Abstract
Mammalian glutamate dehydrogenase (GDH) is an allosterically regulated enzyme that is expressed widely. Its activity is potently inhibited by GTP and thought to be controlled by the need of the cell for ATP. In addition to this housekeeping human (h) GDH1, humans have acquired (via a duplication event) a highly homologous isoenzyme (hGDH2) that is resistant to GTP. Although transcripts of GLUD2, the gene encoding hGDH2, have been detected in human neural and testicular tissues, data on the endogenous protein are lacking. Here, we developed an antibody specific for hGDH2 and used it to study human tissues. Western blot analyses revealed, to our surprise, that endogenous hGDH2 is more densely expressed in testis than in brain. At the subcellular level, hGDH2 localized to mitochondria. Study of testicular tissue using immunocytochemical and immunofluorescence methods revealed that the Sertoli cells were strongly labeled by our anti-hGDH2 antibody. In human cerebral cortex, a robust labeling of astrocytes was detected, with neurons showing faint hGDH2 immunoreactivity. Astrocytes and Sertoli cells are known to support neurons and germ cells, respectively, providing them with lactate that largely derives from the tricarboxylic acid cycle via conversion of glutamate to α-ketoglutarate (GDH reaction). As hGDH2 is not subject to GTP control, the enzyme is able to metabolize glutamate even when the tricarboxylic acid cycle generates GTP amounts sufficient to inactivate the housekeeping hGDH1 protein. Hence, the selective expression of hGDH2 by astrocytes and Sertoli cells may provide a significant biological advantage by facilitating metabolic recycling processes essential to the supportive role of these cells.
Keywords: Dehydrogenase, Enzyme Purification, Enzymes, Immunochemistry, Mitochondria, Astrocytes, Glutamate Dehydrogenase, hGDH2 Antibody, Sertoli Cells
Introduction
Glutamate dehydrogenase (GDH3; EC 1.4.1.3) catalyzes the reversible interconversion of glutamate to α-ketoglutarate and ammonia using NADP(H) and NAD(H) as cofactors, thus interconnecting amino acid and carbohydrate metabolism. Mammalian GDH is allosterically regulated, with GTP and ADP being the main negative and positive modulators, respectively (1, 2).
Although GDH in mammals has long been thought to be encoded by a single gene, studies in human tissues revealed the presence of two GDH activities, differing in their regulatory properties and relative resistance to thermal inactivation (3). These observations led to the cloning of two genes encoding human GDH: an intron-containing GLUD1 gene that is located on the 10th chromosome and is expressed widely (housekeeping) (4) and an X-linked intronless GLUD2 gene that is expressed mainly in retina, brain, and testis (5). There is evidence that the GLUD2 gene originated via a duplication event <23,000,000 years ago, being highly homologous to the conserved GLUD1 gene (6). As a result, the proteins encoded by the GLUD1 and GLUD2 genes (hGDH1 and hGDH2, respectively) share in their mature form all but 15 of their 505 amino acid residues. Recombinant hGDH1 and hGDH2 isoproteins, obtained by expression of the corresponding GLUD1 and GLUD2 cDNAs in Sf21 cells, were found to be heat-stable and heat-labile, respectively, and to differ significantly in their allosteric regulation (7–9).
Although the regulatory properties of hGDH1 (activation by ADP and inhibition by GTP) suggest that its activity is controlled by the need of the cell for ATP (1), the function of hGDH2 has been dissociated from GTP control (7–9). Instead, hGDH2 has developed unique molecular mechanisms for regulating its activity (9), and there is evidence that the enzyme has adapted to conditions that prevail in nerve tissue (10). Moreover, the importance of hGDH2 in nervous system biology is underscored by recent observations showing that a gain-of-function variant in GLUD2 modifies Parkinson disease onset, probably by accelerating neurodegeneration of the disease (11).
Despite the insight gained from structure/function analyses of recombinant hGDH2, data on the endogenous hGDH2 enzyme are largely lacking. As hGDH1 and hGDH2 are highly homologous and given that the few residues that set the two human isoenzymes apart are scattered throughout the 505-amino acid-long polypeptide, detection of hGDH2 in human tissues presents a true challenge. Choi et al. (12) have previously raised monoclonal antibodies against two bovine brain GDH activities, which cross-reacted with recombinant human GDHs, but none of these monoclonal antibodies could discriminate between hGDH1 and hGDH2 (13).
Previous investigations have shown the existence of GLUD2 gene-specific mRNA transcripts in human retina, brain, and testis (5). On the other hand, expressed sequence tag libraries deriving from human tissues are enriched with GLUD1-related sequences. Only a few expressed sequence tag clones encoded by the GLUD2 gene are deposited in public data bases (14). These derived from brain (hippocampus), testis, embryonic tissue, and various tumors. Whether the abundance of the GLUD1-related transcripts leads to their preferential amplification compared with the GLUD2 transcripts or the latter are relatively unstable is presently unclear.
Here, we report that we developed a novel polyclonal antibody that selectively identifies the hGDH2 isoprotein. Using this antibody, we confirm, for the first time at the protein level, the endogenous expression of hGDH2 in both human brain and testis. However, we were surprised to find that, compared with brain, endogenous hGDH2 is more densely expressed in testis, in which the Sertoli cells were strongly labeled by our anti-hGDH2 antibody. On the other hand, using unfixed human cerebral cortical tissue, we found that astrocytes were robustly labeled by the antibody, with neurons showing rather faint hGDH2 immunoreactivity. As astrocytes and Sertoli cells are the supporting cells in the mammalian central nervous system and testis, respectively, these observations suggest that the selective expression of hGDH2 by these cells may confer a biological advantage by facilitating the metabolic recycling processes needed for supplying target cells with nutrients.
EXPERIMENTAL PROCEDURES
Materials
Sf21 cells and the baculovirus expression vectors were obtained from Invitrogen (Carlsbad, CA). The medium for the Sf21 insect cells and fetal calf serum were from Invitrogen. Modified baculovirus (BaculoGold) was obtained from Pharmingen. NADPH and ADP were from Roche Applied Science. Phenyl-Sepharose HP was from Amersham Biosciences, and Bio-Gel hydroxyapatite HT was from Bio-Rad. Ficoll was purchased from Sigma, and nitrocellulose membrane (Porablot NCP) was from Macherey-Nagel (Duren, Germany). Anti-GDH antibody, raised against full-length bovine GDH, was obtained from Biodesign International (Saco, ME). Anti-manganese superoxide dismutase antibody was from Millipore (Billerica, MA), and anti-actin monoclonal antibody from Chemicon. Protein determination was done using the DC protein assay (Bio-Rad).
hGDH2-specific Antibody Production
A 12-amino acid-long hGDH2-specific peptide (PTAEFQDSISGA), corresponding to residues 436–447 of the mature human protein, was selected. This peptide, containing the R443S evolutionary change, was synthesized with the addition of a cysteine at the N terminus (to facilitate conjugation) and injected into rabbits. Serum was collected and used for Western blot and immunohistochemistry experiments. All antibody production-related steps were performed by Innovagen (Lund, Sweden).
Site-directed Mutagenesis and Plasmid Constructions
A GLUD2 and a GLUD1 cDNA, cloned in the pBSKII+ vector, were mutagenized at various sites using the Gene Editor mutagenesis system (Promega) as described previously (15). The orientation of the constructs and the absence of incidental DNA alterations were verified by bidirectional sequencing.
Production of Wild-type and Mutant hGDH1 and hGDH2 Proteins
Wild-type and mutant cDNAs were expressed in Sf21 cells using the baculovirus expression system as described previously (5, 7, 15, 16). Wild-type hGDH1 and hGDH2 proteins were purified from Sf21 cell extracts as described previously (9). GDH activity was assayed spectrophotometrically (at 340 nm) in the direction of reductive amination of α-ketoglutarate (8).
Testis Crude Extract and Purified Enzyme Preparation
GDH was studied in testis, brain, and liver tissue obtained at autopsy after informed consent. About 1 g of tissue was homogenized (glass to glass) in 10 mm Tris-HCl (pH 7.4) containing 0.1 mm EDTA, 0.5 m NaCl, 1% Triton X-100, and protease inhibitors. Crude extracts obtained by centrifugation (11,000 × g) of the cell lysates were used. A 30–55% ammonium sulfate cut of these extracts was partially purified using a phenyl-Sepharose column (15). Fractions containing GDH activity were used.
Testis Subcellular Fractionation
About 1 g of tissue was chopped in ice-cold mitochondrial isolation buffer containing 0.25 m sucrose, 10 mm Tris-HCl (pH 7.4), 0.5 mm EDTA, and protease inhibitors. The tissue was homogenized with a Dounce homogenizer (in 15 ml of buffer) and then centrifuged at 2000 × g for 5 min at 4 °C. The supernatant (whole extract) was centrifuged at 12,500 × g for 10 min at 4 °C. The mitochondrial pellet was resuspended in 4.5 ml of 3% Ficoll medium and loaded carefully onto 8 ml of 6% Ficoll medium (17). Following centrifugation at 11,500 × g for 30 min at 4 °C, the supernatant was discarded, and the mitochondrial pellet was washed with mitochondrial isolation buffer and resuspended and homogenized in lysis buffer containing 1% Triton X-100, 0.5 m NaCl, 10 mm Tris-HCl (pH 7.4), and 0.5 mm EDTA.
Western Blot Analyses
Purified recombinant hGDH1 and hGDH2 proteins, crude tissue extracts, and partially purified GDH fractions were run on an 8.5% SDS-polyacrylamide gel. Proteins were transferred to a nitrocellulose membrane and incubated with anti-GDH polyclonal antibody, the anti-hGDH2 polyclonal antibody, anti-manganese superoxide dismutase antibody, or anti-actin monoclonal antibody. Protein bands were visualized using the ChemiLucent detection system kit (Chemicon). Blocking experiments were performed by preincubating the anti-hGDH2 antiserum with the immunogenic peptide used to raise this antibody.
Immunostaining of Human Testis
Normal testicular specimens derived from orchiectomies, performed for therapeutic purposes due to testis torsion, were used. The specimens were fixed in 10% formalin for 24 h at room temperature. The tissues were prepared in an automatic tissue processor using ascending ethanol concentrations, xylene, and paraffin wax. Serial paraffin sections (4 μm thick) were mounted on glass slides and stored unbaked until immunohistochemistry was performed.
Immunohistochemistry
Primary antibodies used included the novel rabbit anti-hGDH2 polyclonal antibody described above (1:5000), mouse anti-vimentin polyclonal antibody (1:500; Neomarkers MS-129), and mouse anti-calretinin monoclonal antibody (1:50; Dako M7245). The slide-mounted sections were baked for 10 min in 60 °C, deparaffinized with two xylene washes, rehydrated through a series of graded alcohol washes, rinsed in water, and washed with 0.1 m phosphate-buffered saline (pH 7.4) containing 0.01% Tween 20. Heat-induced antigen retrieval was performed in a steamer using target retrieval solution (Dako S1700). Endogenous peroxidase was blocked with 3% hydrogen peroxide for 10 min. The slides were then incubated in blocking solution (serum-free protein block, Dako X0909) for 20 min to block nonspecific binding. The primary antibodies were added to the slides and incubated overnight in a humidified chamber at 4 °C. Detection was accomplished using an LSAB+ horseradish peroxidase kit (Dako K0679). Sections were incubated with biotin-conjugated anti-rabbit IgG for 30 min and finally with streptavidin conjugated to peroxidase for 30 min both in a moist chamber at room temperature. Immunostaining was revealed using 3,3′-diaminobenzidine or AEC+ chromogen. The slides were counterstained with hematoxylin, progressively dehydrated through graded alcohols and xylene (unless AEC+ was used), and finally covered with a coverslip after mounting in DPX or Glycergel mounting medium. Competition studies were performed in parallel using 1) the anti-hGDH2 antibody, 2) the anti-hGDH2 antibody preincubated with the immunogenic peptide for 1 h at room temperature, 3) rabbit preimmune serum, and 4) secondary antibody without preincubation of the tissue with the primary antibody. Slides were examined under an Olympus light microscope that was equipped with a 406 objective.
Double Immunofluorescence of Formalin-fixed Paraffin-embedded Testis Tissue Sections
Deparaffinization and antigen retrieval were performed as described above. Sections were blocked in 10% normal donkey serum and incubated overnight at 4 °C with rabbit antiserum against hGDH2 (1:10,000). After being washed three times with phosphate-buffered saline, sections for double immunofluorescence were incubated with the first secondary antibody (Alexa Fluor 594-labeled donkey anti-rabbit) for 1 h at room temperature. The second blocking step was performed using 3.3% normal goat serum. The slides were then incubated with mouse anti-vimentin antibody or mouse anti-calretinin antibody for 1 h at 4 °C, washed with phosphate-buffered saline, and incubated with the second secondary antibody (Alexa Fluor 488-labeled goat anti-mouse) for 1 h at room temperature. Finally, the slides were counterstained with 4′,6-diamidino-2-phenylindole-containing medium and examined by laser scanning confocal fluorescence microscopy (Leica TCS-NT laser scanning microscope, Leica Microsystems Heidelberg GmbH) using a 40 or 63 oil objective. The images were acquired and merged using the Leica application software.
Double Immunofluorescence of Post-mortem Human Brain
Snap-frozen fixed and unfixed post-mortem human brain tissue samples were provided by the UK Multiple Sclerosis Brain Tissue Bank (Imperial College London). Sections were permeabilized in cold methanol for 8 min, washed three times with phosphate-buffered saline, blocked in 2% normal goat serum for 45 min, and incubated overnight at 4 °C with rabbit antiserum against hGDH2 (1:2000). After washing, sections were incubated with biotinylated goat anti-rabbit secondary antibody (1:200; Vector Laboratories BA-1000) for 1 h at room temperature, followed by incubation with fluorescein isothiocyanate-conjugated streptavidin (1:800) for 1 h at room temperature. Sections for double immunofluorescence were subsequently incubated overnight at 4 °C with mouse antibody against glial fibrillary acidic protein (GFAP; 1:1000; Invitrogen), NeuN (1:300; Millipore), or myelin oligodendrocyte glycoprotein (MOG; 1:200; gift of Prof. Richard Reynolds, Imperial College London). After washing, sections were incubated with TRITC-conjugated anti-mouse secondary antibody (1:100; Jackson ImmunoResearch Laboratories) for 1 h and counterstained with 4′,6-diamidino-2-phenylindole. Finally, sections were treated with an autofluorescence eliminator reagent (Chemicon) for 10 min and imaged as described above. To prove the specificity of hGDH2 antiserum labeling, sections were stained in parallel after preincubation of the rabbit antiserum with the immunogenic peptide for 1 h at room temperature. Images were captured with a Zeiss fluorescence microscope and digital camera (Carl Zeiss MicroImaging) using the Zeiss Axiovision software.
RESULTS
Production and Characterization of hGDH2-specific Polyclonal Antibody
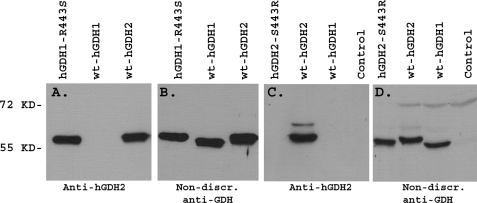
After injecting the synthetic 12-amino acid-long hGDH2-specific peptide into rabbits, we found that the generated antibody was capable of selectively detecting hGDH2 expressed in Sf21 cells. In contrast, the antibody did not recognize hGDH1 expressed in these cells even when as much as 400 ng were loaded per lane (Figs. 1 and 2 and supplemental Fig. 1). This was true using highly purified hGDH2 or hGDH1 preparations (Figs. 1 and 2, A and B), whole homogenates of cultured Sf21 cells expressing hGDH1 or hGDH2 (Fig. 2, C and D), or crude liver extracts (supplemental Fig. 1). Human liver is known to express only the housekeeping GDH (hGDH1) (5). Our Western blot analyses of Sf21 cell homogenates expressing the two human isoenzymes revealed that hGDH2 migrated upon SDS-PAGE slightly higher than hGDH1 (Fig. 2C), as shown previously for purified recombinant hGDH1 and hGDH2 (9). Moreover, Western blot analyses of Sf21 cells expressing mutant hGDH2 proteins revealed that our antibody could not recognize a hGDH2 mutant obtained by substitution of Arg for Ser443 (Fig. 2) or by substitution of Leu for Ser445 (supplemental Fig. 2). Also, the antibody displayed a decreased affinity for the Q441R and S445A hGDH2 mutants (supplemental Fig. 2). All these single amino acid substitutions are inside the 12-amino acid-long peptide used for raising the antibody (supplemental Fig. 3). On the other hand, substitution of Glu for Lys450 or Tyr for His454, residues outside this epitope (supplemental Fig. 3), did not affect the recognition of the hGDH2 mutant by our antibody (supplemental Fig. 2). Inversely, substitution of Ser for Arg443 in hGDH1 enabled the antibody to recognize the recombinant hGDH1 mutant (Fig. 2A). As the 12-residue peptide used to generate our antibody had Ser instead of Arg at residue 443 (as wild-type hGDH2 does), these results confirm the specificity of the antibody for this hGDH2 epitope (Fig. 2). In contrast to the data obtained with our anti-hGDH2 antibody, a commercially available antibody raised against the full-length bovine liver GDH detected with a comparable affinity all wild-type and mutant human GDHs studied here (non-discriminating anti-GDH antibody) (Figs. 1 and 2 (B and D) and supplemental Figs. 1 and 2).
FIGURE 1.
Western blot analysis of purified hGDH1 and hGDH2 proteins. Recombinant GLUD1 and GLUD2 cDNAs were expressed in Sf21 cells, producing hGDH1 and hGDH2 proteins, respectively. These were purified to homogeneity as described under “Experimental Procedures.” Increasing concentrations of the purified proteins were electrophoresed on an 8.5% SDS-polyacrylamide gel and blotted with either our anti-hGDH2 antibody (left panel) or a commercially available antibody raised against bovine liver GDH (right panel). The amount of purified hGDH1 and hGDH2 loaded is shown (in nanograms) above the corresponding lane. Under these conditions, the anti-hGDH2 antibody recognized only purified hGDH2, whereas the commercially available anti-GDH antibody recognized both human isoproteins (non-discriminating). hGDH2 migrated somewhat higher than hGDH1 (estimated molecular masses of 58 and 56 kDa, respectively), as shown previously for purified hGDH1 and hGDH2 separated by SDS-PAGE and visualized with Coomassie Blue (9).
FIGURE 2.
Effect of mutations at residue 443 on the specificity of the anti-hGDH2 antibody. Mutant and wild-type (wt) hGDH1 and hGDH2 were produced in Sf21 cells using the baculovirus expression system. Recombinant human expressed proteins purified to homogeneity (A and B) or crude Sf21 cell extracts (C and D) were electrophoresed on an 8.5% gel and blotted with our anti-hGDH2 antibody (A and C) or the commercially available non-discriminating (non-discr.) anti-GDH antibody (B and D). The anti-hGDH2 antibody, raised against a 12-amino acid-long peptide that corresponds to residues 436–447 of the mature hGDH2 protein, identified the R443S hGDH1 mutant and wild-type hGDH2, both of which carry Ser443 instead of Arg443, which occurs in wild-type hGDH1. In contrast, when Ser443 of hGDH2 was replaced with Arg, the antibody no longer recognized the mutant hGDH2 enzyme.
Detection of hGDH2 in Human Testis and Brain
Using our hGDH2-specific antibody, we performed Western blot analysis of human brain and testis and found that both organs express the hGDH2 protein (Figs. 3 and 4). In contrast, human liver extracts, analyzed by Western blotting under the same conditions, were negative for the presence of hGDH2 (Fig. 4 and supplemental Fig. 1). These results accord with previously reported data showing that human liver expresses the GLUD1 mRNA only (5). In extracts from human testis, our anti-hGDH2 antibody detected a single immunoreactive band (Fig. 3), whereas in extracts from human cerebral cortex (parietal, temporal, and frontal lobe), it revealed two immunoreactive bands differing slightly in their migration pattern (Fig. 4). Preincubation of the anti-hGDH2 antibody with the hGDH2 peptide (used to raise this antibody) almost completely blocked the binding of the antibody to the two bands of human brain extracts (supplemental Fig. 4). Also, using our anti-hGDH2 antibody, we obtained similar results by analyzing partially purified GDH preparations from human testis (Fig. 3) and human brain (supplemental Figs. 4 and 5). Comparison of the two human organs revealed that crude testis extracts contained substantially higher amounts of hGDH2 protein than human brain extracts (Fig. 4). Based on densitometry, we estimated that the relative expression of hGDH2 (amount of hGDH2 protein/mg of total protein loaded onto Western blots) in cerebral cortex was 10–20% of that in testis. When we used the commercial non-discriminating antibody, which recognizes both hGDH1 and hGDH2 (see above), we obtained Western blot patterns that were distinct from those obtained with the anti-hGDH2 antibody. Thus, in addition to the band visualized by the anti-hGDH2 antibody, the non-discriminating antibody detected in human testis extracts a second band representing hGDH1 that migrated slightly below. Moreover, these analyses revealed that crude extracts or partially purified GDH preparations from human testis contained similar amounts of hGDH1 and hGDH2 bands (Fig. 3B). On the other hand, crude extracts or partially purified GDH preparations from human brain contained substantially greater amounts of hGDH1 (Fig. 4). Under these conditions, the non-discriminating antibody identified a dense band that overlapped with those visualized by the hGDH2-specific antibody (Fig. 4). Western blot analyses of human tissues (brain, testis, and liver) from different subjects yielded similar results.
FIGURE 3.
Immunoblots of human testis using the anti-hGDH2 and non-discriminating anti-GDH antibodies. Crude extracts and partially purified GDH preparations from human testis were run on 8.5% gels. Expressed recombinant hGDH1 and hGDH2 were used as controls. The anti-hGDH2 antibody visualized a single hGDH2-specific band in both the purified (Pur.) and crude testis extracts (A). In contrast, the non-discriminating (Non-discr.) antibody revealed two bands of nearly equal amounts, representing hGDH1 and hGDH2 (B).
FIGURE 4.
Western blot analysis of human brain, testis, and liver. A, crude extracts from human parietal, temporal, and frontal lobe (60 μg each) and human testis and liver (20 μg each) were electrophoresed on an 8.5% SDS-polyacrylamide gel and transferred to a nitrocellulose membrane. Blotting was performed using either the anti-hGDH2 antibody (upper panel) or the anti-GDH non-discriminating (non discr.) antibody (lower panel). B, shown are the results from Western blot analyses of human frontal lobe, testis, and liver using different amounts of crude tissue extracts. The total protein (micrograms) loaded is shown above each lane. Blotting was performed using the anti-hGDH2 antibody. These blots show that human testis contains substantially higher levels of hGDH2 than human brain and that human liver has none. This differential expression of hGDH2 contrasts with the fact that human liver extracts contain higher levels of GDH activity (1.301 ± 0.41 μmol of NADPH oxidized per min/mg of protein) than human brain extracts (0.356 ± 0.021, 0.501 ± 0.027, and 0.467 ± 0.014 μmol/min/mg of protein for parietal, temporal, and frontal lobe, respectively), and the crude human testis extracts had even lower GDH activity (0.078 ± 0.003 μmol of NADPH oxidized per min/mg of protein, mean ± S.D.). These enzyme assays measured the sum of hGDH1 and hGDH2 activity.
hGDH2 Localizes Mainly to the Mitochondrial Fraction
Subcellular fractionation of human testis extracts showed that both hGDH2 and hGDH1 localized to the mitochondrial fraction, whereas the cytosolic fraction showed no detectable amounts of these isoforms (Fig. 5). Furthermore, the hGDH1/hGDH2 ratio in the mitochondrial fraction (1:1) was similar to that in the whole tissue lysate and in the phenyl-Sepharose column fractions that contained the highest GDH activity. Similarly, both hGDH1 and hGDH2 were shown to be in the mitochondrial fraction in human frontal and temporal lobe (data not shown).
FIGURE 5.
Distribution of hGDH2 and hGDH1 in mitochondrial and cytosolic fractions prepared from human testis. The mitochondrial fraction was isolated using the Ficoll method (see “Experimental Procedures”). Equal protein amounts of whole lysate and mitochondrial and cytosolic fractions were electrophoresed on an 8.5% SDS-polyacrylamide gel, and immunoblotting was performed using the anti-hGDH2 antibody (A) and the non-discriminating (Non-discr.) anti-GDH antibody (B). Actin (C) and manganese superoxide dismutase (MnSOD; D), visualized by the respective antibodies, served as cytosolic and mitochondrial markers, respectively. Both hGDH1 and hGDH2 were enriched in the mitochondrial fraction, whereas the cytosolic fraction was essentially devoid of GDH immunoreactivity. These immunoblotting data agree with the results of GDH activity assays using these fractions (data not shown).
hGDH2 Immunoreactivity Is Enriched in Sertoli Cells in Human Testis
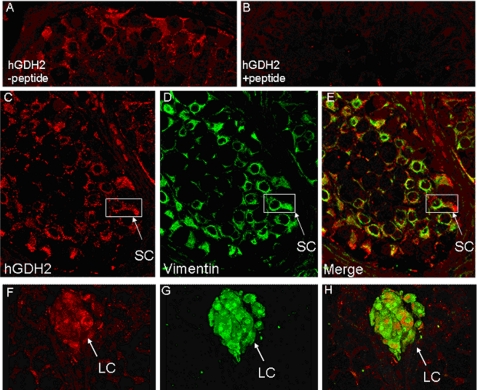
Rabbit anti-hGDH2 polyclonal antibody was tested in increasing dilutions from 1:500 to 1:10,000. The final working dilution was set at 1:5000 for immunohistochemical studies and at 1:10,000 for immunofluorescence studies on human testis. In the seminiferous tubules, a strong expression of hGDH2 was detected in the cytoplasm of the Sertoli cells, with the germ cells being completely devoid of hGDH2 immunoreactivity. Also, no hGDH2 expression was detected in the connective tissue sheath of seminiferous tubules. In the interstitium, hGDH2 was localized in the cytoplasm of Leydig cells. No hGDH2 expression was seen in the other cells of the interstitium, such as myofibroblasts, macrophages, etc. Immunostaining of both Sertoli and Leydig cells had a characteristic punctate appearance consistent with the mitochondrial localization of hGDH2 (Fig. 6A). Staining was visualized after incubation with 3,3′-diaminobenzidine for 1 min (data not shown) or with AEC+ for 15 min (Fig. 6A), and it was markedly attenuated by incubation of the primary antibody with the immunogenic peptide (Fig. 6B). Mild residual staining was observed in the cytoplasm of some Leydig cells after preincubation of the antibody with the peptide (Fig. 6B) and after incubation of the tissue with the serum from the rabbit prior to its immunization with the peptide (preimmune serum), implying that a small part of the Leydig cell staining must be attributed to unspecific binding (Fig. 6, B and C).
FIGURE 6.
Localization of hGDH2 in human testis. Shown are images of paraffin-embedded fixed sections of human testis stained with rabbit antiserum against hGDH2 and visualized with AEC+ chromogen (red pigment). A, punctate immunoreactivity for hGDH2 was found in the cytoplasm of Sertoli cells (SC) inside the seminiferous tubules, as well as in the cytoplasm of Leydig cells (LC) in the intermediate space. Spermatocytes (SPC) were devoid of hGDH2 immunoreactivity. B, preincubation with the immunogenic peptide markedly attenuated the staining. Some residual staining can be observed in the cytoplasm of Leydig cells. C, incubation of the tissue with the rabbit preimmune serum (PIS) revealed mild staining of the Leydig cell cytoplasm, whereas the seminiferous tubules had no hGDH2 immunoreactivity. D, omitting the primary antibody revealed only nonspecific staining attributed to the secondary antibody (Sec Ab).
As vimentin is known to stain the cytoplasm of Sertoli cells (being absent in spermatogonia, germ cells, or spermatides) (18), we also performed immunostaining of human testis using mouse anti-vimentin antibody. The results revealed that the expression of vimentin inside the seminiferous tubules was similar to that obtained after staining for hGDH2 (data not shown). A double immunofluorescence study of vimentin and hGDH2 confirmed that the hGDH2 punctate immunoreactivity was located within the cytoplasm of Sertoli cells (Fig. 7, A and C–E). On the other hand, calretinin is an intracellular calcium-binding protein known to be expressed in both the cytoplasm and nucleus of Leydig cells but not in germ cells or in well differentiated Sertoli cells. Accordingly, we performed double immunofluorescence for hGDH2 and calretinin and found that, as with the calretinin immunoreactivity, the hGDH2 punctate immunoreactivity was located within the cytoplasm of Leydig cells; however, the nucleus of Leydig cells was devoid of hGDH2 immunoreactivity (Fig. 7 (F–H) and supplemental Fig. 6). Study of four different human testes yielded similar results.
FIGURE 7.
Localization of hGDH2 in the cytoplasm of Sertoli and Leydig cells in human testis by double immunofluorescence studies. Shown are images of paraffin-embedded formalin-fixed sections of human testis stained with 1) rabbit antiserum against hGDH2 (red staining), 2) mouse monoclonal antibody against vimentin (green staining), and 3) mouse monoclonal antibody against calretinin (green staining). In A, the rabbit antiserum was not preincubated, whereas in B, it was preincubated with the immunogenic peptide prior to staining. There was specific punctate immunoreactivity for hGDH2 in the cytoplasm of Sertoli cells (SC; A and C), which were labeled also with vimentin (D and E). This reactivity was almost completely abolished after preincubation of the rabbit antiserum with the immunogenic peptide (B). There was also hGDH2-specific staining in the cytoplasm of Leydig cells (LC) (F and H), which were also labeled with calretinin (G and H).
hGDH2 Is Enriched in Astrocytes in Human Brain
To determine the cellular distribution of hGDH2 in normal human brain, we immunostained sections of fixed and unfixed human cortex and subcortical white matter with rabbit antiserum raised against hGDH2 combined sequentially with mouse monoclonal antibodies against GFAP (an astrocyte marker), NeuN (a neuronal marker), and MOG (an oligodendrocyte and myelin marker) (Fig. 8). The anti-hGDH2 polyclonal antibody reacted specifically with the antigen (1:2000) in the unfixed brain tissue only, whereas fixed brain tissue showed nonspecific immunoreactivity. In unfixed human brain, hGDH2 immunostaining was present mainly in the perinuclear cytoplasm of astrocytes as well as along their proximal processes in both gray and white matter (Figs. 8 (A–C) and 9A). The staining had the same punctate appearance as observed in testis (Fig. 9A). These results are consistent with the mitochondrial localization of the protein. Preincubation of the antibody with the immunogenic peptide led to a clear attenuation of this punctate cytoplasmic staining of astrocytes throughout the white and gray matter, confirming its specificity (Fig. 9B). Double immunostaining with anti-hGDH2 and anti-NeuN antibodies revealed that neurons were weakly stained by the anti-hGDH2 antibody (Fig. 8, D–F). This staining was localized diffusely in the cytoplasm of neurons, yet it was much weaker than that observed in astrocytes and lacked the characteristic punctate pattern. No expression of hGDH2 in oligodendrocytes or myelinated fibers was revealed by anti-hGDH2 and anti-MOG double immunostaining (Fig. 8, G–I). Study of cerebral cortical tissue from three different human brains yielded similar results.
FIGURE 8.
Localization of hGDH2 mostly in astrocytes in human brain. Shown are images of unfixed human brain gray (A–F) and white (G–I) matter, double-stained with monoclonal antibodies (red staining) to GFAP (marker for astrocytes), NeuN (neuronal marker (n)), and MOG (marker for oligodendrocytes (o) and myelin (m)), in combination with rabbit antiserum against hGDH2 (green staining). Cell nuclei were visualized with 4′,6-diamidino-2-phenylindole (blue staining). Merged images are shown in the right panels. Strong punctate immunoreactivity for hGDH2 was found in the cytoplasm (closed arrowheads in A, D, and G) and proximal processes (open arrowheads) of cells that were identified as astrocytes (a) by their strong GFAP reactivity (B). Much weaker and rather diffuse hGDH2 reactivity was present in the cytoplasm of NeuN-positive neurons (D–F). There was no overlap between MOG and hGDH2 localization (G–I), indicating a lack of hGDH2 expression in oligodendrocytes.
FIGURE 9.
Specific labeling of hGDH2 in human brain. Shown are images of unfixed human brain cortex immunostained with mouse monoclonal antibody against GFAP (red staining) and rabbit antiserum against hGDH2 (green staining) in the absence (A) and presence (B) of antigen (immunogenic peptide). Cell nuclei were visualized with 4′,6-diamidino-2-phenylindole (DAPI; blue staining). There was specific punctate immunoreactivity for hGDH2 in the cytoplasm (arrowheads) of astrocytes (a), which were labeled with GFAP (arrows). hGDH2 immunoreactivity was almost completely abolished after preincubation of the rabbit antiserum with the immunogenic peptide (B).
DISCUSSION
Here, we developed an antibody that specifically recognizes hGDH2 and used it to study human tissues. The results showed that human brain and testis, but not liver, express hGDH2 endogenously; however, to our surprise, this expression was proportionally higher in testis than in brain. Whereas our anti-hGDH2 antibody recognized a single hGDH2 band in human testis extracts, in extracts from human brain, the antibody recognized two such bands, differing slightly in their migration pattern upon SDS-PAGE. Given that previous studies have suggested that GDH undergoes post-translational modification in human tissues (19, 20), it is possible that our observations may reflect the distinct processing of hGDH2 in human brain and testis. In this regard, sequencing of GDH purified from human liver revealed that a fraction of the enzyme was cleaved at a distinct N-terminal site (19). Also, Hussain et al. (20), who studied GDH purified from human brain using two-dimensional PAGE, reported the presence of four electrophoretically distinct isoproteins, differing slightly in their molecular mass and electric charge.
In human testis, Sertoli cells were strongly immunoreactive for hGDH2, whereas in human brain, cortical astrocytes showed a robust immunoreactivity for hGDH2. Within the cytoplasm of these cells, the anti-hGDH2 antibody labeled coarse structures resembling mitochondria. We have observed a similar pattern in cultured cells expressing hGDH1/EGFP and hGDH2/EGFP (21, 22). These data accord with our Western blot results using subcellular fractions, which showed that hGDH2 localizes to mitochondria. Also, previous studies (21–23) on cultured cells expressing hGDH1/EGFP or hGDH2/EGFP revealed that the enzyme localizes to mitochondria and that deletion of a 53-amino acid-long N-terminal mitochondrial targeting sequence prevents GDH from entering the mitochondria (22). However, on the basis of the present data, we cannot exclude that hGDH1 or hGDH2 localizes to a lesser degree to other cellular compartments such as the endoplasmic reticulum (22, 24, 25).
As noted above, our data showed that, in human testis, Sertoli cells strongly express hGDH2 and that Leydig cells are also hGDH2-positive. In contrast, spermatogonia and differentiated germ cells are negative for this protein. It is known that spermatogenesis is the process by which spermatogonia (stem cells) develop into highly specialized cells, the spermatozoa. This depends largely on Sertoli cells, which are known to nourish and support differentiated germ cells (26–28). Specifically, endocrine regulation allows Sertoli cells to develop and reorganize to generate the hemato-testicular barrier through their tight junction complexes and to provide nutrients and survival and regulatory factors to germ cells (26, 27, 29). This time-modeling process leads to the constitution of a specific biochemical and cytoarchitectural microenvironment in the adluminal compartment, where the germ cells will survive, proliferate, and differentiate. Among the identified Sertoli cell products are energy substrates such as lactate, which, as discussed below for astrocytes, derives mainly from the tricarboxylic acid cycle via conversion of glutamate to α-ketoglutarate. Also, we have recently shown that estrogens inhibit hGDH2 selectively (with an affinity that is similar to that of GTP for hGDH1).4 Hence, these observations taken together raise the possibility that regulation of hGDH2 activity by female hormones may constitute one of the mechanisms by which these hormones affect testicular function.
Similar to the supportive role of Sertoli cells in testis, astrocytes are also known to support, nourish, and insulate neuronal cells in the nervous system. Although the role of GDH in these processes needs to be better understood, Sonnewald et al. (30) have shown that glutamate added to primary cultures of cerebral cortical astrocytes is extensively metabolized by the tricarboxylic acid cycle, forming mainly lactate that is exported to neurons. This requires conversion of glutamate to α-ketoglutarate catalyzed partly by GDH. These observations are consistent with existing evidence that GDH in brain functions mainly toward the oxidative deamination of glutamate (31). On the other hand, there is little evidence that GDH is involved in the synthesis of glutamate in brain, as the enzyme has a high Km for ammonia (10–20 mm) and the NAD+/NADH ratio is high in this organ (31). However, local increases in ammonia concentration could facilitate the reductive amination of α-ketoglutarate, as has been suggested for the “glutamate/glutamine-branched chain amino acid shuttle” that is thought to be involved in the cycling of ammonia between neurons and astrocytes (32). In addition, GDH may be engaged in the “alanine shuttle,” which involves translocation of alanine from neurons to astrocytes with the concomitant translocation of lactate from astrocytes to neurons (33).
There is evidence that the properties acquired by hGDH2 permit the enzyme to adapt to the unique conditions that prevail in neural tissue (9, 10). These include resistance to GTP inhibition, dependence on ADP for catalytic function, and ability to function efficiently at relatively low intracellular pH. Resistance to GTP is thought to facilitate hGDH2 function in the GTP-rich environment of nerve tissue by enabling the enzyme to metabolize glutamate released from synaptic endings during excitatory transmission, even when an enhanced tricarboxylic acid cycle generates GTP amounts sufficient to completely inactivate hGDH1. Also, dependence of hGDH2 on cellular ADP levels may be important for regulating the flux of neurotransmitter glutamate through the GDH pathway, as an enhanced hydrolysis of ATP to ADP is known to occur in synapses during excitatory transmission (34). Similarly, the fact that the optimal pH for hGDH2 is somewhat lower than that for hGDH1 may be important for the function of the enzyme under the conditions of relative acidification that prevail in synaptic astrocytes following glutamate uptake (9, 10).
Whereas GDH function in testis has not been as extensively studied as that in nerve tissue, the finding that hGDH2 is densely expressed by Sertoli cells suggests that the particular functional properties of this protein may enable these supporting cells to effectively recycle metabolites that are essential for supplying nutrients to germ cells. Hence, the expression of hGDH2 by Sertoli cells in testis and by astrocytes in brain may confer an important biological advantage.
Supplementary Material
Acknowledgments
We are grateful to the patients who donated biological materials. We thank Giovanna Arianoglou, Konstantinos Kanavouras, Nikolas Borombokas, Dr. George Z. Rassidakis, Dr. Kiki Thermos, and Dr. Martina Samiotaki for help and Iris Plaitakis for editorial assistance. We are indebted to the UK Multiple Sclerosis Tissue Bank for the post-mortem human brain samples.
This work was supported by grants from the Association for Research and Treatment of Neurologic Disorders of Crete (EY ZHN) and by the Cyprus Research Promotion Foundation (to K. A. K.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–6 and additional references.
N. Borompokas, M. Papachatzaki, K. Kanavouras, V. Mastorodemos, and A. Plaitakis, unpublished data.
- GDH
- glutamate dehydrogenase
- hGDH
- human GDH
- GFAP
- glial fibrillary acidic protein
- MOG
- myelin oligodendrocyte glycoprotein
- TRITC
- tetramethylrhodamine isothiocyanate
- EGFP
- enhanced green fluorescent protein.
REFERENCES
- 1.Smith E. (1979) Proc. Am. Philos. Soc. 123, 73–84 [Google Scholar]
- 2.Hudson R. C., Daniel R. M. (1993) Comp. Biochem. Physiol. B 106, 767–792 [DOI] [PubMed] [Google Scholar]
- 3.Plaitakis A., Berl S., Yahr M. D. (1984) Ann. Neurol. 15, 144–153 [DOI] [PubMed] [Google Scholar]
- 4.Mavrothalassitis G., Tzimagiorgis G., Mitsialis A., Zannis V., Plaitakis A., Papamatheakis J., Moschonas N. (1988) Proc. Natl. Acad. Sci. 85, 3494–3498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shashidharan P., Michaelidis T. M., Robakis N. K., Kresovali A., Papamatheakis J., Plaitakis A. (1994) J. Biol. Chem. 269, 16971–16976 [PubMed] [Google Scholar]
- 6.Burki F., Kaessmann H. (2004) Nat. Genet. 36, 1061–1063 [DOI] [PubMed] [Google Scholar]
- 7.Shashidharan P., Clarke D. D., Ahmed N., Moschonas N., Plaitakis A. (1997) J. Neurochem. 68, 1804–1811 [DOI] [PubMed] [Google Scholar]
- 8.Plaitakis A., Metaxari M., Shashidharan P. (2000) J. Neurochem. 75, 1862–1869 [DOI] [PubMed] [Google Scholar]
- 9.Kanavouras K., Mastorodemos V., Borompokas N., Spanaki C., Plaitakis A. (2007) J. Neurosci. Res. 85, 1101–1109 [DOI] [PubMed] [Google Scholar]
- 10.Plaitakis A., Spanaki C., Mastorodemos V., Zaganas I. (2003) Neurochem. Int. 43, 401–410 [DOI] [PubMed] [Google Scholar]
- 11.Plaitakis A., Latsoudis H., Kanavouras K., Ritz B., Bronstein J. M., Skoula I., Mastorodemos V., Papapetropoulos S., Borompokas N., Zaganas I., Xiromerisiou G., Hadjigeorgiou G. M., Spanaki C. (2010) Eur. J. Hum. Genet. 18, 336–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi S. Y., Hong J. W., Song M. S., Jeon S. G., Bahn J. H., Lee B. R., Ahn J. Y., Cho S. W. (1999) J. Neurochem. 72, 2162–2169 [DOI] [PubMed] [Google Scholar]
- 13.Choi M. M., Huh J. W., Yang S. J., Cho E. H., Choi S. Y., Cho S. W. (2005) FEBS Lett. 579, 4125–4130 [DOI] [PubMed] [Google Scholar]
- 14.Zaganas I., Kanavouras K., Mastorodemos V., Latsoudis H., Spanaki C., Plaitakis A. (2009) Neurochem. Int. 55, 52–63 [DOI] [PubMed] [Google Scholar]
- 15.Zaganas I., Plaitakis A. (2002) J. Biol. Chem. 277, 26422–26428 [DOI] [PubMed] [Google Scholar]
- 16.Kanavouras K., Borompokas N., Latsoudis H., Stagourakis A., Zaganas I., Plaitakis A. (2009) J. Neurochem. 109, 167–173 [DOI] [PubMed] [Google Scholar]
- 17.Clark J. B., Nicklas W. J. (1970) J. Biol. Chem. 245, 4724–4731 [PubMed] [Google Scholar]
- 18.Aumüller G., Steinbrück M., Krause W., Wagner H. J. (1988) Anat. Embryol. 178, 129–136 [DOI] [PubMed] [Google Scholar]
- 19.Julliard J. H., Smith E. L. (1979) J. Biol. Chem. 254, 3427–3438 [PubMed] [Google Scholar]
- 20.Hussain M. M., Zannis V. I., Plaitakis A. (1989) J. Biol. Chem. 264, 20730–20735 [PubMed] [Google Scholar]
- 21.Mastorodemos V., Zaganas I., Spanaki C., Bessa M., Plaitakis A. (2005) J. Neurosci. Res. 79, 65–73 [DOI] [PubMed] [Google Scholar]
- 22.Mastorodemos V., Kotzamani D., Zaganas I., Arianoglou G., Latsoudis H., Plaitakis A. (2009) Biochem. Cell Biol. 87, 505–516 [DOI] [PubMed] [Google Scholar]
- 23.Rosso L., Marques A. C., Reichert A. S., Kaessmann H. (2008) PLoS Genet. 4, e1000150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee W. K., Shin S., Cho S. S., Park J. S. (1999) J. Cell Biochem. 76, 244–253 [DOI] [PubMed] [Google Scholar]
- 25.Morand J. P., Macri J., Adeli K. (2005) J. Biol. Chem. 280, 17626–17633 [DOI] [PubMed] [Google Scholar]
- 26.Fawcett D. W. (1973) Adv. Biosci. 10, 83–99 [PubMed] [Google Scholar]
- 27.Sharpe R. (1994) in The Physiology of Reproduction (Knobil E., Neill J. D. eds) pp. 1363–1434, Raven Press, New York [Google Scholar]
- 28.Griswold M. D. (1995) Biol. Reprod. 52, 211–216 [DOI] [PubMed] [Google Scholar]
- 29.Griswold M. D., Solari A., Tung P. S., Fritz I. B. (1977) Mol. Cell. Endocrinol. 7, 151–165 [DOI] [PubMed] [Google Scholar]
- 30.Sonnewald U., Westergaard N., Petersen S. B., Unsgård G., Schousboe A. (1993) J. Neurochem. 61, 1179–1182 [DOI] [PubMed] [Google Scholar]
- 31.McKenna M. C., Tildon J. T., Stevenson J. H., Huang X. (1996) Dev. Neurosci. 18, 380–390 [DOI] [PubMed] [Google Scholar]
- 32.Hutson S. M., Berkich D., Drown P., Xu B., Aschner M., LaNoue K. F. (1998) J. Neurochem. 71, 863–874 [DOI] [PubMed] [Google Scholar]
- 33.Waagepetersen H. S., Sonnewald U., Larsson O. M., Schousboe A. (2000) J. Neurochem. 75, 471–479 [DOI] [PubMed] [Google Scholar]
- 34.Erecinska M., Nelson D. (1990) J. Neurochem. 5, 1335–1343 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.