Abstract
K3 and K5 are Kaposi's sarcoma-associated herpesvirus (KSHV)-encoded E3 ubiquitin ligases that differentially reduce surface expression of various proteins in infected cells. Here we describe their effects on human dermal microvascular endothelial cells (HDMEC), a natural target of KSHV infection. TNF-treated HDMEC transduced to express K5 show reduced capacity to capture effector memory (EM) CD4+ T cells under conditions of venular shear stress. K5 but not K3 transduction significantly reduces ICAM-1 expression and the inhibition of T cell capture was phenocopied by siRNA knockdown of ICAM-1 and by anti-ICAM-1 Ab blocking. Co-transduction with an ICAM-1 truncated construct not subject to K5 ubiquitylation restored EM CD4+ T cell capture. K3 transductants effectively capture EM CD4+ T cells, but fail to support their transendothelial migration (TEM) in response to TCR engagement by superantigen presented by the EC, leaving intact chemokine-dependent TEM. K3 but not K5 transduction significantly reduces PECAM-1 expression and the effect on TCR-induced TEM is phenocopied by siRNA knockdown of PECAM-1 and by anti-PECAM-1 Ab blocking. TCR-dependent TEM was restored in K3 transductants co-transduced to express a mutant of PECAM-1 not subject to K3-induced ubiquitylation. EM CD4+ T cells lack any known PECAM-1 counter receptor, but heterophilic engagement of PECAM-1 may involve glycosaminoglycans, and TCR-induced TEM, but not chemokine-induced TEM, appears to involve a heparan- or chondroitin-like molecule on T cells. These results both identify specific roles of K5 and K3 in immune evasion and further differentiate the processes of inflammatory chemokine- versus TCR-dependent recruitment of human EM CD4+ T cells.
Keywords: Endothelial cells, T cells, viral infection
Introduction
Kaposi's sarcoma-associated herpesvirus (KSHV), also known as human herpesvirus 8 (HHV8), is the etiological agent of Kaposi's sarcoma. Viral-induced cancers are highly immunogenic and KS is largely a disease of severely immunosuppressed patients, e.g., due to drugs given to transplant recipients or HIV-induced AIDS. The natural targets of KSHV are microvascular endothelial cells (EC), a cell population that is directly exposed to circulating effector elements of the host immune system. KSHV has evolved multiple mechanisms of immune-evasion to persist in immunocompetent hosts; indeed, 25% of the proteins encoded by KSHV genes have been shown to modulate different aspects of the host immune response (1). KSHV proteins encoded by ORFK3 (K3) and ORFK5 (K5), also known as modulator of immune response 1 (MIR-1) and MIR-2, respectively, are E3 ubiquitin ligases that can selectively downregulate cell surface proteins that participate in immune responses. These include MHC class I, B7-2, ICAM-1 (CD54), CD1d, PECAM-1 (CD31), ALCAM (CD166), IFNγR1, MICA/B, AICL, and VE-cadherin (2–11). Most of these targets have been investigated in BJAB B lymphoblastoid cells or HeLa cells. EC specific targets of K3 and K5, e.g., CD31 and VE-cadherin, have been investigated in immortalized EC (7, 11). Infection of primary EC with KSHV down-regulates both ICAM-1 and PECAM-1, which has been attributed to K5 since K5, but not K3, down-regulates ICAM-1 and PECAM-1 in BJAB cells and immortalized EC, respectively (5, 7, 12). However, the functional effects of overexpressing K3 and K5 in untransformed human microvascular EC, the natural target of the virus, have not been described.
Microvascular EC are active participants in the effector phase of the adaptive immune response. We have been interested in mechanisms by which microvascular EC may recruit circulating effector memory (EM) CD4+ T cells. Unlike naïve or central memory (CM) T cells, freshly isolated EM CD4+ T cells can rapidly transmigrate across cultured HUVEC or human dermal microvascular EC (HDMEC) displaying TNF-induced adhesion molecules such as ICAM-1 and VCAM-1 plus the inflammatory chemokine IP-10 (13). More recently, we have shown that EM CD4+ T cells, but again not CM nor naïve CD4+ T cells, will transmigrate in response to signals that engage the TCR such as superantigen presented by TNF-treated HDMEC expressing class II MHC molecules (14). While both modes of TEM require venular type flow to provide shear stress, TCR-dependent (antigen-driven) TEM of EM CD4+ T cells is somewhat delayed compared to chemokine-driven TEM, and may be further differentiated by its requirement for EC fractalkine. Moreover, cells receiving a TCR signal are unresponsive to chemokines such as IP-10 or SDF-1. In this report, we show that over-expressing the KSHV genes K3 or K5 in HDMEC inhibit EM CD4+ T cell recruitment at different steps. Specifically, we find that K5 inhibits capture of flowing EM CD4+ T cells by HDMEC, while K3 inhibits TCR-dependent but not rapid chemokine-dependent transendothelial migration of EM CD4+ T cells. Using siRNA and blocking antibodies, as well as reconstitution experiments, we present evidence that K5-mediated inhibition of capture of EM CD4+ T cells may be explained by ubiquitin-dependent down-regulation of ICAM-1 whereas K3-mediated inhibition of TCR-driven TEM may be explained by ubiquitin-dependent down-regulation of PECAM-1. PECAM-1 has not previously been implicated in the TEM of T cells and we find that EM CD4+ T cells lack any known counter-receptor for PECAM-1. Heterophilic PECAM-1 engagement has previously been shown to induce binding of heparan- or chondroitin-like glycosaminoglycans and we further show that a heparan- or chondroitin-like glycosaminoglycan present on EM CD4+ T cells is also important for TCR-initiated, but not chemokine-driven, TEM. These data identify specific mechanisms that may be employed by KSHV to evade the human immune system, and concomitantly expand our understanding of the differences between TCR- versus chemokine-driven TEM of human EM CD4+ T cells.
Materials and Methods
Cells and reagents
CIITA-HDMEC were generated using a retroviral vector and characterized as described (14). GFP, K5, K5Em, and K3Em lentiviral transductants were prepared by transducing HDMEC or CIITA HDMEC with lentivirus produced from cotransfection of NIH3T3 cells with lentiviral vector plasmid pHRSIN-K3 or -K5 (based on the vector pHR'SINcPPT-SGW, a kind gift from Yasuhiro Ikeda) or pHRSIN-UbEmerald-K3, -K5 or empty vector (co-expressing the Emerald variant of GFP under a seperate ubiquitin promoter), packaging plasmid psPAX2 (a kind gift of Didier Trono), and envelop protein plasmid pLP/VSVG. HDMEC were transduced with retrovirus to express ICAM-1 lacking the cytoplasmic tail as described (15). Site-directed mutagenesis was used to create the PECAM-1 construct with all 11 cytoplasmic domain lysine residues changed to arginine, and one cysteine to alanine. This construct (PECAM 12 mut) or the unmodified cDNA (PECAM wt, a kind gift of Joe Madri, Yale University) were subcloned into retroviral vector LZRSpBMN-Z (kind gift of Gary Nolan) and retroviral supernatants, produced by transfecting phoenix cells, were used to transduce CIITA HDMEC. For most experiments, EC were incubated in the presence of 10 ng/ml recombinant human TNF (TNFα, R&D Systems) for 18–26 hours. PE-conjugated monoclonal antibodies (mAb) to PECAM-1, ICAM-1, ALCAM, HLA-A,B,C control IgG (BD Pharmingen) and VE-cadherin (eBioscience) and a mouse anti-human VCAM-1 mAb (clone E16.5) or a mouse anti FLAG (clone M2) conjugated with Alexa fluor 647 (Zenon Alexafluor 647, Invitrogen) were used to stain cells for FACS. A mAb to K5, a kind gift from Klaus Früh (Oregon Health and Science University, Portland, OR), was used to stain samples for immunofluorescence microscopy. For the blocking antibody experiments, EC were incubated in the presence of 10 μg/ml blocking antibodies to ICAM-1 (R&D systems) or PECAM-1 (hec7) for 30 minutes prior to the flow assay. For siRNA experiments, HDMEC were treated with siRNAs targeting ICAM-1 (Dharmacon) or PECAM-1 (Qiagen) or negative control (Allstar negative control siRNA, Qiagen) as described (15). All reagents used in the glycosaminoglycan experiments were from Sigma. EC were preincubated with 100 μg/ml heparan sulfate (sodium salt, from bovine kidney), 100 μg/ml chondroitin-6-sulfate (sodium salt, from shark cartilage), 100 μg/ml hyaluronic acid (sodium salt, from Streptococcus equi) and T cells were treated with 50 mU/ml chondroitinase ABC or 100 mU/ml hyaluronidase (from Streptomyces hyalurolyticus).
CD4+ T cells were isolated by positive selection with magnetic beads and released with Detachabead (Dynal) from PBMCs prepared by Ficoll gradient of blood collected from healthy donors. Memory (CD4+CD45RA−) T cells were isolated by depletion of CD45RA+ cells from CD4+ T cells using anti-CD45RA mAb (eBiosciences) and pan-mouse IgG beads. EM cells were further enriched by depleting CD4+ memory cells with anti-CCR7 mAb (R&D Systems) and pan-mouse IgG beads. Isolated EM CD4+ T cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum, 2 mM glutamine, penicillin/streptomycin, and nonessential amino acids overnight prior to assays.
FACS analysis
Flow Cytometry Standard (FCS) files of trypsinized EC stained for 30 minutes on ice with PE-conjugated mAb to ICAM-1, PECAM-1, ALCAM, VE-cadherin, HLA-A,B,C, and HLA-DR or anti-VCAM-1 mAb followed by Alexa fluor 647 conjugated goat anti-mouse then washed 2 times with 1 % BSA in PBS, were acquired using FACSCAN or LSRII flow cytometers with Cellquest or FACSDiva software, respectively, and analyzed using Flowjo software. For intracellular staining of FLAG-tagged K3, fixed EC permeabilized with 0.1% saponin were stained with anti-FLAG mAb M2 precomplexed with alexafluor 647 conjugated Fab fragment goat anti-mouse IgG. Total CD4+ or EM CD4+ T cells were stained with FITC-conjugated mouse IgG, mouse anti-CD38, mouse anti-CD31, or mouse anti-CD177, washed 2 times with 1 % BSA in PBS and acquired using LSRII flow cytometer with FACSDiva software and analyzed using Flowjo software.
TEM assays
CIITA HDMEC and CIITA + GFP, CIITA + K3Em, CIITA + K5Em co-transductants, grown to confluence on 35 mm fibronectin-coated coverglasses, were incubated with 100 ng/ml TSST-1 (Toxin Technologies) 30 minutes prior to the flow assay, then 3–5 minutes with 3 μg/ml IP-10, washed twice with RPMI/10% FBS, and assembled with a parallel plate flow chamber apparatus (Glycotech) using the 0.01 inch height, 5 mm wide slit gasket provided by the manufacturer. On a 37°C heating surface, CD4+CD45RA−CCR7low (EM) T cells (106 cells/500 μl) suspended in the same medium were loaded onto the EC monolayer at 0.75 dyne/cm2 for 2 minutes, followed by medium only at 1 dyne/cm2 for 15 or 60 minutes. Samples were then fixed with 3.7% formaldehyde in PBS, stained with anti-Vβ2TCR mAb (Immunotech) followed by Alexafluor 594-or 488-conjugated donkey anti-mouse IgG (Molecular Probes), mounted on slides using mounting medium containing DAPI (Molecular Probes), and examined by microscopy. A FITC filter was used to detect FITC or Alexafluor 488-stained cells, a TRITC filter was used to detect Alexafluor 594-stained cells, a DAPI filter used to detect DAPI-stained nuclei, and a Cy5 filter was used to detect Alexafluor 647 stained cells. Using a 40×/0.60 korr Ph2 objective, phase contrast optics were used to determine whether CD4+ T cells were either on top or underneath the HDMEC monolayer. T cells that were captured and not spread were round and bright when viewed under phase contrast. CD4+ T cells that were spread, but still on top of the HDMEC monolayer were surrounded by a bright corona of light in contrast to those that had transmigrated. The percentage of transmigrated CD4+ T cells were calculated for 200 cells per sample by analyzing ten groups of 20 cells each, calculating the percentage for each group, and calculating the mean and s.e.m. for the ten groups. For cell capture experiments, samples were stained with FITC-conjugated anti CD45, Alexafluor 488 conjugated rabbit anti-FITC, and Alexafluor 488 conjugated goat anti rabbit IgG. The number of CD45+ cells in ten fields (1000 × 1000 pixels) viewed with a 10× objective were counted to determine cells/field.
Statistics
For experiments in which more than two groups were compared, statistical significance was determined by one-way ANOVA using a 95% confidence interval and the Tukey post-test (Prism 4.0 for Macintosh). Statistical error is expressed as s.e.m. For experiments in which two groups were compared, a t-test was used.
Results
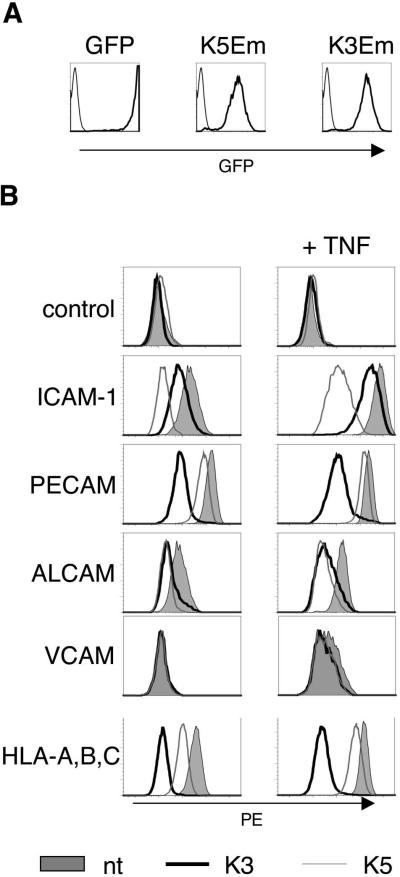
Microvascular EC are a natural target of KSHV infection. To study the immuno-evasive effects of K3 and K5 in this cell type, we transduced cultured HDMEC with lentiviral contructs encoding GFP (as control), K3 and K5; the K3 and K5 vectors also express GFP, but under the control of the ubiquitin promoter (Figure 1A). Expression of K3 and K5 in transduced EC was confirmed by immunostaining (Figures S2 and S3). We then examined the effects of K3 and K5 over-expression in HDMEC on several surface proteins relevant for interactions with T cells and other leukocytes. K3 strongly down-regulates MHC Class I, while K5 has less effect on MHC Class I but strongly down-regulates ICAM-1 (CD54) (Figure 1B). Both K3 and K5 downregulate ALCAM (CD166) and VCAM-1 in HDMEC to a similar extent, which indicates that the targets of these E3 ubiquitin ligases partly overlap. In contrast to previous studies that utilized immortalized HDMEC (7, 11), we find that K3 down-regulates PECAM-1 much more effectively than K5, and that neither K5 nor K3 downregulates VE-cadherin (Figures 1B, S1 and data not shown).
Figure 1.
Phenotype of HDMEC transduced with lentivirus encoding GFP, KSHV gene K3 and KSHV gene K5 (K3 and K5 vectors have GFP driven by the ubiquitin promotor, K3 Em and K5 Em, respectively). A. Histograms showing GFP expression (thick line) compared to background fluorescence of non-transduced HDMEC (thin line). Greater than 95% of HDMEC are transduced by GFP, K3 Em, and K5 Em lentivirus. B. Expression of cell surface proteins on transduced HDMEC. Note that K5 reduces ICAM-1 as well as MHC Class I (HLA-A,B,C), while K3 predictably reduces MHC Class I (HLA-A,B,C) but also severely reduces PECAM-1 as compared to K5. Control refers to staining with IgG. In all histogram plots shown, the x axis scale is log10, and the y axis scale is linear.
We then tested the effects of K3 and K5 over-expression on TEM of EM CD4+ T cells across HDMEC monolayers under conditions of venular flow (1 dyne/cm2). We had established that there are two separate routes of TEM taken by EM CD4+ T cells, both of which require the application of venular levels of shear stress and TNF pretreatment of the HDMEC: (1) chemokine (e.g., IP-10 or SDF-1 supplied exogenously or produced by EC)-dependent TEM which occurs within 15 minutes and (2) TCR-dependent TEM, which prevents rapid chemokine responses but results in TEM with a slightly slower time course (within 60 minutes) (13, 14). To determine if KSHV affects either of these two pathways, we generated CIITA plus K3 or K5 HDMEC co-transductants (which express class II MHC molecules) and overlaid a TNF-treated monolayer of these cells with TSST-1, which binds to MHC class II and is presented by the EC to T cells expressing Vβ2 gene segment positive TCRs; addition of exogenous chemokine was not necessary to stimulate TEM of EM CD4+ T cells due to the high level of endogenous chemokine expression capable of stimulating spontaneous rapid (and pertussis toxin-sensitive) TEM on HDMEC (as compared to HUVEC)(14). (It should be noted that although K3 does block MHC Class II expression in HDMEC in response to IFN-γ, probably via down-regulation of the IFN-γ receptor, a known target of K3 (data not shown), MHC Class II expression is unaffected by K3 or K5 in CIITA HDMEC.) EM CD4+ T cells that utilize Vβ2 gene segments to form their TCR respond to TSST-1 displayed by the CIITA HDMEC by undergoing slow TCR-dependent TEM; those EM CD4+ T cells in the same sample that do not use Vβ2 gene segments to form their TCR do not respond to TSST-1 and instead rapidly undergo TEM in response to endogenous chemokine (the Vβ2 + T cells are identified by staining with an anti-Vβ2 antibody, as described in Materials and Methods; T cells that do or do not stain with the anti-Vβ2 antibody are designated VB2+ and VB2−, respectively, in the figures). As previously noted, cells receiving a TCR signal will not rapidly transmigrate in response to chemokine, although they will do so if TSST-1 is excluded from the system (16). The TEM assay is conducted under conditions of venular flow and in both processes of TEM, the T cells must first adhere to the HDMEC monolayer. K5 transduction reduced the number of cells captured (Figure 2A), but of those cells that did bind, the percentage found to transmigrate, whether by rapid chemokine-dependent or TCR-dependent mechanisms was comparable to that seen in control HDMEC monolayers (and immunostaining confirmed that TEM occurred at the junctions between EC expressing K5) (Figures 2B and S3). However, the reduced capture of T cells does result in a profound net reduction of T cells undergoing TEM in response to either chemokine or TCR signals. K3 transduction did not affect the number of cells captured under flow (Figure 2A) or that undergo rapid, chemokine-dependent TEM, but did inhibit TCR-driven TEM (Figure 2B).
Figure 2.
K5 reduces capture, and K3 reduces TCR-driven TEM, of EM CD4+ T cells. A. K5 reduces capture. EM CD4+ T cells captured in flow TEM assay across TNF-treated, TSST-1 preloaded CIITA HDMEC co-transduced with lentivirus encoding GFP (GFP CIITA), K3 Em K3Em CIITA), K5 Em (K5Em CIITA), or not co-transduced (CIITA); mean +/− standard error of the mean (SEM) of one representative experiment of three is shown. K5Em CIITA binds 17% as many cells as controls expressing CIITA only. p<0.001 between K5Em CIITA co-transductants and all other samples, and p>0.05 between all other samples. B. K3 reduces TCR-driven TEM. Mean +/− SEM of EM CD4+ T cell % TEM in flow TEM assay across cells described above. “VB2+” and “VB2−“ refer to those T cells that do or do not bind to TSST-1 on the CIITA HDMEC, respectively. ***, p<0.001 **, p<0.01. One representative experiment of three is shown.
Our previous studies of TEM of EM CD4+ T cells had revealed that capture of EM CD4+ T cells to TNF-treated EC could be fully recapitulated by ICAM-1 and VCAM-1 expressed on HUVEC (13). Since K5 but not K3 reduces T cell capture and since both K3 and K5 similarly reduce VCAM-1 while K5 much more profoundly reduces ICAM-1, we hypothesized that down-regulation of ICAM-1 could be responsible for the reduced binding of EM CD4+ T cells to EC cells transduced with K5. We investigated this possibility by examining the binding of these T cells to EC treated with ICAM-1 blocking antibody or ICAM-1 siRNA; we included K3 transductants in this assay since two adhesion molecules, namely, ALCAM and VCAM-1, were similarly reduced in K3 and K5 transductants (Figure 1). Blocking antibody as well as strong reduction of ICAM-1 by one siRNA but not a weaker down-regulation by another siRNA (97% and 81% reduction by 741 and 747, respectively) inhibited capture of EM CD4+ T cells by TNF-treated HDMEC (Figure 3A,B). Furthermore, binding of EM CD4+ T cells was rescued in K5 transductants engineered to express ICAM-1 lacking the cytoplasmic domain and therefore not subject to ubiquitylation by K5 (Figure 3C). Together, these experiments support the hypothesis that the marked K5-mediated down-regulation of ICAM-1 in HDMEC accounts for the reduced binding of EM CD4+ T cells, thereby diminishing the capacity for immunosurveillance by the CD4+ T cell population.
Figure 3.
K5 reduction of ICAM-1 reduces capture of EM CD4+ T cells. A. ICAM-1 blocking antibody reduces capture of EM CD4+ T cells to TNF-treated HDMEC. Blocking Ab to PECAM-1 or ICAM-1 was preincubated with TNF-treated HDMEC before 15' flow assay. Shown is the mean +/− SEM of the number of EM CD4+ T cells per field. One representative experiment of three is shown. B. Strong knockdown of HDMEC ICAM-1 by siRNA inhibits capture of EM CD4+ T cells. TNF-treated HDMEC transfected with negative control siRNA (neg), ICAM-1-specific siRNAs 741 or 747 were analyzed by FACS for ICAM-1 expression (histograms) or used in 15' flow assays with EM CD4+ T cells (graph). Histograms show cells stained with isotype control IgG-FITC (filled) or anti-ICAM-1-FITC (line). Knockdown of ICAM-1 by 741 and 747 was consistently about 97% and 81% based on median fluorescence intensity, respectively, in three different experiments. K5 neg refers to K5 tranducatants transfected with negative control siRNA. Graph shows mean +/− SEM of the number of EM CD4+ T cells per field. ***, p<0.001 compared to either non transduced cells transfected with negative control siRNA or ICAM-1 747 siRNA; p>0.05 for all other comparisons. C. Expression of ICAM-1 cytoplasmic deletion mutant in K5 transductants restores binding. Contour plots (left) of the EC used in the capture assay of EM CD4+ T cells on TNF-treated HDMEC transduced with K5 or GFP and transduced or not with ICAM-1 cytoplasmic deletion construct (ICAM). The numbers in the FACS plots refer to the median PE fluorescence intensity. Graph shows mean +/− SEM of the number of cells per field from three separate samples combined, ten fields per sample. p<0.001 between K5 and all others, p>0.05 between K5, ICAM and GFP, ICAM.
PECAM-1 is important for TEM of neutrophils and monocytes, but has not been positively associated with the TEM of T cells (17, 18). However, such studies have focused on chemokine-driven T cell TEM and the role of PECAM-1 in TCR-driven TEM has not previously been studied. To investigate whether the strong reduction of PECAM-1 by K3 could be responsible for the inhibition of TCR-dependent TEM, we treated CIITA HDMEC with anti-PECAM-1 blocking antibody (hec7) or PECAM-1 siRNA prior to TEM assays. Anti-PECAM-1 blocked TCR-driven TEM of EM CD4+ T cells, but had no effect on rapid TEM (Figure 4A). CIITA HDMEC treated with two different siRNAs targeting PECAM-1 consistently reduced PECAM-1 expression by about 95% (like K3) and by about 80% (P1 and P2, respectively, Figure 4B). PECAM-1 knockdown resulted in a dose response inhibition of TCR driven TEM, but had no effect on rapid chemokine-dependent TEM (Figure 4B). TCR-dependent TEM was rescued in K3 transductants by expressing a PECAM-1 mutated to escape targeted destruction by K3 (containing 11 arginines rather than lysines, as well as one alanine rather than cysteine, in the cytoplasmic domain)(Figure 4C). These results suggest that the strong ubiquitin-dependent reduction of PECAM-1 by K3 is sufficient to inhibit TCR-driven TEM, and provides another example of how KSHV immunomodulatory genes may function to evade the host response. These results also identify PECAM-1 as a key EC molecule that is utilized by EM CD4+ T cells specifically in TCR-driven, but not rapid chemokine-dependent, TEM, further differentiating these processes.
Figure 4.
PECAM-1 dependence of TCR-driven TEM of EM CD4+ T cells. A. PECAM-1 blocking antibody blocks TCR-driven TEM. Blocking Ab to PECAM-1 (hec7) or control IgG was preincubated with TNF-treated, TSST-1 preloaded CIITA HDMEC before flow TEM assays. Graphs show mean +/− SEM of % TEM of VBTCR-(left) and VB2TCR+ (right) EM CD4+ T cells. Left graph shows %TEM at 15'. B. Knockdown of HDMEC PECAM-1 by siRNA inhibits TCR-driven TEM of EM CD4+ T cells. TNF-treated CIITA HDMEC transfected with negative control (neg), PECAM-1_1 (P1), or PECAM-1_2 (P2) siRNA were analyzed by FACS for PECAM-1 expression (upper panels) or used in flow TEM assays with EM CD4+ T cells (lower panels). Upper panels show results of one representative experiment of cells stained with isotype control IgG-FITC (filled) or anti-PECAM-FITC (line). Knockdown of PECAM-1 by P1 and P2 was consistently about 95% and 80% for P1 and P2, respectively, in three different experiments. Bottom panels show mean +/− SEM of data from three different experiments. The lower left shows % TEM of the Vβ2− cells at 15 min of flow, lower right shows %TEM of Vβ2+ cells at 15 and 60 min. ***, p<0.001. C. Expression of PECAM-1 without K3 target residues restores TCR-driven TEM to K3 transduced CIITA HDMEC. TEM assay of EM CD4+ T cells on CIITA HDMEC transduced to express K3 alone or in combination with PECAM-1 lacking cytoplasmic domain lysines and cysteines (12 mut) or non-mutant PECAM-1 (PECAM wt). Contour plots show FACS analysis of cells stained with PE-conjugated anti-PECAM-1. Graph shows mean +/− SEM % TEM of VB2TCR+ cells after 60' flow, combined data from 2 separate experiments in duplicate. P<0.001 for comparisons between all data sets except CIITA, K3Em vs CIITA, K3Em, PECAM wt (P<0.05). MFI of cells stained with PE-conjugated anti-PECAM: CIITA = 1028; CIITA, K3Em = 274: CIITA, K3Em, PECAM 12 mut = 641; CIITA, K3Em, PECAM wt = 276.
The role of PECAM-1 in TEM of EM CD4+ T cells triggered by TCR signaling was unexpected. PECAM-1 is a ligand for several molecules on leukocytes, including PECAM-1 itself, ADP-ribose cyclase (CD38), and CD177 (19–21). However, PECAM-1, CD38, and CD177 are not expressed on EM CD4+ T cells (Figure 5A). PECAM-1 does not bind directly to glycosaminoglycans, but PECAM-1 engagement has been reported to trigger heparan- or chondroitin-like glycosaminoglycan binding in PECAM-1 transfected cells (22–25). We therefore tested if TCR-driven TEM of EM CD4+ T cells involved HDMEC binding of a T cell-expressed glycosaminoglycan. To do so, we either incubated the EC with a variety of glycosaminoglycans to block the putative binding site, or we enzymatically removed various glycosaminoglycans from the T cells with specific glycosaminoglycan degrading enzymes prior to TEM assays. TCR-dependent TEM (but not chemokine-dependent TEM) of EM CD4+ T cells was blocked by EC treatment with chondroitin and heparan, but not hyaluronate, or by T cell treatment with chondroitinase but not hyaluronidase (Figure 5B). While these data do not identify a specific PECAM-1 ligand, they support the idea that PECAM-1 engagement on HDMEC is functional in TCR-initiated TEM, as first suggested by the effects of K3.
Figure 5.
A. EM CD4+ T cells do not express known ligands of PECAM-1. Histograms showing total CD4+ T cells (top row) and EM CD4+ T cells (bottom row) stained with isotype control (filled peak) or stained with FITC-conjugated anti-CD31, CD38, and CD177 (solid lines). The x axis scale is log10, and the y axis scale is linear. B. Dependence of TCR-driven TEM on an EM CD4+ T cell glycosaminoglycan. CIITA HDMEC were preincubated with vehicle, heparan sulfate, chondroitin-6-sulfate, or hyaluronic acid, or T cells were pre-treated with chondroitinase or hyaluronidase prior to 15 or 60 minutes flow TEM assay. Shown is mean +/− SEM of % TEM. ***, p<0.001 compared to vehicle, *, p<0.05 compared to vehicle. One representative experiment of three is shown.
Discussion
The focus of the present study was to determine the effect of expressing KSHV immune-modulatory proteins K3 and K5 in microvascular cells on the interactions of these cells with EM CD4+ T cells. Functionally, K5 and K3 expression each inhibit the ability of HDMEC to recruit EM CD4+ T cells, but these viral proteins act at different steps, namely by inhibiting T cell capture under flow for K5, and by inhibiting TCR-driven TEM by K3. By probing the targets of K5 and K3 in blocking antibody and siRNA experiments, as well as by expression of proteins mutated to avoid ubiquitin-dependent proteolysis, we attribute the reduced capture of EM CD4+ T cells to the strong downregulation of ICAM-1 by K5, and, for the first time, identify PECAM-1 as a key molecule in TCR-dependent TEM, which is potently targeted by K3 in untransformed HDMEC.
The reduced binding of EM CD4+ T cells to K5 transductants is not surprising, since it has previously been reported to reduce ICAM-1 expression in other cell types and ICAM-1 is the principal ligand for LFA-1 on T cells. In HUVEC, we had found that VCAM-1 could also support T cell capture. However, HDMEC express lower quantities of VCAM-1 than HUVEC and our siRNA experiments support a non-redundant role for ICAM-1 expression in this cell type. In contrast, the finding that PECAM-1 plays a role in TCR-dependent TEM was not anticipated since PECAM-1, which is required for monocyte and neutrophil TEM, had previously been shown to be unimportant for the TEM of T cells (17, 18). However, prior experiments had focused on chemokine-driven responses. The requirement of PECAM-1 in EM CD4+ T cell recruitment thus further distinguishes TCR-dependent TEM from rapid chemokine-mediated TEM. EM CD4+ T cells do not express any of the known counter-receptors for PECAM-1. Spurred on by previous reports suggesting PECAM-1 engagement may promote binding of heparan- or chondroitin-like glycosaminoglycans, we further demonstrate that such molecules may also participate in a non-redundant way in the TCR-driven TEM of EM CD4+ T cells. Our data suggest, therefore, a role for three as yet unidentified molecules in this process: a novel counter-receptor for PECAM-1 on T cells, a glycosaminoglycan-containing proteoglycan on T cells, and a glycosaminoglycan receptor on EC. While further studies are needed to identify the specific molecules involved, it is interesting to note that the glycosaminoglycan inhibition profile (heparan and chondroitin inhibit, hyaluronate does not) resembles that shown earlier for L cells, although the treatment with chondroitinase ABC did not have as robust an effect as inhibition by the competitor (23). This may be due to incomplete digestion of glycosaminoglycan on the T cells by chondroitinase ABC, which leaves a stub of chondroitin sulfate. Residual binding of chondroitinase ABC treated neurocan to transmembrane protein tyrosine phosphatase (PTP) sigma, a recently described receptor for chondroitin sulfate proteoglycan, has similarly been observed (26). One further characteristic of the PECAM-1 ligand on EM CD4+ T cells that may be inferred from these studies is that, since hec7 binds to and blocks the amino-terminus of PECAM-1 previously implicated in homophilic adhesion (IgG domains 1 and/or 2), the receptor on EM CD4+ T cells may bind to a similar region, unlike the previously described heterophilic counter-receptors that bind to IgG domain 6 of PECAM-1 (27).
The strong downregulation of PECAM-1 by K3 in HDMEC was unexpected, since previous work using immortalized EC had instead identified PECAM-1 as a K5 target. Indeed, we have found in the course of these studies that the functions of K3 and K5 are cell type-specific. This is particularly evident in comparing various primary EC cells and EC cell lines. For example, we noticed that HUVEC transduced with K5 but not K3 stopped proliferating while K5-transduced HDMEC proliferate at least as well if not better than nontransduced HDMEC or K3 and GFP transductants and proliferation of the immortalized EaHy.926 cell line is unaffected by K5 (unpublished observations, TDM). We believe that we are the first investigators to assess the effects of K3 and K5 in untransformed microvascular EC, the natural target of KSHV. The nature of our HDMEC also warrants some consideration. Freshly isolated HDMEC are a mixture of vascular and lymphatic EC that converge to a common phenotype with both vascular and lymphatic features in cell culture. KS cells also display both vascular and lymphatic characteristics, possibly reflecting the common physiological origin of these EC types, and the actual target of infection in vivo is a matter of some controversy (28, 29). We also note that we have studied K3 and K5 in cells not infected by KSHV. It is possible that other KSHV genes will modulate the actions of these gene products. Furthermore, there is no simple way to assess if the levels of K3 or K5 expressed in our system are appropriate to that found in infected cells, but the lentiviral vectors used tend to produce lower, more physiological levels of expression than adenovirus or plasmids. Despite this caveat, the reductionist approach used in our study is useful for understanding the specific properties of these viral proteins.
In summary, our results further extend the list of mechanisms by which KSHV acts to evade the human immune system to include inhibition of T cell recruitment to an infected site. At the same time, analysis of these effects have revealed further differences between TCR-driven and chemokine-driven TEM of EM CD4+ T cells, which may aid in developing effective treatment for not just KS but a variety of conditions involving EM CD4+ T cells, such as autoimmunity, atherosclerosis and allograft rejection.
Supplementary Material
Acknowledgments
We thank William Sessa for use of the immunofluorescence microscope, and Louise Benson, Gwendoline Davis, and Lisa Gras for excellent assistance in cell culture. We thank Klaus Früh (Oregon Health and Science University, Portland, OR) for the K5 mAb.
This work is funded by National Institute of Health grants P01-HL070295 and HL51014 (to JSP and TDM), R01 HL046849 and R37 HL064774 (to WAM), and by the Wellcome Trust (to SH and PJL).
Abbreviations
- (EM)
effector memory
- (EC)
endothelial cell
- (HDMEC)
human dermal microvascular endothelial cell
- (KSHV)
Kaposi's sarcoma-associated herpes virus
- (TEM)
transendothelial migration
Footnotes
Disclosures The authors have no conflicting financial interests.
References
- 1.Coscoy L. Immune evasion by Kaposi's sarcoma-associated herpesvirus. Nat Rev Immunol. 2007;7:391–401. doi: 10.1038/nri2076. [DOI] [PubMed] [Google Scholar]
- 2.Coscoy L, Ganem D. Kaposi's sarcoma-associated herpesvirus encodes two proteins that block cell surface display of MHC class I chains by enhancing their endocytosis. Proc Natl Acad Sci U S A. 2000;97:8051–8056. doi: 10.1073/pnas.140129797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stevenson PG, Efstathiou S, Doherty PC, Lehner PJ. Inhibition of MHC class I-restricted antigen presentation by gamma 2-herpesviruses. Proc Natl Acad Sci U S A. 2000;97:8455–8460. doi: 10.1073/pnas.150240097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ishido S, Choi JK, Lee BS, Wang C, DeMaria M, Johnson RP, Cohen GB, Jung JU. Inhibition of natural killer cell-mediated cytotoxicity by Kaposi's sarcoma-associated herpesvirus K5 protein. Immunity. 2000;13:365–374. doi: 10.1016/s1074-7613(00)00036-4. [DOI] [PubMed] [Google Scholar]
- 5.Coscoy L, Ganem D. A viral protein that selectively downregulates ICAM-1 and B7-2 and modulates T cell costimulation. J Clin Invest. 2001;107:1599–1606. doi: 10.1172/JCI12432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanchez DJ, Gumperz JE, Ganem D. Regulation of CD1d expression and function by a herpesvirus infection. J Clin Invest. 2005;115:1369–1378. doi: 10.1172/JCI24041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mansouri M, Douglas J, Rose PP, Gouveia K, Thomas G, Means RE, Moses AV, Fruh K. Kaposi sarcoma herpesvirus K5 removes CD31/PECAM from endothelial cells. Blood. 2006;108:1932–1940. doi: 10.1182/blood-2005-11-4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartee E, McCormack A, Fruh K. Quantitative membrane proteomics reveals new cellular targets of viral immune modulators. PLoS Pathog. 2006;2:e107. doi: 10.1371/journal.ppat.0020107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Q, Means R, Lang S, Jung JU. Downregulation of gamma interferon receptor 1 by Kaposi's sarcoma-associated herpesvirus K3 and K5. J Virol. 2007;81:2117–2127. doi: 10.1128/JVI.01961-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas M, Boname JM, Field S, Nejentsev S, Salio M, Cerundolo V, Wills M, Lehner PJ. Down-regulation of NKG2D and NKp80 ligands by Kaposi's sarcoma-associated herpesvirus K5 protects against NK cell cytotoxicity. Proc Natl Acad Sci U S A. 2008;105:1656–1661. doi: 10.1073/pnas.0707883105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mansouri M, Rose PP, Moses AV, Fruh K. Remodeling of endothelial adherens junctions by Kaposi's sarcoma-associated herpesvirus. J Virol. 2008;82:9615–9628. doi: 10.1128/JVI.02633-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tomescu C, Law WK, Kedes DH. Surface downregulation of major histocompatibility complex class I, PE-CAM, and ICAM-1 following de novo infection of endothelial cells with Kaposi's sarcoma-associated herpesvirus. J Virol. 2003;77:9669–9684. doi: 10.1128/JVI.77.17.9669-9684.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manes TD, Pober JS, Kluger MS. Endothelial cell-T lymphocyte interactions: iP-10 stimulates rapid transendothelial migration of human effector but not central memory CD4+ T cells. Requirements for shear stress and adhesion molecules. Transplantation. 2006;82:S9–14. doi: 10.1097/01.tp.0000231356.57576.82. [DOI] [PubMed] [Google Scholar]
- 14.Manes TD, Pober JS. Antigen Presentation by Human Microvascular Endothelial Cells Triggers ICAM-1-Dependent Transendothelial Protrusion by, and Fractalkine-Dependent Transendothelial Migration of, Effector Memory CD4+ T Cells. J Immunol. 2008;180:8386–8392. doi: 10.4049/jimmunol.180.12.8386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clark PR, Manes TD, Pober JS, Kluger MS. Increased ICAM-1 expression causes endothelial cell leakiness, cytoskeletal reorganization and junctional alterations. J Invest Dermatol. 2007;127:762–774. doi: 10.1038/sj.jid.5700670. [DOI] [PubMed] [Google Scholar]
- 16.Manes TD, Shiao SL, Dengler TJ, Pober JS. TCR signaling antagonizes rapid IP-10-mediated transendothelial migration of effector memory CD4+ T cells. J Immunol. 2007;178:3237–3243. doi: 10.4049/jimmunol.178.5.3237. [DOI] [PubMed] [Google Scholar]
- 17.Muller WA. Mechanisms of transendothelial migration of leukocytes. Circ Res. 2009;105:223–230. doi: 10.1161/CIRCRESAHA.109.200717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bird IN, Spragg JH, Ager A, Matthews N. Studies of lymphocyte transendothelial migration: analysis of migrated cell phenotypes with regard to CD31 (PECAM-1), CD45RA and CD45RO. Immunology. 1993;80:553–560. [PMC free article] [PubMed] [Google Scholar]
- 19.Sun QH, DeLisser HM, Zukowski MM, Paddock C, Albelda SM, Newman PJ. Individually distinct Ig homology domains in PECAM-1 regulate homophilic binding and modulate receptor affinity. J Biol Chem. 1996;271:11090–11098. doi: 10.1074/jbc.271.19.11090. [DOI] [PubMed] [Google Scholar]
- 20.Deaglio S, Morra M, Mallone R, Ausiello CM, Prager E, Garbarino G, Dianzani U, Stockinger H, Malavasi F. Human CD38 (ADP-ribosyl cyclase) is a counter-receptor of CD31, an Ig superfamily member. J Immunol. 1998;160:395–402. [PubMed] [Google Scholar]
- 21.Sachs UJ, Andrei-Selmer CL, Maniar A, Weiss T, Paddock C, Orlova VV, Choi EY, Newman PJ, Preissner KT, Chavakis T, Santoso S. The neutrophil-specific antigen CD177 is a counter-receptor for platelet endothelial cell adhesion molecule-1 (CD31) J Biol Chem. 2007;282:23603–23612. doi: 10.1074/jbc.M701120200. [DOI] [PubMed] [Google Scholar]
- 22.Muller WA, Berman ME, Newman PJ, DeLisser HM, Albelda SM. A heterophilic adhesion mechanism for platelet/endothelial cell adhesion molecule 1 (CD31) J Exp Med. 1992;175:1401–1404. doi: 10.1084/jem.175.5.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeLisser HM, Yan HC, Newman PJ, Muller WA, Buck CA, Albelda SM. Platelet/endothelial cell adhesion molecule-1 (CD31)-mediated cellular aggregation involves cell surface glycosaminoglycans. J Biol Chem. 1993;268:16037–16046. [PubMed] [Google Scholar]
- 24.Sun QH, Paddock C, Visentin GP, Zukowski MM, Muller WA, Newman PJ. Cell surface glycosaminoglycans do not serve as ligands for PECAM-1. PECAM-1 is not a heparin-binding protein. J Biol Chem. 1998;273:11483–11490. doi: 10.1074/jbc.273.19.11483. [DOI] [PubMed] [Google Scholar]
- 25.Newman PJ. The biology of PECAM-1. J Clin Invest. 1997;99:3–8. doi: 10.1172/JCI119129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shen Y, Tenney AP, Busch SA, Horn KP, Cuascut FX, Liu K, He Z, Silver J, Flanagan JG. PTPsigma is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science. 2009;326:592–596. doi: 10.1126/science.1178310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liao F, Huynh HK, Eiroa A, Greene T, Polizzi E, Muller WA. Migration of monocytes across endothelium and passage through extracellular matrix involve separate molecular domains of PECAM-1. J Exp Med. 1995;182:1337–1343. doi: 10.1084/jem.182.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hong YK, Foreman K, Shin JW, Hirakawa S, Curry CL, Sage DR, Libermann T, Dezube BJ, Fingeroth JD, Detmar M. Lymphatic reprogramming of blood vascular endothelium by Kaposi sarcoma-associated herpesvirus. Nat Genet. 2004;36:683–685. doi: 10.1038/ng1383. [DOI] [PubMed] [Google Scholar]
- 29.Wang HW, Trotter MW, Lagos D, Bourboulia D, Henderson S, Makinen T, Elliman S, Flanagan AM, Alitalo K, Boshoff C. Kaposi sarcoma herpesvirus-induced cellular reprogramming contributes to the lymphatic endothelial gene expression in Kaposi sarcoma. Nat Genet. 2004;36:687–693. doi: 10.1038/ng1384. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.