Abstract
The microtubule-associated protein Tau plays a crucial role in regulating the dynamic stability of microtubules during neuronal development and synaptic transmission. In a group of neurodegenerative diseases, such as Alzheimer disease and other tauopathies, conformational changes in Tau are associated with the initial stages of disease pathology. Folding of Tau into the MC1 conformation, where the amino acids at residues 7–9 interact with residues 312–342, is one of the earliest pathological alterations of Tau in Alzheimer disease. The mechanism of this conformational change in Tau and the subsequent effect on function and association to microtubules is largely unknown. Recent work by our group and others suggests that members of the Hsp70 family play a significant role in Tau regulation. Our new findings suggest that heat shock cognate (Hsc) 70 facilitates Tau-mediated microtubule polymerization. The association of Hsc70 with Tau was rapidly enhanced following treatment with microtubule-destabilizing agents. The fate of Tau released from the microtubule was found to be dependent on ATPase activity of Hsc70. Microtubule destabilization also rapidly increased the MC1 folded conformation of Tau. An in vitro assay suggests that Hsc70 facilitates formation of MC1 Tau. However, in a hyperphosphorylating environment, the formation of MC1 was abrogated, but Hsc70 binding to Tau was enhanced. Thus, under normal circumstances, MC1 formation may be a protective conformation facilitated by Hsc70. However, in a diseased environment, Hsc70 may preserve Tau in a more unstructured state, perhaps facilitating its pathogenicity.
Keywords: Aging, Chaperone Chaperonin, Heat Shock Protein, Microtubules, Phosphorylation Enzymes, Tau
Introduction
Deposition of Tau after its sequestration from microtubules is a neuropathological hallmark of a group of diseases termed tauopathies. This family of diseases includes Alzheimer disease (AD),3 hereditary frontotemporal dementia with parkinsonism linked to chromosome 17, progressive supranuclear palsy, and corticobasal degeneration (1–4). The Tau protein is normally expressed in the cytoplasm of neurons and performs the critical function of stabilizing and maintaining the microtubule networks that are essential for axonal transport. During the development of Tau pathology, Tau dissociates from microtubules, and unknown factors converge to cause abnormal polymerization of Tau into highly insoluble paired helical filaments (PHF) and forms neurofibillary tangles. The number of neurofibillary tangles directly correlates with the degree of dementia seen in the progressing stages of AD (5, 6); however, it is now speculated that it is intermediate species of Tau rather than the pathologically visible tangles that are the most toxic (7, 8). It has been hypothesized that in disease, hyperphosphorylation and conformational changes lead to the loss of function and pathogenic assembly of Tau (9–11). This idea of Tau adopting a conformation, or “fold,” is somewhat paradoxical because Tau is almost entirely an unstructured protein. However, evidence for a folded Tau species emerged from studies that used paired helical filaments from AD brain as haptens for monoclonal antibody production (10–12). Several antibodies were generated that had discontinuous epitopes within the Tau molecule. One of these antibodies, MC1, recognizes Tau when its N terminus is positioned near residues of the ninth exon. Pathological studies with this antibody revealed that formation of this species was an early event in Tau pathology in AD (10–12). Thus, detection of Tau in the MC1 conformation could result from either folding of a single Tau molecule, or it could be due to anti-parallel stacking/filament formation of Tau.
The emergence of molecular chaperones as key regulators of Tau processing suggests that conformational changes to the structure of Tau may indeed be key events in the pathogenesis of AD and other tauopathies (13–17). Aggregation of Tau in vitro is negligible and typically requires accelerants such as heparin (18, 19), an indication that conformation plays an important role in its assembly. In a cellular environment, post-translational processing of Tau is likely essential for facilitating its aggregation, and certainly this could be regulated by the chaperone network. One of the first described chaperone regulators of Tau is the Hsp70 chaperone family. This family is comprised of 13 members; however, the cytoplasm is predominated by two members, the constitutive heat shock cognate (Hsc) 70 protein and the inducible Hsp70. Hsc70 and Hsp70 share 92% primary sequence homology. Both have highly conserved N-terminal ATPase domains as well as substrate-binding domains located just upstream of a more variable/regulatory domain (20). The cyto-protective role of Hsc70 may be particularly relevant in neurons where an attenuated Hsp70 response has been correlated with aging. Hsc70 and Hsp70 can each bind Tau in the nervous system, but endogenous Hsc70 is typically in much higher abundance than Hsp70 in the cytosol (21, 22). Based on previous work, we hypothesized that the highly abundant Hsc70 protein would be the most likely member of the Hsp70 family to regulate Tau processing. We also speculated that the time when Hsc70 would be most engaged with Tau would be following its release from microtubules (17). Lastly, we investigated what affect Hsc70 might have on Tau in distinct cellular environments. For the first time, we have connected microtubule dysfunction with chaperone-mediated regulation of Tau, as well as conformational changes to and hyperphosphorylation of Tau in a comprehensive way. These findings could be useful for implementing therapeutic strategies based on Hsc70, given its apparent early involvement in Tau pathogenicity. These studies may also point to a mechanism of how Tau dysfunction begins in AD and other tauopathies.
MATERIALS AND METHODS
Antibodies and Chemicals
MC1 and PHF1 (anti-S396/S404 p-Tau) were provided by P. Davies (Albert Einstein College of Medicine, Yeshiva University, New York, NY). Anti-Hsc70 was obtained from Stressgen Biotechnologies (Ann Arbor, MI). Anti-actin was obtained from Sigma-Aldrich. Anti-Tau (total Tau) was obtained from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). Secondary antibodies were obtained from Southern Biotech (Birmingham, AL). All of the antibodies were used at a 1:1,000 dilution with the exception of PHF1, which was used at a dilution of 1:200. Secondary antibodies conjugated to fluorophore were purchased from AlexaFluor (Molecular Probes). Albendazole, colchicine, paclitaxel, okadaic acid, and phenothiazine (methylene blue) were purchased from Sigma. Nocodazole was purchased from Tocris Bioscience.
Recombinant Protein Purification
Wild type 4R Tau and Hsc70 gene sequences cloned into pET28 vector with His tag and were transformed into the Escherichia coli strain BL21 (DE3) codon Plus. Bacterial culture condition and protein purification protocol were followed as described earlier (23). The purity of all proteins was verified on a Coomassie Brilliant Blue-stained SDS-polyacrylamide gel.
Xenopus Oocytes
S phase Xenopus egg extracts were prepared according to a standard protocol (24) and supplemented with 5% Me2SO to stimulate microtubule polymerization as described (23). Purified recombinant wild type Tau and Hsc70 proteins were added at a final concentration of 80 ng/μl. The extracts were treated with nocodazole and incubated for 30 min at room temperature, and newly formed microtubule structures were visualized by fluorescence of incorporated rhodamine-tubuline. Image quantification was performed as previously described (25). Briefly, tubulin-positive signal was detected using the same threshold for each image in ImageJ (26, 27), and the fraction of the total area of the field covered by the tubulin signal was collected for statistical analysis.
Cell Culture and Western Blot Analysis
HeLa cells stably transfected with V5-tagged 4R0N Tau were generated using G418 selection. HeLa cells were grown in Opti-Mem plus 10% fetal bovine serum (complete media; Invitrogen) and passaged every 3–5 days based on 90% confluence. IMR32 cells were maintained in Opti-Mem plus 10% fetal bovine serum and 2% of 200 mm l-glutamine (Cellgro, Mediatech, Inc., Herndon, VA). The cells were harvested in M-PER buffer (Pierce) containing 1× protease inhibitor mixture (Calbiochem), 1 mm phenylmethylsulfonyl fluoride, and 1× phosphatase inhibitor I and II cocktails (Sigma). The measurements of Tau levels in cell culture were performed by Western blot analysis. Plasmid transfections were done utilizing Lipofectamine 2000 reagent from Invitrogen. HeLa cells stably transfected with V5-tagged 4R human Tau were transfected with 3 μg of DNA. The cells were incubated for 4 h with the Lipofectamine/plasmid mixture in Opti-MEM medium, and this was replaced with fresh complete medium for an additional 44–48 h. For the immunoprecipitation (IP) studies, cell culture lysates were incubated with 2 μg of antibody for 2–3 h at 4 °C with rocking. Then 50 μl of protein G were added and rocked at 4 °C for overnight. The protein G beads were pelleted and washed five times with phosphate-buffered saline.
Drug Preparations
All of the drugs for the cell culture experiments were solubilized in Me2SO to get a 20 mm stock. For the in vivo study in mice, albendazole used in the hippocampal injections in Fig. 3C was prepared by dissolving 3 g of albendazole in 40% hydroxypropyl-β-cyclodextrin in water.
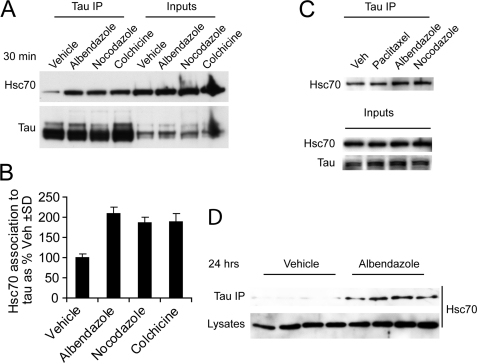
FIGURE 3.
In vitro and in vivo enhanced association of Hsc70 to microtubule-destabilized Tau. A, IMR32 cells treated with microtubule-destabilizing agents albendazole, nocodazole, colchicine or vehicle Me2SO as a control for 30 min. Treated cell lysates were immunoprecipitated (IP) with total Tau antibody and immunoblotted with Hsc70 antibody. B, quantitation of immunoblot from A after normalization to corresponding inputs shows increased binding of Hsc70 to Tau upon destabilization of microtubules by various drugs compared with vehicle control. The values are presented as Hsc70 association to Tau as a percentage of vehicle-treated cells ± S.D. C, In HeLa cells stably transfected with Tau were treated with microtubule stabilizer paclitaxel and destabilizer albendazole or nocodazole for 30 min. An IP with total Tau antibody followed by immunoblotting with Hsc70 antibody, showed only increased association of Hsc70 with Tau upon treatment with microtubule destabilizer not with stabilizer treatment. D, in vivo study was performed in mice. Brain hippocampus of mice (n = 4) were injected with albendazole or vehicle as a control. 24 h after injection, the mouse brains were homogenized. An IP with total Tau antibody followed by immunoblot analysis showed significant increased of Tau association to Hsc70 upon treatment with albendazole compared with vehicle-injected animals.
Immunocytochemistry
IMR32 cells were plated onto a chamber slide (Fisher) and maintained in the medium described above. The cells were treated with nocodazole for 30 min and were fixed in high pressure liquid chromatography grade methanol for 15 min. Blocking and permeabilization were done by incubating samples with Tris-buffered saline containing 5% normal goat serum, 0.1% Tween 20, and 0.03 NaN3 for 60 min at 25 °C. Primary antibodies rabbit anti-Tau and rat anti-Hsc70 (1:100) were added and incubated overnight at 4 °C. The slide was washed five times for 5 min in 1× Tris-buffered saline. Secondary antibodies AlexaFluor 488 (anti-rabbit; 1:1000) and 594 (anti-rat; 1:1000) were added to the slides and incubated for 60 min at 25 °C. The slides were washed and coverslipped. A Leica confocal microscope was used to take images. Significance was assessed by Pearson's correlation coefficient.
Albendazole Injections in Mouse Brains
All of the procedures using mice were done in accord with the guidelines set forth by the University of South Florida's Institutional Animal Care and Use Committee. The wild type FVB/N background mice brain hippocampus were injected with 2 μl of vehicle and 3 mg/ml albendazole. The surgical procedure was performed as previously described by Carty et al. (28) with some modifications. The mice were anesthetized using isofluorane, and the cranium was exposed with a small incision along the skin covering the medial sagittal plane. Holes were drilled through the cranium over the desired injection sites as measured with a stereotaxic apparatus. Burr holes were drilled using a dental drill bit (SSW HP-3; SSWhiteBurs) or a 21-gauge needle (BD). A 2-μl total volume of vehicle or albendazole were dispensed into the each injection site. The animals were recovered within 10 min of post-surgery and were singly housed. 24 h after the injections, the brains were harvested as described earlier (29) and processed for immunoprecipitation as described above.
Enzyme-linked Immunosorbent Assay
Tau and HSP complex containing Hsc70, DNAJB2, and ATP were preincubated for 30 min. Paraformaldehyde was added into the samples and coated on to 96-well plate for 1 h at 37 °C followed by washing five times with wash buffer containing 0.45% NaCl and 0.05% Tween 20. Blocking of plate was done for 1 h at 37 °C by using starting blocking solution (Pierce and Thermo Fisher Scientific), followed by a wash. MC1 antibody was added at 1:50 dilutions and incubated at 37 °C for 1 h, and the plate was washed again. Horseradish peroxidase-linked secondary antibody was added and incubated at 37 °C for 1 h. The plate was washed. 3,3′,5,5′-Tetramethylbenzidine dihydrochloride (Sigma) solution was added, and the reaction was stopped by adding 2 m H2SO4. The plate was read at 450 nm in spectrophotometer.
RESULTS
Hsc70 Facilitates Tau-mediated Microtubule Stabilization
Previous work from our lab and others suggested that the Hsp70 chaperone network plays a critical role in Tau biology and could be used as a therapeutic target for tauopathies (13, 14, 17, 30, 31). Of the Hsp70 family members, the Hsc70 protein expression level was found to be highest in the neurons from adult brain (21, 32–34). Based on this evidence, we investigated the role of Hsc70 on Tau-mediated microtubule polymerization. We used our recently optimized assay for the analysis of microtubule polymerization in oocyte extract (23). Using purified recombinant Hsc70 and Tau, we incubated Xenopus oocyte extracts with Tau, Hsc70, or a combination of both Tau and Hsc70 for 30 min. These extracts were then treated with 20 μm nocodazole or vehicle for an additional 30 min. Microtubule formation was analyzed using fluorescent microscopy (Fig. 1A). Tubulin-positive signal was detected using the same threshold for each image as determined by ImageJ software (26, 27), and the fraction of the total area of the field covered by the tubulin signal was collected for statistical analysis (Fig. 1B). The extracts incubated with Tau alone and Hsc70 alone showed similar levels of microtubule formation (Fig. 1). However, we observed a significant increase in microtubule formation when Tau was supplemented with Hsc70. Nocodazole was able to abrogate microtubule formation under all the previously mentioned conditions as seen in the representative image (Fig. 1A). These findings suggest that Hsc70 could be a potent enhancer of Tau-mediated microtubule polymerization, suggesting that microtubule dynamics could be critical for facilitating the Tau/Hsc70 interface.
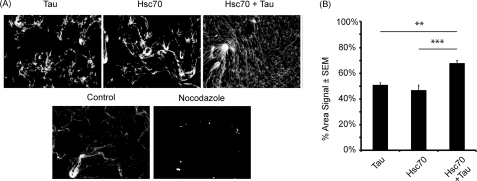
FIGURE 1.
Hsc70 facilitates Tau-mediated microtubule stabilization. A, in vitro microtubule assembly assays were performed using Xenopus oocyte extracts supplemented with rhodamine-labeled tubulin. Recombinant proteins, Tau, Hsc70, or both in combination, were added to the Xenopus extracts, and microtubules were detected with fluorescent microscopy. Control indicates no protein added. As a negative control, microtubules were disrupted using nocodazole in the presence of Hsc70 and Tau. B, microtubules were quantified as the percentage of area distributed throughout the total area of the field; see details under “Materials and Methods” (n = 19; **, p (hsc70 versus both) < 0.001; and ***, p (Tau versus both) < 0.0001).
Tau Associates with Hsc70 upon Microtubule Destabilization
Although Hsc70 facilitated Tau-mediated microtubule assembly in normal cellular oocyte extracts, we found that microtubule destabilization with nocodazole overrode this activity (Fig. 1). Based on this result, we wanted to determine how microtubule destabilization may impact the association of Hsc70 with endogenous Tau in an intact cellular environment. First, we performed fluorescent immunostaining on neuroblastoma cells (IMR32) following microtubule destabilization to determine whether colocalization of these two proteins was increased. The cells were grown on chamber slides and treated with nocodazole for 30 min and then fixed, and the levels of Tau and Hsc70 were assessed by immunofluorescent confocal microscopy. Image analysis showed no significant colocalization between Tau and Hsc70 in the vehicle-treated cells as determined by Pearson's correlation coefficient; however, Hsc70 and Tau were found to be colocalized by Pearson's correlation coefficient after nocodazole treatment (Fig. 2). These data suggested that Hsc70 and Tau do have a greater propensity to interact following microtubule destabilization. However, we wanted to further confirm these data using coimmunoprecipitation studies.
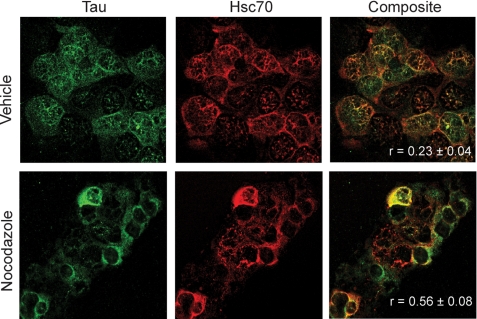
FIGURE 2.
Dramatic increase in Tau colocalization with Hsc70 upon microtubule destabilization. IMR32 cells were treated with 20 μm nocodazole or vehicle control for 30 min. Immunofluorescent staining of Tau (green) and Hsc70 (red) in cells shows enhanced colocalization of these two proteins upon treatment with microtubule destabilize nocodazole.
We tested whether the interaction of Tau with Hsc70 could be enhanced with microtubule destabilization in cells overexpressing Tau, as well as cells with endogenous Tau levels. We treated IMR32 cells that have endogenous levels of Tau with three microtubule-destabilizing drugs: albendazole, nocodazole, and colchicine. These compounds did not increase Hsc70 levels; however, consistent with our colocalization data, they did increase the binding of Tau with HSc70 (Fig. 3, A and B). A similar result was found with HeLa cell overexpressing wild type human Tau (Fig. 3C). As a control in this overexpressing cell model, we also treated with the microtubule stabilizer, paclitaxel, and saw no increase in Tau association to Hsc70 (Fig. 3C). We then tested whether the association of Hsc70 with Tau could be increased by microtubule destabilization in the brain. We injected albendazole and vehicle control into the hippocampal region of mice. Brain tissue was harvested 24 h after injection, and homogenates were analyzed by an IP with Tau antibody followed by Western blot probing with Hsc70 antibody. Again, we found that microtubule destabilization increased the association of Tau with Hsc70 (Fig. 3D).
ATPase Activity of Hsp70/Hsc70 Regulates Tau upon Microtubule Destabilization
Based on these findings and our previous work with Hsp/Hsc70 chemical inhibitors (14), we speculated that chemically inhibiting Hsc70 activity when the number of Hsc70·Tau complexes is highest (after microtubule destabilization) would lead to enhanced efficacy for reducing Tau levels. To determine this, we performed dose-response studies with an Hsp70 ATPase inhibitor (methylene blue) in the presence or absence of a 30-min pretreatment with albendazole. Indeed we found that reductions in both endogenous and overexpressed Tau by Hsc70 inhibition were enhanced following microtubule destabilization in IMR32 cells and HeLa cells, respectively (Fig. 4). These findings provide further evidence that the association of Hsc70 with Tau is rapidly enhanced following microtubule destabilization, which forms more Hsc70·Tau complexes that can be targeted by inhibitors.
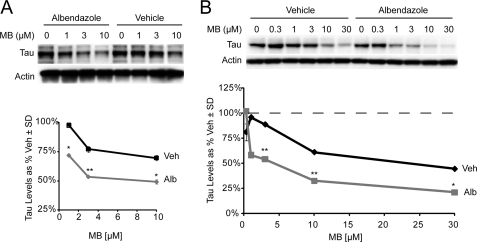
FIGURE 4.
Tau released from microtubules is regulated by ATPase activity of Hsc70. A, IMR32 cells were treated with 20 μm albendazole or vehicle for 30 min, followed by 1 h of treatment with various doses of methylene blue (MB). B, a similar experiment as in A was performed in HeLa cells stably transfected with Tau. Quantitation plots of the Western blots after actin normalization illustrates microtubule destabilization favors further reduction of Tau by the Hsc70 ATPase inhibitor methylene blue. The gray lines depict the efficacy of the Hsp70 complex ATPase modulators alone, and black lines depict the efficacy of the ATPase modulators in conjunction with albendazole treatment. The values are presented as Tau levels as percentages of vehicle-treated cells ± S.D. *, p < 0.05; **, p < 0.001 by Student's t test.
Hsc70 Folds Tau into MC1 Conformation
Based on our results, we hypothesized that Hsc70 could be contributing to some of the early pathological modifications to Tau depending on the cellular environment. Because Hsc70 largely functions as a protein “foldase” (35), and Tau folding is thought to be an early event in disease progression (11, 12, 36), we investigated what impact Hsc70 could be having on Tau folding.
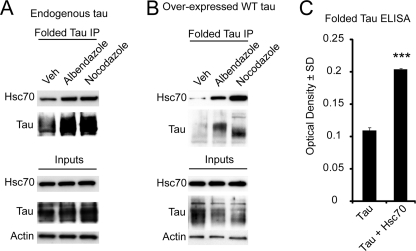
We first tested whether the folding of Tau into the MC1 conformation coincided with Hsc70 binding to Tau. Again we treated two cell models, one with endogenous Tau and the other with overexpressed Tau, with microtubule destabilizers for 30 min and performed IPs with the MC1 antibody, which recognizes folded Tau, and a total Tau antibody. We found that MC1-reactive Tau was increased within 30 min in both cell models, similar to the time frame of Hsc70 binding to Tau (Fig. 5, A and B); however, this effect was more pronounced in cells overexpressing Tau (Fig. 5B). Although this suggested that Hsc70 and Tau folding were occurring in a similar time frame, we still were not certain that Hsc70 was facilitating the MC1 conformation for Tau. To test this more directly, we performed an in vitro enzyme-linked immunosorbent assay experiment using purified recombinant proteins to look for the amount of MC1 reactive Tau in the presence and absence of Hsc70. Impressively, we found more MC1-reactive Tau species when Tau was incubated with Hsc70 versus bovine serum albumin (Fig. 5C). Based on our findings that the MC1 Tau conformation could be rapidly facilitated by Hsc70, we speculated that Tau hyperphosphorylation might be impacted as well when microtubules are destabilized.
FIGURE 5.
Hsc70 folds Tau into MC1 conformation. A, IMR32 cells having endogenous Tau were treated with 20 μm albendazole or nocodazole for 30 min prior to an IP using a MC1 (folded) Tau antibody. An increased binding of Hsc70 to folded Tau was observed upon treatment with drugs. B, HeLa cells stably transfected with Tau (overexpressing Tau) were treated with 20 μm albendazole or nocodazole for 30 min prior to an IP using a MC1 (folded) Tau antibody. An increased binding of Hsc70 to folded Tau was observed upon treatment with drugs. C, an enzyme-linked immunosorbent assay (ELISA) for folding of Tau (folded, MC1) was performed using recombinant Tau and Hsc70 in a buffer containing ATP. The plate was read at 450 nm, and the data are represented as black bars. A significant increase in Tau folding by Hsc70 suggests that Hsc70 folds Tau. The values are presented as Tau levels as percentages of vehicle (Veh)-treated cells ± S.D. ***, p < 0.0001 by Student's t test.
Microtubule Destabilization Affects Tau Phosphorylation Pattern
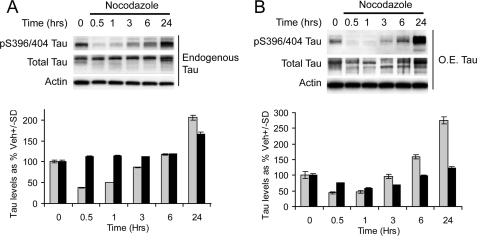
We first investigated the phosphorylation of Tau within the first 24 h following microtubule destabilization. Again we investigated this for both endogenous Tau and overexpressed Tau. Interestingly, we observed an initial decrease in Tau phosphorylation at 30 min, the same time that MC1 Tau formation and Hsc70 binding to Tau were increased. Tau phosphorylation then began to recover over the next several hours and eventually was increased by 24 h in both cell lines (Fig. 6). Total Tau levels remained largely unaffected. Thus, microtubule destabilization decouples Tau phosphorylation from MC1 formation and Hsc70 binding. Perhaps more importantly, MC1 formation and Hsc70 actually preceded endogenous phosphorylation of Tau following microtubule destabilization. This led us to consider what would happen to both the MC1 conformation of Tau and Hsc70 binding to Tau if microtubules were destabilized in the face of aberrant kinase activation.
FIGURE 6.
Microtubule destabilization affects Tau phosphorylation pattern. A, IMR32 cells were treated with nocodazole for 0, 0.5, 1, 3, 6, and 24 h. Tau phosphorylation was found to be reduced initially and in later on phosphorylation was increased. B, HeLa cells stably transfected with Tau were treated with nocodazole for 0, 0.5, 1, 3, 6, and 24 h. Tau phosphorylation was found to be reduced initially, and later on phosphorylation was increased. Quantitation plots of the Western blots after actin normalization illustrates a time-dependent initial reduction and later on up-regulation of Tau phosphorylation. The gray bars represent phospho-Tau, and the black bars represent total Tau. The values are presented as Tau levels as percentages of vehicle-treated cells ± S.D.
A Hyperphosphorylating Environment Blocks Folding of Tau but Enhances Hsc70 Binding
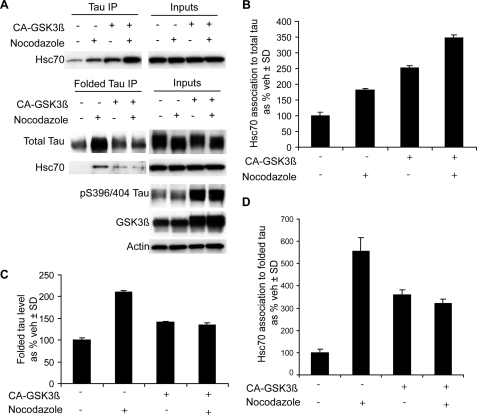
Although we have demonstrated that Hsc70 associates to Tau upon microtubule destabilization, we wanted to determine how this association is affected in a hyperphosphorylating environment. Tau protein can be phosphorylated by a number of proline-directed serine/threonine kinases at several sites. The prevalence and chronology of these phosphorylation events have been rigorously correlated with AD tangle pathology (5, 6). GSK3β is a primary kinase for several key sites that are associated with Tau pathology, and it can lead to Tau hyperphosphorylation (37). Here, we have created a hyperphosphorylating environment for Tau by overexpressing a constitutively active form of GSK3β. Lysates from cells overexpressing either the constitutively active form of GSK3β or empty vector treated with nocodazole for 30 min were analyzed by IP as described above. Although we found that phosphorylation of Tau (as measured by increased immunoreactivity to PHF1 antibody) did increase its association with Hsc70, we were surprised to find that hyperphosphorylation of Tau actually blocked MC1 formation (Fig. 7). Thus, when microtubule destabilization occurs in a hyperphosphorylating environment, MC1 formation is blocked, but Hsc70 binding goes up. In this way, Hsc70 may be preserving hyperphosphorylated Tau in a more exposed, linear conformation that may be more prone to toxic intracellular interactions than it would be in the condensed MC1 conformation.
FIGURE 7.
A hyperphosphorylating environment reduces folding of Tau but enhances Hsc70 binding. A, HeLa cells stably expressing Tau were transfected with and without constitutively active GSK-3β followed by a treatment with nocodazole for 30 min. Two separate IPs were performed: one with total Tau antibody and another with MC1 antibody (folded Tau). B–D, quantitation of the immunoblots from A was performed after input normalization. The values are presented as percentages of vehicle (veh)-treated cells ± S.D. for Hsc70 binding to total Tau (B), total Tau following MC1 IP (C), and Hsc70 binding to MC1 Tau (D).
DISCUSSION
Apart from the known association of the members of the Hsp70 family of chaperones with Tau in the brain, their role in the initiation and progression of AD and other tauopathies is not clear. Here, we aimed to explore the role of the most prevalent cytosolic member of the Hsp70 family, Hsc70, in the early events of Tau pathogenesis. We developed a model using microtubule-destabilizing compounds to explore the consequences of loss of function for Tau and then coupled this with a toxic gain of function by promoting Tau hyperphosphorylation with GSK3β overexpression (14, 38, 39). Our results were surprising: we found that the MC1 conformation of Tau can be decoupled from Tau hyperphosphorylation. Moreover, Hsc70 facilitated MC1 formation while also enhancing Tau-mediated microtubule polymerization. Although MC1 has indeed been shown to be an early pathological structure of Tau pathology, as assessed by post-mortem tissue analysis (10) and a conformation that emerges during fibrillarization (11), our results suggest that there might be multiple phases of MC1 formation: one at the onset of Tau dysfunction, as described here, and others after Tau tangles have been seeded and become self-perpetuating (29, 40).
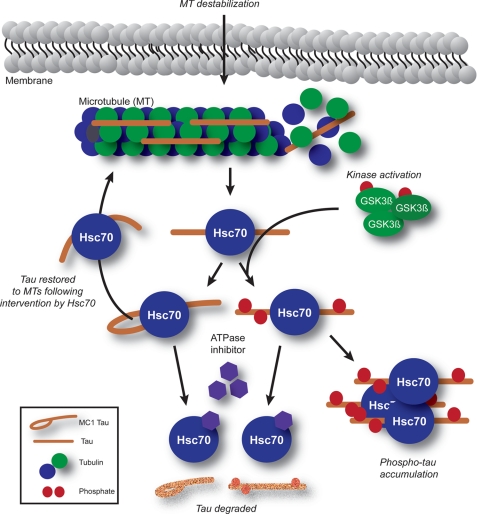
Based on our results, we speculate that Tau folding into the MC1 conformation after microtubule destabilization could be a protective mechanism facilitated by Hsc70 to control the disordered nature of Tau and prevent its self-assembly in the neuron. In fact, this would be expected given the known “foldase” functionality of Hsc70 and previous work showing that it prevents disordered proteins from aggregating (41–44). However, when microtubules are destabilized in a hyperphosphorylating environment, Hsc70 still binds to Tau, but this phospho-Tau can no longer be protectively folded into the MC1 conformation. In this way, Hsc70 may be a double-edged sword; when Tau loses its function, Hsc70 can hold it in a labile folded state, returning it to the microtubule once the insult on microtubule stability has resolved. But when Tau loses its function and gains a toxic modification such as aberrant phoshorylation, Hsc70 still holds Tau but fails to protect it, perhaps inadvertently contributing to the preservation of hyperphosphorylated Tau in the cell (Fig. 8). This notion that unfolded proteins are intrinsically prone to aggregation is an emerging principle for the field of protein chemistry, as recently reviewed (45).
FIGURE 8.
Model for the early stages of Tau dysfunction. Microtubule (MT) destabilization causes rapid association and folding of Hsc70 with Tau. In a hyperphosphorylating environment, as promoted by GSK3β activation, folding is subverted, but Hsc70 binding is enhanced, and Tau accumulates. In a normal environment, Hsc70 may attempt to restore Tau to the microtubule by establishing a protective conformation (in this case, MC1). Inhibition of Hsc70 ATPase function facilitates degradation of either Tau species. In both cases, Hsc70 can facilitate degradation independent of ATPase inhibition; however, the properties of phospho-Tau may make it more prone to aggregation.
This necessity for Tau to both lose its function and gain toxic post-translational modifications could be precisely why single mechanisms linking amyloid accumulation to Tau pathogenesis have been challenging to prove. The Tau dysfunction seen in AD might require a pleiotropic activation strategy, where a number of pathways activated by the pluripotency of amyloid must converge to promote Tau pathogenesis. In fact, amyloid is known to destabilize microtubules and activate kinases (37, 46). Thus, our model may recapitulate some aspects of amyloid-induced Tau pathogenesis and describe the very earliest part of this cascade.
Our study also shows that regardless of the neuronal environment, Hsc70 is engaged with Tau when microtubule stability is altered. Thus, inhibitors of Hsc70 ATPase activity that facilitate the release and degradation of Tau, such as those that we have recently described (14, 35), could be broadly applicable throughout the Tau pathogenic cycle. Although not investigated here, we would anticipate that Hsp90 and other chaperone proteins would also be involved in Tau processing when it disengages from the microtubule. Therefore developing diverse therapeutic strategies aimed at modulating distinct chaperone functions could be highly applicable for AD.
In summary, our data suggest that the dissociation of Tau from microtubules enhances its association with the chaperone network, specifically with Hsc70. This association, as our data suggest, seems to be crucial for Tau folding and restoration of Tau function. However, abnormal phosphorylation of Tau, coupled with its loss of function, circumvents the protective surveillance of Hsc70, and Hsc70 may actually become an unwitting accomplice to Tau pathogenesis by holding onto abnormal Tau. Based on these data, pharmacological manipulation of the Hsp70 family of proteins may prevent the disease progression in certain Tau-related neurodegenerative diseases where microtubule disruption has occurred in a hyperphosphorylating environment, a likely scenario in AD. However, in circumstances where disease arises only from a loss of function for Tau, then inhibiting Hsc70 may accelerate pathogenicity by subverting the attempts of Hsc70 to restore Tau to the microtubule. By understanding the mechanisms involved in Tau pathogenesis, we can continue to identify therapeutic targets and strategies that may be customizable based on disease etiology.
Acknowledgments
We thank Dr. Huntington Potter for providing invaluable insights through discussions. We thank Dr. Peter Davies (Albert Einstein College of Medicine) for PHF1 (pS396/S404) and MC1 Tau antibodies. We thank Dr. Benjamin Wolozin for providing the GSK3β expression plasmid.
This work was supported, in whole or in part, by National Institutes of Health Grants NS059690 (to J. E. G.) and AG031291. This work was also supported by the Rosalinde and Arthur Gilbert Foundation/American Federation for Aging Research and CurePSP.
- AD
- Alzheimer disease
- Hsp
- heat shock protein
- Hsc
- heat shock cognate
- PHF
- paired helical filament(s)
- IP
- immunoprecipitation
- GSK3β
- glycogen synthase kinase 3β.
REFERENCES
- 1.Hutton M., Lendon C. L., Rizzu P., Baker M., Froelich S., Houlden H., Pickering-Brown S., Chakraverty S., Isaacs A., Grover A., Hackett J., Adamson J., Lincoln S., Dickson D., Davies P., Petersen R. C., Stevens M., de Graaff E., Wauters E., van Baren J., Hillebrand M., Joosse M., Kwon J. M., Nowotny P., Che L. K., Norton J., Morris J. C., Reed L. A., Trojanowski J., Basun H., Lannfelt L., Neystat M., Fahn S., Dark F., Tannenberg T., Dodd P. R., Hayward N., Kwok J. B., Schofield P. R., Andreadis A., Snowden J., Craufurd D., Neary D., Owen F., Oostra B. A., Hardy J., Goate A., van Swieten J., Mann D., Lynch T., Heutink P. (1998) Nature 393, 702–705 [DOI] [PubMed] [Google Scholar]
- 2.Goedert M., Spillantini M. G., Crowther R. A., Chen S. G., Parchi P., Tabaton M., Lanska D. J., Markesbery W. R., Wilhelmsen K. C., Dickson D. W., Petersen R. B., Gambetti P. (1999) Nat. Med. 5, 454–457 [DOI] [PubMed] [Google Scholar]
- 3.Spillantini M. G., Murrell J. R., Goedert M., Farlow M. R., Klug A., Ghetti B. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 7737–7741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hardy J., Orr H. (2006) J. Neurochem. 97, 1690–1699 [DOI] [PubMed] [Google Scholar]
- 5.Braak H., Braak E. (1991) Acta Neuropathol. 82, 239–259 [DOI] [PubMed] [Google Scholar]
- 6.Grundke-Iqbal I., Iqbal K., Tung Y. C., Quinlan M., Wisniewski H. M., Binder L. I. (1986) Proc. Natl. Acad. Sci. U.S.A. 83, 4913–4917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spires-Jones T. L., de Calignon A., Matsui T., Zehr C., Pitstick R., Wu H. Y., Osetek J. D., Jones P. B., Bacskai B. J., Feany M. B., Carlson G. A., Ashe K. H., Lewis J., Hyman B. T. (2008) J. Neurosci. 28, 862–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolfe M. S. (2009) J. Biol. Chem. 284, 6021–6025 [DOI] [PubMed] [Google Scholar]
- 9.Uboga N. V., Price J. L. (2000) Neurobiol. Aging 21, 1–10 [DOI] [PubMed] [Google Scholar]
- 10.Weaver C. L., Espinoza M., Kress Y., Davies P. (2000) Neurobiol. Aging 21, 719–727 [DOI] [PubMed] [Google Scholar]
- 11.Carmel G., Mager E. M., Binder L. I., Kuret J. (1996) J. Biol. Chem. 271, 32789–32795 [DOI] [PubMed] [Google Scholar]
- 12.Jicha G. A., Bowser R., Kazam I. G., Davies P. (1997) J. Neurosci. Res. 48, 128–132 [DOI] [PubMed] [Google Scholar]
- 13.Dickey C. A., Kamal A., Lundgren K., Klosak N., Bailey R. M., Dunmore J., Ash P., Shoraka S., Zlatkovic J., Eckman C. B., Patterson C., Dickson D. W., Nahman N. S., Jr., Hutton M., Burrows F., Petrucelli L. (2007) J. Clin. Invest. 117, 648–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jinwal U. K., Miyata Y., Koren J., 3rd, Jones J. R., Trotter J. H., Chang L., O'Leary J., Morgan D., Lee D. C., Shults C. L., Rousaki A., Weeber E. J., Zuiderweg E. R., Gestwicki J. E., Dickey C. A. (2009) J. Neurosci. 29, 12079–12088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tortosa E., Santa-Maria I., Moreno F., Lim F., Perez M., Avila J. (2009) J. Alzheimer's Dis. 17, 319–325 [DOI] [PubMed] [Google Scholar]
- 16.Sevcik J., Skrabana R., Kontsekova E., Novak M. (2009) Protein Pept. Lett. 16, 61–64 [DOI] [PubMed] [Google Scholar]
- 17.Dou F., Netzer W. J., Tanemura K., Li F., Hartl F. U., Takashima A., Gouras G. K., Greengard P., Xu H. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 721–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pérez M., Arrasate M., Montejo De Garcini E., Muñoz V., Avila J. (2001) Biochemistry 40, 5983–5991 [DOI] [PubMed] [Google Scholar]
- 19.Barghorn S., Davies P., Mandelkow E. (2004) Biochemistry 43, 1694–1703 [DOI] [PubMed] [Google Scholar]
- 20.Mayer M. P., Bukau B. (2005) Cell Mol. Life Sci. 62, 670–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manzerra P., Rush S. J., Brown I. R. (1997) J. Cell. Physiol. 170, 130–137 [DOI] [PubMed] [Google Scholar]
- 22.Sarkar M., Kuret J., Lee G. (2008) J. Neurosci. Res. 86, 2763–2773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jinwal U. K., Koren J., 3rd, Borysov S. I., Schmid A. B., Abisambra J. F., Blair L. J., Johnson A. G., Jones J. R., Shults C. L., O'Leary J. C., 3rd, Jin Y., Buchner J., Cox M. B., Dickey C. A. (2010) J. Neurosci. 30, 591–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Desai A., Verma S., Mitchison T. J., Walczak C. E. (1999) Cell 96, 69–78 [DOI] [PubMed] [Google Scholar]
- 25.Abisambra J. F., Fiorelli T., Padmanabhan J., Neame P., Wefes I., Potter H. (2010) PLoS One 5, e8556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bolte S., Cordelières F. P. (2006) J. Microsc. 224, 213–232 [DOI] [PubMed] [Google Scholar]
- 27.Rasband M. N. (2009) PLoS Genet 5, e1000340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carty N. C., Nash K., Lee D., Mercer M., Gottschall P. E., Meyers C., Muzyczka N., Gordon M. N., Morgan D. (2008) Mol. Ther. 16, 1580–1586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dickey C., Kraft C., Jinwal U., Koren J., Johnson A., Anderson L., Lebson L., Lee D., Dickson D., de Silva R., Binder L. I., Morgan D., Lewis J. (2009) Am J. Pathol. 174, 228–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dickey C. A., Koren J., Zhang Y. J., Xu Y. F., Jinwal U. K., Birnbaum M. J., Monks B., Sun M., Cheng J. Q., Patterson C., Bailey R. M., Dunmore J., Soresh S., Leon C., Morgan D., Petrucelli L. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 3622–3627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petrucelli L., Dickson D., Kehoe K., Taylor J., Snyder H., Grover A., De Lucia M., McGowan E., Lewis J., Prihar G., Kim J., Dillmann W. H., Browne S. E., Hall A., Voellmy R., Tsuboi Y., Dawson T. M., Wolozin B., Hardy J., Hutton M. (2004) Hum. Mol. Genet. 13, 703–714 [DOI] [PubMed] [Google Scholar]
- 32.D'Souza S. M., Brown I. R. (1998) Cell Stress Chaperones 3, 188–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen S., Brown I. R. (2007) J. Neurosci. Res. 85, 402–409 [DOI] [PubMed] [Google Scholar]
- 34.Tohgi H., Utsugisawa K., Yoshimura M., Yamagata M., Nagane Y. (1995) Neurosci. Lett. 196, 89–92 [DOI] [PubMed] [Google Scholar]
- 35.Koren J., 3rd, Jinwal U. K., Jin Y., O'Leary J., Jones J. R., Johnson A. G., Blair L. J., Abisambra J. F., Chang L., Miyata Y., Cheng A. M., Guo J., Cheng J. Q., Gestwicki J. E., Dickey C. A. (2010) J. Biol. Chem. 285, 2498–2505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jeganathan S., von Bergen M., Mandelkow E. M., Mandelkow E. (2008) Biochemistry 47, 10526–10539 [DOI] [PubMed] [Google Scholar]
- 37.Hernandez F., Gomez de Barreda E., Fuster-Matanzo A., Lucas J. J., Avila J. (2009) Exp. Neurol. 10.1016/j.expneurol.2009.09.011 [DOI] [PubMed] [Google Scholar]
- 38.Khalilzadeh A., Wangoo K. T., Morris D. L., Pourgholami M. H. (2007) Biochem. Pharmacol. 74, 407–414 [DOI] [PubMed] [Google Scholar]
- 39.Liang Z., Liu F., Iqbal K., Grundke-Iqbal I., Wegiel J., Gong C. X. (2008) J. Alzheimer's Dis. 13, 295–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clavaguera F., Bolmont T., Crowther R. A., Abramowski D., Frank S., Probst A., Fraser G., Stalder A. K., Beibel M., Staufenbiel M., Jucker M., Goedert M., Tolnay M. (2009) Nat. Cell Biol. 11, 909–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morano K. A. (2007) Ann. N.Y. Acad. Sci. 1113, 1–14 [DOI] [PubMed] [Google Scholar]
- 42.Meriin A. B., Zhang X., He X., Newnam G. P., Chernoff Y. O., Sherman M. Y. (2002) J. Cell Biol. 157, 997–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ryu K., Kim C. W., Kim B. H., Han K. S., Kim K. H., Choi S. I., Seong B. L. (2008) Biochem. Biophys. Res. Commun. 373, 74–79 [DOI] [PubMed] [Google Scholar]
- 44.Ben-Zvi A. P., Goloubinoff P. (2001) J. Struct. Biol. 135, 84–93 [DOI] [PubMed] [Google Scholar]
- 45.Hartl F. U., Hayer-Hartl M. (2009) Nat. Struct. Mol. Biol. 16, 574–581 [DOI] [PubMed] [Google Scholar]
- 46.King M. E., Kan H. M., Baas P. W., Erisir A., Glabe C. G., Bloom G. S. (2006) J. Cell Biol. 175, 541–546 [DOI] [PMC free article] [PubMed] [Google Scholar]