Abstract
Photosynthetic organisms are able to adapt to changes in light conditions by balancing the light excitation energy between the light-harvesting systems of photosystem (PS) II and photosystem I to optimize the photosynthetic yield. A key component in this process, called state transitions, is the chloroplast protein kinase Stt7/STN7, which senses the redox state of the plastoquinone pool. Upon preferential excitation of photosystem II, this kinase is activated through the cytochrome b6f complex and required for the phosphorylation of the light-harvesting system of photosystem II, a portion of which migrates to photosystem I (state 2). Preferential excitation of photosystem I leads to the inactivation of the kinase and to dephosphorylation of light-harvesting complex (LHC) II and its return to photosystem II (state 1). Here we compared the thylakoid phosphoproteome of the wild-type strain and the stt7 mutant of Chlamydomonas under state 1 and state 2 conditions. This analysis revealed that under state 2 conditions several Stt7-dependent phosphorylations of specific Thr residues occur in Lhcbm1/Lhcbm10, Lhcbm4/Lhcbm6/Lhcbm8/Lhcbm9, Lhcbm3, Lhcbm5, and CP29 located at the interface between PSII and its light-harvesting system. Among the two phosphorylation sites detected specifically in CP29 under state 2, one is Stt7-dependent. This phosphorylation may play a crucial role in the dissociation of CP29 from PSII and/or in its association to PSI where it serves as a docking site for LHCII in state 2. Moreover, Stt7 was required for the phosphorylation of the thylakoid protein kinase Stl1 under state 2 conditions, suggesting the existence of a thylakoid protein kinase cascade. Stt7 itself is phosphorylated at Ser533 in state 2, but analysis of mutants with a S533A/D change indicated that this phosphorylation is not required for state transitions. Moreover, we also identified phosphorylation sites that are redox (state 2)-dependent but independent of Stt7 and additional phosphorylation sites that are redox-independent.
The primary photochemical reactions of photosynthesis are catalyzed by the pigment-protein complexes photosystem II (PSII)1 and PSI (PSI), which are linked in series through the plastoquinone pool, the cytochrome b6f complex, and plastocyanin in the thylakoid membranes. Upon light absorption by the antenna systems of PSII and PSI, charge separations occur across the membrane that lead to the oxidation of water by PSII and electron flow to PSI and ultimately to the reduction of NADP+. Because the antenna systems of PSII and PSI have different pigment composition, they are differentially sensitized upon changes in light quality and quantity. However, photosynthetic organisms have the ability to adapt to changes in light. They balance energy input and consumption in the short term through dissipation of excess absorbed light energy into heat through non-photochemical quenching and regulate absorption of excitation energy between PSII and PSI through state transitions (supplemental Fig. 1). This reversible redistribution leads to an overall increase in photosynthetic quantum yield. State transitions occur when preferential excitation of PSII reduces the plastoquinone pool. This leads to the activation of a thylakoid protein kinase as a result of the docking of plastoquinol to the Qo site of the cytochrome b6f complex (1, 2) and to the phosphorylation of the polypeptides of the light-harvesting complex II (LHCII), a part of which migrates to PSI (state 2) (3–5). The process is reversible as preferential excitation of PSI inactivates the kinase and allows for dephosphorylation of LHCII and its return to PSII (state 1) (3, 6). In the green alga Chlamydomonas reinhardtii, the LHCII protein set consists of Type I (Lhcbm3, Lhcbm4, Lhcbm6, Lhcbm8, and Lhcbm9), Type II (Lhcbm5), Type III (Lhcbm2 and Lhcbm7), and Type IV (Lhcbm1 and Lhcbm10) proteins and of Lhcb7, CP26, and CP29 (7). Because of their nearly identical sequences and sizes, several of these Lhcbm proteins cannot be distinguished by SDS-PAGE. Most of them fractionate into four bands called P11 and P13 (Type I), P16 (Type IV), and P17 (Type III). Whereas P16 is not phosphorylated, phosphorylation events occur on P11, P13, and P17 (7, 8).
The association of the mobile part of LHCII to PSI during a transition from state 1 to state 2 requires the PsaH subunit (9) and CP29, which also moves to PSI and is essential for docking LHCII to PSI (10–12). The lateral displacement of LHCII from the PSII-rich grana to the PSI-rich lamellar thylakoid regions results in transfer to PSI of about 80% of the excitation energy absorbed by LHCII in C. reinhardtii (13), a considerably higher amount than in land plants in which only 15–20% of LHCII is mobile (3). In C. reinhardtii, state transitions are associated with a reorganization of the photosynthetic electron transfer chain with a switch from linear to cyclic electron flow during a transition from state 1 to state 2 (14, 15). Thus, cells produce ATP and NADPH in state 1 but only ATP in state 2. It appears that the major function of state transitions in this alga is to adjust the level of ATP and the ATP/NADPH ratio to cellular demands (5).
Thylakoid membranes contain appressed grana and nonappressed stromal domains in which PSII and PSI are enriched, respectively. Because LHCII is a major stabilizer of the grana structure (16), the movement of LHCII from PSII to PSI is expected to lead to major rearrangements of these membranes during state transitions. Indeed, based on extensive electron microscope studies, it was proposed that fusion and fission events occur at the interface between the grana and stroma lamellar domains that lead to a remodeling of the membranes (17).
Mapping of in vivo protein phosphorylation sites in photosynthetic membranes of Chlamydomonas revealed a total of 19 sites corresponding to 15 genes (18). It was shown that the major changes are clustered at the interface between the PSII core and the associated LHCII proteins during state transitions. Phosphorylation of the PSII core subunits D2 and PsbR and multiple phosphorylations of the minor LHCII antenna subunit CP29 were detected as well as phosphorylation of Lhcbm1, which belongs to the major LHCII complex (18).
Although the phosphorylation of LHCII was observed many years ago (6), it is only recently that kinases involved in this process were uncovered. Fleischmann et al. (19) and Kruse et al. (20) used a genetic approach in C. reinhardtii with the aim of dissecting the signal transduction chain of state transitions. Two allelic mutants blocked in state 1 were identified that are affected in the Stt7 gene encoding a thylakoid Ser-Thr protein kinase that is required for LHCII phosphorylation during a transition from state 1 to state 2 (21). This Stt7 kinase is conserved in land plants and has an ortholog, STN7, in Arabidopsis (22).
The 754-amino acid Stt7 kinase has a catalytic domain characteristic of Ser-Thr kinases (21). It contains a putative 41-amino acid transit peptide at its N-terminal end, and the protein is localized on the thylakoid membrane. Stt7 is associated with photosynthetic complexes including LHCII, PSI, and the cytochrome b6f complex (23). Stt7 also contains a transmembrane region that separates its catalytic kinase domain on the stromal side from its N-terminal end in the thylakoid lumen with two conserved Cys residues that are critical for its activity and state transitions (23). Moreover, the level of Stt7 decreases considerably under state 1 conditions, and the kinase acts in catalytic amounts (23). However, it is not yet known whether this kinase directly phosphorylates LHCII or whether it is part of a kinase cascade involved in the signaling pathway of state transitions.
In this work, we used a mass spectrometry-based approach (24) to map the in vivo Stt7-dependent protein phosphorylation sites within thylakoid membranes isolated from the green alga C. reinhardtii subjected to state 1 and state 2 conditions. In contrast with the earlier studies via direct MS/MS sequencing of the IMAC-enriched phosphorylated peptides from thylakoid proteins (18, 25), we performed additional LC-MS/MS-based analyses using alternating collision-induced dissociation and electron transfer dissociation of peptide ions. This approach revealed novel phosphorylation sites in LHCII polypeptides, in several other membrane and membrane-associated proteins, and in the thylakoid protein kinases Stt7 and Stl1, suggesting the existence of a thylakoid protein kinase cascade. Relative quantification of phosphorylated peptides labeled with stable isotopes determined the specific Stt7-dependent phosphorylation site in CP29 linker protein under state 2. Moreover, we also identified phosphorylation sites that are redox-dependent but independent of Stt7 and additional phosphorylation sites that are redox-independent. This mapping provides new insights into the regulatory network of protein phosphorylation in algal photosynthetic membranes during state transitions.
EXPERIMENTAL PROCEDURES
Strains and media
C. reinhardtii wild-type and mutant cells were grown as described (26). The stt7 mutant and stt7 complemented with Stt7-HA were used (23). The HA tag consists of six copies of the HA peptide YPYDVPDYA inserted at the C-terminal end of Stt7. In some experiments, mutated versions of Stt7-HA (S533A and S533D) were obtained by mutating the TCA codon of Ser533 to GCG or GAC to replace Ser533 by alanine or aspartic acid, respectively. Strains were maintained on Tris acetate-phosphate medium at 25 °C in dim light (10 μmol·m−2·s−1). State 1 and state 2 were induced in cells in high salt medium (26) as described (23).
Isolation of Thylakoid Membranes and Phosphorylated Peptides from Membrane Proteins
Thylakoid membranes were isolated from the algal cells as described (11). All buffers contained 10 mm NaF to inhibit the activities of endogenous protein phosphatases. The isolated thylakoids were washed twice with 25 mm NH4HCO3, 10 mm NaF; resuspended in the same buffer to a concentration of 2 mg of chlorophyll/ml; and incubated with sequencing grade modified trypsin (Promega) (5 μg of enzyme/mg of chlorophyll) at 22 °C for 3 and 16 h. The digestion products were frozen, thawed, and centrifuged at 15,000 × g. The supernatant containing peptides released from the membranes was collected, and the peptides were methyl-esterified by methanolic HCl (27) and enriched for phosphorylated peptides by the IMAC procedure described previously (28).
Identification of Phosphorylated Peptides by LC-MS
The IMAC-enriched mixtures of phosphorylated peptides were separated using an Agilent 1100 HPLC system with the flow splitter and analyzed by electrospray ionization MS in positive ionization mode using the ion trap “HCTultra PTM Discovery System” (Bruker Daltonics, Bremen, Germany). The peptides were separated on C18 reverse phase column (5 μm; 0.3 × 150 mm) at a flow rate of 7 μl/min. A gradient of 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B) was distributed as follow: 0–5% B in the first min, 5–40% B in 1–46 min, 40–100% B in 46–53 min, and 100% B in 53–60 min. The automated on-line tandem MS analyses were performed using alternating collision-induced dissociation and electron transfer dissociation of peptide ions. A full-scan MS acquisition was performed in “standard-enhanced” mode (8,100 m/z/s) followed by two MS/MS scans in “ultra scan” mode (26,000 m/z/s) on the five most abundant ions.
The raw data files were processed using Bruker Daltonics DataAnalysis 3.4 (Bruker Daltonics). The resulting MGF files were used for searches in the C. reinhardtii genome v3.0 database at the Joint Genome Institute on an in-house MASCOT server (version: 2.2.06) licensed to Umea University by Matrix Science. The search parameters allowed mass errors up to 0.4 Da for MS data and up to 0.6 Da for MS/MS data in the case of collision-induced dissociation of peptide ions and 1.5 Da in the case of electron transfer dissociation of peptide ions. The charge states of the peptides were varied. Additional settings permitted three missed cleavage sites for trypsin. Cysteine carbamidomethylation, methylation of aspartic and glutamic acids, and methylation of C-terminal carboxylic groups were selected as fixed modifications. N-terminal acetylation and serine or threonine phosphorylation were selected as variable modifications. For identification of phosphorylated peptides we used the following criteria: a MASCOT score above 30 for MS/MS spectra of the peptides identified by collision-induced dissociation or electron transfer dissociation fragmentation was used, the significance threshold was chosen to be 0.05, and redundant identifications were excluded using the bold red function. The sequence of each phosphorylated peptide was verified by manual analysis of MS/MS spectra.
Relative Quantification of Protein Phosphorylation
Lyophilized tryptic peptides from thylakoids that contained 50 μg of chlorophyll from cells in either state 1 or state 2 were methyl-esterified (27, 29) with either 200 μl of 2 n methanolic HCl or DCl prepared by addition of 160 μl of acetyl chloride to 1 ml of anhydrous d0-methyl alcohol or to 1 ml of anhydrous d3-methyl d-alcohol (Sigma-Aldrich). The reactions were performed at room temperature for 2 h, the peptides were lyophilized, and the esterification procedure was repeated once more. Light and heavy isotope-labeled peptides were mixed 1:1 and enriched for phosphopeptides by the IMAC method as described above. The reverse labeling of the corresponding peptides was performed as an internal control. The IMAC-enriched mixtures of phosphorylated peptides were vacuum-dried, reconstituted in 0.1% formic acid in water, and separated using an on-line nanoflow HPLC system (EASY-nLC from Bruker Daltonics), and data were acquired using an electrospray ionization MS ion trap (HCTultra PTM Discovery System, Bruker Daltonics). The peptides were separated by reverse phase chromatography on a 20-mm × 100-μm (particle size, 5 μm) C18 precolumn followed by a 100-mm × 75-μm C18 column (particle size, 5 μm) at a flow rate of 300 nl/min. The total time for a single LC run was 120 min. A gradient of 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B) was distributed as follows: starting with 0% B, linear gradient of 0–30% B in 3–100 min, 30–100% B in 100–115 min, and 100% B in 115–120 min. The automated on-line tandem MS analyses were performed using alternating collision-induced dissociation and electron transfer dissociation of peptide ions.
For the comparative quantification of differentially labeled phosphorylated peptides, the extracted ion chromatograms acquired through an LC-MS run were computed for the selected peptides. The extracted ion chromatogram peak area represented the total ion abundance of the selected peptide. Analyses were performed using Bruker Daltonics DataAnalysis 3.4. After statistical analysis, the data were presented as the mean ± S.D. Statistical significance was set at p < 0.05. All mass spectrometry assays were performed three times unless otherwise specified.
Protein Separation and Immunoblotting Analyses of Phosphorylated Proteins during State Transitions
Proteins from thylakoid membranes isolated from cells in either state 1 or state 2 were separated on a 15% SDS-polyacrylamide 6 m urea gel and transferred to nitrocellulose membranes. The membranes were blocked with bovine serum albumin and incubated with rabbit anti-phosphothreonine antibody (Cell Signaling Technology). As a loading control, the same samples were analyzed by SDS-PAGE and immunoblotting. The antibodies used were raised against CP43, CP29, D2, and LHCII.
Site-directed Mutagenesis of Ser533 of Stt7
The genomic Stt7 clone was digested with NruI and AscI to subclone the fragment containing the TCA codon of Ser533. This codon was then mutated either to GCG or GAC, coding alanine or aspartic acid, respectively, by fusion PCR. These fragments containing mutated Ser533 were cloned back in the initial genomic Stt7 clone. All new plasmids were sequenced to check the presence of the mutation. Transformation of stt7 cells was obtained by selecting for paromomycin resistance.
Fluorescence Spectra during State Transitions
Measurements of fluorescence emission spectra were performed as described (23). Cells in state 1 were transferred to the dark in a closed vessel of a pulse amplitude modulation fluorometer (Hansatech). Under these conditions, respiration in the absence of photosynthesis leads to anaerobiosis. The increase of F0 (the fluorescence yield when the electron acceptor of PSII is fully oxidized; lower trace) indicates a more reduced state of the plastoquinone pool. The decrease of maximal fluorescence (measured by 0.7-s saturating light pulses given every 2 min and shown as spikes) indicates the occurrence of state 2. If the maximal fluorescence does not decrease, cells are blocked in state 1.
Protein Stability Measurements during State Transitions
Cultures were grown in Tris acetate-phosphate medium to a density of 2 × 106 cells/ml. After a 10-fold concentration, cells were incubated in high salt medium under anaerobic conditions in the dark for 2 h. Cells were then maintained under state 2 conditions or cultured under dim light under strong aeration (state 1 conditions). Total cell extracts were analyzed by SDS-PAGE and immunoblotting using antibodies raised against HA and cytochrome f.
Graphical Pattern of Aligned Phosphorylated Peptides
The characters representing the sequence were stacked on top of each other for each position in the aligned sequences. The height of each letter is proportional to its frequency, and the letters are sorted with the most common one at the bottom. The height of the entire stack is then adjusted to signify the information content (measured in bits) of the sequences at that position. The logo displays both significant residues and subtle sequence patterns (30). Basic amino acids (His, Lys, and Arg), acidic amino acids (Asp and Glu), neutral amino acids, and amino acids with uncharged, polar residues are shown in blue, red, black, and green, respectively.
Bioinformatics Search for Other Targets of Stt7
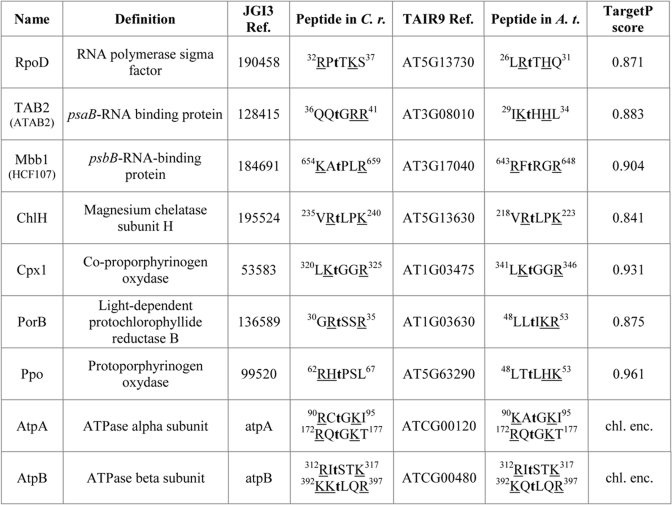
To search for putative targets of Stt7 in the chloroplast, it is necessary to predict which proteins of Chlamydomonas are targeted to the chloroplast. Unfortunately, prediction algorithms for chloroplast localization are not reliable for C. reinhardtii in contrast to Arabidopsis thaliana. Among 33,410 putative proteins in the TAIR9 database for Arabidopsis (31), 2,830 are predicted to be targeted to the chloroplast using the TargetP algorithm, which detects chloroplast transit peptides (32). In addition, other proteins without obvious transit peptide were experimentally shown to be targeted to the chloroplast (33). Together with 88 polypeptides directly encoded by the chloroplast genome, this yields a total of 3,107 proteins predicted to be localized in chloroplasts. To identify the orthologs in C. reinhardtii, the JGI3 database (34), which predicts all together 15,212 proteins in Chlamydomonas (15,143 encoded by the nuclear genome and 69 encoded by the chloroplast genome), was screened using the sequences of the above 3,107 polypeptides from Arabidopsis as a query for a protein BLAST homology search (matrix BLOSUM62 and expectation value fixed at e−20) (35). In this way, we found 1,986 Chlamydomonas proteins showing similarity with the 3,107 predicted chloroplast proteins of A. thaliana. Next, a search for the presence of at least two basic residues (Lys, Arg, or His) surrounding a threonine residue of the (K/R/H)(K/R/H)TX(K/R/H)(K/R/H) motif in all these proteins was performed using the R project software (36). Peptides matching these parameters but containing at least one acidic residue (Asp or Glu) were not considered in the results. Moreover, assuming that Stt7 and STN7 share the same signature motif, only the pairs of orthologs in which both proteins possess the signature motif in the same peptide region were considered.
RESULTS
Involvement of Protein Kinase Stt7 in Phosphorylation of Thylakoid Proteins during State Transitions in C. reinhardtii
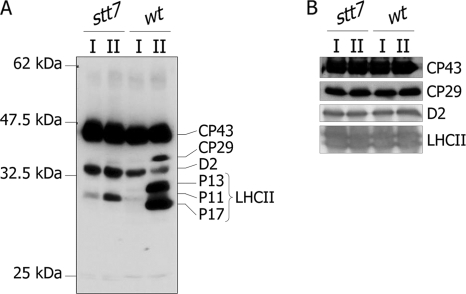
To examine changes in protein phosphorylation during state transitions, cells from wild type and stt7 were subjected to state 1 and state 2 conditions that were induced by strong agitation of the cells under low light (state 1) and by transferring cells to anaerobia in the dark (state 2) (23). Thylakoids from wild-type and stt7 cells were isolated under state 1 or state 2 conditions and subjected to immunoblotting using an anti-phosphothreonine antibody (Fig. 1A). This antibody detects phosphorylation of CP43, CP29, D2, and LHCII proteins. As expected, there was a massive increase in phosphorylation of CP29, P13, and P17 when wt cells were transferred from state 1 to state 2 conditions. As reported earlier (37), phosphorylation of the PSII D2 reaction center protein decreased under these conditions. The phosphorylation patterns observed in stt7 were strikingly different from those of wild type. Only CP43, D2, and P11 were phosphorylated in thylakoids isolated from stt7 cells. The phosphorylation patterns did not change in stt7 thylakoids between state 1 and state 2; in particular, no phosphorylation was detected for P13 and P17, and there was only a slight increase in phosphorylation of P11 in state 2. CP43 phosphorylation was the same under state 1 and state 2 conditions both in wild type and stt7. These different phosphorylation patterns are not due to differences in the amount of proteins loaded as shown by immunoblotting of the same samples with antibodies against several thylakoid membrane proteins (Fig. 1B). These observations confirm the involvement of the Stt7 kinase in the phosphorylation of major and minor LHCII proteins during state transitions (21).
Fig. 1.
Protein phosphorylation in thylakoids from wt and stt7 C. reinhardtii cells in state 1 and state 2. A, isolated thylakoid membranes from C. reinhardtii wt and stt7 cells in state 1 (I) and state 2 (II) were subjected to SDS-PAGE and immunoblotted using anti-phosphothreonine antibody from Cell Signaling Technology. The identity of the phosphorylated proteins was determined by immunoblotting with antisera from the indicated proteins. B, as a loading control, the same protein extracts were immunoblotted with antisera against CP43, CP29, D2, and LHCII as indicated.
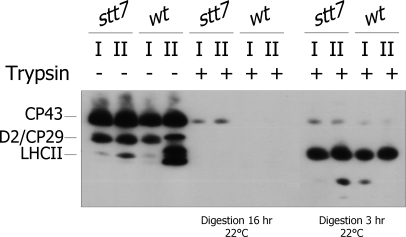
A limitation with immunoblot analysis is that protein phosphorylation patterns observed with anti-phosphothreonine antibodies depend not only on the amount of phosphorylated protein but also to a significant degree on the source of the antiserum, and it is not possible to detect multiple phosphorylation sites within the same protein (29). We therefore extended this study on phosphorylation patterns of LHCII proteins with a mass spectrometric analysis, which allows one to identify the phosphorylation sites within these proteins. Thylakoid membranes from stt7 or wild-type cells in state 1 or state 2 were prepared for phosphoproteomics analysis as described (18). Digestion of the membranes with trypsin resulted in cleavage of the surface-exposed phosphorylated domains of membrane proteins as confirmed by immunoblotting with anti-phosphothreonine antibody (Fig. 2). A 3-h digestion was not sufficient to release all the phosphopeptides from the membranes. Thus, samples were reloaded with trypsin and subjected to an overnight (16-h) digestion at 22 °C. Supernatants from both digestions were pooled, and phosphopeptides were methyl-esterified and affinity-enriched by IMAC (10, 18, 28). The peptides were then analyzed by LC-MS/MS using an ion trap performing alternating collision-induced dissociation and electron transfer dissociation of peptide ions. The electron transfer dissociation is a mild fragmentation technique that does not destroy phosphorylated amino acids (38). To identify the corresponding phosphopeptides, the LC-MS/MS data analysis was done using MASCOT searches against protein and genomic databases of C. reinhardtii. Sequences of all phosphorylated peptides were confirmed by manual validation of the fragmentation spectra.
Fig. 2.
Shaving of thylakoid phosphorylated proteins using trypsin. Isolated thylakoids from wt and stt7 cells in state 1 (I) and state 2 (II) were treated with (+) or without (−) trypsin at 22 °C for 3 or 16 h for achieving partial or complete digestion, respectively. The proteins were subsequently separated by SDS-PAGE and immunoblotted with an anti-phosphothreonine antibody. The identity of the phosphorylated proteins is indicated on the left.
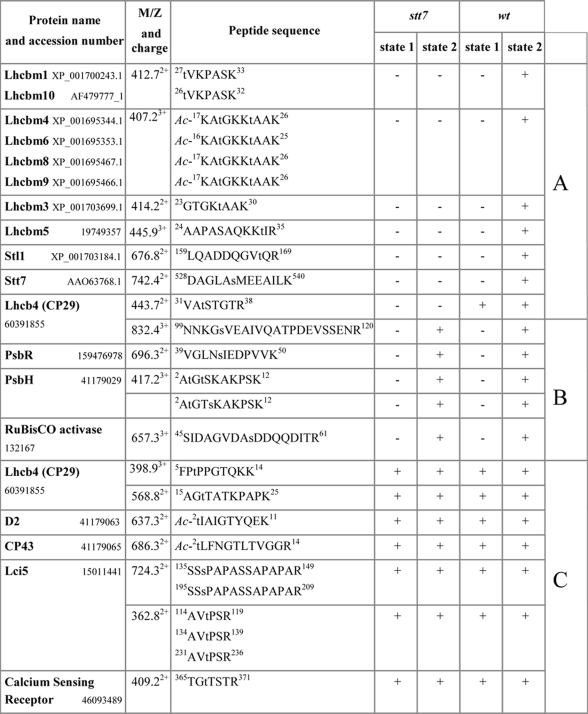
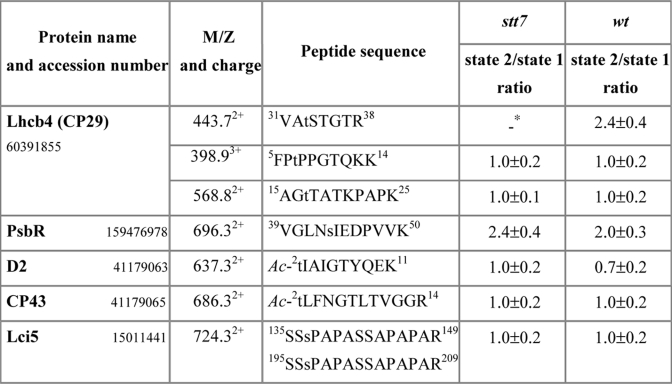
The protein sites phosphorylated under state 1 and state 2 conditions in wild type are shown in Table I. A total of 21 sites was identified including 15 Thr and 6 Ser residues in the peptides corresponding to proteins encoded by 18 different genes in the Chlamydomonas genome (Table I). In this way, we identified several previously unknown phosphorylation sites. Among the major light-harvesting proteins, we found novel phosphorylation sites at the positions corresponding to Thr27 and Thr33 in the primary translation products of Lhcbm3 and Lhcbm5, respectively (supplemental Fig. 2). In addition to the previously known single phosphorylation site at Thr3 in the PsbH protein of photosystem II (39), we also found alternative phosphorylation of either Thr5 or Ser6 (supplemental Fig. 3). Two doubly phosphorylated peptides had slightly different retention times during LC separation, whereas their fragmentation by electron transfer dissociation demonstrated unambiguous localization of the second phosphorylation site to either Thr5 or Ser6 (supplemental Fig. 3). Notably, although phosphorylation of Thr5 was known in plant PsbH (24), the alternative phosphorylation at Ser6 has not been seen before. The other previously unknown phosphorylation site was found in a protein homologous to the calcium-sensing receptor in A. thaliana. The phosphorylated threonine residue in the C-terminal region of this protein (supplemental Fig. 4) was localized at the site with high sequence similarity to the phosphorylated region in the Arabidopsis protein (40). Moreover, one additional previously unknown phosphorylation site was localized by electron transfer dissociation at Ser53 in the Rubisco activase (supplemental Fig. 5). Although this enzyme is mostly localized in the chloroplast stroma, a portion has also been found associated with the thylakoid membranes (41).
Table I. Protein phosphorylation sites identified in thylakoids from wt and stt7 cells in state 1 and state 2.
The names of the proteins corresponding to the sequenced peptides are shown. The sequences of the peptides are typed in single letter amino acid code with the lowercase t and s designating phosphorylated threonine and serine residues, respectively. Ac- indicates N-terminal acetylation. The superscript numbers in the sequences correspond to the amino acid positions in the initial translation product of the corresponding protein. Carboxylic groups in all peptides were methyl-esterified. Plus or minus signs in the four columns of stt7 and wt indicate whether the corresponding phosphorylated peptide was detected or not, respectively, in the sample from stt7 and wt algal cells in state 1 or state 2. A refers to Stt7-dependent phosphorylation in state 2, B refers to Stt7-independent phosphorylation in state 2, and C refers to Stt7-independent phosphorylation both in state 1 and state 2.
Seven of the 21 sites phosphorylated under state 2 conditions were also phosphorylated in state 1 (Table I). They include the N-terminal threonine residues of the PSII core proteins D2 and CP43 (18, supplemental MS/MS fragmentation data), Thr7 and Thr17 of the minor light-harvesting protein CP29 (Lhcb4) (18), a phosphorylated threonine and a serine residue in peptides derived from tandem repeat sequences of the Lci5 protein previously found to be phosphorylated under low CO2 conditions (25, supplemental MS/MS fragmentation data), and Thr367 of the calcium-sensing receptor protein.
Thirteen sites were found to be specifically phosphorylated in thylakoid proteins from cells in state 2 (Table I). Phosphorylation of two threonines was detected in the N-terminally acetylated peptide, which is identical in the sequences of Lhcbm4, Lhcbm6, Lhcbm8, and Lhcbm9 (18). Four additional phosphorylation sites specific to Chlamydomonas cells or showing enhanced phosphorylation in state 2 were found in other LHCII antenna proteins (Lhcbm1/Lhcbm10, Lhcbm3, and Lhcbm5 and Thr33 and Ser103 in CP29). Phosphorylation of the PsbR protein of PSII at Ser43 as well as of the PsbH protein at three different positions, Thr3, Thr5, and Ser6, was also only detected under state 2 conditions (Table I). Moreover, phosphorylation of Ser53 in the Rubisco activase only occurred in state 2 (Table I).
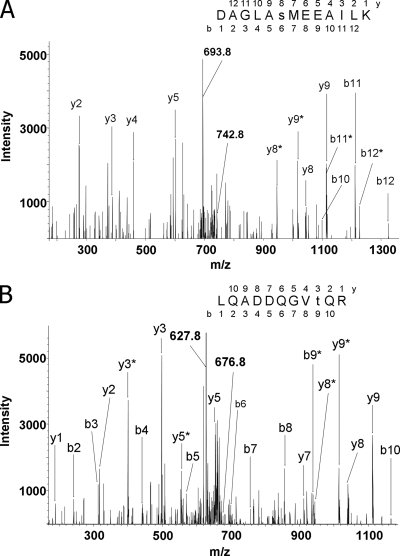
The sequential application of phosphopeptide enrichment by IMAC and the following LC-MS analyses allowed us to identify not only phosphorylation sites in proteins of the major photosynthetic complexes but also in low abundance thylakoid proteins. The most interesting finding was the identification of phosphorylation sites in two protein kinases, Stt7 and Stl1. Fig. 3 shows tandem mass spectra of the phosphorylated peptides, which mapped the sites to Ser533 and Thr167 in Stt7 and Stl1, respectively. Both kinases were only found phosphorylated in cells in state 2 (Table I). The role of the Stl1 protein kinase, which is highly homologous to Stt7 and also localized in thylakoid membranes of Chlamydomonas, is still largely unknown (21).
Fig. 3.
Mass spectrometric sequencing of phosphorylated peptides from Stt7 and Stl1 protein kinases. Peptides were obtained by tryptic cleavage of isolated thylakoid membranes of the wild-type C. reinhardtii cells exposed to state 2 conditions. Peptides were then methylated and subjected to IMAC enrichment of phosphopeptides. The collision-induced fragmentation spectra of the peptides are shown. The b (N-terminal) and y (C-terminal) fragment ions are labeled in the spectra and in the displayed peptide sequences, which were deduced from the spectra. Lowercase s and t in the sequences designate phosphorylated serine and threonine residues, respectively. Fragment ions that contained a phosphorylated residue and underwent the neutral loss of phosphoric acid (H3PO4; mass, 98 Da) are marked with asterisks. Phosphorylation sites were localized according to the pattern of the fragment ions containing phosphate and corresponding fragments with the neutral loss. A, the fragmentation spectrum of the doubly protonated peptide ion with m/z = 742.8 (indicated). The peptide corresponds to amino acids 528–540 from Stt7 protein. The doubly charged ion indicated at m/z = 693.8 corresponds to the parent ion after the neutral loss of phosphoric acid (742.8 × 2 − 693.8 × 2 = 98). Phosphorylation of the serine residue is evident from the distinct set of the fragment ions: y8, y8*, and y5. B, the fragmentation spectrum of the doubly protonated peptide ion with m/z = 676.8 (marked). The peptide corresponds to amino acids 159–169 from Stl1 protein. The doubly charged ion indicated at m/z = 627.8 corresponds to the peptide with the neutral loss of phosphoric acid. The site of phosphorylation has been localized according to the pattern of the ions containing phosphate and the satellite fragments with the neutral loss of phosphoric acid: y3, y3*, b8, and b9*.
Analysis of the phosphorylation patterns in stt7 revealed that among all the phosphorylated residues detected in wild-type cells in state 2 seven were not detected in thylakoids from the stt7 mutant (Table I). These sites include Thr residues in Lhcbm1/Lhcbm10, Lhcbm4/Lhcbm6/Lhcbm8/Lhcbm9, Lhcbm3, and Lhcbm5 and Thr167 in Stl1. The phosphorylation of these sites depends on the Stt7 kinase, directly or indirectly. The Stt7-dependent phosphorylation of Stl1 strongly suggests the existence of a functional link between these two kinases. In the case of the Stt7 kinase, which was also phosphorylated under state 2 conditions (Table I), it is not known whether this is due to autophosphorylation or whether another kinase is involved. The presence of a phosphorylated residue in Stt7 was expected from the study of Depège et al. (21) that revealed that treatment of thylakoid proteins with phosphatase converted a slower to a faster migrating form of Stt7.
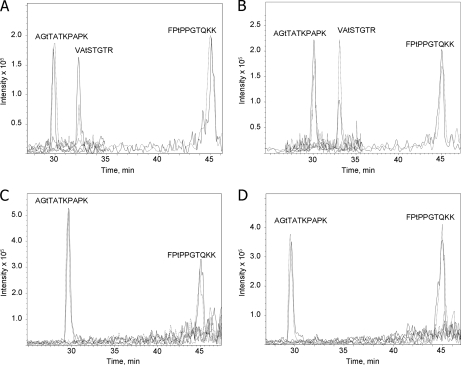
To obtain quantitative data on relative protein phosphorylation under state 1 and state 2 conditions, we analyzed thylakoid proteins using differential stable isotope labeling and mass spectrometry. Carboxyl groups of the tryptic peptides from thylakoid membranes isolated from cells in either state 1 or state 2 were esterified with anhydrous d0-methyl alcohol or d3-methyl d-alcohol as described (27, 29). As a result, a mass increment of 14 or 17 Da per each esterified carboxyl group was obtained for d0-methanol or d3-methanol, correspondingly. Light isotope-labeled peptides from state 1 sample were mixed 1:1 with heavy isotope-labeled peptides from state 2 sample. The same procedure was done for the peptides from the wild-type and stt7 thylakoids. In both cases, we performed the reverse labeling of the wild-type and mutant peptides as an internal control. The methyl esters of the phosphorylated peptide mixtures were then enriched by IMAC and analyzed by LC-MS, which allowed for simultaneous measurements of intensities for light and heavy isotope-labeled phosphopeptide pairs and comparative quantification of the phosphorylation level. This resulted in reliable quantitative data for three sites in CP29 (Fig. 4). Thr33 was phosphorylated 2.4 times more in state 2 than in state 1, and phosphorylation was dependent on Stt7 (Table II and Fig. 4). Phosphorylation of Ser103 only occurred in state 2 but was independent of Stt7. In contrast, Thr7 and Thr17 were phosphorylated under both state 1 and state 2 conditions in the wild type and stt7 (Table II and Fig. 4). Similar results were obtained for PsbR, CP43, and Lci5 (Table II). Interestingly, the lower phosphorylation of D2 under state 2 in comparison with state 1 conditions in the wild type that was observed earlier (37) was not apparent in stt7 as observed in Fig. 1 (Table II). No phosphopeptides from LHCII were detected in stt7 mutant samples or in the state 1 samples from the wild type. Factors that could hinder detection of possible low amounts of these peptides are the following: only single modification by labeled methanol in each peptide, high charge state, and low m/z in the region of the high background.
Fig. 4.
Quantitative mass spectrometric analysis of phosphorylated peptides of CP29. A, ion chromatograms of the phosphorylated peptides from wild type labeled with light isotope (dashed line) in state 1 and with heavy isotope (continuous line) in state 2 analyzed by LC-MS. The sequences of three N-terminal phosphorylated peptides from CP29 are shown above the corresponding ion intensity peaks. B, same as A except that the wild-type phosphorylated peptides were labeled with heavy isotope in state 1 (continuous line) and with light isotope in state 2 (dashed line). C, ion chromatograms of the phosphorylated peptides from the stt7 mutant labeled with light isotope (dashed line) in state 1 and with heavy isotope (continuous line) in state 2. D, same as C except that the phosphorylated peptides from stt7 were labeled with heavy isotope in state 1 (continuous line) and with light isotope in state 2 (dashed line). Lowercase t in the sequences designates phosphorylated threonine residues.
Table II. Mass spectrometric quantification of thylakoid protein phosphorylation from wt and stt7 cells in state 1 and state 2.
The state 2/state 1 ratios for mutant and wild type were determined in every LC-MS experiment from the areas of extracted ion chromatograms for each of the differentially labeled phosphorylated peptides corresponding to a particular protein. The m/z values are shown only for the light isotope-labeled peptide ions. The data are averages (±S.D.) from three different preparations. The state 1 and state 2 samples from each preparation were analyzed by both forward and reversed stable isotope labeling.
* The VAtSTGTR phosphopeptide signal was not detected in the stt7 sample in all three experiments.
Phosphorylation of Ser533 of Stt7 Is Not Required for State Transitions
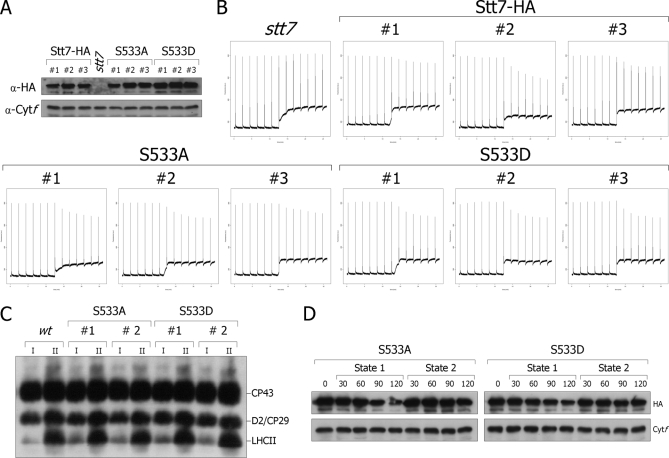
The finding that Stt7 is phosphorylated at Ser533 in wild type under state 2 conditions raises the question whether this phosphorylation is required for regulation or activation of Stt7 during transition from state 1 to state 2. To answer this question, this serine residue was changed to either alanine or aspartic acid by site-directed mutagenesis and transformation of the stt7 mutant with either the Stt7-HA-S533A or the Stt7-HA-S533D construct carrying an HA epitope at its C-terminal end. A change from Ser to Ala prevents phosphorylation of this residue, whereas a change to Asp mimics the phosphorylated form of Ser. Three independent transformants for each construct were analyzed (Fig. 5). The HA tag was included in all these constructs to check the expression level of the mutant kinases by immunoblotting (Fig. 5A). A loading control was performed with anti-cytochrome f antibody. In all cases, the mutant kinase accumulated as in cells transformed with wild-type Stt7 (Stt7-HA). Transition from state 1 to state 2 as assayed by the decrease in maximum fluorescence measured by pulse amplitude modulation fluorometry was nearly identical in all these mutants and in the strain rescued with wild-type Stt7 (Fig. 5B). Thus, these mutants are still able to perform state transitions in the absence of phosphorylation at Ser533. Moreover, phosphorylation of LHCII under state 2 conditions in the Ser533 mutants occurred as in wild type (Fig. 5C). We conclude that phosphorylation at Ser533 is not required for the activation of the kinase and state transitions. We cannot exclude that there are other phosphorylated residues in Stt7 that can compensate for the lack of phosphorylation of Ser533.
Fig. 5.
Analysis of Ser533 mutants of Stt7. A, immunoblots of total extracts from transformants of the stt7 mutant (stt7) containing wild-type Stt7-HA (Stt7-HA), Stt7-S533A-HA (S533A), or Stt7-S533D-HA (S533D) with HA and cytochrome f (Cytf) antibodies as a loading control. B, the strains described in A were transferred to the dark in a closed vessel of a pulse amplitude modulation fluorometer (Hansatech). Under these conditions, respiration in the absence of photosynthesis leads to anaerobiosis. The increase of F0 (the fluorescence yield when the electron acceptor of PSII are fully oxidized; lower trace) indicates increased reduction of the plastoquinone pool. The decrease of maximal fluorescence (measured by 0.7-s saturating light pulses given every 2 min and shown as spikes) indicates the occurrence of state 2 in all rescued strains but not in the mutant that is blocked in state 1. For each version of Stt7-HA, three independent clones were analyzed. a.u., arbitrary units. C, immunoblots of total extracts from wild type, S523A, and S533D in state 1 (I) or state 2 (II) with Thr(P) antiserum. D, immunoblots of total extracts from S533A and S533D with HA and cytochrome f antibodies as loading control.
Another possible role of this state transition-dependent phosphorylation of Stt7 is a change in stability of this kinase. Recently, we showed that Stt7 accumulates stably under state 2 conditions but is degraded under state 1 conditions (23). We observed a similar decrease in stability of the Stt7-HA-S533A and Stt7-HA-S533D kinases under state 1 conditions, whereas the amounts of the Stt7-HA-S533A and Stt7-HA-S533D kinases did not change under state 2 conditions (Fig. 5D). It thus appears that phosphorylation of the Ser533 residue does not play a significant role for the accumulation/degradation of the Stt7 kinase during state transitions.
Substrate Specificity of Stt7 Kinase
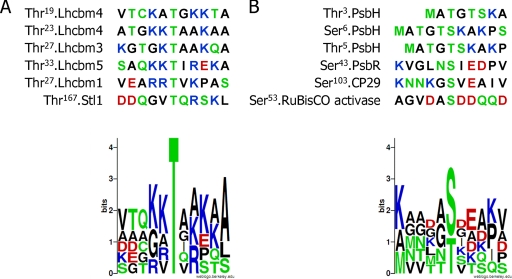
In this work, we accomplished the first comparative mapping of in vivo protein phosphorylation sites within photosynthetic membranes of wild type and the stt7 mutant. Overall, we identified eight Stt7-dependent phosphorylation sites corresponding to 11 genes in the Chlamydomonas genome (Table I). However, because the phosphopeptides are identical in Lhcbm4/Lhcbm6/Lhcbm8/Lhcbm9, we cannot determine whether all or a subset of these proteins are phosphorylated under state 2 conditions. The phosphorylated residue in all these peptides is always a threonine residue that is surrounded on each side by basic residues (Fig. 6A). This conserved motif (K/R/H)(K/R/H)TX(K/R/H)(K/R/H) could represent a signature for substrate specificity of the Stt7 kinase. The same kind of motif was found by Vainonen et al. (29) in two phosphorylated peptides, Ac-RKtVAKPK and Ac-RRtVK (where lowercase t represents phosphorylated threonine), and proposed for the substrate specificity of STN7, the ortholog of Stt7 from Arabidopsis. The phosphorylated Thr167 in Stl1, which is surrounded by only one arginine residue, is not closely related to the consensus motif. Maybe this Stt7-dependent phosphorylation site in Stl1 is phosphorylated by Stt7 only when Stt7 reaches a particular state of activation. Another possibility is that Stl1 is not phosphorylated directly by Stt7 but by another kinase acting downstream of Stt7. If true, this would imply the existence of a kinase cascade in the chloroplast. This possibility is compatible with the fact that we were not able to detect any direct interaction between Stt7 and Stl1 (data not shown). However, it is equally possible that a transient interaction occurs between these two protein kinases. Similarly, the phosphorylation site of Thr33 from CP29 does not follow the consensus motif, although phosphorylation at this residue is strictly Stt7-dependent (Table II and Fig. 4). Thus, other sites appear to be recognized by Stt7. We also searched for the presence of a consensus sequence in peptides phosphorylated in an Stt7-independent way under state 2 conditions but were unable to identify such a signature (Fig. 6B).
Fig. 6.
Alignment of peptides phosphorylated only under state 2 conditions. Amino acid sequences of peptides phosphorylated specifically under state 2 conditions were aligned to determine which amino acids are surrounding the phosphorylated threonine or serine residues. A, six Stt7-dependent phosphorylated residues corresponding to peptides from Lhcbm1/Lhcbm10, Lhcbm4/Lhcbm6/Lhcbm8/Lhcbm9, Lhcbm3, Lhcbm5, and Thr167 of Stl1 were aligned. The graphical representation of the sequence conservation of the flanking amino acids was obtained using WebLogo (55). B, six Stt7-independent phosphorylated residues corresponding to peptides from PsbR; Thr3, Thr5, and Ser6 of PsbH; Ser53 of Rubisco activase; and Ser103 of CP29 were aligned. The graphical representation of the sequence conservation of amino acids was obtained using WebLogo (55).
DISCUSSION
Identification of New Phosphorylation Sites in LHCII Proteins
Here we mapped for the first time the in vivo phosphorylation sites of Lhcbm3 (supplemental Fig. 2A) and Lhcbm5 (supplemental Fig. 2B). Both of these proteins are major LHCII proteins. Lhcbm3 is an LHCII type I protein (11) that is a part of LHCII trimers. Lhcbm5 is an LHCII type II protein that was already shown to be phosphorylated under state 2 conditions (11), but the phosphorylation site was not mapped. In Arabidopsis, LHCII trimers are connected to PSII through the minor LHCII proteins CP24, CP26, and CP29 (16, 42). However, no CP24 could be identified in Chlamydomonas. Takahashi et al. (11) placed Lhcbm5 in the position suggested for CP24 in PS-LHCII supercomplexes of higher plants (16). It is therefore likely that phosphorylation of Lhcbm5 is important for state transitions in Chlamydomonas. This is supported by the fact that Lhcbm5 does not have any ortholog in higher plants, although it has a sequence similar to that of many major LHCII proteins. This difference between Chlamydomonas and Arabidopsis should be put in relation with the difference in lateral displacement of the mobile LHCII from the PSII-rich grana to the PSI-rich lamellar thylakoid regions in these two organisms. The mobile LHCII represents about 80% of the total LHCII in C. reinhardtii (13), whereas in land plants only 15–20% of LHCII is mobile (3).
We also found two new phosphorylated peptides from PsbH protein under state 2 conditions that were also detected in the absence of Stt7 (supplemental Fig. 3 and Table I). Interestingly, Thr3 was phosphorylated in both peptides, but the second phosphorylation site maps either on Thr5 or Ser6. In Arabidopsis, Vainonen et al. (29) showed that Thr4 phosphorylation was dependent on Thr2 phosphorylation. They proposed that phosphorylation of other residues in PsbH depends on the phosphorylation of Thr2 and that this second phosphorylation requires the STN8 kinase. These sequential phosphorylation events raise the question of a cross-talk between thylakoid protein kinases at the substrate level.
A new phosphorylation site, Thr367, was also found in the calcium-sensing receptor protein (supplemental Fig. 4) that was detected under both state 1 and state 2 conditions and in the absence of Stt7. The same site was found phosphorylated in the calcium-sensing receptor from Arabidopsis, and the phosphorylation of this residue involves the STN8 kinase (40).
Our results on the mapping of in vivo phosphorylation sites during state transitions differ slightly from those of Turkina et al. (18). These differences may be due in part to the fact that state 1 conditions induced through aerobic agitation in the dark by Turkina et al. (18) are less stringent than those obtained in this study through vigorous agitation in the presence of 3-(3,4-dichlorophenyl)-1,1-dimethylurea under low light. The differences could also arise from the use of different protocols and of more sensitive methods for identifying phosphorylated peptides. Whereas these authors found Thr2 of D2 protein phosphorylated only under state 2 conditions, we detected phosphorylation of this residue also in state 1. This result agrees with the phosphorylation pattern under state 1 and state 2 conditions observed by immunoblotting (Fig. 1) and with earlier studies (37). Turkina et al. (18) also found Thr33 of CP29 phosphorylated both in state 1 and state 2, whereas we found that phosphorylation of this site is increased under state 2 conditions. However, without quantitative analysis, this difference would have escaped detection. In this study, we found 12 new phosphorylation sites that were not detected in the earlier studies. Two phosphorylated threonine residues from Lhcbm4, Lhcbm6, Lhcbm8, and Lhcbm9; Thr27 from Lhcbm3; Thr33 from Lhcbm5; Thr3, Thr5, and Ser6 from PsbH; Thr2 of CP43; Thr367 of calcium-sensing receptor; Ser533 of Stt7; Thr167 of Stl1; and Ser53 from Rubisco activase.
Taken together, our results indicate that phosphorylation of thylakoid membrane proteins is regulated in at least three different ways corresponding to groups A, B, and C of Table I. Group A, comprising mostly LHCII proteins, is phosphorylated in an Stt7-dependent manner. Group B is phosphorylated in a redox-dependent but Stt7-independent way, and for group C, phosphorylation is independent of the redox state of the plastoquinone pool, i.e. it occurs under both state 1 and state 2 conditions.
Search for Other Targets of Stt7
The comparative analysis of several Stt7 target sites in the LHCII proteins allowed us to establish a consensus motif for these sites in which the phosphorylated Thr is flanked on each side by two basic residues (Fig. 6A). This motif was also proposed to be the signature for specific STN7 phosphorylation sites (29). To identify additional potential Stt7/STN7 target sites, we first searched for predicted chloroplast proteins from Chlamydomonas according to their sequence similarity with the predicted chloroplast proteins from Arabidopsis for the presence of the (K/R/H)(K/R/H)TX(K/R/H)(K/R/H) motif. Interestingly, motifs of this sort were found in several predicted chloroplast proteins of Chlamydomonas and Arabidopsis (Table III). Several of these proteins are low abundance nucleus-encoded chloroplast proteins involved in post-transcriptional steps of plastid gene expression such as Tab2 required for psaB mRNA translation (43) and Mbb1 involved in psbB mRNA stability (44, 45). These proteins are present in the stroma and/or are not tightly bound to the thylakoid membrane and were therefore missed in this study, which focused on thylakoid membrane proteins. The finding of the σ factor RpoD as a possible target of Stt7 is particularly interesting. It is noteworthy that differential phosphorylation of plastid σ factors of land plants has profound effects on plastid transcription. It was shown that phosphorylation and dephosphorylation of the σ factors confer etioplast and chloroplast type-specific promoter usage, respectively (46). These findings raise the question whether Stt7 may have some role in the modulation of the synthesis of chloroplast proteins involved in photosynthesis (Fig. 7). Potential Stt7 target sites were also found among proteins involved in chlorophyll biosynthesis (ChlH, Cpx1, PorB, and Ppo) and the two ATP synthase subunits AtpA and AtpB. However, it remains to be seen whether these proteins are indeed phosphorylated under specific conditions.
Table III. Potential targets of Stt7.
When the name is not conserved between Chlamydomonas and Arabidopsis, the name of the related protein in Arabidopsis is indicated in parentheses. The sequences of the phosphopeptides are typed in single letter amino acid code with the lowercase t designating probable phosphorylated threonine. The superscript numbers in the sequences correspond to the amino acid positions in the initial translation product of the corresponding protein. Amino acids corresponding to the consensus motif (K/R/H)(K/R/H)TX(K/R/H)(K/R/H) are underlined. The TargetP score corresponds to the chloroplast localization prediction of the Arabidopsis protein. C.r., C. reinhardtii, A.t., A. thaliana, chl. enc., chloroplast-encoded, JGI3 Ref. and TAIR9 Ref. are the accession numbers of Chlamydomonas and Arabidopsis peptide sequences, respectively.
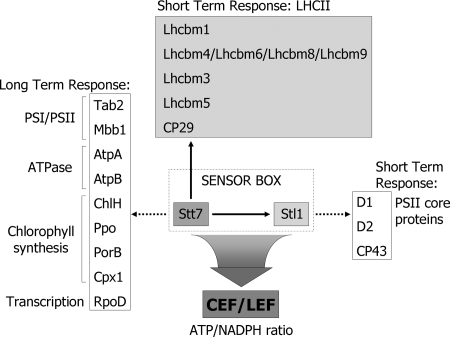
Fig. 7.
Target proteins of Stt7 protein kinase in C. reinhardtii. The target proteins identified in this study are marked with arrows. Stt7 is required for the switch from cyclic (CEF) to linear electron flow (LEF) during state transitions that modulate the ATP/NADPH ratio. The dotted arrows indicate that the proteins are potential targets predicted by the search for proteins with an Stt7 substrate site shown in Fig. 6. In the case of Stl1, the likely targets are the same as those of the STN8 kinase from Arabidopsis that is presumably the ortholog of Stl1.
Ser533 of Stt7 Is Phosphorylated Only under State 2 Conditions
Our earlier studies suggested that Stt7 is phosphorylated (21). Here we have shown that Stt7 is phosphorylated during a state 1 to state 2 transition at Ser533 (Fig. 3). However, cells in which this residue was changed to either alanine or aspartic acid residues were not impaired in state transitions. Moreover, proteolysis of Stt7, which occurs under state 1 conditions, was not altered in these mutants. It is noteworthy that the phosphorylation site of Stt7, Ser533, is located in a region that is not conserved between Stt7 and STN7 from Arabidopsis. Thus, it is possible that the phosphorylation is involved in another function of Stt7 that is distinct from state transitions and that either is different in Chlamydomonas and Arabidopsis or performs a similar function that has considerably diverged during evolution. Another possibility is that this phosphorylation event affects the kinetics and/or efficiency of the Stt7 kinase, but these changes are undetectable under the conditions used for activation of the kinase. Moreover, it is worth noting that the phosphorylation site of Stt7 is not similar to the consensus motif (K/R/H)(K/R/H)TX(K/R/H)(K/R/H). This raises the possibility that Stt7 could be phosphorylated by another kinase in a signal transduction chain that remains to be elucidated.
Multiple Phosphorylations and Interplay between Thylakoid Protein Kinases
The light-dependent multiple and differential phosphorylation of CP29 linker protein in green algae was suggested to control both photosynthetic state transitions and uncoupling of light-harvesting proteins from photosystem II under high light (47). CP29 has emerged as an essential component in state transitions mainly based on the observation that state transitions are blocked in a Chlamydomonas RNA interference strain in which expression of CP29 was knocked down (12). This protein, which lies at the interface between the PSII core and the LHCII antenna, was shown to migrate from PSII to PSI during a transition from state 1 to state 2 (10, 11). During this process, four sites are phosphorylated (18), but our study revealed that only one of them, Thr33, is phosphorylated through Stt7. Apparently, another unidentified kinase is responsible for the phosphorylation of the other three sites of CP29, suggesting that there is a cooperative interplay between different chloroplast protein kinases. Given the fact that both Stt7 and CP29 are required for state transitions and that they specifically interact with each other under state 2 conditions (23), it is tempting to propose that phosphorylation of Thr33 plays a key role in this process. Surprisingly, Thr33 is already phosphorylated under state 1 conditions in an Stt7-dependent manner, but phosphorylation is enhanced upon transition to state 2. This increase in phosphorylation could trigger the dissociation of CP29 from PSII and thereby promote the detachment of LHCII trimers from PSII. Alternatively, it could mediate the proper association of CP29 to PSI as it has been shown that once CP29 has moved to PSI in state 2 it also plays an essential role for the association of LHCII to PSI (12). CP29 is a unique protein because it is the only known nucleus-encoded thylakoid polypeptide in Chlamydomonas that retains the transit peptide in the mature form (48). It is worth noting that the importance of the phosphorylation site at Thr33, found to be Stt7-dependent in this study, could be the reason for the unique retention of the transit peptide.
Stt7/Stl1 Interaction
Among the unidentified kinases involved in thylakoid protein phosphorylation, Stl1 is a promising candidate. It is closely related to the Arabidopsis STN8 kinase (21). Both STN7 (22) and STN8 (29) are activated in thylakoid membranes by light and function synergistically (49, 50). Loss of STN8 kinase leads to a specific decrease in phosphorylation of the PSII core proteins D1, D2, and CP43 (29, 49). The function of Stl1 in Chlamydomonas may be similar to that of STN8 in Arabidopsis based on the sequence similarity. The synergistic functions of STN8 and STN7 in Arabidopsis were the basis for the proposal of the “sensor box” model in which both kinases interact directly or indirectly to adapt photosynthetic activity to changes in light quality and intensity (51). A major role of this sensor box in C. reinhardtii is most likely to adapt to changes in metabolic state by modulating the contributions of cyclic and linear electron flow and thereby adjusting the ATP/NADPH ratio (Fig. 7). The fact that Stl1 probably has a function similar to that of STN8 raises the question of a possible functional interaction between Stt7 and Stl1. This hypothesis is supported not only by the synergistic phenotype of the stn7stn8 mutant compared with the phenotypes of stn7 and stn8 (49, 50) but also by the Stt7-dependent phosphorylation of Thr167 in Stl1 under state 2 conditions. This strongly suggests that Stt7 acts upstream of Stl1 in a signal transduction chain that remains to be elucidated. Unfortunately, no Chlamydomonas stl1 mutant is available. Co-immunoprecipitations of Stt7 with Stl1 failed perhaps because these kinases only interact transiently or because their interaction is too weak.2
Some Proteins Are Phosphorylated Independently from Stt7 under State 2 Conditions
Although the PSII proteins PsbH and PsbR are phosphorylated under state 2 conditions, this occurs independently from Stt7 and is mediated through another protein kinase (Table I). A similar pattern was observed for the calcium-sensing receptor and Rubisco activase (Table I). The latter is mainly localized in the stroma where it is involved in releasing inhibitory sugar phosphates from the active site of Rubisco in an ATP-dependent manner (52). A smaller portion is also associated with the thylakoid membrane (41). Moreover, upon heat treatment, the distribution of Rubisco activase changes from the stroma to the thylakoid membrane where this protein has been proposed to act as a chaperone associated with thylakoid-bound ribosomes (53, 54). Because transition to state 2 in Chlamydomonas is induced when the cellular ATP level declines, it is possible that under these conditions, when it is phosphorylated, Rubisco activase binds to the thylakoid membrane and that one of its roles may be to participate in the protection of Stt7 against proteolysis that occurs under state 1 conditions (23). Because transition from state 1 to state 2 in Chlamydomonas leads to a switch from linear to cyclic electron flow (15), the relocation of Rubisco activase from the stroma to the thylakoid membrane would also serve as an adaptive mechanism to reduce the activity of Rubisco under these conditions.
Acknowledgments
We thank N. Roggli for help with the figures and the Umeå Protein Analysis Facility for the use of the in-house MASCOT server licensed to Umea University by Matrix Science.
* This work was supported by grants from the Swedish Research Council, the Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (to A. V. V.) and from the National Centre of Competence in Research (NCCR) Plant Survival and the Swiss National Foundation (Grant 3100AO-117712 to J.-D. R.).
 This article contains supplemental Figs. 1–5 and MS/MS fragmentation data.
This article contains supplemental Figs. 1–5 and MS/MS fragmentation data.
2 S. Lemeille and J.-D. Rochaix, unpublished results.
1 The abbreviations used are:
- PSII
- photosystem II
- PSI
- photosystem I
- LHCII
- light-harvesting chlorophyll a/b-binding proteins of photosystem II
- Rubisco
- ribulose-1,5-bisphosphate carboxylase/oxygenase
- HA
- hemagglutinin
- wt
- wild-type.
REFERENCES
- 1.Vener A. V., van Kan P. J., Rich P. R., Ohad I., Andersson B. (1997) Plastoquinol at the quinol oxidation site of reduced cytochrome bf mediates signal transduction between light and protein phosphorylation: thylakoid protein kinase deactivation by a single-turnover flash. Proc. Natl. Acad. Sci. U.S.A 94, 1585–1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zito F., Finazzi G., Delosme R., Nitschke W., Picot D., Wollman F. A. (1999) The Qo site of cytochrome b6f complexes controls the activation of the LHCII kinase. EMBO J 18, 2961–2969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen J. F. (1992) Protein phosphorylation in regulation of photosynthesis. Biochim. Biophys. Acta 1098, 275–335 [DOI] [PubMed] [Google Scholar]
- 4.Rochaix J. D. (2007) Role of thylakoid protein kinases in photosynthetic acclimation. FEBS Lett 581, 2768–2775 [DOI] [PubMed] [Google Scholar]
- 5.Wollman F. A. (2001) State transitions reveal the dynamics and flexibility of the photosynthetic apparatus. EMBO J 20, 3623–3630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bennett J. (1977) Phosphorylation of chloroplast membrane proteins. Nature 269, 344–346 [Google Scholar]
- 7.Minagawa J., Takahashi Y. (2004) Structure, function and assembly of Photosystem II and its light-harvesting proteins. Photosynth. Res 82, 241–263 [DOI] [PubMed] [Google Scholar]
- 8.Wollman F. A., Delepelaire P. (1984) Correlation between changes in light energy distribution and changes in thylakoid membrane polypeptide phosphorylation in Chlamydomonas reinhardtii. J. Cell Biol 98, 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lunde C., Jensen P. E., Haldrup A., Knoetzel J., Scheller H. V. (2000) The PSI-H subunit of photosystem I is essential for state transitions in plant photosynthesis. Nature 408, 613–615 [DOI] [PubMed] [Google Scholar]
- 10.Kargul J., Turkina M. V., Nield J., Benson S., Vener A. V., Barber J. (2005) Light-harvesting complex II protein CP29 binds to photosystem I of Chlamydomonas reinhardtii under State 2 conditions. FEBS J 272, 4797–4806 [DOI] [PubMed] [Google Scholar]
- 11.Takahashi H., Iwai M., Takahashi Y., Minagawa J. (2006) Identification of the mobile light-harvesting complex II polypeptides for state transitions in Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. U.S.A 103, 477–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tokutsu R., Iwai M., Minagawa J. (2009) CP29, a monomeric light-harvesting complex II protein, is essential for state transitions in Chlamydomonas reinhardtii. J. Biol. Chem 284, 7777–7782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delosme R., Olive J., Wollman F. A. (1996) Changes in light energy distribution upon state transitions: an in vivo photoacoustic study of the wild type and photosynthesis mutants from Chlamydomonas reinhardtii. Biochim. Biophys. Acta 1273, 150–158 [Google Scholar]
- 14.Finazzi G., Furia A., Barbagallo R. P., Forti G. (1999) State transitions, cyclic and linear electron transport and photophosphorylation in Chlamydomonas reinhardtii. Biochim. Biophys. Acta 1413, 117–129 [DOI] [PubMed] [Google Scholar]
- 15.Finazzi G., Rappaport F., Furia A., Fleischmann M., Rochaix J. D., Zito F., Forti G. (2002) Involvement of state transitions in the switch between linear and cyclic electron flow in Chlamydomonas reinhardtii. EMBO Rep 3, 280–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dekker J. P., Boekema E. J. (2005) Supramolecular organization of thylakoid membrane proteins in green plants. Biochim. Biophys. Acta 1706, 12–39 [DOI] [PubMed] [Google Scholar]
- 17.Chuartzman S. G., Nevo R., Shimoni E., Charuvi D., Kiss V., Ohad I., Brumfeld V., Reich Z. (2008) Thylakoid membrane remodeling during state transitions in Arabidopsis. Plant Cell 20, 1029–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turkina M. V., Kargul J., Blanco-Rivero A., Villarejo A., Barber J., Vener A. V. (2006) Environmentally modulated phosphoproteome of photosynthetic membranes in the green alga Chlamydomonas reinhardtii. Mol. Cell. Proteomics 5, 1412–1425 [DOI] [PubMed] [Google Scholar]
- 19.Fleischmann M. M., Ravanel S., Delosme R., Olive J., Zito F., Wollman F. A., Rochaix J. D. (1999) Isolation and characterization of photoautotrophic mutants of Chlamydomonas reinhardtii deficient in state transition. J. Biol. Chem 274, 30987–30994 [DOI] [PubMed] [Google Scholar]
- 20.Kruse O., Nixon P. J., Schmid G. H., Mullineaux C. W. (1999) Isolation of state transition mutants of Chlamydomonas reinhardtii by fluorescence video imaging. Photosynth. Res 61, 43–51 [Google Scholar]
- 21.Depège N., Bellafiore S., Rochaix J. D. (2003) Role of chloroplast protein kinase Stt7 in LHCII phosphorylation and state transition in Chlamydomonas. Science 299, 1572–1575 [DOI] [PubMed] [Google Scholar]
- 22.Bellafiore S., Barneche F., Peltier G., Rochaix J. D. (2005) State transitions and light adaptation require chloroplast thylakoid protein kinase STN7. Nature 433, 892–895 [DOI] [PubMed] [Google Scholar]
- 23.Lemeille S., Willig A., Depège-Fargeix N., Delessert C., Bassi R., Rochaix J. D. (2009) Analysis of the chloroplast protein kinase Stt7 during state transitions. PLoS Biol 7, e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vener A. V., Harms A., Sussman M. R., Vierstra R. D. (2001) Mass spectrometric resolution of reversible protein phosphorylation in photosynthetic membranes of Arabidopsis thaliana. J. Biol. Chem 276, 6959–6966 [DOI] [PubMed] [Google Scholar]
- 25.Turkina M. V., Blanco-Rivero A., Vainonen J. P., Vener A. V., Villarejo A. (2006) CO(2) limitation induces specific redox-dependent protein phosphorylation in Chlamydomonas reinhardtii. Proteomics 6, 2693–2704 [DOI] [PubMed] [Google Scholar]
- 26.Harris E. H. (1989) The Chlamydomonas Source Book: a Comprehensive Guide to Biology and Laboratory Use, Academic Press, Inc., San Diego, CA: [DOI] [PubMed] [Google Scholar]
- 27.Ficarro S. B., McCleland M. L., Stukenberg P. T., Burke D. J., Ross M. M., Shabanowitz J., Hunt D. F., White F. M. (2002) Phosphoproteome analysis by mass spectrometry and its application to Saccharomyces cerevisiae. Nat. Biotechnol 20, 301–305 [DOI] [PubMed] [Google Scholar]
- 28.Turkina M. V., Vener A. V. (2006) Identification of phosphorylated proteins, in Plant Proteomics: Methods and Protocols Series: Methods in Molecular Biology 355, 305–316 [DOI] [PubMed] [Google Scholar]
- 29.Vainonen J. P., Hansson M., Vener A. V. (2005) STN8 protein kinase in Arabidopsis thaliana is specific in phosphorylation of photosystem II core proteins. J. Biol. Chem 280, 33679–33686 [DOI] [PubMed] [Google Scholar]
- 30.Schneider T. D., Stephens R. M. (1990) Sequence logos: a new way to display consensus sequences. Nucleic Acids Res 18, 6097–6100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swarbreck D., Wilks C., Lamesch P., Berardini T. Z., Garcia-Hernandez M., Foerster H., Li D., Meyer T., Muller R., Ploetz L., Radenbaugh A., Singh S., Swing V., Tissier C., Zhang P., Huala E. (2008) The Arabidopsis Information Resource (TAIR): gene structure and function annotation. Nucleic Acids Res 36, D1009–D1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Emanuelsson O., Brunak S., von Heijne G., Nielsen H. (2007) Locating proteins in the cell using TargetP, SignalP and related tools. Nat. Protoc 2, 953–971 [DOI] [PubMed] [Google Scholar]
- 33.Armbruster U., Hertle A., Makarenko E., Zuhlke J., Pribil M., Dietzmann A., Schliebner I., Aseeva E., Fenino E., Scharfenberg M., Voigt C., Leister D. (2009) Chloroplast proteins without cleavable transit peptides: rare exceptions or a major constituent of the chloroplast proteome? Mol. Plant 2, 1325–1335 [DOI] [PubMed] [Google Scholar]
- 34.Merchant S. S., Prochnik S. E., Vallon O., Harris E. H., Karpowicz S. J., Witman G. B., Terry A., Salamov A., Fritz-Laylin L. K., Maréchal-Drouard L., Marshall W. F., Qu L. H., Nelson D. R., Sanderfoot A. A., Spalding M. H., Kapitonov V. V., Ren Q., Ferris P., Lindquist E., Shapiro H., Lucas S. M., Grimwood J., Schmutz J., Cardol P., Cerutti H., Chanfreau G., Chen C. L., Cognat V., Croft M. T., Dent R., Dutcher S., Fernández E., Fukuzawa H., González-Ballester D., González-Halphen D., Hallmann A., Hanikenne M., Hippler M., Inwood W., Jabbari K., Kalanon M., Kuras R., Lefebvre P. A., Lemaire S. D., Lobanov A. V., Lohr M., Manuell A., Meier I., Mets L., Mittag M., Mittelmeier T., Moroney J. V., Moseley J., Napoli C., Nedelcu A. M., Niyogi K., Novoselov S. V., Paulsen I. T., Pazour G., Purton S., Ral J. P., Riaño-Pachón D. M., Riekhof W., Rymarquis L., Schroda M., Stern D., Umen J., Willows R., Wilson N., Zimmer S. L., Allmer J., Balk J., Bisova K., Chen C. J., Elias M., Gendler K., Hauser C., Lamb M. R., Ledford H., Long J. C., Minagawa J., Page M. D., Pan J., Pootakham W., Roje S., Rose A., Stahlberg E., Terauchi A. M., Yang P., Ball S., Bowler C., Dieckmann C. L., Gladyshev V. N., Green P., Jorgensen R., Mayfield S., Mueller-Roeber B., Rajamani S., Sayre R. T., Brokstein P., Dubchak I., Goodstein D., Hornick L., Huang Y. W., Jhaveri J., Luo Y., Martínez D., Ngau W. C., Otillar B., Poliakov A., Porter A., Szajkowski L., Werner G., Zhou K., Grigoriev I. V., Rokhsar D. S., Grossman A. R. (2007) The Chlamydomonas genome reveals the evolution of key animal and plant functions. Science 318, 245–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Altschul S. F., Madden T. L., Schäffer A. A., Zhang J., Zhang Z., Miller W., Lipman D. J. (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25, 3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.R Development Core Team (2009) R: a Language and Environment for Statistical Computing, R Foundation for Statistical Computing, Vienna, Austria [Google Scholar]
- 37.De Vitry C., Wollman F. A. (1988) Changes in phosphorylation of thylakoid membrane proteins in light-harvesting complex mutants from Chlamydomonas reinhardtii. Biochim. Biophys. Acta 933, 444–449 [Google Scholar]
- 38.Syka J. E., Coon J. J., Schroeder M. J., Shabanowitz J., Hunt D. F. (2004) Peptide and protein sequence analysis by electron transfer dissociation mass spectrometry. Proc. Natl. Acad. Sci. U.S.A 101, 9528–9533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dedner N., Meyer H. E., Ashton C., Wildner G. F. (1988) N-terminal sequence analysis of 8 kDa protein in Chlamydomonas reinhardtii. Localization of the phosphothreonine. FEBS Lett 236, 77–82 [Google Scholar]
- 40.Vainonen J. P., Sakuragi Y., Stael S., Tikkanen M., Allahverdiyeva Y., Paakkarinen V., Aro E., Suorsa M., Scheller H. V., Vener A. V., Aro E. M. (2008) Light regulation of CaS, a novel phosphoprotein in the thylakoid membrane of Arabidopsis thaliana. FEBS J 275, 1767–1777 [DOI] [PubMed] [Google Scholar]
- 41.Jin S. H., Hong J., Li X. Q., Jiang D. A. (2006) Antisense inhibition of Rubisco activase increases Rubisco content and alters the proportion of Rubisco activase in stroma and thylakoids in chloroplasts of rice leaves. Ann. Bot 97, 739–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yakushevska A. E., Keegstra W., Boekema E. J., Dekker J. P., Andersson J., Jansson S., Ruban A. V., Horton P. (2003) The structure of photosystem II in Arabidopsis: localization of the CP26 and CP29 antenna complexes. Biochemistry 42, 608–613 [DOI] [PubMed] [Google Scholar]
- 43.Dauvillée D., Stampacchia O., Girard-Bascou J., Rochaix J. D. (2003) Tab2 is a novel conserved RNA binding protein required for translation of the chloroplast psaB mRNA. EMBO J 22, 6378–6388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vaistij F. E., Boudreau E., Lemaire S. D., Goldschmidt-Clermont M., Rochaix J. D. (2000) Characterization of Mbb1, a nucleus-encoded tetratricopeptide-like repeat protein required for expression of the chloroplast psbB/psbT/psbH gene cluster in Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. U.S.A 97, 14813–14818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vaistij F. E., Goldschmidt-Clermont M., Wostrikoff K., Rochaix J. D. (2000) Stability determinants in the chloroplast psbB/T/H mRNAs of Chlamydomonas reinhardtii. Plant J 21, 469–482 [DOI] [PubMed] [Google Scholar]
- 46.Tiller K., Link G. (1993) Phosphorylation and dephosphorylation affect functional characteristics of chloroplast and etioplast transcription systems from mustard (Sinapis alba L.). EMBO J 12, 1745–1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vener A. V. (2007) Environmentally modulated phosphorylation and dynamics of proteins in photosynthetic membranes. Biochim. Biophys. Acta 1767, 449–457 [DOI] [PubMed] [Google Scholar]
- 48.Turkina M. V., Villarejo A., Vener A. V. (2004) The transit peptide of CP29 thylakoid protein in Chlamydomonas reinhardtii is not removed but undergoes acetylation and phosphorylation. FEBS Lett 564, 104–108 [DOI] [PubMed] [Google Scholar]
- 49.Bonardi V., Pesaresi P., Becker T., Schleiff E., Wagner R., Pfannschmidt T., Jahns P., Leister D. (2005) Photosystem II core phosphorylation and photosynthetic acclimation require two different protein kinases. Nature 437, 1179–1182 [DOI] [PubMed] [Google Scholar]
- 50.Frenkel M., Bellafiore S., Rochaix J. D., Jansson S. (2007) Hierarchy amongst photosynthetic acclimation responses for plant fitness. Physiol. Plant 129, 455–459 [Google Scholar]
- 51.Dietzel L., Bräutigam K., Pfannschmidt T. (2008) Photosynthetic acclimation: state transitions and adjustment of photosystem stoichiometry-functional relationships between short-term and long-term light quality acclimation in plants. FEBS J 275, 1080–1088 [DOI] [PubMed] [Google Scholar]
- 52.Portis J. A. (1995) The regulation of Rubisco by Rubisco activase. J. Exp. Bot 46, 1285–1291 [DOI] [PubMed] [Google Scholar]
- 53.Rokka A., Aro E. M., Herrmann R. G., Andersson B., Vener A. V. (2000) Dephosphorylation of photosystem II reaction center proteins in plant photosynthetic membranes as an immediate response to abrupt elevation of temperature. Plant Physiol 123, 1525–1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang X., Liang Z., Lu C. (2005) Genetic engineering of the biosynthesis of glycinebetaine enhances photosynthesis against high temperature stress in transgenic tobacco plants. Plant Physiol 138, 2299–2309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Crooks G. E., Hon G., Chandonia J. M., Brenner S. E. (2004) WebLogo: a sequence logo generator. Genome Res 14, 1188–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]