rappaport Fellowship and the National Institutes of Health: NIH-NS062092 to SSC.; NIH-NS018741 to EH.; EE was supported by NEI R01EY017658, NIDA R01NS063249, NSF IOB 0645886, the HHMI, and the Klingenstein Foundation; ENB was supported by DP1-0D003646. The information contained herein does not necessarily reflect the position or policy of the Government, and no official endorsement should be inferred.
Supplementary material
Supplementary material is available at Brain online.
Supplementary Material
Acknowledgements
The authors thank Alex Chan, Andrew Dykstra, Nima Dehghani, Justine Cormier, Chris Sherman, Jacob Donoghue, Caroline Raclin and Rodrigo Zepeda for their invaluable comments during the preparation of the manuscript. The authors are enormously indebted to the patients that participated in this study, as well as the nursing and physician staff at each facility.
Glossary
Abbreviations
- EEG
electroencephalogram
References
- Altafullah I, Halgren E, Stapleton JM, Crandall PH. Interictal spike-wave complexes in the human medial temporal lobe: typical topography and comparisons with cognitive potentials. Electroencephalogr Clin Neurophysiol. 1986;63:503–16. doi: 10.1016/0013-4694(86)90138-0. [DOI] [PubMed] [Google Scholar]
- Amirnovin R, Williams ZM, Cosgrove GR, Eskandar EN. Visually guided movements suppress subthalamic oscillations in Parkinson's disease patients. J Neurosci. 2004;24:11302–6. doi: 10.1523/JNEUROSCI.3242-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amzica F, Steriade M. Electrophysiological correlates of sleep delta waves. Electroencephalogr Clin Neurophysiol. 1998;107:69–83. doi: 10.1016/s0013-4694(98)00051-0. [DOI] [PubMed] [Google Scholar]
- Babb TL, Carr E, Crandall PH. Analysis of extracellular firing patterns of deep temporal lobe structures in man. Electroencephalogr Clin Neurophysiol. 1973;34:247–57. doi: 10.1016/0013-4694(73)90252-6. [DOI] [PubMed] [Google Scholar]
- Babb TL, Crandall PH. Epileptogenesis of human limbic neurons in psychomotor epileptics. Electroencephalogr Clin Neurophysiol. 1976;40:225–43. doi: 10.1016/0013-4694(76)90147-4. [DOI] [PubMed] [Google Scholar]
- Babb TL, Pretorius JK, Kupfer WR, Crandall PH. Glutamate decarboxylase-immunoreactive neurons are preserved in human epileptic hippocampus. J Neurosci. 1989;9:2562–74. doi: 10.1523/JNEUROSCI.09-07-02562.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babb TL, Wilson CL, Isokawa-Akesson M. Firing of human limbic neurons during stereoencephalography (SEEG) and clinical temporal lobe seizures. Electroencephalogr Clin Neurophysiol. 1987;66:467–82. doi: 10.1016/0013-4694(87)90093-9. [DOI] [PubMed] [Google Scholar]
- Baumgartner C, Lindinger G, Ebner A, Aull S, Sesles W, Olbrich A, Lurger S, Czech T, Burgess R, Lüders H. Propagation of interictal epileptic activity in temporal lobe epilepsy. Neurology. 1995;45:118–22. doi: 10.1212/wnl.45.1.118. [DOI] [PubMed] [Google Scholar]
- Blume WT. Current trends in electroencephalography. Curr Opin Neurol. 2001;14:193–7. doi: 10.1097/00019052-200104000-00010. [DOI] [PubMed] [Google Scholar]
- Blume WT, Holloway GM, Wiebe S. Temporal epileptogenesis: localizing value of scalp and subdural interictal and ictal EEG data. Epilepsia. 2001;42:508–14. doi: 10.1046/j.1528-1157.2001.02700.x. [DOI] [PubMed] [Google Scholar]
- Blume WT, Kaibara M. Localization of epileptic foci in children. Can J Neurol Sci. 1991;18:570–2. doi: 10.1017/s0317167100032728. [DOI] [PubMed] [Google Scholar]
- Bower MR, Buckmaster PS. Changes in granule cell firing rates precede locally recorded spontaneous seizures by minutes in an animal model of temporal lobe epilepsy. Neurophysiol. 2008;99:2431–42. doi: 10.1152/jn.01369.2007. [DOI] [PubMed] [Google Scholar]
- Cameron KA, Yashar S, Wilson CL, Fried I. Human hippocampal neurons predict how well word pairs will be remembered. Neuron. 2001;30:289–98. doi: 10.1016/s0896-6273(01)00280-x. [DOI] [PubMed] [Google Scholar]
- Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, et al. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–7. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash SS, Halgren E, Dehghani N, Rossetti AO, Thesen T, Wang C, et al. The human K-complex represents an isolated cortical down-state. Science. 2009;324:1084–7. doi: 10.1126/science.1169626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatrian GE, Bergamini L, Dondey M, Klass DW, Lennox-Buchthal M, Petersen I. A glossary of terms most commonly used by clinical electroencephalographers. Electroenceph Clin Neurophysiol. 1974;37:538–48. doi: 10.1016/0013-4694(74)90099-6. [DOI] [PubMed] [Google Scholar]
- Colder BW, Frysinger RC, Wilson CL, Harper RM, Engel J., Jr Decreased neuronal burst discharge near site of seizure onset in epileptic human temporal lobes. Epilepsia. 1996;37:113–21. doi: 10.1111/j.1528-1157.1996.tb00001.x. [DOI] [PubMed] [Google Scholar]
- de Curtis M, Avanzini G. Interictal spikes in focal epileptogenesis. Prog Neurobiol. 2001;63:541–67. doi: 10.1016/s0301-0082(00)00026-5. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–94. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Delgado-Escueta AV, Walsh GO. The selection process for surgery of intractable complex partial seizures: Surface EEG and depth electrography. In: Ward AA Jr, Penry JK, Purpura DP, editors. Epilepsy. New York: Raven press; 1983. pp. 295–326. [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Ekstrom AD, Kahana MJ, Caplan JB, Fields TA, Isham EA, Newman EL, et al. Cellular networks underlying human spatial navigation. Nature. 2003;425:184–8. doi: 10.1038/nature01964. [DOI] [PubMed] [Google Scholar]
- Engel J, Crandall PH, Rausch P. Surgical treatment of partial epilepsies. In: Rosenburg RN, Grossman RG, Schoclet S, editors. The clinical Neurosciences. New York: Churchill Livingston; 1983. pp. 1349–80. [Google Scholar]
- Engel J, Rausch R, Lieb JP, Kulh DE, Crandall PH. Correlation of criteria used for localizing epileptic foci in patients considered for surgical therapy of epilepsy. Ann Neurol. 1981;9:215–24. doi: 10.1002/ana.410090303. [DOI] [PubMed] [Google Scholar]
- Fabó D, Maglóczky Z, Wittner L, Pék A, Erö L, Czirják S, et al. Properties of in vivo interictal spike generation in the human subiculum. Brain. 2008;131:485–99. doi: 10.1093/brain/awm297. [DOI] [PubMed] [Google Scholar]
- Gale JT, Shields DC, Jain FA, Amirnovin R, Eskandar EN. Subthalamic nucleus discharge patterns during movement in the normal monkey and Parkinsonian patient. Brain Res. 2009;1260:15–23. doi: 10.1016/j.brainres.2008.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons J. Nonparametric Statistical Inference. New York: Marcel Dekker; 1985. [Google Scholar]
- Gibbs FA, Davis H, Lennox WG. The electroencephalogram in epilepsy and conditions with impaired consciousness. Archives of Neurology and Psychiatry. 1935;34:1133–48. [Google Scholar]
- Goldensohn ES, Purpura DP. Intracellular potentials of cortical neurons during focal epileptogenic discharges. Science. 1963;139:840–2. doi: 10.1126/science.139.3557.840. [DOI] [PubMed] [Google Scholar]
- Gotman J. Quantitative measurements of epileptic spike morphology in the human EEG. Electroenceph Clin Neurophysiol. 1980;48:551–7. doi: 10.1016/0013-4694(80)90290-4. [DOI] [PubMed] [Google Scholar]
- Hochberg LR, Serruya MD, Friehs GM, Mukand JA, Saleh M, Caplan AH, et al. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature. 2006;442:164–71. doi: 10.1038/nature04970. [DOI] [PubMed] [Google Scholar]
- Hogg RV LJ. Engineering statistics. New York: MacMillan Publishing; 1987. [Google Scholar]
- Hollander M, Wolfe DA. Nonparametric statistical methods. New York: Wiley; 1999. [Google Scholar]
- Holmes MD, Kutsy RL. Interictal, unifocal spikes in refractory extratemporal epilepsy predict ictal origin and postsurgical outcome. Clin Neurophysiol. 2000;111:1802–8. doi: 10.1016/s1388-2457(00)00389-8. [DOI] [PubMed] [Google Scholar]
- Isokawa-Akesson M, Wilson CL, Babb TL. Inhibition in synchronously firing human hippocampal neurons. Epilepsy Res. 1989;3:236–47. doi: 10.1016/0920-1211(89)90030-2. [DOI] [PubMed] [Google Scholar]
- Keller CJ, Cash SS, Narayanan S, Wang C, Kuzniecky R, Carlson C, et al. Intracranial microprobe for evaluating neuro-hemodynamic coupling in unanesthetized human neocortex. J Neurosci Methods. 2009;179:208–18. doi: 10.1016/j.jneumeth.2009.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooi KA. Voltage-time characteristics of spikes and other rapid electroencephalographic transients: semantic and morphological considerations. Neurology. 1966;16:59–66. doi: 10.1212/wnl.16.1.59. [DOI] [PubMed] [Google Scholar]
- Lewicki MS. Bayesian modeling and classification of neural signals. Neural Comput. 1994;6:1005–30. [Google Scholar]
- Lieb JP, Woods SC, Siccardi A, Crandall PH, Walter DO, Leake B. Quantitative analysis of depth spiking in relation to seizure foci in patients with temporal lobe epilepsy. Electroencephalogr Clin Neurophysiol. 1978;44:641–63. doi: 10.1016/0013-4694(78)90130-x. [DOI] [PubMed] [Google Scholar]
- Marsaglia G, Wang J. Evaluating Kolmogorov's Distribution. J Stat Software. 2003;8:1–4. [Google Scholar]
- Massey FJ. The Kolmogorov-Smirnov test for goodness of fit. J Am Stat Association. 1951;46:68–78. [Google Scholar]
- Matsumoto H, Ajmone Marsan C. Cellular mechanisms in experimental epileptic seizures. Science. 1964;144:193–4. doi: 10.1126/science.144.3615.193. [DOI] [PubMed] [Google Scholar]
- Merchant H, Naselaris T, Georgopoulos AP. Dynamic sculpting of directional tuning in the primate motor cortex during three-dimensional reaching. J Neurosci. 2008;28:9164–72. doi: 10.1523/JNEUROSCI.1898-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedermeyer E, Lopes da Silva F. Electroencephalography: Basic Principles, Clinical Applications and Related Fields. New York: Lippincott Williams and Wilkins; 1982. [Google Scholar]
- Pedley T. Epilepsy and the human electroencephalogram. In: Schwartzkroin P, Wheal H, editors. Pathophysiology of Epilepsy. London: Academic Press; 1984. p. 130. [Google Scholar]
- Penfield W, Jasper H. Epilepsy and the functional anatomy of the human brain. Boston: Little Brown; 1954. [Google Scholar]
- Prince DA, Wong RK. Human epileptic neurons studied in vitro. Brain Res. 1981;210:323–33. doi: 10.1016/0006-8993(81)90905-7. [DOI] [PubMed] [Google Scholar]
- Rayport M, Waller HJ. Technique and results of micro-electrode recording in human epileptogenic foci. Electroencephalogr Clin Neurophysiol Suppl. 1967;25:143+. [PubMed] [Google Scholar]
- Raz A, Vaadia E, Bergman H. Firing patterns and correlations of spontaneous discharge of pallidal neurons in the normal and the tremulous 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine vervet model of parkinsonism. J Neurosci. 2007;20:8559–71. doi: 10.1523/JNEUROSCI.20-22-08559.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schevon CA, Ng SK, Cappell J, Goodman RR, McKhann G, Jr, Waziri A, et al. Microphysiology of epileptiform activity in human neocortex. J Clin Neurophysiol. 2008;25:321–30. doi: 10.1097/WNP.0b013e31818e8010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzkroin PA, Turner DA, Knowles WD, Wyler AR. Studies of human and monkey “epileptic” neocortex in the in vitro slice preparation. Ann Neurol. 1983;13:249–57. doi: 10.1002/ana.410130305. [DOI] [PubMed] [Google Scholar]
- Staba RJ, Wilson CL, Bragin A, Fried I, Engel J., Jr Sleep states differentiate single neuron activity recorded from human epileptic hippocampus, entorhinal cortex, and subiculum. J Neurosci. 2002;22:5694–704. doi: 10.1523/JNEUROSCI.22-13-05694.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Amzica F. Coalescence of sleep rhythms and their chronology in corticothalamic networks. Sleep Res Online. 1998;1:1–10. [PubMed] [Google Scholar]
- Steriade M, Contreras D. Relations between cortical and thalamic cellular events during transition from sleep patterns to paroxysmal activity. J Neurosci. 1995;15:623–42. doi: 10.1523/JNEUROSCI.15-01-00623.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swadlow HA. Fast-spike interneurons and feedforward inhibition in awake sensory neocortex. Cereb Cortex. 2003;13:25–32. doi: 10.1093/cercor/13.1.25. [DOI] [PubMed] [Google Scholar]
- Truccolo W, Friehs GM, Donoghue JP, Hochberg LR. Primary motor cortex tuning to intended movement kinematics in humans with tetraplegia. J Neurosci. 2008;28:1163–78. doi: 10.1523/JNEUROSCI.4415-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulbert I, Halgren E, Heit G, Karmos G. Multiple microelectrode-recording system for human intracortical applications. J Neurosci Methods. 2001;106:69–79. doi: 10.1016/s0165-0270(01)00330-2. [DOI] [PubMed] [Google Scholar]
- Ulbert I, Heit G, Madsen J, Karmos G, Halgren E. Laminar analysis of human neocortical interictal spike generation and propagation: current source density and multiunit analysis in vivo. Epilepsia. 2004;45(Suppl 4):48–56. doi: 10.1111/j.0013-9580.2004.04011.x. [DOI] [PubMed] [Google Scholar]
- Ulbert I, Karmos G, Heit G, Halgren E. Early discrimination of coherent versus incoherent motion by multiunit and synaptic activity in human putative MT+ Hum Brain Mapp. 2001;13:226–38. doi: 10.1002/hbm.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verzeano M, Crandall PH, Dymond A. Neuronal activity of the amygdale in patients with psychomotor epilepsy. Neuropsychologia. 1971;9:331–44. doi: 10.1016/0028-3932(71)90029-7. [DOI] [PubMed] [Google Scholar]
- Walczac TS, Jayakar P. Interictal Electroencephalography. In: Engel J, Pedley TA, editors. Epilepsy: a comprehensive textbook. New York: Raven Press; 1997. pp. 831–48. [Google Scholar]
- Wang C, Ulbert I, Schomer DL, Marinkovic K, Halgren E. Responses of human anterior cingulate cortex microdomains to error detection, conflict monitoring, stimulus-response mapping, familiarity, and orienting. J Neurosci. 2005;25:604–13. doi: 10.1523/JNEUROSCI.4151-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward AA, Thomas LB. The electrical activity of single units in the cerebral cortex of man. Electroencephalogr Clin Neurophysiol. 1955;7:135–6. doi: 10.1016/0013-4694(55)90067-5. [DOI] [PubMed] [Google Scholar]
- Waziri A, Schevon CA, Cappell J, Emerson RG, McKhann GM, 2nd, Goodman RR. Initial surgical experience with a dense cortical microarray in epileptic patients undergoing craniotomy for subdural electrode implantation. Neurosurgery. 2009;64:540–5; discussion 545. doi: 10.1227/01.NEU.0000337575.63861.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson A, Spencer DD. Electrophysiological characterization of CA2 pyramidal cells from epileptic humans. Hippocampus. 1994;4:226–37. doi: 10.1002/hipo.450040213. [DOI] [PubMed] [Google Scholar]
- Worrell GA, Gardner AB, Stead SM, Hu S, Goerss S, Cascino GJ, et al. High-frequency oscillations in human temporal lobe: simultaneous microwire and clinical macroelectrode recordings. Brain. 2008;131:928–37. doi: 10.1093/brain/awn006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyler AR, Ojemann GA, Ward AA., Jr Neurons in human epileptic cortex: correlation between unit and EEG activity. Ann Neurol. 1982;11:301–8. doi: 10.1002/ana.410110311. [DOI] [PubMed] [Google Scholar]
- Ylinen A, Bragin A, Nadasdy Z, Jando G, Szabo I, Sik A, et al. Sharp wave-associated high-frequency oscillation (200 Hz) in the intact hippocampus: network and intracellular mechanisms. J Neurosci. 1995;15:30–46. doi: 10.1523/JNEUROSCI.15-01-00030.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.