Abstract
Although the literature contains many studies on the function of UCP3, its role is still being debated. It has been hypothesized that UCP3 may mediate lipid hydroperoxide (LOOH) translocation across the mitochondrial inner membrane (MIM), thus protecting the mitochondrial matrix from this very aggressive molecule. However, no experiments on mitochondria have provided evidence in support of this hypothesis. Here, using mitochondria isolated from UCP3-null mice and their wild-type littermates, we demonstrate the following. (i) In the absence of free fatty acids, proton conductance did not differ between wild-type and UCP3-null mitochondria. Addition of arachidonic acid (AA) to such mitochondria induced an increase in proton conductance, with wild-type mitochondria showing greater enhancement. In wild-type mitochondria, the uncoupling effect of AA was significantly reduced both when the release of O2˙̄ in the matrix was inhibited and when the formation of LOOH was inhibited. In UCP3-null mitochondria, however, the uncoupling effect of AA was independent of the above mechanisms. (ii) In the presence of AA, wild-type mitochondria released significantly more LOOH compared with UCP3-null mitochondria. This difference was abolished both when UCP3 was inhibited by GDP and under a condition in which there was reduced LOOH formation on the matrix side of the MIM. These data demonstrate that UCP3 is involved both in mediating the translocation of LOOH across the MIM and in LOOH-dependent mitochondrial uncoupling.
Keywords: Bioenergetics, Cell Metabolism, Metabolism, Mitochondria, Mitochondrial Metabolism, Lipid Hydroperoxide, Proton Leak, Uncoupling Protein
Introduction
Uncoupling proteins (UCPs)3 are homologous proteins belonging to a subfamily of mitochondrial anion carriers. Although both the function and mechanism of action of the first cloned UCP (UCP1) are quite well established, those of the UCP1 homologs are still far from clear. Because of its homology to UCP1 and its prevalent skeletal muscle expression, UCP3 has been hypothesized to be a thermogenic protein. However, the literature contains conflicting results, and an increased expression of UCP3 has not always been associated with mitochondrial uncoupling. This may suggest that uncoupling is not its primary function but rather a consequence of its real function (for reviews, see Refs. 1–3).
Hypotheses attributing physiological roles to UCP3 other than thermogenesis are present in the literature; however, only some of these hypotheses attribute to UCP3 the capacity to mediate mitochondrial uncoupling, and none of them has received universal acceptance (for reviews, see Refs. 1–3). One such hypothesis supposes that UCP3 mediates the transport of fatty acid anions from the mitochondrial matrix (4, 5), allowing high fatty acid oxidation rates (4) or protecting against lipotoxicity (5). However, using complementary mechanistic approaches on mitochondria from wild-type and UCP3-null mice, Seifert et al. (6) excluded a role for UCP3 in catalyzing the export of fatty acid anions from the matrix.
Another hypothesis suggests the involvement of UCP3 in the import of Ca2+ into mitochondria. Initially, using both UCP-overexpressing endothelial cells and small interfering RNA against UCP2/3-transfected cells, Trenker et al. (7) reported the involvement of UCP2 and UCP3 in mitochondrial calcium uptake induced by physiological stimuli. However, Brookes et al. (8) failed to find a difference in the calcium transport rate between UCP3-null skeletal muscle mitochondria and their wild-type controls, thus casting doubt on the ability of UCP3 to mediate Ca2+ import.
Bouillaud (9) suggested that by acting as a pyruvate anionic uniporter and promoting the extrusion of pyruvate, UCP3 inhibits its oxidation at the mitochondrial level and induces compensatory utilizations of fatty acid and glutamine. However, experimental support for the existence of such a UCP3-mediated biochemical pathway is still lacking.
That UCP3 can protect cells against oxidative damage has been widely reported (1–3, 10, 11) either by attenuating the mitochondrial production of reactive oxygen species (12, 13) or by extruding fatty acid hydroperoxides (LOOH) (14). Actually, the ability of UCP3 to reduce mitochondrial reactive oxygen species production seems to be related to its ability to lower the mitochondrial proton-motive force by inducing mild mitochondrial uncoupling because proton-motive force is a key factor influencing mitochondrial O2− release (13). Echtay et al. (15) were the first to note the ability of superoxide released into the matrix to activate UCP3 uncoupling activity, and the mechanism proposed for this process takes into account the formation of membrane lipid peroxidation products such as 4-hydroxynonenal (HNE) (16). By activating UCPs, HNE would provide a negative feedback loop that would limit the mitochondrial production of reactive oxygen species and the damage they induce (16).
An involvement of fatty acid peroxidation in the activation of UCP3 also formed part of a hypothesis proposed by Goglia and Skulachev (14), who suggested the participation of UCP3 in the export of LOOH outside the matrix. In this way, the inner leaflet of the mitochondrial inner membrane (MIM) would be cleared of LOOH that might otherwise trigger a cascade leading to oxidative damage to the mitochondrial DNA and enzymes and to other mitochondrial matrix-localized components of vital importance. Evidence supporting and indeed extending this hypothesis comes from Jaburek et al. (17), who, by performing experiments on UCP2-reconstituted liposomes, showed that UCP2 could catalyze the extrusion of LOOH and the ability of this compound to re-enter by flip-flopping. The existence of these two processes in mitochondria would be expected to lead to dissipation of proton-motive force and uncoupling. However, data obtained from a more physiological system, such as isolated mitochondria, are lacking.
In this context, we reported recently evidence supporting the idea that an interrelated role between the presence of polyunsaturated fatty acid and mitochondrial O2− production might exist in the induction of skeletal muscle mitochondrial uncoupling and that the formation of mitochondrial LOOH might be a key event in the polyunsaturated fatty acid-induced GDP-dependent inhibition of mitochondrial uncoupling (18). However, to date, there is no published evidence either that UCP3 is involved in the extrusion of LOOH from the mitochondrial matrix or that this process is associated with mitochondrial uncoupling.
This study addresses the putative roles of UCP3 in (i) LOOH-induced mitochondrial uncoupling and (ii) LOOH export across the MIM. To try to establish unambiguously whether UCP3 is involved in mediating the above processes, we employed mitochondria isolated from wild-type and UCP3-null mice.
EXPERIMENTAL PROCEDURES
Mice (Swiss Black, Taconic) harboring a truncated Ucp3 gene were originally constructed by Gong and co-workers (19) and were a kind gift from Dr. Michael N. Sack (Translational Medicine Branch, National Institutes of Health, Bethesda, MD).
Wild-type and UCP3-null mice were separated by gender, and littermates were housed in cages at 24 °C. Water was available ad libitum, as was a standard chow. Mice were anesthetized by intraperitoneal injection of chloral hydrate (40 mg/100 g of body weight) and then killed by decapitation.
Isolation of Mitochondria
Mouse skeletal muscle was gently homogenized in 10 volumes of isolation medium consisting of 220 mm mannitol, 70 mm sucrose, 20 mm Tris-HCl (pH 7.2–7.4), 1 mm EDTA, 5 mm EGTA, and 0.5% bovine serum albumin (BSA). Homogenates were centrifuged at 500 × g, and the resulting supernatant was centrifuged at 4000 × g. The mitochondrial pellet was then washed twice, resuspended in a minimal volume, and kept on ice. Mitochondria prepared for Western blot analysis were kept in the same medium supplemented with protease inhibitors. Mitochondrial protein concentration was determined by the method of Hartree (20).
Western Immunoblot Analysis
Mitochondrial lysate was prepared by resuspending the mitochondria in SDS loading buffer, followed by heating for 3 min at 95 °C. Mitochondrial lysates containing 20 μg of protein were loaded in each lane and electrophoresed on a 13% SDS-polyacrylamide gel. A polyclonal antibody against UCP3 (raised against the C-terminal region of the human UCP3 protein (AB3046) and purchased from Chemicon International (Temecula, CA)) and an anti-rabbit antibody were used as primary and secondary antibodies, respectively, in a chemiluminescence protein detection method (PerkinElmer Life Sciences). Equal loading was verified by Ponceau S staining.
Proton Leak Kinetic Assay
The kinetic response of the proton leak to a change in membrane potential was evaluated by performing malonate titration of the oligomycin-inhibited respiration rate. Respiration rate and membrane potential were measured simultaneously using a Clark-type electrode and a triphenylmethylphosphonium (TPMP+)-sensitive electrode, respectively. For these measurements, 0.75 mg of muscle mitochondrial protein was incubated in 1 ml of respiration medium (80 mm KCl, 50 mm HEPES (pH 7), 1 mm EGTA, 5 mm K2HPO4, 5 mm MgCl2, 1 μg/ml oligomycin, 80 ng/ml nigericin, and 0.5% BSA).
To measure the free fatty acid (FFA)-independent proton leak, mitochondria were incubated in the respiratory medium for 2 min to allow calibration of the TPMP+-sensitive electrode, with calibration being achieved by sequential additions of up to 2 μm TPMP+. Mitochondria were then energized using 6 mm succinate, and respiration was titrated with increasing amounts of malonate (up to 1 mm).
We chose to test the effect of arachidonic acid (AA) on the proton leak because (i) it is a polyunsaturated fatty acid and thus displays a very high sensitivity to oxidative damage (21); (ii) free radical damage and lipid peroxidation each increase as a function of the degree of unsaturation of the fatty acid (21); and (iii) Beck et al. (22) reported that among the free fatty acids tested, AA had the greatest capacity to induce proton conductance in planar lipid membranes reconstituted with purified recombinant human UCPs. To test the effect of AA on mitochondrial uncoupling, malonate titrations of the respiration rate were performed in the presence of 240 μm AA. (According to Richieri et al. (23), because of the presence of BSA, the ratio between free AA and milligrams of mitochondrial protein corresponds to 159 pmol/mg of mitochondrial protein.) To exclude the involvement of adenine nucleotide translocase (ANT) in the AA-induced proton leak, carboxyatractyloside (CAT; 10 μm) was added to the incubation medium.
To test whether O2− production via reverse electron transport from complex II to complex I is involved in the AA-induced proton leak, rotenone (4 μm) was present in the respiration medium where indicated. To test the effect of phenylbutylnitrone (PBN) on the AA-induced proton leak, it was present in the respiration medium at a concentration of 1 mm where indicated. The effect of GDP on the FFA-induced proton leak was tested by adding it to the incubation medium at a concentration of 0.5 mm. Each of the above compounds was added to the incubation medium before the mitochondria were added.
Base-line correction was obtained by the addition of 0.2 μm carbonyl cyanide p-trifluoromethoxyphenylhydrazone, which induces complete release of TPMP+. A TPMP+-binding correction of 0.4 (μl/mg of protein) was applied. Under all experimental conditions, EGTA was present in the incubation medium to prevent the opening of transition pores (24).
Detection of Lipid Hydroperoxide Release by Mitochondria
We detected mitochondrial LOOH release using the probe Amplex Red (Molecular Probes, Eugene, OR), which is known to be unable to permeate mitochondria (27), in the presence of both peroxidase and catalase. Actually, horseradish peroxidase catalyzes both the H2O2- and LOOH-dependent oxidation of non-fluorescent Amplex Red to fluorescent resorufin red (27). Consequently, to evaluate the LOOH-dependent oxidation, an excess of catalase in the incubation medium is required (i) to remove rapidly the H2O2 released by mitochondria and (ii) to allow us to attribute to mitochondrial LOOH release the detected increase in fluorescence. Under our experimental conditions, we detected mitochondrial LOOH release employing Amplex Red, horseradish peroxidase, and catalase using the methods suggested by Bhattacharya et al. (27).
To test the ability of UCP3 to translocate LOOH from the inner to the outer leaflet of the MIM, we modified the experimental condition reported by Bhattacharya et al. (27) to allow us to compare mitochondrial LOOH release between two situations: when high levels of LOOH are formed at the matrix side of the MIM and when this process is inhibited. To achieve the formation of a high level of LOOH at the matrix side of the MIM, we added AA to the mitochondria, and we energized them with succinate in the absence of rotenone. Under this condition, mitochondria release high levels of O2− into the matrix. Inhibition of LOOH formation can be achieved by adding rotenone to the incubation medium because it inhibits the reverse electron transport from complex II to complex I and the subsequent release of O2− into the matrix. We avoided the eventual reduction of LOOH to LOH in the outer leaflet of the inner membrane by inhibiting glutathione peroxidase, which has been reported to be localized within the intermembrane space (for some indications, see Refs. 25 and 26). This was done using diethyl malate. Under this condition, if UCP3 exports LOOH across the MIM, it will be released by the mitochondria. To evaluate the role played by UCP3 in the release of LOOH, we performed studies on wild-type and UCP3-null mice, and we inhibited UCP3 with GDP. However, because GDP binds ANT (28), we inhibited the latter with CAT.
Mouse mitochondria (0.1 mg/ml) were incubated at 37 °C for a total of 12 min in a final volume of 2 ml of respiration medium supplemented with 10 μm CAT, 120 μm AA (which, according to Richieri et al. (23), corresponds to a free concentration of 15.9 nm; thus, the ratio between AA and milligrams of mitochondrial protein was 159 (pmol/mg of mitochondrial protein)), 80 μm Amplex Red, 4 units/ml catalase, 2 units/ml horseradish peroxidase, and 2 mm diethyl malate in a fluorometer from Jansko. In some experiments, where indicated in the relevant figure legends, 0.5 mm GDP and 4 μm rotenone (alone or in combination) were added to the assay medium.
For all incubation periods, fluorescence was detected using an excitation wavelength of 545 nm and an emission wavelength of 590 nm. After 8 min of incubation, mitochondrial respiration was started by the addition of succinate. The increase in fluorescence that preceded succinate addition (detected between 7 and 8 min) was then subtracted from that detected following succinate addition, allowing us to evaluate energization-dependent mitochondrial hydroperoxide release. Calibration curves were obtained by the addition of known amounts of tert-butylhydroperoxide (0–2500 pmol) to the assay medium in the presence of the reactants (Amplex Red and horseradish peroxidase).
As AA easily undergoes peroxidation processes, for our measurements, we used only freshly prepared AA solution. In addition, to exclude the possibility that the AA solution might already have undergone peroxidation, we tested its ability to induce Amplex Red oxidation when progressively added to the incubation medium in the presence of reactants (peroxidase and catalase). In fact, our freshly prepared AA did not exhibit any reaction with Amplex Red.
RESULTS
Mitochondrial UCP3 Protein Content
To determine whether mitochondrial UCP3 protein is really absent in the skeletal muscle of UCP3-null mice, we performed Western blot analysis on protein isolated from freshly prepared mitochondria. A UCP3-specific signal was detected in wild-type mitochondria but not in UCP3-null mitochondria (Fig. 1).
FIGURE 1.
Mitochondrial UCP3 protein levels in gastrocnemius muscles from three wild-type and three UCP3-null mice. The Western blot (using 20 μg of protein isolated from gastrocnemius muscle mitochondria) shows 35-kDa UCP3 protein.
FFA-independent Proton Leak Kinetics in Wild-type and UCP3-null Mitochondria
With mitochondrial endogenous FFA chelated by BSA, there was no difference in proton leak kinetics between wild-type and UCP3-null succinate-energized mitochondria (Fig. 2). The contribution of ANT to the FFA-independent proton leak was similar between wild-type and UCP3-null mitochondria; indeed, when ANT was inhibited by CAT, a reduction in the proton leak rate was observed in both wild-type and UCP3-null mitochondria, and overlapping proton leak kinetics were still detected (data not shown).
FIGURE 2.
Proton leak kinetics of mitochondria isolated from wild-type and UCP3-null mice. The proton leak rate (expressed as the respiration rate that drives the leak) is plotted as a function of membrane potential. This was varied by titration with malonate. Skeletal muscle mitochondria were from wild-type (●) and UCP3-null (□) mice. Values are means ± S.E. of three independent experiments, each one performed in duplicate. prot, protein.
Ability of Arachidonic Acid to Induce the Mitochondrial Proton Leak in Wild-type and UCP3-null Mitochondria and Involvement of O2− Release into the Matrix and the Lipid Peroxidation Process
AA induced the proton leak in both wild-type and UCP3-null mitochondria (Fig. 3). In the presence of AA, mitochondria have to respire at a higher level to maintain the same membrane potential, the effect being greater in wild-type than UCP3-null mitochondria (Fig. 3, compare A and B). Part of the uncoupling effect of AA was abolished by GDP in wild-type mitochondria (Fig. 3A) but not in UCP3-null mitochondria (Fig. 3B).
FIGURE 3.

Effect of AA and GDP on proton leak kinetics of skeletal muscle mitochondria isolated from wild-type (A) and UCP3-null (B) mice. The proton leak rate (expressed as the respiration rate that drives the leak) is plotted as a function of membrane potential and was detected in the presence of CAT. The membrane potential was varied by titration with malonate. The insets show the -fold proton leak induction (evaluated by measuring the increase in the respiration rate at the maximal common membrane potential (144 mV)). Error bars labeled with different letters are significantly different (p < 0.05). Black circles and black bars, mitochondria with no addition; white circles and white bars, mitochondria with AA; gray circles and gray bars, mitochondria with AA plus GDP. Values are means ± S.E. of four independent experiments, each one performed in duplicate. prot, protein.
Induction of the proton leak by AA can be evaluated by quantifying the increase in respiration rate observed at a fixed membrane potential. We chose to calculate it at the highest common potential (144 mV). At that membrane potential, in wild-type mitochondria, AA induced an increase in respiration rate (which is an index of the proton leak rate) of +303%, and GDP reduced this value to +135% (Fig. 3A, inset). In the presence of AA with or without GDP, the corresponding value in UCP3-null mitochondria, again at 144 mV, was about +160% (Fig. 3B, inset).
We next tested the involvement of O2− (released into the matrix via reverse electron transport from complex II to complex I) in the AA-induced proton leak and also the sensitivity of such uncoupling to GDP. In wild-type mitochondria, AA-induced uncoupling was weaker in the presence of rotenone than in its absence (compare Figs. 3A and 4A), and in the presence of rotenone, it was insensitive to GDP (Fig. 4A). In contrast, in UCP3-null mitochondria, rotenone did not affect the ability of AA to induce uncoupling (compare Figs. 3B and 4C). Next, we quantified the AA-induced increase in the proton leak at 144 mV; this allowed us to make a comparison with data obtained in the absence of rotenone. In wild-type mitochondria, AA induced an increase in the proton leak of about +130% (at 144 mV), with the addition of GDP proving ineffective (Fig. 4C, inset). Quantitatively similar results were obtained in UCP3-null mitochondria under the same experimental condition (Fig. 4A, inset). Collectively, these data show that an interrelated role between O2− and AA exists in the induction of UCP3-mediated mitochondrial uncoupling and that lipid peroxidation may be involved in this process.
FIGURE 4.
Ability of AA to induce the mitochondrial proton leak in skeletal muscle mitochondria isolated from wild-type (A and B) and UCP3-null (C and D) mitochondria when the release of O2− into the matrix is inhibited by rotenone (A and C) and when lipid peroxidation is inhibited by PBN (B and D) in the presence or absence of GDP. The proton leak rate (expressed as the respiration rate that drives the leak) is plotted as a function of membrane potential and was detected in the presence of CAT. The membrane potential was varied by titration with malonate. The insets show -fold proton leak induction (evaluated by measuring the increase in the respiration rate at the membrane potential 144 mV). Error bars labeled with different letters are significantly different (p < 0.05). Black circles and black bars, mitochondria with no addition; white circles and white bars, mitochondria with arachidonic AA; gray circles and gray bars, mitochondria with AA plus GDP. Values are means ± S.E. of three independent experiments, each one performed in duplicate. prot, protein.
To test whether the AA-induced increase in the proton leak involved lipid peroxidation processes, we inhibited these processes by adding PBN (a chain-breaking spin trap able to quench carbon-centered radical intermediates) to the respiratory medium. In wild-type mitochondria, AA-induced uncoupling was weaker in the presence of PBN than in its absence (compare Figs. 3A and 4B), and the addition of GDP was ineffective (Fig. 4B). Under the same conditions, in UCP3-null mitochondria, the addition of PBN was ineffective (compare Figs. 3B and 4D). Quantifying the increase in the proton leak at 144 mV revealed that in wild-type mitochondria, AA induced an increase of +135% whether PBN was present alone or in combination with GDP (Fig. 4B, inset). Similarly, the AA-induced increase was not significantly different between PBN with GDP and PBN without GDP in UCP3-null mitochondria (Fig. 4D, inset).
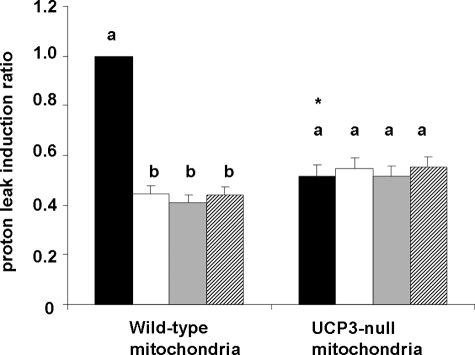
To assess the contribution made by UCP3 to the AA-induced proton leak, we related the induced proton leak observed under each of the experimental conditions used in both wild-type and UCP3-null mitochondria to the maximal observed induction. Actually, the maximal AA-induced proton leak was detected in wild-type mitochondria in the absence of any inhibitors. As shown in Fig. 5, in wild-type mitochondria, a significant part of the AA-induced proton leak seemed to be mediated by UCP3 because (i) in wild-type mitochondria, ∼55% of AA-induced uncoupling was abolished by GDP, and (ii) the AA-induced proton leak observed in UCP3-null mitochondria was ∼50% of that observed in wild-type mitochondria. In addition, in wild-type mitochondria, ∼50% of the uncoupling effect of AA was abolished by either rotenone or PBN, yet in UCP3-null mitochondria, these inhibitors were ineffective. These data show that the release of O2− into the matrix and the formation of LOOH are crucial processes to show AA-induced UCP3-mediated uncoupling.
FIGURE 5.
Induction of the proton leak by AA under various experimental conditions (expressed relative to maximal induction). Values represent the ratio between the AA-induced proton leak under each experimental condition (i.e. AA alone or in combination with one of the inhibitors: GDP, rotenone, or PBN) and the maximal induction (i.e. that observed in wild-type mitochondria in the absence of any inhibitors (black bar in left panel)). Black bars, mitochondria with AA; white bars, mitochondria with AA plus GDP; gray bars, mitochondria with AA plus rotenone; hatched bars, mitochondria with AA plus PBN. Values are means ± S.E. of three independent experiments, each one performed in duplicate. For a given mitochondrial type, error bars labeled with different letters are significantly different (p < 0.05). *, significant difference versus the same experimental condition in wild-type mitochondria (p < 0.05).
Mitochondrial Lipid Hydroperoxide Release
We evaluated the ability of wild-type and UCP3-null mitochondria to release LOOH when a high level of this compound was formed at the matrix side of the MIM. This situation is obtained through the exogenous addition of AA to mitochondria and the energization of the mitochondria with succinate in the absence of rotenone. We found that wild-type mitochondria released a significantly greater amount of LOOH (+55%) compared with UCP3-null mitochondria (Fig. 6) and that GDP significantly reduced the release of LOOH from wild-type mitochondria (to the levels observed in UCP3-null mitochondria). Moreover, GDP was ineffective at inhibiting LOOH release in UCP3-null mitochondria (Fig. 6). These data show that UCP3 plays a role in mitochondrial LOOH release.
FIGURE 6.
Ability of mitochondria to release LOOH: comparison between wild-type and UCP3-null mitochondria and effects of rotenone and GDP on this process. Results are means ± S.E. of six (wild-type) and four (UCP3-null) independent experiments, each one performed in duplicate. For a given mitochondrial type, error bars labeled with different letters are significantly different (p < 0.01). Black bars, mitochondria with AA; white bars, mitochondria with AA plus GDP; gray bars, mitochondria with AA plus rotenone; hatched bars, mitochondria with AA plus rotenone and GDP. *, significant difference versus the same experimental condition in wild-type mitochondria (p < 0.01). prot, protein.
In the presence of rotenone (low level of O2˙̄ release into the matrix and low level of LOOH formation), wild-type mitochondria showed a reduction in their ability to release LOOH (Fig. 6), the level being similar to that observed in UCP3-null mitochondria. Moreover, under such conditions, GDP lost its ability to inhibit LOOH release from wild-type mitochondria. In contrast, the addition of rotenone did not significantly affect LOOH release from UCP3-null mitochondria (Fig. 6). These data show that O2− release into the matrix is fundamental if involvement of UCP3 in mitochondrial LOOH release is to be evident and that UCP3 plays a role in the export of the LOOH produced at the level of the matrix side of the MIM.
DISCUSSION
Despite ever expanding literature documenting the many attempts to elucidate the function of UCP3, it has not been established, and none of the hypotheses formulated to ascribe a role to UCP3 has universal acceptance (1–3). Our results support (a) a role for UCP3 in the export of LOOH from the inner to the outer leaflet of the MIM and (b) lipid peroxidation as a key event in UCP3-mediated uncoupling.
Lipid Hydroperoxide Formation Is a Key Event in Activation of the UCP3-mediated Proton Leak
Despite the fact that the mechanism by which UCP3 catalyzes the proton leak is unclear, there is a broad consensus that UCP3 catalyzes the proton leak only in the presence of specific cofactors, with FFA and reactive oxygen species playing crucial roles (29, 30). Our data show that O2− per se, independent of FFA, was unable to induce UCP3-mediated uncoupling. In fact, despite a high level of O2− release into the matrix, we did not detect a difference in proton leak kinetics between wild-type and UCP3-null mitochondria in the absence of FFA (Fig. 2). These data conflict with those published by others (19, 31), who reported a reduced basal proton leak in UCP3-null mitochondria versus wild-type mitochondria. However, they are in agreement with the results of Cadenas et al. (32), who reported an overlap in basal proton leak kinetics between wild-type and UCP3-null mitochondria. More recently, Parker et al. (33) described an endogenous activation of proton conductance (catalyzed partly by UCP3 and partly by ANT) that increased with the time of mitochondrial energization and that disappeared when a high level of BSA was present in the respiratory medium. The lack of a difference in the proton leak between UCP3-null and wild-type mitochondria, as reported here, is probably due to (i) the duration of the substrate energization (<2 min) probably not being enough for endogenous activation of the proton leak and (ii) the presence of a high concentration of BSA during mitochondrial isolation and in the respiratory medium.
When we excluded the contribution of ANT to basal proton conductance (by inhibiting it with CAT), we still did not observe any difference in proton leak kinetics between wild-type and UCP3-null mitochondria (data not shown), suggesting that the contribution made by ANT to the basal proton leak is not affected by the presence of UCP3. These data fit well with the results of Parker et al. (33), who found no difference in ANT levels between UCP3-null and wild-type mitochondria.
Our data suggest that an interrelated role between O2− and AA exists in the induction of UCP3-mediated uncoupling and that lipid peroxidation is involved in this process. Indeed, in wild-type mitochondria, the ability of AA to induce the proton leak appears to be related to the capacity of the mitochondria to produce O2−, being significantly reduced when O2− production was inhibited by rotenone (compare Figs. 3A and 4A and see Fig. 5). This indicates that a peroxidative process is responsible for the uncoupling effect of AA.
The involvement of such a process is also suggested by data we obtained when lipid peroxidation was inhibited by PBN. In the presence of PBN, AA lost a significant part of its capacity to induce mitochondrial uncoupling (compare Figs. 3A and 4B and see Fig. 5). In addition, our evidence that GDP was able to exert its inhibitory effect on AA-induced uncoupling in wild-type mitochondria only when rotenone and PBN were absent in the incubation medium (compare Figs. 3A and 4, A and B) supports the involvement of a peroxidative process in UCP3-mediated uncoupling. This conclusion was further supported by our findings that in UCP3-null mitochondria, (i) the capacity of AA to induce uncoupling was reduced versus that in wild-type mitochondria (Figs. 3 and 5), and (ii) rotenone and PBN did not affect AA-induced uncoupling, in contrast to their suppressive effects in wild-type mitochondria (compare Figs. 3 and 4). In addition, about half of the uncoupling induced by AA was abolished (i) when UCP3 was unable to contribute to the proton leak (i.e. in GDP-treated wild-type mitochondria or in UCP3-null mitochondria) or (ii) when AA could not undergo peroxidation (Fig. 5). Collectively, the above data strongly indicate that lipid peroxidation is a key event in the activation of UCP3-mediated uncoupling.
Some authors have reported that the LOOH metabolite HNE can activate UCP3-mediated uncoupling (16, 33, 34), but it requires a very high membrane potential to act (34) because it was ineffective in inducing the UCP3-mediated proton leak following a decrease of 10–20 mV in the mitochondrial membrane potential. Under our experimental conditions, the membrane potential of wild-type mitochondria was reduced by ∼20 mV by the presence of AA (as shown in Fig. 4A), so we can attribute to LOOH itself the ability to activate UCP3, i.e. we can rule out the involvement of HNE. However, our data do not exclude HNE being able to activate UCP3-mediated uncoupling at a high membrane potential.
UCP3 Is Involved in the Release of LOOH by Mitochondria
To examine the involvement of UCP3 in the translocation of LOOH across the MIM, we investigated mitochondrial LOOH release when the formation of LOOH at the matrix side of the MIM was high and also when such formation was inhibited (details of the system and reasons for choosing the inhibitors used are provided under “Experimental Procedures”). Our data show that mitochondria were able to release LOOH into the incubation medium, confirming the report by Bhattacharya et al. (27). Moreover, it was evident that a significant part of that release was attributable to UCP3 (Fig. 6). Indeed, in wild-type mitochondria, GDP significantly reduced such LOOH release, whereas in UCP3-null mitochondria, which displayed a limited capacity to release LOOH compared with their wild-type littermate controls, GDP was ineffective.
O2− released into the matrix plays a role in UCP3-mediated mitochondrial LOOH release. In fact, when the production of O2− and its release into the matrix were inhibited by rotenone, a reduced mitochondrial LOOH release from wild-type mitochondria but not from UCP3-null mitochondria was detected. Moreover, the inhibitory effect of rotenone did not summate with that of GDP (Fig. 6). This indicates that both the O2− release into the matrix and (presumably) the consequent formation of LOOH at the level of the matrix side of the MIM are crucial factors in UCP3-mediated LOOH release and suggests that UCP3 mediates the extrusion of LOOH across the MIM.
Hypothesis of How UCP3-mediated LOOH Export Is Related to UCP3-mediated Uncoupling
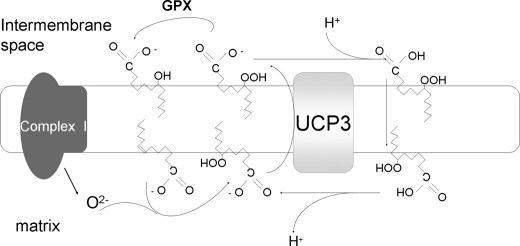
Our results are consistent with LOOH being translocated by UCP3 from the inner to the outer leaflet of the MIM. They thereby lend experimental support to the hypothesis proposed by Goglia and Skulachev (14), which had been supported previously only by results of experiments performed on UCP2-reconstituted liposomes (17). Our data also indicate that the formation of LOOH at the matrix side of the MIM is a key event in the activation of UCP3-mediated uncoupling. The questions remains as to how the two events are interrelated. We hypothesize (see Fig. 7 for a possible schema) that when mitochondria produce and release a high level of O2− at the matrix side of their inner membrane, a high level of LOOH is also produced at this site. LOOH (in its anionic form) is then translocated across the MIM. Once in the outer leaflet part of the MIM, some LOOH can be metabolized by lipid glutathione peroxidase to LOH, and the remainder (in the protonated form) can flip back into the matrix. Such LOOH extrusion and the associated flipping back of LOOH into the matrix would lead to uncoupling. The notion that LOOH can cross membranes is supported by the data obtained using liposomes by Jaburek et al. (17).
FIGURE 7.
Proposed mechanism of action of UCP3. When a high level of O2− is produced and released into the matrix, the LOOH formed at the inner leaflet of the MIM is translocated (in the anionic form) across the MIM. Once in the outer leaflet, some LOOH acts as a substrate for glutathione peroxidase (GPX), whereas the remainder (in the protonated form) flips back across the membrane toward the matrix. This cycle protects the mitochondrial DNA, enzymes, and other vitally important mitochondrial matrix-localized components against LOOH-induced oxidation. The same cycle causes dissipation of proton-motive force.
This work was supported by Ministero dell'Istruzione, dell'Università e della Ricerca COFIN-MIUR 2008.
- UCP
- uncoupling protein
- LOOH
- lipid hydroperoxide(s)
- HNE
- 4-hydroxynonenal
- MIM
- mitochondrial inner membrane
- BSA
- bovine serum albumin
- TPMP+
- triphenylmethylphosphonium
- FFA
- free fatty acid
- AA
- arachidonic acid
- ANT
- adenine nucleotide translocase
- CAT
- carboxyatractyloside
- PBN
- phenylbutylnitrone.
REFERENCES
- 1.Brand M. D., Esteves T. C. (2005) Cell Metab. 2, 85–93 [DOI] [PubMed] [Google Scholar]
- 2.Echtay K. S. (2007) Free Radic. Biol. Med. 43, 1351–1371 [DOI] [PubMed] [Google Scholar]
- 3.Cioffi F., Senese R., de Lange P., Goglia F., Lanni A., Lombardi A. (2009) Biofactors 35, 417–428 [DOI] [PubMed] [Google Scholar]
- 4.Himms-Hagen J., Harper M. H. (2001) Exp. Biol. Med. 226, 8–84 [DOI] [PubMed] [Google Scholar]
- 5.Schrauwen P., Hesselink M. K. (2004) Proc. Nutr. Soc. 63, 287–292 [DOI] [PubMed] [Google Scholar]
- 6.Seifert E. L., Bézaire V., Estey C., Harper M. E. (2008) J. Biol. Chem. 283, 25124–25131 [DOI] [PubMed] [Google Scholar]
- 7.Trenker M., Malli R., Fertschai I., Levak-Frank S., Graier W. F. (2007) Nat. Cell Biol. 9, 445–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brookes P. S., Parker N., Buckingham J. A., Vidal-Puig A., Helestrap A. P., Gunter T. E., Nicholls D. G., Bernardi P., Lemasters J. J., Brand M. D. (2008) Nat. Cell Biol. 10, 235–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bouillaud F. (2009) Biochim. Biophys. Acta 1787, 377–383 [DOI] [PubMed] [Google Scholar]
- 10.Brand M. D., Pamplona R., Portero-Otín M., Requena J. R., Roebuck S. J., Buckingham J. A., Clapham J. C., Cadenas S. (2002) Biochem. J. 368, 597–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Talbot D. A., Brand M. D. (2005) Biochim. Biophys. Acta 1709, 150–156 [DOI] [PubMed] [Google Scholar]
- 12.Skulachev V. P. (1996) Q. Rev. Biophys. 29, 169–202 [DOI] [PubMed] [Google Scholar]
- 13.Korshunov S. S., Skulachev V. P., Starkov A. A. (1997) FEBS Lett. 416, 15–18 [DOI] [PubMed] [Google Scholar]
- 14.Goglia F., Skulachev V. P. (2003) FASEB J. 17, 1585–1591 [DOI] [PubMed] [Google Scholar]
- 15.Echtay K. S., Murphy M. P., Smith R. A., Talbot D. A., Brand M. D. (2002) J. Biol. Chem. 277, 47129–47135 [DOI] [PubMed] [Google Scholar]
- 16.Echtay K. S., Esteves T. C., Pakay J. L., Jekabsons M. B., Lambert A. J., Portero-Otín M., Pamplona R., Vidal-Puig A. J., Wang S., Roebuck S. J., Brand M. D. (2003) EMBO J. 22, 4103–4110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaburek M., Miyamoto S., Di Mascio P., Garlid K. D., Jezek P. (2004) J. Biol. Chem. 279, 53097–53102 [DOI] [PubMed] [Google Scholar]
- 18.Lombardi A., Grasso P., Moreno M., de Lange P., Silvestri E., Lanni A., Goglia F. (2008) Biochim. Biophys. Acta 1777, 826–833 [DOI] [PubMed] [Google Scholar]
- 19.Gong D. W., He Y., Karas M., Reitman M. (1997) J. Biol. Chem. 272, 24129–24132 [DOI] [PubMed] [Google Scholar]
- 20.Hartree E. F. (1972) Anal. Biochem. 48, 422–427 [DOI] [PubMed] [Google Scholar]
- 21.Pamplona R., Portero-Otín M., Riba D., Requena J. R., Thorpe S. R., López-Torres M., Barja G. (2000) J. Gerontol. A Biol. Sci. Med. Sci. 55, B286–B291 [DOI] [PubMed] [Google Scholar]
- 22.Beck V., Jabůrek M., Demina T., Rupprecht A., Porter R. K., Jezek P., Pohl E. E. (2007) FASEB J. 21, 1137–1144 [DOI] [PubMed] [Google Scholar]
- 23.Richieri V. G., Anel A., Kleinfeld A. M. (1993) Biochemistry 32, 7574–7580 [DOI] [PubMed] [Google Scholar]
- 24.Crompton M. (1999) Biochem. J. 341, 233–249 [PMC free article] [PubMed] [Google Scholar]
- 25.Imai H., Nakagawa Y. (2003) Biol. Med. 34, 145–169 [DOI] [PubMed] [Google Scholar]
- 26.Nomura K., Imai H., Koumura T., Kobayashi T., Nakagawa Y. (2000) Biochem. J. 351, 183–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhattacharya A., Muller F. L., Liu Y., Sabia M., Liang H., Song W., Jang Y. C., Ran Q., Van Remmen H. (2009) J. Biol. Chem. 284, 46–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khailova L. S., Prikhodko E. A., Dedukhova V. I., Mokhova E. N., Popov V. N., Skulachev V. P. (2006) Biochim. Biophys. Acta 1757, 1324–1329 [DOI] [PubMed] [Google Scholar]
- 29.Brand M. D., Affourtit C., Esteves T. C., Green K., Lambert A. J., Miwa S., Pakay J. L., Parker N. (2004) Free Radic. Biol. Med. 37, 755–767 [DOI] [PubMed] [Google Scholar]
- 30.Lanni A., Beneduce L., Lombardi A., Moreno M., Boss O., Muzzin P., Giacobino J. P., Goglia F. (1999) FEBS Lett. 444, 250–254 [DOI] [PubMed] [Google Scholar]
- 31.Vidal-Puig A. J., Grujic D., Zhang C. Y., Hagen T., Boss O., Ido Y., Szczepanik A., Wade J., Mootha V., Cortright R., Muoio D. M., Lowell B. B. (2000) J. Biol. Chem. 275, 16258–16266 [DOI] [PubMed] [Google Scholar]
- 32.Cadenas S., Echtay K. S., Harper J. A., Jekabsons M. B., Buckingham J. A., Grau E., Abuin A., Chapman H., Clapham J. C., Brand M. D. (2002) J. Biol. Chem. 277, 2773–2778 [DOI] [PubMed] [Google Scholar]
- 33.Parker N., Affourtit C., Vidal-Puig A., Brand M. D. (2008) Biochem. J. 412, 131–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parker N., Vidal-Puig A., Brand M. D. (2008) Biosci. Rep. 28, 83–88 [DOI] [PMC free article] [PubMed] [Google Scholar]