Abstract
Regulation of cell volume is of great importance because persistent swelling or shrinkage leads to cell death. Tissues experience hypertonicity in both physiological (kidney medullar cells) and pathological states (hypernatremia). Hypertonicity induces an adaptive gene expression program that leads to cell volume recovery or apoptosis under persistent stress. We show that the commitment to apoptosis is controlled by phosphorylation of the translation initiation factor eIF2α, the master regulator of the stress response. Studies with cultured mouse fibroblasts and cortical neurons show that mutants deficient in eIF2α phosphorylation are protected from hypertonicity-induced apoptosis. A novel link is revealed between eIF2α phosphorylation and the subcellular distribution of the RNA-binding protein heterogeneous nuclear ribonucleoprotein A1 (hnRNP A1). Stress-induced phosphorylation of eIF2α promotes apoptosis by inducing the cytoplasmic accumulation of hnRNP A1, which attenuates internal ribosome entry site-mediated translation of anti-apoptotic mRNAs, including Bcl-xL that was studied here. Hypertonic stress induced the eIF2α phosphorylation-independent formation of cytoplasmic stress granules (SGs, structures that harbor translationally arrested mRNAs) and the eIF2α phosphorylation-dependent accumulation of hnRNP A1 in SGs. The importance of hnRNP A1 was demonstrated by induction of apoptosis in eIF2α phosphorylation-deficient cells that express exogenous cytoplasmic hnRNP A1. We propose that eIF2α phosphorylation during hypertonic stress promotes apoptosis by sequestration of specific mRNAs in SGs in a process mediated by the cytoplasmic accumulation of hnRNP A1.
Keywords: Cell Death, Protein Synthesis, Signal Transduction, Stress Granule, Translation, IRES, hnRNPs
Introduction
The regulation of cell volume in response to changes in extracellular osmolarity is an important function. Hypertonic conditions induce responses that protect cells by inducing transport of osmolytes referred to as compatible solutes. Intermediate levels of hypertonic stress cause cell cycle arrest followed by cell survival, but higher levels induce apoptosis (1).
The master regulator of this response is the tonicity-responsive enhancer-binding protein, TonEBP, which translocates to the nucleus and induces transcription of genes encoding osmolyte transporters and heat shock proteins (1). A remarkable example of adaptation is seen in kidney medulla, which is exposed to hypertonic urine. Mice deficient in TonEBP show atrophy of the renal medulla and deficiencies in immune system function (2). In addition, brain cells have adaptive mechanisms for cell volume recovery that involve the TonEBP target gene SNAT2 (3). This is further supported by the induction of TonEBP in neurons during systemic hypertonicity (4). Neurological disorders have also been associated with hypertonic blood plasma, as in diseases of water imbalance (5).
The cellular response to stress involves regulation of mRNA translation (6–8). Diverse stresses limit protein synthesis, mostly via inhibition of translation initiation. Much of this regulation involves eIF2,5 which delivers the initiator tRNA (Met-tRNAiMet) to cellular mRNAs that use an AUG initiation codon. Phosphorylation of the α subunit on Ser51 sequesters eIF2 in an inactive complex, thus decreasing translation initiation (9).
The outcome of the stress response depends on the intensity and duration of the stress. Events during the early response promote the recovery of translation and the synthesis of proteins essential for adaptation (10). However, prolonged and severe stress promotes apoptosis. The regulated synthesis of pro-survival and pro-death proteins plays an important role in determining cell fate during stress. It has been suggested that persistent inhibition of protein synthesis can lead to apoptosis by inhibiting translation of mRNAs encoding anti-apoptotic proteins (11, 12). Translation of some pro-apoptotic and anti-apoptotic mRNAs is mediated by internal ribosome entry sites (IRESs) in the mRNA leaders. IRES-mediated translation initiation involves recruitment of the ribosome to the mRNA independently of the 5′-cap. In most cases, this requires IRES trans-acting factors that mediate ribosome recruitment. The activity of many IRESs can be positively or negatively regulated by IRES trans-acting factors depending on the type of stress (6, 13).
We previously showed that mild osmotic stress (400 mosmol/liter) causes a pro-survival response without induction of eIF2α phosphorylation (14). In this study, we show that severe osmotic stress induces apoptosis in a manner dependent on eIF2α phosphorylation. In contrast to the current view, decreased protein synthesis in the absence of eIF2α phosphorylation does not induce apoptosis. Rather, eIF2α phosphorylation is required for the translocation of the RNA-binding protein hnRNP A1 from the nucleus to the cytoplasm, which blocks translation of mRNAs encoding inhibitors of apoptosis. The pro-apoptotic function of eIF2α phosphorylation during osmotic stress is also demonstrated in primary cortical neurons. This mechanism of translational control may have direct implications in many diseases that involve undesirable apoptosis such as heart failure and neurodegeneration.
EXPERIMENTAL PROCEDURES
Cell Cultures
MEFs were cultured in high glucose Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 2 mm glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin. Human embryonic kidney (HEK 293) cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% heat-inactivated fetal calf serum, 2 mm glutamine, and antibiotics as above. Media were supplemented with sucrose to give the desired osmolarity, assuming that standard medium is 300 mosmol/liter. Unless otherwise specified, hypertonic medium was 600 mosmol/liter obtained by addition of 300 mm sucrose to standard medium. Endoplasmic reticulum stress was induced with 400 nm thapsigargin.
All animal studies were approved by the Institutional Animal Care and Use Committee of Case Western Reserve University. Mice were housed individually in microisolator cages with a 12:12-h light/dark cycle with free access to food and water at 23 ± 1 °C. Genotyping was performed by PCR as described previously (9) using primers in supplemental Table I.
Mice heterozygous for the S51A mutation of eIF2α were mated for two continuous nights. Pregnant mice were sacrificed on the 17th day after first mating to obtain 16–17-day embryos. Cultures of primary neurons were prepared from cerebral cortices (15). Cells were plated at 1 × 105 cells/cm2 on 8-well chamber slides coated with poly-d-lysine/laminin in Neurobasal Medium (Invitrogen) supplemented with 2% B27 (Invitrogen), 2 mm GlutaMAX (Invitrogen). Half the culture medium was replaced every 3 days. More than 90% of the cells were neurons after 7 days of culture. TUNEL assays were performed using an in situ cell death detection kit, TMR Red (Roche Applied Science). Confocal images were captured with a Zeiss LSM 510 confocal microscope (Zeiss, Oberkochen, Germany). Apoptosis was scored as the ratio of TUNEL-positive cells to total cells (DAPI staining). Approximately 500 cells were counted for each condition.
Plasmids and Transfections
Expression vectors for eIF2α S51A and S51D were a generous gift from Dr. J. Pelletier. These vectors were generated by inserting S51A into the BamHI-XhoI and S51D into the EcoRI sites of the MSCV-IRES-GFP vector. Plasmids expressing wild-type and F1 mutant FLAG-tagged hnRNP A1 were described previously (16). The Bcl-xL 5′-UTR was cloned into the modified pβgal/CAT reporter plasmid (17) NheI (5′) and XhoI (3′) restriction sites. The Bcl-xL 5′-UTR was amplified from the Bcl-xL (full length cDNA) (−446 to −1) template (18) using the primers in supplemental Table I. Transfections used Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol, and transiently transfected cells were used for experiments after 48 h. Stable mass cultures were generated by cotransfecting an expression vector for hygromycin resistance (pTK-Hyg) and selecting in 200 μg/ml hygromycin B. The authenticity of the Bcl-xL IRES was confirmed using transfections of promotorless and hairpin-containing monocistronic reporter expression vectors in S/S cells (data not shown). Bcl-xL IRES activity was measured by assaying chloramphenicol acetyltransferase and β-galactosidase activities 48 h after transfection (19). IRES activity is expressed as the ratio of chloramphenicol acetyltransferase and β-galactosidase activities. Neuronal cultures were transfected 5 days after plating using NeuroFECT (Genlantis) according to the manufacturer's protocol and used 2 days later.
Cell Viability Assays
Cells were seeded in 24-well plates at 5 × 104 cells/well. After 24 h, cells were incubated in test media and then in the same media supplemented with 44 μm resazurin (Sigma) for an additional 2 h, and fluorescence was measured (14).
Metabolic Labeling
Cells were seeded in 24-well plates at 5 × 104 cells/well, grown for 24 h, and subjected to hypertonic treatment. 35S-Met/Cys was added (30 μCi/ml Expre35S35S protein labeling mix, PerkinElmer Life Sciences) for an additional 30 min. Cells were washed twice with ice-cold phosphate-buffered saline and incubated in three changes of 5% trichloroacetic acid, 1 mm Met for 10 min on ice. Precipitates were dissolved in 200 μl of 1 n NaOH, 0.5% sodium deoxycholate, and radioactivity was determined by liquid scintillation counting. Protein was assayed in aliquots as described previously (20).
Immunofluorescence Microscopy
Cells were seeded on gelatin-coated coverslips, grown overnight, and subjected to hypertonic stress. They were then fixed with 4% paraformaldehyde (30 min at room temperature), permeabilized in 70% ethanol (overnight at 4 °C), incubated in 300 mm NaCl, 30 mm sodium citrate, pH 7, and blocked with phosphate-buffered saline, 10% bovine serum albumin. Samples were incubated with primary antibodies (supplemental Table II) in phosphate-buffered saline, 10% bovine serum albumin overnight at 4 °C. After three washes with phosphate-buffered saline, samples were incubated with Alexa Fluor 488- or 594-conjugated secondary antibodies (Invitrogen) (1:300) for 1 h at 37 °C in the dark, except for cytochrome c where a fluorophore-conjugated antibody was used. The preparations were mounted in SlowFade Gold with DAPI (Invitrogen) and visualized on a Leica DMI4000 B microscope or with a Zeiss LSM 510 confocal microscope (Zeiss, Oberkochen, Germany).
Immunoprecipitation
S/S cells were transiently transfected with FLAG-tagged WT hnRNP A1 and incubated for 3 h in control or hypertonic medium. Cytoplasmic extracts (500 μg of protein) were immunoprecipitated with 4 μg of anti-FLAG or mouse control antibody (Santa Cruz Biotechnology) as described previously (21), except that Dynabeads protein G (Invitrogen) were used. mRNAs were analyzed by RT-qPCR as described below.
Other Methods
Nuclear and cytosolic fractions were prepared from cultured cells as described previously (21). Immunoblot analysis was performed using standard procedures. Antibodies are listed in supplemental Table II. Analysis of polyribosomes by sucrose gradient sedimentation was carried out as described previously (22). Total RNAs from fractions of polyribosome analysis were prepared using TRIzol (Invitrogen). Ribosomal RNAs were visualized by agarose gel electrophoresis, and mRNAs were monitored by RT-qPCR using Power SYBR Green PCR Master Mix (Applied Biosystem) and primers in supplemental Table I. Northern blot analysis was performed as described previously (23). Probes for Bcl-xL and HSP70 mRNAs were generated by PCR using the primer sets listed in supplemental Table I. Probes for 18 S ribosomal RNA and glyceraldehyde-3-phosphate dehydrogenase were generated as described previously (23).
RESULTS
Induction of Apoptosis by Osmotic Stress Requires eIF2α Phosphorylation
Phosphorylation of eIF2α by diverse stress conditions has pro-survival and/or pro-apoptotic functions (24). We tested if induction of apoptosis by severe hypertonic stress requires eIF2α phosphorylation. Mouse embryonic fibroblasts (MEFs) from C57BL/6 mice (S/S) treated for 1 h with hypertonic media showed increased eIF2α phosphorylation with increasing stress intensity, without a change in total eIF2α (Fig. 1A). As shown in earlier studies, osmotic stress reduced the phosphorylation of ribosomal protein S6 and 4EBP1 consistent with the inhibition of the mammalian target of rapamycin pathway (25).
FIGURE 1.
Induction of apoptosis by severe hypertonic stress is dependent on eIF2α phosphorylation. Wild-type MEFs (S/S) were treated with media of the indicated osmolarity for 1 h (A), or S/S and A/A MEFs were treated with 600 mosmol/liter medium for the indicated times (B), and proteins were detected by Western blot analysis of total extracts and were quantified by densitometry. The eIF2α-P/eIF2α ratio increased by 7 ± 1.9-fold (n = 4) after 5 h of hypertonic treatment in wild-type MEFs. Casp, caspase. C, viability was determined by measuring the metabolic activity of S/S and A/A cells treated with 600 mosmol/liter medium for the indicated times using resazurin. D, 35S-Met/Cys incorporation into protein in S/S and A/A treated with 600 mosmol/liter medium for the indicated times was evaluated. The error bars in C and D represent the mean ± S.E. of two independent experiments with six determinations each. E, S/S and A/A cells were treated for 3 h with hypertonic medium, and polyribosome profiles were analyzed by sucrose gradient centrifugation. Gradients were fractionated, and absorbance at 254 nm was recorded (upper panels). The positions of 80 S ribosomes and polyribosomes are indicated. Total RNA samples were run on agarose gels to analyze ribosomal RNAs (lower panels). The significance of differences among means in C and D were evaluated using Student's t test; *, p < 0.05; ***, p < 0.001; ns, not significant.
Severe osmotic stress is known to induce apoptosis (1). The role of eIF2α phosphorylation in this process was tested by comparing the response of A/A MEFs, which have a homozygous mutation in eIF2α (S51A) that abolishes the phosphorylation site and the congenic WT S/S cells. In S/S cells treated with 600 mosmol/liter hypertonic medium, eIF2α phosphorylation increased gradually over 7 h (Fig. 1B). This stress also induced apoptosis, as shown by cleavage of the execution caspase 3 and its substrate, poly(ADP-ribose) polymerase. In contrast, A/A cells did not show this cleavage. In addition, cell viability was significantly lower in S/S than in A/A cells (Fig. 1C). These data suggest that A/A cells are protected from apoptosis by the lack of eIF2α phosphorylation. Cellular protein synthesis rates decreased by >80% in both stressed S/S and A/A cells (Fig. 1D). In agreement, hypertonic stress caused a dramatic decrease in the abundance of polyribosomes in both cell lines (Fig. 1E) suggesting that reduced translation initiation is one of the causes of decreased rates of protein synthesis in S/S and A/A cells. These data also suggest that the inhibition of global protein synthesis involves more factors than eIF2α phosphorylation, and this inhibition is not the sole cause of the induction of apoptosis in S/S cells.
To examine the reason for the decreased protein synthesis in A/A cells, we determined the phosphorylation status of elongation factor 2 and 4EBP1. Phosphorylation of elongation factor 2 at Thr56 decreases translation elongation, whereas phosphorylation of 4EBP1 stimulates translation initiation (26). We found that treatment with hypertonic medium induced elongation factor 2 phosphorylation (Fig. 1B) and dephosphorylation of 4EBP1 (supplemental Fig. 1A) to similar extents in S/S and A/A cells. These data suggest the following: (i) eIF2α phosphorylation is required for osmotic stress-induced apoptosis, and (ii) changes in the phosphorylation of elongation factor 2 and 4EBP1 can contribute to the dramatic decrease of protein synthesis in S/S and A/A cells during osmotic stress, although other mechanisms may also be involved (27). These findings are in agreement with the idea that hypertonic stress induced apoptosis in S/S but not in A/A cells, despite the dramatic decrease in protein synthesis in both cells.
To confirm the linkage of eIF2α phosphorylation to apoptosis in hypertonic stress, we tested the effects of two eIF2α mutants on induction of apoptosis by hypertonic stress. We predicted that a dominant negative mutant (S51A) should attenuate apoptosis in S/S cells during stress and that a phosphomimetic mutant (S51D) should promote apoptosis in A/A cells. Expression of HA-tagged eIF2α S51A in stably transfected S/S mass cultures caused attenuation of hypertonic stress-induced apoptosis as shown by the reduced cleavage of caspase 3 (Fig. 2A). Conversely, expression of the HA-tagged eIF2α S51D in A/A cells induced apoptosis, as evident by the cleavage of caspase 3 (this experiment was performed in transiently transfected cells because the S51D mutant inhibits cell growth). Immunofluorescence microscopy confirmed these findings (Fig. 2B). GFP, which was expressed from the plasmids carrying the mutant eIF2α, was used to identify transfected cells. In S/S cells expressing eIF2α S51A, hypertonic stress did not induce cleaved caspase 3. Conversely, hypertonic stress induced caspase 3 cleavage in all A/A cells expressing eIF2α S51D. These data support the conclusion that eIF2α phosphorylation is required for the induction of apoptosis during severe hypertonic stress.
FIGURE 2.
Expression of a mutant eIF2α that cannot be phosphorylated attenuates induction of apoptosis by hypertonic stress. A, stable mass culture expressing HA-tagged eIF2α harboring the S51A mutation was established as described under “Experimental Procedures.” A/A cells were transiently transfected with an empty vector or an HA-tagged eIF2α S51D expression vector. Both cell lines were treated with 600 mosmol/liter medium for 3 h, and the indicated proteins were detected by Western blot analysis of total extracts. B, S/S and A/A cells were transiently transfected with expression vectors for eIF2α S51A or eIF2α S51D, respectively, that also express GFP to allow visualization of transfected cells. Cells were cultured in hypertonic medium for 3 h and then processed for immunofluorescence microscopy. Nuclei were visualized with DAPI. Arrowheads show transfected cells. All S/S cells positive for GFP expression (37 cells in 25 fields) were negative for cleaved caspase 3. All A/A cells positive for GFP expression (24 cells in 15 fields) were positive for cleaved caspase 3. Representative confocal pictures are shown. Bar, 10 μm.
eIF2α Phosphorylation Attenuates Translation of the Bcl-xL mRNA during Hypertonic Stress
The induction of apoptosis in S/S but not A/A cells during hypertonic stress led us to hypothesize that eIF2α phosphorylation attenuates expression of anti-apoptotic proteins and promotes cell death. They include Bcl-2 family members (Bcl-2, Bcl-xL, and Mcl-1), which prevent mitochondrial outer membrane permeabilization (28), and the caspase inhibitor, X-linked inhibitor of apoptosis (XIAP) (29). We first determined the effect of osmotic stress on the levels of XIAP and Bcl-xL proteins in S/S and A/A cells (Fig. 3A). Both proteins increased during early stress in S/S cells followed by decreases. The levels of XIAP showed a small increase followed by a 50% decrease at 7 h of stress, and the levels of Bcl-xL increased by 5-fold before decreasing below the levels in untreated cells at 7 h. In contrast, the levels of both proteins did not decrease in A/A cells up to 5 h of treatment, demonstrating that eIF2α phosphorylation is required for repression of these anti-apoptotic proteins during stress. Moreover, it suggests that decreased levels of these proteins in S/S cells play a role in hypertonic stress-induced apoptosis.
FIGURE 3.
eIF2α phosphorylation during hypertonic stress induces translational inhibition of Bcl-xL mRNA. A, wild-type (S/S) and A/A MEFs were cultured in hypertonic medium for the indicated times, and proteins were detected on Western blots of total cell extracts. B and C, equal volumes of fractions from Fig. 1E were analyzed for mRNAs by RT-qPCR. Bcl-xL mRNA distribution is shown in B, and the percentage of the indicated mRNAs on polyribosomes (pooled fractions 7–12, disomes and greater) is shown in (C). Data were expressed as mean ± S.E. of three independent experiments. The significance of differences among means was evaluated using Student's t test. *, p < 0.05 was considered significant; ns, not significant. D and E, S/S and A/A MEFs were cultured in hypertonic medium for the indicated times, and mRNA levels for the indicated genes were detected by RT-qPCR (D) and Northern blot analysis (E). GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
The translation of some anti-apoptotic mRNAs, including XIAP and Bcl-xL, is initiated at IRESs. eIF2α phosphorylation has been suggested to affect the translation of many IRES-containing mRNAs (21), and this regulation is probably independent of the effects on global translation. It was previously shown that osmotic stress decreases translation of the XIAP mRNA (16) in agreement with the decreased levels of XIAP in this study (Fig. 3A). Because the levels of Bcl-xL protein showed a dramatic decrease during osmotic stress, we tested the hypothesis that eIF2α phosphorylation targets Bcl-xL mRNA translation. We evaluated the distribution of the Bcl-xL mRNA on polyribosome profiles from control and stressed S/S and A/A cells. It was shown earlier that hypertonic stress decreased the abundance of polyribosomes in both cells (Fig. 1E), in agreement with the decrease in protein synthesis rates (Fig. 1D). Stress had different effects on the association of the Bcl-xL mRNA with polyribosomes in S/S and A/A cells (Fig. 3, B and C). Hypertonic stress caused attenuation of the translation of the Bcl-xL mRNA in S/S cells, as indicated by a decrease in the amount of mRNA in the polyribosome region (fractions 7–12) and an increase in the amount of free mRNA (fractions 1–6) (Fig. 3B). In contrast, the distribution of Bcl-xL mRNA in A/A cells was not affected by stress despite the dramatic decrease in global translation. Quantitative analysis showed that stress induced an 18% decrease in the polyribosome-associated Bcl-xL mRNA in S/S cells but no significant change in A/A cells (Fig. 3C). These data support the hypothesis that hypertonic stress reduces the translation efficiency of the Bcl-xL mRNA during stress in S/S but not A/A cells, which contributes to the decreased levels of this protein in the former cells.
The levels of Bcl-xL mRNA also declined by 30–50% in S/S cells during stress, whereas smaller declines were seen in A/A cells (Fig. 3D), suggesting that the control of mRNA levels by hypertonic stress is important in regulating the levels of this protein. It should be noted that in contrast to the Bcl-xL mRNA levels that decreased during hypertonic stress, we observed a 5-fold increase in the levels of the HSP70 mRNA (Fig. 3E), which is known to be up-regulated by these conditions (1).
We also examined the translation of the IRES-containing c-Myc and the cap-dependent anti-apoptotic Mcl-1 mRNAs. We found that 55% of the c-Myc mRNA was in polyribosomes in control and 59% in treated S/S cells (Fig. 3C), suggesting that not all IRES-containing mRNAs are translationally repressed during osmotic stress. It is known that translation of the Mcl-1 mRNA is repressed during stresses that cause eIF2α phosphorylation (11). The amount of this cap-dependent mRNA in polyribosomes decreased during stress by ∼30% in both S/S and A/A cells (Fig. 3C). These data are consistent with the global decrease of protein synthesis and with previous reports. These data suggest that osmotic stress inhibits the cap-dependent translation of the Mcl-1 mRNA independently of eIF2α phosphorylation.
The Bcl-xL IRES is stimulated by a member of the eIF4G family, DAP5 (p97), and its caspase-cleaved fragment, p86 (30, 31). Interestingly, DAP5 mRNA is also translated via an IRES, whose activity is enhanced by the DAP5 protein (32). Osmotic stress caused a decrease in DAP5 protein levels in both S/S and A/A cells (Fig. 3A), in agreement with the decreased translation of DAP5 mRNA (Fig. 3C). In addition, cleaved DAP5 accumulated at 5–7 h of osmotic stress in S/S cells (Fig. 3A), which should positively regulate translation of the Bcl-xL mRNA (30, 33). However, Bcl-xL mRNA translation declined in S/S cells at 5 h of stress (data not shown), suggesting that there is attenuation of translation by other mechanisms. In summary, our analysis shows that the reduction in the levels of the anti-apoptotic protein Bcl-xL during osmotic stress requires eIF2α phosphorylation and that this reduction involves decreased translation of its mRNA.
Finally, as an additional test of the role of eIF2α phosphorylation and reduced Bcl-xL levels in apoptosis induced by hypertonic stress, we examined the release of cytochrome c from mitochondria by immunofluorescence microscopy. As expected, hypertonic stress caused cytochrome c release from mitochondria in S/S but not in A/A cells (supplemental Fig. 1B) consistent with the increase in mitochondria-induced apoptosis in the former cells.
Accumulation of hnRNP A1 in the Cytoplasm during Hypertonic Stress Depends on eIF2α Phosphorylation
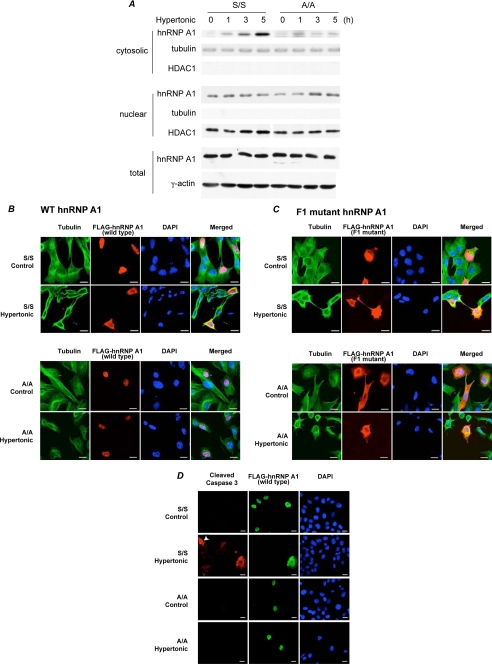
The RNA-binding protein hnRNP A1 shuttles between the nucleus and cytoplasm. Hypertonic stress induces accumulation of this protein in the cytoplasm (34). Moreover, hnRNP A1 is a trans-acting factor for the XIAP IRES, and inhibition of IRES activity during hypertonic stress was shown to be mediated by hnRNP A1 binding to the IRES (16). This suggests a role for hnRNP A1 as a translational inhibitor of a subset of IRES-containing mRNAs during stress (34). We hypothesized that the cytoplasmic accumulation of hnRNP A1 during hypertonic stress depends on eIF2α phosphorylation. To test this hypothesis, we compared the effect of hypertonic stress on hnRNP A1 localization in S/S and A/A cells by probing cytoplasmic and nuclear fractions on Western blots. hnRNP A1 levels increased in the cytoplasm of stressed S/S, but not A/A cells (Fig. 4A), showing that the cytoplasmic accumulation of hnRNP A1 during osmotic stress requires eIF2α phosphorylation. There was no change in total cellular hnRNP A1 levels (Fig. 4A). To confirm this finding, we expressed a FLAG-tagged hnRNP A1 protein in S/S and A/A cells and determined the effect of hypertonic stress on protein distribution by confocal microscopy. Osmotic stress induced the cytoplasmic localization of the FLAG-hnRNP A1 to a greater extent in S/S than in A/A cells (Fig. 4B), with 78% of S/S and 31% of A/A cells showing cytoplasmic accumulation. A/A cells that showed cytoplasmic localization of hnRNP A1 also expressed high levels of the transfected protein. All the S/S and A/A cells that showed high levels of cytoplasmic localization of FLAG-tagged hnRNP A1 during osmotic stress also contained cleaved caspase 3 (Fig. 4D), supporting the idea that induction of apoptosis involves the cytoplasmic accumulation of hnRNP A1. Because endogenous hnRNP A1 accumulated in the cytoplasm of S/S cells during osmotic stress (Fig. 4A), cleaved caspase 3 was also present in untransfected cells, but at lower intensity than in overexpressing cells (Fig. 4D, arrowhead).
FIGURE 4.
Cytoplasmic accumulation of hnRNP A1 during hypertonic stress depends on eIF2α phosphorylation. A, WT (S/S) and A/A cells were treated with hypertonic medium for the indicated times, and levels of endogenous hnRNP A1 protein were detected in cytoplasmic, nuclear, and total extracts by Western blotting. B–D, confocal microscopy of S/S and A/A MEFs transfected with wild-type (B and D) or F1 mutant (C) FLAG-tagged hnRNP A1. Cells were cultured in hypertonic medium for 3 h and then processed for immunofluorescence. Nuclei were visualized with DAPI. B and C, localization of the FLAG-tagged protein and tubulin. To evaluate cytoplasmic redistribution of FLAG-tagged hnRNP A1, for each condition >100 FLAG-positive cells were analyzed from two independent experiments. D, localization of FLAG-tagged protein and cleaved caspase 3. Arrowhead shows an untransfected cell positive for cleaved caspase 3. Bars, 20 μm.
The cytoplasmic accumulation of hnRNP A1 during hypertonic stress has been shown to require phosphorylation of Ser residues in the C-terminal region (F peptide) of the protein by a pathway that involves p38/Mnk kinases (35). The importance of the F peptide was demonstrated using the phosphomimetic F1 mutant in which all the Ser residues in the F peptide were changed to Asp; this mutant is constitutively located in the cytoplasm (34). We showed that the F1 mutant hnRNP A1 accumulates in the cytoplasm of both stressed and unstressed S/S and A/A cells (Fig. 4C).
Our data suggest that hypertonic stress induces apoptosis via mechanisms that involve the eIF2α phosphorylation-dependent accumulation of hnRNP A1 in the cytoplasm and translational repression of specific anti-apoptotic mRNAs. As a test of this hypothesis, we evaluated whether the constitutive cytoplasmic expression of F1 mutant FLAG-tagged hnRNP A1 in A/A cells restores stress-induced apoptosis. Osmotic stress caused caspase 3 cleavage in A/A cells transiently transfected with the F1 hnRNP A1 mutant but not in untransfected cells (Fig. 5A). The increased apoptosis in the stressed F1 mutant FLAG-tagged hnRNP A1-transfected A/A cells was also in agreement with the decreased viability in these cells as compared with untransfected stressed cells (Fig. 5B). In agreement with our hypothesis, Bcl-xL protein levels decreased in these cells (Fig. 5A), suggesting that cytoplasmic hnRNP A1 repressed Bcl-xL mRNA translation during osmotic stress. This hypothesis was tested directly by examining the effect of F1 mutant hnRNP A1 on Bcl-xL IRES activity (Fig. 5C). Expression of this protein inhibited IRES activity in S/S and A/A cells, as well as in HEK 293 human fibroblasts, which have been used extensively to study translation of mRNAs for anti-apoptotic proteins. We also examined the association of Bcl-xL mRNA with hnRNP A1 by immunoprecipitating FLAG-tagged hnRNP A1 from cytoplasmic extracts of transfected S/S cells. We found Bcl-xL mRNA associated with hnRNP A1, and the amount increased more than 2-fold during hypertonic stress (Fig. 5D). This effect was specific because the association of hnRNP A1 with the c-Myc mRNA was not altered by stress, in agreement with the lack of stress-induced translational inhibition of this mRNA (Fig. 5E).
FIGURE 5.
Cytoplasmic expression of hnRNP A1 in A/A cells induces apoptosis. A, A/A cells were transiently transfected with F1 mutant FLAG-tagged hnRNP A1 and treated with hypertonic medium for the indicated times, and proteins were detected by Western blot analysis of total cell extracts. B, cell viability was determined by measuring the metabolic activity of A/A cells untransfected or stably expressing F1 mutant (mut) FLAG-tagged hnRNP A1 treated with 600 mosmol/liter medium for the indicated times using resazurin. C, WT (S/S) MEFs, A/A MEFs, and HEK 293 cells were cotransfected with the Bcl-xL IRES bicistronic vector along with expression vectors for F1-mutant hnRNP A1. Chloramphenicol acetyltransferase and β-galactosidase were measured in cell extracts, and IRES activity was calculated as described under “Experimental Procedures.” Activities have been normalized to the level in control S/S cells. D, cytoplasmic extracts from S/S cells expressing FLAG-tagged WT hnRNP A1 incubated for 3 h in control or hypertonic medium were immunoprecipitated (IP) with the indicated antibodies. Bcl-xL and c-Myc mRNAs in the input samples and immunoprecipitates were monitored by RT-qPCR. Values are normalized to the level in control cells or control cells precipitated with anti-FLAG. E, S/S and A/A cells (untransfected or expressing FLAG-tagged F1 mutant hnRNP A1) were incubated in control or hypertonic medium for 3 h. Polyribosomes were fractionated by centrifugation, and the percentage of total c-Myc and Bcl-xL mRNAs in the polyribosome fraction was analyzed. F, S/S and A/A cells transfected with control siRNA or siRNA to Bcl-xL were treated with hypertonic medium for the indicated times, and proteins were detected by Western blot analysis of total cell extracts. PARP, poly(ADP-ribose) polymerase. G, S/S and A/A MEFs were transiently transfected with F1 mutant FLAG-tagged hnRNP A1 and cultured in hypertonic medium for 3 h. FLAG-tagged protein and cleaved caspase 3 were detected by immunofluorescence microscopy. Arrowheads show an untransfected cell positive for cleaved caspase 3. H, quantitative analysis of the experiments in G and Fig. 4D. The graph shows the fraction of FLAG-expressing cells that contained cleaved caspase 3. For each condition, >500 cells were scored from two independent experiments. Bars, 20 μm. The error bars in B–E represent the mean ± S.E. of at least two independent experiments with at least three determinations each. The significance of differences among means was evaluated using the Student's t test; *, p < 0.05; **, p < 0.01; ***, p < 0.001; ns, not significant. The brackets in E indicate significant differences of the compared experimental samples.
To further support the importance of cytoplasmic hnRNP A1 in the response to hypertonic stress, we examined Bcl-xL mRNA translation in cells expressing the F1 mutant. Expression of this protein in mass cultures of stably transfected S/S and A/A cells caused a 40% decrease in the association of Bcl-xL mRNA with polyribosomes in both cell types during osmotic stress (compared with an 18% decrease in untransfected S/S and no decrease in untransfected A/A) but had no effect on translation of the c-Myc mRNA (Fig. 5E). These data suggest that the cytoplasmic accumulation of hnRNP A1 during hypertonic stress promotes apoptosis by reducing Bcl-xL protein levels (Figs. 3A and 5A).
Although Bcl-xL levels correlated with protection of A/A cells from apoptosis during osmotic stress, cleavage of caspase 3 and its target poly(ADP-ribose) polymerase in Bcl-xL-depleted A/A cells was much lower than in S/S cells (Fig. 5F), suggesting that regulation of Bcl-xL levels is not the sole determinant of cell fate during osmotic stress.
Further evidence for the importance of cytoplasmic hnRNP A1 in induction of apoptosis was obtained by using confocal microscopy and scoring the FLAG-hnRNP A1-expressing cells, which are positive for cleaved caspase 3 (Figs. 4D and 5G). We found that hypertonic stress induced apoptosis in 50% of S/S and 15% of A/A cells overexpressing WT hnRNP A1 (Fig. 4D and Fig. 5H). Expression of the F1 mutant hnRNP A1, which is constitutively located in the cytoplasm, caused induction of apoptosis in both cell lines in unstressed conditions (Fig. 5, G and H). More significantly, expression of this mutant caused induction of caspase 3 cleavage in 55% of A/A cells during hypertonic stress conditions (Fig. 5H). Cleaved caspase 3 in unstressed A/A cells expressing the F1 mutant hnRNP A1 was not detected on Western blots, presumably because microscopy has greater sensitivity. The ability of F1 mutant hnRNP A1 to restore induction of apoptosis in A/A cells suggests that this protein is a critical factor for decreased Bcl-xL protein levels and induction of apoptosis. These data support our hypothesis that eIF2α phosphorylation controls cell fate during hypertonic stress by modulating the subcellular distribution of hnRNP A1 and in part regulating translation of IRES-containing anti-apoptotic mRNAs.
Hypertonic Stress Induces hnRNP A1-containing Stress Granules in S/S but Not in A/A Cells
HnRNP A1 is found in cytoplasmic SGs during osmotic stress (36). SGs are dynamic structures that harbor translationally arrested mRNAs and proteins such as HuR and TIAR during stress (37). Phosphorylation of eIF2α by diverse stress conditions has been associated with formation of SGs (38, 39). Therefore, we hypothesized that the absence of translational repression of hnRNP A1 target mRNAs in A/A cells was due to their inability to form SGs. However, ∼50% of S/S and A/A cells contained granules positive for the SG markers HuR and TIAR during hypertonic stress (Fig. 6, A and C, and data not shown). This suggests that formation of SGs during severe osmotic stress is the result of global translational inhibition and is independent of eIF2α phosphorylation. An alternative explanation for differences in the translational repression of hnRNP A1 target mRNAs is that hnRNP A1 is localized in SGs in S/S but not in A/A cells. Our data support this hypothesis; during hypertonic stress hnRNP A1 was found in SGs in ∼20% of S/S but not in A/A cells (Fig. 6, B and C, and supplemental Fig. 4). All SGs positive for hnRNP A1 were also positive for TIAR (Fig. 6C). These data suggest that hnRNP A1 is present in a subset of SGs during osmotic stress. The absence of cytoplasmic hnRNP A1 in A/A cells correlates with the absence of hnRNP A1 in SGs. Therefore, eIF2α phosphorylation may inhibit translation of a group of anti-apoptotic mRNAs via the hnRNP A1-mediated sequestration of the mRNAs in SGs.
FIGURE 6.
Osmotic stress induces eIF2α-dependent localization of hnRNP A1 in SGs. S/S and A/A MEFs cultured on glass coverslips were left untreated (Control) or exposed to osmotic stress for 3 h (Hypertonic). Cells were fixed and immunostained for HuR (A), hnRNP A1 (B), or TIAR and hnRNP A1 (C) to visualize formation of SGs (A and C) and hnRNP A1 subcellular localization (B and C) as described under “Experimental Procedures.” Nuclei were visualized with DAPI. Arrowheads in C show cells with SGs that contain both TIAR and hnRNP A1.
Hypertonic Stress Activates TonEBP in S/S and A/A Cells
The adaptive response to hypertonic conditions involves the Ras-MAPK cascade and activation of TonEBP (40). TonEBP is found in the nucleus and the cytoplasm of normal cells, but hypertonic conditions cause its phosphorylation and nuclear translocation (1). Treatment of S/S and A/A cells with hypertonic medium increased both extracellular signal-regulated kinase (ERK) phosphorylation and TonEBP nuclear translocation (supplemental Fig. 2, A and B). In agreement with the nuclear translocation of TonEBP, transcription of the target gene HSP70 was increased in both cell types (Fig. 3E). These data suggest that signaling through TonEBP in response to osmotic stress is independent of eIF2α phosphorylation.
Signaling Pathways of the Unfolded Protein Response Are Not Fully Activated by Hypertonic Stress
eIF2α phosphorylation is induced by a variety of stresses, including unfolded proteins in the endoplasmic reticulum and amino acid starvation (41). Moreover, the unfolded protein response (UPR) is known to induce apoptosis (42, 43). eIF2α phosphorylation causes increased translation of the mRNA for the transcription factor ATF4, which in turn induces transcription of genes that encode pro-survival or pro-apoptotic proteins (44). Hypertonic stress increased ATF4 levels in S/S cells and induced mRNA levels for the downstream targets of ATF4: ATF3, and CHOP (supplemental Fig. 3A and data not shown). However, other features of the response to hypertonic stress are distinct from the response to other stresses that also induce eIF2α phosphorylation and ATF4. The ATF4 target genes ATF3 and CHOP were not induced by hypertonic stress at the protein level but were induced by thapsigargin, which triggers the UPR (supplemental Fig. 3A). The absence of induction of ATF4-target proteins during hypertonic stress can be explained by the inability of the newly transcribed mRNAs to be translated during this stress condition. In contrast, during the UPR, newly transcribed mRNAs are translated due to translational recovery that follows translational repression (45). Therefore, the observed differences in the cellular response between the two stress conditions can be explained by the sustained inhibition of global protein synthesis during hypertonic stress, in contrast to translational recovery during the UPR. Other differences between the response to hypertonic stress and the UPR are as follows: (i) the spliced XBP1 isoform (XBP1s) that results from UPR-induced RNA splicing was not observed in hypertonic stress (supplemental Fig. 3A); (ii) the transcription factor C/EBPβ, which is induced in the UPR, showed a gradual decline during osmotic stress (supplemental Fig. 3A); and (iii) eIF2α phosphorylation is required for caspase 3 cleavage during osmotic stress but not during the UPR (Fig. 1B and supplemental Fig. 3B). Moreover, ATF4 is not required for induction of apoptosis by hypertonic stress because apoptosis was induced in ATF4−/− cells (not shown). Therefore, we conclude that hypertonic stress induces apoptosis via a novel mechanism that requires eIF2α phosphorylation but not ATF4 induction.
Absence of eIF2α Phosphorylation Protects Cortical Neurons from Apoptosis
The Bcl-2 family members play an important role in neuronal survival during stress conditions (46). We used primary cortical neurons as a model to demonstrate the physiological significance of our findings. We therefore tested whether induction of apoptosis by hypertonic stress depends on eIF2α phosphorylation. Because A/A mice die within a few hours after birth (9), we cultured neurons from 16-day embryos and treated them with hypertonic media after 7 days in culture. S/S and A/A neurons showed similar morphology and neurite outgrowth, suggesting that eIF2α phosphorylation is not important for cortical neuron differentiation (supplemental Fig. 5). A/A neurons were protected from hypertonic stress-induced apoptosis. TUNEL staining was seen in 22% of S/S neurons compared with 11% of the A/A neurons (Fig. 7A). In addition, transfected FLAG-tagged hnRNP A1 redistributed in the cytoplasm of S/S but not A/A neurons during stress (supplemental Fig. 5), similar to MEFs (Fig. 4B). These data suggest that the eIF2α phosphorylation-dependent cytoplasmic accumulation of hnRNP A1 during hypertonic stress is linked with increased apoptosis in cortical neurons. These findings highlight the potential importance of eIF2α phosphorylation in neuronal cell survival during different stress or pathological conditions.
FIGURE 7.
Primary cortical neurons from A/A mice are protected from apoptosis induced by severe hypertonic stress. A, cultured primary cortical neurons from S/S and A/A mice were cultured for 7 days and treated for 5 h with hypertonic medium, and TUNEL staining was performed and quantified as described under “Experimental Procedures.” Representative confocal images are shown. Bars, 100 μm. B, schematic representation of the importance of eIF2α phosphorylation in shifting the balance toward apoptosis during severe osmotic stress.
DISCUSSION
Acute hypertonic conditions cause cell shrinkage and induce an adaptive response that leads to volume recovery. However, severe and prolonged hypertonic stress induces apoptosis. We show here that the balance between survival and apoptosis involves signaling mediated by phosphorylation of the translation initiation factor eIF2α (summarized in Fig. 7B). A critical factor that shifts the balance from survival to apoptosis is the eIF2α phosphorylation-dependent accumulation of hnRNP A1 in cytoplasmic SGs. hnRNP A1 is likely to sequester anti-apoptotic mRNAs in SGs, leading to their translational attenuation. This will shift the balance toward activation of the execution caspases and cleavage of survival factors, among them members of the eIF4G family (data not shown). These findings challenge the current view (11, 12) that induction of apoptosis during osmotic stress is due to depletion of short lived anti-apoptotic proteins caused by the inhibition of global protein synthesis that is triggered by eIF2α phosphorylation (11).
The phosphorylation of eIF2α on Ser51 is an important regulator of the decision between survival and apoptosis in response to diverse stress conditions (44). This phosphorylation is carried out by four stress-regulated kinases (GCN2, PERK, PKR, and HRI), suggesting that eIF2α is a master regulator of the stress response (44). The initial response to eIF2α phosphorylation is inhibition of global protein synthesis that may have protective (47) or proapoptotic functions. In most stress conditions that activate PKR (viral infection, UV irradiation, exposure to interferon, or tumor necrosis factor-α), eIF2α phosphorylation has a proapoptotic role (48). This is also supported by several studies showing that dephosphorylation of eIF2α during diverse stress conditions correlates with better survival (49).
The translation inhibition induced by eIF2α phosphorylation has been suggested to induce apoptosis by inhibiting the expression of anti-apoptotic proteins such as the Bcl-2 family member, Mcl-1, which inhibits the proapoptotic proteins Bax and Bak (50). It was suggested that down-regulation of Mcl-1 enables but does not cause apoptosis and that eIF2α phosphorylation induced by osmotic stress inhibits Mcl-1 mRNA translation and causes mitochondrial apoptosis (11). However, we have shown that Mcl-1 mRNA translation was repressed in both S/S and A/A cells, suggesting that decreased Mcl-1 protein levels do not induce apoptosis. We show here that the eIF2α phosphorylation-mediated shift to apoptosis involves reduced translation of the IRES-containing Bcl-xL mRNA.
Regulation of IRES-mediated translation can play a critical role in cell fate during severe stress by controlling the synthesis of pro-apoptotic or anti-apoptotic proteins. IRES-mediated translation of these mRNAs is regulated by positive and negative factors. The mRNA-binding protein hnRNP A1 has been shown to inhibit translation of the prototypic member of the inhibitors of apoptosis proteins, XIAP, by binding and decreasing the activity of the XIAP IRES. Osmotic stress was shown to increase the cytoplasmic accumulation of hnRNP A1, thus inhibiting translation of the XIAP mRNA (16). We show here that hnRNP A1 also represses the activity of the Bcl-xL IRES, in agreement with the decreased translation efficiency of this mRNA during osmotic stress in S/S cells. The eIF4G member DAP5 has also been shown to positively regulate XIAP (30) and Bcl-xL (51) IRES activities. Because DAP5 is cleaved during osmotic stress and hnRNP A1 cytoplasmic levels increase, the balance between positive and negative regulators may favor attenuation of translation. These examples underscore the complex regulation of IRES-mediated translation and show how the balance between positive and negative IRES trans-acting factors leads to regulation during stress.
Our studies pose the following important question. How does eIF2α phosphorylation regulate hnRNP A1 nucleocytoplasmic trafficking? eIF2α phosphorylation could enhance nuclear export or prevent nuclear import, possibly by stimulating phosphorylation in the cytoplasm. Phosphorylation of hnRNP A1 during osmotic stress is carried out by the p38/Mnk-1 pathway (35). Because both kinases were activated in S/S and A/A cells during osmotic stress (supplemental Fig. 2C), additional factors should contribute to its cytoplasmic retention. The latter could involve post-translational modifications, such as phosphorylation, ubiquitination, sumoylation, and methylation that occur on hnRNP A1. Methylation of hnRNP proteins has been associated with their nuclear export (52), and several arginines within the RGG repeats of hnRNP A1 are methylated (34). We are currently testing the hypothesis that a post-translational modification of hnRNP A1 in the nucleus controls its nuclear exit and/or phosphorylation in the cytoplasm.
A striking finding of our studies was that cytoplasmic hnRNP A1 was required for the induction of apoptosis by osmotic stress. This was shown by the ability of transfected F1 mutant protein, which is constitutively located in the cytoplasm, to enable hypertonic stress-induced apoptosis in A/A cells. This finding is consistent with a report showing that cytoplasmic but not nuclear hnRNP A1 protein causes translational inhibition of IRES-mediated translation (53). We therefore suggest that modulation of IRES-mediated translation by hnRNP A1 during osmotic stress controls cell fate. In addition to its role in mRNA translation, hnRNP A1 increases turnover of some anti-apoptotic mRNAs during stress via the AU-rich elements in their 3′-UTRs (54). Although we did not investigate this mechanism, it is another level of control that modulates the cellular response to stress. Apoptosis in S/S cells is likely to be controlled by several proteins with mRNAs targeted by hnRNP A1, including the anti-apoptotic proteins XIAP (16) and Bcl-xL studied here. The importance of Bcl-xL in protecting cells from apoptosis by inhibiting mitochondrial outer membrane permeabilization and cytochrome c release has been described previously (55). Although Bcl-xL was shown to be important for protection of A/A cells from apoptosis during osmotic stress, regulation of Bcl-xL levels cannot solely determine cell fate during osmotic stress (Fig. 5F).
A novel finding of this study is the absence of hnRNP A1 in cytoplasmic SGs in A/A cells during hypertonic stress. hnRNP A1 has been shown to localize in SGs during osmotic stress (36). We show here that the absence of translational repression of hnRNP A1 target mRNAs in A/A cells was not due to their inability to form SGs. However, hnRNP A1 was absent from SGs in A/A cells, suggesting that eIF2α phosphorylation is required for this process. This finding suggests that a group of mRNAs are translationally repressed during osmotic stress by hnRNP-A1-mediated sequestration in SGs. XIAP (16) and Bcl-xL (this study) are two candidate mRNAs for this regulation.
In contrast to the proapoptotic function of hnRNP A1 described here, Caceres and co-workers (36) demonstrated a protective role of this protein during osmotic stress by mechanisms that involve both its nuclear and cytoplasmic functions. Because hnRNP A1 has multiple functions in protein synthesis and RNA metabolism, it is likely that one of these functions promotes survival. We suggest that hnRNP A1 promotes apoptosis by inhibiting the IRES-mediated translation of a group of anti-apoptotic mRNAs. This experimental approach provides a system for studying how osmotic stress modulates the diverse functions of hnRNP A1.
The goal of osmoadaptation to hypertonic or hypotonic conditions is the recovery of cell volume (1). Mild hypertonic stress induces transcription of osmoprotective genes via increased levels and nuclear localization of TonEBP and translation of the induced mRNAs. Significantly, this response does not involve increased eIF2α phosphorylation (14) or sustained decrease of global protein synthesis (56), consistent with the translation of the induced stress response mRNAs. In contrast, we show that severe osmotic stress induces eIF2α phosphorylation and inhibits osmoadaptation by inhibiting global protein synthesis and attenuating the anti-apoptotic program. This response guarantees elimination of severely damaged cells. It is noteworthy that A/A cells escape apoptosis during severe osmotic stress. Because global protein synthesis is inhibited in these cells, it is likely that survival requires IRES-containing mRNAs that are translated efficiently in A/A but not S/S cells. The identification of mRNAs that are differentially translated in S/S and A/A cells during severe osmotic stress will shed light on the role of eIF2α phosphorylation in the induction of apoptosis.
We used primary cortical neurons from S/S and A/A mouse embryos to demonstrate the importance of eIF2α phosphorylation and hnRNP A1 localization in the response to severe osmotic stress. Neurons were chosen as a model because the intrinsic pathway of apoptosis is very important for neuronal apoptosis in late embryonic development (4, 57). We show here that cerebral cortex progenitor cells do not require eIF2α phosphorylation for differentiation in culture. However, severe hypertonic stress, which also causes mitochondrial apoptosis, required eIF2α phosphorylation for the induction of neuronal apoptosis. It will be interesting to determine the importance of eIF2α phosphorylation in neuronal apoptosis during embryogenesis.
The findings in this study may have far-reaching applications in the treatment of human diseases, including viral infection and cancer. In agreement with our model, infection with vesicular stomatitis virus causes cytoplasmic accumulation of hnRNP A1, leading to induction of apoptosis (58). In addition, hnRNP A1 levels and cytoplasmic accumulation of hnRNP A1 increase in several cancers, but the subcellular distribution of the protein has not been correlated with survival of cancer cells. Intriguingly, the p38 signaling during osmotic stress that mediates cytoplasmic hnRNP A1 phosphorylation is also important for induction of apoptosis in cancer cells treated with chemotherapeutic drugs. It is therefore likely that phosphorylation of eIF2α plays an important role in regulating the balance between survival and apoptosis in a variety of physiological stresses via modulation of nucleocytoplasmic trafficking of hnRNP A1.
Supplementary Material
Acknowledgments
We thank Drs. Anton Komar, Calin-Bogdan Chiribau, Renata Franchi-Gazzola, and Ovidio Bussolati for stimulating discussions. We also thank Dr. H. Moo Kwon for antibodies to TonEBP and Dr. Jerry Pelletier for the eIF2α S51A and S51D expression vectors. We also want to thank Dr. Samantha Morley for assisting us with the immunofluorescence microscopy.
This work was supported, in whole or in part, by National Institutes of Health Grants DK60596 and DK53307 (to M. H.) and DK42304, HL52173, and HL057346 (to R. J. K.). This work was also supported by the Canadian Cancer Society Research Institute Grant CCRSI 17285 (to A. E. K.) and the Canadian Institutes of Health Research Grants MOP 89737 and FRN 74740 (to M. H.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–5 and Tables I and II.
- eIF2
- eukaryotic initiation factor 2
- hnRNP A1
- heterogeneous nuclear ribonucleoprotein A1
- IRES
- internal ribosome entry site
- SG
- stress granule
- MEF
- mouse embryonic fibroblast
- UTR
- untranslated region
- RT-qPCR
- reverse transcription-quantitative PCR
- TUNEL
- terminal dUTP nick end-labeling
- HA
- hemagglutinin
- GFP
- green fluorescent protein
- DAPI
- 4′,6-diamidino-2-phenylindole
- WT
- wild type
- XIAP
- X-linked inhibitor of apoptosis
- UPR
- unfolded protein response.
REFERENCES
- 1.Burg M. B., Ferraris J. D., Dmitrieva N. I. (2007) Physiol. Rev. 87, 1441–1474 [DOI] [PubMed] [Google Scholar]
- 2.Go W. Y., Liu X., Roti M. A., Liu F., Ho S. N. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 10673–10678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maallem S., Mutin M., González-González I. M., Zafra F., Tappaz M. L. (2008) Neuroscience 153, 95–107 [DOI] [PubMed] [Google Scholar]
- 4.Loyher M. L., Mutin M., Woo S. K., Kwon H. M., Tappaz M. L. (2004) Neuroscience 124, 89–104 [DOI] [PubMed] [Google Scholar]
- 5.Lin M., Liu S. J., Lim I. T. (2005) Emerg. Med. Clin. North Am. 23, 749–770, ix [DOI] [PubMed] [Google Scholar]
- 6.Graber T. E., Holcik M. (2007) Mol. Biosyst. 3, 825–834 [DOI] [PubMed] [Google Scholar]
- 7.Komar A. A., Hatzoglou M. (2005) J. Biol. Chem. 280, 23425–23428 [DOI] [PubMed] [Google Scholar]
- 8.Silvera D., Arju R., Darvishian F., Levine P. H., Zolfaghari L., Goldberg J., Hochman T., Formenti S. C., Schneider R. J. (2009) Nat. Cell Biol. 11, 903–908 [DOI] [PubMed] [Google Scholar]
- 9.Scheuner D., Song B., McEwen E., Liu C., Laybutt R., Gillespie P., Saunders T., Bonner-Weir S., Kaufman R. J. (2001) Mol. Cell 7, 1165–1176 [DOI] [PubMed] [Google Scholar]
- 10.Rutkowski D. T., Arnold S. M., Miller C. N., Wu J., Li J., Gunnison K. M., Mori K., Sadighi Akha A. A., Raden D., Kaufman R. J. (2006) PLoS Biol. 4, e374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fritsch R. M., Schneider G., Saur D., Scheibel M., Schmid R. M. (2007) J. Biol. Chem. 282, 22551–22562 [DOI] [PubMed] [Google Scholar]
- 12.Scheuner D., Patel R., Wang F., Lee K., Kumar K., Wu J., Nilsson A., Karin M., Kaufman R. J. (2006) J. Biol. Chem. 281, 21458–21468 [DOI] [PubMed] [Google Scholar]
- 13.Spriggs K. A., Bushell M., Mitchell S. A., Willis A. E. (2005) Cell Death Differ. 12, 585–591 [DOI] [PubMed] [Google Scholar]
- 14.Gaccioli F., Huang C. C., Wang C., Bevilacqua E., Franchi-Gazzola R., Gazzola G. C., Bussolati O., Snider M. D., Hatzoglou M. (2006) J. Biol. Chem. 281, 17929–17940 [DOI] [PubMed] [Google Scholar]
- 15.Kaech S., Banker G. (2006) Nat. Protoc. 1, 2406–2415 [DOI] [PubMed] [Google Scholar]
- 16.Lewis S. M., Veyrier A., Hosszu Ungureanu N., Bonnal S., Vagner S., Holcik M. (2007) Mol. Biol. Cell 18, 1302–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holcik M., Lefebvre C., Yeh C., Chow T., Korneluk R. G. (1999) Nat. Cell Biol. 1, 190–192 [DOI] [PubMed] [Google Scholar]
- 18.Yoon A., Peng G., Brandenburger Y., Brandenburg Y., Zollo O., Xu W., Rego E., Ruggero D. (2006) Science 312, 902–906 [DOI] [PubMed] [Google Scholar]
- 19.Fernandez J., Yaman I., Mishra R., Merrick W. C., Snider M. D., Lamers W. H., Hatzoglou M. (2001) J. Biol. Chem. 276, 12285–12291 [DOI] [PubMed] [Google Scholar]
- 20.Franchi-Gazzola R., Visigalli R., Dall'Asta V., Sala R., Woo S. K., Kwon H. M., Gazzola G. C., Bussolati O. (2001) Am. J. Physiol. Cell Physiol. 280, C1465–C1474 [DOI] [PubMed] [Google Scholar]
- 21.Majumder M., Yaman I., Gaccioli F., Zeenko V. V., Wang C., Caprara M. G., Venema R. C., Komar A. A., Snider M. D., Hatzoglou M. (2009) Mol. Cell. Biol. 29, 2899–2912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y., Bevilacqua E., Chiribau C. B., Majumder M., Wang C., Croniger C. M., Snider M. D., Johnson P. F., Hatzoglou M. (2008) J. Biol. Chem. 283, 22443–22456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aulak K. S., Mishra R., Zhou L., Hyatt S. L., de Jonge W., Lamers W., Snider M., Hatzoglou M. (1999) J. Biol. Chem. 274, 30424–30432 [DOI] [PubMed] [Google Scholar]
- 24.Harding H. P., Novoa I., Bertolotti A., Zeng H., Zhang Y., Urano F., Jousse C., Ron D. (2001) Cold Spring Harbor Symp. Quant. Biol. 66, 499–508 [DOI] [PubMed] [Google Scholar]
- 25.Morley S. J., Naegele S. (2002) J. Biol. Chem. 277, 32855–32859 [DOI] [PubMed] [Google Scholar]
- 26.Hay N., Sonenberg N. (2004) Genes Dev. 18, 1926–1945 [DOI] [PubMed] [Google Scholar]
- 27.Yamasaki S., Ivanov P., Hu G. F., Anderson P. (2009) J. Cell Biol. 185, 35–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Danial N. N., Korsmeyer S. J. (2004) Cell 116, 205–219 [DOI] [PubMed] [Google Scholar]
- 29.Srinivasula S. M., Ashwell J. D. (2008) Mol. Cell 30, 123–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henis-Korenblit S., Shani G., Sines T., Marash L., Shohat G., Kimchi A. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 5400–5405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marash L., Kimchi A. (2005) Cell Death Differ. 12, 554–562 [DOI] [PubMed] [Google Scholar]
- 32.Lewis S. M., Cerquozzi S., Graber T. E., Ungureanu N. H., Andrews M., Holcik M. (2008) Nucleic Acids Res. 36, 168–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Henis-Korenblit S., Strumpf N. L., Goldstaub D., Kimchi A. (2000) Mol. Cell. Biol. 20, 496–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Allemand E., Guil S., Myers M., Moscat J., Cáceres J. F., Krainer A. R. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 3605–3610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van der Houven van Oordt W., Diaz-Meco M. T., Lozano J., Krainer A. R., Moscat J., Cáceres J. F. (2000) J. Cell Biol. 149, 307–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guil S., Long J. C., Cáceres J. F. (2006) Mol. Cell. Biol. 26, 5744–5758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kedersha N., Anderson P. (2002) Biochem. Soc. Trans. 30, 963–969 [DOI] [PubMed] [Google Scholar]
- 38.Kedersha N., Stoecklin G., Ayodele M., Yacono P., Lykke-Andersen J., Fritzler M. J., Scheuner D., Kaufman R. J., Golan D. E., Anderson P. (2005) J. Cell Biol. 169, 871–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kimball S. R., Horetsky R. L., Ron D., Jefferson L. S., Harding H. P. (2003) Am. J. Physiol. Cell Physiol 284, C273–C284 [DOI] [PubMed] [Google Scholar]
- 40.Jeon U. S., Kim J. A., Sheen M. R., Kwon H. M. (2006) Acta Physiol. 187, 241–247 [DOI] [PubMed] [Google Scholar]
- 41.Zhang P., McGrath B. C., Reinert J., Olsen D. S., Lei L., Gill S., Wek S. A., Vattem K. M., Wek R. C., Kimball S. R., Jefferson L. S., Cavener D. R. (2002) Mol. Cell. Biol. 22, 6681–6688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rao R. V., Ellerby H. M., Bredesen D. E. (2004) Cell Death Differ. 11, 372–380 [DOI] [PubMed] [Google Scholar]
- 43.Urano F., Wang X., Bertolotti A., Zhang Y., Chung P., Harding H. P., Ron D. (2000) Science 287, 664–666 [DOI] [PubMed] [Google Scholar]
- 44.Wek R. C., Jiang H. Y., Anthony T. G. (2006) Biochem. Soc. Trans. 34, 7–11 [DOI] [PubMed] [Google Scholar]
- 45.Novoa I., Zhang Y., Zeng H., Jungreis R., Harding H. P., Ron D. (2003) EMBO J. 22, 1180–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benn S. C., Woolf C. J. (2004) Nat. Rev. Neurosci. 5, 686–700 [DOI] [PubMed] [Google Scholar]
- 47.Jiang H. Y., Wek S. A., McGrath B. C., Scheuner D., Kaufman R. J., Cavener D. R., Wek R. C. (2003) Mol. Cell. Biol. 23, 5651–5663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gil J., Esteban M. (2000) Apoptosis 5, 107–114 [DOI] [PubMed] [Google Scholar]
- 49.Boyce M., Bryant K. F., Jousse C., Long K., Harding H. P., Scheuner D., Kaufman R. J., Ma D., Coen D. M., Ron D., Yuan J. (2005) Science 307, 935–939 [DOI] [PubMed] [Google Scholar]
- 50.Kim H., Rafiuddin-Shah M., Tu H. C., Jeffers J. R., Zambetti G. P., Hsieh J. J., Cheng E. H. (2006) Nat. Cell Biol. 8, 1348–1358 [DOI] [PubMed] [Google Scholar]
- 51.Liberman N., Marash L., Kimchi A. (2009) Cell Cycle 8, 204–209 [DOI] [PubMed] [Google Scholar]
- 52.Bedford M. T., Richard S. (2005) Mol. Cell 18, 263–272 [DOI] [PubMed] [Google Scholar]
- 53.Cammas A., Pileur F., Bonnal S., Lewis S. M., Lévêque N., Holcik M., Vagner S. (2007) Mol. Biol. Cell 18, 5048–5059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao T. T., Graber T. E., Jordan L. E., Cloutier M., Lewis S. M., Goulet I., Côté J., Holcik M. (2009) Cell Death Differ. 16, 244–252 [DOI] [PubMed] [Google Scholar]
- 55.Chipuk J. E., Bouchier-Hayes L., Green D. R. (2006) Cell Death Differ. 13, 1396–1402 [DOI] [PubMed] [Google Scholar]
- 56.Brigotti M., Petronini P. G., Carnicelli D., Alfieri R. R., Bonelli M. A., Borghetti A. F., Wheeler K. P. (2003) Biochem. J. 369, 369–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Johnson C. E., Huang Y. Y., Parrish A. B., Smith M. I., Vaughn A. E., Zhang Q., Wright K. M., Van Dyke T., Wechsler-Reya R. J., Kornbluth S., Deshmukh M. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 20820–20825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pettit Kneller E. L., Connor J. H., Lyles D. S. (2009) J. Virol. 83, 770–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.