Abstract
Background
We tested the hypothesis that patients recovered from leptospirosis have peripheral blood memory T cells specific for Leptospira or Leptospira protein antigens.
Methods
Peripheral blood mononuclear cells (PBMC) were obtained from recovered leptospirosis patients, and from control individuals. PBMC were assessed for in vitro proliferation, phenotyping and cytokine production following stimulation with different strains of Leptospira, recombinant LipL32, or overlapping synthetic peptides of different outer membrane proteins
Results
Both control and patient PBMC produced significant proliferative responses to all Leptospira strains. Proliferation from control PBMC were significantly higher than responses produced by patient PBMC. Select strains of Leptospira expanded both TCRαβ and TCRγδ T cells in both control and patient PBMC. Patient and control PBMC produced equivalent levels of TNFα and IFNγ, but patient PBMC produced significantly less IL10 than control PBMC following stimulation by different strains of Leptospira. PBMC from patients failed to respond to recombinant LipL32 or to any of the Leptospira peptides.
Conclusion
Leptospira induced significant proliferative responses, TCRγδ T cell expansion and cytokine production in both control and patient PBMC. Patient PBMC failed to recognize Leptospira protein antigens. Leptospirosis does not seem to generate memory T cells that can be activated by in vitro stimulation.
Keywords: Leptospirosis, Zoonotic Diseases, Cellular Immunology, Human
Introduction
Leptospirosis is a zoonotic disease caused by spirochetes of the genus Leptospira (1). This disease is found worldwide with a disproportionate impact in tropical climates (2). Infection by Leptospira spp. can lead to widely divergent clinical outcomes: symptomatic infection, common in endemic regions (3); an undifferentiated febrile illness or an aseptic meningitis syndrome with low morbidity (4); or fulminant disease that has diverse manifestations including a septic shock-like syndrome, jaundice, renal failure, myocarditis/heart failure, hemorrhage, and death (5).
The role of cell-mediated immunity in host defense to Leptospira remains poorly understood in both animal models and human disease. One study has shown that cattle immunized with a killed Leptospira vaccine develop protective immunity associated with CD4+ T cells and γδT cells that produce in vitro proliferative responses and IFNγ secretion following stimulation with a Leptospira Ag preparation (6). Naturally acquired immunity that protects against re-infection by Leptospira does occur, but this has been shown to be serovar-specific in animal models (7), and has been suggested to be mediated primarily by LPS-specific antibody (7, 8, 9, 10). There is also evidence that antibodies specific to Leptospira membrane-associated proteins may play a role in host defense (7, 11) in animal models of vaccination.
Nonetheless, little is known about the human immune response to Leptospira. We have reported that in vitro stimulation of human peripheral blood mononuclear cells (PBMC), from leptospirosis-naïve individuals, with a pathogenic Leptospira isolate results in the expansion of not only TCRαβ-T cells but also TCRγδ-T cells and high production of IFNγ and other Th1 cytokines (12). Additionally, an uncharacterized leptospiral glycolipoprotein has also been shown to induce in vitro production of TNF-α and IL-10 as well as up-regulate the expression of CD69 and HLA DR on PBMC (13). Elevated levels of soluble IL-2R, IL-6, and TNFα have been demonstrated in sera obtained from patients treated for acute leptospirosis (14). Heat-killed Leptospira have been shown to induce IFNγ and IL-12 production from human whole blood cultures (15). Still, it is unknown if people living in leptospirosis endemic regions develop naturally acquired protective immunity against severe disease or whether cellular immune responses develop following recovery from leptospirosis. A key question--whether spontaneously recovered Leptospira-infected patients have protective immunological memory--is a fundamentally important question yet to be resolved. In this study, we tested the hypothesis that patients recovered from leptospirosis have peripheral blood memory T cells specific for Leptospira whose function could be demonstrated by in vitro recall responses to Leptospira or leptospiral proteins.
Materials and Methods
Reagents
Purified protein derivative (PPD) was obtained from Mycos Research. FITC/PE-conjugated anti-human TCRαβ, TCRγδ from Caltag Laboratories. Overlapping peptides for in vitro stimulation of human PBMC were from the following Leptospira: 1) L. interrogans L1130 LipL32, AAS69953.1, 2) L. interrogans L1130 LipL21, AAS68648.1, 3) L. interrogans L1130 OmpL1 AY461983, and 4) L. interrogans L1130 LipL41,AE017298. All peptides were 15aa in length, with 10 aa overlaps between sequential peptides. The overlapping peptides covered the full length of each protein: 1) 53 peptides for LipL32, 2) 36 peptides for LipL21, 3) 62 peptides for OmpL1, and 4) 69 peptides for LipL41. Pools containing 10 – 12 peptides were prepared and used for in vitro stimulation. His6-LipL32 fusion protein was purified by affinity chromatography and dialyzed against PBS containing 10% glycerol, 0.3% Triton X-100 (16). The purity of the His6-LipL32 fusion protein was verified by SDS-PAGE and shown to be free of LPS using an LAL.
Patients and controls
There were 23 patients and 30 controls enrolled. Isolates were not obtained from all patients. Patients were diagnosed by microscopic agglutination. While serology does not necessarily identify an infecting serovar (17) and there is broad cross- reactivity for different serovars (hence the percentages add to greater than 100%), the frequency of serovar identification was as follows: Icterohaemorrhagiae (30%), Copenhageni (26%), Bratislava (26%), Var10 (65%), Australis (17%), Autumnalis (8.7%), Canicola (13.0%), Cynopteri (4.3%). In patients recovered from leptospirosis, the blood sample was collected 0.5–2.8 years after diagnosis by MAT. Controls (n=23) were healthy volunteers with no history of Leptospira exposure (lived in Lima, seronegative). For cell proliferation/ phenotyping/cytokine production, PBMC were stimulated with different Leptospira. Var10, RGA and HAI188 were assessed for inducing cell proliferation, phenotyping and/or cytokine production in PBMC from 13 patients and 13 controls. Seven different controls were stimulated with varying numbers of live versus formalin- fixed Leptospira (Var10 and RGA), and 6 different controls were also assessed for proliferation following stimulation with HAI024, HAI864 and HRL280 strains. Finally, PBMC from 10 patients and 10 controls were stimulated with recombinant proteins and synthetic peptides and tested for cell proliferation. The study was approved by the Committee of Ethics of the Universidad Peruana Cayetano Heredia and the University of California San Diego Human Subjects Protections Program; informed consent was provided by all subjects.
Bacteria
For in vitro stimulation of PBMC, Leptospira were grown in modified EMJH liquid to log phase, counted using a Petroff-Hauser counting chamber under the dark field microscopy, and then washed once and adjusted to the correct density. In some experiments, the bacteria were formalin-fixed, 0.5% by 2 h then washed 4 times and adjusted to the correct number in sterile PBS. The following isolates were used in this study: RGA was obtained from ATCC and is a standard reference strain of L. interrogans serovar Icterohaemorrhagiae and has been continuously cultured for decades (i.e high passage). Fresh isolates included Leptospira licerasiae serovar Var10 (18) ( confirmed to infect rats, Vinetz, unpublished) and several strains of L. interrogans serovar Icterohaemorragia: 1) strain HAI188, newly isolated strain of L. interrogans serovar Icterohaemorrhagiae (19), 2) HAI024 (L. interrogans serovar Icterohaemorrhagiae), 3) HAI864 (L. interrogans serovar Icterohaemorrhagiae), and 4) HRL280 (L. santarosai serovar unique as determined by PFGE).These strains were isolated from symtomatic patients in the Iquitos region-Perú and identified by sequencing of the16S ribosomal gene and pulse field gel electrophoresis (20).
PBMC culture
PBMC were isolated using Ficoll-Paque™Plus (Pharmacia, Uppsala, Sweden). Cells were set up in 96-well plates at 2×105 cells /well (cell proliferation and cytokines) or in 24-well plates at 1×106 cells /well (cell phenotyping) with one of the following: 1) medium only, 2) varying numbers of live Leptospira, 3) varying quantities of LipL32 recombinant, 4) 2µg/well from each peptide pool, with each pool containing no more than 12 peptides, or 5) PPD Mycobacterium tuberculosis (5µg/ml). PPD was used as a positive control for measuring an in vitro memory response, since a majority of the population had been immunized with BCG. Cell cultures were incubated in RPMI 1640 medium (Gibco) containing 5% heat-inactivated human serum and penicillin / streptomycin.
Cell Proliferation Assay
Cell proliferation was measured from 96 well plate cultures after varying times in culture. Each well was pulsed with 1 µCi of [3H] thymidine (SIGMA St. Louis, MO), and cultured for 5 hrs. The total incorporated counts per minute of [3H] thymidine were determined by a 1205 Liquid Scintillation Counter, Betaplate, Wallac. The mean counts per minute (CPM) of triplicate cultures was calculated. Results are expressed as CPM or proliferation index, which is the ratio of the CPM of cultures exposed to antigen to CPM from control cultures treated with medium alone. A proliferation index ≥ 2.0 was considered positive.
Flow Cytometry Analysis
The phenotype of different T cell populations present in PBMC stimulated by different antigens was determined using flow cytometry. PBMC were cultured in 24 well plates (1×106/mL) with different antigens and then assessed at 6 days for the percentages of T cells bearing TCRαβ versus TCRγδ. The PBMC were incubated with a panel of monoclonal FITC-or PE conjugated mAbs specific for T cells expressing TCRαβ versus TCRγδ . In parallel, additional cells were also incubated with the corresponding isotype control monoclonals. After washing, cells were analyzed using a FACSCalibur (Becton Dickinson, San Jose CA). The percentages of positive cells were obtained using the software Cellquest (BD).
Cytokine measurements
PBMC cultures, as described above, were incubated for 6 days. At the end of culture, supernatants were collected. TNFα, IFNγ, IL-2 and IL-10 were measured in culture supernatants by ELISA (BD OptEIA™ kit, BDBioscience)
Statistical Analysis
Data are expressed as mean ± standard deviation. The proliferation index, cell surface phenotypes (TCR αβ vs. γδ), and cytokine profiles in the in vitro response to Leptospira antigens between PBMC from recovered leptospirosis patients versus controls subjects were compared with the U-test Mann-Whitney. To compare treatments with different antigens (live or formalin- fixed bacteria), the Wilcoxon Test was used. Probabilities (p value) less than 0.05 indicate a significant statistical difference. STATA TM 9.0 2005 was used for the statistical analysis.
Results
Leptospira induce proliferative responses in PBMC from healthy humans
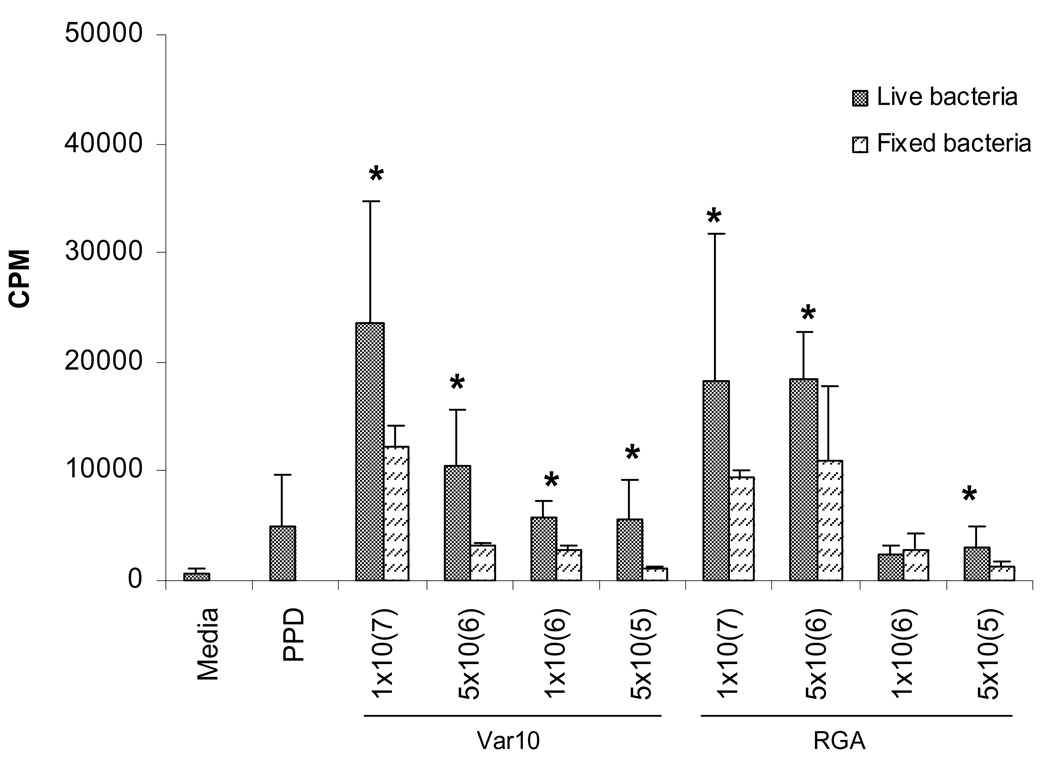
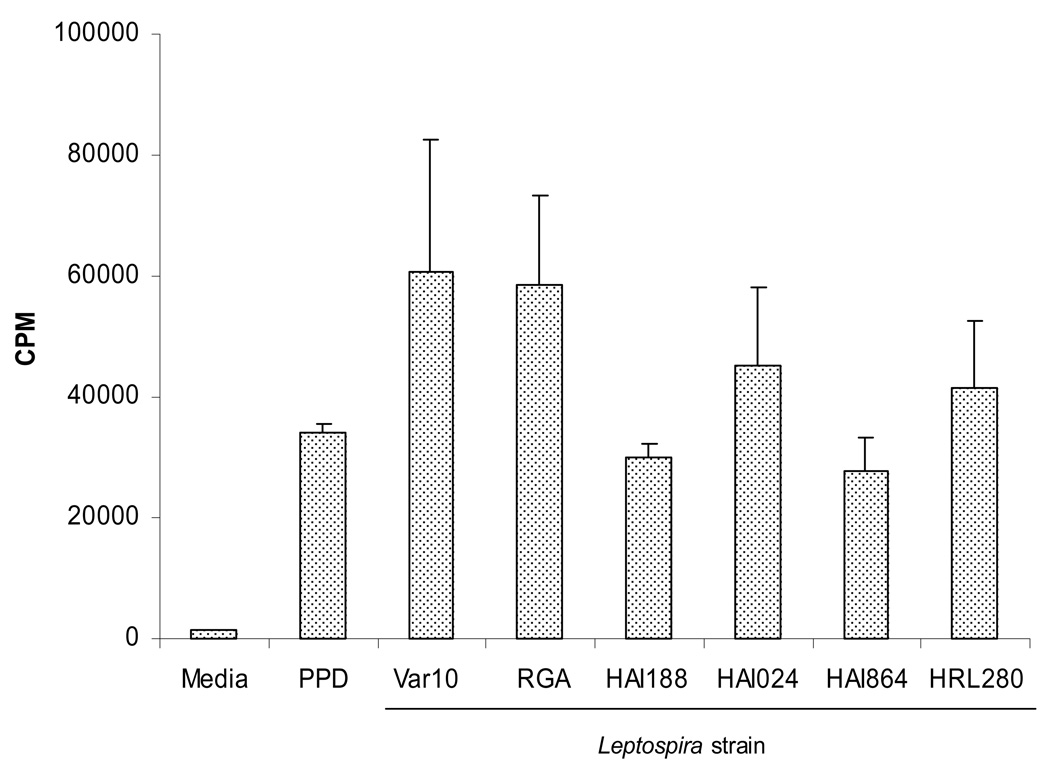
We have previously shown that PBMC from normal healthy volunteers, with no previous infection by Leptospira, gave high in vitro proliferative responses upon exposure to Leptospira interrogans (strain M20) (12). These vigorous proliferative responses were associated with the production of high levels of Th1 cytokines and expansion of both TCRαβ and TCRγδ T cells. As an essential first step towards determining whether PBMC from recovered leptospirosis patients could give an in vitro memory response, we initiated a series of experiments to investigate whether PBMC from normal humans responded in a similar fashion to other Leptospira. PBMC were assessed for proliferation following in vitro exposure to Var10 versus RGA. Data presented in Figure 1 show the results from a representative experiment determining the best dose of bacteria for inducing a proliferative response and comparing fixed versus live Leptospira. Optimal proliferative responses were found using high numbers of Leptospira (107), and were maximal at day six of culture (data not shown). Live Leptospira were superior to fixed bacteria in inducing a maximal response. To further explore the ability of PBMC from normal volunteers to respond to Leptospira, we assessed PBMC from a number of control donors for their ability to give proliferative responses to a number of different strains of Leptospira. PBMC gave significant proliferative responses when stimulated by four other Leptospira strains (Fig. 2), but there were differences produced by fresh isolates vs. strains in long-term culture (RGA) and between even recently obtained strains. Thus, Leptospira can induce significant proliferative responses in PBMC obtained from individuals without a history of Leptospira infection. In fact, similar results were obtained from experiments performed at the University of Texas Medical Branch in Galveston, Texas using normal volunteer PBMC (data not shown).
Figure 1.
Live Leptospira- induced proliferation of control PBMC. PBMC were cultured in microtiter wells at 2×105/well with one of the following: 1) medium only, 2) PPD (5µg/mL), or 3) Leptospira, or 4) formalin- fixed Leptospira. Cell proliferation was assessed at 6 days by measuring the amount of [3H] thymidine incorporation. Results are from one experiment that was representative of 7 independent experiments (7 different controls). * P<0.05 live vs fixed bacteria.
Figure 2.
Different Leptospira strains induce proliferation of control PBMC. PBMC were cultured in microtiter wells at 2×105/well with one of the following: 1) medium only, 2) PPD (5µg/mL) or 3) different strains of Leptospira (1×107). Cell proliferation was assessed at 6 days by measuring the amount of [3H] thymidine incorporation. Results are from one experiment that was representative of 6 independent experiments (6 different controls).
PBMC from recovered Leptospirosis patients fail to give enhanced in vitro proliferation to Leptospira
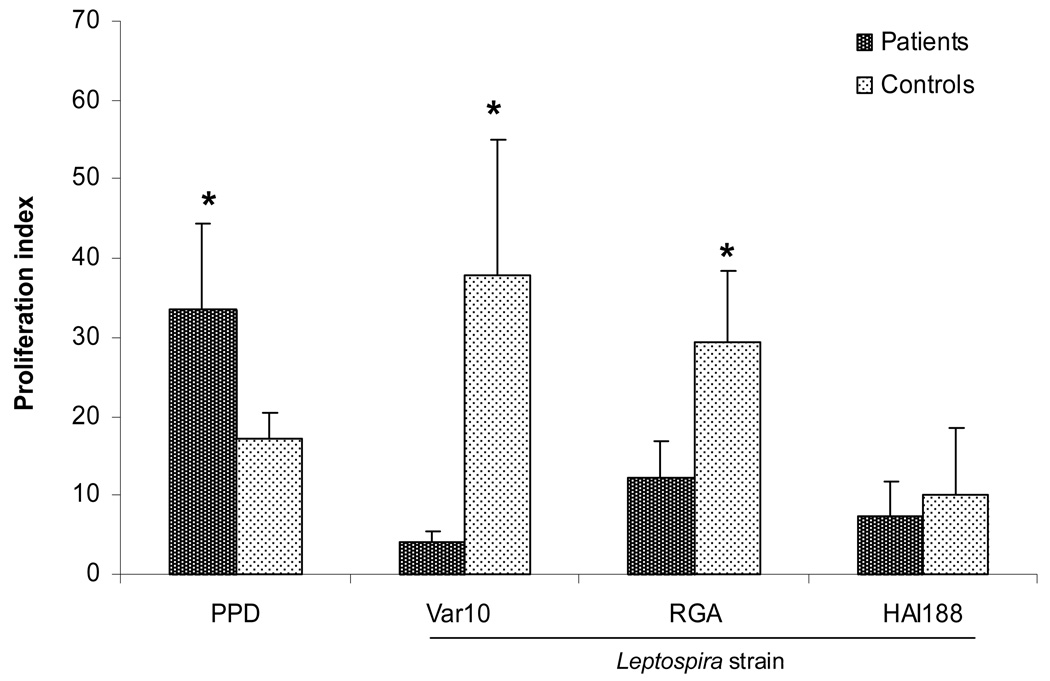
A series of experiments were then performed using PBMC from patients who had been identified as having recovered from leptospirosis. The goal was to directly test the hypothesis that such patients would have peripheral blood memory T cells specific for Leptospira, and whose function could be demonstrated by enhanced in vitro proliferative responses to Leptospira. We predicted that PBMC from these patients would give a significantly higher proliferative response to Leptospira when compared to that of PBMC from control individuals who had never been exposed or infected with Leptospira. We then compared the in vitro proliferative responses of PBMC from 13 different patients versus 13 different control volunteers to three different strains of Leptospira: Var10, RGA, and HAI188 (Fig. 3). Surprisingly, when stimulated by Var10 and RGA, PBMC from patients produced significantly lower responses compared to responses given by control PBMC. Control PBMC reproducibly produced significantly higher responses to these Leptospira. However, HAI188 induced lower, but identical proliferative responses from both patient and control PBMC. This finding was also observed at three days post stimulation and with varying numbers of either live or fixed Leptospira (data not shown). Even though PBMC from patients gave a lower response to Leptospira, they produced higher responses to the recall antigen PPD, when compared to PBMC from controls. Thus, recovered leptospirosis patients seem to have a diminished or suppressed in vitro proliferative response to Leptospira.
Figure 3.
Different Leptospira strains induced proliferation of control versus patients PBMC. PBMC were cultured in microtiter wells at 2×105/well with one of the following: 1) medium only, 2) PPD (5µg/mL) or 3) Var10, RGA, or HAI188 (1×107). Cell proliferation was assessed at 6 days by measuring the amount of [3H] thymidine incorporation. Results are mean of 13 experiments (13 different patients and 13 different controls) . * P<0.05 Patients vs Controls.
Leptospira-induced expansion of TCRαβ versus TCRγδ T cells
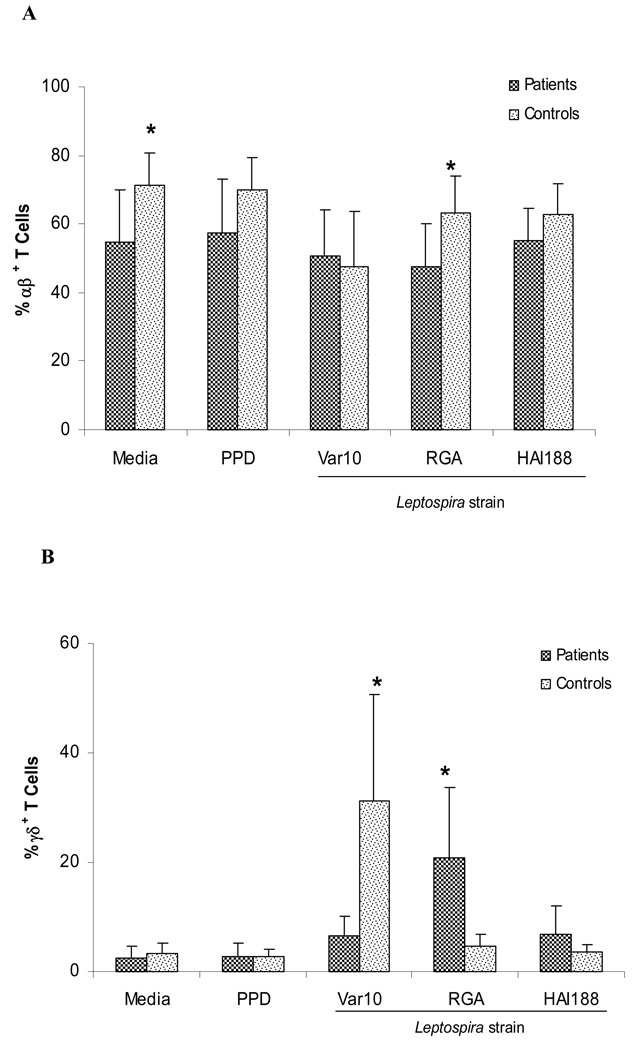
To further investigate the TCR phenotypes associated with proliferative responses induced by different Leptospira, we performed a series of experiments to characterize the T cells present at six days of culture following exposure to three different Leptospira strains (Var10, RGA, and HAI188). Figure 4A and 4B show the percentages of TCRαβ versus TCRγδ T cells present in cultures at six days post stimulation with the different Leptospira strains. TCRαβ T cells were the majority of T cells present in all cultures. The only significant difference between patients and controls for these T cells (TCRαβ) were cultures stimulated with RGA. In these cultures, control PBMC contained slightly more TCRαβ T cells then the patient PBMC. However, there was a significant difference in the percentage of TCRγδ T cells in Leptospira cultures when comparing the different strains and between patients versus controls. Var10 consistently induced high percentages of TCRγδ T cells in control cultures. Surprisingly, this was not observed in PBMC from patient cultures. In contrast, PBMC from patients yielded a high percentage of TCRγδ T cells in cultures stimulated with RGA when compared to control PBMC, which yielded significantly fewer TCRγδ T cells at six days of culture. The significance of these differences is unclear, but seem to indicate that there is preferential expansion of TCRγδ T cells in response to different serovars.
Figure 4.
Preferential expansion of TCRαβ+ (A) and TCRγδ+ (B) T cells. Patient and control PBMC were set up in 24 well culture wells (106/well) and stimulated with Var10 vs RGA vs HAI188 (1×108 ). Cultures were terminated on day 6, and cells obtained from each culture were phenotyped by flow cytometry. Data shown are the mean of 13 experiments (13 different patients and 13 different controls) . * P<0.05 difference between responses of patient PBMC versus control PBMC. Panel A presents TCRαβ T cells Panel B presents TCRγδ T cells.
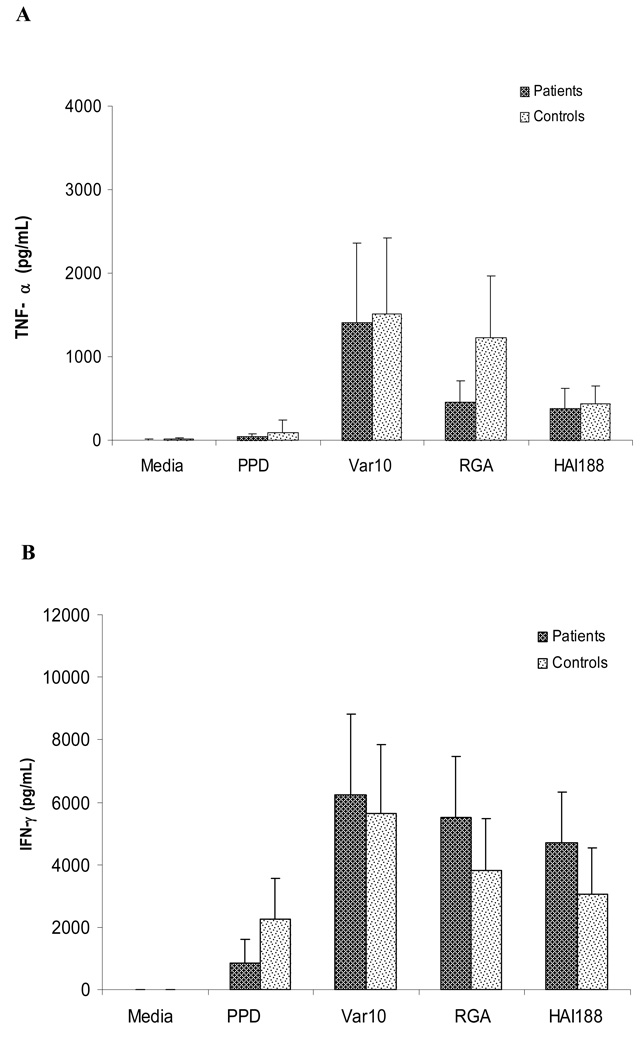
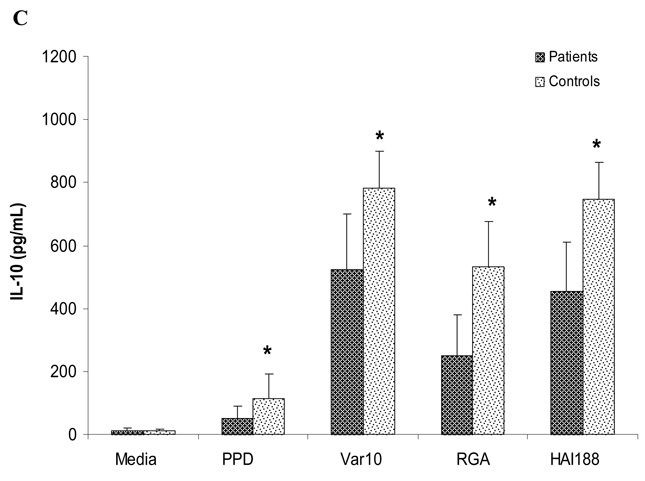
Cytokine production by PBMC from patient versus controls
To explore whether antigen-induced cytokine production could be used to assess in vitro memory T cell responses, we compared cytokine production by PBMC from patients versus controls following in vitro stimulation with Var10, RGA, or HAI188. PBMC were established as described above for measuring proliferation, except culture supernatants were harvested at 48hrs and 6 days of culture. Levels of IFNγ, TNFα and IL10 were assessed in supernatants by ELISA (Fig. 5A, B and C), which used supernatants from cultures at 6 days. There was no difference in the levels of IFNγ or TNFα produced by PBMC from patients versus controls when stimulated with any of the three different Leptospira strains. However, PBMC from patients always produced significantly less IL10 than PBMC from controls, regardless of which Leptospira strain was used for stimulation; this finding held true after PPD stimulation as well. Identical results were also obtained from supernatants obtained from cultures at 48hrs post stimulation (data not shown). The significance of lower levels of IL10 being produced by PBMC from patients is unclear. However, the absence of elevated levels of cytokine production by PBMC from patients is compatible with the proliferation data which also showed no difference between patient versus control PBMC.
Figure 5.
Cytokine profile produced by PBMC obtained from controls versus patients. PBMC were stimulated with one of the following: 1) medium only, 2) PPD (5µg/mL) or 3) Leptospira (1×108): Var10 vs RGA vs HAI188. Culture supernatants were collected at six days of culture and assessed for different cytokine levels. Panel A: TNF-α, Panel B: IFN-γ and Panel C: IL-10. Data presented are the mean of 13 experiments (13 different patients and 13 different controls) . * P<0.05 difference between patients and controls.
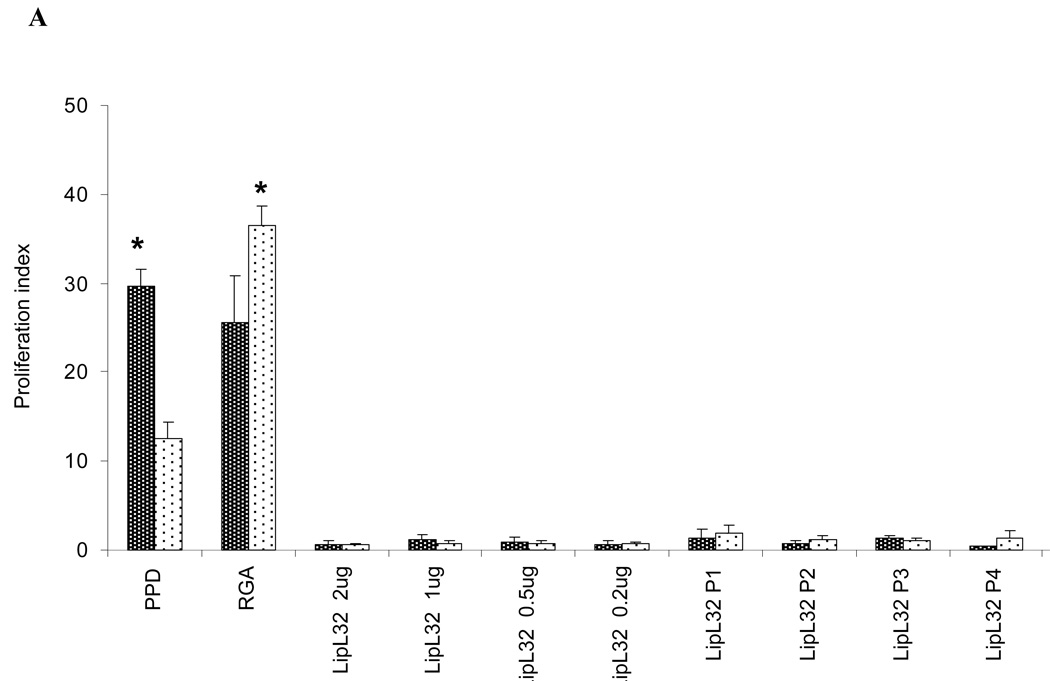
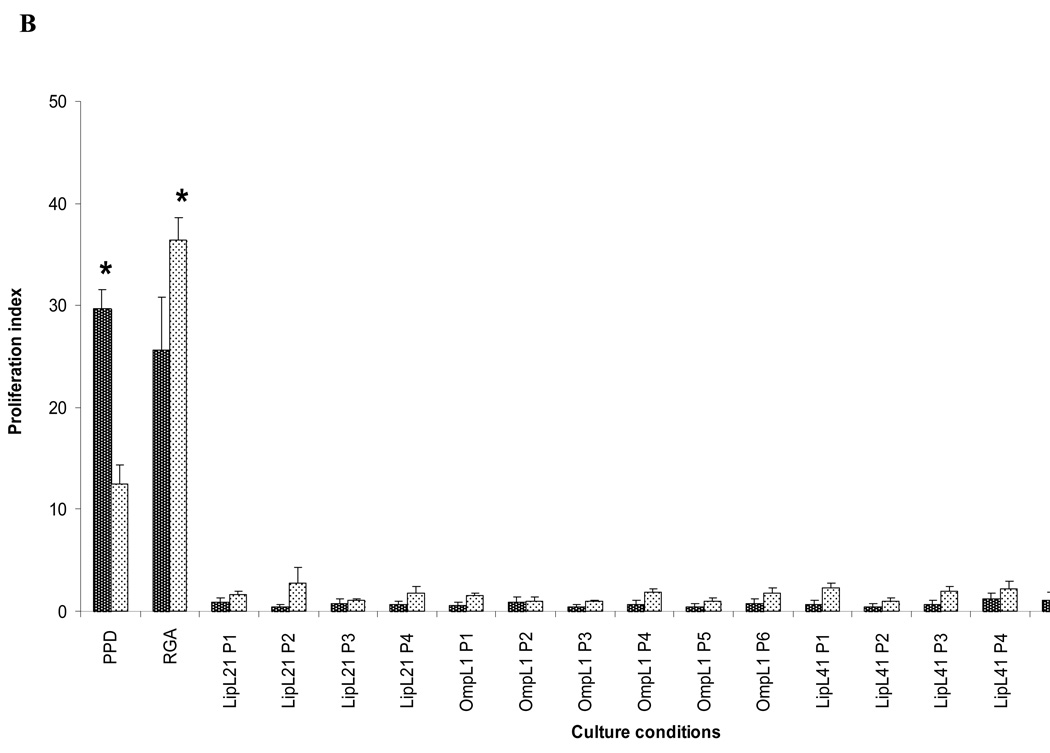
Stimulation of PBMC by Leptospira proteins and peptides
Whole Leptospira, either fixed or live, induced proliferation in PBMC from normal control individuals (Figs. 1 and 2). Since this phenomenon could complicate or mask memory T cell responses from patient PBMCs, we next investigated whether purified Leptospira proteins or synthetic peptides derived from previously recognized protein antigens could preferentially induce a high proliferative response in PBMC from patients. Thus, PBMC from patients and controls were assessed for proliferation at six days following incubation with one of the following: 1) medium, 2) PPD (positive control for recall responses), 3) recombinant LipL32, or 4) overlapping peptides from LipL32 (Figure 6A), Lip21, OmpL1 or LipL41 (Fig 6B). Although PBMC from both patients and controls gave strong proliferative responses to the recall antigen PPD, no increase in proliferation was observed from either control or patient PBMC when exposed to any of the peptides or LipL32. These results suggest that no T cell memory to these antigens developed during infection and recovery from leptospirosis.
Figure 6.
PBMC from patients fail to respond to Leptospira antigens. PBMC from leptospirosis recovering patients and control subjects were cultured with one of the following: 1) medium only, 2) PPD, 3) RGA (1×107), 4) LipL32 recombinant protein, or 5) overlapping peptides to LipL32, LipL21, OmpL1, and LipL41. Data presented is the Proliferation Index at day 6 of culture. Panel A: LipL32 recombinant and synthetic peptides, Panel B: LipL21, OmpL1 and LipL41 synthetic peptides. Peptides and recombinant LipL32 were used a 10 µg/ml. Data presented are from one experiment that was representative of 10 different experiments (10 different patients and 10 different controls). P=pool *P<0.05 Patients vs Control PBMC.
Discussion
The role of the T cells in protection and recovery from leptospirosis remains poorly understood in humans. In the present study we demonstrated that PBMC from humans, who spontaneously recovered from leptospirosis in the hyperendemic setting of the Peruvian Amazon, fail to mount an antigen-specific T cell proliferative response to leptospiral antigens. The lack of PBMC proliferation was in response to a variety of leptospiral antigens including intact whole Leptospira cells as well as recombinant and synthetic peptide antigens, previously shown to be recognized by antibodies from human and animal model infections. We also observed that whole leptospiral cells stimulated PBMCs from both previously infected and Leptospira-naïve humans. This observation could reflect a superantigen-like property associated with Leptospira, and appeared to be more pronounced with long-term in vitro cultivated Leptospira than with fresh leptospiral isolates.
A major goal of this study was to compare PBMC from recovered leptospirosis patients with PBMC from Leptospira-naïve individuals. Surprisingly, we found that PBMC from recovered patients gave a significantly lower proliferative response to Var10 and RGA when compared to the response given by PBMC from control individuals. However, TCRγδ+ T cells expanded from patient PBMC following in vitro exposure to the long-term in vitro cultivated L. interrogans serovar Icterohaemorrhagiae RGA strain, which was not the case for PBMC from controls. The significance of this difference is unclear, but might suggest that RGA is a common strain associated with this particular area of Perú and that the expansion of TCRγδ+ T cells reflected a novel type of memory response in this T cell lineage. We also compared cytokine production by PBMC from patients vs. naïve controls following in vitro stimulation by the three different Leptospira strains, Var10, RGA, and HAI188. PBMC from controls and patients produced equivalent levels of TNFα and IFNγ following in vitro expose to the three Leptospira strains tested. We cannot rule out the possibility that patient PBMC might have developed memory but only to the infecting Leptospira strain. However, we believe this to be unlikely, since patient PBMC failed to respond to any of the proteins or peptides. Interestingly, patient PBMC always produced less IL10 than PBMC from control individuals. The significance of this difference is not clear but could reflect a difference in the cytokine profiles produced by PMBC from patients versus controls, and will be further investigated.
In a final attempt to detect memory T cells after recovery from leptospirosis, PBMC from patients vs. naive controls were tested for in vitro proliferation following exposure to recombinant and synthetic peptide Leptospira antigens. This strategy would hopefully address the possibility that in vitro stimulation of PBMC by whole Leptospira might be complicated by non-specific or superantigen-like activity of Leptospira, which was capable of inducing high proliferation in control PBMC. However, PBMC from patients failed to give any profilerative response to either recombinant, purified, LPS-free LipL32, or to any of the overlapping peptides from four Leptospira outer membrane proteins.
Whether people living in leptospirosis hyperendemic regions such as Iquitos, Perú develop naturally acquired protective immunity against leptospirosis remains unknown. If immunity develops in these patients, then, one potential way to assess this immunity would be to show in vitro recall responses to either Leptospira or leptospiral antigens by PBMC from immune, recovered patients. In vitro recall responses have been demonstrated using PBMC from individuals immune to many different pathogens or antigens. Of direct relevance to the present study, Burkholderia pseudomallei is able to stimulate in vitro memory T cell-mediated cytokine and proliferative responses (21), in contrast to our findings here. Thus, we reasoned that if immunity to leptospirosis develops in recovering patients then PBMC from these patients should give enhanced in vitro proliferative responses, when compared to the responses from PBMC from control individuals. Our previous studies showed that naïve PBMC gave high proliferative responses and produced high levels of Th1 cytokines in response to in vitro exposure to Leptospira interrogans serovar Copenhageni, strain M20 (12). This strain induced the expansion of TCRαβ CD4+ T cells and a 10–50 fold expansion of TCRγδ+ T cells. This observation was surprising and suggested that this strain might contain superantigen-like traits. Thus, a major goal of this study was to investigate other strains of Leptospira for their ability to induce PBMC proliferative responses and cytokine production.
This study is the first to investigate the ability of PBMC from spontaneously recovered leptospirosis patients to give in vitro recall responses to Leptospira and individual leptospiral antigens. We show that PBMC from normal volunteers respond vigorously to in vitro exposure to whole Leptospira and that some Leptospira strains can induce expansion of both TCRαβ CD4+ and TCRγδ+ T cells. More importantly, we present evidence that suggests that leptospirosis does not lead to the generation of memory T cells specific for Leptospira that can be demonstrated by in vitro stimulation.
Acknowledgments
We thank the following members of the Alexander von Humboldt Institute of Tropical Medicine for their support of this work: Sonia Torres and Raul Chuquiyauri for help with patient enrollment and sample collection in Iquitos, Peru; Kalina Campos for help in diagnosis of leptospirosis; Beronica Infante for technical support in the experiments; and Professors Jorge Arevalo, Humberto Guerra and Eduardo Gotuzzo for overall help and guidance. We also thank Judith Haithcoat at UTMB for technical help in the experiments.
Funding
This work was supported by U.S. Public Health Services grants R01AI053422(GK), K24AI068903 (JMV) and R01TW005860 (JMV). IT was a Fogarty Scholar supported by NIH/Fogarty International Center Global Infectious Diseases Training Grant D43TW007120 (JMV).
Footnotes
Conflict of interest statement
None of the authors has any conflict of interest in the work presented.
This work was presented in part at the 5th International Meeting of the International Leptospirosis Society, Quito, Ecuador, 17–20 September 2007, supported in part by U.S. Public Health Service grant R13AI075971.
References
- 1.Vinetz JM. Leptospirosis. Curr. Opin. Infect. Dis. 2001;14:527. doi: 10.1097/00001432-200110000-00005. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Leptospirosis worldwide, 1999. Wkly. Epidemiol. Rec. 1999;74:237. [PubMed]
- 3.Ashford DA, Kaiser RM, Spiegel RA, et al. Asymptomatic infection and risk factors for leptospirosis in Nicaragua. Am. J. Trop. Med. Hyg. 2000;63:249. [PubMed] [Google Scholar]
- 4.Berman SJ, Tsai CC, Holmes K, Fresh JW, Watten RH. Sporadic anicteric leptospirosis in South Vietnam. A study in 150 patients. Ann. Intern. Med. 1973;79:167. doi: 10.7326/0003-4819-79-2-167. [DOI] [PubMed] [Google Scholar]
- 5.Levett PN. Leptospirosis. Clin. Microbiol. Rev. 2001;14:296. doi: 10.1128/CMR.14.2.296-326.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naiman BM, Alt D, Bolin CA, Zuerner R, Baldwin CL. Protective killed Leptospira borgpetersenii vaccine induces potent Th1 immunity comprising responses by CD4 and γ-δ T lymphocytes. Infect. Immun. 2001;69:7550. doi: 10.1128/IAI.69.12.7550-7558.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adler B, Fame S. Host immunological mechanisms in the resistance of mice to leptospiral infections. Infect. Immun. 1977;17:67. doi: 10.1128/iai.17.1.67-72.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sonrier C, Branger C, Michel V, Ruvoen-Clouet N, Ganiere JP, Andre-Fontaine G. Evidence of cross-protection within Leptospira interrogans in an experimental model. Vaccine. 2000;19:86. doi: 10.1016/s0264-410x(00)00129-8. [DOI] [PubMed] [Google Scholar]
- 9.Adler B, Faine S. Serological cross-reactions of leptospiral lipopolysaccharide (F4) antigen. Zbl. Bakt. Hyg. I. Abt. Orig. 1979;A244:291. [PubMed] [Google Scholar]
- 10.Kalambaheti T, Bulach DM, Rajakumar K, Adler B. Genetic organization of the lipopolysaccharide O-antigen biosynthetic locus of Leptospira borgpetersenii serovar Hardjobovis. Microb. Pathog. 1999;27:105. doi: 10.1006/mpat.1999.0285. [DOI] [PubMed] [Google Scholar]
- 11.Haake DA, Mazel MK, McCoy AM, et al. Leptospiral outer membrane proteins OmpL1 and LipL4l exhibit synergistic immunoprotection. Infect. Immun. 1999;67:65. doi: 10.1128/iai.67.12.6572-6582.1999. 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klimpel GR, Mathias MM, Vinetz JM. Leptospira interrogans activation of human peripheral blood mononuclear cells: preferential expansion of TCRγδ+ T cells vs TCRαβ+ T cells. The Journal of Immunology. 2003;171:1447–1477. doi: 10.4049/jimmunol.171.3.1447. [DOI] [PubMed] [Google Scholar]
- 13.Diament D, Brunialti MK, Romero EC, Kallas EG, Salomao R. Peripheral blood mononuclear cell activation induced by Leptospira interrogans glycolipoprotein. Infect. Immun. 2002;70:16. doi: 10.1128/IAI.70.4.1677-1683.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petros SLU, Engelmann L. Serum procalcitonin and proinflammatory cytokines in a patient with acute severe leptospirosis. Scan J Infect Dis. 2000;32:104. doi: 10.1080/00365540050164362. [DOI] [PubMed] [Google Scholar]
- 15.de Fost M, Hartskeeri RA, Groenendijik MR, van der Poll T. Interleukin 12 in part regulates γ interferon release in human whole blood stimulated with Leptospira interrogans. Clin Diagn Lab Immunol. 2003;10:233. doi: 10.1128/CDLI.10.2.332-335.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haake DA, Chao G, Zuerner RL, et al. The Leptospiral Major Outer Membrane Protein LipL32 Is a Lipoprotein Expressed during Mammalian infection. Infection and Immunity. 2000;68:2276–2285. doi: 10.1128/iai.68.4.2276-2285.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levett PN. Usefulness of Serologic Analysis as Predictor of the Infecting Serovar in Patients with Severe Leptospirosis. Clin. Infect. Diseases. 2003;36:447–452. doi: 10.1086/346208. [DOI] [PubMed] [Google Scholar]
- 18.Matthias MA, Ricaldi JN, Cespedes M, et al. Human Leptospirosis caused by a New, Antigenically Unique Leptospira Associated with a Rattus Species Reservoir in the Peruvian Amazon. Plos Negl Trop Dis. 2008;2(4):e213. doi: 10.1371/journal.pntd.0000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Segura ER, Ganoza CA, Campos K, et al. Clinical spectrum of pulmonary involvement in leptospirosis in a region of endemicity, with quantification of leptospiral burden. Clin Infect Dis. 2005;40(3):343–351. doi: 10.1086/427110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ganoza CA, Matthias MA, Collins-Richards D, et al. Determining Risk for severe leptospirosis by molecular analysis of environmental surface waters for pathogenic Leptospira. PLOS Medicine. 2005;l3 doi: 10.1371/journal.pmed.0030308. 1329-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tippayawat P, Saenwongsa W, Mahawantung J, et al. Phenotypic and Functional Characterizatin of Human Memory T Cell Responses to Burkholderia pseudomallie. PLOS Neglected Tropical Diseases. 2009;3:1–10. doi: 10.1371/journal.pntd.0000407. [DOI] [PMC free article] [PubMed] [Google Scholar]