Abstract
The present work demonstrates the ability of CO to prevent apoptosis in a primary culture of astrocytes. For the first time, the antiapoptotic behavior can be clearly attributed to the inhibition of mitochondrial membrane permeabilization (MMP), a key event in the intrinsic apoptotic pathway. In isolated non-synaptic mitochondria, CO partially inhibits (i) loss of potential, (ii) the opening of a nonspecific pore through the inner membrane, (iii) swelling, and (iv) cytochrome c release, which are induced by calcium, diamide, or atractyloside (a ligand of ANT). CO directly modulates ANT function by enhancing ADP/ATP exchange and prevents its pore-forming activity. Additionally, CO induces reactive oxygen species (ROS) generation, and its prevention by β-carotene decreases CO cytoprotection in intact cells as well as in isolated mitochondria, revealing the key role of ROS. On the other hand, CO induces a slight increase in mitochondrial oxidized glutathione, which is essential for apoptosis modulation by (i) delaying astrocytic apoptosis, (ii) decreasing MMP, and (iii) enhancing ADP/ATP translocation activity of ANT. Moreover, CO and GSSG trigger ANT glutathionylation, a post-translational process regulating protein function in response to redox cellular changes. In conclusion, CO protects astrocytes from apoptosis by preventing MMP, acting on ANT (glutathionylation and inhibition of its pore activity) via a preconditioning-like process mediated by ROS and GSSG.
Keywords: Apoptosis, Glutathione, Subcellular Organelles/Mitochondria, Brain, Mitochondrial Apoptosis, Adenine Nucleotide Translocase, Carbon Monoxide, Glutathionylation, Preconditioning, Reactive Oxygen Species
Introduction
Preconditioning (PC)2 is induced by stimulation below the threshold of injury, resulting in subsequent tissue protection, or tolerance, which is defined as a condition of transiently increased resistance to injury. PC was first found to be triggered by short episodes of ischemia, called ischemic preconditioning; however, the stimuli can also be pharmacological or chemical (1). Understanding the preconditioning phenomenon can be a tool to elucidate the cellular endogenous protective mechanisms. Many factors are involved in the signaling, transducing or executing the PC response. Reactive oxygen species (ROS) are crucial signaling molecules during PC development and are mostly generated in the mitochondria (2). The four protein complexes associated with the respiratory chain are the primary source of ROS by handling the bulk of oxygen metabolism (3). Furthermore, respiratory chain inhibition induces preconditioning and cytoprotection against focal cerebral ischemia via ROS generation (4).
Apoptosis occurs via two distinct pathways: an extrinsic pathway (relying on cell surface membrane receptors) and an intrinsic pathway, which is triggered by several conditions of intracellular stress, leading to mitochondrial membrane permeabilization (MMP). In many models, MMP induces (i) mitochondrial transmembrane potential dissipation, (ii) respiratory chain uncoupling, (iii) ROS overproduction, (iv) ATP synthesis arrest, and (v) the release of several death-regulating molecules (activating proteases and nucleases), making the cell death process irreversible (5, 6). Depending on the cell type and apoptosis stimuli, MMP can occur only in the outer membrane or in both mitochondrial membranes (inner and outer membranes) via the mitochondrial permeability transition pore (PTP). Permeability transition consists of a sudden increase in the inner membrane permeabilization to solutes up to 1500 Da; PTP can include the dynamic interaction between voltage-dependent anion channel, cyclophilin D, and ANT (7, 8). Apoptotic or necrotic cell death due to hypoxia-ischemia and reperfusion injury clearly involves the process of mitochondrial permeability transition (5).
ANT is the most abundant inner membrane protein responsible for the vital function of stoichiometric ADP/ATP exchange on the inner membrane. However, ANT can switch to a lethal function, corresponding to its pore-forming activity. ANT can interact with different proteins, depending on the cell type or in response to apoptotic stimuli. Anti- or proapoptotic members of the Bcl-2 family, such as Bcl-2 or Bax, can physically interact with ANT, facilitating its antiporter or pore-forming activity, respectively (9, 10). On the other side, glutathione S-transferase (GST) interacts with ANT in normal tissue, in colon carcinoma cells and in vitro. This interaction is lost during apoptosis induction, suggesting that GST behaves as an endogenous repressor of PTP and of ANT pore activity (11). The ANT pore forming property is also modulated by oxidation of critical thiol groups in cysteine residues (cysteines 56, 159, and 256) facing the matrix side (12, 13). Several thiol-cross-linking agents (such as diamide, dithiodipyridine, or phenylarsine oxide) enforce PTP opening and pore-forming activity of ANT, which is not prevented by Bcl-2 (13, 14).
Carbon monoxide (CO) is an endogenous product of heme degradation by heme oxygenase, which also generates free iron and biliverdin (15). Heme oxygenase plays an important role in cell redox state, acting as an antioxidant enzyme, which can be especially important for tissues with weak endogenous antioxidant defenses, such as the myocardium and the nervous system (16). Heme oxygenase activity has been suggested to modulate and prevent cerebral cell death in several models; this neuroprotective property has been mainly attributed to bilirubin antioxidant activity (17, 18); however, little data are available for CO in the central nervous system.
Low concentrations of CO confer an increased resistance to apoptosis triggered by several stimuli in different models, including endothelial cells, vascular smooth muscle cells, liver, or lung. CO also mediates other biological functions, such as anti-inflammation, arrest of proliferation, or vasodilatation (15), and this molecule presents a strong potential in therapeutic applications (19). More recently, it has been shown that ROS, generated at mitochondria (20, 21), are imperative signaling molecules for CO biological functions, such as anti-inflammation, cardioprotection, anti-proliferation, or anti-apoptosis in several systems (22). Carbon monoxide is described as binding to cytochrome c oxidase (complex IV), which slows down the rate of electron transport enabling electron to accumulate, including at complex III. Thus, the lifetime of the ubisemiquinone state of coenzyme Q is prolonged, increasing the propensity to reduce O2 into superoxide (O2˙̄), which is enzymatically converted to other ROS (22–24). In the literature, CO biological properties are prevented by the addition of antioxidants, inhibition of complex III, or the use of respiration-deficient ρ° cells (22–25). In neuronal primary cultures, CO exposure provides cytoprotective PC with an increased resistance against apoptosis, and ROS are crucial signaling molecules (25).
On one hand, CO presents antiapoptotic properties in several models. On the other hand, mitochondria are central executors of the programmed cell death process, via the MMP. In the literature, the most described role of CO in mitochondria is the generation of ROS, which are signaling factors. Because no data concerning the direct action of CO on MMP are available, the present work has explored the direct effect of CO on non-synaptic mitochondria (MMP modulation) and, consequently, its ability to prevent apoptosis in astrocytes. The involvement of ROS as signaling molecules for CO-PC triggering was investigated as well as the biochemical mechanisms involved in the MMP control by CO.
EXPERIMENTAL PROCEDURES
Materials
All of the chemicals were of analytical grade and were obtained from Sigma unless stated otherwise. Plastic tissue culture dishes were from Nunc; fetal bovine serum, glutamine, penicillin/streptomycin solution, and Dulbecco's minimum essential medium were obtained from Invitrogen; and Wistar rats were purchased from Instituto de Higiene e Medicina Tropical (Lisboa, Portugal).
Cell Culture
Primary cultures of astrocytes were prepared from 2-day-old rat cortex, as described (26). Briefly, cerebral hemispheres were carefully freed of the meninges, washed in ice-cold phosphate-buffered saline (PBS), and mechanically disrupted. Single-cell suspensions were plated in T-flasks (three hemispheres/175 cm2) in Dulbecco's minimum essential medium supplemented with 10% (v/v) fetal bovine serum (heat-inactivated), 100 units/ml penicillin/streptomycin solution, and glucose (to obtain a final concentration of 10 mm). Cells were maintained in a humidified atmosphere of 7% CO2 at 37 °C. After 8 days, the phase dark cells growing on the astrocytic cell layer were separated by vigorous shaking and removed. The remaining astrocytes were detached by mild trypsinization using trypsin/EDTA (0.25%, w/v) and subcultured in T-flasks for another 2 weeks. Growth medium was changed twice a week.
Isolation of Non-synaptic Mitochondria from Cortex
Mitochondria were isolated from 300–350-g male Wistar adult rats according to Ref. 27. Briefly, the cortex was removed and washed in a ice-cold isolation buffer containing 225 mm manitol, 75 mm sucrose, 1 mm EGTA, and 5 mm HEPES, pH 7.4. The tissue was minced with scissors and manually homogenized with Potter-Elvehjem in isolation buffer. The homogenate was centrifuged at 1300 × g for 3 min, and the resuspended pellet was recentrifuged a 1300 × g for 3 min. Both supernatants were pooled together and centrifuged at 21,200 × g for 10 min. The remaining pellet was resuspended in 3.5 ml of 15% Percoll solution and layered into centrifuge tubes containing a preformed two-step discontinuous density gradient consisting of 3.7 ml of 24% Percoll on top of 1.7 ml of 40% Percoll. The gradient was centrifuged at 31,700 × g for 9 min. The mitochondrial fraction, located between the layers of 24 and 40% Percoll, was removed, diluted 1:8 in isolation buffer and centrifuged at 16,700 × g for 10 min. The pellet was resuspended in 10 ml of isolation buffer containing 5 mg/ml bovine serum albumin (to remove lipids) and centrifuged at 6800 × g for 10 min. The mitochondrial pellet was resuspended in 100 μl of isolation buffer, and the total amount of protein was quantified using a BCA assay (Pierce). All of the steps were carried out at 4 °C.
All isolated mitochondria analyses were performed on modified brain buffer (27) containing 125 mm KCl, 2 mm K2HPO4, 1 mm MgCl2, 15 μm EGTA, 20 mm Tris, 5 mm glutamate, and 5 mm malate, pH 7.3, unless stated otherwise. All CO treatment in isolated mitochondria was performed with a final concentration of 10 μm for 15 min at room temperature, unless stated otherwise.
Preparation of CO Solutions
Fresh stock solutions of CO gas were prepared each day and carefully sealed. PBS was saturated by bubbling 100% of CO gas for 30 min to produce 10−3 m stock solution. The concentration of CO in solution was determined spectrophotometrically by measuring the conversion of deoxymyoglobin to carbon monoxymyoglobin, as described previously (28). 100% CO was purchased as compressed gas (Linde).
Apoptosis Induction/Prevention
Astrocytes were treated with CO (50 μm) for 3 h or with ethacrynic acid (EA; 50 μm) or carmustine (BCNU; 100 μm) for 1 h, followed by medium exchange. Then apoptosis was induced with diamide at concentrations ranging from 50 to 250 μm or with tert-butylhydroperoxide (t-BHP) at concentrations from 80 to 280 μm for 18 h. In some cases, β-carotene (1 μm) was used to modulate CO effect by applying this antioxidant to cells 1 h prior to CO treatment.
Assessment of Apoptosis-associated Parameters
To detect apoptosis induced by diamide or t-BHP, cell samples were collected by trypsinization, and cells were gated by the forward and side scatter. Two dyes were used: 3,3′-dihexyloxacarbocyanine iodide (DiOC6(3); 20 nm) (Invitrogen) to quantify the mitochondrial transmembrane potential (ΔΨm) and propidium iodide (PI; 1 μg/ml) (Invitrogen) to determine cell viability, based on plasma membrane integrity. A flow cytometer (Partec, Germany) was used to analyze apoptosis-associated parameters. This cytometer contains a blue solid state laser (488 nm) with FL1 green fluorescence channel for DiOC6(3) at 530 nm and a FL3 red fluorescence channel for PI detection at 650 nm. The acquisition and analysis of the results were performed with FlowMax® (Partec) software.
Measurement of ROS Generation
ROS generation was followed by the conversion of 2′,7′-dichlorofluorescein diacetate (H2DCFDA) (Invitrogen) to fluorescent 2′,7′-dichlorofluorescein (DCF). Astrocytes were treated for 3 h with CO, supernatant was removed, and cells were incubated for 20 min with 10 μm H2DCFDA prepared in PBS. Cells were washed twice, and fluorescence was measured (λex, 485 nm; λem, 530 nm) using a FL500 96-well spectrofluorimeter. β-Carotene (1 μm) was added 1 h prior to CO treatment. In the case of isolated mitochondria, 25 μg of mitochondrial protein was incubated with 5 μm H2DCFDA and 10, 50, or 250 μm CO in modified brain buffer. Fluorescence (λex, 485 nm; λem, 530 nm) was measured using a Biotek Synergy 2 spectrofluorimeter for 30 min at 37 °C. ROS generation was calculated as an increase over base-line levels, determined for untreated cells (100%). In some cases, β-carotene (1 μm) was added to isolated mitochondria 10 min prior to CO treatment.
Quantification of Mitochondrial Swelling
25 μg of mitochondrial protein was diluted in modified brain buffer containing or not containing 10 μm CO. After 15 min of incubation at room temperature, 5 or 15 μm Ca2+ was added, and the decrease in optical density at 540 nm was immediately measured for 30 min at 37 °C, using a Biotek Synergy 2 spectrofluorimeter. 100% of swelling is calculated based on the optical density decrease after 30 min between non-treated and 15 μm Ca2+-treated mitochondria.
Cytochrome c Release Detection
25 μg of protein from isolated mitochondria was diluted in modified brain buffer containing or not containing 10 μm CO. After 15 min of incubation at room temperature, 5 or 15 μm Ca2+ was added, and mitochondria were incubated for 30 min at 37 °C. Samples were centrifuged for 10 min at 10,000 × g, and the mitochondrial pellet was analyzed by immunoblotting with α-cytochrome c.
Mitochondrial Depolarization Detection
For depolarization measurements, isolated mitochondria (25 μg) containing 1 μm rhodamine 123 in modified brain buffer were pretreated with 10 μm CO, 5 μm cyclosporine A, 1 μm β-carotene, or 10 μm EA. To depolarization assessment by rhodamine 123 dequenching several MMP inducers were added: 5 or 7.5 μm Ca2+, 300 μm atractyloside, or 250 μm diamide. The fluorescent measurements (λex, 485 nm; λem, 535 nm) (Biotek Synergy 2 spectrofluorimeter) were followed for 30 min at 37 °C and are expressed as a percentage relative to the positive control, 5 μm Ca2+ (100%), at the indicated time point.
Inner Membrane Permeabilization Assay
A citrate synthase activity assay is used to assess the inner membrane permeability, as described in Ref. 29. Upon inner mitochondrial membrane permeabilization, acetyl-CoA is able to enter into the mitochondrial matrix, reacting with citrate synthase. A 5,5′-dithiobis-2-nitrobenzoic acid and deacetyled acetyl-CoA reaction gives 5-thio-2-nitrobenzoate, which can be followed by absorbance at 412 nm.
Briefly, 25 μg of protein from isolated mitochondria was incubated with CO (10 μm), GSSG (1 μm), or EA (25 μm), in modified brain buffer containing 100 μm 5,5′-dithiobis-2-nitrobenzoic acid, 300 μm acetyl-CoA, and 1 mm oxaloacetate. Inner membrane permeabilization was induced by atractyloside at 300 μm or Ca2+ at 15 μm. In either case, β-carotene (1 μm) was added 10 min prior to CO treatment. The absorbance at 412 nm was recorded for 20 min, using a Biotek Synergy 2 spectrofluorimeter.
ADP/ATP Translocase Activity Assessment
ADP/ATP translocase activity was assessed accordingly with Ref. 29. The ADP/ATP exchange rate was evaluated on 75 μg of protein from isolated mitochondria diluted in ATP buffer (20 mm HEPES, pH 7.2, 5 mm succinate, 300 mm sucrose, 10 mm KCl, 1 mm MgCl2, 1 mm Pi, 10 μm EGTA, 2 μm rotenone). Isolated mitochondria were treated with 10 μm CO, 1 or 100 μm GSSG, or 10 μm EA. ATP efflux triggered by externally added ADP was monitored by fluorescence (λex, 360 nm; λem, 465 nm) following NADP+ reduction occurring in a solution containing 2.5 mm glucose, 1 enzyme unit of hexokinase (EC 2.7.1.1), 0.5 enzyme unit of glucose-6-phosphate-dehydrogenase, and 0.2 mm NADPH, as described (29). Influence of adenylate kinase-dependent ATP synthesis was evaluated after treatment of isolated mitochondria by 10 μm adenylate kinase-specific inhibitor Ap5A (P1P5-diadenosine-5′-pentaphosphate). The values obtained, using a Biotek Synergy 2 spectrofluorimeter, for 30 min at 37 °C are expressed in percentage relative to control (100%) at the time point indicated.
Glutathione Content Quantification
After treatment with CO at 10 or 50 μm, in the presence or absence of β-carotene at 1 μm, reduced and oxidized glutathione in isolated mitochondria were determined spectrophotometrically (SpectraMax 340, Molecular Devices) using the procedure described previously (30). The assay measured the rate of formation of 5-thio-2-nitrobenzoate from 5,5′-dithiobis-2-nitrobenzoic acid, in the presence of NADPH and glutathione reductase, at 412 nm, for 5 min. GSSG was measured as described earlier after derivatization of GSH with 2-vinylpiridine (31). The values are expressed as the GSSG/GSH ratio.
Mitochondrion Isolation from Primary Culture of Astrocytes
Primary cultures of astrocytes were washed with ice-cold PBS collected by trypsinization. The samples were centrifuged at 200 × g for 10 min, and cells were washed in PBS by centrifugation at 200 × g for 10 min. The supernatant was discarded, and the pellet (cells) was incubated in 3.5 ml of hypotonic buffer (0.15 mm MgCl2, 10 mm KCl, 10 mm Tris-HCl, pH 7.6) at 4 °C for 5 min. After the addition of an equal volume of homogenization buffer (0.15 mm MgCl2, 10 mm KCl, 10 mm Tris-HCl, 0.4 mm phenylmethylsulfonyl fluoride, 250 mm saccharose, pH 7.6) twice concentrated, samples were manually homogenized with a Dounce Potter homogenizer. Cell extracts were centrifuged at 900 × g for 10 min, followed by supernatant centrifugation at 10,000 × g for 10 min. The mitochondrial pellet was resuspended in 100 μl of homogenization buffer, and the total amount of protein was quantified using BCA assay (Pierce). All of the steps were carried out at 4 °C.
Immunoprecipitation
100 μg of mitochondrial protein (isolated from astrocytes or from rat cortex) was incubated in 100 μl of homogenization buffer containing 0.5% of Triton X-100 in the presence of 20 μl of α-GSH (1 mg/ml; ViroGen) or 20 μl of α-ANT monoclonal antibody (Mitosciences) for 90 min at 37 °C, followed by immunoprecipitation with 15 μl of protein A/G PLUS-agarose beads (Santa Cruz Biotechnology) for 30 min at 37 °C. After 10 min of 10,000 × g centrifugation, supernatant was discarded, and pellet was washed four times with PBS. Proteins attached to the beads were solubilized by Laemmli buffer for further Western blot analysis.
Immunoblotting
Several samples (from cell extracts, mitochondria, or immunoprecipitated protein) were separated under reducing electrophoresis on a 1-mm NuPAGE® Novex BisTris gel (Invitrogen) and electrically transferred to a nitrocellulose membrane (HybondTM-C extra, Amersham Biosciences). ANT, caspase-3, GST, or cytochrome c protein was stained with α-ANT monoclonal antibody (Mitosciences), α-caspase-3 (Sigma), α-GST (GE Healthcare), or α-cytochrome c (Abcam), all of them at a 1:1000 dilution and 2 h of room temperature incubation. Blots were developed using the ECL (enhanced chemiluminescence) detection system after incubation with horseradish peroxidase-labeled anti-mouse IgG antibody (Amersham Biosciences) at 1:5000 dilution and 1 h of room temperature incubation. The area and intensity of bands were quantified by densitometry analysis (GraphPad Prism 4) and are presented as a percentage relative to the positive control (100%). These experiments have been repeated three times with similar results.
Statistical Analyses
The data concerning intact cells were from experiments carried out in at least three independent preparations (cell isolation). All data related to isolated mitochondria were derived in triplicate from at least three independent animals. Mitochondrial data are presented as a representative result of three independent assays. For the Western blot technique, a representative image of three independent assays is shown. All values are mean ± S.D., n ≥ 3. Error bars, corresponding to S.D., are represented in the figures. Statistical comparisons were performed using analysis of variance, single factor with replication, with p < 0.05, n ≥ 3. p < 0.05 means that samples are significantly different at a confidence level of 95%.
RESULTS
Carbon Monoxide Prevents Apoptosis in Astrocytes
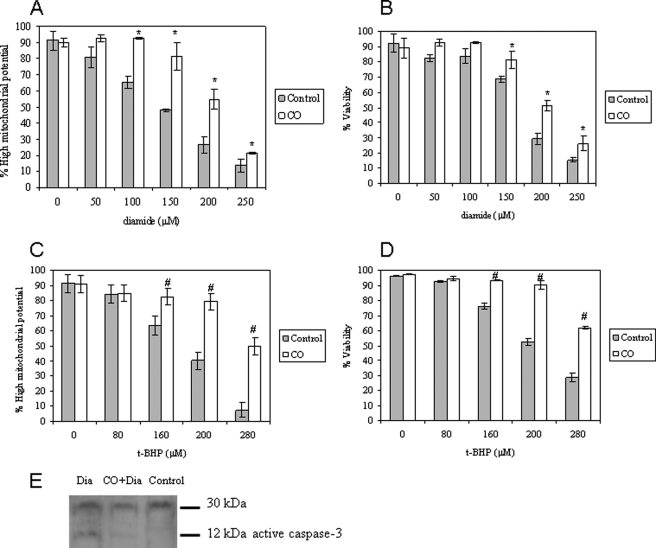
Astrocytic apoptosis was induced by oxidative stress with thiol cross-linker diamide (Fig. 1, A and B) and pro-oxidant tert-butylhydroperoxide (Fig. 1, C and D) treatment during 18 h. Both reagents have been shown to induce cell death by acting at the mitochondrial level (14). Primary cultures of cortical astrocytes were treated with CO-saturated PBS with a final concentration of 50 μm 3 h prior to apoptosis induction. CO partially prevents the dissipation of ΔΨm (quantified by DiOC6(3); Fig. 1, A and C), followed by plasma membrane permeabilization (detected by propidium iodide fluorescence, Fig. 1, B and D), a marker for loss of viability. These features were assessed by flow cytometry. CO is not toxic up to 100 μm; however, at this concentration, its cytoprotection decreases (supplemental Fig. 1). In addition, carbon monoxide prevents astrocytic cell death up to 48 h after induction (supplemental Fig. 2). Thus, the role of CO is not limited to delaying apoptosis in a short time window.
FIGURE 1.
Carbon monoxide confers protection against apoptosis. Primary cultures of astrocytes cultured in 24-well plates were pretreated with 50 μm CO for 3 h, following apoptosis induction by 18-h exposure to the thiol cross-linker diamide (Dia; 0–250 μm) (A and B) and to the pro-oxidant t-BHP (0–280 μm) (C and D). The apoptotic hallmarks were assessed by flow cytometry. In A and C, the percentage of cells presenting high mitochondrial potential, detected by DiOC6(3), is expressed. In B and D, the percentage of cells containing intact plasma membrane (viable cells) is presented, assessed with PI fluorochrome. All values are mean ± S.D. (error bars), n = 4. *, p < 0.05 compared with control and CO-treated cells for each concentration of diamide (A and B); #, p < 0.05 compared with control and CO-treated cells for each concentration of t-BHP (C and D). E, immunodetection of caspase-3 activation by its cleavage into 12-kDa fractions. The first lane corresponds to astrocytes treated with diamide at 200 μm (18 h); the second lane shows astrocytes pretreated with 50 μm CO (3 h), followed by diamide induction of apoptosis; and the third lane shows control astrocytes.
In addition, carbon monoxide prevents caspase-3 activation induced by diamide (Fig. 1E). In conclusion, CO does protect astrocytes from cell death induced by oxidative stress; furthermore, CO presents an extended time window of action.
ROS Generation Is Crucial for CO-induced Cytoprotection
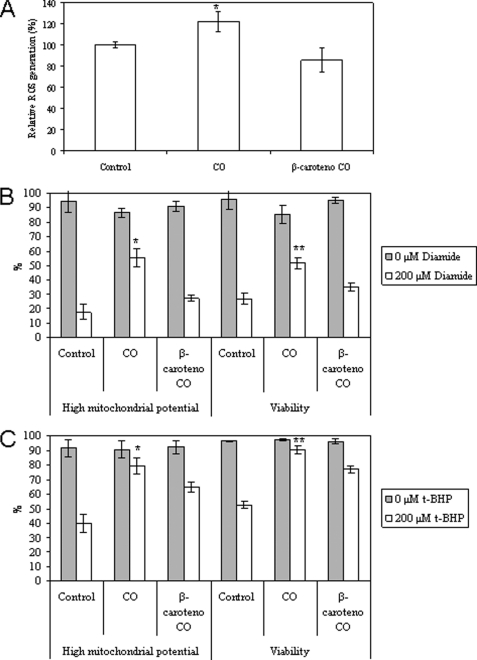
Astrocytes were treated with 50 μm carbon monoxide, and after 3 h, intracellular ROS generation (specifically H2O2) was measured by the conversion of H2DCFDA to fluorescent DCF. CO induces an increase in intracellular ROS levels of about 20%, which is prevented by 1 h of pretreatment with the antioxidant β-carotene at 1 μm (Fig. 2A). In order to verify the role of ROS and, thus, the preconditioning mode of action of CO, 1 μm β-carotene was added to primary culture of astrocytes previous to CO treatment. Indeed, inhibition of ROS generation decreases the antiapoptotic effect of CO for diamide (Fig. 2B) and for t-BHP (Fig. 2C) inductions. Thus, in intact cells, ROS are imperative signaling molecules for prevention of apoptosis by CO, indicating that a preconditioning-like mechanism is involved.
FIGURE 2.
Relevance of ROS to a carbon monoxide protective role. A, CO induces intracellular ROS generation, and it is prevented by the antioxidant β-carotene. Primary cultures of astroglial cells were pretreated for 1 h with 1 μm β-carotene, followed by the addition of 50 μm CO for 3 h. ROS quantification was performed using H2DCFDA. B and C, astrocytes were subjected to 1 μm β-carotene for 1 h, followed by treatment with 50 μm CO for 3 h, and apoptosis was induced with diamide (B) or t-BHP (C) for 18 h. Mitochondrial potential and viability were assessed by flow cytometry, using DiOC6(3) and PI, respectively. All values are mean ± S.D. (error bars), n = 3. A, *, p < 0.05 compared with control or with β-carotene and CO-treated cells. B, *, p < 0.05 compared with control or with β-carotene and CO-treated cells for high ΔΨm; **, p < 0.05 compared with control or with β-carotene and CO-treated cells for viability. C, *, p < 0.05 compared with control or with β-carotene and CO-treated cells for high ΔΨm; **, p < 0.05 compared with control or with β-carotene and CO-treated cells for viability.
CO Inhibits MMP in Isolated Non-synaptic Mitochondria
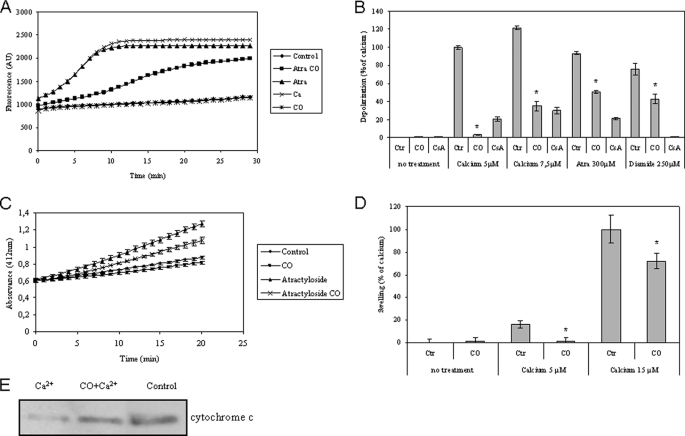
Several different approaches were used to assess direct or indirectly mitochondrial membrane permeabilization: mitochondrial depolarization, inner membrane permeabilization, cytochrome c release, and mitochondrial swelling. Non-synaptic mitochondria isolated from brain cortex (27) were treated with 10 μm CO during 15 min prior to the addition of diamide, atractyloside (a ligand of ANT that prevents ADP/ATP translocation and induces its pore forming function), or calcium to induce MMP. Loss of ΔΨm, or mitochondrial depolarization, was measured using the methodology based on dequenching of the fluorescent probe rhodamine 123 (29). Loss of ΔΨm induced by atractyloside was prevented by the prior addition of CO (Fig. 3A). CO also inhibits mitochondrial depolarization induced by diamide and Ca2+ (Fig. 3B). To quantify this effect, Ca2+ at 5 μm was normalized to 100% of depolarization (Fig. 3B). Moreover, cyclosporine A also prevents ΔΨm loss, indicating the involvement of the permeability transition pore (Fig. 3B) (32). Changes in the inner membrane permeability (the opening of a large channel for molecules up to ∼800 Da) were assessed via an enzymatic assay based on the accessibility of citrate synthase, which is a soluble matrix enzyme (29). The atractyloside induction of inner membrane permeabilization is partially prevented by CO (Fig. 3C). Finally, CO also inhibits mitochondrial swelling triggered by 5 or 15 μm Ca2+ (Fig. 3D), and there is a partial prevention of cytochrome c release from mitochondria pretreated with CO when challenged with Ca2+ at 15 μm (Fig. 3E). CO treatment at 10 μm (alone) in isolated mitochondria had no effect on swelling, mitochondrial depolarization, or pore formation through the inner membrane (Fig. 3). In conclusion, CO inhibits mitochondrial swelling, cytochrome c release, loss of ΔΨm, and inner membrane permeabilization. Thus, CO prevents apoptosis via a direct effect on mitochondria (i.e. by reducing MMP).
FIGURE 3.
Carbon monoxide effect on the mitochondrial membrane depolarization, inner membrane permeabilization, mitochondrial swelling, and cytochrome c release. All four experimental assays were performed using isolated non-synaptic mitochondria in modified brain buffer. A, representative micrograph for rhodamine 123 fluorescence change (λex, 485 nm; λem, 535 nm), measured for 30 min at 37 °C, in the absence or presence of CO at 10 μm and atractyloside at 300 μm or Ca2+ at 5 μm. B, quantitative expression of rhodamine 123 fluorescent measurements at 15 min of incubation. Isolated mitochondria were pretreated with CO at 10 μm or cyclosporine A (CsA) at 1 μm, and then Ca2+ at 0, 5, or 7.5 μm, atractyloside at 300 μm, or diamide at 250 μm was added. The values are expressed in relative percentage to 5 μm Ca2+ (100%). All values are mean ± S.D. (error bars), n = 3. *, p < 0.05 compared with control mitochondria for each inducer. C, an enzymatic assay based on citrate synthase activity was used to follow inner membrane permeabilization. Measurements were performed at 412 nm in the absence or presence of 10 μm CO and 300 μm atractyloside, at 37 °C for 20 min. All values are mean ± S.D., n = 3. D, mitochondrial swelling was measured by absorbance at 540 nm at 37 °C for 30 min, and the effect of calcium at 15 μm was normalized to 100% of swelling. Mitochondria were treated in the presence or absence of Ca2+ at 5 or 15 μm and/or CO at 10 μm. Experiments were done in triplicate and repeated three times. All values are mean ± S.D. (error bars), n = 3. *, p < 0.05 compared with control and CO-treated mitochondria. E, mitochondria, in the absence or presence of 10 μm CO, were treated with Ca2+ at 15 μm at 37 °C for 30 min, followed by centrifugation to separate mitochondrial pellet for immunodetection of cytochrome c release.
ROS Are Important Molecules for CO Prevention of MMP
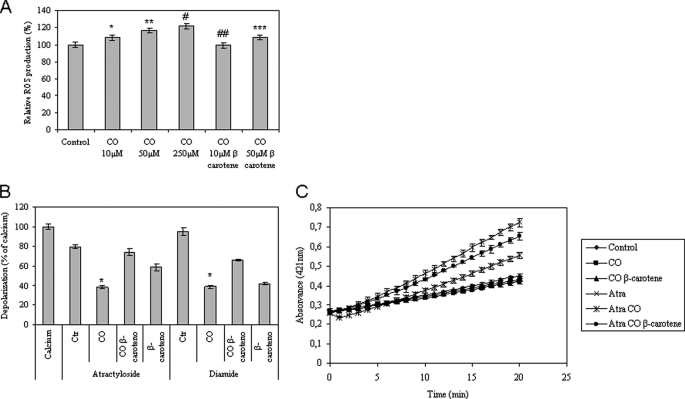
In isolated non-synaptic mitochondria, treatment with CO at 10, 50, or 250 μm induced ROS generation in a dose-response manner, and pretreatment with β-carotene (1 μm) prevented ROS formation by CO (Fig. 4A). Inhibition of mitochondrial depolarization by CO was lost when mitochondria were treated with the antioxidant β-carotene prior to CO exposure (Fig. 4B). Still, CO became unable to prevent the atractyloside-induced opening of a large channel in the inner membrane when β-carotene was added to mitochondria before CO treatment (Fig. 4C). These findings support the hypothesis that ROS generation by CO is necessary for its MMP prevention in isolated mitochondria. Therefore, ROS are also imperative CO-signaling molecules at the mitochondrial level.
FIGURE 4.
Influence of ROS on CO effect at mitochondrial level. In A, mitochondria were treated with 10, 50, or 250 μm CO in the presence or absence of 1 μm β-carotene, followed by ROS quantification using H2DCFDA (λex, 485 nm; λem, 530 nm). The values are expressed in percentage relative to control (100%). All values are mean ± S.D., n = 4. *, p < 0.05 compared with control; **, p < 0.05 compared with control; #, p < 0.05 compared with control; ##, p < 0.05 compared with 10 μm CO; ***, p < 0.05 compared with 50 μm CO. B, mitochondria were pretreated with 1 μm β-carotene and 10 μm CO, and then atractyloside at 300 μm or diamide at 250 μm was added. The fluorescent measurements (λex, 485 nm; λem, 535 nm) are expressed in relative percentage to 5 μm Ca2+ (100%) at 15 min of incubation. All values are mean ± S.D., n = 3; *, p < 0.05 compared with control and with β-carotene and CO-treated mitochondria. C, inner membrane permeabilization was assessed according to Ref. 29. Measurements were performed at 412 nm in the absence or presence of 10 μm CO and 300 μm atractyloside for 20 min at 37 °C. All values are mean ± S.D. (error bars), n = 3.
CO Facilitates ADP/ATP Translocation Function of ANT
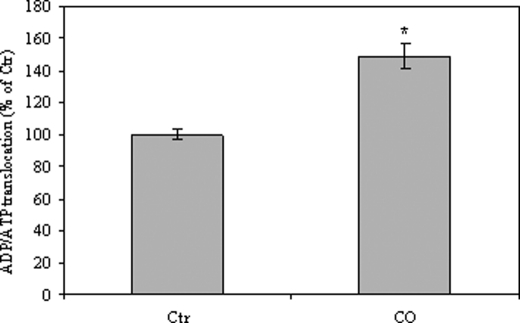
In order to clarify the mechanisms involved in CO inhibition of MMP, the influence of this gas on ANT activity was measured using an enzyme-based assay (29). ANT is a double function protein located in the mitochondrial inner membrane. The physiological role of ANT consists in exchanging ADP against ATP in a stoichiometric manner; in contrast, in response to diverse stimuli, its activity can switch to that of a pore-forming protein, modulating MMP (9). Mitochondria treated with CO present an increased ANT translocase activity (Fig. 5), providing evidence that CO prevents the opening of a nonspecific pore through the inner membrane by directly acting on ANT and enforcing its physiological activity.
FIGURE 5.
Carbon monoxide effect on ADP/ATP translocase activity of ANT. The results were obtained using isolated non-synaptic mitochondria treated with 10 μm CO, and ADP/ATP translocation was assessed according to Ref. 29. ADP was added to mitochondria and diffused into the intermembrane space through the voltage-dependent anion channel. Once into the intermembrane space, ADP can be transformed by adenylate kinase (AK) in AMP and ATP or exchanged against ATP by adenine nucleotide translocator (ANT). The values are expressed in relative percentage to control (100%) at 15 min of incubation and are mean ± S.D. (error bars), n = 3. *, p < 0.05 compared with control mitochondria.
CO Augments GSSG/GSH Ratio in Isolated Mitochondria
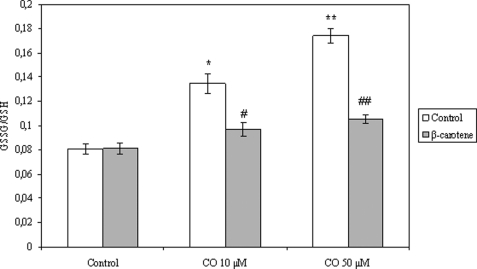
Based on the fact that (i) CO generates ROS in isolated mitochondria and (ii) glutathione is one of the most efficient antioxidant systems in the cell, as well as at the mitochondrial level (33), GSH and GSSG were measured after CO treatment in isolated mitochondria. The total amount of mitochondrial GSH is not altered in the presence of carbon monoxide; this result correlates with the fact that glutathione synthesis occurs in the cytosol, whereas at the mitochondrial level, only GSH recycling takes place. In contrast, mitochondrial levels of GSSG increase after CO exposure in a dose-response manner, which enhances the GSSG/GSH ratio (Fig. 6). In addition, mitochondrial pretreatment with β-carotene prevents GSSG/GSH ratio augmentation due to the presence of CO (Fig. 6). These data suggest that GSSG is also a candidate factor for signaling preconditioning and apoptosis prevention triggered by CO.
FIGURE 6.
Carbon monoxide effect on mitochondrial GSSG/GSH ratio. After CO treatment (10 or 50 μm) in the presence or absence of β-carotene (1 μm), oxidized and reduced glutathione quantification was performed using a microtiter plate assay, as described under “Experimental Procedures.” The values are mean ± S.D. (error bars), n = 3. *, p < 0.05 compared with control; **, p < 0.05 compared with mitochondria treated with 10 μm CO; #, p < 0.05 compared with mitochondria without β-carotene treatment and 10 μm CO treatment; ##, p < 0.05 compared with mitochondria without β-carotene treatment and 50 μm CO treatment.
GSSG Signaling and Protein Glutathionylation Are Involved in the Modulation of ANT Activity and MMP by CO
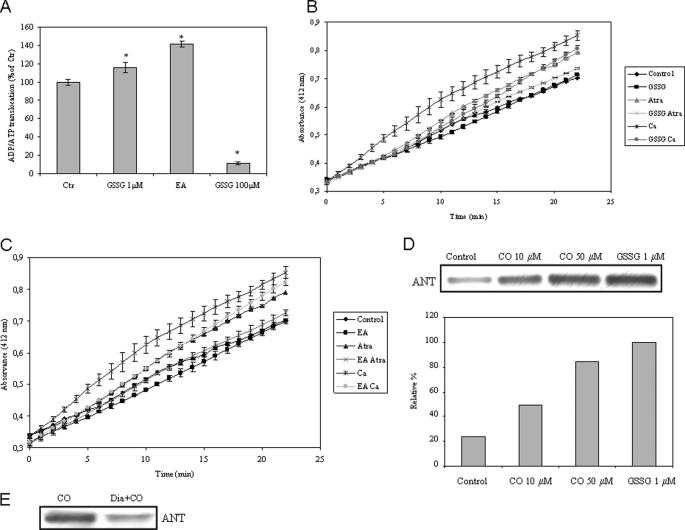
In order to challenge the hypothesis of GSSG as a signaling molecule in the CO cytoprotective pathway, three distinct approaches were studied: (i) the effect of GSSG on ADP/ATP translocation by ANT, (ii) modulation of inner membrane permeability by small amounts of GSSG, and (iii) the covalent modification of ANT by glutathionylation of thiol groups. Small amounts of oxidized glutathione (1 μm) increase the translocase activity of ANT (Fig. 7A), which is similar to the CO effect upon this enzyme. Furthermore, mitochondria treated with EA, which prevents glutathione recycling (34) and increases GSSG levels, also facilitates ADP/ATP translocation by ANT (Fig. 7A). Thus, there might be a direct effect of GSSG on ANT. However, higher concentrations of GSSG (100 μm) prevent ADT/ATP exchange activity (Fig. 7A). Still, oxidized glutathione (1 μm) and EA partially prevent inner membrane permeabilization challenged by atractyloside and Ca2+ (Fig. 7, B and C). Thus, small amounts of GSSG seem to modulate ANT activity and prevent MMP.
FIGURE 7.
Role of ANT glutathionylation in MMP modulation. A, ADP/ATP translocation was followed in isolated mitochondria in the presence of 1 or 100 μm GSSG or 10 μm EA. The values are expressed in relative percentage to control (100%) at 15 min of incubation at 37 °C and are mean ± S.D. (error bars), n = 3. *, p < 0.05 compared with control. B and C, isolated non-synaptic mitochondria were treated with GSSG at 1 μm (B) or with EA at 25 μm (C) for 10 min, followed by atractyloside (Atra; 300 μm) or Ca2+ (5 μm) addition in order to induce inner membrane permeabilization, which was assessed according to Ref. 29. Measurements were performed at 412 nm for 20 min at 37 °C. All values are mean ± S.D., n = 3. D, primary cultures of astrocytes were treated with 0, 10, or 50 μm CO following mitochondria isolation; additionally, 1 μm GSSG was added to mitochondria isolated from control astrocytes. Glutathionylated proteins (α-GSH) were immunoprecipitated in mitochondria isolated from astrocytes, and ANT was immunodetected by Western blot from the immunoprecipitated proteins. The area and intensity of bands were quantified by densitometry analysis (GraphPad Prism 4) and are presented as relative percentage to the positive control (100%). This experiment was repeated three times with similar results. E, isolated non-synaptic mitochondria were treated in the presence or absence of diamide at 100 μm for 15 min, followed by CO (10 μm) incubation for 15 min, and then glutathionylated proteins were immunoprecipitated, and ANT was immunodetected by Western blot from the immunoprecipitated proteins. This experiment was repeated three times with similar results.
The GSSG/GSH ratio was calculated for different conditions in order to correlate the effect of CO on glutathione levels and the addition of 1 μm GSSG. GSSG/GSH ratio in non-treated mitochondria is 0.080, and in CO (10 μm)-treated mitochondria, it increases to 0.135, whereas the addition of 1 μm GSSG in a mitochondrial preparation corresponds to a GSSG/GSH ratio of 0.426, which is 3 times higher than the ratio induced by 10 μm CO. However, oxidized glutathione is added into the media, and it does not correspond to glutathione concentration in the mitochondrial matrix. Because of this, and based on these similar values (the same order of magnitude), one can consider that the addition of GSSG at 1 μm might mimic the augmentation of the GSSG/GSH ratio due to CO induction. These data are an additional support for the hypothesis that GSSGs are signaling molecules in the CO-induced prevention of MMP.
In addition, an increase of GSSG levels allows the formation of protein mixed disulfides, or protein glutathionylation. Reversible protein glutathionylation is a post-translational process involved in the cellular response to redox changes, which can protect cysteine residues against irreversible damage due to oxidative stress (35). In addition, ANT presents critical thiol residues (14) that are important for the control of protein function and are candidates to be glutathionylated. Purified mitochondria from control astrocytes or from CO-treated astrocytes were incubated with α-GSH to immunoprecipitate glutathionylated proteins, followed by immunodetection of ANT in a Western blot assay (Fig. 7D). Moreover, purified mitochondria from non-treated astrocytes were incubated with small amounts of GSSG (1 μm), followed by immunoprecipitation with α-GSH, and detected with α-ANT by Western blot (Fig. 7D). The presence of CO or GSSG augments the levels of glutathionylated ANT (Fig. 7D).
In order to verify if diamide-targeted thiol residues of ANT are protected by glutathionylation, a competition assay was performed. Isolated mitochondria were treated with diamide and followed by CO exposure. In fact, diamide pretreatment decreased the ANT glutathionylation levels induced by CO (Fig. 7E), suggesting that diamide acts on the same cysteine residue(s) that is glutathionylated by CO. Taken all together, these data provide evidence that CO increases GSSG levels, which covalently modifies ANT by glutathionylation, facilitating ADP/ATP translocation and preventing inner membrane permeabilization.
In contrast, and to further confirm the functional role of ANT glutathionylation by CO, the same experimental conditions used to test CO modulation of MMP (Fig. 3) were used to detect ANT glutathionylation. Isolated non-synaptic mitochondria were first pretreated with CO (10 or 50 μm) or GSSG (1 μm) at room temperature for 15 min, followed by diamide addition (100 μm) at 37 °C for 30 min. Immunoprecipitation was performed, showing that ANT glutathionylation still occurs after diamide treatment (supplemental Fig. 3).
Role of GST
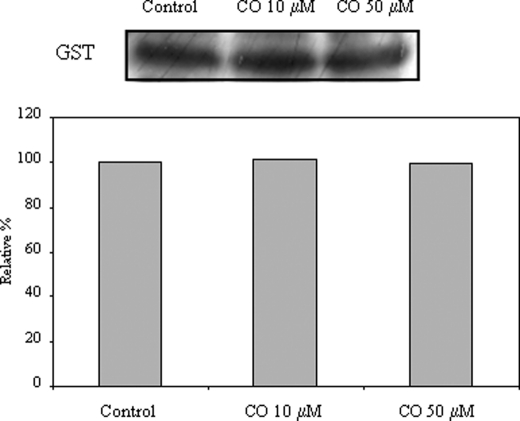
In the literature, GST is described to physically interact with ANT and to be an endogenous repressor of apoptosis (11). ANT has been co-purified with GST from rat brain and co-immunoprecipitated from a colon carcinoma cell line, and the functional cooperation between both proteins has been mimicked by reconstitution into proteoliposomes (11). On the other hand, GST is a potential candidate enzyme to be involved in the ANT glutathionylation. Thus, the effect of CO in the interaction between ANT and GST was assessed. Purified mitochondria from control or from CO-treated astrocytes were immunoprecipitated with α-ANT, followed by GST immunodetection in a Western blot assay (Fig. 8). However, CO does not alter the physical interaction of ANT-GST. Further future work about CO implications for GST activity induction must be performed to disclose the role of GST in CO-induced ANT glutathionylation.
FIGURE 8.
Effect of CO in ANT-GST interaction. Primary cultures of astrocytes were treated with 0, 10, or 50 μm CO following mitochondria isolation; ANT (α-ANT) was immunoprecipitated in mitochondria isolated from astrocytes, and GST was immunodetected by Western blot from the immunoprecipitated proteins. This experiment was repeated three times with similar results.
Prevention of Mitochondrial GSH Recycling Protects Astrocytes from Cell Death and Inhibits MMP
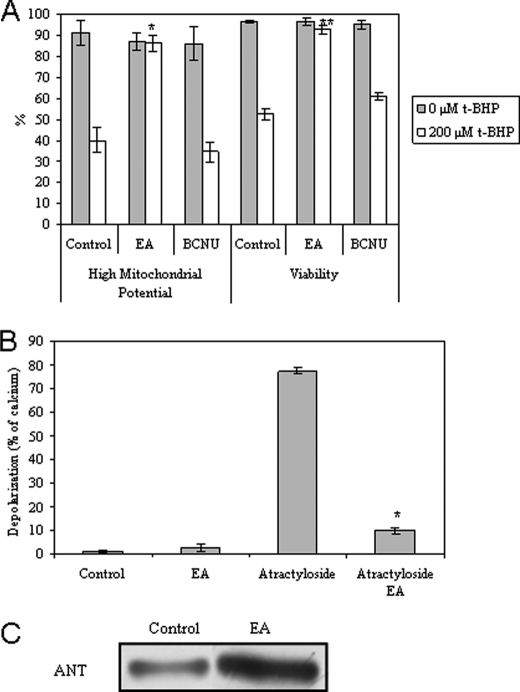
To verify the role of mitochondrial GSSG as signaling molecules in the modulation of apoptosis, inhibitor factors of GSH recycling were used: EA (specific for mitochondrial recycling) (34) or BCNU. Astrocytes were incubated with EA or BCNU prior to apoptosis induction with t-BHP (Fig. 9A) or diamide (data not shown). EA inhibits mitochondrial depolarization and loss of viability in astrocytes, whereas BCNU presents no effect (Fig. 9A). Furthermore, in isolated non-synaptic mitochondria, the presence of EA also prevents dissipation of ΔΨm induced by atractyloside (Fig. 9B). It is important to highlight that the EA concentration used here for intact cells (50 μm) is half of that used by Muyderman et al. (34) (100 μm), where they have shown a total prevention of mitochondrial GSH recycling. Thus, the protective effect of EA in the present work might be due to a partial inhibition of GSH recycling, slightly increasing GSSG levels. In addition, isolated mitochondria from EA-treated astrocytes revealed higher levels of ANT glutathionylation than from non-treated astrocytes (Fig. 9C). Taken all together, these results indicate that slight levels of GSSG in the mitochondria are important for prevention of MMP and cell survival signaling, probably by targeting ANT.
FIGURE 9.
Effect of mitochondrial GSSG on cell death, MMP, and post-translational ANT modifications. A, the percentage of astrocytic survival when subjected to 50 μm EA or 100 μm BCNU for 1 h, followed by medium exchange and treatment with t-BHP (200 μm) for 18 h. Mitochondrial potential and viability were assessed by flow cytometry, using DiOC6(3) and PI, respectively. All values are mean ± S.D. (error bars), n = 4. *, p < 0.05 compared with control cells treated with t-BHP for ΔΨm high. **, p < 0.05 compared with control cells treated with t-BHP for viability. B, depolarization assay. Isolated mitochondria were pretreated with 10 μm EA for 10 min, followed by 300 μm atractyloside or 5 μm Ca2+ treatment at 37 °C. The fluorescent measurements (λex, 485 nm; λem, 535 nm) are expressed in relative percentage to 5 μm Ca2+ (100%) at 15 min of incubation. All values are mean ± S.D., n = 4. *, p < 0.05 compared with atractyloside-treated mitochondria. C, primary cultures of astrocytes were treated with 0 or 50 μm EA following mitochondria isolation; glutathionylated proteins (α-GSH) were immunoprecipitated in mitochondria isolated from astrocytes, and ANT was immunodetected by Western blot from the immunoprecipitated proteins. This experiment was repeated three times with similar results.
DISCUSSION
The present study has demonstrated that CO confers protection against oxidative stress-induced apoptosis in primary culture of astrocytes (Fig. 1). For the first time, it has been shown that there is a direct antiapoptotic effect of CO upon mitochondria, by preventing MMP, which is a key event in the intrinsic apoptotic pathway. The data reported here revealed that CO inhibits mitochondrial swelling, cytochrome c release, dissipation of ΔΨm, and the opening of a nonspecific pore through the inner membrane in isolated non-synaptic mitochondria (Fig. 3).
Previously, we have shown that CO prevented neuronal apoptosis by inducing a preconditioning-like effect, a model in which reactive oxygen species appeared to be signaling molecules (25). Herein, it has been demonstrated that (i) CO induces ROS production in astrocytes and (ii) inhibition of ROS generation by an antioxidant addition (β-carotene) reverses the antiapoptotic effect of CO. In intact cells, ROS are necessary for CO to delay apoptosis (Fig. 2). Also, at the subcellular level (isolated mitochondria), ROS appear to be critical for CO to reduce MMP (Fig. 4). Thus, ROS generation is crucial for CO signaling.
ANT is a key protein involved in the control of the permeability transition pore, leading to the release of proapoptotic factors into the cytosol (36). ANT interacts with either the proapoptotic protein Bax or the antiapoptotic protein Bcl-2, which both influence ANT in opposite ways. Bax facilitates the opening of a pore through the inner membrane, whereas Bcl-2 increases translocation activity of ANT (9, 10, 37). CO increases the ADP/ATP translocation by ANT (Fig. 5), which is comparable with the Bcl-2-ANT model of MMP regulation. CO appears to change ANT conformation, which stimulates translocation activity and inhibits channel function. This conformation might be the c-conformation first described by Vignais and co-workers (38). This is in agreement with the literature because it has been demonstrated that heme oxygenase-1 expression increases the activity of ADP/ATP transporter in renal mitochondria in experimental diabetes (39). Therefore, CO modulates MMP by acting on ANT.
CO augments the mitochondrial GSSG/GSH ratio in a dose-response manner (Fig. 6). This GSSG/GSH ratio increase is prevented by β-carotene addition. Additionally, glutathione redox changes can lead to the regulated formation of mixed disulfides between protein thiol and glutathione disulfide (protein glutathionylation). The progressive glutathionylation of key proteins is proposed as a molecular switch by which cells respond in an immediate and reversible fashion to oxidative stress (35). Protein glutathionylation can be considered as a physiological signaling function, in the same way as the phosphorylation process. On the other hand, critical thiol residues of ANT can be oxidized and/or derivatized in order to modulate permeability transition (13, 14). Herein it was shown that CO and GSSG induce ANT glutathionylation (Fig. 7D). Moreover, in a functional approach, it has been demonstrated that small concentrations of GSSG (1 μm) or EA (inhibitor of mitochondrial GSH recycling) stimulate ADP/ATP translocation activity of ANT (Fig. 7A) and prevent inner membrane permeabilization (Fig. 7, B and C), as does CO. Therefore, one can speculate that glutathionylation of ANT protects critical thiol groups against oxidation and/or cross-linking between residues, avoiding conformation changes, such as the formation of ANT dimers, which are responsible for a nonspecific pore formation (14). In fact, diamide pretreatment decreases ANT glutathionylation levels induced by CO in isolated mitochondria (Fig. 7E), which suggests that the diamide-targeted cysteine residue(s) might be the same residue(s) glutathionylated by CO. In contrast, higher concentrations of GSSG (100 μm) inhibit ADP/ATP translocation by ANT (Fig. 7A), which is in agreement with the findings of Vesce et al. (40), who have shown that acute GSH depletion decreases ATP transport through the inner membrane.
Furthermore, one can state that a 1 μm addition of GSSG mimics the effect of CO (10 μm) treatment in isolated mitochondria. In mitochondria, the GSSG/GSH ratio increases from 0.080 to 0.135 upon CO exposure, whereas the addition of 1 μm GSSG corresponds to a GSSG/GSH ratio of 0.426. Although there is a 3-fold increase, these values have the same order of magnitude. Moreover, the addition of GSSG is done into the medium, and the GSSG/GSH ratio is calculated in the mitochondrial matrix. In conclusion, the addition of a small amount of GSSG mimics endogenous GSSG generation triggered by CO, acting as signaling factors.
A slight increase in mitochondrial GSSG levels is also demonstrated to be important for cell signaling in functional approaches. Partial inhibition of GSH recycling at the mitochondrial level, by EA addition, protects astrocytes from cell death induced by oxidative stress (Fig. 9A) and protects mitochondria from MMP induced by atractyloside (Fig. 9B). However, when a general inhibitor of GSH recycling (BCNU) is used, no protection is found (Fig. 9B). Furthermore, EA increases ANT glutathionylation levels (Fig. 9C). Taken together, these data suggest that little amounts of mitochondrial GSSG can act as signaling molecules, in much the same way as ROS do.
Several examples of protein glutathionylation or deglutathionylation involved in apoptosis control can be found in the literature: (i) glutathionylation prevents caspase-3 activation (41); (ii) glutathionylation of complex II decreases after myocardial ischemia and reperfusion, limiting the electron transfer activity of this complex (42); or (iii) reversible glutathionylation of complex I increases mitochondrial superoxide formation (43). In accordance with our data, Piantadosi et al. (44) have found that rats exposed to small amounts of CO presented higher levels of protein mixed disulfides in liver mitochondria. Further data are necessary to clarify the existence of other target proteins to be glutathionylated in the CO modulation of apoptosis. Some possible targets are Bcl-2 family proteins or the different isoforms of ANT because ANT1 and ANT3 have been identified as proapoptotic isoforms, whereas ANT2 is antiapoptotic (45).
GST is considered as an endogenous repressor of apoptosis because its interaction with ANT is lost during the apoptotic process and, on the other hand, cancer cell lines present higher levels of GST interacting with ANT (11). Ablation of GST expression increases apoptosis induced by oxidative stress with 4-hydroxynonenal (46). Moreover, GST is also a promising candidate enzyme to be involved in the ANT glutathionylation process. By immunoprecipitation assays, it was confirmed that ANT physically interacts with GST; however, CO does not alter this interaction (Fig. 8). Whether CO induces an augmentation of GST activity remains to be determined.
In this work, CO protects astrocytes from cell death by inhibiting MMP, and the process can be divided into at least three main steps: (i) ROS as signaling molecules, (ii) mitochondrial GSSG as transducing factors, and (iii) ANT as effector protein. CO appears to trigger a preconditioning-like event, by activating the cellular endogenous protective mechanisms. It is important to highlight that the mechanisms described in the present study are related to early preconditioning response because the time frame window analyzed is short. ROS generation, mitochondrial GSSG increase, and ANT glutathionylation were assessed between 5 minutes and 3 h after CO treatment. However, late preconditioning is not excluded because when apoptosis is induced 24 h after CO treatment, this molecule is still able to confer cell protection.3 In addition, it is expected that late preconditioning involves the expression of cellular antioxidant enzymes. Indeed, hypoxia-induced PC increases expression of superoxide dismutase and/or glutathione peroxidase (47, 48), and continuous CO exposure in rats increases SOD2 expression (44). Thus, in future work, the cellular antioxidant machinery behavior in response to CO will be explored. Still, it can be speculated that oxidized glutathione increases locally in mitochondria in a first time window of cellular response to CO, and in a second step (late preconditioning), cellular reduced glutathione levels might increase to prevent oxidative injury.
In summary, CO presents antiapoptotic properties in astrocytes by directly preventing mitochondrial membrane permeabilization, with GSSG as transducing factor, and acting on ANT function via critical thiol residue glutathionylation. CO is a potential antiapoptotic factor against cerebral hypoxia-ischemia and reperfusion; furthermore, it can be used as a tool to disclose the cellular pathways involved in the preconditioning phenomenon able to confer cytoprotection.
Supplementary Material
Acknowledgments
We thank João Seixas (Alfama, Portugal) for measurements of CO in solution, Dr. Lígia Martins (Instituto de Tecnologia Química e Biológia) for providing the Biotek Synergy 2 Spectrofluorimeter.
This work was supported by the Portuguese Fundação para a Ciência e Tecnologia Project PTDC/SAU-NEU/64327/2006, Helena L. A. Vieira Fellowship SFRH/BPD/27125/2006, and Cláudia S. F. Queiroga Fellowship SFRH/BD/43387/2008.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–3.
C. S. F. Queiroga, A. S. Almeida, C. Martel, C. Brenner, P. M. Alves, and H. L. A. Vieira, unpublished data.
- PC
- preconditioning
- ROS
- reactive oxygen species
- MMP
- mitochondrial membrane permeabilization
- t-BHP
- tert-butylhydroperoxide
- EA
- ethacrynic acid
- ΔΨm
- mitochondrial membrane potential
- H2DCFDA
- 2′,7′-dichlorofluorescein diacetate
- DiOC6(3)
- 3,3′ dihexyloxacarbocyanine iodide
- PI
- propidium iodide
- PTP
- permeability transition pore
- GST
- glutathione S-transferase
- PBS
- phosphate-buffered saline
- DCF
- 2′,7′-dichlorofluorescein
- BisTris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol
- BCNU
- 1,3-bis(2-chloroethyl)-1-nitrosourea or carmustine.
REFERENCES
- 1.Kirino T. (2002) J. Cereb. Blood Flow Metab. 22, 1283–1296 [DOI] [PubMed] [Google Scholar]
- 2.Dirnagl U., Meisel A. (2008) Neuropharmacology 55, 334–344 [DOI] [PubMed] [Google Scholar]
- 3.Busija D. W., Gaspar T., Domoki F., Katakam P. V., Bari F. (2008) Adv. Drug Deliv. Rev. 60, 1471–1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiegand F., Liao W., Busch C., Castell S., Knapp F., Lindauer U., Megow D., Meisel A., Redetzky A., Ruscher K., Trendelenburg G., Victorov I., Riepe M., Diener H. C., Dirnagl U. (1999) J. Cereb. Blood Flow Metab. 19, 1229–1237 [DOI] [PubMed] [Google Scholar]
- 5.Kroemer G., Galluzzi L., Brenner C. (2007) Physiol. Rev. 87, 99–163 [DOI] [PubMed] [Google Scholar]
- 6.Galluzzi L., Blomgren K., Kroemer G. (2009) Nat. Rev. Neurosci. 10, 481–494 [DOI] [PubMed] [Google Scholar]
- 7.Zoratti M., Szabò I. (1995) Biochim. Biophys. Acta 1241, 139–176 [DOI] [PubMed] [Google Scholar]
- 8.Bernardi P., Petronilli V., Di Lisa F., Forte M. (2001) Trends Biochem. Sci. 26, 112–117 [DOI] [PubMed] [Google Scholar]
- 9.Belzacq A. S., Vieira H. L., Verrier F., Vandecasteele G., Cohen I., Prévost M. C., Larquet E., Pariselli F., Petit P. X., Kahn A., Rizzuto R., Brenner C., Kroemer G. (2003) Cancer Res. 63, 541–546 [PubMed] [Google Scholar]
- 10.Marzo I., Brenner C., Zamzami N., Jürgensmeier J. M., Susin S. A., Vieira H. L., Prévost M. C., Xie Z., Matsuyama S., Reed J. C., Kroemer G. (1998) Science 281, 2027–2031 [DOI] [PubMed] [Google Scholar]
- 11.Verrier F., Deniaud A., Lebras M., Métivier D., Kroemer G., Mignotte B., Jan G., Brenner C. (2004) Oncogene 23, 8049–8064 [DOI] [PubMed] [Google Scholar]
- 12.Costantini P., Chernyak B. V., Petronilli V., Bernardi P. (1996) J. Biol. Chem. 271, 6746–6751 [DOI] [PubMed] [Google Scholar]
- 13.McStay G. P., Clarke S. J., Halestrap A. P. (2002) Biochem. J. 367, 541–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Costantini P., Belzacq A. S., Vieira H. L., Larochette N., de Pablo M. A., Zamzami N., Susin S. A., Brenner C., Kroemer G. (2000) Oncogene 19, 307–314 [DOI] [PubMed] [Google Scholar]
- 15.Ryter S. W., Alam J., Choi A. M. (2006) Physiol. Rev. 86, 583–650 [DOI] [PubMed] [Google Scholar]
- 16.Doré S. (2002) Free Radic. Biol. Med. 32, 1276–1282 [DOI] [PubMed] [Google Scholar]
- 17.Boehning D., Snyder S. H. (2003) Annu. Rev. Neurosci. 26, 105–131 [DOI] [PubMed] [Google Scholar]
- 18.Schipper H. M. (2004) Free Radic. Biol. Med. 37, 1995–2011 [DOI] [PubMed] [Google Scholar]
- 19.Bannenberg G. L., Vieira H. L. (2009) Expert Opin. Ther. Pat. 19, 663–682 [DOI] [PubMed] [Google Scholar]
- 20.D'Amico G., Lam F., Hagen T., Moncada S. (2006) J. Cell Sci. 119, 2291–2298 [DOI] [PubMed] [Google Scholar]
- 21.Sandouka A., Balogun E., Foresti R., Mann B. E., Johnson T. R., Tayem Y., Green C. J., Fuller B., Motterlini R. (2005) Cell Mol. Biol. 51, 425–432 [PubMed] [Google Scholar]
- 22.Bilban M., Haschemi A., Wegiel B., Chin B. Y., Wagner O., Otterbein L. E. (2008) J. Mol. Med. 86, 267–279 [DOI] [PubMed] [Google Scholar]
- 23.Chin B. Y., Jiang G., Wegiel B., Wang H. J., Macdonald T., Zhang X. C., Gallo D., Cszimadia E., Bach F. H., Lee P. J., Otterbein L. E. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 5109–5114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zuckerbraun B. S., Chin B. Y., Bilban M., d'Avila J. C., Rao J., Billiar T. R., Otterbein L. E. (2007) FASEB J. 21, 1099–1106 [DOI] [PubMed] [Google Scholar]
- 25.Vieira H. L., Queiroga C. S., Alves P. M. (2008) J. Neurochem. 107, 375–384 [DOI] [PubMed] [Google Scholar]
- 26.Sá Santos S., Fonseca L. L., Monteiro M. A., Carrondo M. J., Alves P. M. (2005) J. Neurosci. Res. 79, 26–32 [DOI] [PubMed] [Google Scholar]
- 27.Kristián T., Gertsch J., Bates T. E., Siesjö B. K. (2000) J. Neurochem. 74, 1999–2009 [DOI] [PubMed] [Google Scholar]
- 28.Motterlini R., Clark J. E., Foresti R., Sarathchandra P., Mann B. E., Green C. J. (2002) Circ. Res. 90, E17–E24 [DOI] [PubMed] [Google Scholar]
- 29.Belzacq-Casagrande A. S., Martel C., Pertuiset C., Borgne-Sanchez A., Jacotot E., Brenner C. (2009) Front. Biosci. 14, 3550–3562 [DOI] [PubMed] [Google Scholar]
- 30.Hirrlinger J., Dringen R. (2005) Methods Enzymol. 400, 395–409 [DOI] [PubMed] [Google Scholar]
- 31.Dringen R., Hamprecht B. (1996) J. Neurochem. 67, 1375–1382 [DOI] [PubMed] [Google Scholar]
- 32.Crompton M., Virji S., Doyle V., Johnson N., Ward J. M. (1999) Biochem. Soc. Symp. 66, 167–179 [DOI] [PubMed] [Google Scholar]
- 33.Dringen R. (2000) Prog. Neurobiol. 62, 649–671 [DOI] [PubMed] [Google Scholar]
- 34.Muyderman H., Nilsson M., Sims N. R. (2004) J. Neurosci. 24, 8019–8028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gallogly M. M., Mieyal J. J. (2007) Curr. Opin. Pharmacol. 7, 381–391 [DOI] [PubMed] [Google Scholar]
- 36.Vieira H. L., Haouzi D., El Hamel C., Jacotot E., Belzacq A. S., Brenner C., Kroemer G. (2000) Cell Death Differ. 7, 1146–1154 [DOI] [PubMed] [Google Scholar]
- 37.Brenner C., Cadiou H., Vieira H. L., Zamzami N., Marzo I., Xie Z., Leber B., Andrews D., Duclohier H., Reed J. C., Kroemer G. (2000) Oncogene 19, 329–336 [DOI] [PubMed] [Google Scholar]
- 38.Brandolin G., Le Saux A., Trezeguet V., Lauquin G. J., Vignais P. V. (1993) J. Bioenerg. Biomembr. 25, 459–472 [DOI] [PubMed] [Google Scholar]
- 39.Di Noia M. A., Van Driesche S., Palmieri F., Yang L. M., Quan S., Goodman A. I., Abraham N. G. (2006) J. Biol. Chem. 281, 15687–15693 [DOI] [PubMed] [Google Scholar]
- 40.Vesce S., Jekabsons M. B., Johnson-Cadwell L. I., Nicholls D. G. (2005) J. Biol. Chem. 280, 38720–38728 [DOI] [PubMed] [Google Scholar]
- 41.Huang Z., Pinto J. T., Deng H., Richie J. P., Jr. (2008) Biochem. Pharmacol. 75, 2234–2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen Y. R., Chen C. L., Pfeiffer D. R., Zweier J. L. (2007) J. Biol. Chem. 282, 32640–32654 [DOI] [PubMed] [Google Scholar]
- 43.Taylor E. R., Hurrell F., Shannon R. J., Lin T. K., Hirst J., Murphy M. P. (2003) J. Biol. Chem. 278, 19603–19610 [DOI] [PubMed] [Google Scholar]
- 44.Piantadosi C. A., Carraway M. S., Suliman H. B. (2006) Free Radic. Biol. Med. 40, 1332–1339 [DOI] [PubMed] [Google Scholar]
- 45.Le Bras M., Borgne-Sanchez A., Touat Z., El Dein O. S., Deniaud A., Maillier E., Lecellier G., Rebouillat D., Lemaire C., Kroemer G., Jacotot E., Brenner C. (2006) Cancer Res. 66, 9143–9152 [DOI] [PubMed] [Google Scholar]
- 46.Vaillancourt F., Fahmi H., Shi Q., Lavigne P., Ranger P., Fernandes J. C., Benderdour M. (2008) Arthritis Res. Ther. 10, R107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stroev S. A., Gluschenko T. S., Tjulkova E. I., Rybnikova E. A., Samoilov M. O., Pelto-Huikko M. (2005) Neurosci. Res. 53, 39–47 [DOI] [PubMed] [Google Scholar]
- 48.Choi Y. S., Cho K. O., Kim E. J., Sung K. W., Kim S. Y. (2007) Exp. Mol. Med. 39, 556–563 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.