Abstract
Progression through mitosis requires the coordinated regulation of Cdk1 kinase activity. Activation of Cdk1 is a multistep process comprising binding of Cdk1 to cyclin B, relocation of cyclin-kinase complexes to the nucleus, activating phosphorylation of Cdk1 on Thr161 by the Cdk-activating kinase (CAK; Cdk7 in metazoans), and removal of inhibitory Thr14 and Tyr15 phosphorylations. This dephosphorylation is catalyzed by the dual specific Cdc25 phosphatases, which occur in three isoforms in mammalian cells, Cdc25A, -B, and -C. We find that expression of Cdc25A leads to an accelerated G2/M phase transition. In Cdc25A-overexpressing cells, Cdk1 exhibits high kinase activity despite being phosphorylated on Tyr15. In addition, Tyr15-phosphorylated Cdk1 binds more cyclin B in Cdc25A-overexpressing cells compared with control cells. Consistent with this observation, we demonstrate that in human transformed cells, Cdc25A and Cdc25B, but not Cdc25C phosphatases have an effect on timing and efficiency of cyclin-kinase complex formation. Overexpression of Cdc25A or Cdc25B promotes earlier assembly and activation of Cdk1-cyclin B complexes, whereas repression of these phosphatases by short hairpin RNA has a reverse effect, leading to a substantial decrease in amounts of cyclin B-bound Cdk1 in G2 and mitosis. Importantly, we find that Cdc25A overexpression leads to an activation of Cdk7 and increase in Thr161 phosphorylation of Cdk1. In conclusion, our data suggest that complex assembly and dephosphorylation of Cdk1 at G2/M is tightly coupled and regulated by Cdc25 phosphatases.
Keywords: CDK (Cyclin-dependent Kinase), Cell Cycle, Cell Division, Mitosis, Phosphatase, Cdc25A, Cdk1-Cyclin B, Cdk7
Introduction
In eukaryotic cells, progression through the cell division cycle is driven by sequential activation of cyclin-dependent kinases. The activity of the Cdk1 kinase is required for mitotic progression in human cells (1, 2). One of the pivotal events, occurring at the time of mitotic onset and dramatically increasing the kinase activity of Cdk1, is its binding to cyclin B (3, 4). Phosphorylation of the Thr161 residue within the T-loop of Cdk1 stabilizes its interaction with cyclins and leads to further activation of the kinase (5). In mammalian cells, this phosphorylation is carried out by a Cdk-activating kinase (CAK)2 (6, 7). Selective inhibition of the CAK catalytic subunit Cdk7 in human cells blocks mitotic entry due to impaired assembly of Cdk1-cyclin B complexes, indicating that the activating phosphorylation is a prerequisite for formation of stable complexes in vivo (8). Cyclin-kinase complexes can be inactivated via inhibitory phosphorylation of conserved Thr14 and Tyr15 residues within the ATP-binding pocket of the Cdk1 kinase (9). A cytoplasmic kinase, Myt1, predominantly phosphorylates Thr14 (10), whereas nuclear Wee1 is mainly active toward Tyr15 (11). Upon entry into mitosis, these inhibitory phosphate groups must be removed by Cdc25 dual specific phosphatases to achieve full activation of Cdk1 (12). Inhibition of Cdc25 phosphatases, which takes place in response to DNA damage or other stress conditions, decreases Cdk activity and leads to a cell cycle block (13–15).
In mammalian cells, the Cdc25 phosphatase family occurs in three isoforms, namely Cdc25A, -B, and -C (for a review, see Ref. 16). The Cdc25A and Cdc25B phosphatases act as oncogenes; they can cause oncogenic transformation of rodent cells lacking Rb1 or expressing activated Ras (17). Overexpression of Cdc25A or Cdc25B was detected in a variety of human cancers, including breast, lung, prostate cancer (18). Transient repression of Cdc25A or Cdc25B in cancer cells by small interfering RNA delays the G2/M transition (19), whereas overexpression of wild type phosphatases induces premature Cdk1 activation and entry into mitosis (20–22). In contrast, overexpression of Cdc25C does not lead to oncogenic transformation (17), and small interfering RNA-mediated repression of this phosphatase has no effect on G2/M progression in human cells (19). Furthermore, Cdc25A activates both Cdk1- and Cdk2-cyclin A and Cdk1-cyclin B complexes, whereas Cdc25B seems to be involved only in activation of Cdk1-cyclin B (23). These differences between closely related enzymes suggest a diversity in the regulation and, perhaps, mode of action of Cdc25 phosphatases. Therefore, a better insight into the functions of Cdc25A and Cdc25B phosphatases during cell cycle regulation is crucial for understanding how the onset of mitosis is regulated and might serve for development of new approaches for cancer therapy.
In this study, we further investigated the role of Cdc25A and B phosphatases at the G2/M transition. We found that Cdc25A and -B but not Cdc25C expression affects Cdk1-cyclin B complex formation. Furthermore, we provide evidence that both Cdc25A and Cdc25B phosphatases are required for timely assembly of Cdk1-cyclin B complexes. Our results suggest that the processes of complex formation and activating dephosphorylation are tightly coupled.
EXPERIMENTAL PROCEDURES
Cell Culture
U2OS Tet-Off cells, a human osteosarcoma cell line expressing a chimeric tetracycline activator, was obtained from Clontech and used for generation of stable cell lines with inducible expression of Cdc25A1 and Cdc25C1. The U2OS Tet-Off cell line expressing HA-Cdc25B3 was a kind gift from Dr. B. Ducommun (University of Toulouse, France). Cells were grown in Dulbecco's modified Eagle's medium (Sigma) supplemented with 10% (v/v) fetal bovine serum (PAA Laboratories GmbH), 2 mm l-glutamine (Sigma), and antibiotic-antimycotic (Invitrogen) in a humidified incubator at 37 °C in 5% CO2. For transfection of U2OS cells, the Lipofectamine 2000 reagent (Invitrogen) was used according to the manufacturer's protocols; HeLa cells were transfected using the calcium phosphate method as described previously (24). To generate stable cell lines expressing Cdc25 phosphatases, the corresponding plasmids were cotransfected with pPuro vector (Clontech) containing a marker of puromycin resistance. Selection of stable clones was performed in the presence of 1 mg/ml puromycin. The Dulbecco's modified Eagle's medium for U2OS Tet-off cells were supplemented with 2 μg/ml tetracycline to suppress induction, and cells were washed three times with phosphate-buffered saline before the addition of tetracycline-free medium to induce Cdc25 expression. To synchronize cells at the G1/S boundary, a double thymidine block was performed as follows. Thymidine was added to the medium at a final concentration of 2 mm for 17 h, and then cells on plates were washed two times with phosphate-buffered saline and released into fresh medium for 10–12 h; afterward, thymidine was added again for 17 h until final wash/release. Expression of Cdc25 was induced at the first release (27–29 h before the final release).
Construction of Plasmids and Site-directed Mutagenesis
Short hairpin RNAs (shRNA) were annealed and cloned into the pSuperGFPneo vector (Oligoengine Inc.) according to the manufacturer's protocols. The following pairs of oligonucleotides were used: 5′-GATCCCGGCGCTATTTGGCGCTTCATTCAAGAGATGAAGCGCCAAATAGCGCCTTTTTA and 5′-AGCTTAAAAAGGCGCTATTTGGCGCTTCATCTCTTGAATGAAGCGCCAAATAGCGCCGGG (underlined sequences correspond to positions 84–102 of the human Cdc25A open reading frame); 5′-GATCCCGGAAAATGAAGCCTTTGAGTTCAAGAGACTCAAAGGCTTCATTTTCCTTTTTA and 5′-AGCTTAAAAAGGAAAATGAAGCCTTTGAGTCTCTTGAACTCAAAGGCTTCATTTTCCGGG (underlined sequences correspond to positions 423–441 of the human Cdc25A open reading frame); 5′-GATCCCAATCCTCCCTGTCGTCTGATTCAAGAGATCAGACGACAGGGAGGATTTTTTTA and 5′-AGCTTAAAAAAATCCTCCCTGTCGTCTGATCTCTTGAATCAGACGACAGGGAGGATTGGG (underlined sequences correspond to positions 287–305 of the human Cdc25B open reading frame); 5′-GATCCCTCCTAGAGAGTGACTTAAATTCAAGAGATTTAAGTCACTCTCTAGGATTTTTA and 5′-AGCTTAAAAATCCTAGAGAGTGACTTAAATCTCTTGAATTTAAGTCACTCTCTAGGAGGG (underlined sequences correspond to positions 698–716 of the human Cdc25B open reading frame). The Cdk1 mutants (T14A/Y15F, T14A/Y15E, and T14E/Y15E) were generated using the GeneTailorTM site-directed mutagenesis system (Invitrogen) following the protocols of the manufacturer. The pCDNA3.1(+)-HA-cyclin B1 construct was generated by subcloning of the full-length human cyclin B1 from pGEX-2TKP-cyclin B1 (kind gift from J. Pines (Cambridge, UK)). GST-tagged catalytically inactive Cdk2 was obtained by subcloning from the pSM-HA-Cdk2 K33T/K34S plasmid (D. Morgan (University of California, San Francisco).
Expression and Purification of Recombinant Proteins
Wild-type and mutated GST-Cdk1 (T14A/Y15F, T14A/Y15E, T14E/Y15E), GST-cyclin B1, and catalytically inactive GST-Cdk2 K33T/K34S were expressed in the E. coli strain BL21 and purified as described previously (25).
Immunoblotting and Immunoprecipitations
Preparation of cell extracts, immunoprecipitations, and immunoblotting were performed as described previously (26). Nuclear, cytosolic, and detergent-resistant fractions were prepared as described (27, 28). For Western blotting and immunoprecipitations, the following antibodies were used: rabbit polyclonal antibodies against Cdc25A (M-191), Cdc25B (C-20), Cdc25C (C-20), and cyclin A (H-432) and mouse monoclonal anti-Cdc25A (F6), anti-Cdc2 (p34), anti-APC3 (AF3.1), and anti-cyclin A (BF683) from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA); monoclonal anti-cyclin B1 antibody from Upstate Biotechnology; polyclonal anti-Thr(P)14 Cdc2, anti-Tyr(P)15 cdc2, anti-Thr(P)161 Cdc2, anti-Wee1, and anti-MEK1/2 antibodies from Cell Signaling; anti-Thr(P)244 APC3 from Rockland Inc.; mouse monoclonal antibodies against α-tubulin, anti-β-actin, anti-FLAG (M2), and anti-Cdk7 (MO1.1) from Sigma; and mouse monoclonal anti-HA antibody (Babco) and anti-poly(ADP-ribose) polymerase (BD Biosciences). The rabbit polyclonal anti-Cdc25A and anti-cyclin B antibodies were described previously (26) as well as the anti-Cdc25B (29). Densitometric analyses of immunoblots were performed using ImageJ software.
Gel Electrophoresis
SDS-PAGE was performed as described previously (26). For two-dimensional gel electrophoresis separations, immunoprecipitated proteins were uncoupled from beads in rehydration buffer, containing 8 m urea, 0.4% dithiothreitol, 1% CHAPS, and 2.5% Pharmalyte (pH 3–10). Samples were then applied to immobilized linear pH gradient (pH 3–10) strips (Amersham Biosciences) for in-gel rehydration for 10 h at room temperature. Proteins were resolved by isoelectric focusing on the IPGphor apparatus (Amersham Biosciences), following the protocols of the manufacturer. Afterward, the immobilized linear pH gradient strips were equilibrated as described elsewhere (30) and loaded onto 12% SDS-polyacrylamide gel slabs for separation in the second dimension.
Kinase Assays
The activity of Cdk1 kinase (immunoprecipitated directly using anti-Cdk1 or anti-Tyr(P)15 antibodies or indirectly using anti-cyclin B antibodies) was measured in histone H1 kinase assays as described previously (25). To determine Cdk7 and Wee1 kinase activities, the proteins were immunoprecipitated using the respective antibodies, and beads were incubated with 2 μg of purified recombinant catalytically inactive GST-Cdk2 K33T/K34S (31) in 30 μl of kinase buffer (50 mm Tris-HCl, pH 7.5, 10 mm MgCl2, 100 μm ATP, 1 mm dithiothreitol), supplemented with 2.5 μCi of [γ-32P]ATP. Reactions were stopped by the addition of 4× Laemmli buffer, and samples were denaturated for 5 min at 95 °C before loading to a 12% SDS-polyacrylamide gel. Phosphorylation was detected and quantified with a PhosphorImager (Amersham Biosciences) and ImageJ software and normalized according to the amount of the immunoprecipitated protein.
Immunofluorescence and Time Lapse Microscopy
For immunofluorescent stainings, cells were seeded on acid-washed coverslips and fixed with 3% paraformaldehyde (Sigma) in phosphate-buffered saline for 10 min at room temperature and permeabilized with 100% MeOH at −20 °C for 3 min. Anti-cyclin B1 (Upstate Biotechnology), anti-Thr(P)244 APC3 (Rockland Inc.), anti-Thr(P)161 Cdc2 (Cell Signaling), and anti-mouse Alexa 488 and anti-rabbit Alexa 594 (Invitrogen) antibodies were used. DNA was stained with Hoechst 33342 (Molecular Probes). Pixel intensity quantifications were performed using Adobe Photoshop and ImageJ software. For live cell imaging, cells were seeded onto thin glass bottom dishes (Ibidi) and placed into a Tokai Hit environmental chamber (Tokai Hit Co.). Video recording was performed using a Nikon TIRF TE2000-U inverted microscope equipped with the Nikon Perfect Focus System, S Fluor ×20 and Plan Apo TIRF ×40 objectives, and a Hamamatsu ORCA-AG camera. Images of differential interference contrast microscopy were collected every 2 min and processed using the NIC Elements (Nikon) software.
Flow Cytometry
Pulse-labeling of synchronized cells with BrdUrd was performed by the addition of BrdUrd to the medium to a final concentration of 10 mm for 1 h. Cells were then harvested and fixed in 70% ice-cold ethanol and stored at −20 °C. After washing cell pellets in phosphate-buffered saline, DNA was stained using propidium iodide at a final concentration of 50 mg/ml in 38 mm sodium citrate buffer, pH 7.5, containing RNase A at 2 mg/ml. Incorporated BrdUrd or phosphorylated Ser10 histone H3 was labeled first by anti-BrdUrd (BD Biosciences) or anti-Ser(P)10 H3 antibodies (Cell Signaling) in accordance to the manufacturer's guidelines and then with fluorescent secondary antibody (Alexa 488 from Invitrogen). Bivariate FACS analyses were performed on a FACSCalibur flow cytometer (BD Biosciences). Data were analyzed using the Cell Quest (BD Biosciences) software; statistical analyses (when done) were performed using the GraphPad Prism software.
RESULTS
Overexpression of Cdc25A Leads to an Accelerated G2/M Transition
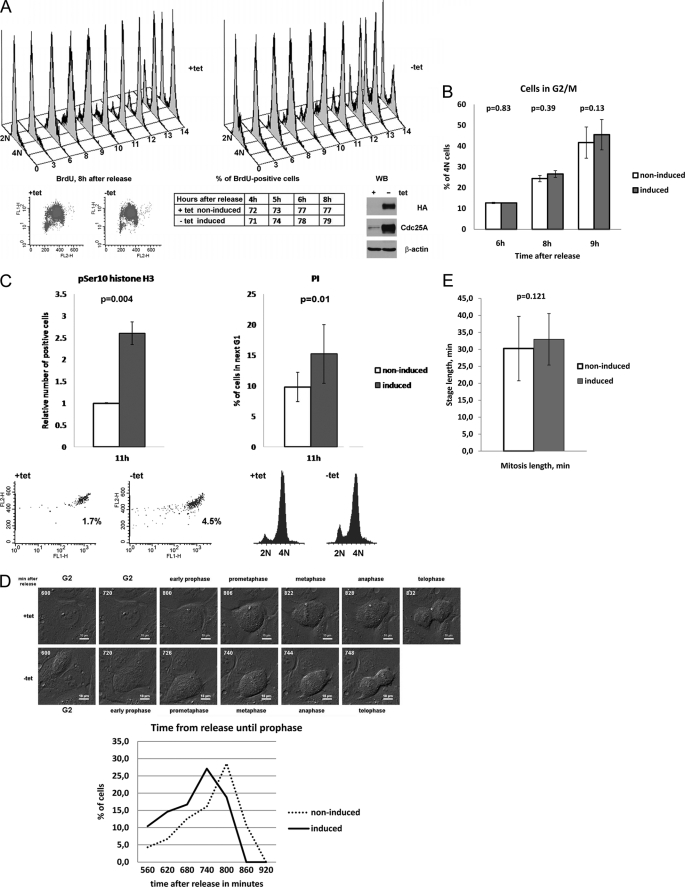
Recent publications point to a potential importance of Cdc25A in mitosis (20–22). In order to further investigate the role of Cdc25A phosphatase in an unperturbed cell cycle, we made use of a stable inducible U2OS cell line, expressing Cdc25A in a tetracycline-dependent manner. To assess the dynamics of S phase progression in non-induced and induced cells, they were synchronized at the G1/S boundary using a double thymidine block and pulse-labeling with BrdUrd at different time intervals after release. Incorporated BrdUrd was detected by anti-BrdUrd antibodies, whereas the DNA was stained with propidium iodide, and cells were analyzed by FACS. The results show a similar rate of BrdUrd incorporation in induced and non-induced cells in S phase (Fig. 1A), suggesting that overexpression of Cdc25A did not accelerate S phase progression. Concomitantly, analysis of the DNA content until 9 h after release (beginning of G2 phase in U2OS, as can be estimated by FACS) revealed no significant difference in growth of the 4 n cell population between induced and non-induced cells (Fig. 1B). However, in Cdc25A-overexpressing cells, collected 11 h after release, the mitotic index was significantly higher in comparison with control cells, as detected by FACS analysis of cells labeled with anti-Ser(P)10 histone H3 antibodies (Fig. 1C, left). Correspondingly, induced cells seemed to pass through G2 or mitosis faster because they appeared earlier in the next G1 phase (Fig. 1C, right). To investigate whether G2 phase or mitosis was shortened upon Cdc25A overexpression, we used time lapse microscopy. Synchronized non-induced and induced U2OS cells were monitored for 16 h after release from the double thymidine block. We found that in induced cells, the mitotic onset, detected as a characteristic rounding up of cells (32), started ∼1 h earlier than in non-induced cells (Fig. 1D). However, the total length of mitosis, estimated as the time from the beginning of prometaphase (rounding of cells, preceding DNA condensation) until cytokinesis, was not changed upon Cdc25A overexpression (not shown). Thus, the FACS analysis and time lapse data show that overexpression of Cdc25A did not affect substantially S phase progression or the length of mitosis but markedly shortened the duration of G2 phase.
FIGURE 1.
Overexpression of Cdc25A leads to shortened G2 phase and early mitotic onset. A, U2OS cells with stable inducible expression of Cdc25A were synchronized at G1/S using a double thymidine block and then released as described under “Experimental Procedures.” After pulse-labeling with BrdUrd, cells were collected, fixed, and subjected to staining with anti-BrdUrd antibodies and PI. The top panels show DNA content-based cell cycle profiles in non-induced (+tet; left) and induced (−tet; right) cells. The lower left panels demonstrate the absence of a significant difference between BrdUrd-positive populations in non-induced and induced cells at 8 h after release; the middle lower table shows quantified data for sequential time points from 4 to 8 h after release; a WB in the lower right panel illustrates levels of Cdc25A protein in non-induced and induced states. Representative results of one of three experiments are shown. B, quantifications of four independent FACS-assisted measurements of DNA content during cell cycle progression in synchronized U2OS cells. Results were analyzed using a paired t test, p for 95% confidence interval indicated above the graphs. Note the absence of a significant difference in rate of accumulation of cells with completed DNA replication (4N). C, U2OS Cdc25A cells were collected at different time intervals after release, fixed, and stained with the anti-phospho-Ser10 histone H3 antibody and propidium iodide. The quantification of three independent series of FACS-assisted measurements is shown (a representative time point, 11 h, was selected) for phospho-H3 (pH3)-positive cells (upper left panel) and 2 n cells in the next G1 phase (upper right panel). The lower panels represent the volume of pH3-positive population (left) and DNA profiles (right) in non-induced (+tet) and induced (−tet) cells at 11 h after release. D, U2OS Cdc25A cells were released from a double-thymidine block and monitored using differential interference contrast microscopy. The beginning of characteristic rounding of cells was considered as the onset of mitosis because soon after that, condensation of DNA was observed. Top, still photographs of one representative non-induced and induced cell are shown. The numbers indicate the time from release in minutes (scale bars, 10 μm). Bottom, durations of time from release to prophase for each individual cell were grouped into six intervals. The percentage of prophase cells at each interval is shown (non-induced, n = 51; induced, n = 46). pSer10, Ser(P)10. E, U2OS Cdc25A cells were released from a double-thymidine block and monitored using differential interference contrast microscopy. Duration of mitosis was measured for 51 induced and 46 non-induced cells as described in the text. Results were analyzed using paired t-test; p for 95% confidence interval is indicated above the graphs.
Cdc25A Overexpression Causes Accumulation of Tyr15-phosphorylated Cdk1 at G2/M
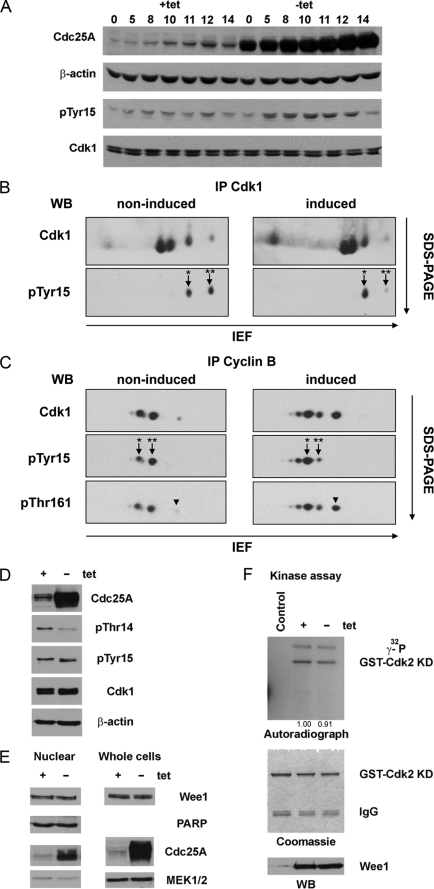
When samples collected at sequential time points after release from a double thymidine block were analyzed by WB, we observed a pronounced accumulation of phospho-Tyr15 Cdk1 in Cdc25A-overexpressing cells, particularly at the time of late G2 and early mitosis. This phenomenon was repeatedly observed in Cdc25A-overexpressing U2OS and HeLa cells (Fig. 2A) (data not shown). To investigate this in more detail, we performed immunoprecipitations of Cdk1 from lysates of synchronized cells collected at 10 h after release and then separated proteins by two-dimensional electrophoresis. Using anti-Tyr(P)15-specific and anti-Cdk1 antibodies for immunoblotting, we noticed that when Cdc25A was overexpressed, the pool of phospho-Cdk1 was represented almost exclusively by Tyr(P)15 Cdk1, whereas in non-induced cells, the two spots representing Tyr(P)15 Cdk1 and Thr(P)14/Tyr(P)15 Cdk1 were equally abundant (Fig. 2B). Similar results were obtained when we analyzed the cyclin B-bound fractions of Cdk1. Immunoprecipitations were performed using anti-cyclin B antibodies, and immunocomplexes were resolved on two-dimensional electrophoresis. At 10 h after release, in cells with normal protein levels of Cdc25A, this pool consisted of double-phosphorylated Thr(P)14/Tyr(P)15 and monophosphorylated Tyr(P)15 Cdk1, whereas in induced cells, the majority of Cdk1 bound to cyclin B was phosphorylated on Tyr15 (Fig. 2C, middle). This accumulation of Tyr15-phosphorylated Cdk1 in Cdc25A-overexpressing cells was surprising because it is anticipated that Cdc25 phosphatases activate Cdk1 via removal of inhibitory phosphates from both Thr14 and Tyr15 residues. However, it has been shown that in vitro Cdc25A catalyzes dephosphorylation of Cdk2-cyclin A in two steps and has more than 10-fold higher preference toward Thr(P)14 over Tyr(P)15 (33). Moreover, in starfish oocytes at the prophase-to-metaphase transition, Cdk1 was shown to be dephosphorylated sequentially (first Thr(P)14 and then Tyr(P)15), and the Tyr15-phosphorylated Cdk1 was described as a prophase-specific form (34). Therefore, the observed accumulation of Tyr(P)15 Cdk1 in human cells upon overexpression of Cdc25A might be due to increased removal of phosphate from Thr(P)14, whereas Tyr(P)15 remains intact. Indeed, using the anti-Thr(P)14-specific antibody, we detected a substantial reduction in Thr(P)14 level in induced cells (Fig. 2D). On the other hand, it is known that Wee1 kinase counteracts Cdc25 phosphatases and phosphorylates Cdk1 on the Tyr15 residue (11, 35). We could not rule out the possibility that hyperactive Cdc25A induces a negative feedback loop that leads to activation of Wee1. To verify this, we analyzed Wee1 protein levels and kinase activity. Neither in lysates prepared from whole cells nor in nuclear fractions was any change in protein levels of Wee1 observed (Fig. 2E). We also found no significant difference in kinase activities of Wee1 immunoprecipitated from lysates of non-induced and Cdc25A-overexpressing cells (Fig. 2F). Thus, we concluded that accumulation of Tyr(P)15 at G2/M and in early mitosis upon overexpression of Cdc25A occurs because Cdc25A preferentially dephosphorylates Thr14 of Cdk1.
FIGURE 2.
In Cdc25A-overexpressing cells, the major pool of cyclin B-bound Cdk1 kinase is phosphorylated on Tyr15 at G2/M. A, U2OS Cdc25A cells were synchronized as in Fig. 2, and samples for WB were collected at the indicated time points and probed with respective antibodies. Note the increase in the Tyr(P)15 (pTyr15) band intensity in induced cells. B, Cdk1 was immunoprecipitated from lysates of U2OS Cdc25A cells collected at 10 h after release. Proteins were resolved on two-dimensional PAGE and analyzed using anti-Cdk1 and anti-Tyr(P)15 antibodies. Asterisks correspond to double-phosphorylated Thr(P)14/Tyr(P)15 (**) and monophosphorylated Tyr(P)15 Cdk1 (*). The arrowheads mark phosphorylation of Cdk1 on Thr161 (pThr161). C, cyclin B-bound Cdk1 was co-immunoprecipitated using the anti-cyclin B antibody, and protein complexes were resolved and analyzed as in A using the indicated antibodies. D, the same lysates as in B and C were resolved using SDS-PAGE and analyzed by WB with the respective antibodies. E, nuclear extracts and lysates of detergent-resistant fractions of non-induced and induced U2OS Cdc25A cells were analyzed by immunoblotting. The figure represents WB of lysates prepared from cells collected at 10 h after release. F, the same lysates as in E were used for IP with the anti-Wee1 antibody or rabbit IgG as a control. After binding to Protein A-Sepharose beads (Amersham Biosciences), immunoprecipitates were split for kinase assay (top) and WB (bottom). The middle panel shows equal loading of the substrate (catalytically inactive purified GST-Cdk2 K33T/K34S). IEF, isoelectric focusing. PARP, poly(ADP-ribose) polymerase.
Cdk1 Phosphorylated on Tyr15 Has High Kinase Activity in Cdc25A-overexpressing Cells
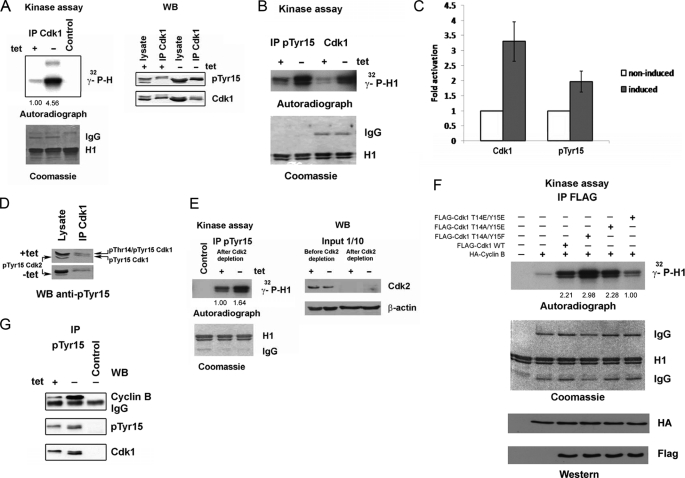
In our FACS and time lapse experiments presented above, we observed an earlier mitotic onset in Cdc25A-overexpressing cells. Therefore, we expected to find an elevated Cdk1 kinase activity in those cells in comparison with non-induced ones. Indeed, when we measured the kinase activity of Cdk1 immunoprecipitated from lysates collected at 10 h after release, it proved to be significantly elevated in induced, Cdc25A-overexpressing cells (Fig. 3A, left). Interestingly, activation of Cdk1 occurred despite high levels of Tyr15 phosphorylation (Fig. 3A, right; see also Fig. 2, A and D). Because in vitro experiments published previously demonstrated that this phosphorylation strongly inhibited the activity of recombinant Cdk1-cyclin B complexes (11, 35), we suggest that enhanced kinase activity of immunoprecipitates, obtained from induced U2OS Cdc25A cells using the anti-Cdk1 antibody, might be due to an increased content of dephosphorylated Cdk1. If this hypothesis is true, immunoprecipitations using phospho-specific antibodies against Tyr(P)15 should not detect any difference between induced and non-induced cells. To our surprise, we repeatedly observed that the kinase activity of immunoprecipitated Tyr(P)15 Cdk1 was substantially elevated in Cdc25A-overexpressing cells (Fig. 3, B and C). The phospho-specific antibody used recognizes both Cdk1 and Cdk2 phosphorylated on Tyr15. Although overexpression of Cdc25A did not cause an accumulation of phospho-Tyr15 Cdk2 but rather its reduction (Fig. 3D), we decided to use experimental conditions where Cdk2 kinase activity was absent. Therefore, we depleted endogenous Cdk2 using double immunoprecipitations with the respective antibody followed by immunoprecipitations with anti-Tyr(P)15 antibodies. Reduction in Cdk2 level did not abrogate the difference in kinase activity of Tyr(P)15 immunoprecipitates between non-induced and induced cells (Fig. 3E), confirming that monophosphorylated Tyr(P)15 Cdk1 from Cdc25A-overexpressing cells is more active than the Thr(P)14/Tyr(P)15 Cdk1 from non-induced ones. We also analyzed kinase activities of FLAG-tagged wild-type Cdk1 or the phospho-mimicking mutants T14A/Y15F, T14A/Y15E, and T14E/Y15E, co-expressed with HA-cyclin B in 293T cells. Exogenous FLAG-Cdk1 and mutant Cdks in complex with cyclin B were immunoprecipitated using anti-FLAG antibodies, and the kinase activities toward histone H1 were measured in vitro. As expected, the Cdk1 T14A/Y15F mutant exhibited the highest kinase activity, whereas the activity of Cdk1 T14E/Y15E was just slightly above the background (Fig. 3F). More interestingly, although the kinase activity of the T14A/Y15E mutant (mimicking singly phosphorylated Tyr(P)15 Cdk1) was lower in comparison with Cdk1 T14A/Y15F, it was at least as active as the wild-type FLAG-Cdk1. Thus, our results show that phosphorylation on Tyr15 did not lead to inactivation of Cdk1. In order to uncover the cause of elevated activity of Tyr15-phosphorylated Cdk1, we analyzed by WB a part of the same Tyr(P)15 immunoprecipitates from non-induced and induced U2OS Cdc25A cells that were used for kinase assays. Notably, in immunocomplexes obtained from induced cells, the level of co-immunoprecipitated cyclin B was substantially higher than in those from non-induced cells, although the amount of Cdk1 was only slightly increased (Fig. 3G).
FIGURE 3.
Partially dephosphorylated Tyr(P)15 Cdk1 is catalytically active. A, Cdk1 was immunoprecipitated from 500 μg of lysates of U2OS Cdc25A cells, collected at 10 h after release. Samples were split and used for immunoblotting (right) or kinase assays (left). Note the high levels of Tyr15 (pTyr15) phosphorylation and Cdk1 kinase activity in induced cells. B, same lysates as in A were used for IP with the anti-Tyr(P)15 or anti-Cdk1 antibody and split for subsequent H1 kinase assays and WB (G). C, quantifications of relative kinase activities of Cdk1 in non-induced and induced U2OS cells. The kinase was immunoprecipitated using anti-Cdk1 or anti-Tyr(P)15 antibodies. Activity in non-induced cells was considered as 1; n = 3. Error bars, S.D. D, lysates and Cdk1 immunoprecipitates prepared as in A were immunoblotted with the anti-Tyr(P)15 antibody. E, the same lysates as above were depleted of Cdk2 kinase by two rounds of immunoprecipitations, and efficiency of depletion was controlled by immunoblotting (right). After depletion, IP with the anti-Tyr(P)15 antibody was performed, and the kinase activity of immunoprecipitates was assessed in an H1 kinase assay (left). F, 293T cells were co-transfected with pCDNA3.1(+)-FLAG-Cdk1 WT or the indicated mutants and pCDNA3.1(+)-HA-cyclin B1; amounts of DNA were equilibrated using the empty vector pCDNA3.1(+). After 24 h post-transfection, cells were lysed, and immunoprecipitations using the anti-FLAG M2 antibody were performed and used for H1 kinase assays. G, immunoprecipitates obtained using the anti-Tyr(P)15 (B) were probed in WB with the indicated antibodies. Note the substantial increase in amounts of cyclin B co-immunoprecipitated with Tyr(P)15 Cdk1 from induced cells.
Overexpression of Cdc25A and Cdc25B Causes a Shift in the Timing of Cyclin B-Cdk1 Complex Assembly
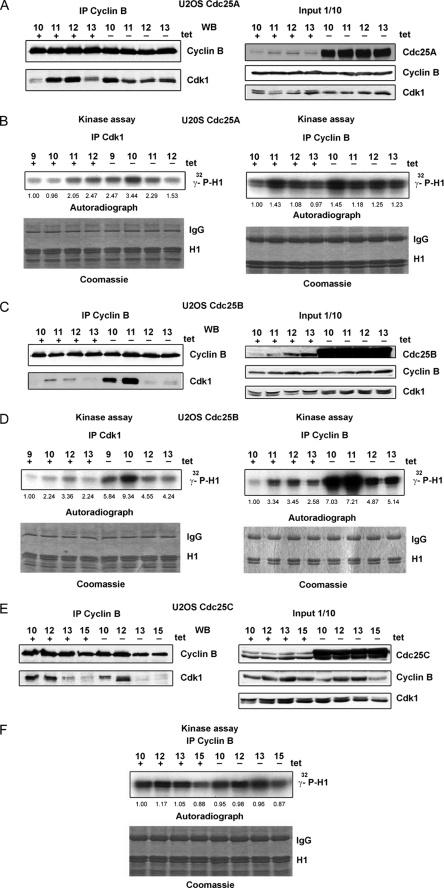
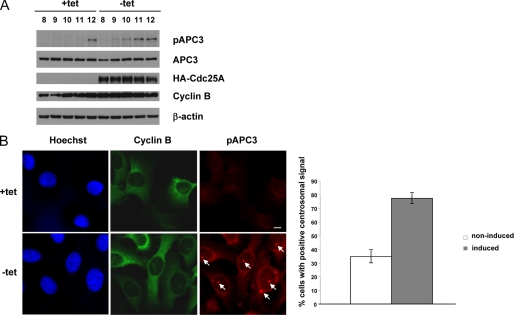
The fact that upon overexpression of Cdc25A, more cyclin B was detected in complex with Cdk1 at 10 h after release from a double thymidine block prompted us to examine the levels of Cdk1-cyclin B complexes at different time points from G2 to M phase. We observed that the amount of Cdk co-immunoprecipitated using the anti-cyclin B antibodies markedly varied in non-induced cells (gradually increased, reached the maximum, and then decreased again). More notably, the highest amounts of cyclin B bound to Cdk1 were detected in Cdc25A-overexpressing cells approximately 1 h earlier than in non-induced cells, despite similar levels of cyclin B in immunoprecipitates and lysates in non-induced and induced cells (Fig. 4A). In accordance with the WB results, the peak of the cyclin B-associated kinase activity was shifted in induced cells toward earlier time points (Fig. 4B). Reciprocal immunoprecipitations with anti-Cdk1 antibodies exhibited analogous results; the amount of co-immunoprecipitated cyclin B1 varied similarly, despite a constant level of Cdk1 (Fig. 4B) (data not shown). Thus, an excess of Cdc25A protein caused changes in the timing of Cdk1-cyclin B complex assembly and activation. To test whether overexpression of Cdc25B or Cdc25C might have analogous effects, we performed immunoprecipitations of cyclin B from lysates of synchronized U2OS Cdc25B and Cdc25C cells in induced and non-induced states. As shown in Fig. 4, C and D, overexpression of Cdc25B caused similar changes in timing of Cdk1-cyclin B complex formation, whereas high levels of the Cdc25C protein affected neither abundance nor activity of cyclin B-bound Cdk1 in G2 and mitosis (Fig. 4, E and F). To further confirm an earlier activation of Cdk1-cyclin B in response to Cdc25A induction, we made use of an antibody that recognizes APC3 phosphorylated on Thr244, a well established Cdk1 target in vivo at the centrosome (36). We find that overexpression of Cdc25A leads to an earlier phosphorylation on APC3-Thr244 compared with control cells, as shown in Western blots (Fig. 5A) and by monitoring the accumulation of Tyr(P)244-APC3 at the centrosome (Fig. 5B).
FIGURE 4.
Overexpression of Cdc25A or Cdc25B but not Cdc25C induces an earlier Cdk1-cyclin B complex assembly and activation. A, immunoprecipitations obtained with anti-cyclin B antibodies from 500 μg of lysates of synchronized U2OS Cdc25A cells were used for WB. The right panel shows protein levels in 50 μg of lysates used for IP, detected by WB with the indicated antibodies. B, kinase assays were performed with immunoprecipitations obtained using anti-cyclin B or anti-Cdk1 antibodies from the same lysates as in A. C and D, similar to A and B, but lysates from synchronized inducible U2OS cells expressing Cdc25B were used. E and F, similar to A and B, but lysates from synchronized inducible U2OS cells expressing Cdc25C were used.
FIGURE 5.
Overexpression of Cdc25A correlates with earlier phosphorylation of APC3 on Thr244. A, U2OS Cdc25A cells were released from a double thymidine block (left), and samples for WB were collected at the indicated time points (hours) and probed with the respective antibodies. Note the earlier appearance of the APC3-Thr(P)244 signal in induced cells. B, induced and non-induced U2OS Cdc25A cells were fixed on coverslips 9 h after release from a double thymidine block (left). Cells were stained with anti-cyclin B (green) and anti-APC3-Thr(P)244 (red) antibodies. DNA was stained with Hoechst (blue). Scale bar, 10 μm. The arrows indicate the Thr(P)244 signal decorating the centrosomes. Right, the percentage of cells with a positive centrosomal Thr(P)244 signal for Cdc25A induced and non-induced cells. Error bars, S.D. in three independent experiments (n = 200).
Simultaneous Inhibition of Both Cdc25A and -B Phosphatases Markedly Affects Cdk1-Cyclin B Complex Assembly
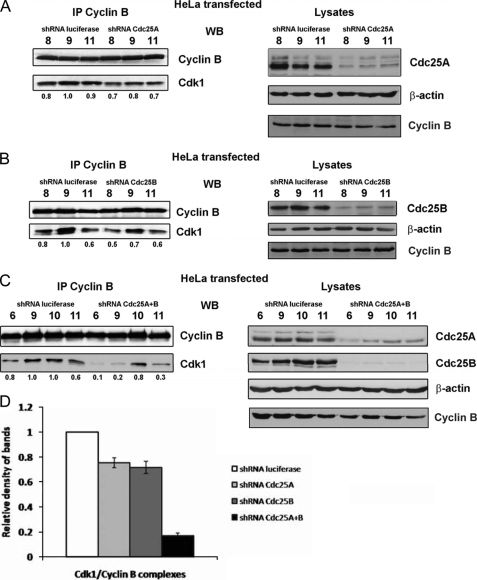
To test whether repression of Cdc25A might have a reverse effect on the abundance of Cdk1-cyclin B complexes or the time of complex assembly, we transfected HeLa cells with plasmids expressing shRNA against Cdc25A or firefly luciferase as a control. After synchronization by a double thymidine block, cells were collected at different intervals after release, and cyclin B was immunoprecipitated. Although the Cdc25A protein was not completely abolished and protein levels of cyclin B in repressed and control cells were similar, we could observe in Cdc25A-depleted cells a slight reduction in the amounts of Cdk1, co-immunoprecipitated with cyclin B (Fig. 6A). We performed the same experiments but this time using shRNA-mediated repression of Cdc25B and detected similar effects (Fig. 6B). Thus, when Cdc25A or Cdc25B were repressed separately, the decrease in Cdk1-cyclin B complexes levels was modest although reproducible. However, when HeLa cells were co-transfected with shRNA targeting both Cdc25A and Cdc25B, we observed a dramatic drop in amounts of cyclin B-bound Cdk1 at time points corresponding to G2/M transition and mitosis (i.e. time points 6 and 9) (Fig. 6, C and D). Taken together, our results demonstrate that normal levels of Cdc25A and Cdc25B proteins are important for the timely assembly of complexes between Cdk1 and cyclin B.
FIGURE 6.
Repression of Cdc25A or Cdc25B by shRNA impairs Cdk1-cyclin B complex assembly. A, HeLa cells were transfected with plasmids, encoding shRNA targeting Cdc25A or firefly luciferase as a control and, 24 h after transfection, were synchronized by a double thymidine block. After release, cells were collected at different time intervals and used for FACS to confirm synchronization and trace stages of the cell cycle (not shown) and for preparation of lysates. Left, cyclin B was immunoprecipitated from 500 μg of lysates using the antibodies indicated, and proteins were resolved on SDS-PAGE and analyzed by WB. Density of Cdk1 bands was measured using ImageJ software, and their relative intensities were calculated as the ratio to the density of the Cdk1 band at 9 h after release (mitosis), considered as 1. Results are indicated below the Cdk1 blots. Right, 50 μg of lysates used for IP analyzed by WB to monitor repression of Cdc25A and evenness of loading. B, similar to A, but plasmids expressing shRNA against Cdc25B phosphatase were used. C, similar to A and B, but both Cdc25A and Cdc25B were repressed using corresponding shRNA-expressing constructs. D, data of relative density measurements at time point 9 h were used for statistical analysis in three independent experiments. Error bars, S.D.
Cdc25A Overexpression Leads to Increased Thr161 Phosphorylation on Cdk1 and Activation of Cdk7
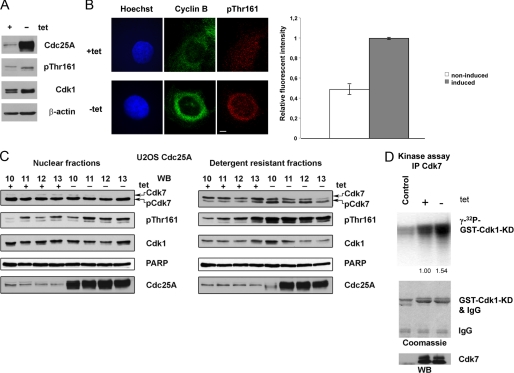
CAK phosphorylates Cdk1 and Cdk2 on a conserved threonine residue within the T-loop of the catalytic domain (6). This phosphorylation (in mammalian Cdk1 the Thr161 residue) leads to activation of Cdk and can improve its binding to cyclins (5, 37). It has been shown recently that Cdk7 (the catalytic subunit of CAK) plays an important role in complex assembly between Cdk1 and cyclin B at the G2/M transition (8). We assumed that Cdk7 may be more active in Cdc25A-overexpressing cells because we noticed that Cdk1 co-immunoprecipitated with cyclin B from lysates of induced cells was more extensively phosphorylated on Thr161, as was shown by two-dimensional electrophoresis and subsequent immunoblotting (Fig. 2C). However, this could be due to higher amounts of co-immunoprecipitated Cdk1, existing in complexes with cyclin B at this time point in induced cells (Fig. 4A). This prompted us to analyze WB blots with anti-Thr(P)161 antibodies in crude lysates prepared from cells taken 10–11 h after release from a double thymidine block. In these experiments, we observed only little difference between non-induced and induced cells (Fig. 7A). As further readout of an earlier Cdk1-cyclin B complex formation in response to Cdc25A overexpression, we then analyzed Thr(P)161 accumulation directly in cells in response to Cdc25A overexpression. As shown in Fig. 7B, we observed a clear increase of the Thr161 signal around the cell nucleus, which was similar to cyclin B localization. It is known that Cdk7 has a predominantly nuclear localization, and a part of its cellular pool is also associated with some poorly soluble intracellular structures (28). Therefore, we decided to test lysates prepared from nuclear and detergent-resistant subcellular fractions of U2OS cells, collected at sequential time points after release. In detergent-resistant fractions (and less pronounced in nuclear fractions), higher levels of Cdk1 Thr161 phosphorylation were clearly detectable in Cdc25A-overexpressing cells (Fig. 6B). Correspondingly, a faster migrating, presumably Thr(P)164/Thr(P)170-phosphorylated and active fraction of Cdk7 (28) was more pronounced in induced cells in comparison with non-induced cells (Fig. 7C). It has been shown that Cdk7 mutants mimicking phosphorylation of Thr164/Thr170 can slightly increase Cdk7 kinase activity toward Cdk2 (28). To detect a difference in the activity of Cdk7 between non-induced and induced synchronized U2OS cells, we performed immunoprecipitations from whole cell lysates and detergent-resistant fractions using the same anti-Cdk7 antibody as in the previous experiment. In accordance with the increase in the activating phosphorylation of Cdk7, we also observed an increase in Cdk7 activity (Fig. 7D) in Cdc25A-overexpressing cells using GST-Cdk1-KD as a substrate in in vitro kinase assays. Taken together, our findings show that Cdc25A phosphatase induces complex assembly of cyclin B and Cdk1 through activation of Cdk7. This then leads to an increase in Thr161 phosphorylation, thus stabilizing the complex.
FIGURE 7.
Overexpression of Cdc25A correlates with increased electrophoretic mobility of Cdk7. A, lysates of U2OS Cdc25A synchronized cells, collected at 10 h after release from a double thymidine block, were analyzed by immunoblotting with the indicated antibodies. Note a shift of the Thr(P)161 (pThr161) band in induced cells, corresponding to Tyr15/Thr161-phosphorylated Cdk1, in comparison with Thr(P)14/Tyr(P)15/Thr(P)161 phosphorylated in non-induced cells, and a slight increase in Thr161 phosphorylation upon overexpression of Cdc25A. B, induced and non-induced U2OS Cdc25A cells were fixed on coverslips 9 h after release from a double thymidine block (left). Cells were stained with anti-cyclin B (green) and anti-Cdk1-Thr(P)161 (red) antibodies. DNA was stained with Hoechst (blue). Scale bar, 10 μm. On the right, relative signal intensities of anti-Thr(P)161 stainings for ∼50 cells were determined. Error bars, S.D. in three independent experiments. C, nuclear (left) and detergent-resistant fractions (right) from cells synchronized as in A. Extracts from U2OS Cdc25A cells were resolved using SDS-PAGE and probed with denoted antibodies. Note that in detergent-resistant fractions obtained from induced cells, the faster migrating Cdk7 bands are more abundant than in those from non-induced cells. Phospho-Thr161 Cdk1 (upper band) is also increased in these extracts upon Cdc25A overexpression. The lower bands in Thr(P)161 immunoblots correspond to Thr(P)160 Cdk2, also recognized by the antibody used. D, Cdk7 was immunoprecipitated from 500 μg of lysates prepared as in A and IP were split for immunoblotting (bottom) and kinase assays using catalytically inactive GST-Cdk1 (GST-Cdk1-KD) as a substrate (top). As a control, mouse IgGs were used.
DISCUSSION
It is known that Cdc25A can activate Cdk1 in vivo, whereas repression of Cdc25A leads to a decreased Cdk1 kinase activity and delayed G2/M transition (19, 21). Here we show that overexpression of Cdc25A in transformed human cells does not affect the duration of S or M phases but markedly shortens G2 phase and leads to an early mitotic entry. Surprisingly, upon elevated protein levels of Cdc25A, we detected a pronounced accumulation of Cdk1 kinase phosphorylated on Tyr15. This phosphorylation is known to be catalyzed by Wee1 kinase, which inhibits Cdk1-cyclin B catalytic activity (11, 35). However we found that stockpiling of Tyr(P)15 Cdk1 was not due to elevated Wee1 kinase activity but rather the result of incomplete dephosphorylation because overexpressed Cdc25A phosphatase removed phosphates efficiently from Thr14 but not from Tyr15. A preferential dephosphorylation of Cdk2 on Thr14 has been earlier detected in experiments in vitro using recombinant Cdk2-cyclin A and Cdc25A (33). In starfish oocytes, a sequential dephosphorylation of Cdk1 (first Thr14 and then Tyr15) has been observed in vivo, and the monophosphorylated Tyr(P)15 Cdk1 was characterized as prophase-specific (34). A gradual accumulation of Tyr15-phosphorylated, presumably cyclin-bound Cdk1 has also been described at the G2/M transition in HeLa cells (36). Notably, in our experiments, Tyr(P)15 Cdk1 immunoprecipitated from Cdc25A-overexpressing cells was highly active, as well as the phospho-mimicking T14A/Y15E mutant expressed in 293T cells, whereas both dually phosphorylated Thr(P)14/Tyr(P)15 Cdk1 from non-induced cells and the T14E/Y15E mutant exhibited much lower kinase activity. In addition, we observed that Tyr(P)15 Cdk1 immunoprecipitated from G2 cells in response to Cdc25A overexpression bound substantially more cyclin B than Thr(P)14/Tyr(P)15 Cdk1, immunoprecipitated from non-induced cells. These findings raise the question of whether the two-step dephosphorylation has a physiological relevance in mammalian cells.
Regulation of Cdk1-cyclin B complex assembly in human cells remains poorly understood. In many cancer cell lines, the protein levels of cyclin B are relatively high in S phase and do not increase dramatically at the G2/M transition (38, 39). On the other hand, the protein levels of Cdk1 kinase are known to be constant throughout the cell cycle (40). Although both proteins coexist in the cytoplasm during interphase (41, 42), our data confirm other published results (20) and show that the abundance and activity of Cdk1-cyclin B complexes remain low in G1 and S phases. In U2OS and HeLa cells used in our experiments, we detected that the amounts of Cdk1 co-immunoprecipitated with cyclin B were dramatically increased only at G2 and mitosis despite a relatively small change in cyclin B protein levels. Even more interesting is our finding that overexpression of Cdc25A caused a precocious formation of Cdk1-cyclin B complexes, which correlated with shortened G2 phase and early mitotic onset in induced cells. Overexpression of Cdc25B has a similar, if not stronger, effect on cyclin-kinase complex assembly, whereas high levels of exogenous Cdc25C protein did not affect this process. Correspondingly, shRNA mediated inhibition of Cdc25A and Cdc25B phosphatases resulted at G2/M in a substantial decrease of cyclin B levels bound to Cdk1. These findings suggest that Cdc25A and Cdc25B participate (directly or indirectly) in the assembly of Cdk1-cyclin B complexes. The simplest explanation of this phenomenon would be a higher stability of Tyr(P)15-Cdk1-cyclin B in comparison with Thr(P)14/Tyr(P)15-Cdk1-cyclin B, but we could not confirm this using different in vitro approaches.3 Other scenarios are more difficult to investigate, and one of them lies in CAK-mediated stabilization of Cdk1-cyclin B complexes. It is known that an activating phosphorylation of the Thr161 residue within the T-loop of Cdk1 improves binding of the kinase to cyclins (5, 37). In mammalian cells, this phosphorylation is carried out by CAK consisting of catalytic Cdk7, regulatory cyclin H, and accessory Mat1 subunits (6, 7). When the kinase activity of analog-sensitive Cdk7 was eliminated in G2 phase, mitotic entry was blocked due to defects in assembly of Cdk1-cyclin B complexes, indicating that activating phosphorylation is required for stabilization of the complexes in vivo (8). Although the kinase activity of Cdk7 seems constant throughout the cell cycle (43, 44), it is known to be enhanced via phosphorylation on two sites in the T-loop, Thr164 and Thr170 (28, 45). In a manner similar to Cdk1, these phosphorylations improve interactions of Cdk7 with cyclin H and slightly increase its activity toward Cdk2 (28). It has been shown that both Cdk2-cyclin A and Cdk1-cyclin B can phosphorylate and activate Cdk7, but in vivo, mitotic Cdk1-cyclin B does it more efficiently (45), which explains why Cdk7 activity can be restricted until mitosis. Our data provide new insights into the coordination of reciprocal activation of Cdk7 and Cdk1 in G2/M. We observed that overexpression of Cdc25A stimulates phosphorylation of Cdk7 at G2, leading to a corresponding increase in phosphorylated Thr161-Cdk1 levels, which confirms early activation of CAK in induced cells. It is unlikely that this strong activation is caused by Cdk2-cyclin A, because (i) it has low efficiency in Cdk7 phosphorylation in vivo (45) and (ii) overexpression of Cdc25B has a strong effect on Cdk1-cyclin B complex assembly despite its low activity toward Cdk2-cyclin A in vivo (23). We suggest that accumulation of Cdc25A and B phosphatases at the G2/M transition leads to primary activation of low abundant Cdk1-cyclin B complexes via partial dephosphorylation and thus provides the initial signal for launching a Cdk1-Cdk7 positive feedback loop. In addition, removal of the phosphate from Thr14 can facilitate nuclear relocation of Cdk1-cyclin B complexes because overproduction of Myt1 (which phosphorylates Thr14 preferentially (10)) impairs intracellular trafficking of Cdk1-cyclin B (46). Thus, partially dephosphorylated “prophase-specific” Tyr(P)15-Cdk1-cyclin B might have a role as a starter kinase, being a better substrate for CAK (than fully phosphorylated Thr(P)14/Tyr(P)15-Cdk1-cyclin B) or performing initial phosphorylation and activation of Cdk7. Phosphorylation of Cdc25A and Cdc25B by Cdk1 improves its stability (21) or activity toward Cdk1-cyclin B (47), respectively, therefore leading to a further boost of the activating feedback loop. We also cannot rule out the possibility that Tyr15-phosphorylated “prophase” Cdk1 has different substrate specificities than its fully dephosphorylated “metaphase” form; likewise, it has been described in yeast (48). Interestingly, Cdc25C phosphatase cannot accelerate the G2/M transition and Cdk1-cyclin B complex assembly. In fact, in our experiments, overexpression of Cdc25C did not induce accumulation of Tyr(P)15-Cdk1 despite high phosphatase activity,3 which may suggest a role for Cdc25C beyond prophase. However, to unravel this possibility, a separate investigation would be required.
Acknowledgments
We are grateful to D. Morgan, J. Pines, B. Ducommun, and R. Voit for providing reagents. We also thank T. Orlik for technical assistance.
This work was supported by Deutsche Krebshilfe Grant 107058 (to I. H.).
O. Timofeev, O. Cizmecioglu, F. Settele, T. Kempf, and I. Hoffmann, unpublished results.
- CAK
- Cdk-activating kinase
- shRNA
- short hairpin RNA
- GST
- glutathione S-transferase
- CHAPS
- 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonic acid
- BrdUrd
- bromodeoxyuridine
- FACS
- fluorescence-activated cell sorting
- WB
- Western blot
- HA
- hemagglutinin
- IP
- immunoprecipitation.
REFERENCES
- 1.Draetta G., Beach D. (1988) Cell 54, 17–26 [DOI] [PubMed] [Google Scholar]
- 2.Riabowol K., Draetta G., Brizuela L., Vandre D., Beach D. (1989) Cell 57, 393–401 [DOI] [PubMed] [Google Scholar]
- 3.Pines J., Hunter T. (1990) Nature 346, 760–763 [DOI] [PubMed] [Google Scholar]
- 4.Pines J., Hunter T. (1992) Ciba Found. Symp. 170, 187–196; discussion 196–204 [PubMed] [Google Scholar]
- 5.Ducommun B., Brambilla P., Félix M. A., Franza B. R., Jr., Karsenti E., Draetta G. (1991) EMBO J. 10, 3311–3319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larochelle S., Pandur J., Fisher R. P., Salz H. K., Suter B. (1998) Genes Dev. 12, 370–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fisher R. P., Morgan D. O. (1994) Cell 78, 713–724 [DOI] [PubMed] [Google Scholar]
- 8.Larochelle S., Merrick K. A., Terret M. E., Wohlbold L., Barboza N. M., Zhang C., Shokat K. M., Jallepalli P. V., Fisher R. P. (2007) Mol. Cell 25, 839–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atherton-Fessler S., Liu F., Gabrielli B., Lee M. S., Peng C. Y., Piwnica-Worms H. (1994) Mol. Biol. Cell 5, 989–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu F., Stanton J. J., Wu Z., Piwnica-Worms H. (1997) Mol. Cell. Biol. 17, 571–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parker L. L., Atherton-Fessler S., Piwnica-Worms H. (1992) Proc. Natl. Acad. Sci. U.S.A. 89, 2917–2921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strausfeld U., Labbé J. C., Fesquet D., Cavadore J. C., Picard A., Sadhu K., Russell P., Dorée M. (1991) Nature 351, 242–245 [DOI] [PubMed] [Google Scholar]
- 13.Mailand N., Falck J., Lukas C., Syljuâsen R. G., Welcker M., Bartek J., Lukas J. (2000) Science 288, 1425–1429 [DOI] [PubMed] [Google Scholar]
- 14.Falck J., Mailand N., Syljuåsen R. G., Bartek J., Lukas J. (2001) Nature 410, 842–847 [DOI] [PubMed] [Google Scholar]
- 15.Bartek J., Lukas C., Lukas J. (2004) Nat. Rev. Mol. Cell. Biol. 5, 792–804 [DOI] [PubMed] [Google Scholar]
- 16.Boutros R., Dozier C., Ducommun B. (2006) Curr. Opin. Cell Biol. 18, 185–191 [DOI] [PubMed] [Google Scholar]
- 17.Galaktionov K., Lee A. K., Eckstein J., Draetta G., Meckler J., Loda M., Beach D. (1995) Science 269, 1575–1577 [DOI] [PubMed] [Google Scholar]
- 18.Boutros R., Lobjois V., Ducommun B. (2007) Nat. Rev. Cancer 7, 495–507 [DOI] [PubMed] [Google Scholar]
- 19.Lindqvist A., Källström H., Lundgren A., Barsoum E., Rosenthal C. K. (2005) J. Cell Biol. 171, 35–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Molinari M., Mercurio C., Dominguez J., Goubin F., Draetta G. F. (2000) EMBO Rep. 1, 71–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mailand N., Podtelejnikov A. V., Groth A., Mann M., Bartek J., Lukas J. (2002) EMBO J. 21, 5911–5920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen M. S., Ryan C. E., Piwnica-Worms H. (2003) Mol. Cell. Biol. 23, 7488–7497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Timofeev O., Cizmecioglu O., Hu E., Orlik T., Hoffmann I. (2009) FEBS Lett. 583, 841–847 [DOI] [PubMed] [Google Scholar]
- 24.Hassepass I., Voit R., Hoffmann I. (2003) J. Biol. Chem. 278, 29824–29829 [DOI] [PubMed] [Google Scholar]
- 25.Hoffmann I., Clarke P. R., Marcote M. J., Karsenti E., Draetta G. (1993) EMBO J. 12, 53–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blomberg I., Hoffmann I. (1999) Mol. Cell. Biol. 19, 6183–6194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bulavin D. V., Phillips C., Nannenga B., Timofeev O., Donehower L. A., Anderson C. W., Appella E., Fornace A. J., Jr. (2004) Nat. Genet. 36, 343–350 [DOI] [PubMed] [Google Scholar]
- 28.Larochelle S., Chen J., Knights R., Pandur J., Morcillo P., Erdjument-Bromage H., Tempst P., Suter B., Fisher R. P. (2001) EMBO J. 20, 3749–3759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lammer C., Wagerer S., Saffrich R., Mertens D., Ansorge W., Hoffmann I. (1998) J. Cell Sci. 111, 2445–2453 [DOI] [PubMed] [Google Scholar]
- 30.Coulonval K., Bockstaele L., Paternot S., Roger P. P. (2003) J. Biol. Chem. 278, 52052–52060 [DOI] [PubMed] [Google Scholar]
- 31.Montagnoli A., Fiore F., Eytan E., Carrano A. C., Draetta G. F., Hershko A., Pagano M. (1999) Genes Dev. 13, 1181–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Furuno N., den Elzen N., Pines J. (1999) J. Cell Biol. 147, 295–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rudolph J., Epstein D. M., Parker L., Eckstein J. (2001) Anal. Biochem. 289, 43–51 [DOI] [PubMed] [Google Scholar]
- 34.Borgne A., Meijer L. (1996) J. Biol. Chem. 271, 27847–27854 [DOI] [PubMed] [Google Scholar]
- 35.Parker L. L., Piwnica-Worms H. (1992) Science 257, 1955–1957 [DOI] [PubMed] [Google Scholar]
- 36.Lindqvist A., van Zon W., Karlsson Rosenthal C., Wolthuis R. M. (2007) PLoS Biol. 5, e123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Desai D., Wessling H. C., Fisher R. P., Morgan D. O. (1995) Mol. Cell Biol. 15, 345–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu B., Mitra J., van den Heuvel S., Enders G. H. (2001) Mol. Cell Biol. 21, 2755–2766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mitra J., Enders G. H. (2004) Oncogene 23, 3361–3367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Welch P. J., Wang J. Y. (1992) Proc. Natl. Acad. Sci. U.S.A. 89, 3093–3097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pines J., Hunter T. (1991) J. Cell Biol. 115, 1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bailly E., Dorée M., Nurse P., Bornens M. (1989) EMBO J. 8, 3985–3995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tassan J. P., Schultz S. J., Bartek J., Nigg E. A. (1994) J. Cell Biol. 127, 467–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poon R. Y., Yamashita K., Howell M., Ershler M. A., Belyavsky A., Hunt T. (1994) J. Cell Sci. 107, 2789–2799 [DOI] [PubMed] [Google Scholar]
- 45.Garrett S., Barton W. A., Knights R., Jin P., Morgan D. O., Fisher R. P. (2001) Mol. Cell Biol. 21, 88–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu F., Rothblum-Oviatt C., Ryan C. E., Piwnica-Worms H. (1999) Mol. Cell Biol. 19, 5113–5123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gabrielli B. G., Clark J. M., McCormack A. K., Ellem K. A. (1997) J. Biol. Chem. 272, 28607–28614 [DOI] [PubMed] [Google Scholar]
- 48.Crasta K., Huang P., Morgan G., Winey M., Surana U. (2006) EMBO J. 25, 2551–2563 [DOI] [PMC free article] [PubMed] [Google Scholar]