Abstract
We sought to reduce tumor hypoxia by topical application of a vasodilator, benzyl nicotinate (BN), and investigated its effect on the growth of tumors irradiated at times when tumor pO2 increased. EPR oximetry was used to follow the changes in the tissue pO2 of subcutaneous radiation-induced fibrosarcoma (RIF-1) tumors during topical applications of 1.25–8% BN formulations for 5 consecutive days. The RIF-1 tumors were hypoxic with a tissue pO2 of 4.6–7.0 mmHg. A significant increase in tumor pO2 occurred 10–30 min after BN application. The formulation with the minimal BN concentration that produced a significant increase in tumor pO2 was used for the radiation study. The tumors were irradiated (4 Gy × 5) at the time of the maximum increase in pO2 observed with the 2.5% BN formulation. The tumors with an increase in pO2 of greaterthan 2 mmHg from the baseline after application of BN on day 1 had a significant growth inhibition compared to the tumors with an increase in pO2 of less than 2 mmHg. The results indicate that the irradiation of tumors at the time of an increase in pO2 after the topical application of the 2.5% BN formulation led to a significant growth inhibition. EPR oximetry provided dynamic information on the changes in tumor pO2, which could be used to identify responders and non-responders and schedule therapy during the experiments.
INTRODUCTION
The presence of hypoxia in tumors and its impact on therapeutic resistance has been observed in various preclinical and clinical studies (1, 2). Tumor hypoxia also leads to aggressive tumor behavior and tumor metastasis (3, 4). Thus hypoxic regions should be minimized or eliminated by improving tumor oxygenation to gain therapeutic benefits, but so far this approach has yielded mixed results. Raising arterial pO2 (partial pressure of oxygen) by inhalation of hyperoxic gases (usually carbogen) was effective in a large number of tumor models and patients (5–7); however, some studies reported no effect or a worsening of the oxygenation status (8, 9). ARCON (accelerated radiotherapy with carbogen and nicotinamide) combines radiation with hypoxia-modifying methods, with the aim of counteracting tumor cell repopulation and hypoxia resistance mechanisms (10, 11). Promising results have been obtained in several clinical studies using ARCON (12–15); however, the results of a phase I/II study (16), and non-small cell lung cancer (17) and glioblastoma (18) trials were not encouraging. Nicotinamide was used in these studies because it was thought to be a fairly non-toxic drug, but a daily administration of 80 mg/kg nicotinamide during radiotherapy caused nausea and vomiting in most patients (19). Over 30% of the patients discontinued use due to these side effects, which were generally unresponsive to antiemetic drugs (20, 21). Other nicotinate derivatives also have been investigated to achieve better tumor oxygenation to enhance radio-therapeutic response (22, 23). However, in most of these experiments, the drugs were administered systemically. Gallez et al. found an increase in tumor oxygenation by intraperitoneal administration of xanthinol nicotinate using the transplantable mouse liver tumor (TLT) model (22, 23).
To optimize hypoxia-modifying methods and combine these efficaciously with radiotherapy, it is important to measure temporal changes in tumor pO2 during treatments. Therapeutic outcome could be enhanced if the radiation could be scheduled at times of increased tumor oxygenation. However, until recently, the lack of methods that can provide repeated measurement of tumor pO2 noninvasively has restricted these optimizations. In vivo EPR oximetry has the capability to provide repeated measurements of tumor pO2 and is being used increasingly to investigate hypoxia-modifying methods and demonstrate therapeutic optimization (24–28).
To establish an alternative and potentially less toxic approach and to test the effectiveness of monitoring tumor pO2 by EPR oximetry, we focused on the development of formulations with benzyl nicotinate (BN) that could be applied topically to enhance tissue pO2 of peripheral tumors. BN is an ester of nicotinic acid and acts as a prodrug that crosses the skin and releases nicotinic acid upon enzymatic hydrolysis. This provokes an increase in cutaneous blood flow, at least partly by enhancing the formation of vasodilating prostaglandins (29). As a consequence, the level of oxygen in the tissue is augmented. The time of maximal effect and the duration of vasodilation depend on the concentration of the drug, its chemical structure (nicotinic acid and different esters: methyl, ethyl, hexyl, benzyl) (30), and the delivery system. A maximum increase in tissue pO2 of the oral mucosa was observed with 2% BN in polymethyl methacrylate (PMM) ointment (31). The effects of BN formulations with nanosized delivery systems and penetration enhancers on skin pO2 (32) and formulations with liposomes of different sizes and compositions on oral mucosa pO2 have been reported earlier (33).
We used EPR oximetry to determine the effect of different concentrations of BN in the topical formulation on the pO2 of subcutaneous RIF-1 tumors. We also investigated the therapeutic outcome when the tumors were irradiated (4 Gy × 5) at the time of an increase in tumor pO2 observed with the optimal BN formulation determined from the pO2 studies.
MATERIALS AND METHODS
Study Design
The effects of different concentration of BN (1.25%, 2.5%, 5%, 8%) in microemulsion with 1% penetration enhancers (MEPE) on the pO2 of RIF-1 tumors were measured by multisite EPR oximetry for 5 consecutive days. The changes in tumor pO2 were analyzed to determine the BN formulation that resulted in a consistent and significant increase in tumor pO2 over several days. This BN formulation was then used for studies with radiation. The tumor pO2 was assessed before and after BN application, and the tumors were irradiated with 4 Gy at the time of maximum increase in tumor pO2. This protocol was repeated for 5 consecutive days. The same protocol was followed for the control group except that the tumors were treated with the formulaton without BN. The tumor volume was measured each day before irradiation, and the tumor growth was compared between groups to determine the therapeutic efficacy
General Methods
1. Tumor models
The radiation-induced fibrosarcoma (RIF-1) cells were a gift from Dr. J. B. Mitchell’s laboratory at the National Cancer Institute. This well-established subcutaneous tumor model has been used for several studies in our laboratory (24, 34, 35). The cells were cultured in vitro in RPMI 1640 medium supplemented with 10% FBS, glutamine and antibiotics. The procedure for tumor inoculation was described previously (34). Briefly, female C3H/HEJ mice (18–20 g, Charles River Laboratories, Wilmington, MA) were anesthetized (1.2% isoflurane, 30% O2), and a suspension of 2 × 105 cells in 50 μl was injected subcutaneously into the left posterior flank. The tumors were allowed to grow for 12–14 days, and oxygen-sensitive lithium phthalocyanine (LiPc) crystals were implanted into the tumors as described below.
2. Tumor volume measurement
The tumor volumes were estimated using the formula π/6 × length × width2 as described previously (34).
3. Preparation of BN formulations
BN (Fluka, Buchs, Switzerland) was incorporated into the microemulsion with a penetration enhancer (MEPE, vehicle) at different concentrations using the methods described by Abramovic et al. (32). The final BN concentrations in the formulations were 1.25%, 2.5%, 5% and 8%. Briefly, BN was incorporated into the oil phase of a water-in-oil type microemulsion by dissolving in a mixture of surfactant (PEG-8 caprylic/capric glyceride, Labrasol®, Gattefosse, Saint-Priest Cedex, France), cosurfactant (polyglyceryl-6-dioleate, Plurol oleique®, Gattefosse), and oil (caprylic/capric triglyceride, Mygliol 812®, Hüls, Witten, Germany). Finally, a combination of sorbitan laurate and N-lauroyl sarcosine (penetration enhancers, Fluka, Buchs, Switzerland) 3:2 w/w was dissolved in the formulation prepared above. We also added colloidal silica (Aerosil 200®, Degussa AG, Düsseldorf, Germany) at the end to increase the viscosity of the formulation so that formulations could be applied properly on the tumors.
4. Paramagnetic probe for EPR oximetry
LiPc crystals were synthesized in our laboratory; their physico-chemical properties and use for measuring tissue pO2 have been described previously (24, 25, 34, 36). LiPc has a single sharp EPR line whose width is highly sensitive to pO2. The EPR spectra reflect the average partial pressure of oxygen on the surface of the crystals. The high density of unpaired spins combined with the narrow intrinsic line width of LiPc (37) allows the measurements of tissue pO2 using one or more crystals (30–50 μg) with a total diameter of ~200 μm. For LiPc implantation, the mice were anesthetized (1.5% isoflurane, 30% FiO2), and two aggregates of LiPc crystals (30–50 μg each) were implanted in each tumor using 25-gauge needles and wire styluses. The implants were about 0.5–1.0 mm from the tumor surface and were approximately 4.0 mm apart.
5. EPR oximetry
EPR oximetry was performed on an L-band (1.2 GHz) EPR spectrometer with an external loop resonator specifically designed for in vivo experiments (26). A set of coils capable of generating a magnetic-field gradient (3.0 G/cm) was used to separate the EPR spectra of the two implants in each tumor (38). The spectrometer parameters were: incident microwave power, 2 mW; magnetic-field center, 425 gauss; scan range, 2 gauss; modulation frequency, 24 kHz. The modulation amplitude was one-third of the EPR line width with scan time of 10 s. We averaged six scans to enhance the signal-to-noise ratio. The EPR line widths were converted to pO2 using a calibration determined for the LiPc crystals used in this study.
Experiment Protocol
1. Experimental groups
Mice were assigned randomly into seven groups for the determination of an optimal BN formulation: (A) MEPE (no BN, control, N = 10), (B) 1.25% BN (N = 9), (C) 2.5% BN (N = 12), (D) 5% BN (N = 11), (E) 8% BN (N = 10). The results were analyzed to determine the formulation that provided an optimal increase in tumor pO2, and this formulation was used to investigate the effects of radiation in groups (F), MEPE + 4 Gy × 5 (sham control, N = 9), and (G), 2.5% BN + 4 Gy × 5 (N = 10).
2. Procedure for application of BN and irradiation
The mice were anesthetized (1.5% isoflurane, 30% O2) 24 h after LiPc implantation (day 1), and the baseline pO2 was measured for 30 min. Then 0.2 ml of the formulation was applied gently to the tumor for 1 min without moving the mouse from the EPR magnet; the tumor was covered with a thin plastic film to minimize any drying of the formulation. EPR measurements were then continued for 60 min to determine the effect of the BN formulations on tumor pO2. The protocol was repeated for 5 consecutive days. In the control group, the formulation without BN (i.e. MEPE) was applied and the same protocol was followed. The tumor pO2 measured on day 1 prior to any treatment is the baseline pO2, while the pO2 measured on days 2–5 before the application of BN formulations is the pretreatment pO2. Similar terms are also used for tumor volume measurements.
For the irradiation study, a 2.5% BN formulation was applied after baseline tumor pO2 measurement and the measurements were continued for another 20 min. The animals were then moved to the irradiation bed of a Clinac 2100C (Varian Linear Accelerator, 6 MeV, 6 cm × 6 cm applicator), and the beam was focused on the tumor. Appropriate lead shields were used to limit irradiation of the normal tissue of the mice. The approximate time between the end of the EPR measurements and the beginning of the irradiation was 6–8 min. The animals were then immediately moved back to the EPR spectrometer for pO2 measurements (usual time between irradiation and resumption of EPR measurements was 6–10 min), which lasted at least 15 min. In the sham control group, the formulation without BN was applied to the tumor and the same protocol was followed.
3. Physiological control and histological analysis
During EPR measurements, the body temperature of the animals was monitored using a rectal probe and was maintained at 37.0 ± 0.5°C using a thermostatically controlled heated pad and a flow of warm air. The animals were kept warm by an electric heating pad during the transportation to and from the irradiator.
On day 6, the animals were killed humanely, and tumors were removed, fixed and sectioned. Microscopic examination (H&E staining) of the tissue around the LiPc deposits was performed to confirm its location in the tumor. The LiPc deposits were in the interstitial compartment of the tumor tissue with no evidence of edema or infiltration of the inflammatory cells.
Statistical Analysis
The tumor pO2 data were averaged for every 5 min to compare pO2 changes in each group and between groups. A paired t test was used to determine the statistical significance of the changes in pO2 and tumor volume within a group, and an unpaired t test was used to determine the significance of differences between groups. The paired comparison reduces the effects of animal-to-animal heterogeneity and eliminates differences in the baseline pO2. The tests were two-sided, and a change with P < 0.05 was considered significant. All data are expressed as means ± SEM; n is the total number of LiPc implants, and N is the number of animals in each group.
RESULTS
Effect of Formulations with Different Concentrations of BN on Tumor pO2 and Growth
The mean baseline tumor pO2 and tumor volumes on day 1 and the mean pretreatment tumor pO2 and volume on subsequent days for each group are summarized in Table 1. No significant differences in the baseline pO2 and pretreatment pO2 resulting from the two LiPc deposits of each tumor were observed (data not shown); therefore, these were pooled to determine average tumor pO2 on each day. There was no apparent effect of tumor size on tumor pO2, and no significant differences in tumor volume between control (MEPE) and treatment groups were observed.
TABLE 1.
Mean Baseline RIF-1 Tumor pO2 and Mean Tumor Volume Measured on 5 and 6 Consecutive Days, Respectively
| Parameter | Groups | Time (days) |
|||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| Tumor pO2 (mmHg) | MEPE | 5.8 ± 1.0 | 6.0 ± 1.0 | 4.8 ± 0.7 | 5.9 ± 0.9 | 5.3 ± 1.0 | |
| 1.25% BN | 5.1 ± 0.9 | 4.8 ± 1.1 | 5.3 ± 1.0 | 5.6 ± 1.3 | 5.5 ± 1.0 | ||
| 2.5% BN | 6.1 ± 0.6 | 6.0 ± 0.7 | 5.9 ± 0.6 | 5.7 ± 0.6 | 5.2 ± 0.6 | ||
| 5% BN | 6.6 ± 0.7 | 6.7 ± 0.8 | 6.3 ± 0.8 | 7.0 ± 0.8 | 7.0 ± 0.6 | ||
| 8% BN | 5.5 ± 1.0 | 4.6 ± 0.9 | 5.1 ± 0.9 | 4.7 ± 0.7 | 4.8 ± 0.8 | ||
| Tumor volume (mm3) | MEPE | 239 ± 22 | 361 ± 35 | 458 ± 39 | 583 ± 50 | 721 ± 48 | 786 ± 46 |
| 1.25% BN | 202 ± 16 | 307 ± 35 | 430 ± 64 | 538 ± 74 | 647 ± 83 | 781 ± 54 | |
| 2.5% BN | 219 ± 16 | 325 ± 28 | 425 ± 37 | 549 ± 48 | 662 ± 65 | 797 ± 65 | |
| 5% BN | 209 ± 15 | 284 ± 22 | 365 ± 28 | 477 ± 32 | 593 ± 32 | 722 ± 36 | |
| 8% BN | 252 ± 33 | 311 ± 39 | 394 ± 46 | 500 ± 50 | 621 ± 50 | 763 ± 56 | |
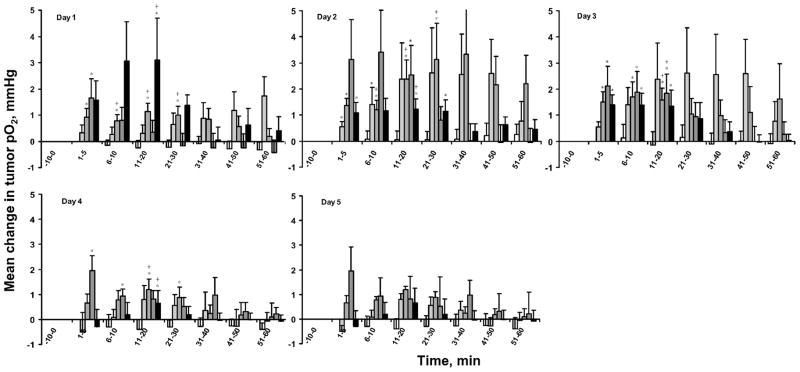
The effect of different concentrations of BN on tumor pO2 varied over days (Fig. 1). The topical application of 1.25% BN resulted in a small but significant increase in tumor pO2 within 10 min only on day 2. In contrast, 2.5% and 5% BN resulted in significant increases in tumor pO2 within 20–30 min from day 1 to day 4, while 8% BN resulted in a significant increase in tumor pO2 on days 1 to 3 only.
FIG. 1.
Mean change in RIF-1 tumor pO2 from baseline/pretreatment (mean pO2 at each time – mean baseline/pretreatment tumor pO2) after the application of MEPE or different concentrations of BN. The pO2 measured for 15 min prior to the application of BN or MEPE were averaged to obtain average baseline pO2/pretreatment pO2. (□): MEPE, ( ) 1.25% BN; (
) 1.25% BN; ( ) 2.5% BN; (
) 2.5% BN; ( ) 5% BN; (■) 8% BN. Means ± SEM. *P < 0.05 compared with baseline on day 1 and pretreatment pO2; +P < 0.05 compared with MEPE.
) 5% BN; (■) 8% BN. Means ± SEM. *P < 0.05 compared with baseline on day 1 and pretreatment pO2; +P < 0.05 compared with MEPE.
The formulation with 2.5% BN provided a significant and consistent increase in tumor pO2 compared to other formulations. Therefore, this formulation was used to investigate the effect on the radiation response when the tumors were irradiated at times of increased tumor pO2.
Tissue pO2 and Growth of Tumors Treated with 2.5% BN or MEPE and 4 Gy for 5 Consecutive Days
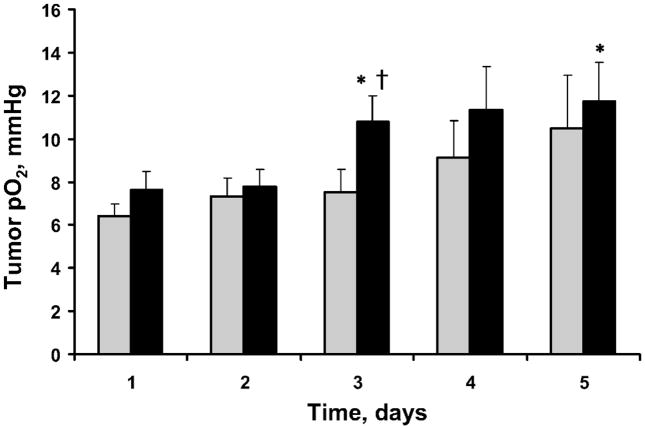
The mean baseline and pretreatment tumor pO2 are summarized in Fig. 2. The pretreatment tumor pO2 of the BN/radiation group increased significantly on days 3 and 5 compared to day 1. No significant changes in the baseline tumor pO2 and pretreatment tumor pO2 were observed in the MEPE/radiation group. The increase in pretreatment pO2 on day 3 was also significantly higher in the BN/radiation group than in the MEPE/radiation group.
FIG. 2.
Mean baseline and pretreatment RIF-1 tumor pO2 before and after the application of 2.5% BN and irradiation (4 Gy) for 5 consecutive days. ( ) MEPE + radiation; (■) 2.5% BN + radiation. Means ± SEM. *P < 0.05 compared with baseline pO2 on day 1; +P < 0.05 compared with MEPE group.
) MEPE + radiation; (■) 2.5% BN + radiation. Means ± SEM. *P < 0.05 compared with baseline pO2 on day 1; +P < 0.05 compared with MEPE group.
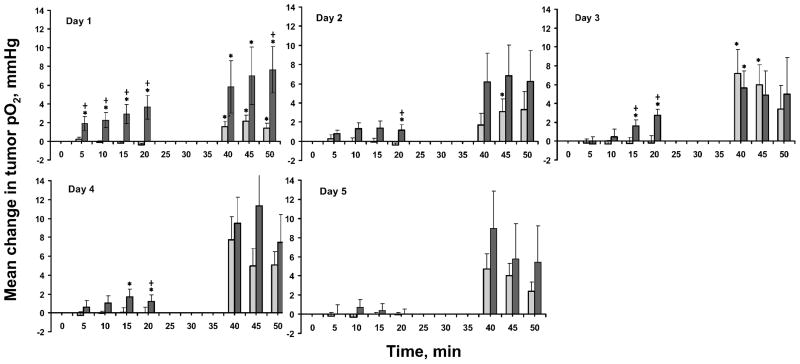
A significant increase in tumor pO2 at 10–30 min was observed with 2.5% BN on day 1 to day 4 compared to MEPE (Fig. 3). A significant increase in tumor pO2 compared to the baseline and pretreatment pO2 was also observed in both the 2.5% BN and MEPE groups after irradiation on days 1 to 5.
FIG. 3.
Mean changes in RIF-1 tumor pO2 from baseline/pretreatment (mean pO2 at each time – mean baseline/pretreatment tumor pO2) before and after application of 2.5% BN and irradiation for 5 consecutive days. ( ) MEPE+ radiation; (■) 2.5% BN + radiation. Means ± SEM. *P < 0.05 compared with baseline/pretreatment; +P < 0.05 compared with MEPE.
) MEPE+ radiation; (■) 2.5% BN + radiation. Means ± SEM. *P < 0.05 compared with baseline/pretreatment; +P < 0.05 compared with MEPE.
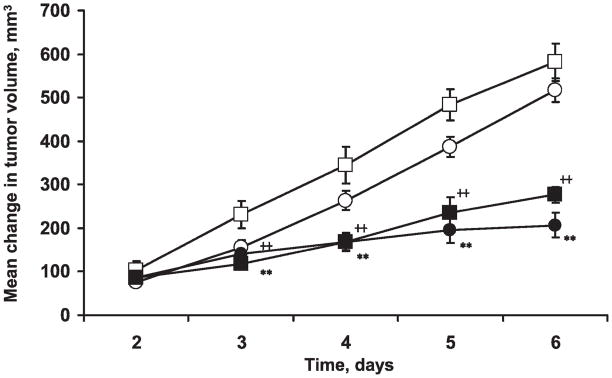
The changes in the tumor volume are shown in Fig. 4. No significant differences in the baseline tumor volumes (tumor volume on day 1) of the 2.5% BN and MEPE groups were observed. We also saw no significant differences in tumor growth between the 2.5% BN and MEPE groups. However, radiotherapy resulted in a highly significant decrease in tumor growth in both the 2.5% BN and MEPE groups.
FIG. 4.
Mean changes in RIF-1 tumor volume (mean tumor volume – mean tumor volume on day 1) after treatment with 2.5% BN or MEPE with or without 4 Gy irradiation for 5 consecutive days. (□) MEPE; (○) 2.5% BN; (■) MEPE + radiation; (●) 2.5%BN+ radiation. Mean ± SEM, ++P < 0.01, compared to the MEPE group (unpaired t test); **P < 0.01 compared with the 2.5% BN group (unpaired t test).
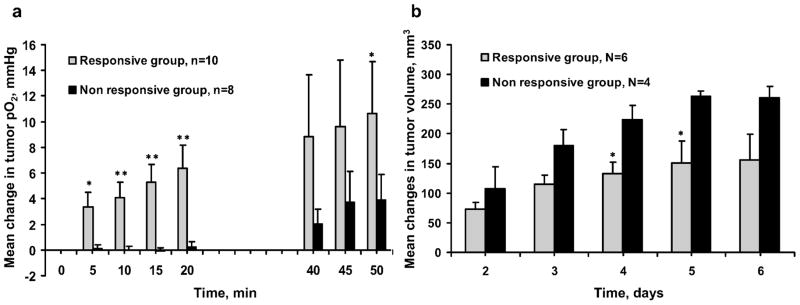
These data were reanalyzed to test the hypothesis that the changes in tumor pO2 observed on day 1 could be used as a marker to identify responders and nonresponders. The tumor volumes of the BN/radiation group were separated into two subgroups based on the tumor pO2 changes observed on day 1: tumors with an increase in pO2 of less than 2 mmHg from baseline (nonresponsive group) and tumors with an increase in pO2 of more than 2 mmHg from baseline (responsive group) (Fig. 5). A significant inhibition of tumor growth was seen on days 4 and 5 in the responsive group, while no such changes were observed in the nonresponsive group.
FIG. 5.
Mean changes in the pO2 (panel a) and volume (panel b) of RIF-1 tumors treated with 2.5% BN or MEPE and 4 Gy irradiation for 5 consecutive days. Treatment groups: (■) nonresponsive group (mean changes in tumor pO2 < 2 mmHg from baseline at 15 to 20 min after BN application on day 1); ( ) responsive group (mean changes in tumor pO2 ≥ 2 mmHg from baseline at 15 to 20 min after BN application on day 1. *P < 0.05, **P < 0.01 compared to the nonresponsive group (unpaired t test).
) responsive group (mean changes in tumor pO2 ≥ 2 mmHg from baseline at 15 to 20 min after BN application on day 1. *P < 0.05, **P < 0.01 compared to the nonresponsive group (unpaired t test).
DISCUSSION
The RIF-1 tumors were hypoxic, and the baseline and pretreatment pO2 were stable over 5 days of repeated measurements during the BN study (without radiation). A significant increase in tumor pO2 was observed after the topical application of the BN formulations, while no such changes were observed with MEPE. The effect of the 2.5% BN formulation on tumor pO2 was most consistent, with a significant increase in pO2 on days 1 to 4. These data are in agreement with a study on the skin pO2 of BALB/c mice by Krzic et al. in which the best results were observed with a 2.5% BN gel formulation (39).
In the 2.5% BN/radiotherapy group, the baseline and pretreatment tumor pO2 were similar to those of the first study. A significant increase in tumor pO2 was observed at 40–50 min after 2.5% BN or MEPE (around 10–20 min after irradiation; Fig. 3). This is likely due to radiation-induced changes in the tumor, such as reduced oxygen consumption or a decrease in interstitial pressure (15, 40, 41). Crokart et al. reported an increase in the tissue pO2 of FSaII tumors 3–4 h after a single dose of 2 Gy due to a decrease in global oxygen consumption and an increase in oxygen delivery (42). Znati et al. reported a decrease in interstitial pressure in experiments with fractionated radiation exceeding 10 Gy, which resulted in an increase in tumor oxygenation (43).
There were no significant differences in tumor growth between the 2.5% BN/radiation and MEPE/radiation groups; however, significant differences were detected when the tumors of the BN/radiation group were grouped as responsive and nonresponsive based on the changes in tumor pO2 observed after the topical application of the BN formulation on day 1. These results suggest that combining 2.5% BN with radiation can provide significant radiosensitization. The increase in tumor oxygenation by BN at the time of irradiation would be expected to decrease the hypoxia-related radioresistance of the tumor, resulting in increased radiosensitivity.
A number of adjuvant treatments have been developed to increase tumor oxygenation such as breathing of oxygen-enriched gases (5–7), inhibitors of tumor oxygen consumption (44, 45), hemoglobin modifier (24, 36) and anti-angiogenic drugs (46, 47). It has been hypothesized that radioresistant hypoxic regions might be minimized or eliminated if delivery of O2 to the tumors could be improved. However, one of the limiting factors is the lack of techniques that could be used to follow the changes in tumor oxygenation during such treatments. EPR oximetry could potentially be used to follow tumor pO2 during such interventions for their optimization and their efficacious combination with other therapeutic modalities. EPR oximetry requires a one-time implantation of the oximetry probe in the tissue of interest using 23–25 gauge needles, but the rest of the measurement procedure is noninvasive and allows repeated measure ments of localized changes in the tissue pO2 (26). This is not feasible with techniques such as polarographic electrodes, because the measurement procedure is invasive and cannot be repeated in the same tumors at different times (or days) due to the tissue damage caused by the electrodes during measurements. The clinical feasibility of EPR oximetry for pO2 measurements in patients with superficial tumors is currently being investigated at our institution (48, 49). The development of techniques such as implantable resonators will extend the application of EPR oximetry to any depth in the body (48, 49).
It is especially interesting and potentially important that the extent of the change in tumor pO2 observed in this study on day 1 could be used as an accurate predictor of outcome. However, further modifications of the BN formulations are necessary to achieve an optimal increase in tumor pO2 during repeated applications. The results also highlight the significance of repeated tumor pO2 measurements by EPR oximetry for the optimization of the BN formulation and for scheduling radio-therapy when an increase in tumor pO2 is observed after the topical application of the formulations.
In conclusion, these results provide quantitative information on the effectiveness of BN formulations in enhancing tumor pO2 and the potential of EPR oximetry to monitor tumor pO2 repeatedly during therapy. The tumor pO2 changes during such protocols could be used to identify responders and nonresponders early during therapy. This vital information could potentially allow clinicians to prescribe alternate therapies to nonresponders and improve clinical outcomes.
Acknowledgments
This work was supported by NIH grants CA118069 and CA120919 to NK and P01EB2180 to HS. We thank David Gladstone, Harriet St. Laurent and Kerry A. Tillson of Radiation Oncology, DHMC, for assistance in the use of the radiation facility. Preliminary results were presented at the 13th International Congress of Radiation Research, San Francisco, CA, August 8–12, 2007.
References
- 1.Rockwell S, Dobrucki IT, Kim EY, Marrison ST, Vu VT. Hypoxia and radiation therapy: past history, ongoing research, and future promise. Curr Mol Med. 2009;9:442–458. doi: 10.2174/156652409788167087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Overgaard J. Hypoxic radiosensitization: adored and ignored. J Clin Oncol. 2007;25:4066–4074. doi: 10.1200/JCO.2007.12.7878. [DOI] [PubMed] [Google Scholar]
- 3.De Jaeger K, Kavanagh MC, Hill RP. Relationship of hypoxia to metastatic ability in rodent tumours. Br J Cancer. 2001;84:1280–1285. doi: 10.1054/bjoc.2001.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Plasswilm L, Tannapfel A, Cordes N, Demir R, Hoper K, Bauer J, Hoper J. Hypoxia-induced tumour cell migration in an in vivo chicken model. Pathobiology. 2000;68:99–105. doi: 10.1159/000055909. [DOI] [PubMed] [Google Scholar]
- 5.Bussink J, Kaanders JH, Strik AM, van der Kogel AJ. Effects of nicotinamide and carbogen on oxygenation in human tumor xenografts measured with luminescence based fiber-optic probes. Radiother Oncol. 2000;57:21–30. doi: 10.1016/s0167-8140(00)00275-9. [DOI] [PubMed] [Google Scholar]
- 6.Rijpkema M, Kaanders JH, Joosten FB, van der Kogel AJ, Heerschap A. Effects of breathing a hyperoxic hypercapnic gas mixture on blood oxygenation and vascularity of head-and-neck tumors as measured by magnetic resonance imaging. Int J Radiat Oncol Biol Phys. 2002;53:1185–1191. doi: 10.1016/s0360-3016(02)02825-0. [DOI] [PubMed] [Google Scholar]
- 7.Thews O, Kelleher DK, Vaupel P. Dynamics of tumor oxygenation and red blood cell flux in response to inspiratory hyperoxia combined with different levels of inspiratory hypercapnia. Radiother Oncol. 2002;62:77–85. doi: 10.1016/s0167-8140(01)00401-7. [DOI] [PubMed] [Google Scholar]
- 8.Brizel DM, Lin S, Johnson JL, Brooks J, Dewhirst MW, Piantadosi CA. The mechanisms by which hyperbaric oxygen and carbogen improve tumour oxygenation. Br J Cancer. 1995;72:1120–1124. doi: 10.1038/bjc.1995.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robinson SP, Rodrigues LM, Ojugo AS, McSheehy PM, Howe FA, Griffiths JR. The response to carbogen breathing in experimental tumour models monitored by gradient-recalled echo magnetic resonance imaging. Br J Cancer. 1997;75:1000–1006. doi: 10.1038/bjc.1997.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bache M, Kappler M, Said M, Staab A, Vordermark D. Detection and specific targeting of hypoxic regions within solid tumors: current preclinical and clinical strategies. Curr Med Chem. 2008;15:322–338. doi: 10.2174/092986708783497391. [DOI] [PubMed] [Google Scholar]
- 11.Kaanders JH, Bussink J, van der Kogel AJ. ARCON: a novel biology-based approach in radiotherapy. Lancet Oncol. 2002;3:728–737. doi: 10.1016/s1470-2045(02)00929-4. [DOI] [PubMed] [Google Scholar]
- 12.Bussink J, Kaanders JH, Van der Kogel AJ. Clinical outcome and tumour microenvironmental effects of accelerated radiotherapy with carbogen and nicotinamide. Acta Oncol. 1999;38:875–882. doi: 10.1080/028418699432563. [DOI] [PubMed] [Google Scholar]
- 13.Hoskin PJ, Saunders MI, Dische S. Hypoxic radiosensitizers in radical radiotherapy for patients with bladder carcinoma: hyperbaric oxygen, misonidazole, and accelerated radiotherapy, carbogen, and nicotinamide. Cancer. 1999;86:1322–1328. [PubMed] [Google Scholar]
- 14.Kaanders JH, Pop LA, Marres HA, Liefers J, van den Hoogen FJ, van Daal WA, van der Kogel AJ. Accelerated radiotherapy with carbogen and nicotinamide (ARCON) for laryngeal cancer. Radiother Oncol. 1998;48:115–122. doi: 10.1016/s0167-8140(98)00043-7. [DOI] [PubMed] [Google Scholar]
- 15.Zhao D, Constantinescu A, Chang CH, Hahn EW, Mason RP. Correlation of tumor oxygen dynamics with radiation response of the Dunning prostate R3327-HI tumor. Radiat Res. 2003;159:621–631. doi: 10.1667/0033-7587(2003)159[0621:cotodw]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 16.Bernier J, Denekamp J, Rojas A, Trovò M, Horiot JC, Hamers H, Antognoni P, Dahl O, Richaud P, Piérart M. ARCON: accelerated radiotherapy with carbogen and nicotin-amide in non small cell lung cancer: a phase I/II study by the EORTC. Radiother Oncol. 1999;52:149–156. doi: 10.1016/s0167-8140(99)00106-1. [DOI] [PubMed] [Google Scholar]
- 17.Bernier J, Denekamp J, Rojas A, Minatel E, Horiot J, Hamers H, Antognoni P, Dahl O, Richaud P, Piérart M. ARCON: accelerated radiotherapy with carbogen and nicotinamide in head and neck squamous cell carcinomas. The experience of the Co-operative Group of Radiotherapy of the European Organization for Research and Treatment of Cancer (EORTC) Radiother Oncol. 2000;55:111–119. doi: 10.1016/s0167-8140(00)00165-1. [DOI] [PubMed] [Google Scholar]
- 18.Miralbell R, Mornex F, Greiner R, Bolla M, Storme G, Hulshof M, Bernier J, Denekamp J, Rojas AM, Mirimanoff RO. Accelerated radiotherapy, carbogen, and nicotinamide in glioblastoma multiforme: report of European Organization for Research and Treatment of Cancer trial 22933. J Clin Oncol. 1999;17:3143–3149. doi: 10.1200/JCO.1999.17.10.3143. [DOI] [PubMed] [Google Scholar]
- 19.Stratford MR, Dennis MF, Hoskin PJ, Saunders MI, Hodgkiss RJ, Rojas A. Nicotinamide pharmacokinetics in normal volunteers and patients undergoing palliative radio-therapy. Acta Oncol. 1996;35:213–219. doi: 10.3109/02841869609098504. [DOI] [PubMed] [Google Scholar]
- 20.Hoskin PJ, Stratford MR, Saunders MI, Hall DW, Dennis MF, Rojas A. Administration of nicotinamide during chart: pharmacokinetics, dose escalation, and clinical toxicity. Int J Radiat Oncol Biol Phys. 1995;32:1111–1119. doi: 10.1016/0360-3016(95)00022-q. [DOI] [PubMed] [Google Scholar]
- 21.Kaanders JH, Stratford MR, Liefers J, Dennis MF, van der Kogel AJ, van Daal WA, Rojas A. Administration of nicotinamide during a five- to seven-week course of radiotherapy: pharmacokinetics, tolerance, and compliance. Radiother Oncol. 1997;43:67–73. doi: 10.1016/s0167-8140(96)01896-8. [DOI] [PubMed] [Google Scholar]
- 22.Gallez B, Jordan BF, Baudelet C, Misson PD. Pharmacological modifications of the partial pressure of oxygen in murine tumors: evaluation using in vivo EPR oximetry. Magn Reson Med. 1999;42:627–630. doi: 10.1002/(sici)1522-2594(199910)42:4<627::aid-mrm2>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 23.Segers J, Crokart N, Danhier P, Gregoire V, Jordan BF, Gallez B. Use of xanthinol nicotinate as a co-treatment for radio- and chemo-therapy in experimental tumors. Int J Cancer. 2010;126:583–588. doi: 10.1002/ijc.24724. [DOI] [PubMed] [Google Scholar]
- 24.Hou H, Khan N, Grinberg OY, Yu H, Grinberg SA, Lu S, Demidenko E, Steffen RP, Swartz HM. The effects of Efaproxyn (efaproxiral) on subcutaneous RIF-1 tumor oxygenation and enhancement of radiotherapy-mediated inhibition of tumor growth in mice. Radiat Res. 2007;168:218–225. doi: 10.1667/RR0962.1. [DOI] [PubMed] [Google Scholar]
- 25.Khan N, Li H, Hou H, Lariviere JP, Gladstone DJ, Demidenko E, Swartz HM. Tissue pO2 of orthotopic 9L and C6 gliomas and tumor-specific response to radiotherapy and hyperoxygenation. Int J Radiat Oncol Biol Phys. 2009;73:878–885. doi: 10.1016/j.ijrobp.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swartz HM, Clarkson RB. The measurement of oxygen in vivo using EPR techniques. Phys Med Biol. 1998;43:1957–1975. doi: 10.1088/0031-9155/43/7/017. [DOI] [PubMed] [Google Scholar]
- 27.Elas M, Williams BB, Parasca A, Mailer C, Pelizzari CA, Lewis MA, River JN, Karczmar GS, Barth ED, Halpern HJ. Quantitative tumor oxymetric images from 4D electron paramagnetic resonance imaging (EPRI): methodology and comparison with blood oxygen level-dependent (BOLD) MRI. Magn Reson Med. 2003;49:682–691. doi: 10.1002/mrm.10408. [DOI] [PubMed] [Google Scholar]
- 28.Gallez B, Baudelet C, Jordan BF. Assessment of tumor oxygenation by electron paramagnetic resonance: principles and applications. NMR Biomed. 2004;17:240–262. doi: 10.1002/nbm.900. [DOI] [PubMed] [Google Scholar]
- 29.Wilkin JK, Fortner G, Reinhardt LA, Flowers OV, Kilpatrick SJ, Streeter WC. Prostaglandins and nicotinate-provoked increase in cutaneous blood flow. Clin Pharmacol Therapeut. 1985;38:273–277. doi: 10.1038/clpt.1985.170. [DOI] [PubMed] [Google Scholar]
- 30.Dowd PM, Whitefield M, Greaves MW. Hexyl-nicotinate-induced vasodilation in normal human skin. Dermatologica. 1987;174:239–243. doi: 10.1159/000249188. [DOI] [PubMed] [Google Scholar]
- 31.Petelin M, Pavlica Z, Bizimoska S, Sentjurc M. In vivo study of different ointments for drug delivery into oral mucosa by EPR oximetry. Int J Pharmaceut. 2004;270:83–91. doi: 10.1016/j.ijpharm.2003.10.030. [DOI] [PubMed] [Google Scholar]
- 32.Abramovic Z, Sustarsic U, Teskac K, Sentjurc M, Kristl J. Influence of nanosized delivery systems with benzyl nicotinate and penetration enhancers on skin oxygenation. Int J Pharmaceut. 2008;359:220–227. doi: 10.1016/j.ijpharm.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 33.Kristl J, Abramovic Z, Sentjurc M. Skin oxygenation after topical application of liposome-entrapped benzyl nicotinate as measured by EPR oximetry in vivo: influence of composition and size. AAPS PharmSci. 2003;5:E2. doi: 10.1208/ps050102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hou H, Lariviere JP, Demidenko E, Gladstone D, Swartz H, Khan N. Repeated tumor pO2 measurements by multi-site EPR oximetry as a prognostic marker for enhanced therapeutic efficacy of fractionated radiotherapy. Radiother Oncol. 2009;91:126–131. doi: 10.1016/j.radonc.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Hara JA, Goda F, Demidenko E, Swartz HM. Effect on regrowth delay in a murine tumor of scheduling split-dose irradiation based on direct pO2 measurements by electron paramagnetic resonance oximetry. Radiat Res. 1998;150:549–556. [PubMed] [Google Scholar]
- 36.Hou H, Khan N, O’Hara JA, Grinberg OY, Dunn JF, Abajian MA, Wilmot CM, Makki M, Demidenko E, Swartz HM. Effect of RSR13, an allosteric hemoglobin modifier, on oxygenation in murine tumors: an in vivo electron paramagnetic resonance oximetry and bold MRI study. Int J Radiat Oncol Biol Phys. 2004;59:834–843. doi: 10.1016/j.ijrobp.2004.02.039. [DOI] [PubMed] [Google Scholar]
- 37.Liu KJ, Gast P, Moussavi M, Norby SW, Vahidi N, Walczak T, Wu M, Swartz HM. Lithium phthalocyanine: a probe for electron paramagnetic resonance oximetry in viable biological systems. Proc Natl Acad Sci USA. 1993;90:5438–5442. doi: 10.1073/pnas.90.12.5438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smirnov AI, Norby SW, Clarkson RB, Walczak T, Swartz HM. Simultaneous multi-site EPR spectroscopy in vivo. Magn Reson Med. 1993;30:213–220. doi: 10.1002/mrm.1910300210. [DOI] [PubMed] [Google Scholar]
- 39.Krzic M, Sentjurc M, Kristl J. Improved skin oxygenation after benzyl nicotinate application in different carriers as measured by EPR oximetry in vivo. J Control Release. 2001;70:203–211. doi: 10.1016/s0168-3659(00)00351-5. [DOI] [PubMed] [Google Scholar]
- 40.Brown JM. Evidence for acutely hypoxic cells in mouse tumours, and a possible mechanism of reoxygenation. Br J Radiol. 1979;52:650–656. doi: 10.1259/0007-1285-52-620-650. [DOI] [PubMed] [Google Scholar]
- 41.Kallman RF. The phenomenon of reoxygenation and its implications for fractionated radiotherapy. Radiology. 1972;105:135–142. doi: 10.1148/105.1.135. [DOI] [PubMed] [Google Scholar]
- 42.Crokart N, Jordan BF, Baudelet C, Ansiaux R, Sonveaux P, Gregoire V, Beghein N, DeWever J, Bouzin C, Gallez B. Early reoxygenation in tumors after irradiation: determining factors and consequences for radiotherapy regimens using daily multiple fractions. Int J Radiat Oncol Biol Phys. 2005;63:901–910. doi: 10.1016/j.ijrobp.2005.02.038. [DOI] [PubMed] [Google Scholar]
- 43.Znati CA, Rosenstein M, Boucher Y, Epperly MW, Bloomer WD, Jain RK. Effect of radiation on interstitial fluid pressure and oxygenation in a human tumor xenograft. Cancer Res. 1996;56:964–968. [PubMed] [Google Scholar]
- 44.Jordan BF, Gregoire V, Demeure RJ, Sonveaux P, Feron O, O’Hara J, Vanhulle VP, Delzenne N, Gallez B. Insulin increases the sensitivity of tumors to irradiation: involvement of an increase in tumor oxygenation mediated by a nitric oxide-dependent decrease of the tumor cells oxygen consumption. Cancer Res. 2002;62:3555–3561. [PubMed] [Google Scholar]
- 45.Snyder SA, Lanzen JL, Braun RD, Rosner G, Secomb TW, Biaglow J, Brizel DM, Dewhirst MW. Simultaneous administration of glucose and hyperoxic gas achieves greater improvement in tumor oxygenation than hyperoxic gas alone. Int J Radiat Oncol Biol Phys. 2001;51:494–506. doi: 10.1016/s0360-3016(01)01654-6. [DOI] [PubMed] [Google Scholar]
- 46.Ansiaux R, Baudelet C, Jordan BF, Beghein N, Sonveaux P, De Wever J, Martinive P, Gregoire V, Feron O, Gallez B. Thalidomide radiosensitizes tumors through early changes in the tumor microenvironment. Clin Cancer Res. 2005;11:743–750. [PubMed] [Google Scholar]
- 47.Segers J, Di Fazio V, Ansiaux R, Martinive P, Feron O, Wallemacq P, Gallez B. Potentiation of cyclophosphamide chemotherapy using the anti-angiogenic drug thalidomide: importance of optimal scheduling to exploit the ‘normalization’ window of the tumor vasculature. Cancer Lett. 2006;244:129–135. doi: 10.1016/j.canlet.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 48.Khan N, Williams BB, Hou H, Li H, Swartz HM. Repetitive tissue pO2 measurements by electron paramagnetic resonance oximetry: current status and future potential for experimental and clinical studies. Antioxid Redox Signal. 2007;9:1169–1182. doi: 10.1089/ars.2007.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khan N, Williams BB, Swartz HM. Clinical applications of in vivo EPR: Rationale and initial results. Appl Magn Reson. 2006;30:185–199. [Google Scholar]