Abstract
Antibodies to the N-methyl-d-aspartate subtype of glutamate receptor have been associated with a newly-described encephalopathy that has been mainly identified in young females with ovarian tumours. However, the full clinical spectrum and treatment responses are not yet clear. We established a sensitive cell-based assay for detection of N-methyl-d-aspartate receptor antibodies in serum or cerebrospinal fluid, and a quantitative fluorescent immunoprecipitation assay for serial studies. Although there was marked intrathecal synthesis of N-methyl-d-aspartate receptor antibodies, the absolute levels of N-methyl-d-aspartate receptor antibodies were higher in serum than in cerebrospinal fluid. N-methyl-d-aspartate receptor antibodies were of the immunoglobulin G1 subclass and were able to activate complement on N-methyl d-aspartate receptor-expressing human embryonic kidney cells. From questionnaires returned on 44 N-methyl-d-aspartate receptor antibody-positive patients, we identified a high proportion without a detected tumour (35/44, 80%: follow-up 3.6–121 months, median 16 months). Among the latter were 15 adult females (43%), 10 adult males (29%) and 10 children (29%), with four in the first decade of life. Overall, there was a high proportion (29%) of non-Caucasians. Good clinical outcomes, as defined by reductions in modified Rankin scores, correlated with decreased N-methyl-d-aspartate receptor antibody levels and were associated with early (<40 days) administration of immunotherapies in non-paraneoplastic patients (P < 0.0001) and earlier tumour removal in paraneoplastic patients (P = 0.02). Ten patients (23%) who were first diagnosed during relapses had no evidence of tumours but had received minimal or no immunotherapy during earlier episodes. Temporal analysis of the onset of the neurological features suggested progression through two main stages. The time of onset of the early features, characterized by neuropsychiatric symptoms and seizures preceded by a median of 10–20 days, the onset of movement disorders, reduction in consciousness and dysautonomia. This temporal dichotomy was also seen in the timing of cerebrospinal fluid, electroencephalographic and in the rather infrequent cerebral imaging changes. Overall, our data support a model in which the early features are associated with cerebrospinal fluid lymphocytosis, and the later features with appearance of oligoclonal bands. The immunological events and neuronal mechanisms underlying these observations need to be explored further, but one possibility is that the early stage represents diffusion of serum antibodies into the cortical grey matter, whereas the later stage results from secondary expansion of the immunological repertoire within the intrathecal compartment acting on subcortical neurons. Four patients, who only had temporal lobe epilepsy without oligoclonal bands, may represent restriction to the first stage.
Keywords: N-methyl-d-aspartate receptor-antibody encephalitis, autoimmunity, non-paraneoplastic, paraneoplastic, immunotherapy-responsive
Introduction
Human auto-antibodies directed against neuronal proteins are of diagnostic importance in defining immunotherapy-responsive neurological diseases. Auto-antibodies with specificity towards intracellular proteins act as diagnostic markers of diseases that are usually associated with an underlying cancer and are rarely immunotherapy-responsive. By contrast, antibodies that target the extracellular domain of surface-expressed neuronal proteins are likely to be pathogenic and their presence usually indicates the possibility of successful immunotherapy. In the peripheral nervous system, it is well established that such antibodies are pathogenic (Vincent et al., 2006).
It is increasingly recognized that some CNS disorders can also be antibody-mediated. Antibodies to voltage-gated potassium channels are often associated with limbic encephalitis, presenting with seizures, amnesia and medial temporal lobe inflammation. These patients do not usually have an underlying neoplasm, and do well following immunotherapies, with a substantial and sometimes complete recovery (Buckley et al., 2001; Thieben et al., 2004; Vincent et al., 2004). Moreover, with the expansion of the voltage-gated potassium channels-antibody-associated phenotype to patients whose diagnosis was drug-resistant epilepsy, new immunotherapy-responsive seizure phenotypes are beginning to be recognized (McKnight et al., 2005; Irani et al., 2008).
More recently, antibodies against the N-methyl-d-aspartate subtype of ionotropic glutamate receptors [N-methyl-d-aspartate receptor (NMDAR) antibodies] have been reported, predominantly in young women who developed a subacute-onset encephalopathy, commonly associated with a prominent movement disorder and frequently an underlying ovarian teratoma (Dalmau et al., 2007), although two recent reports suggest a much higher incidence of non-paraneoplastic cases in children (Dale et al., 2009; Florance et al., 2009). Removal of the teratoma combined with immunotherapy resulted in substantial recovery, but in the minority of patients without a tumour, recovery appeared to be less impressive (Dalmau et al., 2007, 2008). In those studies, the age range was wide, but only 9% of the patients were males.
The full clinical spectrum associated with NMDAR antibodies is likely to widen with increasing recognition. Here we describe an assay suitable for both serum and CSF detection of the antibodies, modified from that of Dalmau et al. (2008), which allowed us to identify 50 patients in the UK and Europe in 2008. Clinical details were gathered on 44 patients and allowed us to correlate serum NMDAR antibodies with clinical outcomes using a novel quantitative fluorescent assay, and to demonstrate the detailed temporal progression of clinical and paraclinical features. Our observations emphasize the importance of early intensive immunotherapies and suggest that the neurological disease occurs in two main stages.
Materials and methods
Clinical data
Ethical approval for this study was from the Oxfordshire Regional Ethical Committee A (07/Q1604/28). Serum and CSF samples were referred for testing by clinicians, mainly from the United Kingdom and Europe. Standardized clinical questionnaires (Supplementary data), patient information sheets and consent forms were distributed to the clinicians referring the first 50 NMDAR-antibody-positive samples from a total of 450 sent for testing. We requested details of clinical features, investigation results and serial modified Rankin scores (Graus et al., 2001). Clinicians were asked to document and detail the timing of specific features such as higher cognitive dysfunction, psychiatric, seizure and movement disorders: we have maintained the categorizations as provided by the clinicians. Eight of the patients have been described in case reports (Schimmel et al., 2009; Zandi et al., 2009; Davies et al., 2010). All data were analysed using GraphPad Prism 5 and the tests used are mentioned in the figures and tables. Thirty-six questionnaires were returned, and eight were completed from clinic letters, emails or telephone conversations.
Cell-based assay for antibodies binding human NMDAR
The NMDAR1 subunit (NR1, Genecopoeia catalogue number EX-XO451-M02) and NMDAR2B subunit (NR2B, IMAGE clone number 8322670) complementary (c)DNAs were modified by in vitro mutagenesis to match the respective GenBank ID consensus sequences NM_000832 (GRIN1-1a) and BC113618 (GRIN2B). The NR2B subunit was subcloned from pCRXL-TOPO into the mammalian expression vector pcDNA3.1hygro(–) using a BamH1/Not1 restriction digest. An enhanced green fluorescent protein (EGFP)-tagged human NR1-1 splice variant was also generated. To create the expression construct for this, the C-terminal stop codon was removed, an NheI restriction site generated and the coding sequence for EGFP inserted with a GT(GS)4 linker between the C-terminus of the NR1-1 and the beginning of the EGFP.
For the cell-based assay, human embryonic kidney (HEK293) cells were grown on glass coverslips in Dulbecco’s modified Eagle’s medium with 10% foetal calf serum and penicillin, streptomycin and amphotericin. After 24 h, cells were transfected, using polyethylenimine and glucose, with untagged-NR1 and NR2B cDNA at a ratio of 3:1. An EGFP expression vector was co-transfected to visualize cells taking-up cDNAs. To prevent cytotoxicity as a result of glutamate in the medium activating the NMDARs, cells were supplemented with 500 µM ketamine 16 h post-transfection. Live cells were incubated with patient sera (1:20) or undiluted CSF for 1 h before fixation (3% formaldehyde) followed by 35 min incubation with Alexa Fluor 568 anti-human immunoglobulin G (IgG). Cells were subsequently washed three times in phosphate buffered saline and mounted on slides in fluorescent mounting medium (DakoCytomation, Cambridge, UK) containing DAPI (4′,6′-diamidino-2-phenlindoledichloride, 1:1000). They were visualized using a fluorescence microscope with a MacProbe v4.3 digital imaging system and the binding scored on a scale from 0–4 by two independent observers (Supplementary Fig. 1A; as in Leite et al., 2008; Waters et al., 2008). All positive samples were retested on muscle-specific-kinase or glycine α1 receptor transfected cells to exclude non-specific binding to the human embryonic kidney cells. For subclass experiments, cells were incubated with isotype-specific mouse antibodies and subsequently with an Alexa Fluor 568 anti-mouse IgG. For immunoabsorption, the sera (1:20 dilution) were preincubated with 5 million trypsinized EGFP-NR1-expressing human embryonic kidney cells for 30 min at 4°C before testing for binding as above.
For detection of deposited complement on transfected cells, the live cells were incubated with heat-inactivated sera (nine NMDAR-antibody positive and eight healthy individuals) and fresh human plasma, as a source of complement (as in Leite et al., 2008; Waters et al., 2008). After 30 min at 37°C the cells were kept on ice, fixed with 3% formaldehyde, incubated with rabbit antibodies against C3b (DAKO; 1:500), or polyclonal rabbit anti-C5b9 (membrane attack complex; kind gift of Prof BP Morgan; 1:100) and Alexa Fluor 568 anti-rabbit IgG (1:1000). Binding to the cells was scored as positive or negative by a blinded observer (PW).
Fluorescent immunoprecipitation assay for antibodies to NMDAR
Human embryonic kidney 293 cells were transfected as above with NR2B and postsynaptic density 95 (PSD95, Open Biosystems, Image Consortium cDNA clones, USA) cDNA expression constructs and with the NR1-EGFP tagged expression construct in a ratio of 1:1:1. PSD95 was used because it improved NR1-EGFP expression in preliminary studies (data not shown). Cells were solubilized using a buffer containing 1% digitonin. A protease inhibitor cocktail (Sigma-Aldrich, UK) was added to this buffer immediately before use. The supernatant was analysed using antibodies to NR1 (Millipore, USA), NR2B (kind gift from Prof F Anne Stephenson, School of Pharmacy, University of London) and PSD95 (Neuromab, USA), and analysis of EGFP fluorescence in fractions following sedimentation on a continuous sucrose gradient (Waters et al., 2008). For serological studies, 25 µl of serum was incubated with 250 µl of solubilized extract overnight at 4°C, as described previously (Waters et al., 2008). Protein-A beads were rotated with the antigen-antibody mixture for 2 h at room temperature. The beads were washed and the captured fluorescence was measured using a fluorescent plate reader (Gemini XS, Molecular Probes). Results were expressed as relative fluorescent units precipitated by 25 µl serum and are reported as fU.
Results
Detection of NMDAR antibodies
We initially used three assays for the detection of NMDAR antibodies (Fig. 1A–C; ‘Materials and methods’ section in Supplementary data) based on those reported previously (Dalmau et al., 2007; Niehusmann et al., 2009). Rat brain sections were incubated with undiluted CSF to look for hippocampal ‘neuropil’ binding (Fig. 1A). To confirm the neuronal cell surface specificity of the antibodies and, thus, their likely clinical relevance, we incubated primary cultures of hippocampal neurons with sera and CSF to observe binding to the surface of live neurons (Fig. 1B). We established an immunofluorescent cell-based assay (Fig. 1C) for detection of NMDAR antibodies, modified from that described previously (Dalmau et al., 2007). Specifically, we employed a 3:1 ratio of NR1:NR2B cDNA (with small amounts of EGFP cDNA) as this gave better results with positive sera (Supplementary Fig. 1B) and the NR1 subunit is thought to contain the main epitope (Dalmau et al., 2008). To observe binding only to the extracellular domain of the NR1/NR2B, we did not permeabilize the cells and applied serum diluted 1:20 and CSF undiluted. Using a semi-quantitative approach similar to that employed for binding of antibodies to other cell surface antigens (Leite et al., 2008; Waters et al., 2008), the binding was scored visually from 0 (no binding) to 4 (very strong binding) by two independent observers. All positive results were retested, and also checked for non-specific binding to the cells using human embryonic kidney cells transfected with other antigens (see ‘Materials and methods’ section). Examples of different scores are shown in Fig. 1C and Supplementary Fig. 1A. Sera from 32 healthy individuals and from 138 disease controls (with other forms of autoimmune encephalopathy, chronic psychosis, multiple sclerosis, lupus, opsoclonus–myoclonus, herpes encephalitis or seizures) gave values of <1 (range 0–0.5, mean 0.01; median 0) (data not shown).
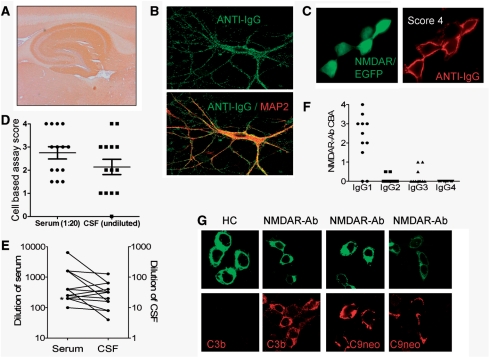
Figure 1.
Detection and characterization of NMDAR antibodies. (A) NMDAR-antibody-positive CSF shows a hippocampal ‘neuropil’ binding pattern on rat brain sections. (B) NMDAR-antibody-positive serum IgG (green) showing surface binding to primary cultures of live hippocampal neurons identified by the neuronal marker microtubule associated protein 2 (MAP2, in red, within merged lower image; 600× magnification). (C) Cell-based assay for NR1/NR2B (NMDAR) antibodies, using transfected human embryonic kidney (HEK293) cells identified by EGFP (enhanced green fluorescent protein) cDNA co-transfection (green), shows surface binding of patient sera (anti-IgG, red). This sample was scored as ‘4’ by visual observation (600× magnification). (D) Cell-based assay scores from 14 paired CSF-serum samples; note one CSF was negative. (E) Values from end-point titrations of the 14 sera and positive paired CSFs in the cell-based assay; note the different vertical scales and that serum levels of NMDAR antibodies were higher than CSF levels. Two of the data pairs overlapped and their values have been slightly adjusted so that the points are visible. Asterisk indicates one data pair could not be plotted as the CSF was negative (D). (F) NMDAR antibodies were found to be predominantly of the IgG1 subclass (n = 11). (G) NMDAR-antibody-positive sera (NMDAR-Ab) but not control sera (HC) were able to deposit complement C3b and C9neo, the membrane attack complex, on human embryonic kidney cells expressing NMDAR (1000× magnification).
We found the cell-based assay to be most sensitive and, as it is specific for NMDAR antibodies, it was used for testing the 450 sera and 35 CSF samples sent for NMDAR-antibody testing over the period of study (results shown in Supplementary Fig. 1C). The scores of 14 paired samples from NMDAR-antibody-positive patients are shown in Fig. 1D; one CSF was negative while the paired serum was positive (the remaining 21 paired samples were negative in both CSF and serum). Overall serum at 1:20 was found to be slightly more sensitive than undiluted CSF (Fig. 1D), and many patients were diagnosed on the basis of the serum antibody alone.
When we serially diluted the 14 available paired samples to find detection endpoints, NMDAR-antibody titres were between 6 and >50 times higher (mean 13.5) in serum than CSF (Fig. 1E). Except in one patient, there was clear evidence of intrathecal synthesis of NMDAR antibodies (median 21.7, range 0–118, n = 10 pairs; Supplementary Table 1).
NMDAR antibodies were found to be predominantly of the complement-fixing IgG1 subclass (Fig. 1F) and were able to deposit complement C3b (9/9 NMDAR antibodies; 0/8 control sera) and C9neo, the membrane attack complex (6/9 patients, 0/8 controls), on NMDAR-transfected human embryonic kidney cells (Fig. 1G), as expected for this IgG subclass.
A quantitative assay for antibodies against the NR1 subunit
In order to measure NMDAR-antibody levels more quantitatively, we established a fluorescent immunoprecipitation assay similar to that reported for AQP4 antibodies (Waters et al., 2008). We found the best expression of EGFP-NR1 occurred when it was co-expressed with NR2B and PSD95. The cells were solubilized in a buffer containing 1% digitonin, and the EGFP-NR1 could be immunoprecipitated by anti-NR1 antibodies but not by anti-NR2B or anti-PSD95 antibodies (Fig. 2A). The main EGFP-tagged material sedimented on a sucrose gradient (Supplementary Fig. 2A) with a peak corresponding to 280 kDa. This suggested that the major component is a dimer of NR1-EGFP (predicted size 274 kDa) rather than a tetramer containing both NR1 and NR2B.
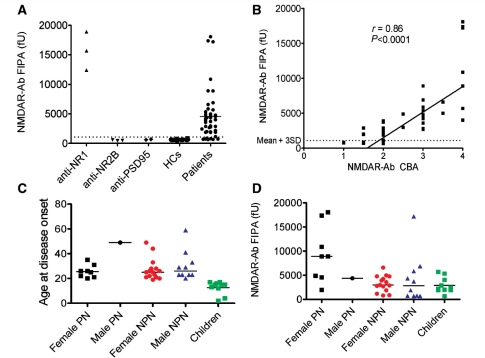
Figure 2.
Quantitative analysis of NMDAR antibodies and subgroups of NMDAR-antibody-positive patients. (A) The fluorescent immunoprecipitation assay. NR1-specific commercial antibodies (anti-NR1) precipitated large amounts of EGFP-NR1 which was not bound by NR2B-specific (anti-NR2B) or PSD95 (anti-PSD95) antibodies (data from three experiments). Eighty-four percent of 44 NMDAR-antibody-positive patients (as determined by the cell-based assay) precipitated EGFP-NR1 at levels greater than the mean plus three standard deviations (mean + 3SD) of results from 20 healthy controls (HCs). (B) There was a strong correlation between the NMDAR-antibody levels determined by the fluorescent immunoprecipitation assay and by the cell-based assay (r = 0.86, P < 0.0001; Spearman rank correlation). (C) The ages at disease onset of the 44 NMDAR-antibody-positive patients shown as male and female paraneoplastic (PN), non-paraneoplastic (NPN), and children (none of whom had tumours detected). (D) The results of the fluorescent immunoprecipitation assay showed the NMDAR-antibody levels were higher in paraneoplastic when compared to all non-paraneoplastic cases (P = 0.0017, Mann-Whitney U-test). fU = relative fluorescence units precipitated by 25 µl of serum.
Eighty-four percent of the cell-based assay-positive sera immunoprecipitated the EGFP-NR1-dimers at levels greater than the mean + 3SD (970 fU) of the healthy control sera (Fig. 2A), and the values correlated with results of the cell-based assay (Fig. 2B, P < 0.0001, r = 0.86). However, the fluorescent immunoprecipitation assay was negative in seven of the sera tested and, therefore, was used only for quantitative serial estimations on individual patients (see below). Because eight of the cell-based assay scores were relatively low (1 or 1.5), including five negative by fluorescent immunoprecipitation assay, we re-analysed the binding to NMDAR (Supplementary Table 2). All eight sera were again positive for binding to NR1/NR2B transfected cells, but they also bound to cells expressing NR1-EGFP alone, and the binding was substantially reduced by prior absorption of the sera with cells expressing NR1 (Supplementary Fig. 2B). These results confirmed the specificity of the antibodies for NMDAR and for the NR1 (Dalmau et al., 2008), but we continue to use the NR1/NR2B combination for diagnostic assays in order not to miss any possible binding to NR2B.
Patients with positive NMDAR antibodies
Overall, 50 of the 450 referred sera were positive for NMDAR antibodies with scores between 1 and 4. Of the 44 patients from whom clinical data were obtained (88%), 10 were identified retrospectively from sera sent over the preceding three years for other antibody tests. The remaining 34 presented over the first 10 months of 2008. Samples were referred from centres in the UK (n = 28), Germany (n = 8), the rest of Europe (n = 6) and elsewhere (n = 2). Interestingly, 8 of the 28 patients (29%) seen in the UK were non-Caucasian (two Chinese and one each from Pakistan, Malaysia, Nigeria, India, Iraq and Singapore).
Using assays in routine clinical use, all NMDAR-antibody-positive sera were negative for antibodies against Hu, Yo, Ri, CV2, Ma/Ma2, amphiphysin, glutamic acid decarboxylase and voltage-gated calcium channels. Two patients had low levels of voltage-gated potassium channel antibodies (197 and 241 pM, normal <100 pM) and four had anti-thyroid peroxidase antibodies (137–416, normal <32 IU/ml).
Demographics and tumour association
The ages at onset, stratified for sex and for presence or absence of tumours, are shown in Fig. 2C. Among 44 patients, 31 were females (70%; ages ranging from 2 to 49 years, median 22 years). These had all undergone intensive whole body/pelvic imaging, but strikingly there were only eight female patients with ovarian teratomas (26%; ages between 20 and 35 years), and no other tumours in females. Among the 13 males (age range 4–59, median 23 years) there was only one tumour, a recurrence of a previously-treated Hodgkin’s lymphoma at 49 years of age. Of the 23 females and 12 males without detectable tumours, 10 (eight females, two males) were under 18 years at presentation. The youngest were a 2-year-old girl and a 4-year-old boy. It is possible that tumours may still be found, but all have been negative to date on whole body CT (35 cases), pelvic ultrasound (13 cases), pelvic MRI (eight cases), whole body PET (14 cases) and often more than one imaging modality (26 cases) (follow-up 3.6-121 months, median 16 months).
Clinical features of 44 patients
The full description (summarized in Table 1) relates to the 44 patients, out of the first 50 identified, on whom detailed clinical data were provided by the referring neurologists. Between 1 and 21 days (median 7) before the onset of neurological disease, 11 (25%) patients, including two adults with ovarian teratomas and seven children, developed an infectious episode. Commonly the prodrome consisted of an upper-respiratory tract infection (n = 6; one mycoplasma IgM, two anti-streptolysin-O antibodies), diarrhoeal illness (n = 2; one with Campylobacter jejuni IgM), one meningitic presentation, and one infected mole. One 13-year-old female received diphtheria/tetanus/pertussis vaccination one day prior to seizure onset. Prodromal headaches or fever were seen in nine of these patients (20%), but more commonly headache and fever occurred after the onset of seizures or neuropsychiatric features (see below for detailed time course).
Table 1.
Typical clinical features in 44 NMDAR-antibody positive patients
| Early features |
| Higher cognitive dysfunction 40/44 (91%): confusion 29, behavioural changes 20, amnesia 14, dysphasia 13 |
| Psychiatric 34/44 (77%): hallucinations 22, psychotic 20, agitation 18, depressive 12, anxiety 10, obsessive 1 |
| Seizures 36/44 (82%): generalized 33, complex partial 16, simple partial 12 |
| Later features |
| Spontaneous reduction in conscious level 20/44 (45%) |
| Movement disorder 39/44 (89%): choreoathetoid 30 (orofacial 27, upper limbs 22, lower limbs 10), parkinsonian 13, rigidity 10, myoclonus 7, oculogyric crises 3, opisthotonus 3, startle 2 |
| Dysautonomia 32/44 (72%): tachy/brady-cardia 22, hyperhidrosis 12, persistent pyrexia 10, central hypoventilation 7, labile/high blood pressure 6, hypersalivation 4, pseudoobstruction 3, cardiac asystole 2 |
Total numbers of patients/total number (%). Anxiety was found as an isolated feature, without psychosis or depression, in only two cases. Data were provided by the referring neurologists. Although shown as early and later, there were some individuals in whom this distinction was not evident. Data from individual patients are shown in Fig. 6 and Supplementary Fig. 3.
As described by Dalmau et al. (2007, 2008), the most common presenting features included seizures, confusion, amnesia, behavioural changes and psychosis. In our series only 8 of 44 patients presented to a psychiatrist, and all were managed by neurologists. Rarer presenting features included hyperacusis, deafness, ataxia and dystonia. The most distinctive clinical features occurred later and included involuntary choreoathetoid orofacial movements, tachy- or bradycardia and a spontaneous fall in conscious level; central hypoventilation occurred in only seven patients. In Table 1, we describe these clinical features divided into six categories with the percentage of patients exhibiting each feature, divided into early and later categories (see below).
Most patients progressed to a severe clinical syndrome and required admission to intensive care. However, three patients had relatively mild syndromes, and four patients, two males (both aged 23) with 4-year histories of drug-resistant temporal lobe epilepsy, and two females (aged 17 and 33 years) who presented with an acute-onset of complex partial status epilepticus, had had minimal or no cognitive involvement and did not develop any movement disorders or other features consistent with the later stages of the disease. None of these four patients had a malignancy detected and they had some of the lower NMDAR-antibody levels (cell-based assay scores 1–2; for details see Supplementary Table 2).
Comparison of patient subgroups
Quantitative antibody titres on serum samples are shown in Fig. 2D. The fluorescent immunoprecipitation assay values were higher in the paraneoplastic than in the non-paraneoplastic patients (P = 0.0017, Mann–Whitney U-test; Table 2). There were also a few clinical features that were less evident in the non-paraneoplastic compared with the paraneoplastic patients (Table 2). Of the 44 patients, 10 including eight females, were under age 18 at onset. The most common presenting features in children were seizures and behavioural changes. All children developed neuropsychiatric features and 80% developed a movement disorder, not obviously different from the adult patients. The incidence of preceding infections was, however, higher in children (P = 0.0008) and none had detectable tumours.
Table 2.
Clinical features that differed in frequency between paraneoplastic and non-paraneoplastic patients
| Paraneoplastic, n = 9 (%) | Non-paraneoplastic, n = 35 (%) | P-valuea | |
|---|---|---|---|
| Initial NMDAR-antibody level (mean and range)b | 7855 (948–17 070) | 2255 (0–16 208) | 0.001 |
| Confusion | 9 (100) | 20 (58) | 0.02 |
| Agitation | 8 (89) | 10 (29) | 0.001 |
| Amnesia | 6 (67) | 8 (24) | 0.02 |
| Spontaneous reduction in conscious level | 8 (89) | 12 (35) | 0.008 |
Relapses were found in 10 of the non-paraneoplastic cases compared with none of the paraneoplastic cases, but this difference did not reach significance.
a Fisher’s exact test (two-tailed) was used for the comparisons (P-values have not been corrected for multiple comparisons).
b Measured using fluorescent immunoprecipitation assay with solubilised enhanced green fluorescent protein-tagged NR1 subunit.
Investigations
CSF analysis revealed lymphocytosis in only 30/44 (68%) of patients. CSF lymphocytosis was absent in samples from 35 days after symptom onset (Fig. 3A), except in those who underwent a clinical relapse (data not shown). By contrast, CSF-specific (unmatched) oligoclonal bands were only present in 9% at first CSF sampling but appeared later in the disease course in another 43% (see below).
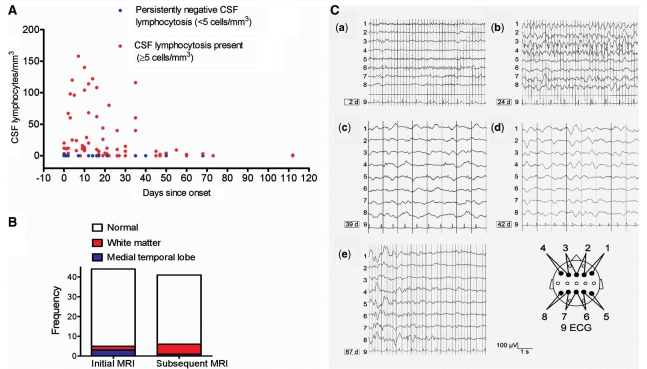
Figure 3.
Imaging, cerebrospinal fluid and EEG results. (A) The extent of CSF lymphocytosis (>5 cells/mm3, red dots) observed at different time points across all patients. Thirt-two percent of patients had persistently negative CSF lymphocytosis (<5 cells/mm3, blue dots). (B) MRI was commonly normal (white) and abnormalities were mainly restricted to white matter tracts (n = 6, red) and hippocampi (n = 4, blue) both at initial (median Day 1) and subsequent (median Day 25) imaging. (C) Serial electroencephalograms (EEGs) of a 17-year-old non-paraneoplastic female NMDAR-antibody-positive patient, not within our cohort of 44 cases. Bipolar transverse EEG recordings are shown in the scheme below. (a) EEG was normal after the patient had experienced a generalized convulsion. From Day 21–24, she suffered from frequent complex partial seizures; (b) EEG showed frequent, in part long-lasting frontal epileptiform spike-wave activity; (c–d) subsequently, there was continuous diffuse high-amplitude slowing without epileptiform potentials. From Day 39 on, no further seizures were observed. On Day 42, anti-epileptic therapy consisted of phenytoin (blood level: 25.4 µg/ml), phenobarbital (29.7 µg/ml) and lorazepam (2 mg daily dose). Glucocorticosteroids were instituted on Day 38. From Day 56, the patient recovered; and (e) normal EEG appearances recurred in sections in between abnormally high waves.
Brain imaging was normal in 39/44 (89%) at initial MRI and remained normal in 34/44 (77%; Fig. 3B). The few imaging abnormalities were in the hippocampi (n = 4) or within white matter regions (n = 6) on T2/fluid attenuated inversion recovery sequences. One non-paraneoplastic male with long-standing drug-resistant epilepsy showed hippocampal sclerosis at 4-year follow-up. Results of brain PET were limited, but it was abnormal in all three patients tested, with frontotemporal, occipital and cerebellar hypermetabolism reported at times when the MRI was normal.
Electroencephalography (EEG) demonstrated epileptiform discharges in 22/44 (50%) patients, usually early during the course of the disease, whereas generalized slowing in the slow theta or delta range was found in 35/44 (80%) patients, generally later during the disorder. Serial EEGs from one additional patient with NMDAR antibodies are shown in Fig. 3C, demonstrating the transition of epileptiform potentials to generalized slowing occurring between 24 and 42 days, followed by normalization at 87 days.
Clinical outcomes and serial estimations of antibodies against the native NR1 subunit
To measure clinical outcomes, the modified Rankin score was reported by the attending clinicians at peak of disease severity and at least two further time points. 37 of 44 (84%) of the patients had a score of 5 [severe disability with total dependency requiring constant attention (Graus et al., 2001)] at peak of disease, and average duration of hospitalization was 160 days (range 16–850). Thirty-five of the patients were treated with immunotherapy: glucocorticosteroids (33), intravenous immunoglobulins (15), plasma exchange (13), cyclophosphamide (4), rituximab (2), azathioprine (1) and mycophenolate mofetil (1), or a combination of the above (23).
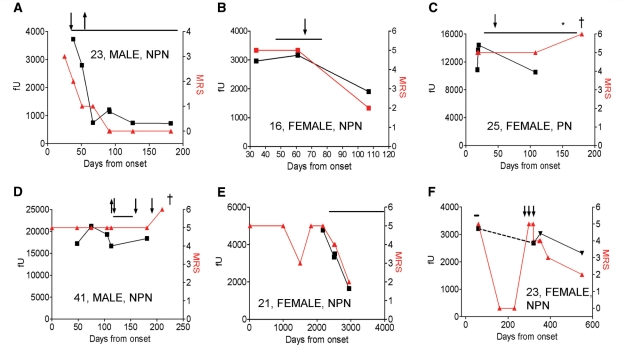
Six examples of serial NMDAR antibodies and modified Rankin scores are shown in Fig. 4 and corresponding clinical vignettes are given in the Supplementary data. Overall there was a good correlation within a patient between the level of serum NMDAR antibody and the modified Rankin scores. Two patients who did well had substantial contemporaneous falls in NMDAR-antibody levels (Fig. 4A and B), whereas two who died had very high absolute antibody levels (>10 000 fU) that persisted despite immunotherapy (Fig. 4C and D). One of these was a patient with an ovarian tumour (Fig. 4C) that was not removed until Day 180. Two patients who were first diagnosed during a relapse (Fig. 4E and F) are discussed below.
Figure 4.
(A–F) Effects of immunotherapy [plasma exchange (downward arrow), glucocorticosteroids (solid lines) and intravenous immunoglobulins (upward arrow)] and oophorectomy (asterisk) on NMDAR-antibody titres [fluorescent units precipitated (fU), black] and on clinical outcomes (modified Rankin scores, red). Death is indicated by ‘dagger’. Age, sex and neoplasm status (paraneoplastic = PN, non-paraneoplastic = NPN) are shown within each figure. (A) and (B) show reductions in NMDAR antibodies correlating with clinical outcome after successful immunotherapy, although antibodies persist in (B). (C) and (D) show two patients who died with very high NMDAR-antibody levels persisted throughout the illness despite immunotherapies (C and D) and late oophorectomy (C). (E) and (F) show patients with clinical relapses after no immunotherapy (E) or only 3 days of intravenous glucocortocosteroid therapy (F) during their first episode. Both patients showed clinical and serological improvement after immunotherapy administered during their second episode. In (F), the dotted line represents unknown titres between episodes. More detailed vignettes of these cases are available in the Supplementary data. MRS = modified Rankin score.
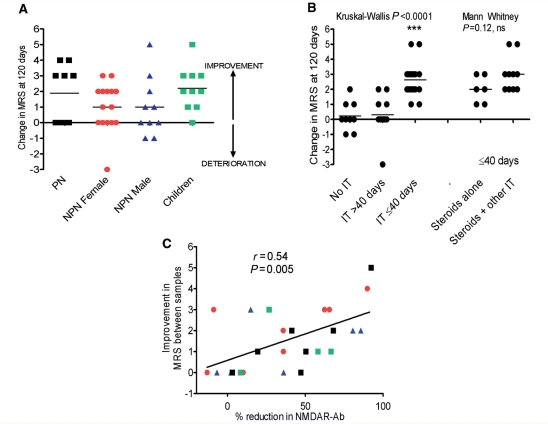
The clinical outcomes in all 44 patients are shown in Fig. 5A. Overall, there was no difference in the outcomes between the paraneoplastic, non-paraneoplastic and childhood cases. However, within the paraneoplastic group, there was a correlation between improvement and time to oophorectomy (r = −0.8, P = 0.02, Spearman rank correlation, data not shown; see Dalmau et al., 2008). Importantly, within the non-paraneoplastic group, those patients administered no immunotherapy or only treated after 40 days, did significantly less well than those treated before 40 days (Kruskal–Wallis, P < 0.0001; Dunn’s multiple comparisons P < 0.05). There was a trend (P = 0.12, Mann–Whitney U-test) towards better outcomes when glucocorticosteroids were combined with at least one other immunotherapy rather than given alone (Fig. 5B; additional immunotherapies are listed in the figure legend). Overall, there was a correlation between clinical improvement and reduction in NMDAR antibodies for all 25 patients on whom sequential samples were available (Fig. 5C; r = 0.54, P = 0.005, Spearman rank correlation).
Figure 5.
Clinical outcomes and therapeutics. (A) The change in modified Rankin score (MRS) at 120 days after onset of symptoms divided into subgroups. (B) The improvement in modified Rankin scores at 120 days after onset of symptoms in non-paraneoplastic patients according to administration of no immunotherapy (No IT), any immunotherapy after 40 days (IT > 40 days), or before or at 40 days (IT ≤ 40 days). The other immunotherapies were intravenous lg (n = 4), plasma exchange (n = 2), intravenous lg and plasma exchange (n = 3) or cyclophosphamide (n = 1). (Kruskal–Wallis, P < 0.0001; ***Dunn’s multiple comparison test P < 0.0001 for no IT or IT > 40 days compared to IT ≤ 40 days. For those administered IT at ≤40 days, there was a trend towards better outcomes when steroids plus other immunotherapies were given (Steroids alone v. Steroids + other IT; Mann Whitney, P = 0.02). (C) The correlation between percentage change in NMDAR-antibody levels determined by fluorescent immunoprecipitation assay and the corresponding change in modified Rankin scores over the time the two samples were taken (r = 0.54, P = 0.005; n = 25). Symbols as in (A).
Relapses
Importantly, 10 of 35 non-paraneoplastic patients experienced relapses (two to four relapses; time between relapses 3 months and 6 years) following some improvement after a previous episode. These were aged between 17 and 44 years (median 25.5 years, two males and eight females) and did not have an occult tumour after at least 14 months of careful follow-up. NMDAR antibodies were detected in all six patients who had samples stored from their previous clinical episode(s). Two examples of relapsing patients, both diagnosed during their second episode after limited immunotherapy had been given in the first episode, are shown in Fig. 4E and F. Overall, during their first episodes, five relapsers received no immunotherapy, three were only administered 3–5 days of intravenous glucocorticosteroids and two relapses occurred immediately after glucocorticosteroid withdrawal. By contrast, most paraneoplastic patients remained severely affected until tumour removal (one improved spontaneously).
Time course of the clinical features
We were struck by the time course of the presenting features and the paraclinical investigations, as shown in Fig. 6A. There was a striking uniformity in the median values of the times at which the seizures, psychiatric and cognitive disorders first appeared, usually within the first few days, whereas the median values for the later features of dysautonomia, movement disorders and fall in consciousness were delayed by ∼10–20 days. Data from individual cases are shown in Supplementary Fig. 3. In the majority, there were at least 5 days between the onset of each of the early features and the subsequent development of the later features. In addition, whereas headache or fever associated with a known infection (upper respiratory tract infection or diarrhoeal) preceded the onset of neurological symptoms by around 5 days, headache or fever without evidence of infection showed a similar time course to the later features.
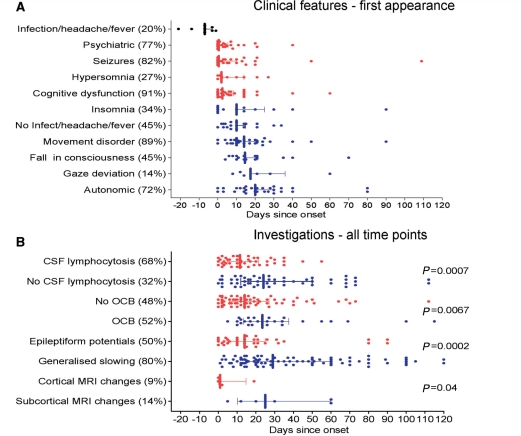
Figure 6.
Temporal progression of clinical features and investigations. (A) The times of first appearance of the main clinical features for each patient, up to 120 days after onset of neurological symptoms. (B) The times of all CSF pleocytosis or oligoclonal bands, and all abnormal EEG or MRI findings. Median and interquartile ranges are shown. P-values in B represent Mann–Whitney U-tests for each blue-red pairing. OCB = CSF-specific oligoclonal bands. Percentages (bracketed) show the frequency of each feature or investigation within the total NMDAR-antibody patient cohort.
To see whether the paraclinical investigations showed a similar dichotomy, we plotted all the available results for each patient against time after onset over the first 120 days (Fig. 6B) (although these data included a variable number of time points from different individuals, the data compare the presence or absence of a particular finding at each time point). Of most interest, was the early appearance of CSF lymphocytosis compared to the later absence of CSF lymphocytosis (P = 0.0007), contrasting with the early absence of CSF-specific oligoclonal bands compared to their later presence (P = 0.0067) (data for individual cases shown in Supplementary Fig. 4). There were also clear differences in the median times at which epileptiform discharges (P = 0.0002) occurred compared to generalized slowing, and the few cortical MRI changes were earlier than the equally uncommon subcortical MRI changes (P = 0.04).
Discussion
This first predominantly European series of NMDAR-antibody encephalitis broadens the published demographic and clinical features, details the timing of different clinical features and describes treatment responses of paraneoplastic and non-paraneoplastic cases. The condition was first identified in 2007 (Dalmau et al., 2007). In 100 cases described by Dalmau et al. (2008) the majority were female (91%) and 59% had ovarian tumours. By contrast, only 9/34 adult patients we studied had tumours, and 11/34 of the adults and 2/10 of the children were male. Here we further characterized the pathogenic potential of the antibodies and show that serial NR1-antibody levels correlate with clinical severity over time within individuals and across the cohort. Moreover, early immunotherapy appeared to be important in improving outcomes, reducing NMDAR-antibody levels and protecting against relapses, which occurred in 23%. By careful analysis of the temporal progression of the clinical features, we found consistent time-lags of 10–20 days between the early/presenting features and the later features that were accompanied by switches in the CSF, MRI and EEG findings, suggesting that the neurological disease occurs in two distinct clinical and neuropathological stages.
For diagnosis, we used a sensitive cell-based assay similar to those we developed for measuring antibodies to other cell surface antigens (Hutchinson et al., 2008; Leite et al., 2008; Waters et al., 2008). The higher proportion of non-paraneoplastic cases in this study compared to the previous report (Dalmau et al., 2008) may reflect the sensitivity of our cell-based assay for serological screening, and the wide referral base of the clinical neuroimmunology service in Oxford, UK. Perhaps partly for this reason, some of the patients had less characteristic syndromes. For instance, relatively low antibody binding scores were found in four adults who presented with seizures and did not progress further (see also one patient in Niehusmann et al., 2009). One of these subsequently developed hippocampal sclerosis, suggesting that NMDAR antibodies may be another cause of limbic encephalitis (Graus et al., 2008) that can progress to hippocampal sclerosis (Bien et al., 2007).
Twenty-three percent of our cases overall were children aged between 2 and 17, confirming the increasing numbers of children, often without tumours, who develop this antibody (Florance et al., 2009), some of whom had been given a previous diagnosis of encephalitis lethargica (Dale et al., 2009). Since some of the features and investigations overlap with those of other disorders, such as Hashimoto’s encephalitis (Castillo et al., 2006) and the paediatric autoimmune neuropsychiatric disorder associated with streptococcal infection (PANDAS, Church et al., 2003), the phenotype associated with this antibody may expand in both children and adults.
For serial measurements on sera we used immunoprecipitation of solubilized native NR1-EGFP dimers, but this assay would need further refinement for use in serological diagnosis or on CSF. An enzyme-linked immunosorbent assay (Dalmau et al., 2008) has been used successfully for serum and CSF studies, although at present we feel that the cell-based assay offers the best sensitivity and specificity for diagnosis. Using our NR1-EGFP immunoprecipitation assay, the nine paraneoplastic patients, eight of whom were females aged between 20 and 35 years with ovarian teratomas, had higher serum NR1-antibody levels than the non-paraneoplastic patients and did well after early tumour removal (as in Dalmau et al., 2008). Follow-up in the non-paraneoplastic patients, who accounted for 80% of our cohort, extend previous data (Dalmau et al., 2008; Florance et al., 2009) that reported a less good response to immunotherapy in the non-paraneoplastic patients. Our best outcomes were in those patients treated early (≤40 days), and given glucocorticosteroids and at least one other immunotherapy, whereas 9/10 of the relapsing patients received insubstantial or no immunotherapy during their previous episode(s). In some cases, positive NMDAR antibodies were still present after treatment of the first episode when only partial clinical remission had been obtained (two examples are shown in Fig. 4). This, together with the finding of high and persistent antibody levels in the three patients who died, and reduction of antibody titres in patients who improved, suggests that the condition needs to be treated more intensively and may need long-term maintenance immunosuppression. This contrasts with voltage-gated potassium channel antibodies which, in general, fall rapidly following similar immunotherapy (Thieben et al., 2004; Vincent et al., 2004), although occasionally patients do relapse (Irani and Vincent, unpublished observations). With an average hospital stay of 160 days, long periods of ventilation and multiple infectious complications, this condition is now becoming a recognized and relatively frequent cause of admission to intensive care (Davies et al., 2010).
The idea that this condition could be ‘multi-stage’ was suggested previously on the basis of four female patients (Iizuka et al., 2008), but the results of our detailed analyses of the timing of clinical features and investigations combine to support a model of the disease occurring in two main stages. The commonest presenting features (seizures, confusion, amnesia and psychosis) seen in 41/44 patients are likely due to cortical temporal lobe dysfunction, as frequently seen in classical limbic encephalitis, and the same applies to the psychotic features (Harrison, 1999; Janssen et al., 2009; Minatogawa-Chang et al., 2009). Clinically, there was a time-lag of 10 to 20 days between the onset of these early features (cognitive, psychiatric, seizure syndromes, CSF lymphocytosis, epileptiform discharges and the few cortical MRI lesions), and the later appearance of basal ganglia and brainstem-localised features (movement disorders, fall in level of consciousness, dysautonomia, CSF oligoclonal bands, generalized slowing or intermittent rhythmic delta activity on EEG, and some subcortical MRI abnormalities). Fever and headache with an obvious infection did precede the neurological features in some cases, but fever and headache without any apparent infection were found later in the disease course, when they might have a subcortical, or perhaps hypothalamic origin. Similarly, whereas hypersomnia was an early feature, insomnia occurred later, possibly reflecting generation by different localisation and mechanisms respectively.
These clinical observations raise many questions regarding the aetiology and the pathogenic mechanisms of this disorder. Firstly, the relatively high proportion of non-Caucasians (but mainly UK resident) individuals in our cohort suggests that there may be human leucocyte antigen or other genetic factors involved in disease susceptibility. Secondly, although numbers of paraneoplastic patients were small, there were no apparent differences in the timing of the CSF, imaging or EEG findings between the paraneoplastic and non-paraneoplastic patients (data not shown) and in all patients, high serum:CSF ratios of NMDAR antibodies suggest that the antigenic stimulus begins initially in the periphery rather than in the CNS. Clearly NMDAR expression by the ovarian tumours provides the antigenic stimulus in young paraneoplastic females (Dalmau et al., 2007; Tuzun et al., 2009), and this strong antigenic stimulus may account for the higher antibody levels. In the more frequent non-paraneoplastic cases, it is possible that occult tumours may have been eliminated by the immune surveillance mechanisms that generated the neurological syndrome, or are still present as has been reported even in some patients who improved spontaneously (Iizuka et al., 2008; Shindo et al., 2009). But in general, for the non-paraneoplastic patients, one needs to invoke some other antigenic stimulus. In our study, preceding infections were common in children, but only two developed their infectious prodrome sufficiently long before the onset of neurological symptoms (i.e. >10 days) to be consistent with the infection generating a novel cross-reacting IgG antibody (one reported in Schimmel et al., 2009) as clearly demonstrated, for instance, in patients with Guillain–Barré syndrome (Willison and Yuki, 2002). Dalmau et al. (2008) reported a higher incidence of preceding infections, but they did not perform such a detailed chronological analysis.
It seems likely that the infectious prodrome, when it occurs, represents an inflammatory event. In many cases this may be associated with CSF lymphocytosis, which was detected within the first few days after onset of neurological symptoms, and is seen frequently in paraneoplastic neurological syndromes (e.g. Psimaras et al., 2010). Between them, these could be responsible for a temporary and/or localized disruption of the blood-brain barrier, allowing antibodies to gain entry to the CNS, possibly in the temporal lobe where many of the presenting features may be localised. It is possible that the CSF lymphocytosis might partly represent an influx of antigen-specific T and B cells (data on lymphocyte subsets is unfortunately not available); the intrathecal synthesis of NMDAR IgG1 antibodies (Tuzun et al., 2009) is likely to require antigen-specific T cell help. Alternatively, our data showing that the antibodies can activate deposition of both C3b and C5b9 (membrane attack complex) on NMDAR expressing cells would be consistent with direct damage and/or complement-dependent chemotaxis being responsible for a subsequent CSF lymphocytosis. Complement deposition was not found at 3 and 4 months, respectively, in two post-mortem studies (Tuzun et al., 2009) but might be important in the early stages. Clearly further studies on serum and CSF taken at different stages of the disease, including looking for specific cellular immune responses, combined with more detailed and investigative imaging, could help unravel the early events but, since the presenting features are not disease-specific, a high suspicion for this condition in young adults and early serological and CSF referral will be important for these studies.
The next questions relate to where and how the antibodies act. It has already been shown that NMDAR-antibody-positive CSF and purified IgG can lead to internalization of NMDARs in hippocampal neurons in culture (Dalmau et al., 2008) and this may play a major role in vivo, particularly if complement levels in the parenchymal extracellular fluid are low. From the time-course we describe, it seems likely that the early features, accompanied by epileptiform discharges and sometimes cortical MRI abnormalities, are due to dysfunction of cortical neurons, perhaps by a combination of reduced NMDARs and complement-mediated damage. But why the later features do not occur at this time is not clear. A recent case series proposed that the involuntary movements seen in NMDAR-antibody encephalitis were the result of reduced corticostriatal disinhibition of autonomous central pattern generators in the striatum (Kleinig et al., 2008), but it is difficult to see why this would occur with such a delayed time-course. It may be that the secondary intrathecal synthesis of NMDAR antibodies and development of oligoclonal bands are required before there is more global cerebral involvement with predominant subcortical dysfunction, slow-wave discharges and white matter lesions. An attractive hypothesis is that, concurrent with the later appearance of unmatched CSF oligoclonal bands, intrathecal antigen spreading with production of different antibody specificities occurs; such non-NMDAR antigen immunization might then be responsible for some of the later features. In support of this concept, the four patients restricted to the early, likely cortical, stage, with predominant epilepsy, never developed oligoclonal bands and only one had CSF lymphocytosis. Further clinical investigations and experimental studies in active and passive transfer models should help unravel some of these challenging questions.
Funding
National Institute for Health Research (NIHR), Department of Health, UK (S.R.I.), the Oxford Biomedical Research Centre (P.W. and A.V.), a Wellcome Trust funded OXION studentship (K.B.), the Medical Research Council, UK (D.B. and S.M.), a European Federation of Neurological Society fellowship (L.Z.) and an Eastern Region Neurosciences Training Fellowship (M.S.Z.).
Conflict of interest: AV and the University Department of Clinical Neurology in Oxford receive royalties and payments for antibody assays. The authors report no other conflict of interests.
Supplementary material
Supplementary material is available at Brain online.
Supplementary Material
Acknowledgements
We are very grateful to Ms P Pettingill for her help and to all of the patients and the clinicians who provided the clinical data and additional samples: Dr M Douglas, Dr R Etti (Birmingham, UK), Dr T Hughes, Dr H Morris (Cardiff, UK), Dr J Miller (Newcastle, UK), Dr M Manford, Prof D Menon, Dr P Molyneux (Cambridge, UK), Dr L Costelloe (Dublin, UK), Dr A Bhattacharya, Dr G Davies, Dr S Slaught, Dr R Walker (London, UK), Dr J Ealing, Dr G Vassallo (Manchester, UK), Dr P Maddison (Nottingham, UK), Prof R Gregory, Dr Y Hart, Dr T McShane (Oxford, UK), Dr Murrigan (Southampton, UK), Dr M Hoppe, Dr J Penzien, Dr B Reuter, Dr M Wolff (Germany), Prof U Aguglia (Italy), Prof E Wilder-Smith (Singapore), Dr A Jiminez Huete (Spain), Dr A Rossetti (Switzerland). We thank Prof F Anne Stephenson (School of Pharmacy, University of London) for the anti-NR2B antibody, and Prof B Paul Morgan for the anti-C5b9 antibody.
Glossary
Abbreviations
- cDNA
complementary DNA
- EGFP
enhanced green fluorescent protein
- IgG
immunoglobulin G
- NMDAR
N-methyl-d-aspartate receptor
References
- Ances BM, Vitaliani R, Taylor RA, Liebeskind DS, Voloschin A, Houghton DJ, et al. Treatment-responsive limbic encephalitis identified by neuropil antibodies: MRI and PET correlates. Brain. 2005;128:1764–77. doi: 10.1093/brain/awh526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bien CG, Urbach H, Schramm J, Soeder BM, Becker AJ, Voltz R, et al. Limbic encephalitis as a precipitating event in adult-onset temporal lobe epilepsy. Neurology. 2007;69:1236–44. doi: 10.1212/01.wnl.0000276946.08412.ef. [DOI] [PubMed] [Google Scholar]
- Buckley C, Oger J, Clover L, Tuzun E, Carpenter K, Jackson M, et al. Potassium channel antibodies in two patients with reversible limbic encephalitis. Ann Neurol. 2001;50:73–8. doi: 10.1002/ana.1097. [DOI] [PubMed] [Google Scholar]
- Castillo P, Woodruff B, Caselli R, Vernino S, Lucchinetti C, Swanson J, et al. Steroid-responsive encephalopathy associated with autoimmune thyroiditis. Arch Neurol. 2006;63:197–202. doi: 10.1001/archneur.63.2.197. [DOI] [PubMed] [Google Scholar]
- Church AJ, Dale RC, Lees AJ, Giovannoni G, Robertson MM. Tourette's syndrome: a cross sectional study to examine the PANDAS hypothesis. J Neurol Neurosurg Psychiatry. 2003;74:602–7. doi: 10.1136/jnnp.74.5.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale RC, Irani SR, Brilot F, Pillai S, Webster R, Gill D, et al. NMDA receptor antibodies in pediatric dyskinetic encephalitis lethargica. Ann Neurol. 2009;66:704–9. doi: 10.1002/ana.21807. [DOI] [PubMed] [Google Scholar]
- Dalmau J, Gleichman AJ, Hughes EG, Rossi JE, Peng X, Lai M, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7:1091–8. doi: 10.1016/S1474-4422(08)70224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmau J, Tuzun E, Wu HY, Masjuan J, Rossi JE, Voloschin A, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61:25–36. doi: 10.1002/ana.21050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies G, Irani SR, Coltart C, Ingle G, Amin Y, Taylor C, et al. NMDA Receptor Antibodies are a Common Cause of Encephalitis Requiring Intensive Care. Crit Care Med. 2010;89:679–82. doi: 10.1097/CCM.0b013e3181cb0968. [DOI] [PubMed] [Google Scholar]
- Florance NR, David RL, Lam C, Szperka C, Zhou L, Ahmad S, et al. Anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis in children and adolescents. Ann Neurol. 2009;66:11–18. doi: 10.1002/ana.21756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graus F, Keime-Guibert F, Rene R, Benyahia B, Ribalta T, Ascaso C, et al. Anti-Hu-associated paraneoplastic encephalomyelitis: analysis of 200 patients. Brain. 2001;124:1138–48. doi: 10.1093/brain/124.6.1138. [DOI] [PubMed] [Google Scholar]
- Graus F, Saiz A, Lai M, Bruna J, Lopez F, Sabater L, et al. Neuronal surface antigen antibodies in limbic encephalitis: clinical-immunologic associations. Neurology. 2008;71:930–6. doi: 10.1212/01.wnl.0000325917.48466.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PJ. The neuropathology of schizophrenia. A critical review of the data and their interpretation. Brain. 1999;122:593–624. doi: 10.1093/brain/122.4.593. [DOI] [PubMed] [Google Scholar]
- Hutchinson M, Waters P, McHugh J, Gorman G, O'Riordan S, Connolly S, et al. Progressive encephalomyelitis, rigidity, and myoclonus: a novel glycine receptor antibody. Neurology. 2008;71:1291–2. doi: 10.1212/01.wnl.0000327606.50322.f0. [DOI] [PubMed] [Google Scholar]
- Iizuka T, Sakai F, Ide T, Monzen T, Yoshii S, Iigaya M, et al. Anti-NMDA receptor encephalitis in Japan: long-term outcome without tumor removal. Neurology. 2008;70:504–11. doi: 10.1212/01.wnl.0000278388.90370.c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irani SR, Buckley C, Vincent A, Cockerell OC, Rudge P, Johnson MR, et al. Immunotherapy-responsive seizure-like episodes with potassium channel antibodies. Neurology. 2008;71:1647–8. doi: 10.1212/01.wnl.0000326572.93762.51. [DOI] [PubMed] [Google Scholar]
- Janssen J, Reig S, Alemán Y, Schnack H, Udias JM, Parellada M, et al. Gyral and sulcal cortical thinning in adolescents with first episode early-onset psychosis. Biol Psychiatry. 2009;66:1047–54. doi: 10.1016/j.biopsych.2009.07.021. [DOI] [PubMed] [Google Scholar]
- Jarius S, Aboul-Enein F, Waters P, Kuenz B, Hauser A, Berger T, et al. Antibody to aquaporin-4 in the long-term course of neuromyelitis optica. Brain. 2008;131:3072–80. doi: 10.1093/brain/awn240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinig TJ, Thompson PD, Matar W, Duggins A, Kimber TE, Morris JG, et al. The distinctive movement disorder of ovarian teratoma-associated encephalitis. Mov Disord. 2008;23:1256–61. doi: 10.1002/mds.22073. [DOI] [PubMed] [Google Scholar]
- Leite MI, Jacob S, Viegas S, Cossins J, Clover L, Morgan BP, et al. IgG1 antibodies to acetylcholine receptors in 'seronegative' myasthenia gravis. Brain. 2008;131:1940–52. doi: 10.1093/brain/awn092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight K, Jiang Y, Hart Y, Cavey A, Wroe S, Blank M, et al. Serum antibodies in epilepsy and seizure-associated disorders. Neurology. 2005;65:1730–6. doi: 10.1212/01.wnl.0000187129.66353.13. [DOI] [PubMed] [Google Scholar]
- Minatogawa-Chang TM, Schaufelberger MS, Ayres AM, Duran FL, Gutt EK, Murray RM, et al. Cognitive performance is related to cortical grey matter volumes in early stages of schizophrenia: a population-based study of first-episode psychosis. Schizophr Res. 2009;113:200–9. doi: 10.1016/j.schres.2009.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niehusmann P, Dalmau J, Rudlowski C, Vincent A, Elger CE, Rossi JE, et al. Diagnostic value of N-methyl-D-aspartate receptor antibodies in women with new-onset epilepsy. Arch Neurol. 2009;66:458–64. doi: 10.1001/archneurol.2009.5. [DOI] [PubMed] [Google Scholar]
- Psimaras D, Carpentier AF, Rossi C. CSF study in paraneoplastic syndromes. J Neurol Neurosurg Psychiatry. 2009 J Neurol Neurosurg Psychiatry. 2010;81:42–5. doi: 10.1136/jnnp.2008.159483. [DOI] [PubMed] [Google Scholar]
- Schimmel M, Bien CG, Vincent A, Schenk W, Penzien J. Successful treatment of anti-N-methyl D-aspartate receptor encephalitis presenting with catatonia. Arch Dis Child. 2009;94:314–6. doi: 10.1136/adc.2008.149021. [DOI] [PubMed] [Google Scholar]
- Shindo A, Kagawa K, Ii Y, Sasaki R, Kokubo Y, Kuzuhara S. Anti-N-methyl-D-aspartate receptor-related grave but reversible encephalitis with ovarian teratoma in 2 Japanese women presenting with excellent recovery without tumor resection. Eur Neurol. 2009;61:50–1. doi: 10.1159/000175122. [DOI] [PubMed] [Google Scholar]
- Thieben MJ, Lennon VA, Boeve BF, Aksamit AJ, Keegan M, Vernino S. Potentially reversible autoimmune limbic encephalitis with neuronal potassium channel antibody. Neurology. 2004;62:1177–82. doi: 10.1212/01.wnl.0000122648.19196.02. [DOI] [PubMed] [Google Scholar]
- Tuzun E, Zhou L, Baehring JM, Bannykh S, Rosenfeld MR, Dalmau J. Evidence for antibody-mediated pathogenesis in anti-NMDAR encephalitis associated with ovarian teratoma. Acta Neuropathol. doi: 10.1007/s00401-009-0582-4. Aug 14 2009 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent A, Lang B, Kleopa KA. Autoimmune channelopathies and related neurological disorders. Neuron. 2006;52:123–38. doi: 10.1016/j.neuron.2006.09.024. [DOI] [PubMed] [Google Scholar]
- Vincent A, Buckley C, Schott JM, Baker I, Dewar BK, Detert N, et al. Potassium channel antibody-associated encephalopathy: a potentially immunotherapy-responsive form of limbic encephalitis. Brain. 2004;127:701–12. doi: 10.1093/brain/awh077. [DOI] [PubMed] [Google Scholar]
- Waters P, Jarius S, Littleton E, Leite MI, Jacob S, Gray B, et al. Aquaporin-4 antibodies in neuromyelitis optica and longitudinally extensive transverse myelitis. Arch Neurol. 2008;65:913–9. doi: 10.1001/archneur.65.7.913. [DOI] [PubMed] [Google Scholar]
- Willison HJ, Yuki N. Peripheral neuropathies and anti-glycolipid antibodies. Brain. 2002;125:2591–625. doi: 10.1093/brain/awf272. [DOI] [PubMed] [Google Scholar]
- Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG. The spectrum of neuromyelitis optica. Lancet Neurol. 2007;6:805–15. doi: 10.1016/S1474-4422(07)70216-8. [DOI] [PubMed] [Google Scholar]
- Zandi MS, Irani SR, Follows G, Moody AM, Molyneux P, Vincent A. Limbic encephalitis associated with antibodies to the NMDA receptor in Hodgkin Lymphoma. Neurology. 2009;73:2039–40. doi: 10.1212/WNL.0b013e3181c55e9b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.