Abstract
Spinophilin regulates excitatory postsynaptic function and morphology during development by virtue of its interactions with filamentous actin, protein phosphatase 1, and a plethora of additional signaling proteins. To provide insight into the roles of spinophilin in mature brain, we characterized the spinophilin interactome in subcellular fractions solubilized from adult rodent striatum by using a shotgun proteomics approach to identify proteins in spinophilin immune complexes. Initial analyses of samples generated using a mouse spinophilin antibody detected 23 proteins that were not present in an IgG control sample; however, 12 of these proteins were detected in complexes isolated from spinophilin knock-out tissue. A second screen using two different spinophilin antibodies and either knock-out or IgG controls identified a total of 125 proteins. The probability of each protein being specifically associated with spinophilin in each sample was calculated, and proteins were ranked according to a χ2 analysis of the probabilities from analyses of multiple samples. Spinophilin and the known associated proteins neurabin and multiple isoforms of protein phosphatase 1 were specifically detected. Multiple, novel, spinophilin-associated proteins (myosin Va, calcium/calmodulin-dependent protein kinase II, neurofilament light polypeptide, postsynaptic density 95, α-actinin, and densin) were then shown to interact with GST fusion proteins containing fragments of spinophilin. Additional biochemical and transfected cell imaging studies showed that α-actinin and densin directly interact with residues 151–300 and 446–817, respectively, of spinophilin. Taken together, we have developed a multi-antibody, shotgun proteomics approach to characterize protein interactomes in native tissues, delineating the importance of knock-out tissue controls and providing novel insights into the nature and function of the spinophilin interactome in mature striatum.
Genomic sequencing has revealed the full repertoire of ∼20,000 proteins that can be expressed in most mammals. Innate biochemical or enzymatic activities of many proteins are critical to their function, but these activities are often modified by interactions with other proteins. Moreover, many proteins have no known catalytic activity and are thought to serve structural roles in assembling protein complexes, greatly increasing the efficiency and fidelity of intracellular processes. Thus, systematic definition of protein interactomes promises tremendous insight into biochemical mechanisms underlying the functions of many proteins.
A prime example of the importance of protein-protein interactions for modifying biological function is the postsynaptic density (PSD),1 an actin-rich organelle localized to neuronal dendritic spines that contains receptors, kinases, phosphatases, and scaffolding proteins (1, 2). Dynamic changes in enzymatic activities and protein-protein interactions underlie changes in the size and shape of both PSDs and dendritic spines as well as the modulation of PSD-targeted neurotransmitter receptors that are critical for synaptic plasticity, learning, and memory. Furthermore, dendritic spine morphology and number are altered in many neurological disorders, including Parkinson disease (PD), Angelman syndrome, and fragile X syndrome (3–7).
Spinophilin (neurabin II) is an F-actin- and protein phosphatase 1 (PP1)-binding protein with no known catalytic function (8–10). It is highly expressed in brain and is localized to dendritic spines and PSDs where it plays a key role targeting PP1 to regulate synaptic plasticity, learning, and memory (11–14). Spinophilin associates with its homolog neurabin, which is also a PP1- and F-actin-binding protein that regulates synaptic plasticity and dendrite morphology (14–16). The interaction between spinophilin and the γ1 isoform of PP1 is enhanced in an animal model of PD (17), perhaps contributing to the altered phosphorylation of synaptic proteins, such as CaMKII and glutamate receptor subunits observed following dopamine (DA) depletion (18–20). DA depletion also decreases the number of dendritic spines on striatal medium spiny neurons (4, 5). Spine density is regulated by dynamic changes in the F-actin cytoskeleton, and spinophilin regulates dendritic spine density during development (21). Indeed, candidate protein or generic protein-protein interaction screens have identified many additional spinophilin-associated proteins (SpAPs) that modulate F-actin dynamics and/or cell morphology (22–27; for a review, see Ref. 28), consistent with the idea that spinophilin is an archetypical scaffolding protein. However, these interactions have mostly been characterized in vitro and/or following protein overexpression in cultured cells, and the inter-relationship of these interactions in vivo is largely unknown. Although the spinophilin interactome appears to dictate the biological roles of spinophilin, the composition of these complexes in the mature brain is poorly understood.
Co-immunoprecipitation is commonly used to confirm the biological relevance of specific bivalent protein-protein interactions in native tissues that were initially identified using generic molecular approaches, such as yeast two-hybrid screening. Prior studies combined this approach with mass spectrometry-based proteomics methods to more broadly characterize the composition of mammalian signaling complexes and the PSD interactome, such as the signalosome associated with synaptic N-methyl-d-aspartate receptors (29) and complexes associated with other PSD-enriched proteins (30). In addition, proteomics methodologies were used to identify over 1100 protein components of the PSD (30). Indeed, the potential for shotgun proteomics studies to provide novel insights into protein function in the brain is increasingly recognized (31). Moreover, computational approaches are being developed to identify potential protein-protein interactions (32). However, validation of specific interactions among the very large data sets of candidates typically identified using these approaches can be daunting. In addition, most proteomics analyses have relied on a single antibody to the target protein of interest with, at best, an unrelated non-immune IgG as a negative control, necessitating the use of very high quality antibodies.
We developed a systematic shotgun proteomics approach to define protein interactomes in a native tissue context. We used this approach to characterize the composition of spinophilin complexes isolated from rodent striatum and confirmed the association of multiple, novel SpAPs. Furthermore, we extensively characterized the interaction of two additional SpAPs, α-actinin and densin, using biochemical and imaging techniques. Our studies directly illustrate the importance of appropriate subcellular fractionation conditions, using multiple antibodies to the protein of interest, and the underappreciated, critical role of analyzing parallel samples prepared from knock-out (KO) animals. Thus, our findings demonstrate a methodological framework with key controls that can be broadly applied to characterizing protein interactomes, in addition to providing novel insights into the role of spinophilin in controlling synaptic signaling.
EXPERIMENTAL PROCEDURES
Antibodies
The following antibodies were used as indicated: spinophilin: mouse monoclonal antibody (BD Biosciences 612166; epitope mapping between rat spinophilin residues 238–348), rabbit polyclonal spinophilin antibody (Millipore 06-852; epitope mapping between rat spinophilin residues 286–390), and goat polyclonal antibody (Santa Cruz Biotechnology SC-14774; epitope mapping to residues ∼50–100 of rat spinophilin); CaMKII: goat polyclonal antibody (33), mouse monoclonal CaMKIIα antibody (Affinity Bioreagents/Thermo Fisher Scientific MA1-048), and mouse monoclonal CaMKIIβ antibody (Zymed Laboratories Inc./Invitrogen 13-9800); pan-α-actinin, rabbit polyclonal antibody (Santa Cruz Biotechnology SC-15335); densin: goat polyclonal antibodies 450 and 650 (34) and rabbit polyclonal antibody BΔN (34); thousand and one amino acid kinase 1 (TAO1): rabbit polyclonal antibody (Bethyl Laboratories A300-524A); TAO3: rabbit polyclonal antibody (Bethyl Laboratories A300-536A); Tiam1: rabbit polyclonal antibody (Santa Cruz Biotechnology SC-872); P70S6 kinase: rabbit polyclonal antibody (Santa Cruz Biotechnology SC-230); doublecortin: rabbit polyclonal antibody (Cell Signaling Technology 4604); RasGrf1: rabbit polyclonal antibody (Santa Cruz Biotechnology SC-224); PP1γ1: sheep polyclonal antibody (35); PSD-95 (NeuroMab 75-028); tyrosine hydroxylase (ImmunoStar 22941); neurabin: mouse monoclonal antibody (BD Transduction Laboratories 611088); myosin Va: rabbit polyclonal antibody (Sigma M4812); neurofilament light polypeptide: rabbit (Cell Signaling Technology 2837) and mouse (Santa Cruz Biotechnology SC-58559) antibodies; Myc: mouse monoclonal antibody (Vanderbilt Monoclonal Antibody Core); HA: mouse monoclonal antibody (Vanderbilt Monoclonal Antibody Core) and rabbit polyclonal antibody (Santa Cruz Biotechnology SC-805); and fluorescent secondary antibodies: donkey anti-mouse Alexa Fluor 546, donkey anti-rabbit Alexa Fluor 488, and donkey anti-goat Alexa Fluor 633 (Molecular Probes).
6-OHDA Lesion of Rat Substantia Nigra
Surgery was performed as described previously (18).
Tissue Preparation and Subcellular Fractionation
Mice were decapitated without anesthesia, brains were removed, and neostriata (referred to as striata) were rapidly dissected, frozen on dry ice, and stored at −80 °C. Two whole frozen mouse striata (one from each hemisphere; ∼20 mg of total tissue) were pooled and homogenized in 2 ml of an isotonic (150 mm KCl, 50 mm Tris-HCl, 1 mm DTT, 0.2 mm PMSF, 1 mm benzamidine, 10 μg/ml leupeptin, 10 μm pepstatin, and 1 μm microcystin) or low ionic strength (2 mm Tris-HCl, pH 7.4, 2 mm EDTA, 2 mm EGTA, 1 mm DTT, 0.2 mm PMSF, 1 mm benzamidine, 10 μg/ml leupeptin, 10 μm pepstatin, and 1 μm microcystin) buffer with no detergent in a Teflon-glass Wheaton tissue grinder with motorized plunger and incubated at 4 °C for 30–60 min. Samples were adjusted to 0.4–1 mg/ml total protein and then centrifuged at 9,000 × g at 4 °C for 10 min. Supernatants (S1) were saved for immunoprecipitation. The pellet (P1) was resuspended in 1 ml of isotonic or low ionic strength buffer containing 0.5% Triton X-100 (v/v; Sigma) in a microcentrifuge tube, and samples were then adjusted to a final volume of 2 ml. Samples were incubated at 4 °C for 30–60 min and then centrifuged at 9,000 × g for 10 min. Supernatants (S2) were saved for immunoprecipitation, and the P2 pellets were resuspended in 2 ml of isotonic or low ionic strength buffer containing 1% Triton X-100 and 1% sodium deoxycholate (w/v; MP Biomedicals) and sonicated. Following incubation at 4 °C for 30 min, samples were then centrifuged at 9,000 × g for 10 min, and the supernatants (S3) were saved for immunoprecipitation. The final pellet (P3) was resuspended in 2 ml of 2× SDS sample buffer. For co-immunoprecipitations from non-fractionated samples, fresh or frozen brain tissue from adult (3–6-month old) male or female mice (WT or spinophilin KO C57/Bl6) or adult male rats were homogenized in low ionic strength buffer containing 0.5–1% Triton X-100 or isotonic strength buffer containing 1% Triton X-100 and 1% sodium deoxycholate.
Co-immunoprecipitations
Solubilized extracts from 1) mouse striata prepared for fractionation studies (S1, S2, or S3) in isotonic or low ionic strength buffer, 2) mouse whole brain homogenized in isotonic (1% Triton X-100 and 1% sodium deoxycholate) or low ionic strength (1% Triton X-100) buffer, or 3) rat or mouse striata or transfected HEK293 cells (see below) prepared directly in low ionic strength buffer containing 0.5–1.0% Triton X-100 were immunoprecipitated essentially as described (17). Antibody concentrations used were as follows: goat spinophilin, 4–8 μg; rabbit spinophilin, 7.5–30 μg; mouse spinophilin, 2.5–15 μg; rabbit α-actinin, 12 μg; and appropriate non-immune IgG control of a similar concentration.
Western Blotting
Western blotting was done as described previously (18).
Proteomics and Mass Spectrometry Methods
Immune complexes were fractionated by SDS-PAGE (10% acrylamide) and stained with colloidal Coomassie Blue G-250 (Invitrogen). Each gel was loaded with only one sample (and molecular weight markers) to limit cross-contamination. Entire gel lanes, with or without prominent IgG bands, were excised in two to three molecular weight ranges, finely chopped, reduced with dithiothreitol, alkylated with iodoacetamide, and digested with trypsin. Tryptic peptides were resolved using a reverse phase packed capillary tip (100 μm × 11 cm) packed with C18 resin (Jupiter C18, 5 μm, 300 Å; Phenomenex, Torrance, CA) and a precolumn (100 μm × 6 cm) with the same resin using a frit generated from liquid silicate Kasil 1 (36) essentially as described previously (37). Mobile phase A was 0.1% formic acid; mobile phase B was acetonitrile with 0.1% formic acid. The peptides were eluted from the column using a 95-min protocol, including a 15-min wash period (100% A for the first 10 min followed by a gradient to 98% A at 15 min) at 1 μl/min to allow for solid phase extraction and removal of residual salts followed by a 35-min linear gradient from 98% A to 75% A at 0.7 μl/min, then a steeper gradient to 10% A at 65 min, and an isocratic phase at 10% A to 75 min. Fractions were analyzed by tandem mass spectrometry using a ThermoFinnigan LTQ ion trap mass spectrometer equipped with a Thermo MicroAS autosampler and Thermo Surveyor HPLC pump, Nanospray source, and Xcalibur 2.0 instrument control software. MS/MS spectra of the peptides were obtained using data-dependent scanning in which one full MS spectra was followed by three MS/MS spectra.
The msconvert tool from ProteoWizard (38) exported mass spectra to the mzML v1.1 format. Intensity and m/z values were reported to 32-bit precision, and both MS and MS/MS scans were exported in centroid format. MyriMatch 1.6.0 (39) identified peptides corresponding to the MS/MS scans. Each scan was assessed as a singly charged precursor if 90% of fragment intensity fell below the precursor m/z; otherwise, the scans were identified under both doubly charged and triply charged precursor assumptions. The sequence database for immunoprecipitations in rat tissue included the IPI rat database (v3.66 with 39,677 sequences), the IPI mouse database (v3.66 with 56,791 sequences), and a database of 71 common contaminant sequences, including five proteases, 42 Ig constant regions, 14 human keratins, and 10 proteins from wool, cotton, and saliva. The sequence database for immunoprecipitations in mouse tissue included all but the IPI rat sequences. In both cases, all sequences were present in both normal and reversed orientations to enable estimation of false discovery rates. Each of the following modifications was allowed to be present or absent on the corresponding residues: carbamidomethyl Cys (+57), oxidized Met (+16), and pyro-Glu from N-terminal Gln (−17). Only one end of each peptide was required to match an expected trypsin cleavage site, and missed cleavages were not counted. Precursor ions were required to fall within 1.25 m/z of peptide average mass, whereas fragments were expected within 0.5 m/z of expected monoisotopic masses.
The IDPicker algorithm (version 2.5.0) filtered raw identifications, conducted protein assembly, and produced tables of spectrum counts for each experiment (40, 41). Immunoprecipitations from rat tissue were assembled separately from those in mouse tissue. In all cases, a peptide-spectrum match false discovery rate of 5% was applied as a limit (estimated by doubling the numbers of reversed matches and dividing by the total number of matches). Two distinct peptide sequences were required of each protein for reporting. Parsimony rules were in effect, grouping together indistinguishable sequences and reporting a minimal list of proteins. For spectral counting (see below), peptides that match multiple proteins are included for each protein they match. The original reports from IDPicker are included in the supplemental material, and all data are publicly available. In all cases, immunoglobulins, keratin, and trypsin were excluded as valid interacting proteins.
Statistical Analysis and Ranking of Peptide Data
Spectral counts for each protein from each antibody immunoprecipitation were used to generate the probability that a more extreme distribution of identified spectra between control (either IgG or KO) and experimental (wild type tissue with spinophilin antibody) would be observed solely by chance. For each antibody, Fisher's exact test (42) computed a p value based on the number of spectra matched to the protein in WT, the number of spectra matched to the protein in KO (or IgG) controls, the number of spectra not matched to the protein in WT, and the number of spectra not matched to the protein in KO (or IgG) controls. These p values were used to rank proteins identified searching both the rat and mouse IPI databases that were detected using a mouse spinophilin antibody and rat tissue (supplemental File RatTissueMouseAntibody.xls). Samples immunoprecipitated from mouse striatum using the mouse, goat, or rabbit (supplemental Tables S1, S2 and S3 respectively) antibodies were searched with the mouse IPI database, and p values from the individual immunoprecipitations are listed (supplemental File MouseTissueAllAntibodies.xls).
Fisher's method then combined the p values (from all five experiments performed in mouse tissue) for the three antibodies into a χ2 value (degrees of freedom, 4). A second set of calculations was performed using only the rabbit and goat antibodies to generate a χ2 value (degrees of freedom, 3), and both values are given in supplemental File MouseTissueAllAntibodies.xls. Only proteins matching two or more peptides from a single immunoprecipitation are listed in any of the tables. All computations were conducted in the R statistical environment (43). Proteins containing more spectra in each individual experimental immunoprecipitation compared with the corresponding control immunoprecipitation were placed into supplemental Table S2 and ranked by their χ2 value (generated from the goat and rabbit immunoprecipitations only) with a higher χ2 meaning a greater likelihood that any difference in peptide matching to WT versus KO is not due to chance alone. Proteins with an equal number (or more) of spectra in the control of any individual immunoprecipitation compared with the experimental condition were placed into supplemental Table S3 and sorted by alphabetical order.
Molecular Biology Constructs
The Myc-tagged spinophilin construct containing cDNAs encoding the full-length rat protein in a pCMV4-myc vector was described previously (9). mCherry (-C1) vector was a kind gift from Dr. D. Piston (Vanderbilt University). GFP-tagged spinophilin was a kind gift from Dr. Q. Wang (University of Alabama-Birmingham). A full-length GFP-densin construct (densin-FL-1–1542 Δ1291–1337), a C-terminal truncated GFP-densin construct missing the PDZ domain (ΔPDZ; Δ1291–1337 Δ1452–1542), a GFP-densin variant missing a larger C-terminal domain (densin-D43; Δ1291–1542), a GFP-densin variant that is missing a large internal fragment (densin-D23; Δ483–1377), a GST-densin construct (residues 1247–1542 Δ1291–1337; CTA), a GST-densin construct (residues 1247–1451 Δ1291–1337; MPD), and a His6-densin construct (residues 1247–1542; CTD) (see Fig. 4A) were described previously (34, 44). Densin-ΔPDZ and densin-D43 were inserted into pEGFPN3 vector between BgIII and SacII sites. GST-spinophilin constructs in PGEX-2T or PGEX-4T were created essentially as described previously (9, 45). The spinophilin 1–300 construct forward primer was created with a BamHI restriction site (CGGGATCCATGATGAAGACGGAGCCTC). The reverse primer was created with an EcoRI restriction site (CGGAATTCTTACACCGGTTTGATCTTGCGTAC). The α-actinin-2 cDNA (human) was a generous gift from Dr. Alan Beggs (Harvard University). The α-actinin-1 cDNA (human) was made by Dr. Carol Otey (46) and obtained from Dr. Donna Webb (Vanderbilt University). Full-length cDNAs were amplified by PCR using oligonucleotide primers containing EcoRI (5′) and XhoI (3′) and SmaI (5′ and 3′) restriction enzyme sites for α-actinin-1 and α-actinin-2, respectively. PCR products were ligated into pGEX4T-1 (Amersham Biosciences) or pET-28b(+) (Novagen) vectors and transformed into BL21 (DE3)pLysS Escherichia coli bacteria. Protein expression was induced by 1 mm isopropyl 1-thio-β-d-galactopyranoside, and bacteria were harvested after 3 h. GST or His6 fusion proteins were purified using glutathione-agarose (Sigma) or nickel-nitrilotriacetic acid-agarose (Qiagen) according to manufacturers' protocols. GST-α-actinin-ΔABD contained residues 248–892 and 255–894 of α-actinin-1 and -2, respectively. FLAG-α-actinin-2 was created in the pFLAG-CMV-2 vector (Sigma) by deleting the spectrin repeats. Purified protein concentrations were determined by Bradford (Bio-Rad) assay.
Fig. 4.
Isolation of immune complexes from WT and spinophilin KO striatum using three spinophilin antibodies. A, domain structure of spinophilin showing the approximate location of epitopes used to raise the commercially available goat, mouse, and rabbit spinophilin antibodies. B–D, each panel shows an immunoblot of a detergent-insoluble and -soluble fractions (DIF and Input, respectively) from striatum of WT and spinophilin KO mice. B, goat (Gt) antibody. C, rabbit (Rbt) antibody. D, mouse (Ms) antibody. E–G, each panel shows a colloidal Coomassie stain of the spinophilin immune complexes isolated from a Triton-soluble fraction of WT and KO striatum. E, goat (Gt) antibody. F, rabbit (Rbt) antibody. G, mouse (Ms) antibody. Ab, antibody; RBD, receptor binding domain.
GST Co-sedimentation
GST proteins (10–32 μg) were incubated with either purified His-tagged proteins or unpurified bacterial, overexpressing HEK293 cell, striatal, or whole brain lysates in 400–500 μl of GST pulldown buffer (50 mm Tris-HCl, pH 7.5, 200 mm NaCl, and 0.5% Triton X-100) or low ionic strength buffer with 0.5–1% Triton X-100 for 60 min. 40 μl of a 1:1 slurry of glutathione-agarose beads (Sigma) were added and incubated for 2 h or overnight. Beads were sedimented by centrifugation and washed in 500 μl of immunoprecipitation wash buffer (150 mM NaCl, 50 mM Tris pH 7.5, 0.5% Triton X-100) three to five times for 5 min each. Sedimented proteins were eluted with 60 μl of 2× SDS-PAGE sample buffer, resolved by SDS-PAGE, and transferred to nitrocellulose membranes for immunoblotting.
Co-localization Studies
Myc-spinophilin, GFP-densin-FL, and/or HA-α-actinin-2 were expressed in STHdh+/Hdh+ striatal cells (47) (a kind gift from Dr. A. Bowman, Vanderbilt University). STHdh+/Hdh+ cells were maintained in 10-cm dishes (Corning) with 10–12 ml of DMEM (Mediatech or Sigma) supplemented with 10% fetal bovine serum (Invitrogen), 1× penicillin, streptomycin, and l-glutamine (Invitrogen); and 400 μg/ml G418 (Mediatech). Cells were incubated with 1.5 ml of trypsin (Mediatech) for 5 min and allowed to detach. 12 ml of medium were added to trypsinized cells, and cells were then plated into a 12-well plate (Falcon) containing an 18-mm round glass coverslip (VWR Scientific) in each well that had been treated with poly-d-lysine for 5 min and subsequently washed five times in double distilled H2O and/or serum-containing DMEM. Cells were allowed to attach overnight and then transfected with DNA (2.5–3 μg) to express Myc-spinophilin, GFP-densin, and/or HA-α-actinin-2 using Lipofectamine LTX (Invitrogen) for 1–2 days. The medium was then removed, and cells were incubated in 1 ml of serum-free DMEM containing either 0.49% dimethyl sulfoxide (Pierce) or a differentiation medium (serum-free DMEM supplemented with 10 ng/ml fibroblast growth factor (Promega), 240 μm isobutylmethylxanthine (Sigma), 20 μm 12-O-tetradecanoylphorbol-13-acetate (Sigma), 48.6 μm forskolin (Sigma), and 5 μm DA (Sigma)) (47). Cells were incubated for 12–14 h, washed in PBS (Invitrogen), and then fixed for 15 min at 37 °C in PBS containing 4% sucrose (EM Science) and 4% paraformaldehyde (Electron Microscopy Sciences). Autofluorescence was quenched by addition of 0.1% NH4Cl (Sigma) in PBS, and cells were permeabilized in 0.1% Triton X-100 in PBS. After blocking in 10% normal donkey serum for 60 min, cells were incubated in a 1:500–1:1,000 dilution of the appropriate primary antibody in PBS containing 2% normal donkey serum (Jackson ImmunoResearch Laboratories) overnight, washed three times in PBS containing 0.01% Triton X-100, and then incubated in an appropriate secondary antibody conjugated to an Alexa Fluor (1:1,000–1:2,000). Cells were washed three times in PBS containing 0.01% Triton X-100, mounted on slides using Aqua Poly/Mount (Polysciences, Inc.), and imaged using an LSM 510 META inverted confocal microscope (Zeiss) essentially as described previously (11). Transfected cells were visualized next to non-transfected cells, and each image channel was linearly adjusted so that background fluorescence from non-transfected cells was not detected. Images within each experiment were collected with minimal adjustment of the gain and laser power settings on the microscope. Z-stack images were acquired, and three-dimensional projections were made using Zeiss Image browser software. Images of optical slices near the bottom of 9–12 undifferentiated or differentiated cells in two to three transfections from each transfection condition were thresholded automatically (48). Intensity correlation analysis was then used to compare normalized pixel intensities in each color channel, calculating an intensity correlation quotient (ICQ). Random overlap or mixed staining (ICQ ∼ 0), dependent staining (0 < ICQ ≤ +0.5), and segregated staining (0 > ICQ ≥ −0.5) can be assessed using this analysis (49). Thresholding and ICQ analysis were done using NIH Image J (downloaded from Wright Cell Imaging Facility with relevant analysis tools). Additional optical slices collected 1 and 2 μm above the initial slice were analyzed separately, yielding similar results (not shown). ICQ scores were compared by analysis of variance followed by Tukey post hoc test.
Quantitation of Blots
For semiquantitative analysis of chemiluminescence Western blot X-ray films exposed in a linear range were scanned using a flatbed scanner (Epson). Densitometric analyses were performed using NIH Image J. To quantify co-immunoprecipitations from control and DA-depleted striatum, immunoreactivity in the DA-depleted pellet was divided by immunoreactivity in the control pellet, and -fold changes in co-precipitating proteins were compared to 1 using either a one-column t test or, if normality was not met or the n was too low to test for normality, a Wilcoxon signed rank test (see (17) for details).
RESULTS
Solubilization of Distinct Pools of Striatal Spinophilin
Spinophilin is typically found in particulate fractions because of its association with multiple subcellular structures, including the PSD. Therefore, increasingly harsher detergents were used to sequentially extract striatal homogenates at two different ionic strengths. Soluble and particulate fractions were Western blotted for multiple synaptic proteins, revealing a dramatic impact of ionic strength on the solubilization properties of multiple dendritic proteins (Fig. 1A).
Fig. 1.
Subcellular fractionation of striatal proteins. A, distribution of proteins between subcellular fractions prepared at different ionic strengths using increasingly stringent detergents. Equivalent volumes of each fraction were fractionated by SDS-PAGE and transferred to nitrocellulose membrane. The membranes were stained for total protein using Ponceau S (top) and then Western blotted for the indicated proteins (lower panels). B, association of PP1γ1 and neurabin with spinophilin immune complexes isolated from each soluble extract. Western blots of proteins present in immune complexes isolated using a goat spinophilin or a control IgG (Sp and IgG, respectively) are shown. STD, protein standard (in kDa); TH, tyrosine hydroxylase.
Effect of Solubilization Conditions on Composition of Spinophilin Complexes
Variable solubilization of spinophilin and other proteins by altering ionic strength, detergent concentration, and detergent type likely reflects differential effects on various protein-protein interactions. Immune complexes were isolated from each solubilized fraction by immunoprecipitation using a goat spinophilin antibody or a control, non-immune IgG. Western blotting revealed that PP1γ1 and neurabin were specifically associated with spinophilin irrespective of extraction condition (Fig. 1B). Neurabin and PP1γ1 were predominantly associated with spinophilin in the S1 fraction obtained using a low ionic strength buffer, whereas in isotonic buffer, both were predominantly associated with spinophilin in Triton/deoxycholate-solubilized extracts (Fig. 1B). PP1γ1 and neurabin were detected in spinophilin immune complexes isolated from striatum of 2–4-month old rats (adult) or 19-day-old (P19; an age that has high spinophilin expression) mice. Interestingly, other known SpAPs tested were not detected in the spinophilin complex (Fig. 2 and data not shown). Therefore, we decided to perform a shotgun proteomics study to identify putative SpAPs that are present in these complexes.
Fig. 2.
Characterization of previously identified SpAPs in striatum. A, immunoprecipitation of adult rat striatal spinophilin using multiple antibodies and Western blot analysis for known SpAPs. B, immunoprecipitation of P19 mouse striatal spinophilin using multiple antibodies and Western blot analysis for known SpAPs. IP, immunoprecipitate; DIF, detergent-insoluble fraction; Sup, supernatant; Pt, pellet.
Characterization of Spinophilin Complexes in Adult Rat Striatum
Initial experiments used rat striatum because it is a larger tissue source than mouse striatum. A monoclonal mouse spinophilin antibody recognized a single band of the expected size in tissue extracts and specifically detected spinophilin immunoprecipitated by a second rabbit spinophilin antibody (Fig. 3A). Coomassie Blue staining detected multiple proteins in the spinophilin immune complex, including a major protein band that migrated at the apparent molecular mass of spinophilin (∼116 kDa), that were not detected in an IgG control complex (Fig. 3B). Entire Coomassie-stained gel lanes from IgG and spinophilin precipitations (excluding IgG bands) were excised, macerated, digested with trypsin, and analyzed using mass spectrometry. The rat and mouse IPI databases (see “Experimental Procedures”) were searched, and 39 total proteins were detected by the presence of two or more peptides with 23 proteins specifically detected in spinophilin immunoprecipitates and 16 detected in both the spinophilin precipitates and the IgG control. Although neurabin and two PP1 isoforms (γ1 and α) were among the specific proteins detected, confirming our immunoblotting data, other known SpAPs were not detected (supplemental Table S1).
Fig. 3.
Nonspecific association of TAO1 with spinophilin immune complexes isolated using mouse spinophilin antibody. A, the mouse spinophilin antibody recognizes a single, strong band and one faint band at a lower molecular weight in rat striatum and in samples immunoprecipitated using a rabbit spinophilin antibody. B, multiple proteins detected in spinophilin immune complexes isolated from rat striatum using the mouse spinophilin antibody are absent from IgG control samples. The gels were stained with colloidal Coomassie Blue. C, TAO1 is strongly detected by Western blotting immune complexes, but not in IgG controls, isolated using the mouse spinophilin antibody from rat striatum (Str), hippocampus (Hip), and cortex (CTX). TAO3, which is structurally similar to TAO1, is not detected in any spinophilin immunoprecipitates. D, total levels of TAO1 as detected by Western blot are unchanged in 6-OHDA-lesioned rats. E, apparent reciprocal co-immunoprecipitation of TAO1 and spinophilin using a mouse spinophilin antibody for immunoprecipitation and Western blotting. The apparent interaction is decreased in 6-OHDA-lesioned rats. The ratios of lesion/intact from normalized values (the mean ± standard error of the mean (error bars)) are compared with a theoretical value of 1 using column statistics. F, use of spinophilin KO striatum demonstrates that association of TAO1, but not PP1γ1, with spinophilin immune complexes isolated using the mouse antibody is nonspecific. L, lesioned; I, intact; Sup, supernatant; Pt, pellet; Spino, spinophilin; IPs, immunoprecipitates; DIF, detergent-insoluble fraction; IgGH, IgG heavy; IgGL, IgG light.
Among the more prominent proteins detected in the rat spinophilin complex (eight peptides) but not in the IgG control was TAO1, a microtubule affinity-regulating kinase kinase (also called MARKK) (50, 51). We confirmed the presence of TAO1 in spinophilin immune complexes isolated from rat striatum, hippocampus, and cortex by immunoblotting, whereas TAO3, a closely related family member, was not detected (Fig. 3C). Striatal dopamine depletion had no effect on TAO1 expression in rat striatum (Fig. 3D), but it significantly reduced the association of TAO1 with spinophilin immune complexes to 49 ± 4% of control levels (Fig. 3E). Probing TAO1 immune complexes using the mouse spinophilin antibody detected a protein that appeared slightly larger than the major spinophilin band detected in whole extracts (Fig. 3E). Dopamine depletion reduced the amount of co-precipitation of this protein with TAO1 to 59 ± 22% of control levels (Fig. 3E). However, TAO1 was not detected in immune complexes isolated using goat or rabbit spinophilin antibodies either by immunoblotting (not shown) or by proteomics analysis (supplemental Table S2). To further test the validity of this interaction, spinophilin was immunoprecipitated from striatum of WT and spinophilin KO mice (21). Spinophilin and PP1γ1 were detected in spinophilin immune complexes isolated from WT but not KO tissue; however, TAO1 was detected in both complexes (Fig. 3F), implying that it is a false positive and illustrating the value of using a KO tissue control.
Identification of Novel SpAPs Using Multiple Antibodies and KO Tissue Control
We then enhanced our proteomics strategy to identify proteins in immune complexes isolated using three different spinophilin antibodies, exploiting the availability of spinophilin KO tissue as a control (Fig. 4A). Each antibody detected an ≈116-kDa protein in extracts from WT, but not KO, tissue with variable detection of nonspecific proteins (Fig. 3, B–D). Moreover, each antibody specifically immunoprecipitated a major ≈116-kDa protein band as detected by Coomassie Blue staining (Fig. 3, E–G) but also immunoprecipitated a variable number of additional proteins in both WT and KO control extracts. Gel lanes containing spinophilin immune complexes isolated from both WT and KO tissue using all three antibodies were excised for proteomics analysis. Data from these analyses, as well as data obtained by analyzing independently isolated spinophilin complexes with an IgG control, were combined, used to search the mouse IPI database using the MyriMatch algorithm, and filtered to a confident and minimal list of proteins using the IDPicker algorithm (see “Experimental Procedures”). We first compared the rat proteins identified using the mouse antibody and an IgG control (see above) to proteins identified in WT and KO immunoprecipitations using the same antibody. Notably, 12 of the 23 proteins specifically detected in rat spinophilin complexes using an IgG control were also present in immune complexes isolated from both WT and KO mouse striata (supplemental Table S1, shaded proteins), including TAO1, inositol trisphosphate receptor, and various subunits of the eukaryotic translation initiation factor 3. Approximately 50% of proteins identified as specific on the basis of an IgG control were eliminated by using a KO tissue control, demonstrating that KO tissue is critical for reducing false positives.
Because the mouse antibody generated numerous false positives and also only weakly detected spinophilin in mouse striatal extracts (data not shown), we next evaluated the proteins identified in complexes isolated from mouse striatum using goat or rabbit spinophilin antibodies with either an IgG or KO tissue control. Supplemental Tables S2 and S3 list the accession numbers, the total number of spectral counts, and unique (isoform-specific) peptide matches along with p values (see “Experimental Procedures”). Furthermore, the p values for proteins detected in a second, independent screen with both antibodies using an IgG control are given. All of these p values were then used to calculate χ2 values, providing an estimate of the probability that the identification of each protein is specific (see supplemental File MouseTissueAllAntibodies.xls for a list of all proteins detected along with probability values). Proteins with more peptide matches in WT compared with KO are arranged in supplemental Table S2 based on the overall probability (χ2 value) that there are more peptides in the experimental compared with the control condition. Proteins with equal or more spectral counts in the controls (KO or IgG) are given in supplemental Table S3 and are arranged alphabetically.
One advantage of IDPicker is that proteins with overlapping peptide matches are clustered. For example, multiple isoforms of protein phosphatase 1 were immunoprecipitated by all three antibodies. A total of six peptides across the three antibodies and two conditions (IgG control and KO control) matched to all isoforms (α, β, and the γ1/2 splice variants). However, one additional peptide matched PP1γ1/γ2 and PP1α, five additional peptides uniquely matched PP1α, three additional peptides uniquely matched PP1γ1/γ2, and one additional peptide uniquely matched PP1β. Superscript letters after the accession number indicate proteins placed in the same cluster by IDPicker because multiple proteins match to one or more peptides, but unique peptides confirm that both proteins are present in the sample. In contrast, asterisks with or without footnotes indicate that multiple protein accession numbers match to all of the peptides detected (e.g. PP1γ1 and PP1γ2). The first IPI accession number from the IDPicker analysis is listed with additional matching IPI accession numbers listed in the footnote.
Initial Validation of Novel SpAPs
Our proteomics approach identified proteins in complex with spinophilin using an antibody-based approach. To test the validity of some of these hits, we used an independent, complementary, recombinant fusion protein-based approach. Solubilized extracts of mouse striatum were incubated with either GST alone or a set of three GST-spinophilin (GST-Sp) fusion proteins containing overlapping fragments spanning the entire amino acid sequence of the protein (Fig. 5A). Fusion proteins were isolated using glutathione-agarose, and the bound proteins were screened by Western blotting for proteins identified in the proteomics studies. We focused on several of the highest confidence protein hits based in part on the χ2 analysis (listed along with their isoforms in Table I). The rank based on the χ2 analysis is listed in parentheses after the protein.
Fig. 5.
Validation of putative novel SpAPs using GST-spinophilin co-sedimentation assays. A, schematic illustrating the overlapping fragments of spinophilin included in three GST-Sp fusion proteins, spanning the entire amino acid sequence. B, CaMKIIα, CaMKIIβ, myosin Va, NF-L, PSD-95, α-actinin, and densin specifically associate with different GST-Sp protein fragments. GAPDH did not precipitate with GST or with any of the GST-Sp constructs. TIF, Triton-insoluble fraction.
Table I. Known SpAPs and proteins selectively identified and validated (along with their isoforms).
Superscript letters mark clusters of proteins with overlapping peptide match(es) (e.g. spinophilinA and neurabinA). In some cases, all peptides matched multiple accession numbers as marked by an asterisk: the first accession number listed by IDPicker is listed with additional matches provided in footnotes. The next columns list numbers of peptides or spectra detected in samples isolated using either the goat or rabbit antibodies, divided into “unique” peptides (specific for a single accession number or group of accession numbers: modified and non-modified versions of the same peptide were only counted once) or “total” spectra (include total spectral counts that match with one or more proteins). A p value was calculated for the enrichment of a protein in each spinophilin immune complex compared with the corresponding control (IgG or KO tissue) and used to calculate an overall χ2 value across all analyses (for details, see “Experimental Procedures”). Proteins were ranked according to the χ2 value (highest value first, lower values last). ND, not detected.
1Calcium/calmodulin-dependent protein kinase type II β (IPI00649778); 68-kDa protein (IPI00875723).
2212-kDa protein (IPI00928007); myosin Va (IPI00928350).
3Myosin-10 (IPI00515398); cDNA, RIKEN full-length enriched library, clone M5C1080G13, product: myosin heavy chain 10, non-muscle, full insert sequence (IPI00757312).
4Protein phosphatase γ2 catalytic subunit (IPI00227773).
5Isoform 4 (IPI00406790); isoform 2 (IPI475044); isoform 3 (IPI00828919); adult male olfactory brain cDNA, RIKEN full-length enriched library, clone 6430579P05, product: calcium/calmodulin-dependent protein kinase II, δ, full insert sequence(IPI00857865.1); calcium/calmodulin-dependent protein kinase type II δ isoform 1 (IPI00858128); 55-kDa protein (IPI00858144).
6Isoform 2 (IPI00228044); isoform 3 (IPI00228045).
7Actin, cytoplasmic 2 (IPI00874482).
8Isoform α CaMKII of calcium/calmodulin-dependent protein kinase type II α chain (IPI00621806).
9Actin, α cardiac muscle 1 (IPI00114593); actin, aortic smooth muscle (IPI00117043); actin, γ enteric smooth muscle (IPI00404804); 14-, 17-day embryo head cDNA, RIKEN full-length enriched library, clone 3200002L22, product: actin, α, cardiac, full insert sequence (IPI00480406); 17-day embryo heart cDNA, RIKEN full-length enriched library, clone I920058J24, product: actin, α, cardiac, full insert sequence (IPI00653007); 17-day embryo heart cDNA, RIKEN full-length enriched library, clone I920072D16, product: actin, α, cardiac, full insert sequence (IPI00654242).
10Isoform 1 (IPI00622720.1); isoform 3 (IPI00626797).
11Leucine-rich repeat-containing 7 (IPI00875695).
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH; protein number 43) was used as a negative control. GAPDH is a highly abundant, soluble protein commonly utilized as a loading control. Initial studies detected one and two total spectra matching GAPDH in spinophilin immunoprecipitates using goat and rabbit antibodies, respectively, with no peptide matches in the IgG control. Moreover, GAPDH matched five and four spectra in the goat and rabbit antibody immune complexes, respectively, from WT tissue with two and one peptides detected in corresponding complexes isolated from KO tissue (p = 0.27 and 0.38, respectively). These statistical analyses, the low number of total spectral matches, and the high abundance of GAPDH suggest that it may be a nonspecific interaction. Consistent with this possibility, we were unable to detect binding of GAPDH to any of the GST-Sp fusion proteins.
CaMKII isoforms are encoded by four genes (α, β, γ, and δ), which can be alternatively spliced and form mixed isoform dodecameric complexes. CaMKIIα is ≈3-fold more abundant in the forebrain than CaMKIIβ (52), and these two isoforms together comprise ≈0.7% of total striatal protein (53). CaMKII plays a key role in regulating multiple dendritic processes that are essential for normal synaptic plasticity (54). Initial studies matched four and eight total spectra in spinophilin immune complexes isolated using the goat and rabbit antibodies, respectively, to CaMKIIβ with one and two total matching spectra in the IgG control. Furthermore, CaMKIIβ (protein number 3) matched five and seven unique (28 and 22 total) peptides in spinophilin immune complexes from WT striatum when using the goat and rabbit antibodies, respectively; whereas zero and one peptides (two and two total spectra) were detected in complexes isolated from KO striatum (p = 2.18e−07 and 1.15e−04, respectively). CaMKIIα (protein number 13) matched seven and four unique peptides (26 and 14 spectra) in spinophilin immune complexes from WT striatum when using the goat and rabbit antibodies, respectively, whereas six and two peptides (eight and two total spectra) were detected in complexes isolated from KO striatum (p = 9.02e−04 and 9.59e−04, respectively). Both CaMKIIα and CaMKIIβ associated with the GST-Sp(817) fusion protein, which contains a C-terminal fragment of spinophilin, but not with GST alone (Fig. 5B). However, both CaMKII isoforms also associated somewhat more weakly with the GST-Sp(300) and GST-Sp(484) proteins. Thus, striatal CaMKII appears to specifically associate either directly or indirectly with multiple domains in spinophilin.
Myosin Va (protein number 4) is a PSD-enriched motor protein (55, 56). It was initially identified in our rabbit spinophilin proteomics (eight matching spectra) using IgG as a control (zero matching peptides) and confirmed in both the rabbit and goat immunoprecipitations using WT (31 and 20 spectral counts, respectively) and KO (five and one spectral counts, respectively) tissue (p = 6.87e−05 and 5.95e−06, respectively). Myosin Va strongly associated with both GST-Sp(300) and GST-Sp(484) with no detectable binding to GST-Sp(817) or GST alone (Fig. 5B). Taken together, these data suggest that myosin Va directly or indirectly associates with N-terminal domains in spinophilin.
Multiple intermediate filament proteins were detected in our screen, including NF-L (protein number 14), neurofilament medium polypeptide (protein number 28), and α-internexin (protein number 16). NF-L also has been shown to bind PP1 (57). We detected four and 19 total spectra matching to NF-L in spinophilin immune complexes isolated using the goat and rabbit antibodies, respectively, with no spectra detected in the KO controls (p = 0.054 and 1.63e−05, respectively). NF-L selectively associated with GST-Sp(300), with much reduced binding to GST-Sp(484) and GST-Sp(817), and with no detectable binding to GST alone (Fig. 5B). Taken together, these data suggest that NF-L directly or indirectly associates with N-terminal domains in spinophilin.
PSD-95 (protein number 36) is an abundant scaffolding protein that is commonly used as a PSD marker. We specifically detected five PSD-95-derived peptides in spinophilin immunoprecipitates using the goat antibody with no peptides detected in the KO control (p = 0.026). However, our proteomics studies failed to detect PSD-95 in the rabbit spinophilin immune complexes. Nevertheless, striatal PSD-95 strongly associated with GST-Sp(300) and GST-Sp(484) but not with GST alone (Fig. 5B). Taken together, these data suggest that PSD-95 directly or indirectly associates with N-terminal domains in spinophilin.
Validation of α-Actinin as Novel SpAPs
In addition to the proteins detected above, we also detected α-actinin in our proteomics screens. The four isoforms of α-actinin are F-actin-binding and spectrin repeat-containing proteins (Fig. 6A). α-Actinin-2 is highly localized to dendritic spines (58, 59) where it can bind to and modulate N-methyl-d-aspartate receptors (60–62) and regulate spine morphology (63). The spinophilin immune complexes isolated using goat and rabbit antibodies contained 12 peptides (14 spectra) and eight peptides (11 spectra), respectively, that matched to α-actinin-2 (protein number 7) (p = 3.63e−05 and 0.00139, respectively). A second isoform, α-actinin-1 (protein number 21), also matched seven and six spectra in complexes isolated using the goat and rabbit antibodies, respectively, with only one peptide being detected in the KO (Table I). Moreover, unidentified α-actinin isoforms were present in complexes isolated from striatal extracts using GST-Sp(300) and GST-Sp(484) but not using GST-Sp(817) or GST alone (Fig. 5B).
Fig. 6.
Characterization of interaction between spinophilin and α-actinin. A, domain structure of α-actinin and the constructs used in these experiments. (SR1–4, spectrin repeats; EF1/2, calcium-binding EF hand domains; ΔABD, deleted actin binding domain). B, spinophilin specifically co-immunoprecipitates with an α-actinin antibody compared with the IgG control (Pellets). C, spinophilin in a detergent-soluble (1% Triton X-100 and 1% deoxycholate fraction) mouse whole brain extract specifically associates with GST-α-actinin-1-FL and GST-α-actinin-2-FL in a co-sedimentation assay. D, Myc-spinophilin expressed in HEK293 cells specifically associates with GST-α-actinin-2, but not GST-α-actinin-2-ΔABD or GST, in a co-sedimentation assay. E, HA-α-actinin-2-FL expressed in HEK293 cells specifically associates with GST-Sp constructs containing residues 1–300 or 151–484, but not residues 446–817, in a co-sedimentation assay. F, FLAG-α-actinin-2-ΔSR1–4 specifically associates with GST-Sp(1–300) and GST-Sp(151–607), but not GST-Sp(286–390) or GST-Sp(427–470) (300, 607, 390, and 470, respectively), in a co-sedimentation assay. G, purified His-α-actinin-2 associates with GST-Sp(300), GST-Sp(484), and GST-Sp(607) but not GST alone or GST-Sp(817). DIF, detergent-insoluble fraction; Sup, supernatant; Act, actinin.
We then further characterized the interaction of α-actinin with spinophilin using additional biochemical approaches. Spinophilin was specifically detected in α-actinin immune complexes isolated from mouse striatum (Fig. 6B). Moreover, spinophilin in a detergent-soluble forebrain extract associated with purified GST-tagged full-length α-actinin-1 or α-actinin-2 but not with GST alone (Fig. 6C). Deletion of the N-terminal actin binding domain (residues 1–254) from GST-α-actinin-2 (ΔABD) substantially reduced the interaction with spinophilin in a brain extract (Fig. 6D). GST-Sp(300) and GST-Sp(484), but not GST-Sp(817), bound HA-α-actinin-2 from extracts of transfected HEK293 cells (Fig. 6E). Furthermore, GST-Sp(300) and GST-Sp(151–607), but not GST-Sp(286–390) or GST-Sp(427–470), bound to an α-actinin-2 mutant lacking the spectrin repeats (Fig. 6F). Given that both spinophilin and α-actinin bind to actin, we used purified proteins to determine whether spinophilin directly interacts with α-actinin-2. A full-length His-α-actinin-2 protein bound to both GST-Sp(300) and GST-Sp(484) but not to GST-Sp(817) or GST alone (Fig. 6G). In combination, these data suggest that an N-terminal domain of α-actinin-2 directly interacts with residues 151–300 of spinophilin.
Probing Depth of Proteomics Data: Validation of Densin as SpAP
A protein that is selectively detected with a low number of peptides, although a lower confidence hit, may still be a valid interacting protein. Densin (protein number 73) matched two peptides in the spinophilin immunoprecipitates using the goat spinophilin antibody and one peptide using the rabbit spinophilin antibody when KO tissue was used as a control (p = 0.233 and 1, respectively). Densin is an interesting candidate because it is a scaffolding protein (Fig. 7A) that can bind Shank1 and Shank3/ProSAP2 (64), which in turn interact with multiple proteins that regulate dendritic functions (for a review, see Ref. 65). Notably, multiple densin- or Shank-binding proteins also were detected in our SpAP screens, such as α-actinin, CaMKII, homer, and ProSAPip1 (66–70) (supplemental Table S2). Moreover, previous studies implicated densin in the modulation of cell-cell contacts and dendritic morphology (64, 65, 71). Thus, densin was selected for further study, allowing us to also gain insight into the useful depth of our proteomics data.
Fig. 7.
Characterization of interaction between spinophilin and densin. A, domain structure of densin and the constructs used for these experiments (LAPSDa/b, leucine-rich repeat and PDZ-specific domains a and b; Mucin, mucin homology domain; TM, transmembrane-like domain; CK, CaMKII binding region). B, MS/MS, collisionally induced dissociation, m/z spectra from the tryptic peptide matching densin is shown. The strong y-5 ion corresponds to the fragmentation after the proline residue. The inset table shows the IPI accession number, charge state of the peptide (z), precursor mass (in Da), calculated mass (in Da), mass error (in Da), and the percent coverage. C, densin is enriched in spinophilin immune complexes from striatum (Pellet) compared with the IgG control. D, GFP-densin-FL expressed in HEK293 cells co-precipitates with the spinophilin immune complex (Pellet) only when spinophilin is co-expressed; furthermore, Myc-spinophilin co-precipitates with the densin immune complex only when densin is co-expressed. E, Myc-spinophilin expressed in HEK293 cells specifically binds to GST-densin-CTA and GST-densin-MPD in a co-sedimentation assay. F, densin-FL expressed in HEK293 cells specifically associates with GST-Sp(817), but not GST-Sp(484) or GST, in a co-sedimentation assay. G, GST-Sp(817) specifically binds to densin-FL, densin-ΔPDZ, and densin-D43, but not densin-D23, in a co-sedimentation assay. H, bacterially expressed His-densin-CTD specifically binds to GST-Sp(817). DIF, detergent-insoluble fraction; Den, densin.
As a first step toward validating densin as an SpAP, manual inspection of one of the MS/MS spectra identified an intense y-5 ion peak corresponding to fragmentation N-terminal to a proline residue in the tryptic peptide (Fig. 7B), a predicted cleavage for this peptide sequence (72). We then confirmed by Western blotting that densin was present in spinophilin immune complexes isolated from striatal extracts (Fig. 7C). In addition, densin in a striatal extract selectively interacted with GST-Sp(817) over GST-Sp(300), GST-Sp(484), or GST alone (Fig. 5B). Experiments using lysates of HEK293 cells expressing densin and/or spinophilin showed that densin was specifically detected in spinophilin immune complexes and that spinophilin was specifically detected in densin immune complexes (Fig. 7D). GST-densin fusion proteins containing the C-terminal domain (densin-CTA and densin-MPD) bound to full-length Myc-tagged spinophilin expressed in HEK cells (Fig. 7E). In addition, GST-Sp(817), but not GST alone or GST-Sp(484), strongly associated with GFP-tagged densin-FL expressed in HEK293 cells (Fig. 7F). The C-terminal truncation of densin to remove only the PDZ domain (densin-ΔPDZ) or both the PDZ and CaMKII binding domains in a natural splice variant (densin-D43) (34) had little effect on binding to GST-Sp(817) (Fig. 7G). In contrast, we could not detect binding of a natural densin splice variant lacking a large central domain (densin-D23) to GST-Sp(817) (Fig. 7G). GST-Sp(817), but not GST alone or GST-Sp(300), was able to directly bind to a His-tagged C-terminal domain fragment of densin (densin-CTD) in vitro (Fig. 7H). Thus, the combined data show that the C-terminal domain of spinophilin interacts directly with a C-terminal domain of densin in vitro and in intact cells, demonstrating the useful depth of our proteomics data.
Co-localization of Spinophilin, α-Actinin, and Densin
We next compared the localization of spinophilin, α-actinin, and densin in a relevant cell system, STHdh+/Hdh+ striatal cells, which can be induced to initiate a neuronal differentiation program, including extension of processes containing the dendritic marker MAP-2 (47). Using confocal microscopy, spinophilin predominantly localized to cortical regions of undifferentiated cells in contrast to diffuse cytosolic staining of the co-expressed mCherry marker (Fig. 8A). The differentiation mixture (see “Experimental Procedures”) induced formation of multiple processes containing GFP-spinophilin, which was often concentrated in the tips of the processes, whereas the mCherry marker was more diffusely localized in the processes and throughout the cell body (Fig. 8A and supplemental Fig. S1). Myc-tagged spinophilin partially co-localized with either HA-α-actinin-2 (Fig. 8B and supplemental Fig. S2) or full-length GFP-densin (Fig. 8C and supplemental Fig. S3) in both undifferentiated and differentiated cells. Using the image correlation analysis method of Li et al. (49) to quantify co-localization (Fig. 8D), there was little or no co-localization of spinophilin with mCherry in undifferentiated or differentiated cells (ICQs of 0.020 ± 0.004 and 0.003 ± 0.010, respectively). In contrast, α-actinin-2 and densin exhibited significantly more co-localization than mCherry with spinophilin in undifferentiated (ICQs of 0.15 ± 0.02 and 0.09 ± 0.01, respectively) and differentiated (ICQs of 0.13 ± 0.02 and 0.09 ± 0.02, respectively) cells. Co-localization of densin with spinophilin appeared modestly weaker than that of α-actinin-2 with spinophilin (p < 0.05 in undifferentiated cells only).
Fig. 8.
Localization of spinophilin and novel SpAPs in STHdh+/Hdh+ striatal cells. A, co-expression of GFP-spinophilin and mCherry shows spinophilin localization in cortical regions of undifferentiated cells and in the neurite-like projections of differentiated cells. Scale bars, 20 μm. B, co-expression of Myc-spinophilin and HA-α-actinin-2 reveals partial co-localization in both undifferentiated and differentiated cells. Scale bars, 20 μm. C, co-expression of Myc-spinophilin and GFP-densin reveals partial co-localization in both undifferentiated and differentiated cells. Scale bars, 20 μm. D, ICQ analysis comparing the distribution of spinophilin with the distribution of mCherry, HA-α-actinin-2, or GFP-densin in undifferentiated (−) and differentiated (+) striatal cells. ICQ values (maximum theoretical range, −0.5 to +0.5) are shown as the mean ± standard error of the mean (error bars). Significant differences from corresponding mCherry-spinophilin ICQ scores are indicated as follows: ***, p < 0.001; and **, p < 0.01. Co-localization of densin-FL with spinophilin is significantly weaker than co-localization of α-actinin-2 with spinophilin in undifferentiated cells (#, p < 0.05). E, localization of Myc-spinophilin, GFP-densin, and HA-α-actinin-2 in triple transfected undifferentiated and differentiated cells. The small panels below show magnified images of the indicated regions of these cells containing colored arrows to point out regions of the cells containing different co-localization patterns: green, GFP-densin alone; red, HA-α-actinin-2 alone; magenta, co-localization of HA-α-actinin-2 with spinophilin; cyan, co-localization of densin with spinophilin; yellow, co-localization of HA-α-actinin-2 with densin; white, co-localization of all three proteins. Scale bar, 5 or 20 μm as noted.
The STHdh+/Hdh+ cells also were transfected to co-express Myc-spinophilin, HA-α-actinin-2, and GFP-densin-FL. Protein localization was then compared in both undifferentiated and differentiated cells, revealing striking differences in the ratios of the three proteins in different subcellular domains (Fig. 8E). Additional images of triple transfected cells in supplemental Fig. S4 show the variability in cell morphology with some triple transfected, undifferentiated cells having “neurite”-like outgrowths. In general, there were few areas in which spinophilin was not co-localized with at least one of the other proteins. However, the co-localization of all three proteins was relatively restricted (white arrows), and spinophilin co-localized with either densin or α-actinin-2 alone in other regions (cyan and magenta arrows, respectively). In contrast, densin and α-actinin-2 did not co-localize in many regions of the cells (yellow arrows), instead adopting quite distinct localizations (green and red arrows, respectively). There was a broad overlap of spinophilin and α-actinin-2 throughout the processes of differentiated cells (magenta arrows).
DISCUSSION
The identification of protein-protein interactions is key to understanding the specificity and fidelity of many physiological processes as well as many diseases (see the Introduction). For example, protein kinases and phosphatases are often assembled into complexes by scaffolding proteins, providing exquisite control of the phosphorylation state of co-assembled proteins (73). Systematic efforts to map protein interactomes using established methods, such as yeast two-hybrid screens or fusion protein pulldowns, have yielded substantial advances. However, these approaches may miss interactions that require post-translational modifications or that are specific to a particular biological context and also demand substantial follow-up to identify the appropriate biological context for each novel interaction. The methodological approach described here (Fig. 9), although initially somewhat time-consuming, circumvents some of these limitations by facilitating direct identification of proteins in an interactome within a defined biological context (e.g. native tissue or disease state) and limits the identification of false positives.
Fig. 9.
Methodological work flow for identification, validation, and characterization of novel SpAPs. A, homogenize a biologically relevant tissue under conditions optimized to solubilize protein(s) of interest with least disruption to protein-protein interactions. B, immunoprecipitate protein of interest using multiple antibodies from WT tissue using a KO tissue control. C, separate precipitated complexes by SDS-PAGE. D, separate enzymatic digests of proteins by HPLC for MS and MS/MS analysis and match the mass spectra to an appropriate protein database. E, validate and further characterize novel interactions using biochemical and co-localization approaches. These studies map the interaction domain and allow for development of novel tools (e.g. fragments or mutated proteins) that may be used to disrupt specific interactions in intact cells. F, the spinophilin interactome characterized in the present studies. Spinophilin directly interacts with α-actinin and densin (in blue). Both of these proteins interact with additional partners, many of which were also identified in our current study (red). Solid lines indicate known direct interactions, whereas dashed lines connect protein pairs that have been previously shown to exist in the same multiprotein complexes (68, 70, 88–90).
Our approach optimally requires a KO animal lacking the protein of interest and multiple antibodies to the native protein ideally raised to distinct epitopes in different species. Systematic efforts are underway to knock out all genes in the mouse (74) and rat. In addition, both commercial (e.g. Santa Cruz Biotechnology) and non-commercial (e.g. NeuroMab) efforts are underway to create or collect antibodies to all of the proteins encoded in the human and mouse genomes. Moreover, it is becoming increasingly cost-effective to reliably generate custom antibodies to protein epitopes of interest. The use of multiple antibodies to different epitopes in the protein of interest with KO tissue controls means that high quality (i.e. specificity and titer) antibodies are not strictly required.
Solubilization of the protein of interest without disrupting interactions with potential partners is a crucial first step in characterizing an interactome. As shown here, the ionic strength and nature of the detergent can have profoundly different effects on the solubilization of different proteins. Our use of a low ionic strength buffer for the proteomics studies tends to destabilize F-actin and its associated proteins, allowing us to efficiently solubilize spinophilin with only Triton X-100, a relatively mild detergent, hopefully with minimal disruption of the spinophilin interactome. However, it may prove interesting to compare spinophilin interactomes extracted under different conditions.
The usefulness of KO tissue to validate interacting proteins from brain and to identify major co-precipitating proteins that can be detected by staining polyacrylamide gels has been demonstrated previously (75). Given the non-selective interactions identified in our initial shotgun proteomics screen of rat tissue using a non-immune IgG control, we expanded this approach to perform a shotgun proteomics analysis of complexes isolated from both WT and KO mouse tissue using three spinophilin antibodies and analyzing entire gel lanes (excluding the IgG heavy and light chains) to allow for identification of proteins that may be present below the detection limit for protein staining. Optimization of protocols to cross-link antibodies to protein G-Sepharose may allow for detection of additional proteins that co-migrate with IgGs using mass spectrometry-based proteomics.
The use of multiple antibodies provided additional dimensions to the proteomics data, identifying novel SpAPs that co-precipitate from native tissue with high fidelity. Although there is a legitimate bias to focus on SpAPs identified using more than one antibody, it is possible that antibodies isolate distinct subpopulations from the total repertoire of spinophilin complexes (e.g. binding of an SpAP may conceal certain epitopes). In addition, antibodies can vary in the efficiency of immunoprecipitation, perhaps affecting the sensitivity for detection of weakly interacting proteins or low abundance complexes. Thus, even proteins identified in a single immune complex and with few peptide matches may be true interactors. For instance, PSD-95 was detected only using the goat antibody. Furthermore, densin had two peptide matches with the goat antibody but only one peptide match when using the rabbit antibody. Given our two-peptide cutoff, this protein may have been missed if only the rabbit antibody was used. Also, although NF-L was detected using both the rabbit and goat antibodies, it had more spectral counts from immunoprecipitations using the rabbit antibody (19 spectral counts) compared with the goat antibody (four spectral counts). Although these studies were not quantitative, the number of spectra (spectral counts) identified in proteomics screens is related to the abundance of the protein in the sample. Therefore, in an initial effort to rank protein hits, we statistically analyzed each set of proteomics data based on the enrichment of spectral counts in experimental versus control samples. The p values from multiple immunoprecipitations were then combined to generate a χ2 value (see “Experimental Procedures”). This technique provided an overall ranking of confidence that each protein is a legitimate SpAP based on the multiple repetitions using different antibodies and controls. Future studies may benefit from merging our methodologies with evolving quantitative technologies that allow for characterization of proteomes using stable isotope labeling (76, 77).
Our co-immunoprecipitation methodology has the advantage of identifying direct or indirect interactions with spinophilin that are relevant in a specific biological context. Given concerns about the specificity of co-immunoprecipitations, especially for abundant proteins, we used an independent GST-spinophilin co-sedimentation approach to test the validity of some of the proteomics data, focusing on proteins that were highly ranked from the χ2 value and also proteins with interesting known biological roles that were not as highly ranked. Because we used three overlapping GST-spinophilin fragments that spanned the entire protein, this approach also provided an initial indication about specific regions/domains on spinophilin that were important for the interaction. Associations of myosin Va (protein number 4), CaMKIIβ and CaMKIIα (protein number 3 and protein number 13), NF-L (protein number 14), PSD-95 (protein number 36), α-actinin (protein number 7 and protein number 21), and densin (protein number 73) with one or more GST-spinophilin fragments were detected by Western blot (Fig. 5B). We then further validated the interactions of spinophilin with α-actinin-2 and densin using a combination of reciprocal co-immunoprecipitations from native rodent striatum and transfected cells, in vitro interactions of purified proteins, and co-localization studies. Taken together, these findings demonstrate the robustness of our methodology and the useful depth of the resulting data.
Although our data show that spinophilin can bind independently to densin and α-actinin, previous studies have shown that the C-terminal PDZ domain of densin can interact directly with the C terminus of α-actinin (67). Both densin and α-actinin function as scaffolding proteins, independently assembling complexes of several other neuronal/synaptic proteins, some of which were selectively detected in our spinophilin immunoprecipitations (Fig. 9F). Spinophilin and α-actinin are known to bind to actin, but the interaction of spinophilin with α-actinin is independent of actin because GST-Sp(300) and GST-Sp(484) directly precipitated purified α-actinin in vitro. Notably, several neurofilament proteins were also detected in spinophilin immune complexes in our proteomics studies, including α-internexin (protein number 16), NF-L (protein number 14), and neurofilament medium polypeptide (protein number 28). Although NF-L was previously shown to interact with PP1 (57), it is unlikely that PP1 is bridging the interaction with spinophilin because we showed that NF-L associates with GST-Sp(300), a spinophilin fragment that does not bind PP1 (78). Spinophilin, α-actinin, densin, and α-internexin have all been independently implicated in regulating cellular morphology and/or synaptic function (21, 63–65, 71, 79). A number of the other candidate SpAPs identified in our proteomics screen have also been implicated in regulating dendrite and/or dendritic spine morphology, including CaMKII isoforms, homer, ProSAPiP, SynGap, and synaptopodin (64, 65, 80–86). Moreover, multiple myosin motor proteins, including myosin Va (protein number 4) and myosin-10 (protein number 5), were detected, and we confirmed that myosin Va associates with N-terminal domains of spinophilin (Fig. 5B). These myosins are PSD-enriched motor proteins that have been implicated in regulating dendritic morphology (55, 56, 87). To our knowledge, this is the first study linking these actin-based motor proteins to spinophilin.
Alterations in dendritic morphology and/or the number of dendritic spines have been linked to numerous neurological disorders, including PD, Angelman syndrome, and fragile X syndrome (3–7), and the interaction between spinophilin and PP1γ1 is altered in an animal model of PD (17). Our data suggest that a novel spinophilin complex(es) associated with neurofilaments and/or motor proteins may be important for physiological and pathological regulation of dendritic morphology and other dendritic processes. It will be important to further investigate the biochemical basis for assembly of these complexes and their biological roles.
In summary, we used an ex vivo, co-immunoprecipitation approach, coupled to shotgun proteomics using tandem mass spectrometry, to identify putative, novel SpAPs that are known regulators of dendritic morphology. Importantly, this work demonstrates a powerful method that can be applied to novel protein targets to rapidly and selectively identify novel interactomes. We focused on native tissue to identify protein-protein interactions that are contextually important (i.e. proteins that reside in the striatum). Yates and co-workers (31) recently discussed potential pitfalls that may occur in proteomics studies, and our data expand this discussion by demonstrating the benefit of using multiple antibodies and the necessity for a KO tissue control to reduce wastage of time and resources chasing false positives. The rapidly increasing availability of antibodies and KO animals suggests that this approach could be systematically applied to gain insight into the biological roles of poorly characterized proteins.
Acknowledgments
Spinophilin KO mouse tissue and GFP-spinophilin were graciously provided by Dr. Q. Wang (University of Alabama-Birmingham). We thank Dr. M. Cobb and Dr M. Raman (University of Texas Southwestern) for discussion of TAO1. We are grateful for excellent technical assistance from the proteomics core of the Vanderbilt Mass Spectrometry Research Center, in particular from Dr. H. MacDonald, K. Cheek, S. Hill, and J. Coleman as well as assistance from G. Wernke for developing the ScanSifter software that was used to extract the data from the raw data files. Finally, the authors thank everyone in the Colbran laboratory for fruitful discussions of the data.
* This work was supported, in whole or in part, by National Institutes of Health Grants R01-MH63232 and P01-NS044282, Training Grant T32-MH65215 in Neurogenomics (to A. J. B.), Fellowship Grant NS061537 (to R. M. G.), and Grant 1R01CA126218 (to D. L. T.). This work was also supported by a Hobbs discovery grant from the Vanderbilt-Kennedy Center. Confocal microscopy was performed in the Vanderbilt University Medical Center Imaging Shared Resource (supported by National Institutes of Health Grants CA68485, DK20593, DK58404, HD15052, DK59637, and EY08126). Mouse anti-Myc and anti-HA antibodies were obtained through the Vanderbilt Monoclonal Antibody Core (supported by the Vanderbilt Ingram Cancer Center through National Institutes of Health Grant P30CA68485).
1 The abbreviations used are:
- PSD
- postsynaptic density
- CaMKII
- calcium/calmodulin-dependent protein kinase II
- CMV
- cytomegalovirus
- DA
- dopamine
- DMEM
- Dulbecco's modified Eagle's medium
- GFP
- green fluorescent protein
- HA
- hemagglutinin
- ICQ
- intensity correlation quotient
- KO
- knock-out
- PD
- Parkinson disease
- PDZ
- PSD-95/disks large/zonula occludens
- PP1
- protein phosphatase 1
- Sp
- spinophilin
- SpAP
- spinophilin-associated protein
- TAO
- thousand and one amino acid kinase
- WT
- wild type
- 6-OHDA
- 6-hydroxydopamine
- IPI
- International Protein Index
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- NF
- neurofilament
- HEK
- human embryonic kidney.
REFERENCES
- 1.Kim E., Sheng M. (2004) PDZ domain proteins of synapses. Nat. Rev. Neurosci 5, 771–781 [DOI] [PubMed] [Google Scholar]
- 2.Kennedy M. B., Beale H. C., Carlisle H. J., Washburn L. R. (2005) Integration of biochemical signalling in spines. Nat. Rev. Neurosci 6, 423–434 [DOI] [PubMed] [Google Scholar]
- 3.Dindot S. V., Antalffy B. A., Bhattacharjee M. B., Beaudet A. L. (2008) The Angelman syndrome ubiquitin ligase localizes to the synapse and nucleus, and maternal deficiency results in abnormal dendritic spine morphology. Hum. Mol. Genet 17, 111–118 [DOI] [PubMed] [Google Scholar]
- 4.Ingham C. A., Hood S. H., Arbuthnott G. W. (1989) Spine density on neostriatal neurones changes with 6-hydroxydopamine lesions and with age. Brain Res 503, 334–338 [DOI] [PubMed] [Google Scholar]
- 5.Ingham C. A., Hood S. H., van Maldegem B., Weenink A., Arbuthnott G. W. (1993) Morphological changes in the rat neostriatum after unilateral 6-hydroxydopamine injections into the nigrostriatal pathway. Exp. Brain Res 93, 17–27 [DOI] [PubMed] [Google Scholar]
- 6.McNeill T. H., Brown S. A., Rafols J. A., Shoulson I. (1988) Atrophy of medium spiny I striatal dendrites in advanced Parkinson's disease. Brain Res 455, 148–152 [DOI] [PubMed] [Google Scholar]
- 7.Wisniewski K. E., Segan S. M., Miezejeski C. M., Sersen E. A., Rudelli R. D. (1991) The Fra(X) syndrome: neurological, electrophysiological, and neuropathological abnormalities. Am. J. Med. Genet 38, 476–480 [DOI] [PubMed] [Google Scholar]
- 8.Allen P. B., Ouimet C. C., Greengard P. (1997) Spinophilin, a novel protein phosphatase 1 binding protein localized to dendritic spines. Proc. Natl. Acad. Sci. U.S.A 94, 9956–9961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacMillan L. B., Bass M. A., Cheng N., Howard E. F., Tamura M., Strack S., Wadzinski B. E., Colbran R. J. (1999) Brain actin-associated protein phosphatase 1 holoenzymes containing spinophilin, neurabin, and selected catalytic subunit isoforms. J. Biol. Chem 274, 35845–35854 [DOI] [PubMed] [Google Scholar]
- 10.Satoh A., Nakanishi H., Obaishi H., Wada M., Takahashi K., Satoh K., Hirao K., Nishioka H., Hata Y., Mizoguchi A., Takai Y. (1998) Neurabin-II/spinophilin. An actin filament-binding protein with one pdz domain localized at cadherin-based cell-cell adhesion sites. J. Biol. Chem 273, 3470–3475 [DOI] [PubMed] [Google Scholar]
- 11.Carmody L. C., Baucum A. J., 2nd, Bass M. A., Colbran R. J. (2008) Selective targeting of the gamma1 isoform of protein phosphatase 1 to F-actin in intact cells requires multiple domains in spinophilin and neurabin. FASEB J 22, 1660–1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stephens D. J., Banting G. (2000) In vivo dynamics of the F-actin-binding protein neurabin-II. Biochem. J 345, 185–194 [PMC free article] [PubMed] [Google Scholar]
- 13.Terry-Lorenzo R. T., Elliot E., Weiser D. C., Prickett T. D., Brautigan D. L., Shenolikar S. (2002) Neurabins recruit protein phosphatase-1 and inhibitor-2 to the actin cytoskeleton. J. Biol. Chem 277, 46535–46543 [DOI] [PubMed] [Google Scholar]
- 14.Allen P. B., Zachariou V., Svenningsson P., Lepore A. C., Centonze D., Costa C., Rossi S., Bender G., Chen G., Feng J., Snyder G. L., Bernardi G., Nestler E. J., Yan Z., Calabresi P., Greengard P. (2006) Distinct roles for spinophilin and neurabin in dopamine-mediated plasticity. Neuroscience 140, 897–911 [DOI] [PubMed] [Google Scholar]
- 15.Nakanishi H., Obaishi H., Satoh A., Wada M., Mandai K., Satoh K., Nishioka H., Matsuura Y., Mizoguchi A., Takai Y. (1997) Neurabin: a novel neural tissue-specific actin filament-binding protein involved in neurite formation. J. Cell Biol 139, 951–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Terry-Lorenzo R. T., Roadcap D. W., Otsuka T., Blanpied T. A., Zamorano P. L., Garner C. C., Shenolikar S., Ehlers M. D. (2005) Neurabin/protein phosphatase-1 complex regulates dendritic spine morphogenesis and maturation. Mol. Biol. Cell 16, 2349–2362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown A. M., Baucum A. J., Bass M. A., Colbran R. J. (2008) Association of protein phosphatase 1 gamma 1 with spinophilin suppresses phosphatase activity in a Parkinson disease model. J. Biol. Chem 283, 14286–14294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown A. M., Deutch A. Y., Colbran R. J. (2005) Dopamine depletion alters phosphorylation of striatal proteins in a model of Parkinsonism. Eur. J. Neurosci 22, 247–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Picconi B., Gardoni F., Centonze D., Mauceri D., Cenci M. A., Bernardi G., Calabresi P., Di Luca M. (2004) Abnormal Ca2+-calmodulin-dependent protein kinase II function mediates synaptic and motor deficits in experimental parkinsonism. J. Neurosci 24, 5283–5291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dunah A. W., Wang Y., Yasuda R. P., Kameyama K., Huganir R. L., Wolfe B. B., Standaert D. G. (2000) Alterations in subunit expression, composition, and phosphorylation of striatal N-methyl-D-aspartate glutamate receptors in a rat 6-hydroxydopamine model of Parkinson's disease. Mol. Pharmacol 57, 342–352 [PubMed] [Google Scholar]
- 21.Feng J., Yan Z., Ferreira A., Tomizawa K., Liauw J. A., Zhuo M., Allen P. B., Ouimet C. C., Greengard P. (2000) Spinophilin regulates the formation and function of dendritic spines. Proc. Natl. Acad. Sci. U.S.A 97, 9287–9292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bielas S. L., Serneo F. F., Chechlacz M., Deerinck T. J., Perkins G. A., Allen P. B., Ellisman M. H., Gleeson J. G. (2007) Spinophilin facilitates dephosphorylation of doublecortin by PP1 to mediate microtubule bundling at the axonal wrist. Cell 129, 579–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsukada M., Prokscha A., Oldekamp J., Eichele G. (2003) Identification of neurabin II as a novel doublecortin interacting protein. Mech. Dev 120, 1033–1043 [DOI] [PubMed] [Google Scholar]
- 24.Tsukada M., Prokscha A., Ungewickell E., Eichele G. (2005) Doublecortin association with actin filaments is regulated by neurabin II. J. Biol. Chem 280, 11361–11368 [DOI] [PubMed] [Google Scholar]
- 25.Buchsbaum R. J., Connolly B. A., Feig L. A. (2003) Regulation of p70 S6 kinase by complex formation between the Rac guanine nucleotide exchange factor (Rac-GEF) Tiam1 and the scaffold spinophilin. J. Biol. Chem 278, 18833–18841 [DOI] [PubMed] [Google Scholar]
- 26.Burnett P. E., Blackshaw S., Lai M. M., Qureshi I. A., Burnett A. F., Sabatini D. M., Snyder S. H. (1998) Neurabin is a synaptic protein linking p70 S6 kinase and the neuronal cytoskeleton. Proc. Natl. Acad. Sci. U.S.A 95, 8351–8356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ryan X. P., Alldritt J., Svenningsson P., Allen P. B., Wu G. Y., Nairn A. C., Greengard P. (2005) The Rho-specific GEF Lfc interacts with neurabin and spinophilin to regulate dendritic spine morphology. Neuron 47, 85–100 [DOI] [PubMed] [Google Scholar]
- 28.Sarrouilhe D., di Tommaso A., Métayé T., Ladeveze V. (2006) Spinophilin: from partners to functions. Biochimie 88, 1099–1113 [DOI] [PubMed] [Google Scholar]
- 29.Husi H., Ward M. A., Choudhary J. S., Blackstock W. P., Grant S. G. (2000) Proteomic analysis of NMDA receptor-adhesion protein signaling complexes. Nat. Neurosci 3, 661–669 [DOI] [PubMed] [Google Scholar]
- 30.Collins M. O., Husi H., Yu L., Brandon J. M., Anderson C. N., Blackstock W. P., Choudhary J. S., Grant S. G. (2006) Molecular characterization and comparison of the components and multiprotein complexes in the postsynaptic proteome. J. Neurochem 97, Suppl. 1, 16–23 [DOI] [PubMed] [Google Scholar]
- 31.Liao L., McClatchy D. B., Yates J. R. (2009) Shotgun Proteomics in Neuroscience. Neuron 63, 12–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goñi J., Esteban F. J., de Mendizábal N. V., Sepulcre J., Ardanza-Trevijano S., Agirrezabal I., Villoslada P. (2008) A computational analysis of protein-protein interaction networks in neurodegenerative diseases. BMC Syst. Biol 2, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McNeill R. B., Colbran R. J. (1995) Interaction of autophosphorylated Ca2+/calmodulin-dependent protein kinase II with neuronal cytoskeletal proteins. Characterization of binding to a 190-kDa postsynaptic density protein. J. Biol. Chem 270, 10043–10049 [DOI] [PubMed] [Google Scholar]
- 34.Jiao Y., Robison A. J., Bass M. A., Colbran R. J. (2008) Developmentally regulated alternative splicing of densin modulates protein-protein interaction and subcellular localization. J. Neurochem 105, 1746–1760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Colbran R. J., Carmody L. C., Bauman P. A., Wadzinski B. E., Bass M. A. (2003) Analysis of specific interactions of native protein phosphatase 1 isoforms with targeting subunits. Methods Enzymol 366, 156–175 [DOI] [PubMed] [Google Scholar]
- 36.Cortes H. J., Pfeiffer C. D., Richter B. E., Stevens T. (1987) Pourous ceramic bed supports for fused silica packed capillary columns used in liquid chromatography. J. High Resolut. Chromatogr. Chromatogr. Commun 10, 446–448 [Google Scholar]
- 37.Licklider L. J., Thoreen C. C., Peng J., Gygi S. P. (2002) Automation of nanoscale microcapillary liquid chromatography-tandem mass spectrometry with a vented column. Anal. Chem 74, 3076–3083 [DOI] [PubMed] [Google Scholar]
- 38.Kessner D., Chambers M., Burke R., Agus D., Mallick P. (2008) ProteoWizard: open source software for rapid proteomics tools development. Bioinformatics 24, 2534–2536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tabb D. L., Fernando C. G., Chambers M. C. (2007) MyriMatch: highly accurate tandem mass spectral peptide identification by multivariate hypergeometric analysis. J. Proteome Res 6, 654–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jadhav V., Solaroglu I., Obenaus A., Zhang J. H. (2007) Neuroprotection against surgically induced brain injury. Surg. Neurol 67, 15–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma Z. Q., Dasari S., Chambers M. C., Litton M. D., Sobecki S. M., Zimmerman L. J., Halvey P. J., Schilling B., Drake P. M., Gibson B. W., Tabb D. L. (2009) IDPicker 2.0: improved protein assembly with high discrimination peptide identification filtering. J. Proteome Res 8, 3872–3881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang B., VerBerkmoes N. C., Langston M. A., Uberbacher E., Hettich R. L., Samatova N. F. (2006) Detecting differential and correlated protein expression in label-free shotgun proteomics. J. Proteome Res 5, 2909–2918 [DOI] [PubMed] [Google Scholar]
- 43.R Development Core Team (2008) R: a Language and Environment for Statistical Computing, R Foundation for Statistical Computing, Vienna, Austria [Google Scholar]
- 44.Robison A. J., Bass M. A., Jiao Y., MacMillan L. B., Carmody L. C., Bartlett R. K., Colbran R. J. (2005) Multivalent interactions of calcium/calmodulin-dependent protein kinase II with the postsynaptic density proteins NR2B, densin-180, and alpha-actinin-2. J. Biol. Chem 280, 35329–35336 [DOI] [PubMed] [Google Scholar]
- 45.Carmody L. C., Bauman P. A., Bass M. A., Mavila N., DePaoli-Roach A. A., Colbran R. J. (2004) A protein phosphatase-1gamma1 isoform selectivity determinant in dendritic spine-associated neurabin. J. Biol. Chem 279, 21714–21723 [DOI] [PubMed] [Google Scholar]
- 46.Edlund M., Lotano M. A., Otey C. A. (2001) Dynamics of alpha-actinin in focal adhesions and stress fibers visualized with alpha-actinin-green fluorescent protein. Cell Motil. Cytoskeleton 48, 190–200 [DOI] [PubMed] [Google Scholar]
- 47.Trettel F., Rigamonti D., Hilditch-Maguire P., Wheeler V. C., Sharp A. H., Persichetti F., Cattaneo E., MacDonald M. E. (2000) Dominant phenotypes produced by the HD mutation in STHdh(Q111) striatal cells. Hum. Mol. Genet 9, 2799–2809 [DOI] [PubMed] [Google Scholar]
- 48.Otsu N. (1979) A threshold selection method from gray-level histograms. IEEE Trans. Syst. Man Cybern 9, 62–66 [Google Scholar]
- 49.Li Q., Lau A., Morris T. J., Guo L., Fordyce C. B., Stanley E. F. (2004) A syntaxin 1, Galpha(o), and N-type calcium channel complex at a presynaptic nerve terminal: analysis by quantitative immunocolocalization. J. Neurosci 24, 4070–4081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hutchison M., Berman K. S., Cobb M. H. (1998) Isolation of TAO1, a protein kinase that activates MEKs in stress-activated protein kinase cascades. J. Biol. Chem 273, 28625–28632 [DOI] [PubMed] [Google Scholar]
- 51.Timm T., Li X. Y., Biernat J., Jiao J., Mandelkow E., Vandekerckhove J., Mandelkow E. M. (2003) MARKK, a Ste20-like kinase, activates the polarity-inducing kinase MARK/PAR-1. EMBO J 22, 5090–5101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miller S. G., Kennedy M. B. (1985) Distinct forebrain and cerebellar isozymes of type II Ca2+/calmodulin-dependent protein kinase associate differently with the postsynaptic density fraction. J. Biol. Chem 260, 9039–9046 [PubMed] [Google Scholar]
- 53.Erondu N. E., Kennedy M. B. (1985) Regional distribution of type II Ca2+/calmodulin-dependent protein kinase in rat brain. J. Neurosci 5, 3270–3277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Colbran R. J., Brown A. M. (2004) Calcium/calmodulin-dependent protein kinase II and synaptic plasticity. Curr. Opin. Neurobiol 14, 318–327 [DOI] [PubMed] [Google Scholar]
- 55.Ishmael J. E., Safic M., Amparan D., Vogel W. K., Pham T., Marley K., Filtz T. M., Maier C. S. (2007) Nonmuscle myosins II-B and Va are components of detergent-resistant membrane skeletons derived from mouse forebrain. Brain Res 1143, 46–59 [DOI] [PubMed] [Google Scholar]
- 56.Walikonis R. S., Jensen O. N., Mann M., Provance D. W., Jr., Mercer J. A., Kennedy M. B. (2000) Identification of proteins in the postsynaptic density fraction by mass spectrometry. J. Neurosci 20, 4069–4080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Terry-Lorenzo R. T., Inoue M., Connor J. H., Haystead T. A., Armbruster B. N., Gupta R. P., Oliver C. J., Shenolikar S. (2000) Neurofilament-L is a protein phosphatase-1-binding protein associated with neuronal plasma membrane and post-synaptic density. J. Biol. Chem 275, 2439–2446 [DOI] [PubMed] [Google Scholar]
- 58.Dunah A. W., Wyszynski M., Martin D. M., Sheng M., Standaert D. G. (2000) Alpha-actinin-2 in rat striatum: localization and interaction with NMDA glutamate receptor subunits. Brain Res. Mol. Brain Res 79, 77–87 [DOI] [PubMed] [Google Scholar]
- 59.Wyszynski M., Kharazia V., Shanghvi R., Rao A., Beggs A. H., Craig A. M., Weinberg R., Sheng M. (1998) Differential regional expression and ultrastructural localization of alpha-actinin-2, a putative NMDA receptor-anchoring protein, in rat brain. J. Neurosci 18, 1383–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Michailidis I. E., Helton T. D., Petrou V. I., Mirshahi T., Ehlers M. D., Logothetis D. E. (2007) Phosphatidylinositol-4,5-bisphosphate regulates NMDA receptor activity through alpha-actinin. J. Neurosci 27, 5523–5532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang S., Ehlers M. D., Bernhardt J. P., Su C. T., Huganir R. L. (1998) Calmodulin mediates calcium-dependent inactivation of N-methyl-D-aspartate receptors. Neuron 21, 443–453 [DOI] [PubMed] [Google Scholar]
- 62.Krupp J. J., Vissel B., Thomas C. G., Heinemann S. F., Westbrook G. L. (1999) Interactions of calmodulin and alpha-actinin with the NR1 subunit modulate Ca2+-dependent inactivation of NMDA receptors. J. Neurosci 19, 1165–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nakagawa T., Engler J. A., Sheng M. (2004) The dynamic turnover and functional roles of alpha-actinin in dendritic spines. Neuropharmacology 47, 734–745 [DOI] [PubMed] [Google Scholar]
- 64.Quitsch A., Berhörster K., Liew C. W., Richter D., Kreienkamp H. J. (2005) Postsynaptic shank antagonizes dendrite branching induced by the leucine-rich repeat protein Densin-180. J. Neurosci 25, 479–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vessey J. P., Karra D. (2007) More than just synaptic building blocks: scaffolding proteins of the post-synaptic density regulate dendritic patterning. J. Neurochem 102, 324–332 [DOI] [PubMed] [Google Scholar]
- 66.Strack S., Robison A. J., Bass M. A., Colbran R. J. (2000) Association of calcium/calmodulin-dependent kinase II with developmentally regulated splice variants of the postsynaptic density protein densin-180. J. Biol. Chem 275, 25061–25064 [DOI] [PubMed] [Google Scholar]
- 67.Walikonis R. S., Oguni A., Khorosheva E. M., Jeng C. J., Asuncion F. J., Kennedy M. B. (2001) Densin-180 forms a ternary complex with the (alpha)-subunit of Ca2+/calmodulin-dependent protein kinase II and (alpha)-actinin. J. Neurosci 21, 423–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hayashi M. K., Tang C., Verpelli C., Narayanan R., Stearns M. H., Xu R. M., Li H., Sala C., Hayashi Y. (2009) The postsynaptic density proteins Homer and Shank form a polymeric network structure. Cell 137, 159–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Spilker C., Acuña Sanhueza G. A., Böckers T. M., Kreutz M. R., Gundelfinger E. D. (2008) SPAR2, a novel SPAR-related protein with GAP activity for Rap1 and Rap2. J. Neurochem 104, 187–201 [DOI] [PubMed] [Google Scholar]
- 70.Wendholt D., Spilker C., Schmitt A., Dolnik A., Smalla K. H., Proepper C., Bockmann J., Sobue K., Gundelfinger E. D., Kreutz M. R., Boeckers T. M. (2006) ProSAP-interacting protein 1 (ProSAPiP1), a novel protein of the postsynaptic density that links the spine-associated Rap-Gap (SPAR) to the scaffolding protein ProSAP2/Shank3. J. Biol. Chem 281, 13805–13816 [DOI] [PubMed] [Google Scholar]
- 71.Apperson M. L., Moon I. S., Kennedy M. B. (1996) Characterization of densin-180, a new brain-specific synaptic protein of the O-sialoglycoprotein family. J. Neurosci 16, 6839–6852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tabb D. L., Friedman D. B., Ham A. J. (2006) Verification of automated peptide identifications from proteomic tandem mass spectra. Nat. Protoc 1, 2213–2222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Scott J. D., Pawson T. (2000) Cell communication: the inside story. Sci. Am 282, 72–79 [DOI] [PubMed] [Google Scholar]
- 74.Austin C. P., Battey J. F., Bradley A., Bucan M., Capecchi M., Collins F. S., Dove W. F., Duyk G., Dymecki S., Eppig J. T., Grieder F. B., Heintz N., Hicks G., Insel T. R., Joyner A., Koller B. H., Lloyd K. C., Magnuson T., Moore M. W., Nagy A., Pollock J. D., Roses A. D., Sands A. T., Seed B., Skarnes W. C., Snoddy J., Soriano P., Stewart D. J., Stewart F., Stillman B., Varmus H., Varticovski L., Verma I. M., Vogt T. F., von Melchner H., Witkowski J., Woychik R. P., Wurst W., Yancopoulos G. D., Young S. G., Zambrowicz B. (2004) The knockout mouse project. Nat. Genet 36, 921–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kabbani N., Woll M. P., Levenson R., Lindstrom J. M., Changeux J. P. (2007) Intracellular complexes of the beta2 subunit of the nicotinic acetylcholine receptor in brain identified by proteomics. Proc. Natl. Acad. Sci. U.S.A 104, 20570–20575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Krüger M., Moser M., Ussar S., Thievessen I., Luber C. A., Forner F., Schmidt S., Zanivan S., Fässler R., Mann M. (2008) SILAC mouse for quantitative proteomics uncovers kindlin-3 as an essential factor for red blood cell function. Cell 134, 353–364 [DOI] [PubMed] [Google Scholar]
- 77.Selbach M., Mann M. (2006) Protein interaction screening by quantitative immunoprecipitation combined with knockdown (QUICK). Nat. Methods 3, 981–983 [DOI] [PubMed] [Google Scholar]
- 78.Hsieh-Wilson L. C., Allen P. B., Watanabe T., Nairn A. C., Greengard P. (1999) Characterization of the neuronal targeting protein spinophilin and its interactions with protein phosphatase-1. Biochemistry 38, 4365–4373 [DOI] [PubMed] [Google Scholar]
- 79.Chien C. L., Liu T. C., Ho C. L., Lu K. S. (2005) Overexpression of neuronal intermediate filament protein alpha-internexin in PC12 cells. J. Neurosci. Res 80, 693–706 [DOI] [PubMed] [Google Scholar]
- 80.Steiner P., Higley M. J., Xu W., Czervionke B. L., Malenka R. C., Sabatini B. L. (2008) Destabilization of the postsynaptic density by PSD-95 serine 73 phosphorylation inhibits spine growth and synaptic plasticity. Neuron 60, 788–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vazquez L. E., Chen H. J., Sokolova I., Knuesel I., Kennedy M. B. (2004) SynGAP regulates spine formation. J. Neurosci 24, 8862–8872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sala C., Piëch V., Wilson N. R., Passafaro M., Liu G., Sheng M. (2001) Regulation of dendritic spine morphology and synaptic function by Shank and Homer. Neuron 31, 115–130 [DOI] [PubMed] [Google Scholar]
- 83.Boeckers T. M., Bockmann J., Kreutz M. R., Gundelfinger E. D. (2002) ProSAP/Shank proteins—a family of higher order organizing molecules of the postsynaptic density with an emerging role in human neurological disease. J. Neurochem 81, 903–910 [DOI] [PubMed] [Google Scholar]
- 84.Pak D. T., Yang S., Rudolph-Correia S., Kim E., Sheng M. (2001) Regulation of dendritic spine morphology by SPAR, a PSD-95-associated RapGAP. Neuron 31, 289–303 [DOI] [PubMed] [Google Scholar]
- 85.Roussignol G., Ango F., Romorini S., Tu J. C., Sala C., Worley P. F., Bockaert J., Fagni L. (2005) Shank expression is sufficient to induce functional dendritic spine synapses in aspiny neurons. J. Neurosci 25, 3560–3570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Deller T., Korte M., Chabanis S., Drakew A., Schwegler H., Stefani G. G., Zuniga A., Schwarz K., Bonhoeffer T., Zeller R., Frotscher M., Mundel P. (2003) Synaptopodin-deficient mice lack a spine apparatus and show deficits in synaptic plasticity. Proc. Natl. Acad. Sci. U.S.A 100, 10494–10499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ryu J., Liu L., Wong T. P., Wu D. C., Burette A., Weinberg R., Wang Y. T., Sheng M. (2006) A critical role for myosin IIb in dendritic spine morphology and synaptic function. Neuron 49, 175–182 [DOI] [PubMed] [Google Scholar]
- 88.Geeves M. A., Fedorov R., Manstein D. J. (2005) Molecular mechanism of actomyosin-based motility. Cell. Mol. Life Sci 62, 1462–1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mundel P., Heid H. W., Mundel T. M., Krüger M., Reiser J., Kriz W. (1997) Synaptopodin: an actin-associated protein in telencephalic dendrites and renal podocytes. J. Cell Biol 139, 193–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Song B., Yan X. B., Zhang G. Y. (2004) PSD-95 promotes CaMKII-catalyzed serine phosphorylation of the synaptic RAS-GTPase activating protein SynGAP after transient brain ischemia in rat hippocampus. Brain Res 1005, 44–50 [DOI] [PubMed] [Google Scholar]



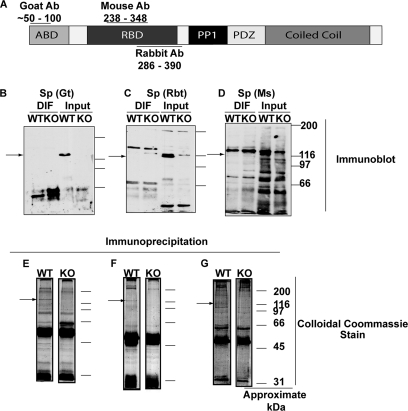
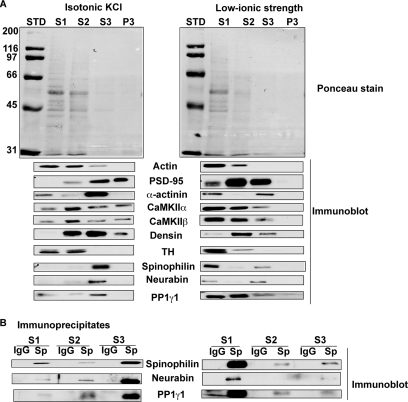
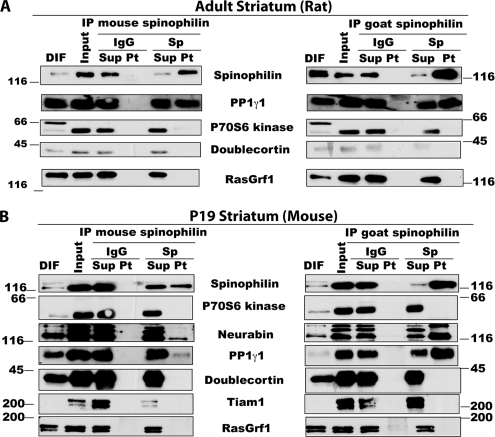
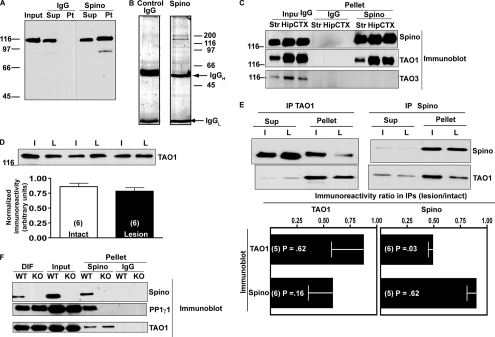
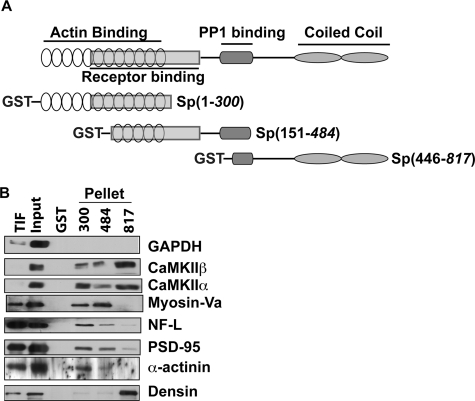
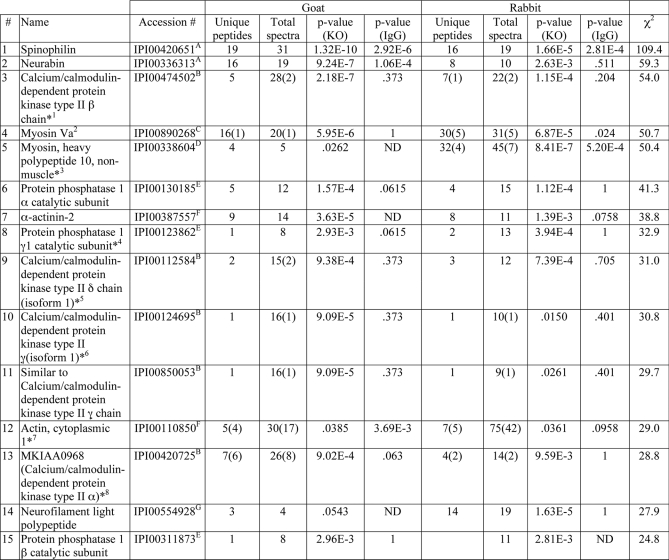
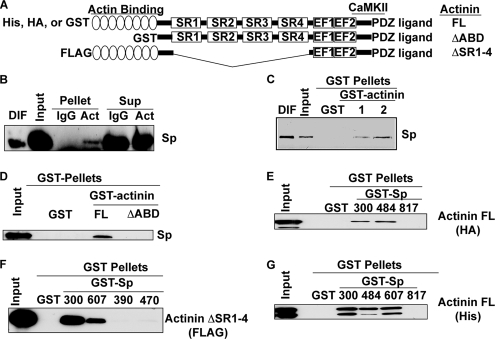
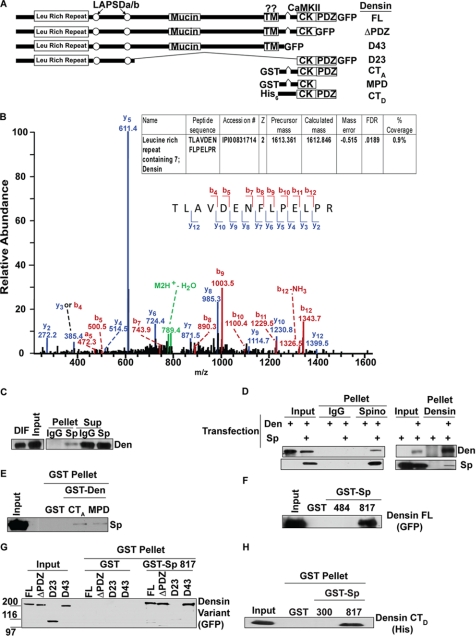
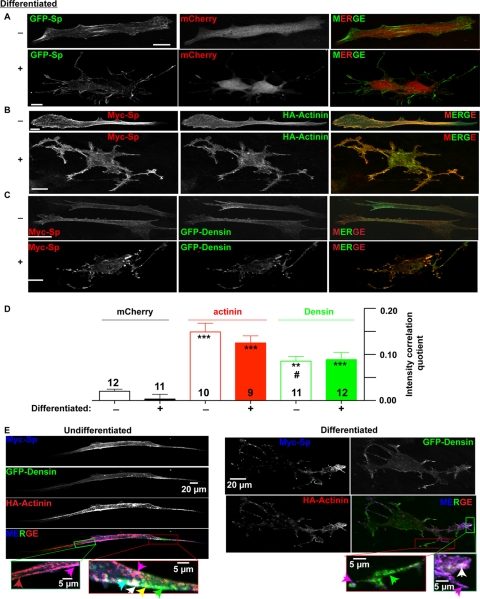
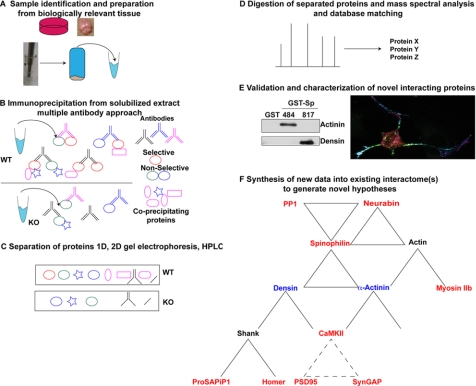
 This article contains
This article contains