Abstract
In addition to its main functions of electron transfer and proton translocation, the cytochrome bc1 complex (bc1) also catalyzes superoxide anion (O2˙̄) generation upon oxidation of ubiquinol in the presence of molecular oxygen. The reaction mechanism of superoxide generation by bc1 remains elusive. The maximum O2˙̄ generation activity is observed when the complex is inhibited by antimycin A or inactivated by heat treatment or proteinase K digestion. The fact that the cytochrome bc1 complex with less structural integrity has higher O2˙̄-generating activity encouraged us to speculate that O2˙̄ is generated inside the complex, perhaps in the hydrophobic environment of the QP pocket through bifurcated oxidation of ubiquinol by transferring its two electrons to a high potential electron acceptor, iron-sulfur cluster, and a low potential heme bL or molecular oxygen. If this speculation is correct, then one should see more O2˙̄ generation upon oxidation of ubiquinol by a high potential oxidant, such as cytochrome c or ferricyanide, in the presence of phospholipid vesicles or detergent micelles than in the hydrophilic conditions, and this is indeed the case. The protein subunits, at least those surrounding the QP pocket, may play a role either in preventing the release of O2˙̄ from its production site to aqueous environments or in preventing O2 from getting access to the hydrophobic QP pocket and might not directly participate in superoxide production.
Keywords: Bioenergetics, Electron Transfer, Membrane Proteins, Superoxide Ion, Ubiquinone
Introduction
It has long been recognized that during mitochondrial respiration, there is a continuous release of electrons from the electron transfer chain to react with molecular oxygen to form a superoxide anion (O2˙̄) (1–3). The generated O2˙̄ is subsequently dismutated to H2O2 spontaneously or by the action of superoxide dismutases (4). Isolated mitochondria in state 4 generate 0.6–1.0 nmol of H2O2/min/mg of protein, accounting for about 2% O2 uptake under physiological conditions (5). Production of O2˙̄ during mitochondrial respiration is closely related to mitochondrial coupling efficiency. More O2˙̄ is produced when the membrane potential of mitochondria is high (6, 7). In the past, most information concerning mitochondrial O2˙̄ generation sites was obtained from studies using intact heart mitochondria with selected electron transfer inhibitors by measuring the H2O2 concentration in the suspending medium (8, 9).
Two segments of the respiratory chain have been demonstrated to be responsible for the generation of O2˙̄ from oxygen. One is located at the NADH-Q3 oxidoreductase (complex I), and the other is at the cytochrome bc1 complex (ubiquinol-cytochrome c oxidoreductase). Production of O2˙̄ by complex I is either via auto-oxidation of the flavine radical in NADH dehydrogenase (10) or via a bound ubisemiquinone radical (11) or the center N-2 (12) of the complex. It was recently suggested (13) that the reversed electron transport through complex I produced more O2˙̄ than the forward transport. Two redox components of the bc1 complex, ubisemiquinone at the QP site (8) and the reduced cytochrome b566 (9, 14), have been implicated as electron donors for molecular oxygen to generate O2˙̄. The production of O2˙̄ by the bc1 complex is greatly enhanced when the complex is inhibited by antimycin (14–16).
In continuing our study of the structural and functional relationship of the cytochrome bc1 complex, it is important to understand the reaction mechanism of superoxide generation in this complex. The fact that antimycin inhibits the electron transfer activity of the cytochrome bc1 complex and stimulates the O2˙̄-generating activity, together with the observation that both activities have a similar activation energy, led investigators to believe that both activities share, at least, a common intermediate (17). According to the Q-cycle mechanism (18–20), during the catalytic reaction of the cytochrome bc1 complex, Q-H2 undergoes bifurcated oxidation by transferring its two electrons, sequentially or simultaneously (concerted), to the iron-sulfur cluster (ISC) of the iron-sulfur protein (ISP) subunit and heme bL of the cytochrome b subunit. In the sequential mechanism (21–23), ubiquinol transfers its first electron to the ISC to become low potential ubisemiquinone that contains a free electron and reduces heme bL instantly. The lack of a functional ubisemiquinone at the QP site (21, 24, 25) undermines this mechanism substantially, although some radicals have been reported under abnormal conditions (26–28). Recently, more compelling evidence against the existence of the semiquinone radical at the QP site has been reported (29). No such radical in a heme bL knock-out mutant complex is detected upon reduction by quinol. Because heme bL is the designated electron acceptor of the semiquinone radical at the QP site in the sequential Q-cycle mechanism, one would expect to see the accumulation of this intermediate when the acceptor is not available, but this is not the case. In the concerted mechanism (29–31), no semiubiquinone is formed, and two electrons of Q-H2 are transferred simultaneously to ISC and heme bL. This bifurcated electron transfer reaction provides a basis for the high efficiency of the bc1 complex and is done inside the cytochrome b subunit buried in the membrane bilayer. It is thus expected that any compromise in the structural integrity of cytochrome b should lead to a decrease in the electron transfer efficiency and to an increase in the production of superoxide. The observation that mutants lacking heme bL or heme bH, respectively, show little electron transfer activity but have high superoxide-generating activity (25) is consistent with the idea that the structural integrity of cytochrome b is required for normal electron transfer activity but not for superoxide generation.
Herein we report a systematic comparison of the electron transfer and O2˙̄-generating activities in various cytochrome bc1 complexes, such as the complexes with varying numbers of supernumerary subunits,4 or with different extents of heat inactivation or proteinase K digestion to see whether or not the intact protein components of the complex are required for O2˙̄-generating activity. We also determined the effect of ubiquinol, phospholipid vesicles, detergent micelles, cytochrome c, and ferricyanide concentration on O2˙̄ generation. Based on the results obtained, we establish that an electron donor (ubiquinol, a high potential electron acceptor), ISC, cytochrome c, or ferricyanide and a hydrophobic environment are required for O2˙̄ production. We formulated a working hypothesis for the reaction mechanism of O2˙̄ production in the bc1 complex.
EXPERIMENTAL PROCEDURES
Materials
Cytochrome c (horse heart, type III), acetylated cytochrome c, and superoxide dismutase were purchased from Sigma. Proteinase K was purchased from Invitrogen. N-Dodecyl-d-maltopyranoside (DM) and N-octyl-d-gluocopyranoside (OG) were obtained from Anatrace. N,N-Dimethyldodecylamine N-oxide (LDAO) was obtained from Sigma. Nickel nitrilotriacetic acid gel and a QIAprep spin miniprep kit were obtained from Qiagen. 2-Methyl-6-(4-methoxyphenyl)-3,7-dihydroimidazol[1,2-α] pyrazin-3-one, hydrochloride (MCLA) was obtained from Molecular Probes, Inc. 2,3-Dimethoxy-5-methyl-6-(10-bromodecyl)-1,4-benzoquinol(Q0C10BrH2) was prepared as reported previously (33). All other chemicals were of the highest purity commercially available.
Enzyme Preparations and Activity Assays
Chromatophores, intracytoplasmic membrane, and the His6-tagged cytochrome bc1 complexes, wild type (34) and mutants (25, 35), were prepared as reported previously. Bovine heart mitochondrial cytochrome bc1 complex was prepared according to the method developed in our laboratory (36, 37).
To assay the cytochrome bc1 complex activity, purified complexes were diluted with 50 mm Tris-Cl, pH 8.0, containing 200 mm NaCl and 0.01% DM to a final concentration of cytochrome c1 of 1 μm. Appropriate amounts of the diluted samples were added to 1 ml of assay mixture containing 100 mm Na+/K+ phosphate buffer, pH 7.4, 300 μm EDTA, 100 μm cytochrome c, and 25 μm Q0C10BrH2. Because Q0C10BrH2 is easily auto-oxidized at neutral or higher pH, the stock solution is kept in 95% ethanol containing 1 mm HCl and diluted in the buffer before use. Activities were determined by measuring the reduction of cytochrome c (the increase of absorbance at 550 nm) in a Shimadzu UV 2101 PC spectrophotometer at 23 °C, using a millimolar extinction coefficient of 18.5 for the calculation. The non-enzymatic oxidation of Q0C10BrH2, determined under the same conditions in the absence of the enzyme, was subtracted from the assay.
Digestion of the Cytochrome bc1 Complex by Proteinase K
A stock solution of proteinase K, 3%, was made in 10 mm Tris-HCl, pH 7.5, containing 20 mm CaCl and 50% glycerol. Two μl of proteinase K solution was added into 200 μl of cytochrome bc1 complex (200 μm cyt b) in 50 mm Tris-HCl, pH 8.0, containing 200 mm NaCl and 0.01% DM. The mixture was incubated at room temperature. The electron transfer and O2˙̄-generating activities were measured during the course of incubation until all the electron transfer activity was diminished. The digested bc1 was then subjected to SDS-PAGE to confirm that all the subunits were digested.
Preparation of Phospholipid Vesicles
Phospholipid vesicles were prepared by the cholate dialysis method (38). Asolectin was dissolved in chloroform and dried as a thin film against the tube by flushing with nitrogen gas while the tube was rotating. The phospholipid was then suspended in 50 mm potassium/sodium phosphate buffer, pH 7.4, containing 1% sodium cholate. The mixture was subjected to sonification intermittently for 30 min until the solution become clear and then dialyzed against the same buffer overnight, with three changes of buffer.
Determination of Superoxide Production
Superoxide production was determined by measuring the chemiluminescence of MCLA-O2˙̄ adduct (39) in an Applied Photophysics stopped-flow reaction analyzer SX.18MV-R (Leatherhead, UK) by leaving the excitation light off and registering light emission (40, 41). Reactions were carried out at 23 °C by mixing 1:1 of solutions A and B. For the determination of O2˙̄ production by the native, heat-inactivated, or proteinase K-digested cytochrome bc1 complexes, Solution A contains 100 mm Na+/K+ phosphate buffer, pH 7.4, 1 mm EDTA, 1 mm NaN3, 0.1% bovine serum albumin, 0.01% DM, and 5.0 μm cytochrome bc1. Solution B contains 125 μm Q0C10BrH2 and 4 μm MCLA in the same buffer. Once the reaction started, the produced fluorescence, in voltage, was consecutively monitored for 2 s. One volt from the Applied Photophysics stopped-flow reaction analyzer SX.18MV-R equals the chemiluminescence (maximum peak height of light intensity) generated by 0.5 unit of xanthine oxidase using 100 μm hypoxanthine as a substrate.
Because MCLA is a neutral, relatively non-polar molecule, it is possible that the efficiency of O2˙̄ production detected by chemiluminescence may be affected by the presence of different detergent micelles, thus complicating the determination of the micelle effect of different detergents on superoxide production. To avoid this possible complication, superoxide productions in the presence of different detergent, micelles were compared by measuring the reduction of acetylated cytochrome c (42) because different detergent micelles do not show significant effect on superoxide production by xanthine oxidase and hypoxanthine determined by the acetylated cytochrome c method. Reduction of acetylated cytochrome c was followed by the increase of absorption at 550 nm in the same stopped-flow reaction analyzer in the normal way. A millimolar extinction coefficient of 18.5 was used for the concentration calculation. Solution A contains 100 mm Na+/K+ phosphate buffer, pH 8.0, 10 μm acetylated cytochrome c, and various amounts of detergents. Solution B contains 250 μm Q0C10BrH2 in 0.5 mm Na+/K+ phosphate buffer, pH 4, in the presence or absence of 300 units/ml superoxide dismutase.
RESULTS AND DISCUSSION
The Inverse Relationship between Superoxide-generating and Electron Transfer Activities in the Cytochrome bc1 Complex
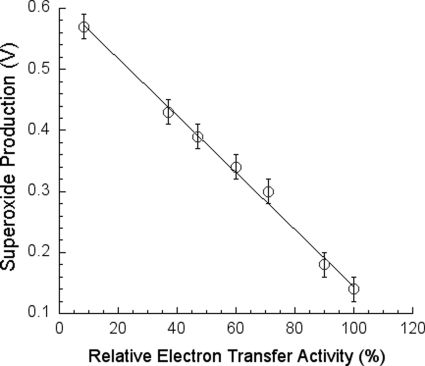
Table 1 summarizes the electron transfer and O2˙̄-generating activities in various bc1 complex preparations. The bovine heart mitochondrial complex has 11 protein subunits. This complex has the highest electron transfer activity and lowest superoxide-generating activity among the complexes tested. The Rhodobacter sphaeroides complex, which contains four protein subunits (three core subunits and one supernumerary subunit), has only about one-twelfth of the electron transfer activity of the bovine complex but has about six times the O2˙̄-generating activity of the bovine enzyme. When the only supernumerary subunit (subunit IV) is deleted from the R. sphaeroides wild-type complex, the resulting three-subunit core complex (RsΔIV) has only a fraction of the electron transfer activity of the wild-type complex but has about four times the O2˙̄-generating activity. When the three-subunit core complex is reconstituted with subunit IV, the electron transfer activity increases, and the O2˙̄-generating activity decreases to the same level as those in the wild-type, four-subunit complex.
TABLE 1.
Comparison of electron transfer and superoxide-generating activities of various cytochrome bc1 complexes
Rsbc1, wild-type, 4 subunit R. sphaeroides bc1; RsΔIV, Rsbc1 lacking subunit IV; Cyt b(H198N), Rsbc1 lacking heme bL; Cyt b(H111N), Rsbc1 lacking heme bH; XO, xanthine oxidase. Each data point represents an average of four experiments.
| Preparation | Activities |
||
|---|---|---|---|
| Electron transfer (μmol of cytochrome c reduced/min/nmol of cytochrome c) | O2˙̄ generation (milliunits of XO/nmol of cytochrome c) |
||
| −Antimycin | +Antimycin | ||
| Mitochondrial bc1 | 43.0 ± 0.3 | 15 ± 2 | 19 ± 2 |
| Rsbc1 | 3.5 ± 0.2 | 91 ± 2 | 300 ± 2 |
| RsΔIV | 0.8 ± 0.2 | 325 ± 2 | 546 ± 5 |
| Cyt b(H198N) | 0.4 ± 0.2 | 510 ± 5 | 510 ± 5 |
| Cyt b(H111N) | 0.3 ± 0.2 | 500 ± 4 | 510 ± 6 |
The differential scanning calorimetric studies indicate that the order of thermal stability among these complexes is: beef > wild-type R. sphaeroides complex = reconstituted complex > RsΔIV complex (data not shown). Therefore, the electron transfer activity of the bc1 complex is in direct proportion to the structural integrity of the complex, whereas the O2˙̄-generating activity has an inverse relationship with the structural integrity of the complex. In other words, the electron transfer activity is inversely proportional to the O2˙̄-generating activity in the bc1 complex.
The finding that the cytochrome bc1 complex with less structural integrity has higher O2˙̄-generating activity encouraged us to speculate that O2˙̄ is generated inside the complex, perhaps in the hydrophobic environment of the QP pocket, and that the protein subunits, at least those surrounding the QP pocket, may play a role either in preventing the release of O2˙̄ from its production site to aqueous environments or in preventing O2 from getting access to the hydrophobic QP pocket.
The Superoxide Anion-generating Activity in the Heat-inactivated Cytochrome bc1 Complex
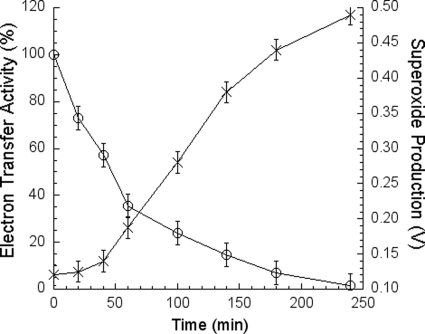
If the above speculation is correct, then one should see an increase in O2˙̄ generation in the complex with denatured protein subunits. To test this speculation, the wild-type R. sphaeroides bc1 complex was incubated at 37 °C to inactivate the complex, and the electron transfer and O2˙̄ generation activities were measured during the course of incubation. As the incubation time increases, the electron transfer activity decreases, whereas the O2˙̄-generating activity increases. Fig. 1 shows the relationship between the electron transfer activity and the O2˙̄-generating activity during the heat inactivation process. Maximum O2˙̄-generating activity is obtained when more than 90% of the electron transfer activity is abolished. This result indicates that the production of O2˙̄ during quinol oxidation by bc1 complex does not require the presence of an intact complex. The impairment of the structural integrity of the complex by the heat denaturalization of the protein subunits leads to the increase in the accessibility of molecular oxygen to the hydrophobic environment of the QP pocket to generate O2˙̄ and to facilitate the release of the produced O2˙̄ to the aqueous medium.
FIGURE 1.
The relationship between the electron transfer activity and superoxide generation during temperature inactivation of cytochrome bc1 complex. 200 μl of cytochrome bc1 complex (200 μm cyt b) in 50 mm Tris-Cl, pH 8.0, containing 200 mm NaCl and 0.01% DM was incubated at 37 °C. At different time intervals, samples were withdrawn and determined for superoxide production (shown as voltage) and electron transfer activities (shown as relative electron transfer activity of the untreated complex; 100% activity is equal to 3.5 μmol of cytochrome c reduced/min/nmol of bc1 complex). Electron transfer activity and the superoxide production were measured as described under “Experimental Procedures.” Solution A contains 100 mm Na+/K+ phosphate buffer, pH 7.4, 1 mm EDTA, 1 mm NaN3, 0.1% bovine serum albumin, 0.01% DM, and 5.0 μm incubated cytochrome bc1. Solution B was the same as Solution A with bc1 complex being replaced with 125 μm Q0C10BrH2 and 4 μm MCLA. Each data point represents an average of four experiments. Error bars indicate S.D.
The notion that an intact protein subunit structure is not required for O2˙̄-generating activity of the bc1 complex is further supported by the observation that mutants H198N and H111N, which lack heme bL and heme bH, respectively, have very little electron transfer activity but show O2˙̄-generating activity equal to that of the antimycin-treated wild-type complex (25) (Table 1). The increased rates of O2˙̄ formation when in the heme bL knock-out complex is strong evidence that although the heme bL may react with oxygen when it is present, a O2˙̄ can also be formed by a route other than by reaction with the heme bL. The loss of either heme bL or heme bH would be expected to have a strong impact on the overall structural integrity of the bc1 complex, leading to the distortion of the QP pocket environment and presumably increasing the accessibility of O2 to the site. It is as expected that the incubation of these two heme b-lacking mutant complexes at 37 °C to denature the protein subunits does not further increase the O2˙̄-generating activity because the structural integrity of these two complexes has already been deteriorated by mutation.
The Superoxide Anion-generating Activity in Proteinase K-digested Complex
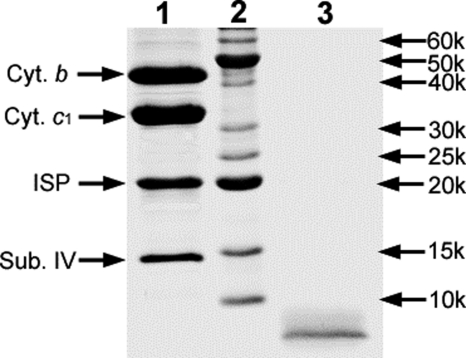
To further confirm that superoxide-generating activity is independent of the presence of an intact protein structure, the bc1 complex was subjected to proteinase K digestion and electron transfer, and superoxide-generating activities were measured during the course of digestion. As shown in Fig. 2, the electron transfer activity diminishes, whereas the O2˙̄-generating activity increases as the digestion time increases. Maximum superoxide-generating activity is observed when electron transfer activity is completely abolished. SDS-PAGE analysis of the proteinase K-digested complex reveals no intact subunits of cytochromes b, c1, or ISP (Fig. 3). The largest peptide band presence in the digested complex has an apparent molecular mass of less than 7 kDa. These results further support our suggestion that the intact protein components of the complex or the intact complex play no direct role in O2˙̄ generation. A Western blotting experiment using anti-ISP indicated that at least a part of this peptide band is derived from ISP. In other words, the ISC detected by EPR is housed in this peptide.
FIGURE 2.
Activity tracings of the electron transfer and O2˙̄ generation during the course of proteinase K digestion of the complex. 200 μl of cytochrome bc1 complex (200 μm cyt b) in 50 mm Tris-Cl, pH 8.0, containing 200 mm NaCl and 0.01% DM was incubated with 60 μg of proteinase K at 37 °C. At the indicated time intervals, samples were withdrawn and determined for superoxide production (Xs) and electron transfer (open circles) activities. Each data point represents an average of four experiments. Error bars indicate S.D.
FIGURE 3.
SDS-PAGE of the cytochrome bc1 complex and its proteinase K-digested products. Lane 1, intact wild-type cytochrome bc1. Lane 2, standard polypeptides. Lane 3, proteinase K-treated wild-type complex. Sub. IV, subunit IV.
If this notion is correct, then what remaining elements in the proteinase K-digested complex contributed to superoxide production from Q-H2? A hydrophobic environment and a high potential electron acceptor ISC in the digested system could contribute to the superoxide formation in the presence of molecular oxygen. The presence of intact ISC in the proteinase K-digested complex was confirmed by the presence of EPR signals of ISC in the digested complex (data not shown). Thus, it is possible that ISC serves as a high potential electron acceptor and oxygen serves as a low potential electron acceptor during bifurcated oxidation of Q-H2 in a hydrophobic environment of the QP pocket to produce superoxide. Because there are no free metal ions in the purified cytochrome bc1 complex and the iron in the heme and iron-sulfur cluster is not released during heat treatment or proteinase digestion, as indicated by absorption and EPR spectra of the tested samples, the possibility that the observed O2˙̄ production is due to free iron can be eliminated.
Generation of Superoxide Anion upon Oxidation of Ubiquinol by Cytochrome c or Ferricyanide in the Presence of Phospholipid Vesicles
Although the addition of ferricytochrome c to the proteinase K-digested complex can increase its superoxide production, oxidation of ubiquinol by ferricytochrome c in the aqueous solution at neutral pH is a very slow reaction with a reduction rate constant K1 of 0.24/s, and little O2˙̄ formation is detected, suggesting that a hydrophobic environment provided by the digested complex is required for superoxide production. Similar results were obtained when potassium ferricyanide was used to replace ferricytochrome c.
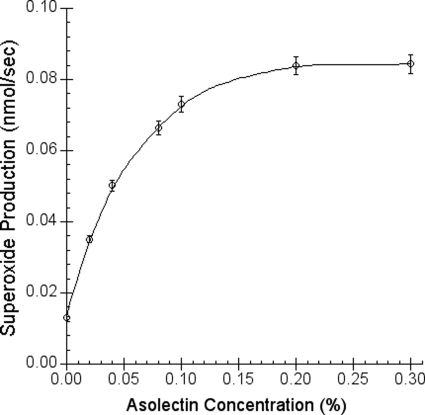
To confirm that a hydrophobic environment is needed for O2˙̄ production, varying amounts of phospholipid vesicles were added to the mixture containing constant amounts of ubiquinol and cytochrome c, and the superoxide anion production was measured by the reduction of acetylated cytochrome c method. In the presence of phospholipid vesicles, the rate of the formation of O2˙̄ is proportional to the amount of vesicles added up to 0.3% (Fig. 4). This result clearly indicates that the formation of O2˙̄ takes place in hydrophobic environments.
FIGURE 4.
Phospholipid vesicle concentration-dependent superoxide formation under constant amounts of cytochrome c and ubiquinol. The superoxide generation was measured by the reduction of superoxide dismutase-sensitive acetylated cytochrome c reduction as described under “Experimental Procedures.” Solution A contains 100 mm Na+/K+ phosphate buffer, pH 7.4, 10.0 μm acetylated cytochrome c, and different concentrations of asolectin vesicle. Solution B contains 0.5 mm Na+/K+ phosphate buffer, pH 4, 250 μm Q-H2 in the presence or absence of 300 units/ml superoxide dismutase. Each data point represents an average of four experiments. Error bars indicate S.D.
Detergents Facilitate Superoxide Generation by QH2 and Cytochrome c
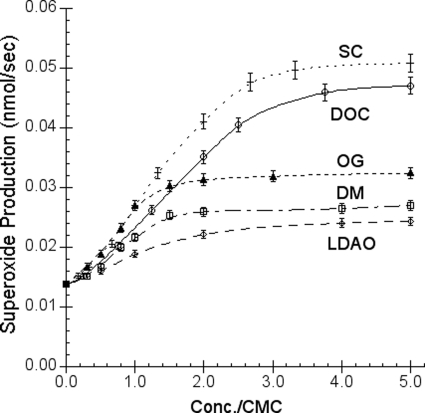
If the hydrophobic environment in the bilayer of phospholipid vesicles can facilitate O2˙̄ generation, one would expect that a micelle solution of detergent should do the same. To test the effects of detergents on the O2˙̄ production by Q-H2 and cytochrome c, various detergents, non-ionic (OG, DM), anionic (sodium cholate (SC) and deoxycholate (DOC)), and cationic (LDAO), were used to substitute phospholipid vesicles. The detergent micelle solution (or phospholipid vesicles), which facilitates superoxide anion production, does not prevent dismutation of the superoxide anion generated by hypoxanthine/xanthine oxidase. Therefore, the superoxide generation-facilitating activity observed is real and not due to the decrease of dismutation. Fig. 5 shows the effect of detergent concentration on the superoxide production during quinol oxidation by cytochrome c. As expected, little O2˙̄ generation is observed when the concentration of detergent used is below its critical micelle concentration because no hydrophobic environment is available. When the concentration of detergent used is higher than its critical micelle concentration, the O2˙̄ production rate increases as the detergent micelle concentration in the system increases. Interestingly, SC or DOC is much more effective in promoting superoxide generation than OG, DM, or LDAO. The critical micelle concentrations for LDAO, OG, DM, DOC, and SC are 1, 25, 0.15, 1.33, and 3 mm (32), respectively, which do not appear to correlate with their abilities to facilitate O2˙̄ generation. However, it is very apparent that detergent with a negatively charged head group such as SC or DOC can provide a better environment for O2˙̄ generation than neutral (OG or DM) or cationic (LDAO) detergents can. There are two simple explanations for the negatively charged detergents to have a better efficiency in promoting O2˙̄ production during the oxidation of quinol. First, the negative charges on the micelle surface may facilitate deprotonation of the 1-hydroxyl group of quinol. Second, the negatively charged detergent micelle surface may attract the positively charged ferricytochrome c better than the neutral or positively charged micelles. Thus, the high potential electron acceptor, ferricytochrome c, has a better accessibility to quinol, which is located near the surface of the detergent micelles. When potassium ferricyanide is used as the high potential electron acceptor, the effect on O2˙̄ generation by the difference in the charge of the micelle surface is less apparent.
FIGURE 5.
Effect of detergents on superoxide generation under constant amounts of cytochrome c and ubiquinol. The superoxide generation was measured by the reduction of superoxide dismutase-sensitive acetylated cytochrome c reduction as described under “Experimental Procedures.” Solution A contains 100 mm Na+/K+ phosphate buffer, pH 8.0, 10 μm acetylated cytochrome c, and different concentrations of detergents (LDAO, DM, OG, SC, and DOC). Solution B contains 0.5 mm Na+/K+ phosphate buffer, pH 4, 250 μm Q-H2 in the presence or absence of 300 units/ml superoxide dismutase. Each data point represents an average of four experiments. Error bars indicate S.D.
Superoxide Anion Generation Is High Potential Oxidant- and Ubiquinol Concentration-dependent
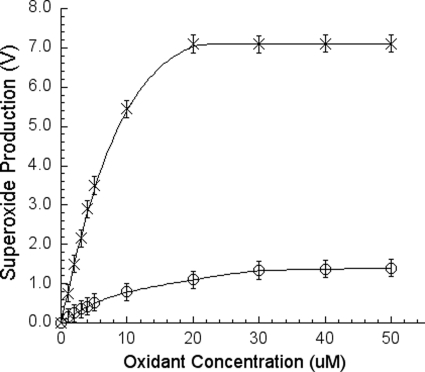
Generation of O2˙̄ requires an electron donor ubiquinol and a high potential oxidant such as ISC, cytochrome c, or ferricyanide as an electron acceptor. To test the cytochrome c concentration dependence of the O2˙̄ generation, various concentrations of cytochrome c were added to a reaction mixture containing 25 μm Q-H2 and 6 mm sodium cholate. As seen in Fig. 6 in the curve with open circles, it is clear that the O2˙̄ production is proportional to the concentration of cytochrome c. In Fig. 6, the curve with Xs shows the effect of ferricyanide concentration on the O2˙̄ generation. Varying concentrations of ferricyanide were added to a reaction mixture containing 25 μm Q-H2 and 6 mm sodium cholate. Like cytochrome c, the O2˙̄ production is proportional to the concentration of ferricyanide added but with five times more efficiency than that of cytochrome c.
FIGURE 6.
High potential oxidant (cytochrome c or ferricyanide) concentration-dependent superoxide generation under a constant amount of ubiquinol. The superoxide production was measured as described under “Experimental Procedures.” The curve with open circles represents cytochrome c, and the curve with Xs represents ferricyanide. Solution A contains 100 mm Na+/K+ phosphate buffer, pH 7.4, 6 mm sodium cholate, and a different concentration of cytochrome c or ferricyanide. Solution B contains 100 mm Na+/K+ phosphate buffer, pH 7.4, 6 mm sodium cholate, 50 μm Q-H2, and 4 μm MCLA. Each data point represents an average of four experiments. Error bars indicate S.D.
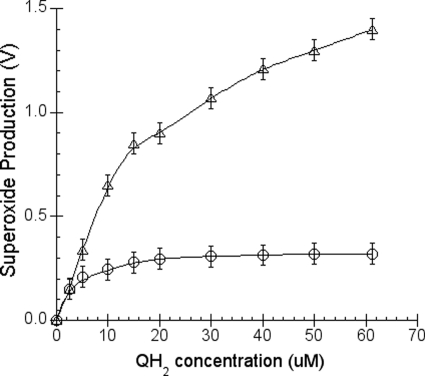
The effect of the Q-H2 concentration on superoxide generation was also studied. Under a constant concentration of cytochrome c (2.5 or 25 μm) and sodium cholate (6 mm), O2˙̄ production increases when Q-H2 concentration increases (Fig. 7). When 2.5 μm cytochrome c is used, maximum O2˙̄ production is obtained when 25 μm Q-H2 is used. When 25 μm cytochrome c is used, maximum O2˙̄ production is not reached until the Q-H2 concentration in the system is about 125 μm or higher (data not shown). These results indicate that at a given concentration of Q-H2, O2˙̄ production is dependent on the concentration of cytochrome c used, confirming the results shown in Fig. 6.
FIGURE 7.
Quinol concentration-dependent superoxide production under a constant amount of sodium cholate. The superoxide production was measured as described under “Experimental Procedures.” Solution A contains 100 mm Na+/K+ phosphate buffer, pH 7.4, and 5 μm (open circles) or 50 μm (Xs) of cytochrome c. Solution B contains 100 mm Na+/K+ phosphate buffer, pH 7.4, 4 μm MCLA, 12 mm sodium cholate, and different concentrations of Q-H2. Each data point represents an average of four experiments. Error bars indicate S.D.
Reaction Mechanism of Superoxide Anion Generation by the Cytochrome bc1 Complex
Based on the results obtained that O2˙̄ is produced during Q-H2 oxidation by cytochrome c or ferricyanide in the presence of phospholipid vesicles or a detergent micelle and that the O2˙̄ is produced by a heat-inactivated or proteinase K-digested complex, a reaction mechanism for O2˙̄ production in the bc1 complex is proposed. In this proposed mechanism, four elements are directly involved in O2˙̄ generation. These are: a hydrophobic environment, a high potential electron acceptor, a low potential electron acceptor, and an electron donor.
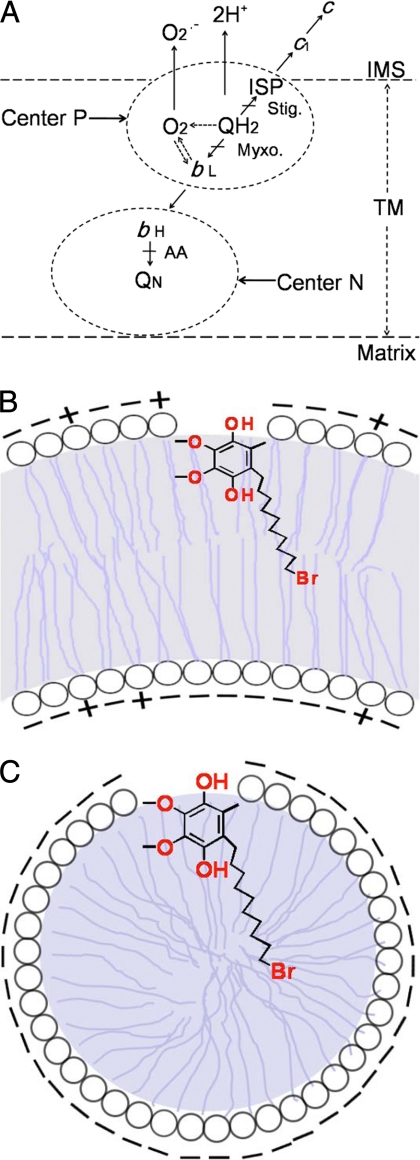
In the intact cytochrome bc1 complex, the protein subunits of the complex directly involved in the superoxide production are ISP, which houses a high potential electron acceptor ISC, and cytochrome b, which provides a hydrophobic environment, a QP pocket, and a low potential electron acceptor heme bL. Based on the concerted Q-cycle mechanism (29–31), quinol undergoes bifurcated oxidation in the QP pocket by simultaneously transferring its two electrons to ISC and heme bL. The molecular oxygen can also receive a hydrogen from quinol to produce a protonated superoxide (O2H), which generates O2˙̄ upon deprotonation. It is also likely that the reduced heme bL can transfer an electron to the molecular oxygen to form O2˙̄, particularly when the oxidant (heme bH) of reduced heme bL is limited or unavailable, such as in the presence of antimycin A (Fig. 8A). According to this proposed O2˙̄ generation mechanism, the generation of only a fraction of O2˙̄ during quinol oxidation catalyzed by intact bc1 complex may result from: (i) the limited accessibility of molecular oxygen to the QP pocket, which is surrounded by structured protein subunits; (ii) molecular oxygen competing unfavorably with heme bL for the electron during bifurcated ubiquinol oxidation, thus ensuring that few electrons are readily available for oxygen to react with; and (iii) the fact that the O2˙̄ being generated within the QP pocket may not easily escape to the aqueous medium.
FIGURE 8.
The schematic depiction of bifurcated oxidation of ubiquinol at QP pocket (A) and the proposed location of Q0C10BrH2 in the phospholipid vesicle (B) or in the detergent micelle (C). Stig., stigmatellin; Myxo, myxothiazol; AA, antimycin A; IMS, the mitochondrial intermembrane space; TM, the transmembrane region.
Although intact protein subunits of the bc1 complex are not directly involved in O2˙̄ generation, they form a barrier for the QP pocket to limit accessibility of molecular oxygen to the pocket. Destruction of protein structural integrity by heme bL or bH deletion, heat inactivation, or proteinase K digestion leads to a loosening of the structural integrity of the QP pocket to facilitate the molecular oxygen to get access to the QP pocket and to ease the release of produced O2˙̄ to the aqueous medium.
In addition, it is possible that in the cytochrome bc1 complex, an oxygen molecule is located between the 4-HO− group of Q-H2 and heme bL to mediate the electron transfer between them in the hydrophobic environment of the QP pocket. The poor hydrogen-bonding nature of the oxygen molecule seems to work against this speculation. However, if this were the case, in a hydrophobic environment, one would expect to see a higher presteady state reduction rate of cytochrome b by ubiquinol in the presence of oxygen than in the absence of it. Our preliminary results seem to support this speculation. Further investigation on the role of oxygen in the reduction of cytochrome b by ubiquinol is currently in progress in our laboratory.
In the protein-free system, our results appear to suggest that the benzoquinol ring of Q-H2 is located at or near the surface of the lipid vesicles or detergent micelles with its 1-hydroxy group extended into the water phase and the 4-hydroxy group together with its alkyl side chain located inside the bilayer or micelle (Fig. 8, B and C). When the ISC is used as an electron acceptor, we speculate that a hydrogen is transferred from the 1-hydroxyl group to the N-3 of the imidazole ring of histidine residue, which is a ligand of the ISC. At the same time, a hydrogen is transferred concurrently from the 4-hydroxy group to a molecule of oxygen, which is dissolved inside the lipid bilayer of vesicles or in the hydrophobic interior of detergent micelles, to generate a protonated superoxide (HO2), which then diffuses to the water phase to become a superoxide anion upon deprotonation.
The high potential electron acceptor ISC can be substituted with cytochrome c or ferricyanide. In this case, we surmise that the 1-hydroxy group of ubiquinone is deprotonated and releases a proton to the water phase before the electron is transferred to ferricyanide or cytochrome c. At the same time, the 4-hydroxy group transfers its hydrogen atom to a molecular oxygen.
In the phospholipid vesicles or detergent micelle systems, we suggest that the electron transfer between cytochrome c and Q-H2 takes place at the surface of the lipid bilayer and that the transfer between Q-H2 and O2 occurs concurrently inside the bilayer. This suggestion is consistent with the fact that the solubility of oxygen is much higher in the hydrophobic environment than that in the aqueous medium and that the rate of reduction of cytochrome c by Q-H2 is much lower under anaerobic conditions than when in the presence of oxygen.
This work was supported, in whole or in part, by National Institutes of Health Grant GM30721 (to C.-A. Y.). This work was also supported by Oklahoma Agricultural Experiment Station Projects 1819 and 2372 from the Oklahoma State University.
“Supernumerary subunits” refer to the subunits of bc1 complex that bear no redox groups.
- Q
- ubiquinone
- Q-H2
- ubiquinol
- ISP
- iron-sulfur protein
- ISC
- iron-sulfur cluster
- cyt
- cytochrome
- DM
- n-dodecyl-β-d-maltopyranoside
- DOC
- deoxycholate
- LDAO
- N,N-dimethyldodecylamine N-oxide
- MCLA
- 2-methyl-6-(4-methoxyphenyl)-3,7-dihydroimidazol[1,2-α]pyrazin-3-one, hydrochloride
- OG
- n-octyl-d-gluocopyranoside
- Q0C10BrH2
- 2,3-dimethoxy-5-methyl-6-(10-bromodecyl)-1,4-benzoquinol
- SC
- sodium cholate.
REFERENCES
- 1.Boveris A., Chance B. (1973) Biochem. J. 134, 707–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loschen G., Azzi A., Flohé L. (1973) FEBS Lett. 33, 84–87 [DOI] [PubMed] [Google Scholar]
- 3.Loschen G., Azzi A., Richter C., Flohé L. (1974) FEBS Lett. 42, 68–72 [DOI] [PubMed] [Google Scholar]
- 4.McCord J. M., Fridovich I. (1969) J. Biol. Chem. 244, 6049–6055 [PubMed] [Google Scholar]
- 5.Boveris A., Oshino N., Chance B. (1972) Biochem. J. 128, 617–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Korshunov S. S., Skulachev V. P., Starkov A. A. (1997) FEBS Lett. 416, 15–18 [DOI] [PubMed] [Google Scholar]
- 7.Rottenberg H., Covian R., Trumpower B. L. (2009) J. Biol. Chem. 284, 19203–19210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turrens J. F., Alexandre A., Lehninger A. L. (1985) Arch. Biochem. Biophys. 237, 408–414 [DOI] [PubMed] [Google Scholar]
- 9.Nohl H., Jordan W. (1986) Biochem. Bioph. Res. Co. 138, 533–539 [DOI] [PubMed] [Google Scholar]
- 10.Galkin A., Brandt U. (2005) J. Biol. Chem. 280, 30129–30135 [DOI] [PubMed] [Google Scholar]
- 11.Ohnishi S. T., Ohnishi T., Muranaka S., Fujita H., Kimura H., Uemura K., Yoshida K., Utsumi K. (2005) J. Bioenerg. Biomembr. 37, 1–15 [DOI] [PubMed] [Google Scholar]
- 12.Genova M. L., Ventura B., Giuliano G., Bovina C., Formiggini G., Parenti Castelli G., Lenaz G. (2001) FEBS Lett. 505, 364–368 [DOI] [PubMed] [Google Scholar]
- 13.Muller F. L., Liu Y., Abdul-Ghani M. A., Lustgarten M. S., Bhattacharya A., Jang Y. C., Van Remmen H. (2008) Biochem. J. 409, 491–499 [DOI] [PubMed] [Google Scholar]
- 14.Zhang L., Yu L., Yu C. A. (1998) J. Biol. Chem. 273, 33972–33976 [DOI] [PubMed] [Google Scholar]
- 15.Muller F., Crofts A. R., Kramer D. M. (2002) Biochemistry 41, 7866–7874 [DOI] [PubMed] [Google Scholar]
- 16.Sun J., Trumpower B. L. (2003) Arch. Biochem. Biophys. 419, 198–206 [DOI] [PubMed] [Google Scholar]
- 17.Forquer I., Covian R., Bowman M. K., Trumpower B. L., Kramer D. M. (2006) J. Biol. Chem. 281, 38459–38465 [DOI] [PubMed] [Google Scholar]
- 18.Mitchell P. (1976) J. Theor. Biol. 62, 327–367 [DOI] [PubMed] [Google Scholar]
- 19.Crofts A. R., Meinhardt S. W., Jones K. R., Snozzi M. (1983) Biochim. Biophys. Acta. 723, 202–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trumpower B. L. (1990) J. Biol. Chem. 265, 11409–11412 [PubMed] [Google Scholar]
- 21.Link T. A. (1997) FEBS Lett. 412, 257–264 [DOI] [PubMed] [Google Scholar]
- 22.Hong S., Ugulava N., Guergova-Kuras M., Crofts A. R. (1999) J. Biol. Chem. 274, 33931–33944 [DOI] [PubMed] [Google Scholar]
- 23.Crofts A. R., Shinkarev V. P., Kolling D. R., Hong S. (2003) J. Biol. Chem. 278, 36191–36201 [DOI] [PubMed] [Google Scholar]
- 24.Jünemann S., Heathcote P., Rich P. R. (1998) J. Biol. Chem. 273, 21603–21607 [DOI] [PubMed] [Google Scholar]
- 25.Yang S., Ma H. W., Yu L., Yu C. A. (2008) J. Biol. Chem. 283, 28767–28776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Vries S., Albracht S. P., Berden J. A., Slater E. C. (1981) J. Biol. Chem. 256, 11996–11998 [PubMed] [Google Scholar]
- 27.Cape J. L, Bowman M. K., Kramer D. M. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 7887–7892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang H., Osyczka A., Dutton P. L., Moser C. C. (2007) Biochim. Biophys. Acta 1767, 883–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu J., Egawa T., Yeh S. R., Yu L., Yu C. A. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 4864–4869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trumpower B. L. (2002) Biochim. Biophys. Acta. 1555, 166–173 [DOI] [PubMed] [Google Scholar]
- 31.Berry E. A., Huang L. S. (2003) FEBS Lett. 555, 13–20 [DOI] [PubMed] [Google Scholar]
- 32.Helenius A., McCaslin D. R., Fries E., Tanford C. (1979) Methods Enzymol. 56, 734–749 [DOI] [PubMed] [Google Scholar]
- 33.Yu C. A., Yu L. (1982) Biochemistry 21, 4096–4101 [DOI] [PubMed] [Google Scholar]
- 34.Tian H., Yu L., Mather M. W., Yu C. A. (1998) J. Biol. Chem. 273, 27953–27959 [DOI] [PubMed] [Google Scholar]
- 35.Tso S. C., Yin Y., Yu C. A., Yu L. (2006) Biochim. Biophys. Acta 1757, 1561–1567 [DOI] [PubMed] [Google Scholar]
- 36.Yu C. A., Yu L. (1980) Biochim. Biophys. Acta 591, 409–420 [DOI] [PubMed] [Google Scholar]
- 37.Yu L., Yang S., Yin Y., Cen X., Zhou F., Xia D., Yu C. A. (2009) Methods Enzymol. 456, 459–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kagawa Y., Racker E. (1971) J. Biol. Chem. 246, 5477–5487 [Google Scholar]
- 39.Nakano M. (1990) Methods Enzymol. 186, 585–591 [DOI] [PubMed] [Google Scholar]
- 40.Denicola A., Souza J. M., Gatti R. M., Augusto O., Radi R. (1995) Free Radic. Biol. Med. 19, 11–19 [DOI] [PubMed] [Google Scholar]
- 41.Gong X., Yu L., Xia D., Yu C. A. (2005) J. Biol. Chem. 280, 9251–9257 [DOI] [PubMed] [Google Scholar]
- 42.Azzi A., Montecucco C., Richter C. (1975) Biochem. Biophys. Res. Commun. 65, 597–603 [DOI] [PubMed] [Google Scholar]