Abstract
A recently developed technique for dilution of the naturally high protein packing density in isolated grana membranes was applied to study the dependence of the light harvesting efficiency of photosystem (PS) II on macromolecular crowding. Slight dilution of the protein packing from 80% area fraction to the value found in intact grana thylakoids (70%) leads to an improved functionality of PSII (increased antenna size, enhanced connectivity between reaction centers). Further dilution induces a functional disconnection of light-harvesting complex (LHC) II from PSII. It is concluded that efficient light harvesting by PSII requires an optimal protein packing density in grana membranes that is close to 70%. We hypothesize that the decreased efficiency in overcrowded isolated grana thylakoids is caused by excited state quenching in LHCII, which has previously been correlated with neoxanthin distortion. Resonance Raman spectroscopy confirms this increase in neoxanthin distortion in overcrowded grana as compared with intact thylakoids. Furthermore, analysis of the changes in the antenna size in highly diluted membranes indicates a lipid-induced dissociation of up to two trimeric LHCII from PSII, leaving one trimer connected. This observation supports a hierarchy of LHCII-binding sites on PSII.
Keywords: Bioenergetics, Membrane/Biophysics, Membrane/Function, Methods/Electron Microscopy, Photosynthesis/Light Reactions, Subcellular Organelles/Chloroplast, Macromolecular Crowding
Introduction
Photosynthetic energy transformation is confronted with the difficulty that even full sunlight is a diluted energy source at the molecular level (1). This constituted a high evolutionary pressure for optimizing light harvesting, which is reflected by the presence of a large number of pigmented light-harvesting complexes (LHC)2 in almost all photosynthetic organisms (with the exception of halobacteria). LHCs enlarge the absorption cross-section of the primary energy converting reaction centers harbored in the photosystems (PS) by coupling them with hundreds or even thousands of pigments (e.g. chlorophylls, carotenoids). These pigments are excitonically well coupled, leading to ultrafast (<1 ps) and thus very efficient energy transfer (2). A biophysical constraint for enlarging the photosynthetic antenna size by a connected pigment system is the steep dependence of the nonradiative excitonic energy transfer rate on the distance between pigments (3). Therefore antenna pigments must be densely packed.
In higher plants and green algae LHCs are integral membrane proteins embedded in the thylakoid membrane, within chloroplast organelles (4). They are tuned to allow very efficient exciton energy transfer at high chlorophyll concentrations (∼0.3 mol·liter−1) (5). This is achieved by an exact and highly ordered positioning of the pigments in the protein scaffold. Slight changes in the pigment arrangement can induce exciton quenching, leading to dissipative loss of harvested light energy (6). In addition to energy transfer between pigments within individual LHCs, light harvesting in thylakoid membranes requires efficient intermolecular transfer of excitations (between proteins). This is because these photosynthetic light-harvesting systems are composed of different LHCs, arranged in a modular way (4). For example the PSII core complex in higher plants is connected to six different LHCs. Current models of PSII in stacked grana thylakoids assume a supercomplex (7) or megacomplex organization (8, 9). Both suggest that PSII is a (core) dimer associated with different sets of bound LHC. In the supercomplex model the core dimer binds four minor LHCs (CP26 and CP29) and two trimeric LHCII. Megacomplexes bind in addition two CP24 and two trimeric LHCII. Although the presence of PSII dimers in native thylakoids has recently been questioned (10), a huge number of data support the dimeric state in intact thylakoid membranes (e.g. 11, 12). However, assuming a dimeric LHCII-PSII supercomplex organization in grana thylakoids, two to six more loosely bound trimeric LHCII are associated to the supercomplex (13, 14), leading to a functional connection of ∼150 to ∼280 chlorophylls/reaction center. The strong distance dependence of the exciton energy transfer suggests that these more loosely bound LHCII trimers must be in close proximity to the LHCII-PSII supercomplex. Indeed the protein density in stacked grana thylakoids is high (15), which is advantageous for efficient intermolecular exciton transfer. On the other hand this high packing density may also lead to the generation of unwanted energy dissipating states. For example, studies on isolated LHCII trimers show aggregation-induced energy quenching (5, 6, 16). Models exist postulating that this “aggregation-induced” quenching is the main mechanism responsible for photoprotective nonphotochemical quenching (17). It is not clear how this dissipative mode is avoided in crowded grana if the system requires very efficient light conversion under nonstressed conditions. These considerations indicate a crucial role for the protein packing density in grana thylakoids in tuning PSII light harvesting. So far no information exists about how the light harvesting efficiency depends on protein packing in grana membranes. A possible reason for this lack of knowledge could be the difficulty of manipulating the lipid/protein ratio and thus the area fraction occupied by proteins in intact plants. As an alternative, in vitro approaches can be used to study the role of protein packing density.
Recently we developed a method of diluting the high protein density in isolated grana membranes by their fusion with liposomes (18). Although this approach has already been applied to intact thylakoids (19), the improvement of our method concerns the use of small unilamellar liposomes that contain native thylakoid lipids rather than artificial ones. Furthermore, clear-cut conclusions are difficult to derive from intact thylakoids because PSI and LHCI contributions interfere with signals generated by PSII and LHCII. In this report we present a detailed structural and functional analysis of isolated grana membranes fused with liposomes to study the interdependency between protein density and light harvesting of PSII.
EXPERIMENTAL PROCEDURES
Preparation of Photosynthetic Membranes with Altered Protein Density
BBY membranes (20) from hydroponically grown (21) Spinacea oleracea var. polka were prepared and fused with small unilamellar vesicles of the native grana thylakoid membrane lipid mixture as described in Ref. 18. The incorporation of lipids was monitored by equilibrium density step gradient ultracentrifugation, whereas the composition and quantification of the lipid:chlorophyll stoichiometry was determined via two-dimensional thin layer chromatography (18). The chlorophyll content was quantified according to Ref. 22. To confirm the functionality of the original BBY membranes, their oxygen evolution was determined polarographically immediately after preparation. For this purpose the membranes were resuspended in BBY measuring medium (40 mm KCl, 7 mm MgCl2, 300 mm sorbitol, 15 mm MES, pH 6.5), yielding a final chlorophyll concentration of 20 μm. All other functional measurements (see below) were performed in the BBY measuring medium. The oxygen evolution measurements were performed in the presence of 0.5 mm 2,6-dichloro-1,4-benzoquinone with a Clark-type electrode (Bachofer GmbH, Reutlingen, Germany) at 20 °C at light intensities of >5000 μmol quanta m−2 s−1.
Steady state electron transport rates of PSII were determined by photometric measurement of the reduction of the PSII electron acceptor 2,6-dichlorophenolindophenol (DCPIP) at 600 nm with a Hitachi U3900 spectrometer. At pH 6.5 (BBY measuring medium), we measured an extinction coefficient for DCPIP of 18.2 mm−1 cm−1. The assay contains BBY measuring medium, membranes corresponding to 10 μm chlorophyll, 70 μm DCPIP, and 2 mm of the PSII electron donor diphenylcarbazide (DPC). PSII was excited by white light at 1000 μmol quanta·m−2 s−1. We confirmed that this light intensity was saturating.
Steady State and Chlorophyll a Fluorescence Induction Spectroscopy
Steady state fluorescence of the fusion products was measured according to Ref. 18 in BBY measuring medium without sorbitol. Oxidizing conditions were established by the addition of 50 μm potassium-hexacyanoferrate III (Fo fluorescence), whereas reduction was induced by 10 mm sodium dithionite (Fm fluorescence). Fluorescence induction curves of dark-adapted membranes were recorded in the above mentioned buffer in the presence of 1 mm NH2OH and 75 μm DCMU. The kinetics were analyzed according to Ref. 23. All of the fluorescence parameters were measured in a laboratory-built fluorometer (21), normalized to the relative chlorophyll content, and corrected for a system-specific small light leak (18).
Absorption Spectroscopy
Absorption spectra of the same samples used for fluorescence spectroscopy were recorded in a Hitachi spectrophotometer U-3010 with a head-on photomultiplier (Hitachi Ltd., Tokyo, Japan). The bandwidth was 1 nm, the scan speed was 300 nm/min, and the optical path length was 10 mm.
77K Fluorescence Spectroscopy
Low temperature fluorescence spectra were obtained as described in Ref. 24. The membranes were resuspended in the BBY measuring medium without sorbitol at a chlorophyll concentration of ∼3 μm, shock frozen in liquid nitrogen, and excited with a broad blue-green light source (400–550 nm) produced with a halogen lamp and Schott BG18, Corning 9782, and LOT heat mirror filters. 25–50 emission spectra/sample were averaged and corrected for the spectral response of the set-up. For spectral deconvolution, these starting spectra were analyzed as follows. To compare absolute fluorescence changes, the spectra were normalized in such a way that the area under the spectrum equals the measured Fm value (see above for steady state Fm measurements). The resulting spectra were fitted with five Gaussian curves, corresponding to free LHCII (F680), CP43 (F685), CP47 (F695), aggregated LHCII (F700), and PSI plus satellite bands of the others (F730). The maximum of each component was deduced from the first derivative of the spectrum. The amplitude and half-width of the Gaussians were free fitting parameters. The positions of the maxima (679.8, 684.2, 692.2, 704.0, and 736.4 nm) and the half-width (9.8, 10.2, 16.2, 21.1, and 57.9 nm) are in the range of published data (25).
PSII Quantification
PSII has been quantified by two different methods. First, chemical difference absorption spectra between 520 and 580 nm were recorded to determine the content of cytochrome b559 (14). Second, the pheophytin concentration was analyzed spectroscopically by reversed phase HPLC (26). The PSII content is expressed relative to the total chlorophyll concentration, i.e. chlorophyll to cytochrome b559 and chlorophyll to two pheophytins.
Freeze-Fracture Electron Microscopy
The membranes were freeze-fractured and examined by electron microscopy as described in Ref. 27. The membranes were placed in a copper sample holder, frozen to −180 °C in liquified ethane, and fractured in a BAF400T (BalTec, Principality Liechtenstein). Platinum/carbon replicas were imaged with an EM208S (FEI Co.).
Time-correlated Single-photon Counting
Time-correlated single-photon counting measurements were performed as described previously (28). The membranes were diluted to 2–10 μm chlorophyll in 10 mm MES (pH 6.5), 7 mm MgCl2, and 40 mm KCl. A combination of low excitation light intensities and the use of ferricyanide guaranteed that ∼100% of the reaction centers stayed open, whereas significant build-up of triplet states was avoided (28). This was confirmed by the measurement of almost identical lifetime components at 10-fold increased light intensities (data not shown).
Resonance Raman Spectroscopy
Resonance Raman spectra were measured essentially as described in Ref. 29. Thylakoid and BBY membranes were measured at 6280 and 4629 μm chl, respectively, whereas the control and fused BBY samples were measured at 100 μm chl after the addition of 7 mm MgCl2. A small drop of sample, maintained at 77 K in a nitrogen flow cryostat (Air Liquide, Sassenage, France), was excited at 488.0 nm with an argon laser (Coherent, Palo Alto, CA). Raman spectra were measured with 90° signal collection using a two-stage monochromator (U1000, Jobin-Yvon, Longjumeau, France) equipped with 1800 groove/mm gratings and a front-illuminated, deep-depleted CCD detector (Jobin-Yvon, Longjumeau, France).
RESULTS
Characterization of Isolated Grana Membranes
The BBY preparations have a chlorophyll a/b ratio of 2.32 ± 0.01 (thylakoids 3.42 ± 0.02) and an oxygen evolution activity of 328 ± 20 μmol O2·(mg chlorophyll·h)−1, indicating the isolation of active grana membranes. From cytochrome b559 difference absorption spectra and from pheophytin quantification by HPLC analysis, total chlorophyll/PSII ratios of 209 ± 26 (n = 3, number of independent measurements) and 199 ± 7 (n = 5) were determined, respectively. Furthermore, in a previous study (30), we measured that in our BBY preparations the PSII/PSI ratio is >30. From these data the trimeric LHCII/PSII-monomer ratio can be calculated from the equation chl/PSII (measured) = R·42 + 63 + 1/30·167; R, LHCII/PSII ratio; the numbers 42, 63, and 167 are the number of chlorophylls bound to trimeric LHCII, PSII (without trimeric LHCII), and PSI (4, 7, 31). Solving this equation for R leads to a LHCII-trimer/PSII ratio of 3.0 to 3.3. The lipid content of the BBY preparation is 1.16 ± 0.15 lipids/chlorophyll. This value is significantly lower than reported for grana membranes prepared by mechanical thylakoid fragmentation (32), indicating that lipids were extracted by the detergent treatment. This is in line with a 20% higher PSII density determined by freeze-fracture electron microscopy: The particle density in the exoplasmic fracture (EF) face (see also below) increases from 1720 ± 63 particles/μm2 in stacked grana regions of intact spinach thylakoids (14) to 2057 ± 22 particles/μm2 in the BBY preparation.
Characterization of BBY Membranes Fused with Unilamellar Liposomes
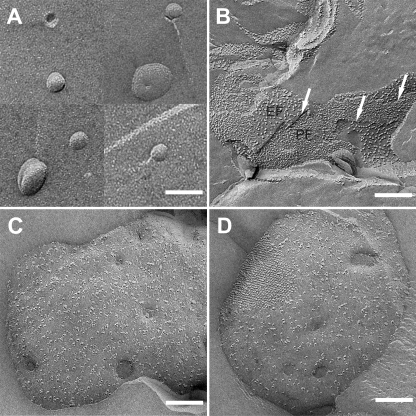
In our previous study (18), we demonstrated that BBY membranes fused with liposomes (we will name these membranes “fused BBY” in the following) have (i) a lower physical density, (ii) a higher lipid content, and (iii) a lipid composition that is virtually indistinguishable from unfused BBY membranes. These observations suggest the incorporation of extra lipids from the liposomes into the BBY membrane bilayer. To further characterize the fusion products, freeze-fracture electron microscopy was applied (Fig. 1). This technique splits the membrane bilayer into two monolayers, which are named the EF face and the protoplasmic fracture (PF) face. EM micrographs of freeze-fractured liposomes (Fig. 1A) confirm the size of ∼60 nm diameter, as previously measured by dynamic light scattering (18). Note that the surfaces of the fractured liposomes are smooth, indicating the absence of nonbilayer lipid phases. This is noteworthy, because the liposomes contain ∼40–50% monogalactosyldiacylglycerol (MGDG), which can form onion-like nonbilayer inverted hexagonal (HII) phases (33). Obviously HII phase formation is suppressed by the presence of the other thylakoid lipids, as already reported for liposomes prepared by the high pressure extruder technique (34).
FIGURE 1.
Electron micrographs of freeze-fracture membranes. A, micrographs of lipid liposomes made of the native thylakoid membrane lipid mixture. Scale bar, 100 nm. B, untreated BBY membranes. Larger particles represent PSII complexes (EF face), smaller particles LHCII (PF face). Arrows, indicate EF-PF transitions, which can occur only in stacked grana membranes. Scale bar, 200 nm. C and D, fused BBY (lipid/chrorophyll 10:1). Note the decreased particle density as compared with B. Semicrystalline protein areas are visible in the upper left part in D. Scale bars, C and D, 200 nm.
Compared with untreated BBY membranes (Fig. 1B), fused BBY reveal a significantly decreased particle density (Fig. 1C). These particles represent photosystem II (larger, less densely packed particles in the EF face in Fig. 1B) and light-harvesting complex II (smaller, densely packed particles in the PF face) (11). This density decrease is a clear indication of the integration of extra lipids from the liposomes into the BBY bilayer. Occasionally, highly ordered semicrystalline protein arrays are visible in fused BBY (Fig. 1D). Statistical analysis reveals that the abundance of these arrays is always less than 20% (percentage of total membrane area). The mean value is ∼10% and independent of the degree of protein dilution (fused BBY with lipid to chlorophyll ratios of 1, 2, and 10 were analyzed). It is noteworthy that no onion-like tubes were observed in fused BBY membranes, indicating the absence of the HII phase.
The fusion protocol was optimized by incubating the membranes in low ionic strength buffer (0.3 mm MgCl2), which gave the highest yield of fused BBY. Comparison of untreated stacked BBY (7 mm MgCl2, 40 mm KCl) with BBY membranes passed through the fusion procedure but without adding liposomes (fusion control) reveals the presence of fracture steps from the EF to the PF face and vice versa in the former (Fig. 1B, arrows), but their absence in fusion controls (supplemental Fig. S1). These EF-PF or vice versa steps can occur only in stacked grana. Thus missing steps in the fusion controls are a clear indication that these grana membranes are destacked. Destacking of grana may be a prerequisite for efficient fusion because the yield of chimeric membranes produced is much lower in buffers with high ionic strength (not shown).
The steady state activity of PSII in fused BBY was probed by the light-induced reduction of the PSII acceptor DCPIP in the presence of the electron donor DPC. The maximal activity of untreated BBY was 135 ± 11 μmol DCPIP·(mg chlorophyll·h)−1 (set as 100%). Relative to this value the activity for BBY that passed through the fusion protocol but without the addition of liposomes drops to 90 ± 2%. In BBY fused with liposomes with a lipid to chlorophyll ratio of 1, the activity is comparable with untreated BBY (97 ± 6%), and at the highest lipid to chlorophyll ratio of 10, the activity is still 67 ± 4%. It can be concluded that liposome fusion of BBY decreased the PSII electron transport rate only slightly.
The intactness of the water splitting apparatus was assessed by comparing DCPIP rates in the absence and presence of DPC. In untreated BBY the activity decreased to 100 ± 6 μmol DCPIP·(mg chlorophyll·h)−1 if DPC was omitted. This indicates that in ∼74% of the PSII the water splitting apparatus is inactive. In fusion controls and in BBYs fused with liposomes at a lipid-to-chlorophyll ratio of 1 and 10, the PSII activity drops to 62 ± 8, 34 ± 3, and 11 ± 9%, respectively. This shows that the fusion protocol induces some damage of the water splitting apparatus (from 74 to 62%). Furthermore, the data reveal that a high protein density is required for efficient water splitting.
Dependence of Stationary Chlorophyll Fluorescence Parameters on the Lipid Content in Fused BBY
The degree of lipid incorporation in BBY membranes can be easily controlled by variation of the liposomes to BBY membrane ratio (expressed as mol % added lipid/mol % chlorophyll in the following). Lipid incorporation can be titrated almost continuously as demonstrated by Ficoll density ultracentrifugation (not shown) because of the large difference in size between liposomes and grana membranes. The influence of increased lipid addition to BBY membranes on chlorophyll a fluorescence parameters is depicted in Fig. 2. The Fo level, indicating the fluorescence level when all reaction centers are open (primary quinone acceptor (QA) is completely oxidized), increases linearly with the addition of lipids and saturates at a lipid/chlorophyll ratio of ∼7. The Fo increase suggests a detachment of LHCII from PSII, i.e. light quanta absorbed by LHCII, that are functionally detached from PSII lead to fluorescence with a high yield. A different dependence is apparent for Fv, which reflects the fluorescence increase associated with the transition from open to closed reaction centers (QA completely reduced). A slight increase in the lipid content of BBY membranes (up to 1 mol % lipid/mol % chlorophyll) induces an increase in Fv, although the data scatter is significant (control point is measured 20 times). At higher lipid contents Fv decreases to a level that is slightly lower than for unfused control membranes. A quantity expressing the maximal photochemical quantum efficiency of PSII is Fv/Fm. Fv/Fm remains almost unchanged up to a lipid/chlorophyll ratio of 1 and declines monotonically at higher protein dilutions. It levels off at a value of ∼0.3 at high lipid contents. The data suggest a critical role for the lipid content in grana thylakoids on light conversion efficiency by LHCII and PSII. Adding lipids above 1 mol % lipid/mol % chlorophyll leads to a significant decline in photochemical quantum efficiency.
FIGURE 2.
Steady state chlorophyll a fluorescence analysis. Black circles, Fo; white triangles, Fv; gray squares, Fv/Fm. The degree of lipid addition from liposomes to BBY membranes is expressed as added lipid/chlorophyll (x axis). The lines serve as a guide to the eye.
Absorption Spectra
The stationary chlorophyll fluorescence data suggest a decoupling of LHCII from PSII. This was further verified by room temperature absorption spectroscopy. Isolated trimeric LHCII and PSII cores reconstituted in liposomes (with the grana lipid composition) have absorption maxima in the Qy region at 676.0 and 674.0 nm, respectively.3 Relative to these values, BBY membranes are red-shifted with a Qy maximum at 678.5 ± 0.5 nm. Because the lipid environment in the proteoliposomes and in BBY is the same, we conclude that the red shift in BBY is due to protein-protein interactions induced by macromolecular crowding. A blue shift in the Qy maximum indicates a separation of the protein complexes that is observed in fused BBY (Fig. 3). Thus room temperature absorption data confirm a lipid-induced separation of granal proteins. Note that the blue shift in Fig. 3 saturates at the same lipid content (7 mol % lipid/mol % chlorophyll) as Fo (Fig. 2).
FIGURE 3.
Shift in the Qy absorption maximum of chlorophyll of fused and control BBY. The absorption maximum of unfused BBY is at 678.5 nm.
Low Temperature Chlorophyll Fluorescence Spectroscopy
Spectral deconvolution of chlorophyll fluorescence spectra at cryogenic temperatures allows the separation of different protein complexes involved in fluorescence emission (25). Fig. 4A shows a measured 77 K emission spectrum and its deconvolution into five components of BBY membranes passed through the fusion procedure but without liposome addition (fusion control). The spectra of untreated BBY are almost identical (not shown). According to the analysis in (25), the various spectral components are assigned to different protein complexes: F680, free nonaggregated LHCII; F685, CP43 of PSII; F695, CP47 of PSII; F700, aggregated LHCII; and F730 comprises LHCI + PSI and vibrational bands of the above-mentioned components.
FIGURE 4.
A and B, 77 K fluorescence spectra of unfused (A) and fused (B) BBY (lipid/chlorophyll = 3). The area under the spectra was normalized to the measured Fm value. Open circles represent measured data, and the lines are fitted curves. Note that the overall increase of the amplitude in B is mainly caused by an increase in F680. C and D, dependence of the amplitudes of the fitted fluorescence components (see A and B) on the added lipid to chlorophyll ratio.
Spectra of fused BBY are significantly different (Fig. 4B). A detailed picture of the changes induced by lipid addition arises from spectral deconvolution. The shapes of the underlying spectra are very similar in all cases, but their relative amplitudes have changed. To compare the amplitudes of the five components between different samples, the area under the spectra are normalized to the corresponding measured Fm values (see “Experimental Procedures”). The dominant change is the marked increase of F680, which indicates the formation of functionally uncoupled nonaggregated LHCII, in line with the interpretations above. The amplitude of F680 increases steadily up to a lipid/chlorophyll of 7. At higher values we cannot resolve whether there is a further increase or saturation. A clear increase is also seen for F695 (CP47) but only up to a lipid/chlorophyll ratio of 1. This increase can be explained by an enlargement of the apparent PSII antenna size, which will be addressed in the next section. All other components are almost unaffected by the addition of lipids.
An important question is whether the supposed signal of aggregated LHCII (F700) is present in unfused BBY (see “Discussion”). The supplemental Fig. S2 demonstrates that spectral deconvolution without this component is significantly worse, indicating that F700 is required for fitting.
Probing the Functional PSII Antenna Organization by Chlorophyll a Fluorescence Induction Analysis
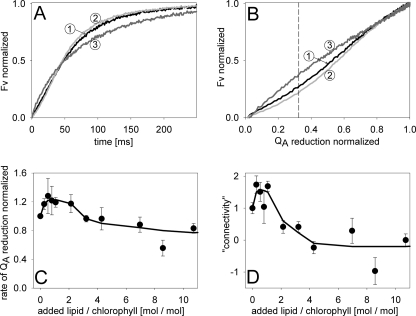
Chlorophyll fluorescence induction measurements in the presence of DCMU form a versatile technique for probing the functional organization of the light-harvesting system of PSII (35). From the rise in Fv (Fig. 5A), QA reduction kinetics were calculated by the “complementary area method” (23). The rate of QA reduction reflects the apparent antenna size of photosystem II. Fig. 5C shows a 25% increase in QA reduction rate in fused BBY for a lipid/chlorophyll ratio of ∼0.5 to 1. Obviously the apparent PSII antenna size increases if the protein density is slightly lowered, which is in agreement with the increase in F695 (Fig. 4D). For higher lipid/chlorophyll ratios, the QA rate decreases to a value of ∼0.8 relative to that of unfused control BBY.
FIGURE 5.
Chlorophyll fluorescence induction analysis. A, normalized Fv induced by broad green excitation light switched on at 0 ms. Curve 1, fusion control, curve 2, fused BBY (lipid/chlorophyll = 1); curve 3, fused BBY (lipid/chlorophyll = 10). Note the faster rise and the stronger sigmoidicity of curve 2 compared with curve 1. B, connectivity plot calculated from A. The deviation from linearity indicates higher cooperativity between PSII centers. The vertical dashed line indicates the 0.33 QA reduction level used for quantifying the connectivity. C, apparent antenna size of PSII expressed as rate of QA reduction (reciprocal half-time) deduced from Fv kinetics as a function of lipid content of BBY membranes (A). D, dependence of the connectivity of PSII on the lipid content. The connectivity parameter is expressed as the Fv value at 0.33 QA reduction (see B) and normalized to the value of unfused BBY. The value of 0 corresponds to linearity in B, equivalent to no connectivity.
Another parameter extracted from the chlorophyll fluorescence induction measurements is the excitonic connectivity between adjacent PSII complexes. It has been long known (36) that PSII complexes can exchange excitons by a functional connection mediated by LHCII. This could be an important strategy to enhance the light harvesting efficiency because an exciton generated at a PSII with a closed reaction center can be used by an adjacent PSII with an open reaction center. The degree of connectivity can be derived from plotting the QA reduction level against Fv (23): the stronger the deviation from linearity the higher the connectivity. Fig. 5B shows examples for fusion control and fused BBY. As for the apparent PSII antenna size (Fig. 5A), the connectivity increases for slightly increased lipid contents (compare curves 1 and 2 in Fig. 5B). For a more quantitative analysis, the degree of deviation from linearity was extracted at a QA reduction level of 0.33 (Fig. 5B, dashed line). The value of Fv at this reduction level was taken as a measure for the connectivity and was plotted as a function of the lipid/chlorophyll ratio (Fig. 5D). At lipid/chlorophyll ratios of 0.5–1, the connectivity is maximal and declines to 0 at high lipid contents. The latter indicates that PSII complexes become functionally disconnected (“puddle” model).
Time-resolved Fluorescence Spectroscopy of Control and Fused BBY
Ultrafast fluorescence relaxation kinetics on control and fused BBY with open reaction centers (see “Experimental Procedures”) were fitted with a sum of four exponentials. The resulting fitting parameters are summarized in Table 1. Overall, increasing the lipid content leads to an increase of the average fluorescence lifetime. At a lipid/chlorophyll ratio of 2, ∼50% of the fluorescence decays with a lifetime of ∼1 ns or longer. At a lipid/chlorophyll ratio of 10, approximately two-thirds of the fluorescence relaxes with a lifetime longer than 1.6 ns. These long lifetime components are indicative of the formation of functionally uncoupled LHCII by protein dilution, consistent with the data presented above. It is important to notice that over the entire range of lipid/chlorophyll ratios, no complete detachment of the outer antenna from the core is observed, because disintegration into PSII cores (D1-D2-CP43-CP47) would have been accompanied by the appearance of a ∼40-ps component (28, 37), which is not seen in our samples.
TABLE 1.
Fluorescence lifetime analysis of unfused and fused BBY
L/Chl indicates the mol% lipid/mol% chlorophyll ratio. The data are the means (with standard deviation) of two independent preparations. Each preparation was measured two times and averaged. The measurements were performed in the Fo state (see “Experimental Procedures”). Amplitude-averaged lifetime (<τ >), is defined as: <τ > = Σ(ai·τ i) and Σ(ai) = 1, with ai and τ i amplitude and lifetime of lifetime component i, respectively.
| Component 1 | Component 2 | Component 3 | Component 4 | Amplitude-averaged lifetime | |
|---|---|---|---|---|---|
| Control | 129 ± 27 ps | 304 ± 61 ps | 607 ± 175 ps | 2762 ± 1016 ps | 287 ps |
| 41 ± 1% | 50 ± 4% | 9 ± 3% | <1% | ||
| L/Chl = 2 | 110 ± 12 ps | 399 ± 73 ps | 950 ± 189 ps | 2535 ± 874 ps | 719 ps |
| 12 ± 5% | 34 ± 7% | 52 ± 2% | 3 ± 1% | ||
| L/Chl = 10 | 121 ± 10 ps | 538 ± 68 ps | 1627 ± 135 ps | 3756 ± 406 ps | 1681 ps |
| 6 ± 5% | 18 ± 1% | 60 ± 3% | 16 ± 3% |
Although the fourth lifetime component can be assigned to completely uncoupled LHCII, the interpretation of the third component is less clear. In unfused control BBY, the third 600-ps component could reflect a small fraction of aggregated LHCII, which have a lifetime between 100 and 600 ps (e.g. 16, 38). The lifetime of the third component in fused BBY is significantly longer (∼1 and ∼1.6 ns) and is possibly caused by a (slight) separation of LHCII from PSII.
Formation of aggregated LHCII in fused BBY or (again) a slight separation of LHCII from PSII core could explain the increase in lifetime of the second component. See “Discussion” for further interpretations.
Resonance Raman Spectroscopy
Carotenoid resonance Raman spectra were measured at 488.0 nm, where neoxanthin contributions dominate (40). In the ν4 region, the spectrum for unfused BBY membranes shows a clear enhancement on the lower frequency side of the envelope (950–960 cm−1) relative to intact thylakoids (Fig. 6A). The difference spectrum (Fig. 6B) has the characteristic shape for distortion of LHC-bound neoxanthin, which has already been observed in aggregated LHCII (40) and during photoprotective energy quenching in vivo (6) and which reflects a conformational change in this protein (41). Thus the difference resonance Raman spectrum reveals the same neoxanthin distortion in unfused BBY compared with intact thylakoids.
FIGURE 6.
488.0 nm resonance Raman spectroscopy in the ν4 region. A, intact thylakoids and BBY membranes. B, xanthophyll distortion in BBY compared with thylakoids is clearly visible in the difference spectrum. C and D, spectra of unfused BBY and BBY fused with liposomes at a lipid to chlorophyll ratio of 1 and 10. The apparent maxima in B are indicated.
When the protein packing density in BBY was reduced by fusion with liposomes, a relaxation of this neoxanthin distortion is observed (Fig. 6, C and D). Indeed for fused BBY at a lipid-to-chlorophyll ratio of 1, the spectrum is very similar to that in thylakoids (the packing density is of the same order). At a fuse ratio of 10, the spectrum resembles that in isolated trimers (40), i.e. very little neoxanthin distortion is present (Fig. 6C, lower trace). This indicates that dilution of the protein density in crowded grana leads to relaxation of this neoxanthin distortion, associated with a conformational change in LHCII.
DISCUSSION
Liposome-fused Grana Thylakoids as a Tool for Studying the Impact of Macromolecular Crowding
Manipulating the lipid to protein ratio by fusion of BBY membranes with liposomes is a straightforward approach to study the functional implications of macromolecular crowding in grana membranes. However, possible artifacts have to be considered. The ideal situation would be if the added lipids from the liposomes (i) are integrated in the BBY bilayer membrane, (ii) are without HII formation of MGDG, (iii) do not induce artificial lipid-protein interactions, (iv) lead to a homogeneous protein dilution, (v) preserve the native protein orientation (i.e. avoiding protein flip-flops), and (vi) do not induce disruption of the protein complexes. Freeze-fracture EM analysis of fused BBY give strong evidence that points (i) and (ii) are realized in our fused BBY. Furthermore, by using the native lipid composition artificial lipid-protein interactions can be ruled out point (iii).
Most of the EM micrographs indicate a homogeneous protein dilution (Fig. 1C; point (iv)), although semicrystalline protein arrays are occasionally visible (Fig. 1D). These highly ordered structures probably represent aggregated LHCII trimers, which are formed if LHCIIs are orientated in an antiparallel way in the membrane (5). This non-native orientation must therefore be caused by an artificial protein flip-flop induced by the fusion. Thus it seems that point (v) could be critical in fused BBY. However, good indications exist that LHCII aggregates are of minor significance in fused BBY. Firstly, two-dimensional semicrystalline arrays were detected in only ∼10% of the membrane areas. Bearing in mind that antiparallel-oriented LHCII trimers have a high tendency to aggregate, this indicates that only a small number of proteins underwent flip-flops. Second, analysis of low temperature fluorescence spectra did not show an increase in the 700-nm band, which is associated with LHCII aggregation (e.g. 39). In contrast, the sharp increase of the 680-nm component (Fig. 4C) is a good indicator of free, nonaggregated LHCII. Third, the interpretation of the low temperature fluorescence data is supported by the Fo data (Fig. 2); LHCII aggregation would lower the Fo level in contrast to the marked increase upon lipid addition. Fourth, LHCII aggregates are characterized by a 100–600-ps lifetime component (e.g. 16, 38). Such aggregates may contribute to the second lifetime component, the value of which increases in fused BBY. However, its amplitude becomes smaller, indicative of a low abundance of semicrystalline arrays.
The integrity of the pigment-protein complexes (point (vi)) is indicated by the absence of emission bands ∼650 and ∼670 nm in fluorescence spectra at cryogenic temperatures (Fig. 4, A and B), which would occur if free, non-protein-bound chlorophyll b (650 nm) or chlorophyll a (670 nm) were formed by the fusion. Furthermore, the absence of a ∼40-ps component (Table 1) in fused BBY indicates that the PSII complex is not disrupted (see also below).
In summary, the detailed functional and structural analysis reveals that fusion of small unilamellar liposomes with BBY leads to a chimeric membrane bilayer with diluted protein packing that avoids disintegration of granal protein complexes and HII formation of MGDG. Artificial protein flip-flops occur but are rare. We conclude that this technique is appropriate to study the elusive field of macromolecular crowding in grana thylakoids.
Suboptimal Light Harvesting at Very High Protein Packing Densities
The addition of ∼0.5–1 mol % lipid/mol % chlorophyll to BBY membranes increases the apparent PSII antenna size detected by fluorescence induction (Fig. 5C) and 77 K fluorescence spectroscopy (F695 increase in Fig. 4D) as well as the excitonic connectivity between PSII centers (Fig. 5D). Obviously light harvesting in highly crowded, unfused BBY membranes is not optimized and becomes more efficient by a slight dilution of the protein density. From our lipid determination (18), we can estimate that the protein density decreases from ∼80% in untreated BBY to ∼70% at a mol % lipid/mol % chlorophyll ratio of 0.5–1 (The protein area fraction for this lipid/chlorophyll range is estimated to be 70–65%). It follows that the PSII particle density decreases from ∼2050 particles/μm2 (freeze fracture EM data) to ∼1800 particles/μm2. Interestingly, the latter density is similar to the PSII density measured in stacked grana of intact thylakoids prepared from spinach plants growing under the same conditions (∼1720 particles/μm2) 14). It seems that the protein density in native grana thylakoids is adjusted to a value of ∼70% area occupation, which ensures efficient light harvesting. The change in protein density from ∼2050 to 1800 PSII dimers/μm2 is illustrated in Fig. 7 (A and B).
FIGURE 7.
Models of protein densities in a grana disc (50 × 50 nm). Structures of the dimeric LHCII-PSII supercomplex and trimeric LHCII are taken from Ref. 7. For the models a trimeric LHCII to PSII ratio of 3 was assumed (see in the text). A, PSII dimer density 2057/μm2, corresponding to unfused BBY. The purple stars indicate putative overlap of protruding neoxanthins with adjacent proteins. B, PSII dimer density 1800/m2 corresponding to fused BBY at lipid/chlorophyll of 075. C, PSII dimer density 1100/m2 corresponding to fused BBY at lipid/chlorophyll of 10.
The question arises of why light-harvesting in overcrowded, untreated BBY membranes is less efficient. A possible explanation can be derived from Fig. 7A. Because of the tight protein packing, part of the neoxanthin molecules protruding outward from trimeric LHCII would overlap with adjacent protein complexes (highlighted by purple stars). Each monomer in LHCII trimers binds one neoxanthin molecule, which sticks out into the hydrophobic membrane and is structurally highly flexible (38). Because a neoxanthin protein overlap is sterically not possible, the flexible carotenoid will be distorted at these contact points. This distortion has also been detected by resonance Raman spectroscopy in tightly packed LHCII aggregates and crystals and is associated with other LHCII structural changes that together induce energy dissipation (6, 40). Thus neoxanthin bending could be forced by protein crowding in unfused BBY causing energy quenching. This in turn would decrease the apparent PSII antenna size and the connectivity between adjacent PSII complexes, i.e. more light quanta are required for photochemistry (affects the apparent antenna size), and energy transfer between PSII will be impeded by the quencher (affects the apparent connectivity).
This “neoxanthin hypothesis” is supported by resonance Raman data (Fig. 6). Unfused, overcrowded BBY membranes show spectral features typical for neoxanthin distortion, which are absent in intact thylakoids (see difference spectrum in Fig. 6B). In addition neoxanthin distortion can be reversed by diluting the protein density (Fig. 6, C and D). Furthermore two pieces of evidence support the existence of energy quenchers in unfused BBY: (i) spectral fitting of 77 K fluorescence spectra is significantly improved if a 700-nm component for quenched LHCII is included (see supplemental Fig. S2), and (ii) Fv increases when the lipid content is slightly increased (∼1 mol % lipid/mol % chlorophyll; Fig. 2). This indicates that Fv was partially quenched in unfused BBY. Importantly, at a protein area occupation of 70% it is possible to pack the proteins without producing a neoxanthin protein overlap (Fig. 7B). Thus protein packing in native grana membranes might be adjusted to ∼70% to avoid neoxanthin bending associated with unwanted energy dissipation.
In parallel with the improvement of light harvesting of PSII by slight dilution of the protein density, the increase of Fo (Fig. 2), the increase of F680 in 77 K fluorescence spectra (Fig. 4), and the blue shift of the Qy chlorophyll absorption band (Fig. 3) indicate the presence of LHCII that are uncoupled from other protein complexes. However, fluorescence lifetime analysis (Table 1) reveals that these changes are not mainly caused by a complete functional uncoupling of LHCII from PSII. Isolated, functionally uncoupled LHCII trimers have a fluorescence lifetime of 2–4 ns (e.g. 16), which corresponds to the fourth component in Table 1. The fluorescence quantum yield of the fourth component (calculated from Table 1) increases from <10% (unfused control) to only ∼11% at a lipid to chlorophyll ratio of 2. Thus this component has a negligible effect on the Fo and F680 increases. A pronounced increase induced by moderate dilution (2 lipids/chlorophyll) is apparent for the third lifetime component, the fluorescence quantum yield of which increases from ∼20% to ∼70% and which is thus mainly responsible for the Fo and F680 increases in this protein dilution range. The increased quantum yield of the third component probably indicates a loosening but not complete uncoupling of functional LHCII-PSII interactions. Because the Förster rate for energy transfer between pigments is highly dependent on the distance between the pigments (3), a slight increase in separation (Fig. 7, compare A with B) could have a pronounced effect on the intermolecular energy transfer probability, expressed by an increased fluorescence lifetime.
Disruption of the Functional Protein Network by Higher Protein Dilution
The strong dependence of the energy transfer probability on the chlorophyll-chlorophyll distance in adjacent LHCIIs or LHCII and PSII-supercomplexes suggests that the protein density in grana thylakoids should not drop under a certain threshold. This is clearly seen in functional PSII parameters. At lipid/chlorophyll ratios above 2, the connectivity parameter (Fig. 4B) and the apparent antenna size (Fig. 5D) clearly decline, going along with a decrease in Fv (Fig. 2). Also the maximal photochemical quantum efficiency of PSII (Fv/Fm) decreases in this lipid/chlorophyll range. However, as demonstrated in Fig. 2, the Fv/Fm decrease is mainly caused by an increased Fo rather than by a Fv decrease. This indicates that the photochemistry of PSII remains intact whereas the lower photochemical quantum yield is due to LHCII separation from PSII. This LHCII separation is consistent with the increase of F680 in 77 K spectra, the blue shift of the Qy absorption band, and the increased contribution of long fluorescence lifetime components (Table 1). The changes of Fo, Fv/Fm, and Qy absorption maximum induced by lipid addition saturate at a lipid/chlorophyll ratio of ∼7. For this value a protein area fraction of 40–50% can be estimated. Obviously at this protein density the functional uncoupling is complete, and further dilution has no effect. Fig. 7C gives an idea of the corresponding protein density.
Closer inspection of the situation at 40–50% protein density reveals interesting details. The connectivity parameter indicates a complete disconnection between photosynthetic units. However, the change in apparent antenna size is moderate. Compared with the maximum value, the functional PSII antenna size decreases by only ∼35% (Fig. 5C). Assuming that all trimeric LHCII are coupled to PSII at a lipid/chlorophyll ratio of 0.5–1 (maximal functional antenna size; Fig. 5C), disconnection of one, two, or three trimeric LHCII would decrease the PSII antenna size by 21, 42, or 64%, respectively (42 LHCII chlorophylls/196 total chlorophylls, 84 LHCII chlorophylls/196 total chlorophylls, or 126 LHCII chlorophylls/196 total chlorophylls). Thus probably only two LHCII trimers can be disconnected from PSII by lipid addition, whereas one remains bound. From these considerations we conclude (as suggested in Fig. 7C) that the LHCII-PSII supercomplex is not disrupted into core particles and outer antennae by the protein dilution. This is in line with the absence of a significant 40-ps component in the time-resolved fluorescence data (Table 1) and with the moderate decrease in Fv. It follows that the supramolecular protein network in grana thylakoids responds differentially to dilution of the protein density in grana membranes by lipid addition. Loosely bound peripheral LHCII can be separated by lipid addition, whereas more strongly bound LHCII trimers in the supercomplex remain connected.
Conclusions
Both the lipid content and the PSII particle density (determined by freeze-fracture EM) indicate a significant lipid depletion in our BBY membranes compared with intact grana thylakoids, leading to a very dense protein packing (∼80% protein area). Decreasing the protein packing to ∼70% area occupation, which resembles the value in intact grana thylakoids, improves the light harvesting efficiency of PSII (larger apparent antenna and higher connectivity). We hypothesize that this improvement is due to an unbending of LHCII-bound neoxanthin, which is distorted in unfused, overcrowded BBYs where it causes energy quenching. This hypothesis is supported by resonance Raman spectroscopy of neoxanthin. A further dilution of the protein density leads to a decreased efficiency of energy transformation, which is due to separation of LHCII from PSII. Thus energy transformation by PSII in grana thylakoids depends on a delicate balance of the protein/lipid stoichiometry. How thylakoid membranes adjust and tune their lipid/protein stoichiometry is unknown. In this respect plastoglobuli could act as a dynamic lipid reservoir. Recently EM tomographic analysis reveals that plastoglobuli are permanently connected to the thylakoid membrane system, thus allowing a flexible lipid exchange (42). Furthermore the lipid content in the thylakoid bilayer could be modulated by HII formation of MGDG (43). An interesting possibility would be that HII formation could determine the lipid content in grana bilayers and in this way control the protein packing density and energy quenching.
Supplementary Material

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
H. Kirchhoff, unpublished results.
- LHC
- light-harvesting complex
- PS
- photosystem
- MES
- 4-morpholineethanesulfonic acid
- DCPIP
- 2,6-dichlorophenolindophenol
- DPC
- diphenylcarbazide
- HPLC
- high pressure liquid chromatography
- chl
- chlorophyll a
- EF
- exoplasmic fracture
- PF
- protoplasmic fracture
- MGDG
- monogalactosyldiacylglycerol
- QA
- primary quinone acceptor of PSII
- Qy
- lowest energy transition along the y axis of chlorophyll.
REFERENCES
- 1.Blankenship (2002) Molecular Mechanisms of Photosynthesis, Blackwell Science, Oxford, UK [Google Scholar]
- 2.van Grondelle R., Novoderezhkin V. I. (2006) Phys. Chem. Chem. Phys. 8, 793–807 [DOI] [PubMed] [Google Scholar]
- 3.Förster V. T. (1949) Z. Naturforsch. 4, 321–327 [Google Scholar]
- 4.Schmid V. H. (2008) Cell. Mol. Life Sci. 65, 3619–3639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barros T., Kühlbrandt W. (2009) Biochim. Biophys. Acta 1787, 753–772 [DOI] [PubMed] [Google Scholar]
- 6.Ruban A. V., Berera R., Ilioaia C., van Stokkum I. H., Kennis J. T., Pascal A. A., van Amerongen H., Robert B., Horton P., van Grondelle R. (2007) Nature 450, 575–578 [DOI] [PubMed] [Google Scholar]
- 7.Nield J., Barber J. (2006) Biochim. Biophys. Acta 1757, 353–361 [DOI] [PubMed] [Google Scholar]
- 8.Dekker J. P., Boekema E. J. (2005) Biochim. Biophys. Acta 1706, 12–39 [DOI] [PubMed] [Google Scholar]
- 9.Caffarri S., Kouril R., Kereïche S., Boekema E. J., Croce R. (2009) EMBO J. 28, 3052–3063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takahashi T., Inoue-Kashino N., Ozawa S., Takahashi Y., Kashino Y., Satoh K. (2009) J. Biol. Chem. 284, 15598–15606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Staehelin L. A., van der Staay G. W. (1996) Oxygenic Photosynthesis: The Light Reactions (Ort D. A., Yocum C. F. eds) pp. 11–30, Kluwer Academic Publishers, The Netherlands [Google Scholar]
- 12.Bumba L., Vácha F. E. (2003) Photosyn. Res. 77, 1–19 [DOI] [PubMed] [Google Scholar]
- 13.Anderson J. M. (1986) Annu. Rev. Plant Physiol. 37, 93–136 [Google Scholar]
- 14.Kirchhoff H., Haase W., Wegner S., Danielsson R., Ackermann R., Albertsson P. A. (2007) Biochemistry 46, 11169–11176 [DOI] [PubMed] [Google Scholar]
- 15.Kirchhoff H. (2008) Trends Plant Sci. 13, 201–207 [DOI] [PubMed] [Google Scholar]
- 16.van Oort B., van Hoek A., Ruban A. V., van Amerongen H. (2007) FEBS Lett. 581, 3528–3532 [DOI] [PubMed] [Google Scholar]
- 17.Horton P., Wentworth M., Ruban A. (2005) FEBS Lett. 579, 4201–4206 [DOI] [PubMed] [Google Scholar]
- 18.Haferkamp S., Kirchhoff H. (2008) Photosynth. Res. 95, 129–134 [DOI] [PubMed] [Google Scholar]
- 19.Siegel C. O., Jordan A. E., Miller K. R. (1981) J. Cell Biol. 91, 113–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berthold D. A., Babcock G. T., Yokum C. F. (1981) Fed. Eur. Biochem. Soc. 134, 231–234 [Google Scholar]
- 21.Kirchhoff H., Borinski M., Lenhert S., Chi L., Büchel C. (2004) Biochemistry 43, 14508–14516 [DOI] [PubMed] [Google Scholar]
- 22.Porra R. J., Thompson W. A., Kriedemann P. E. (1989) Biochim. Biophys. Acta 975, 384–394 [Google Scholar]
- 23.Melis A., Homann P. H. (1976) Photochem. Photobiol. 23, 343–350 [DOI] [PubMed] [Google Scholar]
- 24.Kirchhoff H., Haase W., Haferkamp S., Schott T., Borinski M., Kubitscheck U., Rögner M. (2007) Biochim. Biophys. Acta 1767, 1180–1188 [DOI] [PubMed] [Google Scholar]
- 25.Andreeva A., Stoitchkova K., Busheva M., Apostolova E. (2003) J. Photochem. Photobiol. 70, 153–162 [DOI] [PubMed] [Google Scholar]
- 26.Eijckelhoff C., Dekker J. P. (1997) Photosynth. Res. 52, 69–73 [Google Scholar]
- 27.Kirchhoff H., Tremmel I., Haase W., Kubitscheck U. (2004) Biochemistry 43, 9204–9213 [DOI] [PubMed] [Google Scholar]
- 28.Broess K., Trinkunas G., van der Weij-de Wit C. D., Dekker J. P., van Hoek A., van Amerongen H. (2006) Biophys. J. 91, 3776–3786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sturgis J., Robert B. (1997) J. Phys. Chem. B 101, 7227–7231 [Google Scholar]
- 30.Kirchhoff H., Lenhert S., Büchel C., Chi L., Nield J. (2008) Biochemistry 47, 431–440 [DOI] [PubMed] [Google Scholar]
- 31.Amunts A., Drory O., Nelson N. (2007) Nature 447, 58–63 [DOI] [PubMed] [Google Scholar]
- 32.Duchêne S., Siegenthaler P. A. (2000) Lipids 35, 739–744 [DOI] [PubMed] [Google Scholar]
- 33.Williams W. P. (1998) Lipids in Photosynthesis: Structure, Function and Genetics (Siegenthaler P. A., Murata N. eds) pp. 145–173, Kluwer Academic Publisher, Dordrecht, The Netherlands [Google Scholar]
- 34.Webb M. S., Green B. R. (1989) Biochim. Biophys. Acta 984, 41–49 [Google Scholar]
- 35.Lavergne J., Trissl H. W. (1995) Biophys. J. 68, 2474–2492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joliot P., Joliot A. (1964) C. R. Acad. Sci. 258, 4622–4625 [PubMed] [Google Scholar]
- 37.Miloslavina Y., Szczepaniak M., Müller M. G., Sander J., Nowaczyk M., Rögner M., Holzwarth A. R. (2006) Biochemistry 45, 2436–2442 [DOI] [PubMed] [Google Scholar]
- 38.Barros T., Royant A., Standfuss J., Dreuw A., Kühlbrandt W. (2009) EMBO J. 28, 298–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruban A. V., Horton P. (1992) Biochim. Biophys. Acta 1102, 30–38 [Google Scholar]
- 40.Pascal A. A., Liu Z., Broess K., van Oort B., van Amerongen H., Wang C., Horton P., Robert B., Chang W., Ruban A. (2005) Nature 436, 134–137 [DOI] [PubMed] [Google Scholar]
- 41.Ruban A. V., Pascal A. A, Robert B. (2000) FEBS Lett. 477, 181–185 [DOI] [PubMed] [Google Scholar]
- 42.Austin J. R., 2nd, Frost E., Vidi P. A., Kessler F., Staehelin L. A. (2006) Plant Cell 18, 1693–1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garab G., Lohner K., Laggner P., Farkas T. (2000) Trends Plant Sci. 5, 489–494 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.