Abstract
Translation of localized mRNAs is an important mechanism for controlling spatially discrete cellular processes. The polarity protein Par-3 is locally translated in axons in response to factors such as NGF and netrin-1, and this increased expression is necessary for factor-stimulated axonal outgrowth.
How is cell polarization initiated and regulated in response to extracellular signals? To date, research has focused mainly on the regulation of the polarity proteins that remodel cell morphology, but translation of localized RNAs necessary for growth and morphogenesis can also influence the response of cells to signals. In this issue, an elegant study by Hengst et al. links these two aspects by demonstrating that in certain neurons the localized translation of the Par-3 polarity protein is induced by factors that stimulate axonal growth and is essential for factor-promoted growth1.
Axonal pathfinding can be considered to be a form of cell polarity in which the growth cone extends in a directional manner, in response to attractive or repulsive cues. Outgrowth occurs at variable rates, depending on these cues. For example, both nerve growth factor (NGF) and netrin-1 can stimulate outgrowth above a basal, factor-independent rate, but how they accomplish this stimulation has been unclear.
Immature axons are uniquely capable of local translation from axonally-enriched mRNAs. To study this process, the Jaffrey laboratory1 makes use of a clever experimental system in which rat embryonic dorsal root ganglia (DRG) are cultured in microfluidic chambers that enable the compartmentalization of axons from the cell bodies2. Remarkably, RNA interference is functional in growing axons, and the selective introduction of siRNAs can silence expression in a spatially discrete manner3. Using this microfluidics chamber to inhibit protein synthesis only within the axons, Hengst et al. have demonstrated that this local translation is essential for factor-stimulated outgrowth1. A number of mRNAs have been identified in axons2, and there may be hundreds more, but in this study Hengst et al. focused on polarity proteins that had been implicated in axon specification in vitro. They detected Par-3 mRNA in DRG axons, and found that after severing the cell bodies, treatment of axons with NGF induced a significant increase in axonal Par-3 protein. Moreover, specific depletion of Par-3 mRNA, by the introduction of siRNAs into the axonal compartment, blocked NGF-dependent Par-3 protein expression, and prevented NGF-stimulated axon outgrowth. Importantly, however, depletion of Par-3 had no effect on basal growth rates. Additional experiments using spinal cord explants and dorsal spinal commissural neurons, which respond to netrin-1 supported the conclusion that factor-stimulated axonal outgrowth requires localized Par-3 translation.
Par-3 is a large, multi-domain scaffold protein that binds to another polarity protein, Par-6, and is phosphorylated by atypical protein kinase C (aPKC)4. These proteins have been highly conserved through metazoan evolution and possibly arose in animals to organize an epithelium, and subsequently expanded their repertoire of functions as evolving body plans became more complicated4,5. In addition to establishing apical/basal polarity in epithelial cells, they control asymmetric divisions, and some types of oriented cell migration. In vertebrates, they have also been implicated in axon specification and in the polarization associated with dendritic spine morphogenesis. It is conceivable that they also act in other processes not directly related to polarity, such as apoptosis and the cell cycle6,7.
How can the same proteins have so many effects, and organize changes in the morphology of entire cells? There are likely to be multiple outputs from the Par proteins, although their ultimate effects on the cell often involve the regulation of actin remodeling and microtubule dynamics. Both cytoskeletal elements are intrinsically asymmetric and not only control cell morphology at a structural level, but also regulate intercellular adhesion, vesicle traffic, RNA localization, and cell division. In addition, there are multiple layers of control of Par protein activity. Context-dependent protein interactions allow Par proteins to control one signaling pathway in one cell type and another signaling pathway in a different cell type, or at a different time or place within the cell. For example, Par-3 associates with a protein called Inscuteable in fly neuroblasts and in mammalian keratinocytes, which in turn ensures the correct orientation of the mitotic spindle5. But Par-3 also controls the distribution of cell fate determinants in neuroblasts via a distinct pathway involving Par-6, aPKC and another polarity protein called Lgl5. Second, multiple kinases can phosphorylate Par-3 or Par-6 to modify their activities and some might be specific to particular circumstances, such as stimulation of cells by receptor tyrosine kinases, or intercellular adhesion4. Differential expression of Par proteins could also affect polarization, but to date has not been studied extensively. The par genes appear to be ubiquitously expressed although various isoforms of par-6, apkc and lgl present in vertebrate genomes show distinct tissue distributions8. Hengst et al. highlights one interesting mechanism to control expression – local, signal-activated translation of Par-3. Surprisingly, neither Par-6 nor aPKC mRNAs were detected in the axons.
RNA localization and local translation are important drivers of cell polarization in a number of situations, and have been intensively studied in the Drosophila oocyte, in budding yeast, and in vertebrate neurons9. The local translation of proteins at synapses might play a key role in long-term memory formation10. Recently, RNA localization has also been found to play important roles in apical/basal polarization. The mRNA for the formin Diaphanous is apically localized in Drosophila epithelial cells11 and mRNAs for two epithelial-specific polarity proteins, Crumbs and Stardust, are also enriched near the apical surface12,13. Notably, only certain splice variants of the Stardust mRNA are localized and these are expressed only early in epithelial development12. Translation can, therefore, have a temporal as well as a spatial component, which might be regulated by a variety of signaling pathways.
The results described in the paper by Hengst et al. open up a range of important questions. For instance, why is the translation of Par-3 induced by NGF and netrin but not that of Par-6 or aPKC? One possibility is that the Par-6/aPKC complex in developing axons is normally inhibited by association with Lgl, and that NGF- or netrin-stimulation produces sufficient Par-3 to displace Lgl5. Recently, several groups have demonstrated the phosphorylation of Par-3 by aPKC, and it would be interesting to test if stimulated axonal outgrowth could be rescued in Par-3 siRNA-treated neurons by the expression of a nonphosphorylatable mutant of Par-3, particularly because a constitutively active variant of aPKC is expressed in neurons. Alternatively, increased levels of Par-3 might sequester the Rac exchange factor Tiam1, and alter the spatial distribution of Rac-GTP within the growing axon4. In addition, Par-3 has recently been found to bind the exocyst, so its increased expression might promote the delivery of vesicles to produce new plasma membrane during axonal outgrowth14. The authors suggest that Par-3 regulates the phosphorylation of GSK3β, which is known to control microtubule stability in the growth cone; however, in other systems where it has been studied, the Par-6/aPKC complex and not Par-3 regulates GSK3β phosphorylation7,15, and in the DRG axons, Par-3 is present in puncta along the length of the axon rather than being enriched in the growth cone.
One other puzzling aspect of the data is that Par-3 is constitutively present in the axons and NGF or netrin-1 stimulation induces only a relatively small increase (1.3 - 2-fold) in the axonal concentration of Par-3 protein. It remains unclear how this increase would be sufficient to trigger changes in signaling to increase axonal outgrowth. The localized Par-3 transcript might represent a splice variant that has a unique function in the developing axon. The mammalian Par-3 gene can encode at least 20 splice variants that alter the open reading frame, and an unknown number of others that might alter the 3′ UTR. Nothing is known about most of these variants, although some lack the aPKC phosphorylation sites, and others lack the N-terminal oligomerization domain or the C-terminal region. Alternatively, newly synthesized Par-3 may behave differently than pre-existing Par-3 that has been post-translationally modified. During its synthesis, it might also be available to interact with binding partners that cannot associate with a fully folded form.
Another question concerns how Par-3 expression is regulated in the axons. What is the 3′UTR motif that targets Par-3 mRNA to axons? Is this motif neuron-specific? Is it unique to Par-3 or are other mRNAs localized by the same mechanism? And what are the factors involved in activating translation of Par-3 mRNA downstream of NGF and netrin-1? Whatever the answers, the data in hand confirm that the Par proteins are components of a pivotal signaling node that controls many functions other than classical cell polarization, and that they are subject to many layers of sophisticated regulation. The data also further strengthen the view that the regulated translation of localized mRNAs is a widespread and essential process in morphogenesis.
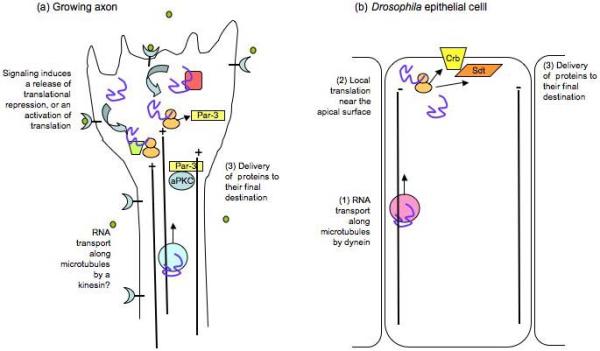
Localized polarity protein translation in axons and epithelial cells. (a) In immature axons of sensory neurons, NGF or netrin-1 (green circles) can stimulate outgrowth, which depends on the localized translation of Par-3 mRNA. Signaling from the receptors might either release a translational repressor, or permit the association of a translational activator to increase protein expression. In nascent axons, Par-3 protein is transported to the growth cone by a kinesin, but it is not known if this also happens in DRG axons. Par-3 may interact with other polarity proteins such as aPKC to regulate outgrowth. (b) In fly embryonic epithelial cells, the transcripts for 2 polarity proteins, Crumbs (crb) and Stardust (Sdt) are transported by dynein to the apical region of the cell, where they are locally translated. A specific splice variant of Sdt is localized.
References
- 1.Hengst U, et al. Axonal elongation triggered by stimulus-coupled local translation of a polarity complex protein. Nature Cell Biology. 2009;11 doi: 10.1038/ncb1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taylor AM, et al. A microfluidic culture platform for CNS axonal injury, regeneration and transport. Nat Methods. 2005;2(8):599. doi: 10.1038/nmeth777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hengst U, Cox LJ, Macosko EZ, Jaffrey SR. Functional and selective RNA interference in developing axons and growth cones. J Neurosci. 2006;26(21):5727. doi: 10.1523/JNEUROSCI.5229-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldstein B, Macara IG. The PAR proteins: fundamental players in animal cell polarization. Dev Cell. 2007;13(5):609. doi: 10.1016/j.devcel.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knoblich JA. Mechanisms of asymmetric stem cell division. Cell. 2008;132(4):583. doi: 10.1016/j.cell.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 6.Aranda V, et al. Par6-aPKC uncouples ErbB2 induced disruption of polarized epithelial organization from proliferation control. Nat Cell Biol. 2006;8(11):1235. doi: 10.1038/ncb1485. [DOI] [PubMed] [Google Scholar]
- 7.Kim M, et al. Polarity proteins PAR6 and aPKC regulate cell death through GSK-3beta in 3D epithelial morphogenesis. J Cell Sci. 2007;120(Pt 14):2309. doi: 10.1242/jcs.007443. [DOI] [PubMed] [Google Scholar]
- 8.Joberty G, Petersen C, Gao L, Macara IG. The cell-polarity protein Par6 links Par3 and atypical protein kinase C to Cdc42. Nat Cell Biol. 2000;2(8):531. doi: 10.1038/35019573. [DOI] [PubMed] [Google Scholar]
- 9.Czaplinski K, Singer RH. Pathways for mRNA localization in the cytoplasm. Trends Biochem Sci. 2006;31(12):687. doi: 10.1016/j.tibs.2006.10.007. [DOI] [PubMed] [Google Scholar]; Martin KC, Ephrussi A. mRNA localization: gene expression in the spatial dimension. Cell. 2009;136(4):719. doi: 10.1016/j.cell.2009.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang DO, et al. Synapse- and stimulus-specific local translation during long-term neuronal plasticity. Science. 2009;324(5934):1536. doi: 10.1126/science.1173205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Massarwa R, Schejter ED, Shilo BZ. Apical secretion in epithelial tubes of the Drosophila embryo is directed by the Formin-family protein Diaphanous. Dev Cell. 2009;16(6):877. doi: 10.1016/j.devcel.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 12.Horne-Badovinac S, Bilder D. Dynein regulates epithelial polarity and the apical localization of stardust A mRNA. PLoS Genet. 2008;4(1):e8. doi: 10.1371/journal.pgen.0040008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Z, Wang L, Hays TS, Cai Y. Dynein-mediated apical localization of crumbs transcripts is required for Crumbs activity in epithelial polarity. J Cell Biol. 2008;180(1):31. doi: 10.1083/jcb.200707007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lalli G. RalA and the exocyst complex influence neuronal polarity through PAR-3 and aPKC. J Cell Sci. 2009;122(Pt 10):1499. doi: 10.1242/jcs.044339. [DOI] [PubMed] [Google Scholar]
- 15.Etienne-Manneville S, Hall A. Cdc42 regulates GSK-3beta and adenomatous polyposis coli to control cell polarity. Nature. 2003;421(6924):753. doi: 10.1038/nature01423. [DOI] [PubMed] [Google Scholar]


