Abstract
Limb girdle muscular dystrophy 2C is caused by mutations in the γ-sarcoglycan gene (gsg) that results in loss of this protein, and disruption of the sarcoglycan (SG) complex. Signal transduction after mechanical perturbation is mediated, in part, through the SG complex and leads to phosphorylation of tyrosines on the intracellular portions of the sarcoglycans. This study tested if the Tyr6 in the intracellular region of γ-sarcoglycan protein (γ-SG) was necessary for proper localization of the protein in skeletal muscle membranes or for the normal pattern of ERK1/2 phosphorylation after eccentric contractions. Viral mediated gene transfer of wild type gsg (WTgsg) and mutant gsg lacking Tyr6 (Y6Agsg) was performed into the muscles of gsg−/− mice. Muscles were examined for production and stability of the γ-SG, as well as the level of ERK1/2 phosphorylation before and after eccentric contraction. Sarcolemmal localization of γ-SG was achieved regardless of which construct was expressed. However, only expression of WTgsg corrected the aberrant ERK1/2 phosphorylation associated with the absence of γ-SG, whereas Y6Agsg failed to have any effect. This study shows that localization of γ-SG does not require Tyr6, but localization alone is insufficient for restoration of normal signal transduction patterns after mechanical perturbation.
Keywords: Diseases/Muscular Dystrophy, Receptors/Membrane, Signal Transduction/Phosphotyrosine, Signal Transduction/Protein Kinases/MAP, Tissue/Organ Systems/Muscle/Skeletal, Adeno-Associated Virus
Introduction
More than 30 genetic disorders of muscle have been identified and arise from mutations in a wide range of genes including those that encode proteins of the dystrophin glycoprotein complex (DGC),2 the nuclear membrane, and extracellular matrix (1). Although each disease manifests in a unique pattern, there is a common set of pathologies associated with the muscular dystrophies. Disease hallmarks include muscle weakness, fragility, and degeneration.
Many of the limb girdle muscular dystrophies are caused by mutations in the sarcoglycans. The sarcoglycan (SG) complex is a subcomplex of the DGC, comprised of α-, β-, γ-, and δ-SG in skeletal muscle (2, 3). These proteins form an integral membrane complex with a short intracellular domain, a single transmembrane focal domain, and a large extracellular domain. The intracellular regions of α-, β-, and γ-SG have potential tyrosine phosphorylation sites. In cell culture studies, adhesion gives rise to phosphorylation of each of these SGs, indicating that the SGs can be modified in response to cell attachment (4). Furthermore, we have been able to identify the SG complex as a player in the mechanical signal transduction process, where γ-SG tyrosine phosphorylation occurs after a series of eccentric contractions, and disruption of the SG complex in mice lacking γ-SG, which is a genetic model for limb girdle muscular dystrophies 2C, leads to an aberrant ERK1/2 response that is distinct from that observed in mdx mice in which the entire DGC is absent (5). Based on these findings, the SG complex has been proposed to be a mechanosensor, which can convert changes in load at the sarcolemma to distinct changes in gene expression and maintenance of muscle survival (6, 7).
Restoration of the SG complex can be achieved by viral mediated gene transfer of the missing protein (8–10). This approach is an attractive therapeutic strategy for the sarcoglycanopathies, for the small size of the gene is within the packaging limits of adeno-associated virus, which has high tropism for muscle with low immunogenicity (11, 12). In addition to providing a method for potentially curing the disease, viral gene transfer can be utilized in combination with site-directed mutagenesis to identify critical residues for the function of the sarcoglycans. Thus, this approach can establish which regions of the sarcoglycans are important for proper assembly (13) and which domains are important for signaling. If the mutated domains play a role in mechanical signal transduction, then absence of the native domain should mimic loss of the protein and not restore the muscle to a healthy state. Alternatively, if the domain is unimportant, then the response of the treated muscle to mechanical stimuli should resemble normal muscle. The goal of this study was to test whether restoration of healthy muscle requires correct assembly of the sarcoglycan complex and whether post-translational modification of γ-SG is important for mediating the intracellular response of skeletal muscle to mechanical load.
EXPERIMENTAL PROCEDURES
Animals
The University of Pennsylvania's Animal Care and Use Committee approved all experiments. Two groups of mice were utilized for this study: γ-SG-null mice (gsg−/−) and the strain-matched control, C57BL/6 (C57). The gsg−/− mouse lacks γ-SG via gene targeting resulting in the additional loss of β- and δ-SG and decrease of α-SG (14). Both male and female mice were utilized in all groups.
Viral Constructs
Human γ sarcoglycan cDNA was utilized to generate recombinant adeno-associated virus serotype 2/8 (rAAV). Site-directed mutagenesis (QuikChange II, Stratagene, La Jolla, CA) was utilized to mutate Tyr6 to Ala (Y6Agsg) blocking the putative phosphorylation site (4, 5). Expression of both wild type (WTgsg) and Y6Agsg cDNAs were regulated by a truncated desmin promoter (15). Vector production was performed at the University of Pennsylvania Vector Core.
Viral Injections
The anterior hind limb muscles of 2-week-old gsg−/− animals were injected with 1 × 1011 rAAV particles diluted in 75–100 μl of phosphate-buffered saline or phosphate-buffered saline alone as described previously (16). Mice were sacrificed at 1 and 5 months post-injection for analysis. At the specified time point, the tibialis anterior (TA) muscles were dissected, washed in phosphate-buffered saline, blotted, weighed, and frozen rapidly in melting isopentane for histological measurements. The extensor digitorum longus (EDL) was utilized for isolated force measurements as described below.
Whole Muscle Mechanics
Isolated whole muscle mechanics were performed on the EDL muscles from 6–7-week-old animals as described previously (5). Muscles were incubated in a bath of Ringer's solution gas, equilibrated with 95% O2 and 5% CO2 and maintained at 22 °C. After determining optimum length, muscles were subjected to three isometric contractions stimulated at 120 Hz for 500 msec, followed by a series of five eccentric contractions with stimulation at 80 Hz for 700 msec, where a stretch of 10% optimum length was imposed on the muscle in the last 200 msec of the contraction. Each contraction was separated by a 5-min rest period. After a series of contractions, the muscles were removed from the mechanics apparatus and placed in oxygenated Ringer's for 30 min (ECC30). A set of unstimulated muscles were kept in oxygenated Ringers for the duration of the experiment and served as unstimulated controls (No Stim). A second set of EDL muscles were subjected to ECC30 or No Stim in the absence or presence of 50 μm PD98059 (Sigma). A third set of EDL muscles were isolated from mice 5 months after viral injection and subjected to isometric force measurements only. At the end of this period, muscles were rapidly frozen in liquid nitrogen and stored at −200 °C for subsequent analysis.
Immunoblotting
Homogenized lysates from treated and control muscles were probed with antibodies that recognize the phosphorylated and total forms of ERK1/2 MAPK (catalog nos. 9101, 9102, and 9107, Cell Signaling, Beverly, MA), γ-SG (NCL-g-SARC, Vector Laboratories, Burlingame, CA), integrin β1D (ab8991, Abcam, Inc., Cambridge, MA), and α-tubulin (Sigma-Aldrich, St. Louis, MO). Muscles were homogenized in 10 volumes/muscle wet weight of modified lysis buffer (50 mm Tris-HCl, pH 7.4, 1% (w/v) Triton X-100, 0.25% sodium deoxycholate, 150 mm NaCl, 1 mm phenylmethylsulfonyl fluoride, 1 mg/ml aprotinin, 1 mg/ml leupeptin, 1 mg/ml pepstatin, 1 mm NaVO4, 1 mm NaF, 1 mm EGTA). Homogenates were centrifuged for 10 min at 10,000 × g to pellet debris, and the total protein was measured in the supernatant (Bio-Rad). A total of 10–30 μg protein from each muscle lysate was separated by 10% SDS-PAGE and transferred to polyvinylidene fluoride membranes (Millipore, Billerica, MA). Membranes were incubated in blocking buffer (5% nonfat dry milk in Tris-buffered saline plus 0.1% Tween 20 (5% milk/TTBS)) and then incubated in primary antibody diluted in 5% milk/TTBS overnight at 4 °C. Membranes were then washed in 5% milk/TTBS and incubated with horseradish peroxidase-conjugated secondary antibody. After a series of washes in milk, TTBS, and TBS, protein detection was performed with enhanced chemiluminescence (ECL) (Perkin Elmer, Boston, MA) and exposure on an imaging system (Kodak MM4000, Eastman Kodak, Rochester, NY), and analysis of band intensity was performed using the associated image analysis software. Multiple exposures of each membrane were utilized in the analysis to ensure signal linearity. The membranes were stained with Coomassie Brilliant Blue R-250 after immunoblotting to confirm equal protein loading.
Immunohistochemistry
Localization of γ-SG was determined by immunohistochemistry on 10 μm cryosections from TA muscles. Sections were incubated with rabbit polyclonal antibody recognizing γ-SG (kind gift from E. M. McNally, University of Chicago), followed by goat anti-rabbit secondary conjugated to Alexa Fluor 555 (Invitrogen). Additional sections were incubated with antibodies recognizing α-SG (IVD3(1)A9, Iowa Hybridoma Bank), δ-SG, β-SG, β-dystroglycan (VP-D501, VP-B206, and VP-B205, Vector Laboratories), and sarcospan (kind gift from R. Crosbie, UCLA). Sections were covered with Vectashield mounting medium containing 4′,6-diamidino-2-phenylindole (Vector Laboratories). Image analysis was performed on a Leica DMR epifluorescence microscope using OpenLab imaging software (Improvision, Perkin Elmer).
Statistic Analysis
Data is presented as mean ± S.E. One-way ANOVA followed by Tukey's multiple comparison test was utilized for comparisons between AAV-injected treatments and mouse strains. Unpaired t tests were utilized for comparisons between stimulated and unstimulated muscles within the same strain or treatment group. Statistical significance was accepted as p < 0.05.
RESULTS
The goal of this study was to determine whether the mutation of Tyr6 in γ-SG prevented proper localization of the protein to the sarcolemma or affected the normal signal transduction pathways following eccentric contractions. Viral delivery of both WTgsg and Y6Agsg were performed in the muscles of gsg−/− mice. Comparisons were made to C57 muscles, which had normal levels of γ-SG, and to gsg−/− muscles, which express no γ-SG.
Restoration of γ-SG Localization by WT and Y6Agsg Expression
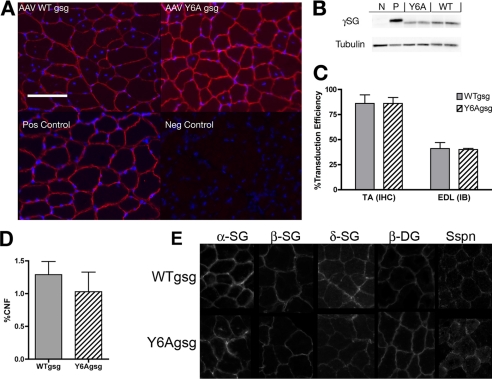
Expression and localization of the viral transgenes was determined by immunoblotting of the EDL muscles and immunohistochemistry of the TA muscles. Almost all fibers in the TA muscles 1 month after viral injection of both WTgsg and Y6Agsg were positive for γ-SG (Fig. 1, A and C). γ-SG staining was restricted to the sarcolemma confirming appropriate localization. Of the γ-SG positive fibers, there was no difference in central nucleation between the constructs (Fig. 1D). There was no difference in γ-SG protein between the viral constructs. Muscle homogenates had approximately half-normal levels of γ-SG for both the WTgsg and Y6Agsg construct compared with C57 controls (Fig. 1, B and C). Localization of the entire SG complex, sarcospan, and β-dystroglycan was preserved after expression of WTgsg and Y6Agsg (Fig. 1E).
FIGURE 1.
Validation of γ-sarcoglycan production after viral injection. A, immunohistochemistry of TA sections demonstrate that both wild type (AAV WTgsg) and mutagenized (AAV Y6Agsg) human γ-SG were properly localized at the sarcolemma in treated gsg−/− mice, compared with C57 (Pos Control) muscle. No expression was observed in uninjected gsg−/− muscles (Neg Control). Costaining by 4′,6-diamidino-2-phenylindole shows that viral expression of either construct stabilized most transduced fibers, which is evident by the peripheral nuclei. Scale bar, 100 μm. B, immunoblotting of EDL muscle lysates show production of both constructs (Y6A, WT) compared with positive control (P, C57BL/6) and negative control (N, gsg−/−) samples. Difference in migration is due to different levels of glycosylation between human and murine γ-SG (Cordier et al., 2001). C, quantification of transduction efficiency 1 month after injection of WTgsg and Y6Agsg. No significant difference in efficiency of expression was observed in muscles treated with either construct by both immunohistochemistry (IHC) or by immunoblotting (IB) (n = 5 for both measurements and each construct). D, quantification of central nucleation (% CNF) in fibers expressing WTgsg or Y6Agsg in the gsg−/− TA muscles. No significant difference was observed between the two treatments. E, immunohistochemistry of TA sections demonstrate that localization of additional members of the sarcolgycan complex, sarcospan (sspn), and β-dystroglycan is restored after injection of WTgsg and Y6Agsg.
Comparisons of mass and force production in young EDL muscles (Table 1) showed no statistical differences in cross-sectional area, tetanic force, or specific force compared with EDL muscles from C57 or gsg−/− animals. Only muscles mass differed between untreated gsg−/− and C57 muscles.
TABLE 1.
Functional properties of young EDL muscles
Data are mean ± S.D. for n = 4–5 male and female mice, age 7–8 weeks. *, p < 0.05 versus C57 by one-way ANOVA followed by Tukey post hoc comparisons. CSA, cross-sectional area; N, newtons.
| Strain | Treatment | Mass | CSA | Tetanic force | Specific force |
|---|---|---|---|---|---|
| mg | mm2 | mN | N/cm2 | ||
| C57 | None | 6.8 ± 0.4 | 1.26 ± 0.07 | 258 ± 45 | 20.4 ± 2.4 |
| gsg−/− | None | 8.8 ± 0.7* | 1.58 ± 0.13 | 323 ± 26 | 20.7 ± 0.7 |
| gsg−/− | AAVWTgsg | 7.9 ± 0.9 | 1.47 ± 0.11 | 313 ± 42 | 20.5 ± 2.4 |
| gsg−/− | AAVY6Agsg | 8.4 ± 1.4 | 1.59 ± 0.29 | 302 ± 26 | 19.4 ± 3.2 |
Loss of the SG and/or DGC from murine skeletal muscle leads to compensatory increases in the α7/β1D integrins (17). To determine whether restoration of γ-SG also affected the integrin complex, immunoblotting for β1D integrin was performed. As shown previously, there was a 2-fold increase in β1D integrin associated with the loss of γ-SG compared with C57 controls (17). Viral expression of WTgsg or Y6Agsg caused a partial decrease in β1D integrin levels compared with gsg−/− muscles (Fig. 2), consistent with the level of γ-SG protein level (Fig. 1). Thus, both WTgsg and Y6Agsg were equivalent in production, localization, and stabilization of γ-SG protein in skeletal muscle.
FIGURE 2.
Changes in Integrin β1D levels after viral expression of γ-SG. A, immunoblot of muscle lysates from untreated C57 and gsg−/− mice, and from gsg−/− mice injected with WTgsg or Y6Agsg. B, quantification of integrin β1D normalized to tubulin for n = 3 muscle lysates per condition. Integrin β1D increased by >2-fold in muscles from gsg−/− mice compared with those from C57 mice. Expression of WTgsg or Y6Agsg in muscles of gsg−/− resulted in a partial reduction of integrin β1D (Int β1D) such that there was no significant difference between treated muscles and C57 muscles or gsg−/− muscles. Comparisons based on one-way ANOVA followed by Tukey's multiple comparison test.
Resting ERK1/2 Phosphorylation Is Not Restored by Y6Agsg Expression
To determine whether viral expression of WTgsg or Y6Agsg affected resting ERK1/2 phosphorylation, total and P-ERK1/2 were measured in EDL muscles of gsg−/− mice 1 month after viral injection of WTgsg and Y6Agsg and also untreated C57 and gsg−/− muscles. As shown in Fig. 3, muscles from gsg−/− mice had elevated ERK1/2 phosphorylation compared with C57 controls. Injection of WTgsg reduced P-ERK1 levels to C57 values but did not affect P-ERK2 levels. In contrast, injection of Y6Agsg did not cause a change in P-ERK1. Furthermore, there was a significant increase in P-ERK2 levels compared with both C57 and gsg−/− muscles. Thus, expression of Y6Agsg failed to restore resting ERK1/2 phosphorylation.
FIGURE 3.
Resting P-ERK1/2 is not restored by expression of Y6Agsg. A, immunoblots of P-ERK1/2 and ERK1/2, muscle lysates from untreated C57 and gsg−/− mice, and from gsg−/− mice injected with WTgsg or Y6Agsg. B, resting P-ERK1 was elevated in untreated muscles from gsg−/− mice compared with muscles from C57 mice. Expression of WTgsg caused a significant decrease in P-ERK1 compared with untreated gsg−/− muscles, whereas P-ERK1 in muscles injected with Y6Agsg remained elevated. C, resting P-ERK2 did not differ significantly between untreated muscles from C57 and gsg−/− mice, and it was not changed after injection of WTgsg. However, injection of Y6Agsg led to a significant elevation of resting P-ERK compared with untreated C57 or gsg−/− muscles. n = 3 for each condition. *, p < .05 for comparisons to C57; †, p < .05 for comparisons to gsg−/− by one-way ANOVA followed by Tukey's multiple comparison test.
ERK1/2 Phosphorylation after Eccentric Contraction Is Aberrant after Y6Agsg Expression
The MAPK pathway is responsive to mechanical perturbation of muscle, where ERK1/2 exhibits a robust transient phosphorylation after eccentric contractions (5, 18–20). Loss of γ-SG modifies the pattern of ERK1/2 phosphorylation; however, it is not clear whether this is the result of SG complex disruption or the effect of a specific region of γ-SG. Examination of ERK1/2 phosphorylation following eccentric contraction was performed in muscles from gsg−/− mice expressing WTgsg or Y6Agsg to determine whether this was also affected by the absence of Tyr6. The results (Fig. 4A) were analyzed in two ways. First, P-ERK1/2 was normalized to total ERK1/2 for each sample (Fig. 4, B and C) for comparisons of the signal intensity. Second, P-ERK1/2 was normalized to the mean No Stim value for each condition (Fig. 4, D and E) to determine the fold change in the response after eccentric contraction.
FIGURE 4.
P-ERK1/2 levels after eccentric contraction are not restored by expression of Y6Agsg. A, immunoblots of P-ERK1/2 and ERK1/2 of muscles lysates from untreated C57 and gsg−/− mice, and from gsg−/− mice injected with WTgsg or Y6Agsg with (+) and without (−) eccentric contraction (ECC). B, P-ERK1 (normalized to ERK1) significantly increases after eccentric contractions (ECC30) compared with unstimulated muscles of the same condition for C57, gsg−/−, and gsg−/− injected with WTgsg, but not for gsg−/− muscles injected with Y6Agsg. For P-ERK1 intensity after eccentric contractions, untreated gsg−/− muscles and those injected with Y6Agsg were significantly higher than C57 muscles. In addition, gsg−/− muscles injected with WTgsg and Y6Agsg had significantly lower P-ERK1 intensity after eccentric contractions compared with untreated gsg−/− muscles. C, P-ERK2 (normalized to ERK2) significantly increases after eccentric contractions (ECC30) compared with unstimulated muscles of the same condition for C57, gsg−/−, and gsg−/− injected with Y6Agsg, but not for gsg−/− muscles injected with WTgsg. For P-ERK2 intensity after eccentric contractions, untreated gsg−/− muscles and those injected with Y6Agsg were significantly higher than C57 muscles. In addition, gsg−/− muscles injected with WTgsg had significantly lower P-ERK2 intensity after eccentric contractions compared with untreated gsg−/− muscles. D, the fold change in P-ERK1 between No Stim and ECC30 shows there is no difference in the relative response to ECC30 between C57, gsg−/−, and gsg−/− injected with WTgsg, but it is significantly lower for gsg−/− muscles injected with Y6Agsg compared with C57 muscles. E, the fold change in P-ERK2 between No Stim and ECC30 is similar in muscles from C57, gsg−/−, and gsg−/− injected with Y6Agsg, but not for gsg−/− muscles injected with WTgsg, where the response is significantly lower in untreated gsg−/− muscles. *, p < .05 for unpaired t tests between ECC30 and No Stim; †, p < .05 for comparisons to C57, and §, p < .05 for comparisons to gsg−/− by one-way ANOVA followed by Tukey's multiple comparison test. n = 3 for all unstimulated samples; n = 4 for all samples subjected to eccentric contraction.
Muscles from gsg−/− mice had elevated P-ERK1 and P-ERK2 30 min after ECC compared with C57 muscles. Expression of WTgsg in gsg−/− muscle restored P-ERK1/2 to levels that were indistinguishable from C57 muscles (Fig. 4, B and C). However, expression of Y6Agsg did not result in a similar change in P-ERK1/2. P-ERK1 levels in the Y6Agsg group remained significantly higher than those in C57 muscles, even though they were reduced from gsg−/− muscles. P-ERK2 levels remained significantly higher in the Y6Agsg group compared with C57 muscles after eccentric contractions, and the levels were similar to untreated gsg−/− muscles.
Even though the intensities of P-ERK1/2 after eccentric contractions differed between C57 and gsg−/− muscles, the relative change between No Stim controls and ECC30 muscles was not significantly different (Fig. 4, D and E). Similarly, the mean fold change of P-ERK1 in WTgsg-injected muscles did not differ from the fold change in either C57 or gsg−/− muscles. All three groups exhibited a significant increase in P-ERK1. In contrast, muscles injected with Y6Agsg had a blunted ERK1 response to eccentric contraction, which was ∼2-fold lower than the response in C57 muscles (Fig. 4D). For P-ERK2, the highest response was observed in the untreated gsg−/− muscles, and muscles injected with Y6Agsg showed a similar response (Fig. 4E). Muscles injected with WTgsg had a reduced P-ERK2 response after eccentric contraction, such that the relative change was not different than No Stim controls. The C57 muscles exhibited a significant increase P-ERK2, which was intermediate to gsg−/− and WTgsg-injected muscles. Thus, expression of WTgsg in gsg−/− muscles partially restored the P-ERK1/2 response to eccentric contraction, whereas expression of Y6Agsg failed to change the phosphorylation pattern found in untreated gsg−/− muscles.
Differential Preservation of Muscle Strength and Transgene after WTgsg and Y6Agsg Injection
The gsg−/− mouse does not exhibit a loss of strength until later in life (5, 21). To determine whether expression of WTgsg or Y6Agsg could preserve muscle force, the function of EDL muscles isolated from gsg−/− mice with and without viral injection was determined at 6 months of age, which was ∼5 months after injection. An additional group of EDL muscles from C57 mice served as a wild type control. There was a significant loss of specific force in the gsg−/− muscles compared with EDL muscles from C57 mice (Table 2). Expression of WTgsg resulted in a recovery of specific forces that were significantly higher than untreated gsg−/− muscles and similar to forces in the C57 controls. However, injection of Y6Agsg did not improve specific force, and strength measurements were not different from untreated gsg−/− muscles.
TABLE 2.
Functional properties of EDL muscles from mature mice
Data are mean ± S.D. for n = 4–5 male and female mice, age 25 weeks. *, p < 0.05 versus C57; †, p < 0.05 vs. gsg−/− by one-way ANOVA followed by Tukey post hoc comparisons. CSA, cross-sectional area; N, newtons.
| Strain | Treatment | Mass | CSA | Tetanic force | Specific force |
|---|---|---|---|---|---|
| mg | mm2 | mN | N/cm2 | ||
| C57 | None | 9.1 ± 0.3 | 1.64 ± 0.11 | 349 ± 16 | 21.4 ± 1.5 |
| gsg−/− | None | 12.2 ± 1.6* | 2.00 ± 0.32 | 316 ± 71 | 15.9 ± 2.7* |
| gsg−/− | AAVWTgsg | 10.0 ± 1.8 | 1.68 ± 0.24 | 357 ± 62 | 21.3 ± 1.7† |
| gsg−/− | AAVY6Agsg | 11.4 ± 1.7 | 1.96 ± 0.22 | 342 ± 80 | 17.3 ± 2.9 |
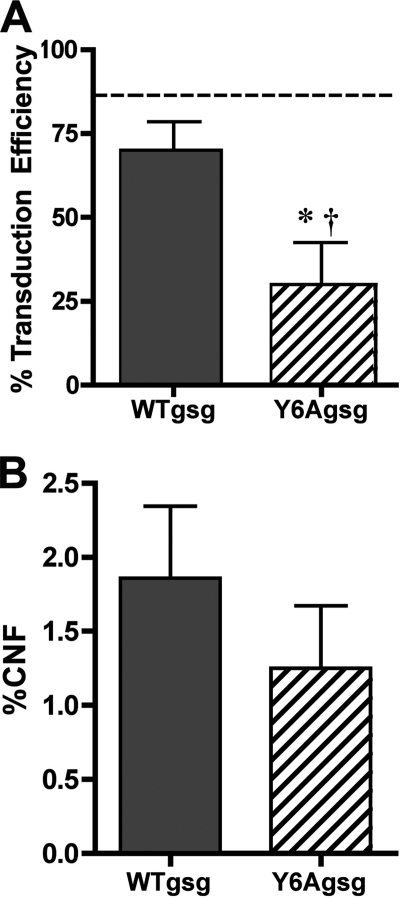
To determine whether the expression of the viral transgenes was maintained for 6 months, the TA muscles from the treatment groups were subjected to immunohistochemistry for γ-SG. As shown in Fig. 5A, transduction efficiency of WTgsg at 6 months of age was similar to that measured at 1 month post-injection. However, there was a significant loss of γ-SG positive fibers between 1 and 5 months after injection of Y6Agsg. Central nucleation of muscle fibers expressing the viral transgenes did not differ significantly from each other (Fig. 5B) or from the values in muscles 1 month after viral injection (Fig. 1D). Thus, it is likely that the lack of strength preservation in muscles injected with Y6Agsg was due to loss of the viral transgene.
FIGURE 5.
Analysis of muscles 5 months after viral injection. A, transduction efficiency measured by immunohistochemistry of TA muscles 5 months after injection of Y6Agsg was significantly lower than muscles 5 months after injection of WTgsg (*) and also lower than muscles injected with the same construct 1 month afterward (†). B, quantification of central nucleation (% CNF) in fibers expressing WTgsg or Y6Agsg in the gsg−/− TA muscles. Although there was a decrease in transduction efficiency, no significant difference in proportion of central nucleation in γ-sarcoglycan positive fibers was observed between the two treatments n = 4 muscles for each condition.
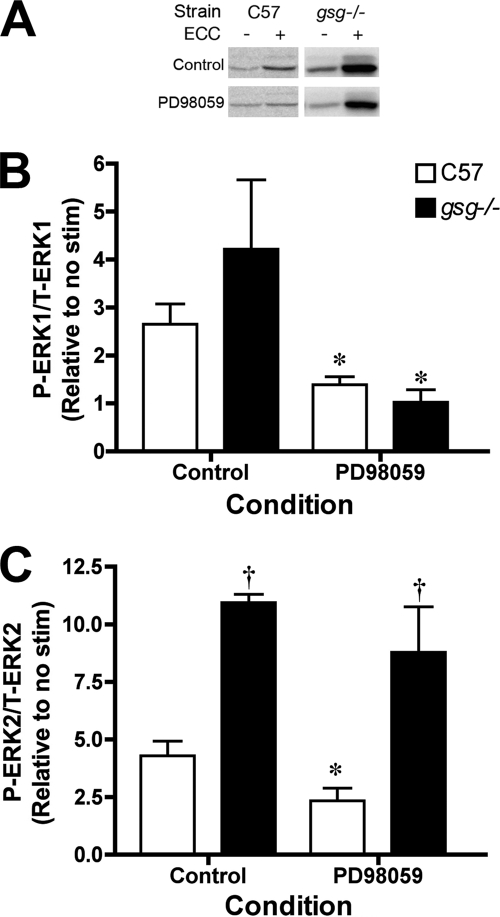
To further elucidate the signaling pathways associated with mechanical signal transduction, muscles from untreated C57 and gsg−/− mice were subjected to eccentric contractions in the presence of the MEK inhibitor, PD98059 (Fig. 6). In muscles from C57 mice, the fold change in P-ERK1/2 was inhibited in the presence of PD98059. In gsg−/− muscles, P-ERK1 changes were also prevented by MEK inhibition. However, there was no significant decrease in P-ERK2 changes with exposure to PD98059, and the heightened fold change in P-ERK2 compared with C57 muscles persisted. Thus, it appears that the changes in P-ERK1 after eccentric contraction are MEK-dependent regardless of the presence or absence of γ-SG. However, the changes in P-ERK2 in the absence of γ-SG are independent of MEK.
FIGURE 6.
P-ERK1/2 levels after eccentric contraction with and without MEK inhibition. A, immunblots of P-ERK1/2 from muscle lysates of C57 and gsg−/− EDL muscles with and without 50 μm PD98059. B, the fold change in P-ERK1 between No Stim and eccentric contraction is significantly decreased by PD98059 in both C57 and gsg−/− muscles. C, the fold change in P-ERK2 between No Stim and eccentric contraction is significantly decreased by PD98059 in C57 muscles but not in gsg−/− muscles, supporting that changes in P-ERK2 in gsg−/− muscle is independent of the MAPK pathway. *, p < .05 for unpaired t tests between no treatment and PD98059 exposure in strain matched samples; †, p < .05 for comparisons to C57 control by one-way ANOVA followed by Tukey's multiple comparison test. n = 3 for all unstimulated samples; n = 4 for all samples subjected to eccentric contraction.
DISCUSSION
The goal of this study was to determine whether localization of γ-SG was necessary and sufficient to correct the aberrant signal transduction response associated with the loss of γ-SG. Using viral mediated gene transfer, it was possible to express equivalent levels of WTgsg and gsg lacking Tyr6 (Y6A) in the muscles of gsg−/− mice. Both gene products were detectable at the sarcolemma and, as an indicator of muscle stability, significantly reduced centrally nucleated fibers. However, even with appropriate membrane localization of Y6Agsg, both resting and mechanically activated P-ERK1/2 were not restored to normal levels, where resting P-ERK1/2 was elevated, and the response to eccentric contractions differed in both intensity and relative change compared with C57 muscles. These results show that localization of γ-SG does not require Tyr6, but localization alone is insufficient for restoration of normal signal transduction patterns after mechanical perturbation. Tyr6 is a critical residue for modulating the response to eccentric contractions.
Most of the known mutations associated with limb girdle muscular dystrophies 2C lead to disruption of the SG complex, and although the genetic defects can cause severe muscle pathology, the mechanisms that underlie the onset of disease have been difficult to identify. For example, an initial observation was that there was little overt membrane fragility, yet there still remained a trigger that caused significant muscle fiber degeneration and increased fibrosis (14, 21). Given the overall structure of the SG complex, an appealing hypothesis was that the complex behaved as a receptor or channel that was critical for muscle fiber survival, and loss of this function led to muscle cell death and fibrotic infiltration. Consistent with this model, cell attachment and dynamic mechanical strain could cause a transient increase in ERK1/2 phosphorylation as well as post-translational modifications of the SG complex (P-Tyr); furthermore, the pattern of the ERK1/2 response was affected by the loss of γ-SG and disruption of the SG complex. This suggested that the SG complex was a mechanoreceptor, and signals stimulated by mechanical perturbations were mediated via the SG complex. However, it has remained unclear where the SG complex lies in the temporal or structural chain of events leading to ERK1/2 phosphorylation after eccentric contractions.
From the current study, it appears that the SG complex serves as a brake on ERK1/2 phosphorylation both at rest and after eccentric contractions, for loss of γ-SG results in an overall increase in P-ERK1/2. Thus, unregulated P-ERK1/2 may be detrimental to muscle fiber stability. In addition, the ability to phosphorylate Tyr6 is required for the regulation of ERK1/2 signals, because the absence of tyrosine 6 in the Y6Agsg construct was no different than the absence of γ-SG in resting and stimulated P-ERK1/2. Consistent with this observation, Y6Agsg expression is lost over the course of 5 months, which may be caused by the heightened ERK1/2 phosphorylation and loss of muscle cell stability.
Because ERK1/2 has been shown by several groups to be responsive to mechanical perturbation, many other signaling pathways were not investigated in great detail. In the initial experiments, P-Akt was also measured because it has been shown that the P13K/Akt pathway can be stimulated by mechanical stretch (22). However, there was no change detected in the resting or stimulated levels of P-Akt between groups (data not shown), and it was not pursued further. Examination of additional signaling pathways may provide insight into the regulation of mechanical signal transduction through the sarcoglycan complex. For example, the pathway responsible for phosphorylation of Tyr6 on γ-SG has not been identified. Candidate motifs were found using the sequence flanking Tyr6 (MVREQYTTVTEG) (23–25) and include the kinases Src (Y(A/G/S/T/E/D)) (26) and JAK2 (YXX(L/I/V)) (27), and SHP1 phosphatase ((E/D)XY) (28), but none of these have been confirmed. Because Src and focal adhesion kinase (FAK) can act together as a signaling complex (29) and FAK has been proposed to interact with both the integrin complex and the DGC (4, 7), it is a distinct possibility that Src phosphorylates γ-SG.
Pharmacological dissection of the pathways leading to ERK1/2 phosphorylation may help to clarify not only the post-translational modifiers of the sarcoglycans but also may identify the downstream effectors of mechanical signal transduction through this complex. An initial test is shown in Fig. 6, where the loss of P-ERK2 regulation in gsg−/− muscles appears to the independent of MEK, whereas in muscles from C57 both P-ERK1 and P-ERK2, changes are MEK-dependent. These results suggest that the disruption of the sarcoglycan complex disrupts the normal mechanical signal transduction. For instance, if Src cannot interact with the native γ-SG tyrosine site, then perhaps it can activate additional signaling cascades more efficiently. At this stage, however, many candidate pathways must be evaluated before understanding how disregulation of P-ERK2 occurs in the absence of γ-SG.
The level of γ-SG expression must be well controlled because both loss of production and increased expression can lead to muscle pathology (14, 30). The truncated desmin promoter provided the tissue specificity needed for this study (15), and the levels of transgene expression were sufficient to stabilize muscle fibers as shown by the reduction of centrally nucleated fibers. It is possible that these levels were insufficient to correct the signaling defect when Y6Agsg was expressed. However, the fact that both constructs had equivalent expression and stabilization but divergent effects on P-ERK1/2 suggests that higher levels of Y6Agsg would still be ineffective in changing modifying P-ERK1/2. The localization of complex members has been confirmed (Fig. 1E), yet one unknown factor in the interpretation of these results is whether all of the proteins that normally associate with γ-SG in particular and the SG complex in general were still present. In addition to transient localization of adaptor proteins to P-Tyr, this could include the other members of the SG complex or associations with filamin, NOS, and dystrobrevin. Thus, the lack of signaling restoration by expression of Y6Agsg may be an indirect effect of loss of an unknown protein that directly mediates the response.
Evidence is accumulating for multifactorial contributions to muscular dystrophy pathology and supports the hypothesis that proteins, in addition to those in the SG complex, affect severity of the disease. Genotype-phenotype correlations in limb girdle muscular dystrophies 2C patients can be variable, even with the same mutation, and suggest that genetic modifiers are involved (31). A more direct test of the genetic modifier hypothesis has been performed in mice and Drosophila. Severity of pathology across multiple parameters in mice lacking γ-SG is dependent upon the specific background strain (32). Proteins that interact with the dystrophin complex have been identified in Drosophila (33) and include members of the Notch, TGF-β, and epidermal growth factor receptor signaling pathways. Whether these pathways also interact with the sarcoglycans has not yet been addressed, but these results provide new candidates for probing the signaling defect in the gsg−/− mouse.
An alternative explanation of the specific loss of the Y6Agsg transgene several months after viral injection is that an immune response mounted against the transgene occurred. This possibility cannot be excluded because it was not directly addressed in this study; however, it seems unlikely for the following reasons. First, transgene expression was restricted by a muscle-specific promoter, which reduces humoral and cellular responses to exogenous γ-SG in the null background (34). Both transgenes would be affected equally and an equivalent loss of transgene expected in the case of a generalized response. Second, the tyrosine residue is in the intracellular portion of the protein, and single residue modifications in this region are unlikely to alter extracellular protein structure. The protein was detected at the sarcolemma with no apparent cytoplasmic staining suggestive of mislocalization (Fig. 1). Furthermore, because the viral transgene is of human origin, there are 35/291 different residues compared with murine γ-SG, many of which are in the extracellular region. In the context of this level of heterogeneity between human and murine γ-SG and the initial localization of both viral transgenes, a more likely explanation for loss of Y6Agsg is due to aberrant ERK1/2 activity and subsequent muscle cell instability.
In conclusion, the SG complex is a critical component of mechanical signal transduction. Restoration of the complex to the sarcolemma is essential for muscle stability. However, without the signal transduction mechanism intact, aberrant signaling persists and contributes to muscle instability. Identification of the pathways regulating sarcoglycan phosphorylation may provide new therapeutic avenues for the muscular dystrophies.
Acknowledgments
I thank Zhiqiang Zhang for the rAAV vector containing the truncated desmin promoter and Zuozhen Tian for technical assistance in isolated muscle mechanical measurements.
This work was supported by American Heart Association Grant 0235157N and the Paul Wellstone Muscular Dystrophy Cooperative Research Center (U54 AR052646).
- DGC
- dystrophin glycoprotein complex
- gsg
- γ-sarcoglycan gene
- SG
- sarcoglycan
- WT
- wild type
- ERK
- extracellular signal-regulated kinase
- TA
- tibialis anterior
- EDL
- extensor digitorum longus
- MAPK
- mitogen-activated protein kinase
- MEK
- mitogen-activated protein kinase/extracellular signal-regulated kinase kinase
- ANOVA
- analysis of variance
- TBS
- Tris-buffered saline
- AAV
- adeno-associated virus serotype 2/8
- rAAV
- recombinant AAV
- No Stim
- unstimulated controls.
REFERENCES
- 1.Wagner K. R. (2002) Neurol. Clin. 20, 645–678 [DOI] [PubMed] [Google Scholar]
- 2.Bönnemann C. G., Finkel R. S. (2002) Semin Pediatr. Neurol. 9, 81–99 [DOI] [PubMed] [Google Scholar]
- 3.Hack A. A., Groh M. E., McNally E. M. (2000) Microsc. Res. Tech. 48, 167–180 [DOI] [PubMed] [Google Scholar]
- 4.Yoshida T., Pan Y., Hanada H., Iwata Y., Shigekawa M. (1998) J. Biol. Chem. 273, 1583–1590 [DOI] [PubMed] [Google Scholar]
- 5.Barton E. R. (2006) Am. J. Physiol. Cell Physiol. 290, C411–419 [DOI] [PubMed] [Google Scholar]
- 6.Lapidos K. A., Kakkar R., McNally E. M. (2004) Circ. Res. 94, 1023–1031 [DOI] [PubMed] [Google Scholar]
- 7.Anastasi G., Amato A., Tarone G., Vita G., Monici M. C., Magaudda L., Brancaccio M., Sidoti A., Trimarchi F., Favaloro A., Cutroneo G. (2003) Cells Tissues Organs 175, 151–164 [DOI] [PubMed] [Google Scholar]
- 8.Cordier L., Hack A. A., Scott M. O., Barton-Davis E. R., Gao G., Wilson J. M., McNally E. M., Sweeney H. L. (2000) Mol. Ther. 1, 119–129 [DOI] [PubMed] [Google Scholar]
- 9.Duclos F., Straub V., Moore S. A., Venzke D. P., Hrstka R. F., Crosbie R. H., Durbeej M., Lebakken C. S., Ettinger A. J., van der Meulen J., Holt K. H., Lim L. E., Sanes J. R., Davidson B. L., Faulkner J. A., Williamson R., Campbell K. P. (1998) J. Cell Biol. 142, 1461–1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holt K. H., Lim L. E., Straub V., Venzke D. P., Duclos F., Anderson R. D., Davidson B. L., Campbell K. P. (1998) Mol. Cell 1, 841–848 [DOI] [PubMed] [Google Scholar]
- 11.Herzog R. W. (2004) Methods Mol. Biol. 246, 179–194 [DOI] [PubMed] [Google Scholar]
- 12.Chamberlain J. S. (2002) Hum. Mol. Genet 11, 2355–2362 [DOI] [PubMed] [Google Scholar]
- 13.Holt K. H., Campbell K. P. (1998) J. Biol. Chem. 273, 34667–34670 [DOI] [PubMed] [Google Scholar]
- 14.Hack A. A., Ly C. T., Jiang F., Clendenin C. J., Sigrist K. S., Wollmann R. L., McNally E. M. (1998) J. Cell Biol. 142, 1279–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Z., Marchand P., Humbert J., Babinet C., Paulin D. (1993) Development 117, 947–959 [DOI] [PubMed] [Google Scholar]
- 16.Barton E. R. (2006) J. Appl. Physiol. 100, 1778–1784 [DOI] [PubMed] [Google Scholar]
- 17.Allikian M. J., Hack A. A., Mewborn S., Mayer U., McNally E. M. (2004) J. Cell Sci. 117, 3821–3830 [DOI] [PubMed] [Google Scholar]
- 18.Aronson D., Violan M. A., Dufresne S. D., Zangen D., Fielding R. A., Goodyear L. J. (1997) J. Clin. Invest. 99, 1251–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carlson C. J., Fan Z., Gordon S. E., Booth F. W. (2001) J. Appl. Physiol. 91, 2079–2087 [DOI] [PubMed] [Google Scholar]
- 20.Hayashi T., Hirshman M. F., Dufresne S. D., Goodyear L. J. (1999) Am. J. Physiol. 277, C701–707 [DOI] [PubMed] [Google Scholar]
- 21.Hack A. A., Cordier L., Shoturma D. I., Lam M. Y., Sweeney H. L., McNally E. M. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 10723–10728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hornberger T. A., Stuppard R., Conley K. E., Fedele M. J., Fiorotto M. L., Chin E. R., Esser K. A. (2004) Biochem. J. 380, 795–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keshava, Prasad T. S., Goel R., Kandasamy K., Keerthikumar S., Kumar S., Mathivanan S., Telikicherla D., Raju R., Shafreen B., Venugopal A., Balakrishnan L., Marimuthu A., Banerjee S., Somanathan D. S., Sebastian A., Rani S., Ray S., Harrys, Kishore C. J., Kanth S., Ahmed M., Kashyap M. K., Mohmood R., Ramachandra Y. L., Krishna V., Rahiman B. A., Mohan S., Ranganathan P., Ramabadran S., Chaerkady R., Pandey A. (2009) Nucleic Acids Res. 37, D767–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mishra G. R., Suresh M., Kumaran K., Kannabiran N., Suresh S., Bala P., Shivakumar K., Anuradha N., Reddy R., Raghavan T. M., Menon S., Hanumanthu G., Gupta M., Upendran S., Gupta S., Mahesh M., Jacob B., Mathew P., Chatterjee P., Arun K. S., Sharma S., Chandrika K. N., Deshpande N., Palvankar K., Raghavnath R., Krishnakanth R., Karathia H., Rekha B., Nayak R., Vishnupriya G., Kumar H. G., Nagini M., Kumar G. S., Jose R., Deepthi P., Mohan S. S., Gandhi T. K., Harsha H. C., Deshpande K. S., Sarker M., Prasad T. S., Pandey A. (2006) Nucleic Acids Res. 34, D411–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peri S., Navarro J. D., Amanchy R., Kristiansen T. Z., Jonnalagadda C. K., Surendranath V., Niranjan V., Muthusamy B., Gandhi T. K., Gronborg M., Ibarrola N., Deshpande N., Shanker K., Shivashankar H. N., Rashmi B. P., Ramya M. A., Zhao Z., Chandrika K. N., Padma N., Harsha H. C., Yatish A. J., Kavitha M. P., Menezes M., Choudhury D. R., Suresh S., Ghosh N., Saravana R., Chandran S., Krishna S., Joy M., Anand S. K., Madavan V., Joseph A., Wong G. W., Schiemann W. P., Constantinescu S. N., Huang L., Khosravi-Far R., Steen H., Tewari M., Ghaffari S., Blobe G. C., Dang C. V., Garcia J. G., Pevsner J., Jensen O. N., Roepstorff P., Deshpande K. S., Chinnaiyan A. M., Hamosh A., Chakravarti A., Pandey A. (2003) Genome Res. 13, 2363–2371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwartz D., Gygi S. P. (2005) Nat. Biotechnol. 23, 1391–1398 [DOI] [PubMed] [Google Scholar]
- 27.Argetsinger L. S., Kouadio J. L., Steen H., Stensballe A., Jensen O. N., Carter-Su C. (2004) Mol. Cell Biol. 24, 4955–4967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang P., Fu H., Snavley D. F., Freitas M. A., Pei D. (2002) Biochemistry 41, 6202–6210 [DOI] [PubMed] [Google Scholar]
- 29.Mitra S. K., Schlaepfer D. D. (2006) Curr. Opin. Cell Biol. 18, 516–523 [DOI] [PubMed] [Google Scholar]
- 30.Zhu X., Hadhazy M., Groh M. E., Wheeler M. T., Wollmann R., McNally E. M. (2001) J. Biol. Chem. 276, 21785–21790 [DOI] [PubMed] [Google Scholar]
- 31.Kefi M., Amouri R., Driss A., Ben Hamida C., Ben Hamida M., Kunkel L. M., Hentati F. (2003) Neuromuscul. Disord. 13, 779–787 [DOI] [PubMed] [Google Scholar]
- 32.Heydemann A., Huber J. M., Demonbreun A., Hadhazy M., McNally E. M. (2005) Neuromuscul. Disord. 15, 601–609 [DOI] [PubMed] [Google Scholar]
- 33.Kucherenko M. M., Pantoja M., Yatsenko A. S., Shcherbata H. R., Fischer K. A., Maksymiv D. V., Chernyk Y. I., Ruohola-Baker H. (2008) PLoS One 3, e2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cordier L., Gao G. P., Hack A. A., McNally E. M., Wilson J. M., Chirmule N., Sweeney H. L. (2001) Hum. Gene Ther. 12, 205–215 [DOI] [PubMed] [Google Scholar]