Abstract
Calcium controls a number of critical events including motility, secretion, cell invasion, and egress by protozoan parasites 1. Compared to animal 2 and plant cells 3, the molecular mechanisms that govern calcium signaling in parasites are poorly understood. Here we demonstrate that the production of the phytohormone abscisic acid (ABA) controls calcium signaling within the apicomplexan parasite Toxoplasma gondii, an important human pathogen. In plants, ABA controls a number of important events including environmental stress responses, embryo development, and seed dormancy 4,5. ABA induces production of the second-messenger cyclic ADP ribose (cADPR), which controls release of intracellular calcium stores in plants 6. cADPR also controls intracellular calcium release in the protozoan parasite T. gondii 7,8; however, previous studies have not revealed the molecular basis of this pathway9. Addition of exogenous ABA induced formation of cADPR in T. gondii, stimulated calcium-dependent protein secretion, and induced parasite egress from the infected host cell in a density-dependent manner. Production of endogenous ABA within the parasite was confirmed by HPLC purification and GC-MS analysis. Selective disruption of ABA synthesis by the inhibitor fluridone (FLU) delayed egress and induced development of the slow-growing, dormant cyst stage of the parasite. Thus, ABA-mediated calcium signaling controls the decision between lytic and chronic stage growth, a developmental switch that is central in pathogenesis and transmission. The pathway for ABA production was likely acquired with an algal endosymbiont that was retained as a non-photosynthetic plastid known as the apicoplast. The plant-like nature of this pathway may be exploited therapeutically as shown by the ability of a specific inhibitor of ABA synthesis to prevent toxoplasmosis in the mouse model.
Calcium-mediated secretion in T. gondii controls both motility and cell invasion and previous studies have demonstrated that these processes utilize the second messenger cADPR, yet the signals triggering this pathway remain unresolved 7,8. In plants 6, hydra 10, and sponges 11, ABA stimulates release of intracellular calcium through elevation of the cyclic nucleotide cADPR. Addition of exogenous ABA proved to be a potent agonist of secretion in T. gondii as shown by the release of the protein MIC2, a parasite adhesin that is discharged into the supernatant in response to increases in intracellular calcium (Fig. 1A). Induction of MIC2 secretion by ABA was highly specific to (±) - ABA and was not induced by (−) - ABA, the precursor β-carotene, or retinoic acid (Fig. 1B). Treatment with ABA lead to a dose-dependent increase in the second messenger cADPR in T. gondii, suggesting ABA may be a natural agonist for calcium signaling in parasites (Fig. 1C). Finally, chelation of intracellular calcium in the parasite blocked secretion induced by ABA, confirming that it acts through release of an intracellular calcium pool (Fig. 1D). Collectively these results indicate that ABA in T. gondii controls release of calcium from intracellular stores through production of cADPR, thus paralleling the pathway seen in plants 6, and early metazoans 10,11.
Fig. 1.
ABA induced cADPR production and calcium-dependent protein secretion by T. gondii. (A) Protein secretion induced by ABA was compared to the positive control ethanol (EtOH) as detected by Western blotting with anti-MIC2 (supernatant) and anti- β-galactosidase antibodies (cell pellet) 7,8. (B) Induction of MIC2 secretion was specific for (±) ABA and was not seen with (−) ABA, β-carotene, or retinoic acid. (C) cADPR production in T. gondii was increased by treatment with ABA. Student’s t -test. *, p < 0.02; **, p < 0.01 vs. control, Mean ± s.e.m., N = 3 experiments. (D) ABA-induced secretion required intracellular calcium. Treatment with the membrane permeable calcium chelator, BAPTA-AM, blocked ABA-induced MIC2 release, while non-permeant BAPTA had no effect. Cellular MIC2 (cMIC2) migrates slower due to absence of processing.
Consistent with its physiological effects, ABA was detected in lysates of T. gondii by direct biochemical analyses. Extracts of T. gondii were purified by HPLC and analyzed by gas chromatography to detect the methyl ester of ABA (methyl-ABA), which migrated with the same retention time (8 min) as the authentic methyl-ABA standard (Fig. 2A). Mass spectrometric analysis of T. gondii samples yielded the expected product of m/z 190 as well as peaks at m/z 91 and 162, characteristic of cis-ABA, and m/z 91 and 134, characteristic of trans-ABA, as described previously 12 (Fig. 2B and Supplementary Fig. 1). The slightly nosier spectrum obtained from T. gondii is consistent with the lower level of ABA found in the sample, nonetheless its signature unambiguously matches ABA. Similar levels of ABA were detected in parasite extracts using a highly specific ELISA kit (Fig. 2C). To confirm that ABA was indeed synthesized by the parasite, we used the herbicide fluridone (FLU) an inhibitor that specifically blocks phytoene destaurase activity, and hence prevents synthesis of ABA by the indirect pathway that predominates in plants5. Treatment with FLU reduced the level of ABA in parasites by more than 4-fold (Fig. 2C). Interestingly, when the level of ABA was monitored during one cycle of intracellular growth, it remained relatively constant during the first 30–36 hrs and then spiked shortly before egress of parasites from the infected host cell, which begins around 40–44 hrs (Fig. 2D).
Fig. 2.
Biochemical evidence for the production of ABA in T. gondii. (A) GC traces of ABA standards and T. gondii samples. A single peak with similar retention time (8.0 min) to authentic methyl-ABA was detected in extracts of the parasite following chemical extraction and methylation. B) Mass spectra of methyl-ABA standard and T. gondii sample from A, showing characteristic fragment at m/z 190, with additional minor peaks in common. (C) Detection of ABA by ELISA in control parasites (T. gondii) or treated with 50 µM of fluridone (FLU) (T. gondii + FLU). Mean ± s.e.m., N = 3 experiments, Student’s t test, P < 0.005 vs. control (*). (D) Time course of ABA production by T. gondii. Values are intracellular concentration in the parasite. Time 0 refers to newly invaded parasites at the start of infection. Mean ± s.e.m., N = 3 experiments
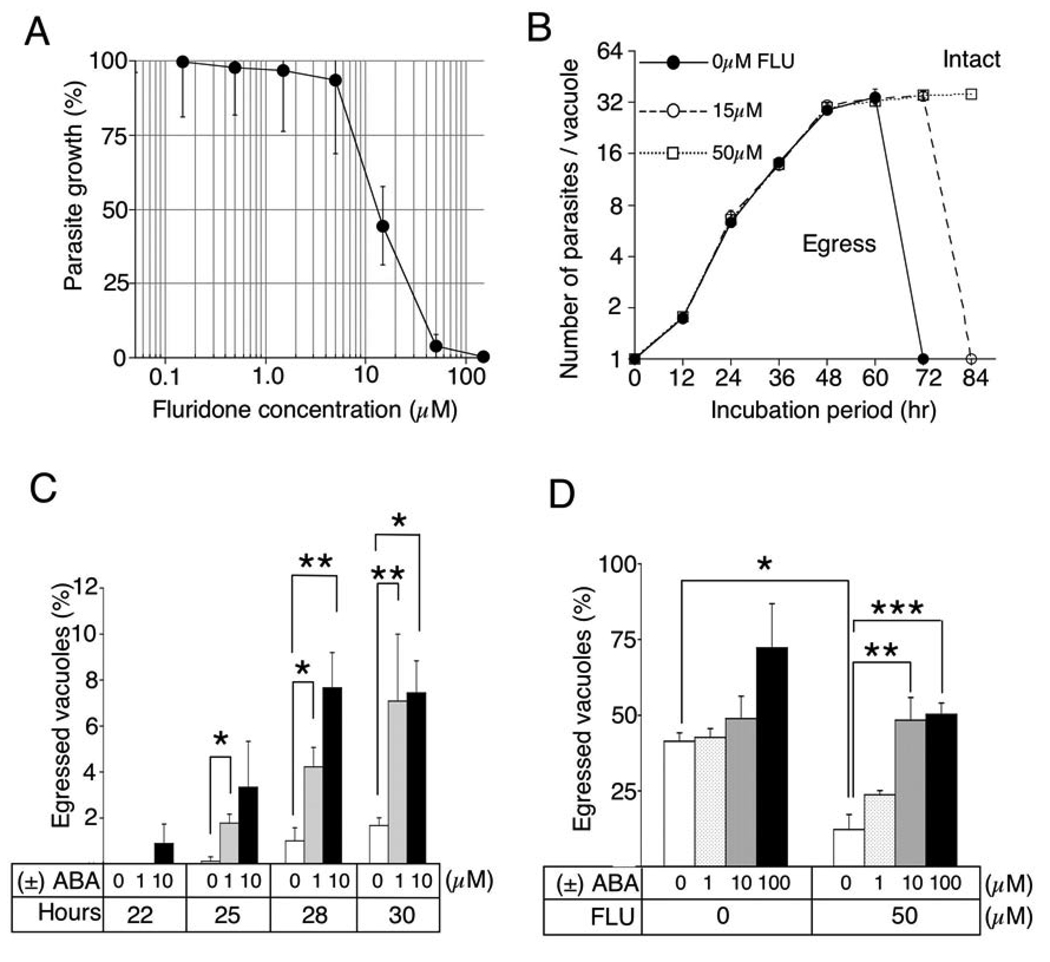
Toxoplasma is an obligate intracellular pathogen and following invasion, the parasite replicates by binary fission within a vacuole, ultimately leaving the depleted host cell by active egress. Intracellular calcium is central for several of these steps, leading us to examine whether ABA-dependent calcium signaling was essential for growth in vitro. Treatment of parasite cultures with increasing concentrations of the ABA inhibitor FLU, lead to inhibition of parasite growth (EC50 ~ 15 µM (Fig. 3A)). Surprisingly, FLU treatment did not block invasion or intracellular replication, but instead inhibited exit of mature parasites from the infected cell. While parasites had naturally egressed by ~ 48 hrs, those treated with 15 µM FLU were delayed, and those treated with 50 µM FLU were completely blocked out to 84 hrs (Fig. 3B), after which the integrity of the monolayer declined (data not shown). Previous studies have implicated calcium signaling in parasite egress from infected cells 13,14 and the rapid increase in ABA concentrations in the parasite just prior to egress (Fig. 2D), suggested this molecule may be the natural signal for activating exit. Consistent with this, when exogenous ABA was added to late-stage vacuoles, it induced premature egress (Fig. 3C). Finally, ABA was able to overcome the inhibition of egress caused by treatment with FLU (Fig. 3D), consistent with it being able to bypass the metabolic block in biosynthesis. While the responses to exogenously added ABA are somewhat modest, this may reflect the relatively low permeability of the charged anion that exists at physiological pH (pKa 4.8), and the fact that it has to cross several membranes to reach the parasite. Whether ABA produced by the parasite accumulates within the parasite or is exported to the parasite-containing vacuole is unknown and the mechanism(s) whereby ABA is sensed remain to be elucidated. Nonetheless, these results indicate that ABA is a natural agonist for host cell egress and that in the absence of its production, parasites remain quiescent within the host cell.
Fig. 3.
Fluridone blocked parasite growth by preventing egress. (A) FLU prevented parasite growth as monitored by β-galactosidase activity in the parasite. (B) Continuous culture in FLU did not affect replication but inhibited egress from the vacuole. (C) Treatment with exogenous ABA induced egress of parasites from mature vacuoles. Hours refers to length of culture prior to addition of ABA for 1 hr and scoring of egress. Student’s t test: *, p <0.02; **, p < 0.05. (D) ABA reversed the inhibition of parasite egress caused by FLU. Parasite infected cells were treated with FLU for 38 hrs, then stimulated with ABA for 1 hr, and evaluated for egress. Student’s t test: *, p < 0.002; **, p < 0.05; ***, p < 0.02. A–D, Data shown are means ± s.e.m., N= 3 experiments.
Toxoplasma undergoes two fundamentally different forms of growth within host cells: 1) the lytic cycle described above, which rapidly amplifies parasite numbers, and 2) differentiation into semi-dormant cysts that are long-lived. These adaptations serve different roles during infection, the first leading to active dissemination and the second resulting in a life-long chronic infection. The decision to undergo this developmental switch is poorly understood, but is thought to be a response to stress. In FLU-treated cultures, parasites that failed to egress from the host cell, commenced to develop into tissue cysts, as shown by staining with the cyst-wall specific lectin Dolichos biflorus (DBL) (Fig. 4A). Tissue cysts produced by treatment with FLU were positive for several stage-specific cyst markers including DBL, the cyst antigens CST1 16, and BAG1 17 (Fig. 4B). Ultrastructural examination of the cysts formed by FLU treatment revealed hallmark features of parasites within cysts including a posterior positioned nucleus, amylopectin granules, dense rhoptries, and a convoluted cyst wall (Fig. 4C). FLU treatment also enhanced the response of parasites to alkaline pH stress (Supplemental Fig. 2), a common experimental method used to induce differentiation 15.
Fig. 4.
Fluridone treatment induced development of tissue cysts and protected mice against toxoplasmosis. (A) FLU treatment enhanced cyst formation, as revealed by Dolichos biflorus lectin (DBL). Student’s t test: *, p <0.02; **, p <0.005. Means ± s.d., N = 3 experiments. (B) FLU-induced cysts stained positively with DBL and expressed cyst-specific antigens, CST-1 and BAG1, similar to control samples. (Scale bar = 5 µm) (C) Cysts induced by FLU contained amylopectin granules (A) and posterior nucleus (N) and prominent cyst wall (W) by EM. (Scale bar 200 nm). (D) Survival following acute challenge with T. gondii and treatment with FLU. Two experiments combined, N= 10 animals per group. (E) Luciferase imaging revealed decreased burdens in mice treated with FLU (†, 4 of 5 control animals died). Mean ± s.d. from a representative experiment, N= 5 animals each. (F) Reduced cyst counts were observed in the brain of mice treated with 2 mg/ kg FLU. Two experiments combined, N = 8 animals / group, except for control where only a single animal survived
In addition to blocking egress in vitro, FLU treatment was also able to prevent lethal infection in laboratory mice, which provide an excellent model for toxoplasmosis (Fig. 4D). Imaging of parasite dissemination in vivo using a luciferase expressing stain revealed that FLU treatment greatly reduced parasite burdens during in vivo infection in the mouse (Fig. 4E, Supplementary Fig. 3), which led to decreased cyst formation in the CNS during chronic infection (Fig. 4F). Fluridone is a registered herbicide that has low toxicity to mammalian cells, owing to the absence of this biosynthetic pathway in animals. Our results, combined with a previous patent indicating that it is also effective against malaria 18, suggest that this selectivity may be exploited for development of improved anti-parasitic drugs.
ABA synthesis in plants proceeds from isoprenoids using an indirect pathway that flows through β-carotene in the plastid 5. T. gondii utilizes a non-mevalonate pathway for isoprenoid biosynthesis 19, which occurs in the apicoplast, a remnant organelle derived from an algal endosymbiont 20. The sensitivity of T. gondii to fluridone indicates that it contains an indirect pathway for ABA production, which depends on the plastid, rather than the direct pathway found in fungi 5, thus providing an additional explanation for the essential nature of the apicoplast 20. While the T. gondii genome (http://ToxoDB.org) contains several candidates with similarity to ABA synthesis genes in higher plants (i.e ABA1, ABA2, and ABA3)5, phylogenetic and domain analyses do not provide unambiguous assignments of these orthologues (data not shown). This divergence is not unexpected given that the plant-like nature of apicomplexans is based on acquisition of an early branching algal endosymbiont 21. While algae have also been shown to make ABA 22,23 and respond to this hormone 24, the precise pathways and genes involved in ABA production by algae are not established. Future studies aimed at defining the pathway for production of ABA in T. gondii may provide further insight into the role of this hormone outside of higher plants.
Our findings demonstrate the conservation of a common plant-like, calcium-signaling pathway in the protozoan parasite T. gondii. Production of ABA is a density-dependent signal that influences the decision for lytic growth vs. dormant development in T. gondii. ABA has recently been shown to regulate gene expression by altering mRNA translation 25, and to activate G-protein coupled receptor signaling 26 in plants, indicating that it has additional important functions beyond calcium signaling. These ABA-response genes are conserved in T. gondii, and the related parasite Plasmodium (http://ApiDB.org), where they may play analogous roles in ABA-mediated signaling and development. Disruption of ABA-mediated signaling in parasites offers a promising new target for development of improved interventions aimed at combating infection with apicomplexan parasites.
Methods Summary
Growth and egress assays
Intracellular parasite growth was monitored using β-galactosidase activity 7, or by microscopic examination determination of the number of parasites per vacuole 13,15. Egress was monitored by the % of intact vacuoles after cultured for defined intervals with and without FLU, followed by treatment with ABA.
Secretion assay
Calcium-dependent secretion was monitored by release of the microneme protein 2 (MIC2)7,27, with β-galactosidase as a cell-loading and non-specific lysis control.
ABA purification and analysis
ABA was extracted from parasites, purified by HPLC, converted to methyl-ABA 28, and analyzed by tandem mass spectrometry using an Agilent Technologies GC-MS instrument to detect the diagnostic fragments 12.
ABA detection by ELISA
ABA concentrations in parasite extracts were determined by ELISA using the Phytodetek® ABA Test Kit (Agdia, Elkhart, IN) after subtraction of the background level of ABA in cultured host cells processed in parallel, and expressed as intracellular concentrations 29.
Cyclic ADPR
Freshly harvested parasites were snap frozen at −80°C, extracted, and cADPR levels determined as described previously 8.
In vitro differentiation
Cyst formation was monitored following treatment with FLU under normal culture conditions as detected using FITC-labeled Dolichos biflorus lectin (DBL) (Sigma) or antibodies to the cyst antigens CST1 16 or BAG1 17. Transmission electron microscopy was performed as described previously 15.
Mouse infections
BALB/c mice were infected with type II strain PTG strain by ip injection and treated with fluridone by daily i.p. injection for 12 days. To monitor tissue burdens, mice were challenged with the luciferase expressing Pru-Luc strain and imaged as described previously 30.
Full Methods accompany this paper
Methods
Chemicals
Chemicals were purchased from Sigma-Aldrich (St. Louis, MO), including: (±) cis, trans abscisic acid (referred to as (± ABA or ABA), (−) cis, trans ABA (- ABA), retinoic acid, and β-carotene. BAPTA and BAPTA-AM were obtained from Molecular Probes (Invitrogen, Carlsbad, CA). Fluridone was obtained from Chem Service Inc., (West Chester, PA). Chemicals were dissolved in DMSO and stored at −80°C.
Protein secretion
To examine the induction of microneme protein secretion, purified 2F strain parasites, which express β-galactodisase, were treated with compounds for 5–10 min at 18°C, and separated into supernatant and cell pellet by centrifugation. MIC2 secretion was detected by Western blotting with anti-MIC2 rabbit antibody (supernatant). Cell pellets were probed with anti- β-galactosidase monoclonal antibody 40a-1 as a control for loading and inadvertent lysis. Secreted MIC2 in the supernatant migrates slightly faster than cellular MIC2 (cMIC2) due to proteolysis during shedding 27. To test the roles of extracellular and intracellular calcium, respectively, purified parasites were treated with 1 mM of BAPTA or 50 µM of BAPTA-AM for 20 min at 18 °C before treatment with 100 nM of ABA for 5 min, as described previously 7.
Growth and egress assays
Parasite replication and egress were monitored by microscopic examination of parasites grown in human foreskin fibroblast (HFF) cells cultured on glass coverslips. Following culture for specific time intervals, the number of intracellular parasites per vacuole, and the % of intact vs. disrupted vacuoles (egress), was determined by examining specific antibody-stained cells (i.e. SAG1 surface straining, DAPI nuclear staining) under epifluorescence microscopy, as described previously 13,15. Egress was monitored following culture in normal medium for defined intervals followed by stimulation with ABA for 1 hr, and microscopic examination to determine the % of intact vacuoles as above. To test the ability of ABA to overcome inhibition of egress by FLU, cells were cultured in control medium or medium containing FLU for 38 hrs, then treated with ABA for 1 hr, and examined microscopically to determine the % of intact vacuoles as above.
GC-MS analysis of ABA
Parasites from heavily infected cultures at 30–36 hrs post inoculation, were snap frozen in an ethanol-dry ice bath, lyophilized, and stored at −80°C. Tissues were extracted in organic solvents and resolved by HPLC using a C18 reverse phase column as described previously 28. Fractions containing ABA were collected, dried under vacuum, methylated using diazomethane, and analyzed by gas chromatography in comparison to standards for methyl-ABA (kindly provided by Jan Zeevaart, Michigan State Univ.), as described previously 28. Tandem mass spectrometry analyses were performed on an Agilent Technologies GC-MS (6890N network GC system). Samples (dissolved in 10 µL) and standards (appropriate dilutions) were solubilized in ethyl acetate, and injected into the GC-MS instrument. The samples were introduced through a DB-5 capillary column (30m × 0.250 mm i.d., film thickness 0.25 µm (J&W Scientific)) with helium as the carrier gas (flow-rate 1.3 mL / min). The GC oven was programmed from 60 to 210°C at 25°C min−1 and from 210 to 280°C at 12°C min−1. The ion-source temperature was 150°C. The mass analyzer was scanned from m/z 50 to 400 at a data rate of 20 Hz for the full scan analyses, and m/z 190 at a data rate of 20 Hz for single ion monitoring (SIM) analyses. Spectra from parasite extracts were compared to the methyl-ABA standard and previously reported literature alues of cis and trans ABA 12.
ABA detection by ELISA
ABA concentrations in parasite extracts were determined by ELISA using the Phytodetek® ABA Test Kit (Agdia, Elkhart, IN) after subtraction of the background level of ABA in cultured host cells processed in parallel. Concentrations of ABA in the parasite were expressed as intracellular concentrations based on previous estimates of the parasite volume 29.
Cyclic ADPR
T. gondii parasites were purified from culture and treated with doses of ABA for 10 min. Parasites were snap frozen at −80°C, extracted in 5% v/v trichloroacetic acid at 4°C, and cADPR levels determined using the cyclase reaction, as described previously 8.
In vitro differentiation
Type II strain ME49 parasites were induced to differentiate by treatment with FLU in normal culture medium (DMEM, 10% FBS, 10 mM HEPES, gentamicin) during culture for 60 hrs at 37°C, 5% CO2. Alternatively, induction was performed by culture 72–96 hrs in RPMI (pH 8.1) in atmospheric air at 37°C. Cysts were stained with FITC-labeled Dolichos biflorus lectin (DBL) (Sigma), DAPI to label DNA, anti-CST1 mAb73.18 16, and anti-BAG1 17 (kindly provided by Louis Weiss, Albert Einstein College of Medicine) followed by fluorescently conjugated secondary antibodies. For ultrastuctural analysis, cultures were induced as described above and processed for transmission electron microscopy, as described previously 15. Cysts were classified based on the presence of a convoluted cyst wall, amylopectin granules, solid-staining rhoptries, and the posterior position of the nucleus 15.
In vivo infections in mice
BALB/c mice were purchased from Charles River Laboratories, Inc. (Wilmington, MA) and infected with tachyzoites of the type II strain PTG, or the type II PRU strain expressing firefly luciferase (LUC) under the control of the T. gondii alpha tubulin promoter (kindly provided by John Boothroyd, Stanford Univ.). Parasites were propagated in vitro in monolayers of HFF cells and tachyzoites were injected by intraperitoneal (i.p.) route. An inoculum capable of resulting in death in 90% of untreated animals (LD90) was used for challenges that consisted of 2 × 103 parasites for PRU-LUC and 2 × 105 for PTG strain parasites. Animals were treated for 12 days by daily i.p. injection with 1 mg/kg or 2 mg/kg of fluridone (Chem Services, West Chester, PA) that was dissolved in DMSO and diluted in PBS. Experiments were conducted with 3–5 animals per group and were repeated 3 or more times. Survival was recorded for 30 days.
For bioluminescence experiments, animals were imaged at two day intervals, following mild anesthesia using isofluorene and i.p. injection of 0.15 mg/kg body weight of firefly luciferin D (Biosynth AG, Switzerland). Animals were imaged using a Xenogen IVIS 100 (Caliper LifeSciences, Hopkinton, MA). Data are expressed in relative light units and are shown as mean ± s.d.. Experiments contained 5 animals per group (except a single animal was used for the non-infected negative control). Animals were followed for 30 days post-infection, after which chronic infection was confirmed by examining homogenates of the brain for tissue cysts, as described previously 15. Briefly, animals were humanely sacrificed, the brains removed and homogenized in 1 ml of sterile PBS. Homogenates were examined by bright field or phase contrast microscopy at 40X and cysts were counted from 4 separate 10 µL aliquotes.
Supplementary Material
Acknowledgements
We thank Jan Zeevaart for conducting the initial purification and analysis of ABA and for supplying standards, John Boothroyd, and Lou Weiss for providing reagents, Wandy Beatty, Silvia Moreno, Boris Striepen, Andy Waters and Liming Xiong for helpful comments, and Julie Nawas and Darcy Gill for expert technical assistance. Supported by the Uehara Medical Foundation (to K.N.), the Mayo Clinic and American Heart Association (to E.N.C.), and a grant from the NIH (to L.D.S.).
Footnotes
Supplementary Information accompanies the paper on www.nature.com/nature.
Author contributions:
K.N. performed the majority of experiments on the affects of ABA and FLU on the parasite, L.M.H. performed the MS studies, B.F. performed the animal studies, F.B. contributed to the analysis of ABA genes, E.N.C. performed the measurements of cADPR, L.D.S. supervised the project and wrote the manuscript with input from all the authors.
References
- 1.Moreno SN, Docampo R. Calcium regulation in protozoan parasites. Curr. Opin. Microbiol. 2003;6:359–364. doi: 10.1016/s1369-5274(03)00091-2. [DOI] [PubMed] [Google Scholar]
- 2.Berridge MJ, Bootman MD, Roderick HL. Calcium signaling: dynamics, homeostasis and remodelling. Nat. Rev. Molec. Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 3.Bothwell JHF, Ng CKY. The evolution of Ca2+ signaling in photosynthetic eukaryotes. New Phytologist. 2005;166:21–38. doi: 10.1111/j.1469-8137.2004.01312.x. [DOI] [PubMed] [Google Scholar]
- 4.Xiong L, Zhu JK. Regulation of abscisic acid biosynthesis. Plant Physiol. 2003;133:29–36. doi: 10.1104/pp.103.025395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwartz SH, Qin X, Zeevaart JAD. Elucidation of the indirect pathway of abscisic acid biosynthesis by mutants, genes, and enzymes. Plant Physiol. 2003;131:1591–1601. doi: 10.1104/pp.102.017921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu Y, et al. Abscisic acid signaling through cyclic ADP ribose in plants. Science. 1997;278:2126–2130. doi: 10.1126/science.278.5346.2126. [DOI] [PubMed] [Google Scholar]
- 7.Lovett JL, Marchesini N, Moreno SN, Sibley LD. Toxoplasma gondii microneme secretion involves intracellular Ca2+ release from IP3 / ryanodine sensitive stores. J. Biol. Chem. 2002;277:25870–25876. doi: 10.1074/jbc.M202553200. [DOI] [PubMed] [Google Scholar]
- 8.Chini EN, Nagamune K, Wetzel DM, Sibley LD. Evidence that the cADPR signaling pathway controls calcium-mediated secretion in Toxoplasma gondii. Biochem. J. 2005;389:269–277. doi: 10.1042/BJ20041971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagamune K, Sibley LD. Comparative genomic and phylogenetic analyses of calcium ATPases and calcium-regulated proteins in the Apicomplexa. Molec. Biol. Evol. 2006;23:1613–1627. doi: 10.1093/molbev/msl026. [DOI] [PubMed] [Google Scholar]
- 10.Puce S, et al. Abscisic acid signaling through cyclic ADP-ribose in hydroid regeneration. J. Bio. Chem. 2004;279:39783–39788. doi: 10.1074/jbc.M405348200. [DOI] [PubMed] [Google Scholar]
- 11.Zocchi E, et al. The temperature-signaling cascade in sponges involves a heat-gated cation channel, abscisic acid, and cyclic ADP ribose. Proc. Natl. Acad. Sci. USA. 2001;98:14859–14864. doi: 10.1073/pnas.261448698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Almeida JAS, Kascheres C, Pereira MDA. Eythylene and abscisic acid in the control of development of the rhizome of Koleria eriantha (Benth.) Hanst. (Gesneriaceae) Braz. J. Plant Physiol. 2005;17:391–399. [Google Scholar]
- 13.Moudy R, Manning TJ, Beckers CJ. The loss of cytoplasmic potassium upon host cell breakdown triggers egress of Toxoplasma gondii. J. Biol. Chem. 2001;276:41492–41501. doi: 10.1074/jbc.M106154200. [DOI] [PubMed] [Google Scholar]
- 14.Endo T, Sethi KK, Piekarski G. Toxoplasma gondii: calcium ionophore A23187-mediated exit of trophozoites from infected murine macrophages. Exp. Parasitol. 1982;53:179–188. doi: 10.1016/0014-4894(82)90059-5. [DOI] [PubMed] [Google Scholar]
- 15.Fux B, et al. Toxoplasma gondii strains defective in oral transmission are also defective in developmental stage differentiation. Infect. Immun. 2007;75:2580–2590. doi: 10.1128/IAI.00085-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang YW, Halonen SK, Ma YF, Wittner M, Weiss LM. Initial characterization of CST1, a Toxoplasma gondii cyst wall glycoprotein. Infect. Immun. 2001;69:501–507. doi: 10.1128/IAI.69.1.501-507.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang YW, et al. Disruption of the Toxoplasma gondii bradyzoite-specific gene BAG1 decreases in vivo cyst formation. Molec. Microbiol. 1999;31:691–701. doi: 10.1046/j.1365-2958.1999.01210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leef JL, Carlson PS. USA: Potomax Ltd. Prtn.; 1999. pp. 1–8. [Google Scholar]
- 19.Jomaa H, et al. Inhibitors of the non-mevalonate pathway of isoprenoid biosynthesis as antimalarial drugs. Science. 1999;285:1573–1576. doi: 10.1126/science.285.5433.1573. [DOI] [PubMed] [Google Scholar]
- 20.Ralph SA, et al. Metabolic maps and functions of the Plasmodium falciparum apicoplast. Nature. 2004;2:203–216. doi: 10.1038/nrmicro843. [DOI] [PubMed] [Google Scholar]
- 21.Waller RF, McFadden GI. The apicoplast: a review of the derived plastid of apicomplexan parasites. Curr. Issues Mol. Biol. 2005;7:57–79. [PubMed] [Google Scholar]
- 22.Hirsch R, Hartung W, Gimmler H. Abscisic acid content of algae under stress. Botanica Acta. 102:326–334. 19889. [Google Scholar]
- 23.Cowan AK, Rose PD. Abscisic acid metabolism in salt stressed cells of Dunaliella salina. Plant Physiol. 1991;97:798–803. doi: 10.1104/pp.97.2.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobayashi M, Hirai N, Kurimura Y, Ohigashi H, Tsuji Y. Abscisic acid-dependent algal morphogenesis in the unicellular green alga Haematococcus pluvialis. Plant Growth Reg. 1997;22:79–85. [Google Scholar]
- 25.Razem FA, El-Kereamy A, Abrams SR, Hill RD. The RNA-binding protein FCA is an abscisic acid receptor. Nature. 2006;439:290–294. doi: 10.1038/nature04373. [DOI] [PubMed] [Google Scholar]
- 26.Liu X, et al. A G protein-coupled receptor is a plasma membrane receptor for the plant hormone abscisic acid. Science. 2007;315:1712–1716. doi: 10.1126/science.1135882. [DOI] [PubMed] [Google Scholar]
- 27.Carruthers VB, Sherman GD, Sibley LD. The Toxoplasma adhesive protein MIC2 is proteolytically processed at multiple sites by two parasite-derived proteases. J. Biol. Chem. 2000;275:14346–14353. doi: 10.1074/jbc.275.19.14346. [DOI] [PubMed] [Google Scholar]
- 28.Tian L, DellaPenna D, Zeevaart JAD. Effect of hydroxylated carotenoid deficiency on ABA accumulation in Arabidopsis. Physiol. Plant. 2004;122:314–320. [Google Scholar]
- 29.Rodrigues CO, Ruiz FA, Rohloff P, Scott DA, Moreno SNJ. Characterization of isolated acidocalcisomes from Toxoplasma gondii tachyzoites reveals a novel pool of hydrolyzable polyphosphate. J. Biol. Chem. 2002;277:48650–48656. doi: 10.1074/jbc.M208990200. [DOI] [PubMed] [Google Scholar]
- 30.Saeij JP, Boyle JP, Grigg ME, Arrizabalaga G, Boothroyd JC. Bioluminescence imaging of Toxoplasma gondii infection in living mice reveals dramatic differences between strains. Infect. Immun. 2005;73:695–702. doi: 10.1128/IAI.73.2.695-702.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.