Abstract
The JAK2-V617F mutation is an important etiologic factor for the development of myeloproliferative neoplasms. The mechanism by which this mutated tyrosine kinase initiates deregulated signals in cells is not completely understood. It is believed that JAK2-V617F requires interactions with homodimeric cytokine receptors to elicit its transforming signal. In this study, we demonstrate that components of heterodimeric cytokine receptors can also activate JAK2-V617F. Expression of IL27Ra, a heterodimeric receptor component, enhanced the activation of JAK2-V617F and subsequent downstream signaling to activation of STAT5 and ERK. In addition, expression of components of the interleukin-3 receptor, IL3Ra and the common β chain, activated JAK2-V617F as well as STAT5 and ERK. Importantly, expression of IL27Ra functionally replaced the requirement of a homodimeric cytokine receptor to promote the activation and transforming activity of JAK2-V617F in BaF3 cells. Tyrosine phosphorylation of IL27Ra was not required to induce activation of JAK2-V617F or STAT5, or to enhance the transforming activity of JAK2-V617F. Expression of IL3Ra or the common β chain in BaF3 cells also enhanced the ability of JAK2-V617F to transform these hematopoietic cells. However, the heterodimeric receptor component IL12RB1 did not enhance the activation or transforming signals of JAK2-V617F in BaF3 cells. IL27Ra also activated the K539L and R683G JAK2 mutants. Together our data demonstrate that in addition to homodimeric receptors, some heterodimeric receptor components can support the activation and transforming signals of JAK2-V617F and other JAK2 mutants. Therefore, heterodimeric receptors may play unappreciated roles in JAK2 activation in the development of hematopoietic diseases including myeloproliferative neoplasms.
Keywords: Oncogene, Phosphorylation/Kinases/Tyrosine, Receptors/Cytokine, Signal Transduction/JAK-STAT, Tyrosine Kinase, JAK2-V617F, Myeloproliferative Disorders, Myeloproliferative Neoplasms
Introduction
Myeloproliferative disorders are diseases affecting the production of myeloid cells of the hematopoietic system. These disorders include, among others, polycythemia vera, essential thrombocytosis, and primary myelofibrosis, resulting in an accumulation of excess red blood cells, an accumulation of platelets, and fibrosis of the bone marrow, respectively (1). In 2008 (2, 3) polycythemia vera, essential thrombocytosis, and primary myelofibrosis were renamed as myeloproliferative neoplasms (MPNs).3 Although these disorders can be deadly on their own, they can also transform to acute myeloid leukemia (1). In 1951, William Dameshek (4) suggested these disorders were related, and in 2005 (5–10), a single point mutation linked these disorders to a potentially common etiological factor. This mutation results in a valine to phenylalanine amino acid change at residue 617 (V617F) in the JAK2 tyrosine kinase, leading to deregulation of this important intracellular signaling protein (5–10).
Members of the Janus kinase (JAK) family of non-receptor tyrosine kinases are important signal transduction regulators downstream of various cell surface receptors (11–14). They interact with the intracellular regions of a large repertoire of cell surface cytokine receptors. This complex mimics the single chain receptor tyrosine kinases that relay signals in response to growth factor stimulation. Ligand binding activates JAK family members through a mechanism that involves receptor dimerization, including both homo- and heterodimeric receptor interactions, receptor conformational changes, conformational changes in receptor-associated JAK family members, and trans-phosphorylation of receptor-bound JAK molecules. Thus, cytokine receptors have a scaffolding as well as a mechanistic role in JAK activation following ligand binding to the receptor. Activated JAK family members subsequently phosphorylate STAT family proteins, which leads to transcriptional regulation of STAT target genes that regulate a diverse array of cellular properties including growth, death, and differentiation (11, 12, 14, 15).
The tyrosine kinase activity of JAK proteins is associated with a functional tyrosine kinase domain (also called the JH1 domain) located at the carboxyl terminus of the protein (11, 13). Just upstream of this domain is the pseudokinase domain (also called the JH2 domain), which has homology to the functional kinase domain but has no apparent kinase activity. Within JAK family members, the pseudokinase domain is believed to function as an autoregulatory region of the kinase. It has been postulated that intramolecular interactions between the pseudokinase domain and the functional kinase domain play an inhibitory role toward the kinase activity of the protein. In addition to limited structural modeling information, this hypothesis is based on work that has observed that mutation or deletion of the pseudokinase domain leads to increased activity of the kinase domain (16–20).
The V617F mutation of JAK2 that is associated with MPNs is located within the pseudokinase domain of JAK2. JAK2-V617F demonstrates deregulated kinase activity and it is believed that the V617F mutation interferes with the ability of the pseudokinase domain to negatively regulate the activity of the kinase domain (10, 16, 19, 20). Recent molecular dynamic simulations have further supported the notion that interactions between the pseudokinase and kinase domains are affected by the V617F mutation and other JAK2 point mutations that have been found in patients (21, 22).
Multiple mouse models have demonstrated that expression of JAK2-V617F can recapitulate MPN-like disease in animals (23–27). Such models have also suggested that gene dosage of JAK2-V617F affects the disease outcome in the animal (25). For example, higher gene dosage tends to result in the development of polycythemia vera over other MPNs. This correlates with clinical studies that indicate in cells from polycythemia vera patients, recombination leads to uniparental disomy resulting in two alleles of the JAK2-V617F gene (8, 9).
Although expression of JAK2-V617F alone induces MPN formation in mouse models, Lu et al. (28) have suggested that JAK2-V617F requires expression of a homodimeric receptor complex to become fully activated and to induce activation of cellular signaling pathways. Although other reports have suggested homodimeric receptors are not needed for JAK2-V617F-induced signaling (6, 8, 29), it was established that higher levels of JAK2-V617F expression could bypass the requirement for homodimeric receptor expression, whereas lower levels of JAK2-V617F needed homodimeric receptor expression to elicit signaling by this mutant JAK2 (30). In either scenario, it is believed that cytokine receptors, either endogenously or exogenously expressed, participate in the activation of JAK2-V617F, presumably by providing a scaffolding function where two JAK2-V617F molecules could properly be juxtaposed to allow for transphosphorylation and subsequent full activation of the tyrosine kinase (30, 31). Lu et al. (28) further suggests that the ability of other receptors, such as heterodimeric receptors, to activate the transforming signals of JAK2-V617F is unknown and would be important to determine.
We have previously shown that expression of a single chain component of a heterodimeric receptor could activate the kinase activity of JAK2-V617F in a ligand-independent manner (32). In these studies, expression of the ligand binding subunit of the receptor for interleukin-27, IL27Ra (also called WSX), supported the activation of JAK2-V617F activity in cells. Therefore, we proposed the possibility that non-homodimeric cytokine receptors may play a role in eliciting signal transduction from JAK2-V617F, and perhaps other activated JAK2 mutants, leading to cellular transformation (33). In this report, we demonstrate that expression of IL27Ra, a single chain component of a heterodimeric receptor, can replace the expression of a homodimeric receptor to support the transforming properties of JAK2-V617F. This may be a unique property of only some heterodimeric receptors, as another heterodimeric receptor component, IL12RB1, is not capable of activating the hematopoietic cell transforming properties of JAK2-V617F. In addition, expression of either of the two components of the interleukin-3 receptor, IL3Ra or the common β subunit, enhanced JAK2-V617F kinase activity and the ability of JAK2-V617F to transform hematopoietic cells. Taken together, our data suggest that certain non-homodimeric receptor components can replace homodimeric receptors in supporting the kinase and transforming activity of JAK2-V617F. Although it is unknown which receptors JAK2-V617F utilizes in cells of MPN patients, our data suggest that in addition to homodimeric receptors, certain heterodimeric receptor components may also contribute to JAK2-V617F activation and signaling.
EXPERIMENTAL PROCEDURES
Expression Vectors
The following expression plasmids were utilized for transient transfection and/or retroviral production: pBabe-Puro-hIL27Ra, pBabe-Puro-hIL27Ra-HA, pBabe-Neo-hIL27R-HA, pBabe-Puro-hIL27R-myc, pBabe-Puro-hEpoR-HA, pBabe-Puro-hIL12RB1, pBabe-Puro-CHA-hIL3Ra, pBabe-Puro-CHA-hIL3/IL5/GM-CSFR common β chain, MSCV-JAK2-WT, and MSCV-JAK2-V617F. Coding sequences for epitope tags were added to the carboxyl terminus of hIL27Ra by PCR. pBabe-Puro-CHA was generated by cloning a coding sequence for the HA tag into the SalI site of pBabe-Puro (34) to allow for expression of carboxyl-terminal HA-tagged proteins. The EpoR and IL12RB1 cDNAs were obtained by PCR of cDNA derived from human acute myeloid leukemia cells. The hIL3Ra cDNA was obtained by PCR from a hIL3Ra containing plasmid purchased from Open Biosystems Inc. The hIL3/IL5/GM-CSFR common β chain cDNA was obtained by PCR from plasmid pKH97, a gift from Dr. Atsushi Miyajima (35). The integrity of all cloned PCR products was confirmed by DNA sequencing. The pBabe-Puro-hIL27Ra, MSCV-JAK2-WT, and MSCV-JAK2-V617F plasmids were previously described (32).
Cell Culture, Transient Transfection, and Retroviral Production and Infection
293T cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% FBS. BaF3 cells were maintained in RPMI 1640 medium supplemented with 10% FBS and 5% WEHI cell-conditioned medium as a source of IL3. All cells were cultured at 37 °C in a humidified incubator with 5% carbon dioxide. Transient transfections were performed via calcium-phosphate precipitation. Ecotropic retrovirus was produced in 293T cells using the pVPack system (Stratagene). Stable cell lines expressing proteins under investigation were generated by retroviral infection using the pBabe-Puro, pBabe-Puro-HA, or MSCVneo retroviral vectors. BaF3 cells (5 × 105) were infected at 37 °C using 0.5–1 ml of retrovirus, 1–1.5 ml of growth medium, and 8 μg/ml of Polybrene (Sigma) in a final volume of 2 ml for 3 h. Growth medium was then added to 10 ml total. Two days later, cells were selected in 0.5 μg/ml of puromycin (Sigma) or 0.5 mg/ml of G418 (Fisher).
Immunoblot Analyses
293T cells were processed for immunoblotting 2 days after transfection. BaF3 cells were washed twice with RPMI 1640 medium supplemented with 10% FBS to remove IL3. These cells were incubated for 3 h in RPMI 1640 medium supplemented with 10% FBS at a concentration of 4 × 105 cells/ml. 293T or BaF3 cells were washed in phosphate-buffered saline and lysed in lysis buffer (25 mm Tris, pH 7.4, 150 mm NaCl, 25 mm NaF, 1% Triton X-100) supplemented with 1 mm sodium vanadate, 2 mm sodium pyrophosphate, 10 μg/ml of leupeptin, 2 μg/ml of aprotinin, and 1 mm phenylmethylsulfonyl fluoride. Protein concentrations of clarified lysates were determined using a BCA protein assay kit (Pierce), and equal amounts of protein were analyzed by SDS-PAGE. The following antibodies were used as primary antibodies for immunoblotting in these studies: pJAK2 (Tyr1007/Tyr1008) (sc-16566-R), STAT5 (sc-835), Akt (sc-8312), ERK1 (sc-93), IL12RB1 (sc-658), and IL3/IL5/GM-CSFRB (sc-678) (Santa Cruz Biotechnology); JAK2 (3229), P-ERK (Thr202/Tyr204) (4370), p-Akt (Ser473) (4060), and Myc tag (2276) (Cell Signaling Technology); pSTAT5 (Tyr694) (611964) (BD Biosciences); HA (to detect IL27Ra, EpoR, and IL3Ra, where indicated) MMS-101R (Covance); and IL27Ra (T5823) (Sigma). Primary antibodies were detected using appropriate secondary antibodies conjugated to horseradish peroxidase (Pierce Thermo Scientific) and blots were developed with chemiluminescent reagents (Pierce Thermo Scientific).
Cell Growth Analyses
BaF3 cells stably expressing proteins of interest were washed two times with RPMI 1640 medium supplemented with 10% FBS to remove IL3 from the growth medium. Washed cells were resuspended at 2 × 105 cells/ml in RPMI 1640 supplemented with 10% FBS. Cell growth was determined by trypan blue exclusion. Data from cell growth experiments were reproducible on multiple (n of at least 3, unless otherwise indicated) independently derived cell lines.
Immunoprecipitation
Immunoprecipitations were performed using antibodies that recognize the Myc tag (2276) (Cell Signaling Technology), the HA tag (MMS-101R) (Covance), actin (A5316), and FLAG (F3165) (Sigma). Immune complexes were captured on protein G-agarose beads (Pierce Thermo Scientific). Immunoprecipitated proteins were analyzed by SDS-PAGE and immunoblotting.
Chemical Cross-linking Studies
Disuccinimidyl suberate (Pierce Thermo Scientific) chemical cross-linking was done essentially as instructed by the supplier. Briefly, cells expressing IL27Ra were washed twice in phosphate-buffered saline and resuspended in phosphate-buffered saline. Disuccinimidyl suberate (Pierce Thermo Scientific) was added to 5 mm and this cell suspension was incubated at room temperature for 30 min. Tris-HCl (pH 7.5) was then added to 20 mm and the cell suspension incubated for 15 min at room temperature. Cells were then lysed and analyzed by immunoblotting.
Site-directed Mutagenesis
Site-directed mutagenesis of IL27Ra and JAK2 was performed using standard PCR-based methods. The complete sequence of the mutated cDNAs was confirmed by DNA sequencing.
RESULTS
IL27Ra Activates the Transforming Properties of JAK2-V617F
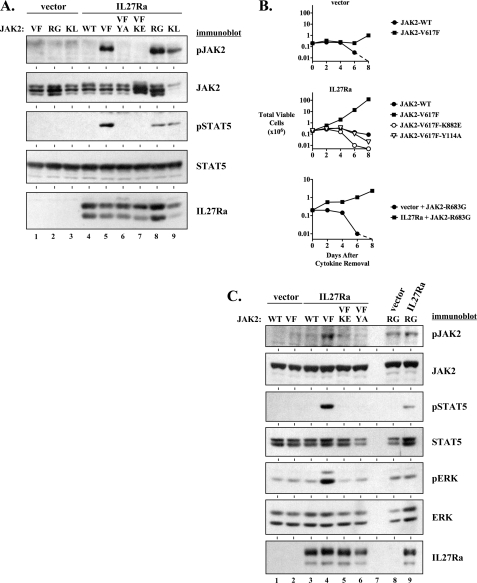
In our previous study we investigated the ability of IL27Ra, a JAK-binding Box 1 motif-containing component of a heterodimeric receptor, to activate JAK2-V617F (32). This work demonstrated that the expression of IL27Ra in 293T cells greatly enhanced the phosphorylation of JAK2-V617F at tyrosines 1007–1008, without significantly affecting the phosphorylation of wild type JAK2. Phosphorylation at these tyrosines is associated with a high catalytic state (36) and is utilized as an indicator of activation of the kinase. We also showed that IL27Ra required a functional Box 1 motif to activate JAK2-V617F (32). It has been suggested that homodimeric receptors are required to support, presumably through a scaffolding role, the activation and transforming function of JAK2-V617F (28, 30). However, IL27Ra is a component of a heterodimeric receptor and our work suggested that heterodimeric receptors might contribute to JAK2-V617F activity (32). We further examined the ability of IL27Ra to activate JAK2-V617F and JAK2-V617F-dependent signals, along with EpoR as the prototypical homodimeric cytokine receptor that supports the activation of this mutant kinase. Expression of IL27Ra, as well as EpoR, activated JAK2-V617F in 293T cells (Fig. 1A, lanes 4 and 6). Neither IL27Ra nor EpoR activated wild type JAK2 (lanes 3 and 5), and JAK2-V617F did not exhibit detectable phosphorylation in the absence of a co-expressed receptor (Fig. 1A, lane 2). IL27Ra-mediated activation of JAK2-V617F was also associated with enhanced phosphorylation of tyrosine 694 of STAT5 as well as threonine 202 and tyrosine 204 of ERK, demonstrating IL27Ra expression was able to support the activation of known downstream components of JAK2-V617F activation (Fig. 1A, lane 4).
FIGURE 1.
Activation of JAK2-V617F signaling and transforming properties by a heterodimeric receptor component, IL27Ra. A, an empty expression vector (lanes 1 and 2), and expression vectors for IL27Ra (lanes 3 and 4) or EpoR (lanes 5 and 6) were transfected into 293T cells along with a wild type JAK2 (WT) expression vector (lanes 1, 3, and 5) or a JAK2-V617F (VF) expression vector (lanes 2, 4, and 6). Two days after transfection, cells were lysed and an equal amount of lysate protein was analyzed by immunoblotting using antibodies to detect pJAK2 (Tyr1007/Tyr1008), pSTAT5 (Tyr694), pERK (Thr202/Tyr204), JAK2, STAT5, ERK, IL27Ra, and EpoR (via HA), as indicated. B, BaF3 cells co-expressing empty vector (lanes 1 and 2), IL27Ra (lanes 3 and 4), or EpoR (lanes 5 and 6) with wild type JAK2 (WT) (lanes 1, 3, and 5) or JAK2-V617F (VF) (lanes 2, 4, and 6) were blotted with antibodies to detect JAK2 and STAT5 (as a loading control). Lysate of BaF3 cells expressing an empty vector control was included (lane 8) to ascertain the approximate level of exogenous JAK2 expression in BaF3 cells expressing either wild type JAK2 or JAK2-V617F in lanes 1–6. C, cytokine-dependent BaF3 cells co-expressing an empty vector (lanes 1 and 2), IL27Ra (lanes 3 and 4), or EpoR (lanes 5 and 6) with wild type JAK2 (WT) (lanes 1, 3, and 5) or JAK2-V617F (VF) (lanes 2, 4, and 6) were starved of cytokine for 3 h. Cells were then lysed and an equal amount of lysate protein was analyzed by immunoblotting for pJAK2 (Tyr1007/Tyr1008), pSTAT5 (Tyr694), pAKT (Ser473), JAK2, STAT5, and AKT. Lysate from cytokine-independent cells expressing IL27Ra with JAK2-V617F and EpoR with JAK2-V617F are shown in lanes 8 and 9, respectively. In the lower panel, similar lysates are immunoblotted for pERK (Thr202/Tyr204) and ERK, with the lysate from cytokine-independent IL27Ra/JAK2-V617F cells shown in lane 6. D, BaF3 cells co-expressing control vector (left), IL27Ra (center), or EpoR (right) with wild type JAK2 (circles) or JAK2-V617F (squares) were washed and plated in the absence of cytokine on day 0. Total viable cells were determined by trypan blue exclusion over time. The broken line indicates the viable cell count dropped below the limit of detection of the hemocytometer.
To determine whether IL27Ra could activate JAK2-V617F in hematopoietic cells, we utilized cytokine-dependent BaF3 cells (37). These cells fail to proliferate and undergo apoptosis in the absence of cytokine. We developed BaF3 cells that express IL27Ra and JAK2-V617F or wild type JAK2 as a control. In these stable cells, the levels of exogenously expressed wild type and JAK2-V617F proteins are less than endogenous levels of JAK2 (Fig. 1B, compare JAK2 immunoblot lane 1-6 to lane 8, which shows the endogenous level of JAK2). This low level of expression is in agreement with previous studies that have utilized JAK2 expression from this same MSCV promoter-driven retroviral construct in these cells (28, 30). In these BaF3 cell lines, co-expression of JAK2-V617F with IL27Ra led to activation of JAK2, as measured by JAK2 tyrosine phosphorylation after a 3-h period of cytokine deprivation (Fig. 1C, top panel, lane 4). Wild type JAK2 was not significantly activated under these same conditions (Fig. 1C, top panel, lane 3). Expression of JAK2-V617F with IL27Ra also led to strong activation of STAT5 as measured by STAT5 tyrosine phosphorylation (Fig. 1C, top panel, lane 4). Similar results were obtained with expression of JAK2-V617F and EpoR (Fig. 1C, lane 6). Although the activation of STAT5 observed in Fig. 1C is higher in IL27R/JAK2-V617F cells than EpoR/JAK2-V617F cells, this relative activation was not consistent across multiple independently derived cell lines. ERK was also activated in cells expressing JAK2-V617F and IL27Ra (Fig. 1C, bottom panel, lane 4). These data suggest IL27R expression can functionally replace a homodimeric receptor, such as EpoR, in mediating activation of JAK2-V617F signaling in hematopoietic cells.
Previous studies have demonstrated that JAK2-V617F, when expressed at low levels, only transforms BaF3 cells to cytokine independence when expressed with a homodimeric receptor (28, 30). Even in the absence of ligand, expression of homodimeric receptors such as EpoR, GCSFR, or TpoR, is sufficient to promote cell transformation by JAK2-V617F (28). Removal of cytokine from BaF3 cells that co-express IL27Ra and JAK2-V617F led to transformation of these cells to cytokine independence (Fig. 1D, middle). This is a standard outcome of activation of JAK2-V617F in these cells. Only BaF3 cells that expressed JAK2-V617F and IL27Ra together resulted in rapid cytokine-independent proliferation, as expression of IL27Ra and wild type JAK2 did not (Fig. 1D, center). This demonstrates that the effect of IL27R on JAK2 activation is dependent on the V617F mutation. The effect of IL27Ra in this assay was similar to EpoR in mediating cytokine-independent transformation of BaF3 cells (Fig. 1D, right). Expression of JAK2-V617F with a control vector did not lead to rapid transformation to cytokine independence, as was expected, due to the low level of exogenous JAK2-V617F expression (Fig. 1D, left). This supports previous work demonstrating that JAK2-V617F expression at or below endogenous levels of JAK2 requires expression of a receptor to induce its transforming activity (28, 30). It is worth noting that we do sometimes see cytokine-independent growth, after an extended period of time, of JAK2-V617F cells that do not express an exogenous receptor, presumably due to the selection for growth of a small fraction of cells that have higher JAK2-V617F levels.
Although we have not detected an increase in Akt phosphorylation in cytokine-dependent cells expressing JAK2-V617F and either IL27Ra or EpoR receptor, enhanced Akt phosphorylation was present in these cells upon transformation to cytokine independence by JAK2-V617F and either IL27Ra or EpoR (Fig. 1C, top panel, lanes 8 and 9). Likewise, there was a significant enhancement of activated JAK2 and STAT5 (Fig. 1C, top panel, lanes 8 and 9) in cytokine-independent cells and elevated ERK activation remained present (Fig. 1C, bottom panel, lane 6). Treatment of BaF3 cells that are transformed to cytokine independence by the expression of IL27Ra and JAK2-V617F with a small molecule JAK inhibitor, JAK inhibitor I (38), led to rapid cell death and loss of proliferation, demonstrating a dependence on JAK activity for the transforming signal (data not shown and supplemental Fig. S1).
IL27Ra-mediated Activation of JAK2-V617F Requires JAK2-V617F Kinase Activity and a Functional FERM Domain
To confirm that activation of JAK2-V617F required its intrinsic kinase activity we mutated lysine 882 in the JAK2 kinase domain to glutamic acid, a mutation that renders the JAK2 kinase inactive (39). As expected, this JAK2-V617F,K882E protein was not activated by expression of IL27Ra as measured by JAK2 phosphorylation and phosphorylation of its downstream signaling mediator, STAT5 (Fig. 2A, lane 7). Because we have previously shown that IL27Ra requires a functional JAK-binding Box 1 motif to activate JAK2-V617F (32), we presumed that JAK2 has to maintain its receptor-binding capacity for its activation by IL27Ra. We further investigated this by introducing a tyrosine to alanine mutation at residue 114 within the FERM domain of JAK2-V617F. This mutation is known to disrupt the binding of JAK2 to receptors and prevents activation of signaling downstream of JAK2-V617F (31). JAK2-V617F,Y114A and STAT5 were not activated by expression of IL27Ra with this mutant JAK2-V617F (Fig. 2A, lane 6).
FIGURE 2.
Activation of JAK2-V617F by IL27Ra requires functional JAK2-V617F kinase and FERM domains. A, an empty expression vector (lanes 1–3) and an expression vector for IL27Ra (lanes 4–9) were transfected into 293T cells along with expression vectors for JAK2-V617F (VF) (lanes 1 and 5), JAK2-R683G (RG) (lanes 2 and 8), JAK2-K539L (KL) (lanes 3 and 9), wild type JAK2 (WT) (lane 4), a FERM domain mutant JAK2-V617F,Y114A (VF-YA) (lane 6), and a kinase domain mutant JAK2-V617F,K882E (VF-KE) (lane 7). Two days after transfection, cells were lysed and an equal amount of lysate protein was analyzed by immunoblotting using antibodies to detect pJAK2 (Tyr1007/Tyr1008), JAK2, pSTAT5 (Tyr694), STAT5, and IL27Ra, as indicated. B, BaF3 cells co-expressing either control vector (top graph) or IL27Ra (center graph), with wild type JAK2 (closed circles), JAK2-V617F (closed squares), JAK2-V617F,K882E (open circles), or JAK2-V617F,Y114A (open triangles) were washed and plated in the absence of cytokine on day 0. Total viable cells were determined by trypan blue exclusion over time. In the same experiment, BaF3 cells co-expressing a control vector with JAK2-R683G (closed circles) or IL27Ra with JAK2-R683G (closed squares) were washed of cytokine and viability was assessed over time (bottom graph). The broken lines indicate the viable cell count dropped below the limit of detection of the hemocytometer. C, cytokine-dependent BaF3 cells co-expressing either an empty vector (lanes 1, 2, and 8) or IL27Ra (lanes 3–6 and 9) with wild type JAK2 (WT) (lanes 1 and 3), JAK2-V617F (VF) (lanes 2 and 4), JAK2-V617F,K882E (lane 5), JAK2-V617F,Y114A (lane 6), or JAK2-R683G (lanes 8 and 9) were starved of cytokine for 3 h. Cells were then lysed and an equal amount of lysate protein was analyzed by immunoblotting for pJAK2 (Tyr1007/Tyr1008), JAK2, pSTAT5 (Tyr694), STAT5, pERK (Thr202/Tyr204), ERK, and IL27Ra, as indicated.
The activation of JAK2-V617F by IL27Ra in BaF3 cells was also dependent on JAK2-V617F kinase activity and a functional FERM domain. These mutations impaired the ability of JAK2-V617F to induce cytokine-independent transformation of BaF3 cells expressing IL27Ra (Fig. 2B). This correlated with a loss of JAK2-V617F activation and a loss of activation of its downstream signaling mediators STAT5 and ERK (Fig. 2C, lanes 5 and 6), in cells expressing IL27Ra and these JAK2-V617F mutants. Likewise, an IL27Ra containing a mutated Box 1 motif (32) is not capable of inducing the transforming activity of JAK2-V617F (supplemental Fig. S2).
IL27Ra Mediates Activation of JAK2-K539L and JAK2-R683G
A lysine to leucine mutation at amino acid 539 of JAK2 is a JAK2 exon 12 mutation that is also present in MPNs. Point mutations of arginine 683 are associated with certain cases of acute lymphoblastic leukemia (ALL) (40, 41). In our experiments in Fig. 2, we also show that IL27Ra could support the activation of JAK2-K539L and JAK2-R683G. This is demonstrated in 293T cells for both of these mutants (Fig. 2A, lanes 8 and 9), as well as in BaF3 cells for JAK2-R683G (Fig. 2, B, bottom graph, and C, lanes 8 and 9). These data demonstrate that IL27Ra-mediated activation of mutant JAK2 is not restricted to JAK2-V617F, because similar results are obtained with the exon 12 JAK2 mutation K539L, as well as the JAK2-R683G mutation that is associated with ALL. However, activation of these mutants and downstream signaling by IL27Ra appears to be less efficient than activation of JAK2-V617F.
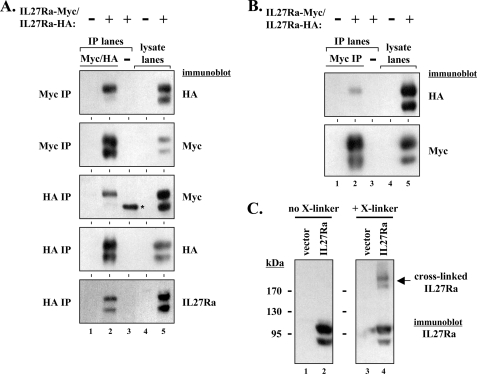
Identification of IL27Ra Homodimeric Complexes
One explanation for the ability of the heterodimeric receptor IL27Ra to replace the requirement of a homodimeric receptor to mediate JAK2-V617F activation is simply that IL27Ra may be capable of forming homodimers. This concept has recently been suggested by Hashimoto et al. (42). To test if IL27Ra could interact with itself, we generated HA and Myc epitope-tagged versions of the protein. Co-expression of these proteins in 293T cells followed by immunoprecipitation with epitope tag antibodies revealed that IL27Ra proteins could interact with each other (Fig. 3A). Similar results were obtained when immunoprecipitations were carried out with either epitope tag antibody and were also obtained utilizing BaF3 cells engineered to express HA- and Myc-tagged IL27Ra proteins (Fig. 3B). This suggests that IL27Ra may form homodimers in cells. Although the IL27Ra protein migrates as a doublet, it is primarily only the higher molecular weight band, which corresponds to the mature, fully glycosylated (endoglycosidase H-resistant) (data not shown) plasma membrane-bound form of the receptor, that co-immunoprecipitates in our experiments. Also, cross-linking of IL27Ra-expressing 293T cells led to the formation of an IL27Ra-antibody reactive protein that is approximately twice the molecular weight of monomeric IL27Ra (Fig. 3C). Immunoblotting for JAK proteins and gp130, the co-receptor for IL27, did not detect these proteins in these higher molecular weight complexes (data not shown). Similar data were obtained using BaF3-expressing IL27Ra cells as well as using a membrane-impermeable cross-linker (data not shown).
FIGURE 3.
Co-immunoprecipitation and cross-linking studies suggest IL27Ra can form homodimers. A, 293T cells were left untransfected (indicated by −) or transfected with expression vectors for both Myc- and HA-tagged versions of IL27Ra (indicated by +). Myc tag immunoprecipitations (IP) (lanes 1 and 2, top two blots) and HA tag immunoprecipitations (lanes 1 and 2, bottom three blots) were performed on untransfected cell lysates (lane 1) and lysates containing epitope-tagged versions of IL27Ra (lane 2). A control immunoprecipitation (−) of IL27Ra-containing lysate is shown in lane 3 using an equal amount of a Myc tag isotype-matched antibody that recognizes actin (top two blots) or an equal amount of a HA tag isotype-matched antibody that recognizes the FLAG epitope tag (bottom three blots). Lysates from untransfected and IL27R-expressing cells used in the immunoprecipitations are shown in lanes 4 and 5, respectively. Immunoblots were performed using HA and Myc epitope tag antibodies as well as IL27Ra antibodies, as indicated. The band (*) in the control immunoprecipitation of the middle blot is a nonspecific band, as it is not detected by IL27Ra antibodies (bottom blot). B, Myc tag antibodies were used in immunoprecipitations of lysates from parental BaF3 cells (−) (lane 1) and cytokine-dependent BaF3 cells co-expressing HA- and Myc-tagged IL27Ra proteins (+) (lane 2). A control immunoprecipitation (−) of lysate of BaF3 cells expressing HA- and Myc-tagged IL27Ra proteins is shown in lane 3 using an equal amount of a Myc tag isotype-matched antibody that recognizes actin. Lysates from parental BaF3 cells and IL27R-expressing cells used in the immunoprecipitations are shown in lanes 4 and 5, respectively. Immunoblots were performed using HA and Myc epitope tag antibodies, as indicated. C, 293T cells were transfected with an empty control expression vector (lanes 1 and 3) or an expression vector for IL27Ra (lanes 2 and 4). Two days after transfection the cells were cross-linked with the chemical cross-linker disuccinimidyl suberate (lanes 3 and 4) or not cross-linked (DMSO treated) (lanes 1 and 3) and lysates were analyzed by immunoblotting for IL27Ra.
IL27Ra-mediated Activation of JAK2-V617F Signaling Does Not Require Cytoplasmic Domain Tyrosine Phosphorylation
Cytokine receptors are believed to play a scaffolding role for activation of the transforming signal of JAK2-V617F (28, 30). JAK2 proteins interacting with cytokine receptor scaffolds are presumed to be properly juxtaposed with other JAK2 proteins to allow for proper trans-phosphorylation and activation of the tyrosine kinase domains upon ligand stimulation of the receptors. However, in the case of the MPN-associated JAK2-V617F mutant, the ligand is not necessary. The combination of the point mutation relieving, to a certain extent, the autoinhibition that is inherent in the kinase, and the juxtaposing of the mutant kinase near another mutant kinase through the scaffolding effect of interacting cytokine receptors, leads to transphosphorylation of the kinases. This results in hyperactivation of the kinase activity and subsequent downstream signaling, all in the absence of ligand stimulation. It has been suggested that a cytokine receptor not only provides a scaffold for interacting proteins but also provides an important substrate for transformation by JAK2-V617F (28). Tyrosine phosphorylation of cytokine receptors by JAK family members provides binding sites for STAT proteins, which are subsequently activated by tyrosine phosphorylation and translocate into the nucleus to regulate target gene expression (11–14). IL27Ra has a single tyrosine (Tyr613) that is predicted to be a phosphorylation site (Fig. 4A). This tyrosine has been suggested to be important for the activation of STAT1 following receptor activation (43). We mutated this tyrosine to phenylalanine, and expressed it along with JAK2-V617F in 293T cells. This mutation did not affect the ability of IL27Ra to activate JAK2-V617F but did reduce the levels of activated/phosphorylated STAT5 (Fig. 4B, lane 7), compared with wild type IL27Ra (lane 6). To ascertain the requirement of this tyrosine in IL27R-mediated activation of JAK2-V617F transforming signals in hematopoietic cells, we expressed it in BaF3/JAK2-V617F cells. This mutant IL27Ra was capable of supporting the transforming signals of JAK2-V617F in BaF3 cells similar to wild type IL27Ra, suggesting this tyrosine residue is not required to be phosphorylated for JAK2-V617F-mediated transformation (Fig. 4C). It is possible an alternative tyrosine is utilized in the absence of tyrosine 613. IL27Ra has an additional tyrosine in its cytoplasmic domain. Like mutation of Tyr613, mutation of this second tyrosine (Tyr543) did not affect the ability of IL27Ra to facilitate the activation of JAK2-V617F in 293T cells (Fig. 4B, lane 8). Unlike mutation of Tyr613, however, it did not reduce the ability of JAK2-V617F to activate STAT5 (Fig. 4B, lane 8). In addition, mutation of Tyr543 did not affect the ability of IL27Ra to support the activation of JAK2-V617F transforming activity in BaF3 cells (Fig. 4C). Mutation of both tyrosine residues (Tyr543 and Tyr613) together did not inhibit the ability of IL27Ra to support JAK2-V617F activation in 293T cells, although again STAT5 activation was lower than when wild type IL27Ra was used to activate JAK2-V617F (Fig. 4B, lane 9). This double tyrosine mutant of IL27Ra was still able to support JAK2-V617F-mediated transformation of BaF3 cells to cytokine-independent growth (Fig. 4C). Although we have not detected tyrosine phosphorylation of IL27Ra, mutation of Tyr613 to phenylalanine prevents IL27Ra/JAK2-V617F-mediated activation of STAT1 in both 293T and BaF3 cells (supplemental Fig. S3) and also diminishes activation of STAT5 (Fig. 4B). This suggests that Tyr613 is required to fully activate downstream STAT proteins, presumably through phosphorylation of this residue, which is consistent with the current knowledge of IL27Ra signaling (43).
FIGURE 4.
Tyrosines in the cytoplasmic region of IL27Ra are not required for activation of JAK2-V617F-mediated signaling and transformation. A, a schematic diagram of IL27Ra is shown. The tyrosines in the cytoplasmic tail of the receptor are indicated. One tyrosine (Y543) is between the transmembrane domain and the JAK-binding Box 1 motif, whereas the other (Y613) is near the carboxyl terminus. B, a control empty expression vector (V) (lanes 1–3), or expression vectors for wild type IL27R (WT) (lanes 4–6), IL27R-Y613F (Y613F) (lane 7), IL27R-Y543F (Y543F) (lane 8), or IL27R-Y613F,Y543F (Y613F,Y543F) (lane 9) were transfected into 293T cells along with either an empty vector (V) (lanes 1 and 4), or expression vectors for wild type JAK2 (WT) (lanes 2 and 5) or JAK2-V617F (VF) (lanes 3 and 6–9). Two days after transfection, cells were lysed and an equal amount of lysate protein was analyzed by immunoblotting using antibodies to detect IL27Ra, pJAK2 (Tyr1007/Tyr1008), JAK2, pSTAT5 (Tyr694), and STAT5, as indicated. The arrow indicates the mobility of phosphorylated JAK2 on the immunoblot. C, BaF3 cells generated to express JAK2-V617F along with an empty control expression vector (closed circles), IL27R-WT (closed squares), IL27R-Y613F (open triangles), IL27R-Y543F (open circles), or IL27R-Y613F,Y543F (closed triangles) were washed and plated in the absence of cytokine on day 0. Total viable cells were determined by trypan blue exclusion over time.
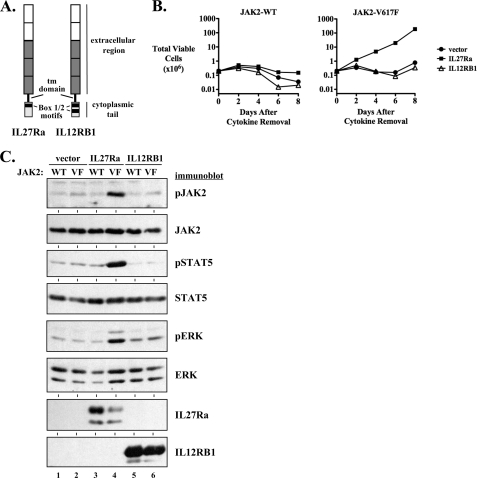
IL12RB1 Does Not Support the Transforming Activity of JAK2-V617F
We next wanted to determine whether the ability of IL27Ra to activate JAK2-V617F was shared with other heterodimeric receptor components or was a unique feature of IL27Ra. To investigate this we utilized interleukin-12 receptor β1 (IL12RB1) because it, like IL27Ra, is a member of the IL6/IL12 receptor family (44). IL12RB1 is structurally similar to IL27R, containing similar organization of extracellular domains, and has a short cytoplasmic tail with JAK-binding motifs (Fig. 5A). Expression of IL12RB1 in JAK2-V617F-expressing BaF3 cells did not lead to an enhanced rate of cytokine-independent transformation of these cells (Fig. 5B). In the same experiment, IL27Ra expression in the same BaF3-JAK2-V617F cells did support JAK2-V617F-mediated transformation (Fig. 5B). Following a brief 3-h period of cytokine deprivation of BaF3-JAK2-V617F cells expressing IL27Ra or IL12RB1, only the cells that express IL27Ra and JAK2-V617F had detectable levels of activated JAK2, STAT5, and ERK (Fig. 5C, lane 4). This correlated with the ability of only this cell line to undergo rapid proliferation in the absence of cytokine (Fig. 5B). Surprisingly, we did detect activation of JAK2-V617F by IL12RB1 in 293T cells. This activation, however, led to significantly weaker STAT5 activation than that observed for IL27Ra (supplemental Fig. S4), yet demonstrates, like IL27Ra and EpoR, IL12RB1 can functionally interact with JAK2-V617F. Nonetheless, unlike IL27Ra, IL12RB1 could not activate the transforming properties of JAK2-V617F in hematopoietic cells.
FIGURE 5.
Transforming properties of JAK2-V617F are not activated by expression of IL12RB1. A, a schematic diagram comparing IL27Ra and IL12RB1 is shown. The white regions of the extracellular region of the receptors represent cytokine receptor homology domains, whereas the gray regions represent fibronectin-like domains. The transmembrane (tm) domain of each receptor is indicated. Within the cytoplasmic tail of each receptor the Box 1 motif for IL27Ra and the Box 1 and 2 motifs of IL12RB1 are indicated. B, BaF3 cells co-expressing wild type JAK2 (JAK2 WT, left) or JAK2-V617F (right) with a control empty vector (filled circles), IL27Ra (filled squares), or IL12RB1 (open triangles) were washed and plated in the absence of cytokine on day 0. Total viable cells were determined by trypan blue exclusion over time. C, BaF3 cells co-expressing empty vector (lanes 1 and 2), IL27Ra (lanes 3 and 4), or IL12RB1 (lanes 5 and 6) with wild type JAK2 (WT) (lanes 1, 3, and 5), or JAK2-V617F (VF) (lanes 2, 4, and 6) were starved of cytokine for 3 h. Cells were then lysed and an equal amount of lysate protein was analyzed by immunoblotting for pJAK2 (Tyr1007/Tyr1008), JAK2, pSTAT5 (Tyr694), STAT5, pERK (Thr202/Tyr204), ERK, IL27Ra, and IL12RB1, as indicated.
Components of the Receptor for IL3 Activate JAK2-V617F and Enhance Its Transforming Properties
We next investigated the ability of heterodimeric receptor components, which are not members of the IL6/IL12 receptor family, to support the activation of JAK2-V617F and its ability to transform cells to cytokine independence. To this end, we utilized IL3Ra and the common β chain for the heterodimeric receptors for IL3, IL5, and GM-CSF (45), and expressed these receptor components in 293T cells. When co-expressed with JAK2-V617F, IL3Ra led to high levels of activated JAK2-V617F as well as activation of the JAK2 downstream signaling targets STAT5 and ERK (Fig. 6A, lane 5). IL3Ra did not activate wild type JAK2, STAT5, or ERK in the presence of wild type JAK2, in the same experiment (Fig. 6A, lane 4). Expression of the common β chain receptor also led to activation of JAK2-V617F, STAT5, and ERK (Fig. 6A, lane 8). The common β chain receptor also activated wild type JAK2 and STAT5 in the presence of wild type JAK2. However, taking into account the relative amount of the common β chain expressed, this activation was less efficient than when the common β chain was expressed with JAK2-V617F (Fig. 6A, lanes 7 and 8). Expression of IL3Ra enhanced JAK2-V617F-mediated transformation of BaF3 cells (Fig. 6B, bottom), whereas co-expression of IL3Ra and JAK2 wild type did not transform these cells (Fig. 6B, top). Transformation of BaF3 cells to cytokine independence by co-expression of JAK2-V617F with IL3Ra occurred at a slower rate than expression with IL27Ra or EpoR (Fig. 6B, bottom, and also see Fig. 1). Expression of the common β chain in BaF3 cells expressing JAK2-V617F had a similar effect as expression of IL3Ra (Fig. 6B, bottom). That is, cytokine-independent transformation was enhanced, but at a slower rate than expression of JAK2-V617F with IL27Ra or EpoR (Fig. 6B, and see Fig. 1). Analysis of STAT5 activation in these cells, prior to cytokine-independent transformation, demonstrated that expression of IL3Ra or the common β chain along with JAK2-V617F led to activation of STAT5 as detected by tyrosine phosphorylation of STAT5 following a brief cytokine deprivation period of 3 h (Fig. 6C, lanes 6 and 8). This STAT5 phosphorylation was less than what was seen in cells co-expressing IL27Ra and JAK2-V617F (Fig. 6C, lane 4). This correlates with the decreased ability of IL3Ra or the common β chain to enhance the rate of JAK2-V617F-mediated transformation compared with IL27Ra (Fig. 6B, bottom). As seen in other experiments (Fig. 1C), significant enhancement of Akt phosphorylation by receptor and JAK2-V617F expression was not evident following transient cytokine removal (Fig. 6C).
FIGURE 6.
Components of the IL3R activate JAK2-V617F signaling and enhance transforming properties of JAK2-V617F. A, an empty expression vector (lanes 1 and 2), an expression vector for IL3Ra-HA (lanes 3–5), and an expression vector for the common β chain (Bc) (lanes 6–8) were transfected into 293T cells along with an empty vector (lanes 3 and 6), a wild type JAK2 (WT) expression vector (lanes 1, 3, and 6), or a JAK2-V617F (VF) expression vector (lanes 2, 5, and 8). Two days after transfection, cells were lysed and an equal amount of lysate protein was analyzed by immunoblotting using antibodies to detect pJAK2 (Tyr1007/Tyr1008), pSTAT5 (Tyr694), pERK (Thr202/Tyr204), JAK2, STAT5, ERK, IL3Ra (via HA), and the common β chain (Bc), as indicated. B, BaF3 cells co-expressing wild type JAK2 (JAK2 WT, top) or JAK2-V617F (bottom) with a control empty vector (filled circles), IL27Ra (filled squares), IL3Ra (filled triangles), or the common β chain (Bc) (open triangles) were washed and plated in the absence of cytokine on day 0. Total viable cells were determined by trypan blue exclusion over time. The growth curve of cells expressing JAK2-V617F and the common β chain overlaps the growth curve for cells expressing JAK2-V617F and IL3Ra as these cells grew at nearly identical rates. Standard deviation error bars derived from triplicate counts are not visible at this scale. C, BaF3 cells co-expressing empty vector (lanes 1 and 2), IL27Ra (lanes 3 and 4), IL3Ra (lanes 5 and 6), or the common β chain (Bc) (lane 7 and 8) along with wild type JAK2 (WT) (lanes 1, 3, 5, and 7), or JAK2-V617F (VF) (lanes 2, 4, 6, and 8) were starved of cytokine for 3 h. Cells were then lysed and an equal amount of lysate protein was analyzed by immunoblotting for JAK2, pSTAT5 (Tyr694), pAKT (Ser473), STAT5, AKT, IL27Ra, IL3Ra (via HA), and the common β chain (Bc), as indicated.
DISCUSSION
The identification of the JAK2-V617F mutation as an important etiologic factor in the development of MPNs has provided an opportunity to attempt to target a mutationally activated disease-causing tyrosine kinase, akin to imatinib treatment for Bcr-Abl-positive leukemias. However, the exact mechanism by which this mutation induces constitutive signaling remains unknown. Although there has been no structural determination of the consequence of the V617F mutation, which resides in the pseudokinase domain, it likely impairs the ability of the pseudokinase domain to impart its negative regulatory function on the tyrosine kinase domain. Recent molecular dynamic simulations of JAK2 mutations that have been identified in MPN patients support this hypothesis (22). However, the mechanism by which JAK2-V617F becomes fully activated is unclear, as it is confounded by the requirement of other cellular proteins, namely, homodimeric cytokine receptors that contain JAK-binding motifs.
There have been contradicting data reported regarding the requirement of a homodimeric cytokine receptor for JAK2-V617F-mediated transformation of hematopoietic cells. Lu et al. (28) suggested a homodimeric receptor was required, whereas earlier studies did not utilize a receptor to demonstrate JAK2-V617F-mediated transformation (6, 8, 29). In a more recent study, Lu et al. (30) demonstrated that the level of JAK2-V617F expression dictates the requirement, or not, of the exogenous expression of a homodimeric receptor. High levels of expression of this mutant kinase can overcome the need for expression of a receptor, whereas low levels of expression requires expression of a homodimeric receptor. The exact reason for this is not known, but it has been suggested that JAK2-V617F has to compete with endogenous wild type JAK2 binding to endogenous receptors (30). If so, enhancing the number of receptors might alleviate this competition and allow for more productive JAK2-V617F·receptor complexes to form. To initiate transforming signals, JAK2-V617F must bind to a Box 1 motif of a cytokine receptor, as mutant JAK2-V617F proteins that cannot interact with Box 1 motifs on cytokine receptors do not exhibit transforming activity (30, 31). Although it appears JAK2-V617F likely requires interactions with receptors to elicit its signaling, the exact receptors that JAK2-V617F utilizes in cells of MPN patients remains unknown.
In a previous study, we demonstrated that a single chain of a heterodimeric receptor, IL27Ra, could enhance the activity of JAK2-V617F in 293T cells (32). This activation was in a ligand- and co-receptor-independent manner and required a functional JAK-binding Box 1 motif of the receptor. Our work was the first to suggest that heterodimeric receptor components were capable of activating JAK2-V617F. In the current study, we extend these observations and investigate the extent to which heterodimeric receptors could functionally replace homodimeric cytokine receptors to mediate activation of the transforming capacity of JAK2-V617F.
In this study, we demonstrate that IL27Ra can activate JAK2-V617F in hematopoietic cells as well as activate transforming signals mediated by JAK2-V617F. The coexpression of IL27Ra with JAK2-V617F in BaF3 cells led to an increase in activated JAK2 signaling (Fig. 1C), correlating with the rapid cytokine-independent outgrowth of these cells (Fig. 1D). IL27Ra-mediated activation of JAK2-V617F was dependent on JAK2-V617F kinase activity and a functional FERM domain of the mutant JAK2 (Fig. 2). Although the transforming effect was greater for JAK2-V617F, IL27Ra also supported the activation of the JAK2-K539L exon 12 mutant and the ALL-associated JAK2-R683G mutant (Fig. 2). These data demonstrate that it is possible for heterodimeric receptors, in addition to homodimeric receptors, to contribute to the activation of mutant JAK2 signaling and transforming activity in hematopoietic cells.
In addition to providing a scaffolding function to properly juxtapose JAK2-V617F molecules near each other to allow for transphosphorylation, JAK2-V617F-bound cytokine receptors also likely provide an important substrate for the kinase activity of the mutant JAK2 (28, 30). Tyrosine phosphorylation of cytokine receptors provides binding sites for downstream effectors of JAK signaling (11, 15). Interestingly, in our experiments in 293T cells, IL27Ra did not activate STAT5 to the same extent as EpoR, even though there was more activated JAK2 in the IL27Ra expressing cells (Fig. 1A, lanes 4 and 6). IL27Ra has a short cytoplasmic tail that has only two tyrosine residues, one of which has been suggested to be a site of importance to STAT signaling (43). EpoR on the other hand has eight tyrosines that may contribute to downstream signaling, at least four of which have been implicated in activation of STAT5 (46). Therefore, in this system, it may be that EpoR is functioning as a more efficient activator of STAT5 than IL27Ra due to the elevated potential to recruit STAT5 to the receptor. Alternatively, the difference in the relative levels of functional JAK2·receptor complexes, or a difference in the negative regulation of such complexes, may be impacting the amount of STAT5 activation observed in these experiments.
We investigated the role of the tyrosines within the cytoplasmic region of IL27Ra in supporting JAK2-mediated STAT5 phosphorylation as well as JAK2-V617F-mediated transformation. Tyrosine to phenylalanine mutants of IL27Ra all activated JAK2-V617F in 293T cells (Fig. 4B) and supported the transforming signal of JAK2-V617F (Fig. 4C). Mutation of the two tyrosines within the cytoplasmic domain of IL27Ra inhibited, but did not prevent STAT5 activation following expression with JAK2-V617F (Fig. 4B). Tyrosine 613 of IL27Ra has been implicated in STAT signaling and in our experiments mutation of this residue to phenylalanine appeared to have a greater effect on STAT5 phosphorylation than mutation of tyrosine 543, which did not decrease STAT5 phosphorylation (43). These data are in line with previous work that demonstrated that removal of EpoR tyrosine phosphorylation sites impaired, but did not prevent, the ability of EpoR to support the transforming capacity of JAK2-V617F in BaF3 cells (28). Thus, whereas STAT5 activation downstream of JAK2-V617F·cytokine receptor complexes can be influenced by tyrosine phosphorylation of the receptor, there appears to be mechanisms that are independent of scaffolding receptor phosphorylation for STAT5 activation downstream of JAK2-V617F. In the scenario where EpoR or IL27Ra have no phosphorylatable tyrosines, but can still support the activation of and transformation by JAK2-V617F, it is possible that JAK2-V617F may phosphorylate STAT5 directly. Also, perhaps other kinases that are activated subsequent to JAK2-V617F activation can phosphorylate STAT5. In addition, other proteins, such as another cytokine receptor, may be phosphorylated by activated JAK2-V617F, resulting in recruitment and phosphorylation of STAT5. Such a scenario of receptors activated by a receptor to receptor cross-talking signal has been suggested in insulin-like growth factor 1 receptor signaling in cells expressing JAK2-V617F (29). Although the α subunit of the GM-CSF receptor is not tyrosine phosphorylated upon GM-CSF stimulation, mutation of all tyrosines in the cytoplasmic region of the common β chain does not inhibit STAT5 phosphorylation in response to GM-CSF (47, 48). In addition, mutation of all tyrosines on the cytoplasmic region of EpoR does not completely prevent Epo-induced STAT5 tyrosine phosphorylation (46). Thus, it appears that receptor scaffold phosphorylation is not necessarily required for JAK2 to transduce a signal to activate STAT5.
The ability of IL27Ra to activate the kinase as well as transforming activity of JAK2-V617F suggested that other heterodimeric receptor components might do the same. Like IL27Ra, IL12RB1 is a member of the IL6/IL12 cytokine receptor family and is expressed in hematopoietic cells of the lymphoid and myeloid lineages (44, 49, 50). However, unlike IL27Ra, IL12RB1 was not able to activate JAK2-V617F to transform BaF3 cells to cytokine independence (Fig. 5, B and C). This suggests not all heterodimeric receptor components can activate JAK2-V617F in hematopoietic cells, and that there may be something unique about certain heterodimeric receptors such as IL27Ra in this regard.
JAK2 has been shown to interact with each single chain component of the receptor for IL3, IL3Ra, and the common β chain (shared among the receptors for IL3, IL5, and GM-CSF) (51, 52). Each of these receptors was able to activate JAK2-V617F when expressed in 293T cells (Fig. 6A). STAT5 was phosphorylated downstream of JAK2-V617F that was activated by either IL3Ra or the common β chain. STAT5 phosphorylation was significantly greater following activation of JAK2-V617F by the common β chain compared with activation by IL3Ra (Fig. 6A). This may be due to the inherent ability of the common β chain to function as the signal transducer in heterodimeric receptors in which it participates. In this respect, the human common β chain has eight tyrosine residues that can participate in signaling, whereas IL3Ra has just one (45). Expression of IL3Ra or the common β chain with JAK2-V617F led to an enhanced rate of cytokine independence compared with JAK2-V617F expression alone (Fig. 6B). This enhancement of cytokine-independent growth was significantly delayed compared with that of IL27Ra, with this delay being consistently reproducible in independently derived lines. Correspondingly, expression of IL27Ra and JAK2-V617F led to significantly greater activation of STAT5 than expression of IL3Ra or the common β chain with JAK2-V617F (Fig. 6C). These data are consistent with a recent report demonstrating that STAT5 activation is required for JAK2-V617F-mediated transformation and that knockdown of STAT5 inhibits this transformation (53). The delay in activation of JAK2-V617F-mediated transformation may be due to JAK2 competition with the endogenous components for the IL3 receptor in these cells. It is difficult to ascertain the relative levels of endogenously versus exogenously expressed IL3Ra in these cells, because we utilized a human cDNA in mouse cells. Cross-reactivity between species is generally not seen for antibodies raised against IL3Ra. Nonetheless, our data suggest that raising the levels of IL3Ra and common β receptors can enhance the activation of JAK2-V617F-mediated signaling and transformation.
It has been shown that even when highly expressed, JAK2-V617F still requires a functional interaction with receptors to transform cells (30). Thus, it has been speculated that signaling by JAK2-V617F, when expressed at high levels in the absence of an exogenously expressed homodimeric receptor, may involve interactions with endogenously expressed receptors such as IL3R. Our work suggests that indeed components of the IL3R are capable of activating JAK2-V617F and that it is possible that transformation to cytokine independence, driven by high levels of JAK2-V617F, could potentially utilize a mechanism involving components of the IL3R.
A possible explanation for the ability of a heterodimeric receptor component to functionally replace a homodimeric receptor in the activation of JAK2-V617F is that certain heterodimeric receptors have the ability to homodimerize or heterodimerize with unknown receptor components. In our current studies, only IL27Ra had a significant ability to activate the transforming signals of JAK2-V617F. It has recently been suggested by Hashimoto et al. (42) that IL27Ra may form signaling competent homodimers. Indeed, we present evidence through co-immunoprecipitation and chemical cross-linking studies that IL27Ra can undergo homodimerization (Fig. 3). This data along with the requirement for the FERM domain of JAK2-V617F (Fig. 2) and the Box 1 motif of IL27R (32) (supplemental Fig. S2) to mediate activation of JAK2-V617F supports the hypothesis that IL27Ra forms dimeric complexes that functionally lead to JAK2-V617F activation.
The ability of the common β chain to homodimerize may explain its ability to functionally replace a homodimeric receptor to activate JAK2-V617F (54–56). However, it has been suggested that the cytoplasmic domains of a common β chain homodimer are too far apart for activation of signaling without association with a ligand-bound IL3Ra subunit (57). It is possible that the autoinhibition relieved by the V617F mutation allows for a more open or extended JAK2 confirmation that can utilize the common β chain as a scaffold for activation. In addition, Hornakova et al. (58) have demonstrated that the α subunit of the IL9 receptor, which normally functions with the common γ chain to form the receptor for IL9, can form homodimers that function to support the activation of clinically derived JAK1 point mutations. Importantly, while our study was under revision, multiple groups reported the aberrant expression of cytokine receptor-like factor 2 (CRLF2) and its ability to activate JAK2-R683 mutations in ALL (59–62). Like the receptors utilized in our study, CRLF2 is a component of a heterodimeric receptor. Thus, these reports strongly support our observations that certain heterodimeric receptor components are capable of supporting the activation of mutated JAK proteins.
As Gakovic et al. (63) suggest, specific interactions of the FERM domains of JAK molecules with various cytokine receptors may differentially position/orient the JAK kinase domain for activation and access to downstream substrates. Thus, interaction of mutated JAK proteins with certain heterodimeric receptor components may allow for proper orientation and activation of kinase activity and downstream signaling, whereas interaction with other heterodimeric receptor components may not. Thus, signaling from mutated JAK proteins could be affected by the repertoire and abundance of JAK-binding cytokine receptors expressed in a given cell.
We recently reported that IL27Ra is expressed on the surface of myeloid cells of AML patients (32). In addition, more recent work has identified IL27Ra expression on hematopoietic stem cells (64). This same study demonstrated that IL27 is a cytokine that can enhance myelopoiesis and CD34+ cell growth. Thus, IL27Ra is a heterodimeric receptor component expressed in various cells within the correct hematopoietic lineage to potentially contribute to JAK2-V617F activation in MPNs. It is unknown whether or not IL27Ra or other heterodimeric receptor components contribute to mutated JAK2 activation in MPNs. However, it is important to note that whereas homodimeric receptors such as EpoR, TpoR, and GCSFR support the activation of JAK2-V671F in cells, the receptors that JAK2-V617F utilize in its causative role in MPNs (as well as the requirement for any specific cytokine receptor) remain to be determined (28).
TpoR is a cytokine receptor that supports the activation of JAK2-V617F and transforming mutations of TpoR are present in a small percent of JAK2-V617F-negative MPN patients (28, 65, 66). Likewise a gain-of-function mutation in CRLF2 has been identified in ALL (59, 62). Interestingly, we have identified in vitro derived mutations in IL27Ra that render it highly transforming.4 These data suggest that unknown mutations in additional cytokine receptors, including heterodimeric receptor components, may activate JAK2 signaling pathways in JAK2 mutation-negative hematopoietic disorders.
In summary, whereas it has been demonstrated that JAK2-V617F requires a cytokine receptor to become functionally activated and to elicit its transforming signal, the exact role of cytokine receptors and the exact repertoire of receptors utilized in JAK2-V617F-positive cells of MPN patients is not known. Our work demonstrates that components of some heterodimeric receptors have the ability to functionally replace homodimeric receptors to activate JAK2-V617F, and other JAK2 mutants, leading to the transformation of hematopoietic cells. Thus, we propose that receptors that are classified as heterodimeric receptor components may participate in activation of mutated JAK2 proteins in MPNs. The very recent reports of CRLF2-mediated activation of JAK2 mutants validate this proposal, albeit in ALL, a lymphoid disease (59–62).
Supplementary Material
Acknowledgments
We thank Dr. Atsushi Miyajima for the generous gift of the pKH97 plasmid and Dr. J. Devon Roll for critically reading the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grant R01CA127250 and the Molecular Biology Core Facility of the Moffitt Cancer Center, American Cancer Society Research Scholar Grant RSG-07-197-01-LIB, the Lauri Strauss Leukemia Foundation, and the Children's Leukemia Research Association (all to G. W. R.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S4.
Q. T. Lambert and G. W. Reuther, unpublished data.
- MPN
- myeloproliferative neoplasm
- JAK
- Janus kinase
- IL27Ra
- interleukin-27 receptor α
- STAT5
- signal transducer and activator of transcription-5
- ERK
- extracellular-regulated kinase
- IL12RB1
- interleukin-12 receptor β-1
- IL3Ra
- interleukin-3 receptor α
- HA
- hemagglutinin
- EpoR
- erythropoietin receptor
- MSCV
- murine stem cell virus
- GM-CSF
- granulocyte macrophage-colony stimulating factor
- FERM
- band 4.1 ezrin radixin moesin
- TpoR
- thrombopoietin receptor
- GCSFR
- granulocyte colony stimulating factor receptor
- ALL
- acute lymphoblastic leukemia
- CRLF2
- cytokine receptor-like factor 2
- FBS
- fetal bovine serum
- IL
- interleukin.
REFERENCES
- 1.Campbell P. J., Green A. R. (2006) N. Engl. J. Med. 355, 2452–2466 [DOI] [PubMed] [Google Scholar]
- 2.Tefferi A., Thiele J., Vardiman J. W. (2009) Cancer 115, 3842–3847 [DOI] [PubMed] [Google Scholar]
- 3.Tefferi A., Vardiman J. W. (2008) Leukemia 22, 14–22 [DOI] [PubMed] [Google Scholar]
- 4.Dameshek W. (1951) Blood 6, 372–375 [PubMed] [Google Scholar]
- 5.Baxter E. J., Scott L. M., Campbell P. J., East C., Fourouclas N., Swanton S., Vassiliou G. S., Bench A. J., Boyd E. M., Curtin N., Scott M. A., Erber W. N., Green A. R. (2005) Lancet 365, 1054–1061 [DOI] [PubMed] [Google Scholar]
- 6.James C., Ugo V., Le Couédic J. P., Staerk J., Delhommeau F., Lacout C., Garçon L., Raslova H., Berger R., Bennaceur-Griscelli A., Villeval J. L., Constantinescu S. N., Casadevall N., Vainchenker W. (2005) Nature 434, 1144–1148 [DOI] [PubMed] [Google Scholar]
- 7.Jones A. V., Kreil S., Zoi K., Waghorn K., Curtis C., Zhang L., Score J., Seear R., Chase A. J., Grand F. H., White H., Zoi C., Loukopoulos D., Terpos E., Vervessou E. C., Schultheis B., Emig M., Ernst T., Lengfelder E., Hehlmann R., Hochhaus A., Oscier D., Silver R. T., Reiter A., Cross N. C. (2005) Blood 106, 2162–2168 [DOI] [PubMed] [Google Scholar]
- 8.Kralovics R., Passamonti F., Buser A. S., Teo S. S., Tiedt R., Passweg J. R., Tichelli A., Cazzola M., Skoda R. C. (2005) N. Engl. J. Med. 352, 1779–1790 [DOI] [PubMed] [Google Scholar]
- 9.Levine R. L., Wadleigh M., Cools J., Ebert B. L., Wernig G., Huntly B. J., Boggon T. J., Wlodarska I., Clark J. J., Moore S., Adelsperger J., Koo S., Lee J. C., Gabriel S., Mercher T., D'Andrea A., Fröhling S., Döhner K., Marynen P., Vandenberghe P., Mesa R. A., Tefferi A., Griffin J. D., Eck M. J., Sellers W. R., Meyerson M., Golub T. R., Lee S. J., Gilliland D. G. (2005) Cancer Cell 7, 387–397 [DOI] [PubMed] [Google Scholar]
- 10.Zhao R., Xing S., Li Z., Fu X., Li Q., Krantz S. B., Zhao Z. J. (2005) J. Biol. Chem. 280, 22788–22792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haan C., Kreis S., Margue C., Behrmann I. (2006) Biochem. Pharmacol. 72, 1538–1546 [DOI] [PubMed] [Google Scholar]
- 12.Ihle J. N. (1995) Nature 377, 591–594 [DOI] [PubMed] [Google Scholar]
- 13.Rane S. G., Reddy E. P. (2000) Oncogene 19, 5662–5679 [DOI] [PubMed] [Google Scholar]
- 14.Yamaoka K., Saharinen P., Pesu M., Holt V. E., 3rd, Silvennoinen O., O'Shea J. J. (2004) Genome Biol. 5, 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baker S. J., Rane S. G., Reddy E. P. (2007) Oncogene 26, 6724–6737 [DOI] [PubMed] [Google Scholar]
- 16.Lindauer K., Loerting T., Liedl K. R., Kroemer R. T. (2001) Protein Eng. 14, 27–37 [DOI] [PubMed] [Google Scholar]
- 17.Luo H., Rose P., Barber D., Hanratty W. P., Lee S., Roberts T. M., D'Andrea A. D., Dearolf C. R. (1997) Mol. Cell. Biol. 17, 1562–1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malinge S., Ben-Abdelali R., Settegrana C., Radford-Weiss I., Debre M., Beldjord K., Macintyre E. A., Villeval J. L., Vainchenker W., Berger R., Bernard O. A., Delabesse E., Penard-Lacronique V. (2007) Blood 109, 2202–2204 [DOI] [PubMed] [Google Scholar]
- 19.Saharinen P., Silvennoinen O. (2002) J. Biol. Chem. 277, 47954–47963 [DOI] [PubMed] [Google Scholar]
- 20.Saharinen P., Takaluoma K., Silvennoinen O. (2000) Mol. Cell. Biol. 20, 3387–3395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee T. S., Ma W., Zhang X., Giles F., Kantarjian H., Albitar M. (2009) Cancer 115, 1692–1700 [DOI] [PubMed] [Google Scholar]
- 22.Lee T. S., Ma W., Zhang X., Kantarjian H., Albitar M. (2009) BMC Struct. Biol. 9, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bumm T. G., Elsea C., Corbin A. S., Loriaux M., Sherbenou D., Wood L., Deininger J., Silver R. T., Druker B. J., Deininger M. W. (2006) Cancer Res. 66, 11156–11165 [DOI] [PubMed] [Google Scholar]
- 24.Lacout C., Pisani D. F., Tulliez M., Gachelin F. M., Vainchenker W., Villeval J. L. (2006) Blood 108, 1652–1660 [DOI] [PubMed] [Google Scholar]
- 25.Tiedt R., Hao-Shen H., Sobas M. A., Looser R., Dirnhofer S., Schwaller J., Skoda R. C. (2008) Blood 111, 3931–3940 [DOI] [PubMed] [Google Scholar]
- 26.Wernig G., Mercher T., Okabe R., Levine R. L., Lee B. H., Gilliland D. G. (2006) Blood 107, 4274–4281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zaleskas V. M., Krause D. S., Lazarides K., Patel N., Hu Y., Li S., Van Etten R. A. (2006) PLoS ONE 1, e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu X., Levine R., Tong W., Wernig G., Pikman Y., Zarnegar S., Gilliland D. G., Lodish H. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 18962–18967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Staerk J., Kallin A., Demoulin J. B., Vainchenker W., Constantinescu S. N. (2005) J. Biol. Chem. 280, 41893–41899 [DOI] [PubMed] [Google Scholar]
- 30.Lu X., Huang L. J., Lodish H. F. (2008) J. Biol. Chem. 283, 5258–5266 [DOI] [PubMed] [Google Scholar]
- 31.Wernig G., Gonneville J. R., Crowley B. J., Rodrigues M. S., Reddy M. M., Hudon H. E., Walz C., Reiter A., Podar K., Royer Y., Constantinescu S. N., Tomasson M. H., Griffin J. D., Gilliland D. G., Sattler M. (2008) Blood 111, 3751–3759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pradhan A., Lambert Q. T., Reuther G. W. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 18502–18507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reuther G. W. (2008) Cell Cycle 7, 714–719 [DOI] [PubMed] [Google Scholar]
- 34.Morgenstern J. P., Land H. (1990) Nucleic Acids Res. 18, 3587–3596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hayashida K., Kitamura T., Gorman D. M., Arai K., Yokota T., Miyajima A. (1990) Proc. Natl. Acad. Sci. U.S.A. 87, 9655–9659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chatti K., Farrar W. L., Duhé R. J. (2004) Biochemistry 43, 4272–4283 [DOI] [PubMed] [Google Scholar]
- 37.Palacios R., Steinmetz M. (1985) Cell 41, 727–734 [DOI] [PubMed] [Google Scholar]
- 38.Thompson J. E., Cubbon R. M., Cummings R. T., Wicker L. S., Frankshun R., Cunningham B. R., Cameron P. M., Meinke P. T., Liverton N., Weng Y., DeMartino J. A. (2002) Bioorg. Med. Chem. Lett. 12, 1219–1223 [DOI] [PubMed] [Google Scholar]
- 39.Feng J., Witthuhn B. A., Matsuda T., Kohlhuber F., Kerr I. M., Ihle J. N. (1997) Mol. Cell. Biol. 17, 2497–2501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bercovich D., Ganmore I., Scott L. M., Wainreb G., Birger Y., Elimelech A., Shochat C., Cazzaniga G., Biondi A., Basso G., Cario G., Schrappe M., Stanulla M., Strehl S., Haas O. A., Mann G., Binder V., Borkhardt A., Kempski H., Trka J., Bielorei B., Avigad S., Stark B., Smith O., Dastugue N., Bourquin J. P., Tal N. B., Green A. R., Izraeli S. (2008) Lancet 372, 1484–1492 [DOI] [PubMed] [Google Scholar]
- 41.Mullighan C. G., Zhang J., Harvey R. C., Collins-Underwood J. R., Schulman B. A., Phillips L. A., Tasian S. K., Loh M. L., Su X., Liu W., Devidas M., Atlas S. R., Chen I. M., Clifford R. J., Gerhard D. S., Carroll W. L., Reaman G. H., Smith M., Downing J. R., Hunger S. P., Willman C. L. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 9414–9418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hashimoto Y., Kurita M., Aiso S., Nishimoto I., Matsuoka M. (2009) Mol. Biol. Cell 20, 2864–2873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takeda A., Hamano S., Yamanaka A., Hanada T., Ishibashi T., Mak T. W., Yoshimura A., Yoshida H. (2003) J. Immunol. 170, 4886–4890 [DOI] [PubMed] [Google Scholar]
- 44.Hunter C. A. (2005) Nat. Rev. Immunol. 5, 521–531 [DOI] [PubMed] [Google Scholar]
- 45.Geijsen N., Koenderman L., Coffer P. J. (2001) Cytokine Growth Factor Rev. 12, 19–25 [DOI] [PubMed] [Google Scholar]
- 46.Klingmüller U., Bergelson S., Hsiao J. G., Lodish H. F. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 8324–8328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Okuda K., Smith L., Griffin J. D., Foster R. (1997) Blood 90, 4759–4766 [PubMed] [Google Scholar]
- 48.Sakamaki K., Miyajima I., Kitamura T., Miyajima A. (1992) EMBO J. 11, 3541–3549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fisher G. M., Iqball S., Knight S. C. (1999) Cytokine 11, 111–117 [DOI] [PubMed] [Google Scholar]
- 50.Grohmann U., Belladonna M. L., Vacca C., Bianchi R., Fallarino F., Orabona C., Fioretti M. C., Puccetti P. (2001) J. Immunol. 167, 221–227 [DOI] [PubMed] [Google Scholar]
- 51.Huang H. M., Lin Y. L., Chen C. H., Chang T. W. (2005) J. Cell. Biochem. 96, 361–375 [DOI] [PubMed] [Google Scholar]
- 52.Quelle F. W., Sato N., Witthuhn B. A., Inhorn R. C., Eder M., Miyajima A., Griffin J. D., Ihle J. N. (1994) Mol. Cell. Biol. 14, 4335–4341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Funakoshi-Tago M., Tago K., Abe M., Sonoda Y., Kasahara T. (2010) J. Biol. Chem. 285, 5296–5307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carr P. D., Gustin S. E., Church A. P., Murphy J. M., Ford S. C., Mann D. A., Woltring D. M., Walker I., Ollis D. L., Young I. G. (2001) Cell 104, 291–300 [DOI] [PubMed] [Google Scholar]
- 55.Murphy J. M., Young I. G. (2006) Vitam. Horm. 74, 1–30 [DOI] [PubMed] [Google Scholar]
- 56.Muto A., Watanabe S., Miyajima A., Yokota T., Arai K. (1996) J. Exp. Med. 183, 1911–1916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martinez-Moczygemba M., Huston D. P. (2003) J. Allergy Clin. Immunol. 112, 653–665; quiz 666 [DOI] [PubMed] [Google Scholar]
- 58.Hornakova T., Staerk J., Royer Y., Flex E., Tartaglia M., Constantinescu S. N., Knoops L., Renauld J. C. (2009) J. Biol. Chem. 284, 6773–6781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hertzberg L., Vendramini E., Ganmore I., Cazzaniga G., Schmitz M., Chalker J., Shiloh R., Iacobucci I., Shochat C., Zeligson S., Cario G., Stanulla M., Strehl S., Russell L. J., Harrison C. J., Bornhauser B., Yoda A., Rechavi G., Bercovich D., Borkhardt A., Kempski H., te Kronnie G. T., Bourquin J. P., Domany E., Izraeli S. (2010) Blood 115, 1006–1017 [DOI] [PubMed] [Google Scholar]
- 60.Mullighan C. G., Collins-Underwood J. R., Phillips L. A., Loudin M. G., Liu W., Zhang J., Ma J., Coustan-Smith E., Harvey R. C., Willman C. L., Mikhail F. M., Meyer J., Carroll A. J., Williams R. T., Cheng J., Heerema N. A., Basso G., Pession A., Pui C. H., Raimondi S. C., Hunger S. P., Downing J. R., Carroll W. L., Rabin K. R. (2009) Nat. Genet. 41, 1243–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Russell L. J., Capasso M., Vater I., Akasaka T., Bernard O. A., Calasanz M. J., Chandrasekaran T., Chapiro E., Gesk S., Griffiths M., Guttery D. S., Haferlach C., Harder L., Heidenreich O., Irving J., Kearney L., Nguyen-Khac F., Machado L., Minto L., Majid A., Moorman A. V., Morrison H., Rand V., Strefford J. C., Schwab C., Tönnies H., Dyer M. J., Siebert R., Harrison C. J. (2009) Blood 114, 2688–2698 [DOI] [PubMed] [Google Scholar]
- 62.Yoda A., Yoda Y., Chiaretti S., Bar-Natan M., Mani K., Rodig S. J., West N., Xiao Y., Brown J. R., Mitsiades C., Sattler M., Kutok J. L., DeAngelo D. J., Wadleigh M., Piciocchi A., Dal Cin P., Bradner J. E., Griffin J. D., Anderson K. C., Stone R. M., Ritz J., Foa R., Aster J. C., Frank D. A., Weinstock D. M. (0000) Proc. Natl. Acad. Sci. U.S.A. 107, 252–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gakovic M., Ragimbeau J., Francois V., Constantinescu S. N., Pellegrini S. (2008) J. Biol. Chem. 283, 18522–18529 [DOI] [PubMed] [Google Scholar]
- 64.Seita J., Asakawa M., Ooehara J., Takayanagi S., Morita Y., Watanabe N., Fujita K., Kudo M., Mizuguchi J., Ema H., Nakauchi H., Yoshimoto T. (2008) Blood 111, 1903–1912 [DOI] [PubMed] [Google Scholar]
- 65.Pardanani A. D., Levine R. L., Lasho T., Pikman Y., Mesa R. A., Wadleigh M., Steensma D. P., Elliott M. A., Wolanskyj A. P., Hogan W. J., McClure R. F., Litzow M. R., Gilliland D. G., Tefferi A. (2006) Blood 108, 3472–3476 [DOI] [PubMed] [Google Scholar]
- 66.Pikman Y., Lee B. H., Mercher T., McDowell E., Ebert B. L., Gozo M., Cuker A., Wernig G., Moore S., Galinsky I., DeAngelo D. J., Clark J. J., Lee S. J., Golub T. R., Wadleigh M., Gilliland D. G., Levine R. L. (2006) PLoS Med. 3, e270. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.