Abstract
The tensile strength of fibrillar collagens depends on stable intermolecular cross-links formed through the lysyl oxidase mechanism. Such cross-links based on hydroxylysine aldehydes are particularly important in cartilage, bone, and other skeletal tissues. In adult cartilages, the mature cross-linking structures are trivalent pyridinolines, which form spontaneously from the initial divalent ketoimines. We examined whether this was the complete story or whether other ketoimine maturation products also form, as the latter are known to disappear almost completely from mature tissues. Denatured, insoluble, bovine articular cartilage collagen was digested with trypsin, and cross-linked peptides were isolated by copper chelation chromatography, which selects for their histidine-containing sequence motifs. The results showed that in addition to the naturally fluorescent pyridinoline peptides, a second set of cross-linked peptides was recoverable at a high yield from mature articular cartilage. Sequencing and mass spectral analysis identified their origin from the same molecular sites as the initial ketoimine cross-links, but the latter peptides did not fluoresce and were nonreducible with NaBH4. On the basis of their mass spectra, they were identical to their precursor ketoimine cross-linked peptides, but the cross-linking residue had an M+188 adduct. Considering the properties of an analogous adduct of identical added mass on a glycated lysine-containing peptide from type II collagen, we predicted that similar dihydroxyimidazolidine structures would form from their ketoimine groups by spontaneous oxidation and free arginine addition. We proposed the trivial name arginoline for the ketoimine cross-link derivative. Mature bovine articular cartilage contains about equimolar amounts of arginoline and hydroxylysyl pyridinoline based on peptide yields.
Keywords: Collagen, Extracellular Matrix Proteins, Hydroxylysine, Mass Spectrometry (MS), Protein Chemistry, Protein Cross-linking, Arginoline, Cartilage, Pyridinoline
Introduction
All fibril-forming collagens, types I, II, III, V, and XI, of vertebrates are cross-linked by the lysyl oxidase mechanism (1, 2). Two reaction pathways can be defined, one based on lysine aldehydes and the other on hydroxylysine aldehydes, which operate tissue-dependently. A combination of both pathways is evident in some tissues, for example in the unique cross-linking profile of bone collagen (3). Cartilage collagens use the hydroxylysine aldehyde route exclusively (4, 5). Newly made type II collagen in hyaline cartilages and other tissues becomes cross-linked almost exclusively by divalent ketoimines, each formed by the addition of a telopeptide, hydroxylysine aldehyde, to a hydroxylysine at either residue 87 or 930 of the triple helix. As fibrils mature, divalent ketoimines interact spontaneously to form a trivalent cross-link, hydroxylysyl pyridinoline (HP)2 (6, 7). The stoichiometry is such that 1 mol of HP is formed from 2 mol of hydroxylysino-ketonorleucine (HLKNL; the ketoimine). This was established by direct amino acid analysis after borohydride reduction of tissue to stabilize the ketoimines to acid hydrolysis (7, 8) and also by radiolabeling of cartilage with [14C]lysine in vivo (9) and in vitro (10).
A reaction mechanism has been proposed (aldol addition followed by ring closure and oxidation) in which two ketoimines condense to form the pyridinoline and release one of the helical domain hydroxylysine side chains (7, 8). An alternative reaction mechanism has been suggested in which the reactants are a ketoimine and a free hydroxylysine aldehyde on a second telopeptide in the same collagen molecule as the first (11). This does not fit the stoichiometry, i.e. the relatively slow reaction kinetics of pyridinoline appearance after fibrils have formed or the low level of free aldehydes in even newly made collagen. Whatever the mechanism, the ketoimines disappear from mature tissues, and pyridinolines become essentially the sole cross-linking residues (12, 13). The content of HP cross-links ranges from 1 to 2 mol/mol of type II collagen in mature human articular cartilage (14) and 1.3 to 1.9 mol/mol in mature bovine articular cartilage (13, 15). Cartilages contain the highest levels of collagen HP of all connective tissues (2).
The chemistry, content, and spatial arrangement of the cross-linking bonds are all thought to be important determinants of collagen fibril properties and overall tissue mechanical behavior. Because mature articular cartilage collagen turns over very slowly at best throughout life (16, 17), most of it is laid down during growth and has to last a lifetime (5). Its resistance to mechanical failure and ability to withstand other biological wear and tear and proteolytic stresses are therefore key determinants of joint longevity.
Determining what drives or enables the initial ketoimine cross-links to convert efficiently (or not) into pyridinoline residues may be important for understanding why joints fail earlier in some individuals than in others. Using a procedure we developed for selecting for the total pool of collagen type II cross-linking domains from protease digests of tissue, we aimed to answer this question in a study of fetal, calf, and adult bovine articular cartilage by mass spectrometry.
EXPERIMENTAL PROCEDURES
Tissue Preparation
Articular cartilage from 18-month steer knee joints (local abattoir) was extracted with 4 m guanidine HCl, 0.05 m Tris-HCl, pH 7.4, for 48 h at 4 °C. The washed residue was digested with pepsin, and solubilized type II collagen was recovered in the 0.7 m NaCl precipitate (12). The isolated type II collagen was digested with recombinant proteomics grade trypsin (Roche Diagnostics) in 0.05 m Tris, 0.15 m NaCl, pH 8.0, for 20 h at 37 °C with an enzyme to substrate ratio of 1:200. Cyanogen bromide (CNBr) peptides were generated by digesting pepsinized collagen with CNBr in 70% formic acid for 24 h at room temperature (18). Type II collagen was similarly extracted from fetal and 3-month calf epiphyseal cartilages with pepsin, precipitated with salt, and then digested with CNBr.
SDS-PAGE
The CNBr digest was run on 12.5% SDS-PAGE, and before staining the gel with Coomassie Blue, it was scanned on a PerkinElmer fluorescence spectrophotometer equipped with a thin layer/slab gel scanning accessory and an E-C Apparatus densitometer (12).
Immobilized Metal Ion Affinity Chromatography (IMAC)
An IMAC column was prepared by charging HiTrap chelating HP beads (Amersham Biosciences) with CuCl2. Tryptic digests of 18-month steer collagen were loaded on the column in 0.05 m Tris, 0.15 m NaCl, pH 8.0. Bound peptides were eluted in three pools: pool A with 0.1 m sodium acetate, pH 6.35; pool B with 0.1 m sodium acetate, pH 4.6; and pool C with 0.1 m sodium acetate, pH 4.6, containing 0.2 m imidazole. Each CNBr digest of pepsin-solubilized type II collagen from fetal and calf cartilage was loaded in 0.05 m Tris-HCl, 0.15 m NaCl, 2 m guanidine HCl, pH 8.0, and the bound peptides were eluted with 0.1 m sodium acetate, pH 4.6, containing 2 m guanidine HCl and 0.2 m imidazole.
Reverse-phase (RP) HPLC
Each tryptic peptide pool from the IMAC column was run on RP-HPLC (C8 RP-300, 25 cm × 4.6 mm, Brownlee columns, Applied Biosystems) (19). Peak tryptic peptide fractions were aliquoted for protein sequencing and/or mass spectrometry.
The CNBr peptides from fetal and calf cartilage type II collagen that bound to the Cu2+ IMAC column consisted mostly of non-cross-linked and cross-linked forms of CB12 and CB9,7 that were resolved into two peaks by C8 RP-HPLC (not shown). The CB12 and CB9,7 pools were digested separately with trypsin and rerun on RP-HPLC.
Protein Microsequencing
Edman N-terminal sequencing was carried out on a Porton 2090E machine equipped with on-line HPLC analysis of phenylthiohydantoin derivatives (20).
Electrospray Mass Spectrometry
Mass spectrometry was performed on an LCQ Deca XP ion trap mass spectrometer (Thermo Scientific) with in-line liquid chromatography (LC) on a C8 capillary column (300-μm inner diameter × 150 mm long; Grace Vydac 208MS5.315) flowing at 4.5 μl/min (21). The mobile phase consisted of buffer A, 0.1% formic acid with 2% buffer B in MilliQ water; and buffer B, 0.1% formic acid in 3:1 (v/v) acetonitrile:N-propanol. The column eluent was introduced into the mass spectrometer with an atmospheric pressure electrospray ionization source with a 3-kV spray voltage and inlet capillary temperature of 160 °C. The mass spectrometer, run in an automatic triple play mode, cycled through a full scan, zoom scan, and MS/MS every few milliseconds.
RESULTS
SDS-PAGE Resolution of Cross-linked Peptides
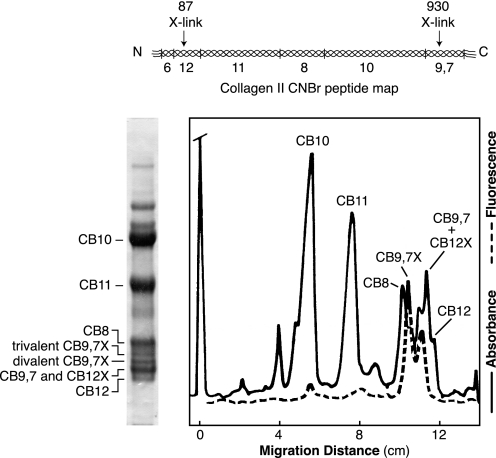
Three forms of the cyanogen bromide peptide CB9,7 from CNBr-digested pepsin-solubilized type II collagen were resolved as distinct bands on SDS-PAGE (Fig. 1). By N-terminal sequencing the fastest of the bands appeared to contain a mixture of linear (i.e. non-cross-linked) CB9,7 mixed with CB12 cross-linked to either one or two C-telopeptides. The middle band is CB9,7 cross-linked to one N-telopeptide and the slowest band is CB9,7 cross-linked to two N-telopeptides. Fig. 1 shows the results of densitometric and fluorometric scans of the gel lane shown. The two fluorescent peaks correspond to the trivalent HP cross-linked forms of CB9,7 and CB12. The divalent cross-links are not fluorescent. Also, as noted above, the divalent and trivalent cross-linked forms of CB12 appeared to comigrate with linear CB9,7 as a single band, but the divalent and trivalent cross-linked forms of CB9,7 separated as distinct bands so that their relative amounts could be assessed. There were about equal amounts of the divalent and trivalent versions of CB9,7. This 1:1 ratio was not predicted from quantitative analysis for the known cross-linking residue HP and the divalent HLKNL in borohydride-reduced and hydrolyzed 18-month bovine cartilage, in which the ratio of HP to HLKNL is 40/1 (13). This implies that most of the divalent cross-linking residue in CB9,7X resolved in the gel is in a form not detectable by the HLKNL assay.
FIGURE 1.
SDS-PAGE of CNBr-digested type II collagen purified from bovine articular cartilage. The collagen was solubilized by pepsin and salt-precipitated before CNBr digestion and running on 12.5% SDS-PAGE. The Coomassie Blue-stained gel is shown on the left and the densitometric and fluorometric (330 nm ex, 395 nm em) scans for pyridinoline-containing peptides on the right. The CNBr peptide map of bovine α1(II), showing the positions of methionines and CB peptides referred to by number in this figure and in the text, is shown above.
Copper Affinity Chromatography
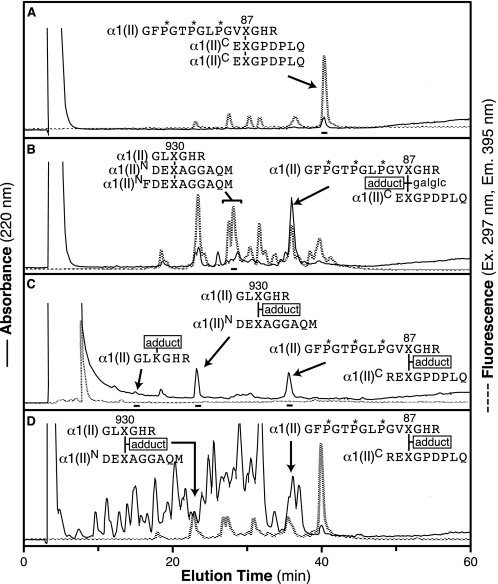
The triple helical domain of bovine α1(II) contains only two histidine residues, both in a GHR sequence immediately C-terminal to the cross-linking hydroxylysine residues at Lys-87 and Lys-930 (Ensembl entry: ENSBTAP00000017505). Because trypsin cleaves after arginine, all cross-linked tryptic peptides will contain histidine. A copper-loaded IMAC column was used to bind the histidine-containing peptides from a tryptic digest of pepsinized type II collagen. Fig. 2, A–C, shows the RP-HPLC profiles of three successive pools eluted from the IMAC column at pH 6.35 (A) and pH 4.6 (B) and 0.2 m imidazole, pH 4.6 (C). Pool C contained the most tightly bound material, which eluted only with the addition of imidazole to displace histidine. Fig. 2D shows the RP-HPLC profile of an aliquot of the total trypsin digest prior to running it on the IMAC column. The trivalent cross-linked peptides eluted in pools A and B, whereas the divalent cross-links were more tightly bound to the IMAC column and eluted in pools B and C.
FIGURE 2.
Reverse-phase HPLC of Cu2+ IMAC affinity column-resolved tryptic peptide pools from bovine type II collagen. Equivalent aliquots of each of the three peptide pools were eluted from a C8 column with a 0–40% gradient of acetonitrile:n-propanol (3:1 v/v) in 0.1% trifluoroacetic acid over 60 min. A, pool A; B, pool B; C, pool C; D, total trypsin digest. The peptides shown in the chromatograms were identified by Edman N-terminal sequencing and ion trap tandem mass spectrometry.
The peptide structures shown against the various peaks identified in Fig. 2 were determined by a combination of N-terminal sequence analysis and mass spectrometry. Variants owing to partial cleavage by pepsin in the telopeptide domains were also identified. For example, the series of fluorescent peaks eluting between 18 and 32 min in Fig. 2B are all N-telopeptide to the Lys-930 pyridinoline isoforms with various missing or present terminal methionine and phenylalanine residues.
Mass Spectrometric Analyses
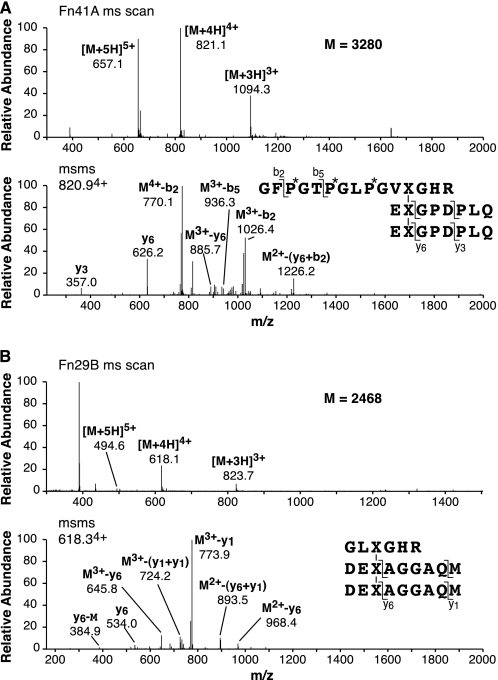
The main fluorescent peptide from pool A in Fig. 2 was shown by mass spectrometry to be the HP-bonded structure linking Lys-87 to two C-telopeptides (Fig. 3A). The early fluorescent peptides from pool B in Fig. 2 were shown by mass spectrometry to be different pepsin cleavage products of the HP-bonded structure linking Lys-930 to two N-telopeptides (Fig. 3B).
FIGURE 3.
Tandem mass spectra identifying the main fluorescent peptides resolved from pools A and B of Fig. 2. A, full-scan mass spectrum from the LC-MS profile of the parent peptide identified in Fig. 2A (upper panel) and the MS/MS fragmentation spectrum of its 4+ ion (lower panel) are shown. The various b (N-terminal)- and y (C-terminal)-fragment ions in conjunction with N-terminal sequencing results and fluorescence properties establish the C-telopeptide to N-helix trivalent structure shown. B, similarly, the parent peptide mass spectrum (upper panel) of the fluorescent peptide eluting at 29 min in Fig. 2B and its MS/MS fragmentation pattern (lower panel) establish the N-telopeptide to C-helix trivalent structure shown.
The two nonfluorescent peptides that were the major components eluting from pool C on RP-HPLC as shown in Fig. 2 were identified by mass spectrometry and N-terminal sequencing to be divalent cross-linked peptides linking residue Lys-930 to a single N-telopeptide (Fig. 4A) and residue Lys-87 to a single C-telopeptide (Fig. 4B). The mass of the peptides (and their MS/MS fragmentation patterns) showed that in each peptide the cross-linking residue had a mass greater by 188 Daltons than the theoretical mass of a ketoimine cross-linking residue (hydroxylysino-ketonorleucine). The same peptides were recovered in the same yield from the total digest (Fig. 2D) without IMAC chromatography and shown to have the +188 adduct yet.
FIGURE 4.
Tandem mass spectra identifying the two main nonfluorescent divalent cross-linked peptides resolved from pool C of Fig. 2. A, full-scan mass spectrum from the LC-MS profile of the parent peptide eluting at 24 min in Fig. 2C (upper panel) and the MS/MS fragmentation pattern of its 3+ ion (lower panel) are shown. B, similarly, the parent peptide mass spectrum of the peptide eluting at 36 min in Fig. 2C (upper panel) and its MS/MS fragmentation pattern (lower panel) establish the peptide structures shown. The molecular mass of each peptide is 188 Daltons more than the theoretical mass of the equivalent ketoimine structure. (See also Fig. 5 for a direct mass spectral comparison of an adduct versus ketoimine pair of otherwise identical tryptic peptides.)
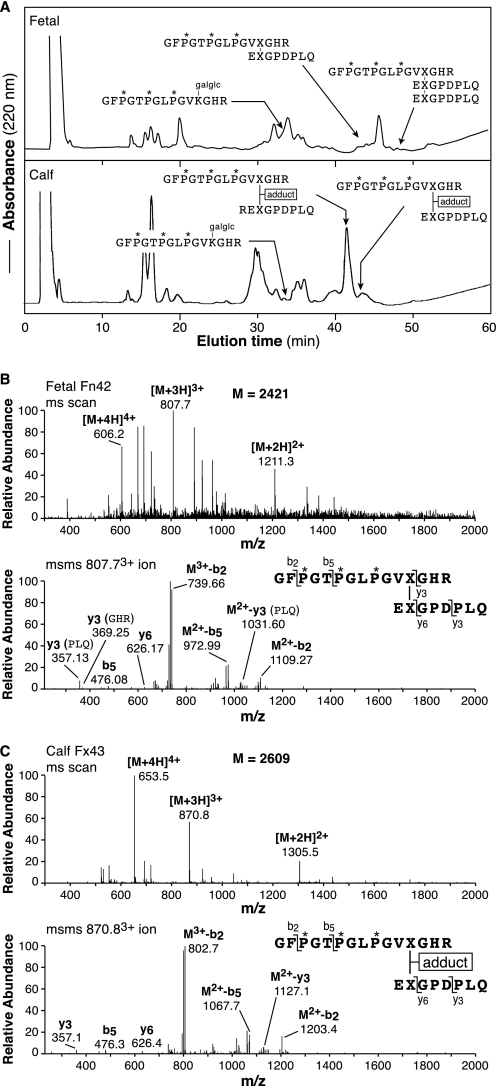
Comparison of Fetal Epiphyseal with 3-Month Calf Articular Cartilage
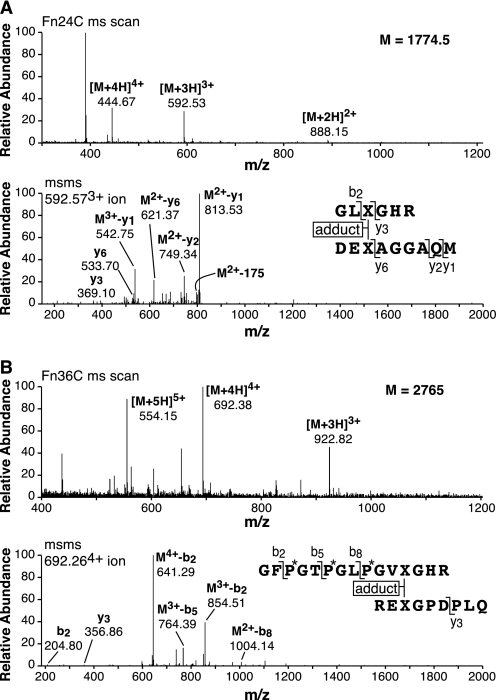
Tryptic peptides from the CB12 pool, isolated by C8 RP-HPLC after Cu2+ IMAC binding, were compared for pepsin-solubilized type II collagen from fetal growth cartilage and 3-month calf articular cartilage (Fig. 5A). From fetal cartilage, essentially the total pool of divalent cross-linked peptides from the Lys-87 site gave a mass matching that of the ketoimine form (Fig. 5B), whereas from 3-month calf cartilage two forms were detected with more than half already in the +188 adduct form. The linear peptide sequence (un-cross-linked) containing Lys-87 was also identified in the chromatograms. Interestingly, Lys-87 in the un-cross-linked peptide was hydroxylysine glycoside, but the cross-linked peptides were largely not enzymically glycosylated. The CB9,7 linear and cross-linked peptide fractions (see “Experimental Procedures”) gave tryptic peptides with the same essential findings, with most having the ketoimine cross-link at Lys-930 from fetal cartilage but over half with the +188 adduct from calf cartilage (results not shown).
FIGURE 5.
RP-HPLC of tryptic peptides from the CB12 pool comparing fetal and 3-month calf cartilages. The C8 RP-HPLC elution profiles (0–30% gradient) of the isolated CB12 pools are shown (A). Tandem mass spectra compare ketoimine (B) and adduct (C) versions of the same divalent cross-linked peptide from fetal and 3-month calf, respectively. It should be noted that this latter peptide differs in lacking an N-terminal arginine on its C-terminal telopeptide sequence compared with the variant that is the major form in the peptide preparation resolved in Fig. 4. Such partial cleavage variants were observed consistently and reflect variable cleavages by pepsin in different digests. (If pepsin cuts immediately before arginine, then trypsin fails to cleave after it.) The other structures shown in A were all identified by their mass spectra together with N-terminal sequencing results.
Identification of a Non-enzymically Glycated Linear Tryptic Peptide from Type II Collagen
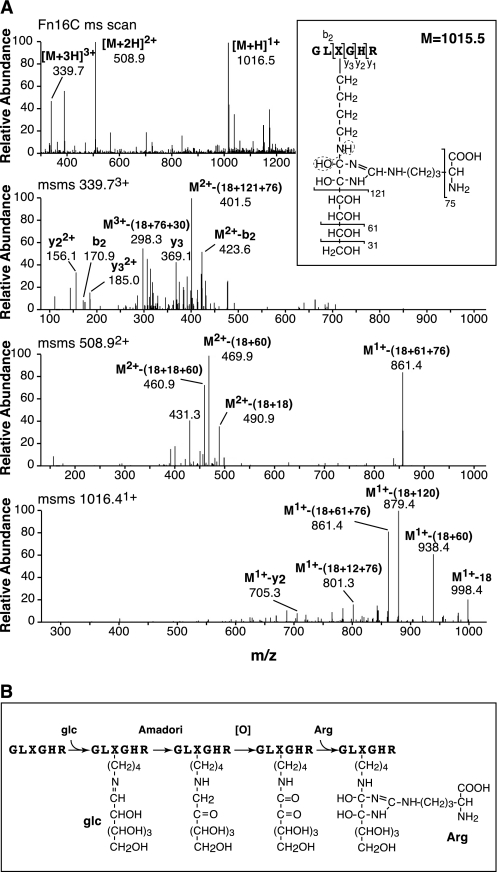
Analysis of additional peptides from the Cu2+ column eluent by N-terminal sequencing and mass spectrometry revealed a hexapeptide (Fig. 2C, fraction 16) containing Lys-930; but the Lys-930 sequencer cycle was blank, and on mass spectrometry (Fig. 6) an additional mass of 350 Daltons on a lysine residue was observed (equals +162 + 188, which matches +hexose + [O] + arginine adduct). The MS/MS fragmentation spectra shown in Fig. 6A were consistent with the structure shown in Fig. 6B, which is predicted to be the ϵ-amino hexosyl (presumably glucosyl) derivative of the lysine side chain of the peptide GLKGHR, in which the ketoimine group has become oxidized to a dicarbonyl that spontaneously had added a free arginine through its guanido group. This maturation reaction is the same mechanism proposed for the +188 adduct found on the lysyl oxidase-mediated ketoimine cross-linked peptides.
FIGURE 6.
Tandem mass spectra of a non-enzymically glycosylated linear peptide from type II collagen. Trypsin digests of mature (18-month steer) type II collagen on RP-HPLC gave a peptide (Fig. 2C, 16-min elution) of N-terminal sequence GLXGHR, where X was a blank cycle. A, tandem mass spectra. Top panel, full-scan mass spectrum across the LC-MS peak. Next three panels, MS/MS fragmentation spectra of the parent peptide 3+, 2+, and 1+ ions, respectively. The boxed dihydroxyimidazolidine structure is predicted from the parent mass, its neutral losses, and fragment ions. In B, proposed mechanism of formation by non-enzymic glucose addition, ketoimine (Amadori) rearrangement, oxidation, and the addition of arginine through its guanido group.
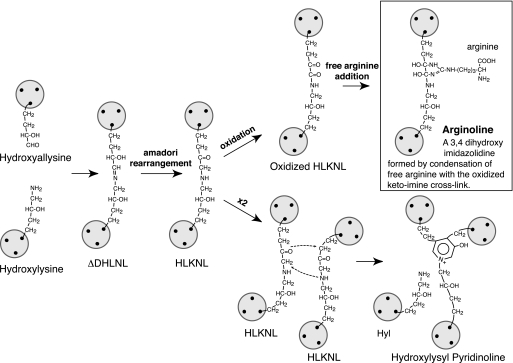
Fig. 7 shows the proposed structure of the ketoimine cross-link adduct, a 3,4 dihydroxy imidazolidine, for which we have suggested the trivial name arginoline. The unusually strong binding of the adduct to the Cu2+ IMAC column (resulting in the fraction shown chromatographed in Fig. 2C) lends support to a structure that embodies an imidazole ring.
FIGURE 7.
Proposed mechanism of formation and the structure and trivial name for the ketoimine cross-link adduct, arginoline. This mechanism parallels that shown in Fig. 6 for the ketoimine resulting from non-enzymic glycosylation of a lysine-containing collagen peptide. Oxidation produces a reactive dicarbonyl that adds free arginine through its guanido group. The proposed structure of arginoline fits exactly the mass of the cross-linking residue in the N-telopeptide-to-helix and C-telopeptide-to-helix structures shown in Fig. 4.
DISCUSSION
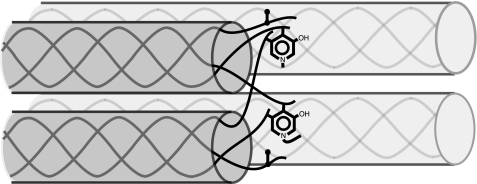
The results show that about half of the initially formed ketoimine cross-links in type II collagen of mature bovine articular cartilage had matured by an alternative chemical reaction to that producing trivalent HP. The product of this alternative reaction path is a nonreducible adduct formed from the ketoimine, which functionally is still a divalent intermolecular cross-link. Quantitatively about equimolar amounts of peptides based on the arginyl ketoimine adduct (arginoline) and HP cross-linked peptides were recovered from each of the two telopeptide-to-helix cross-linking loci of bovine type II collagen. Thus, if two ketoimines effectively condensed to form one HP (see below), then roughly two-thirds of the ketoimines in the cartilage collagen had matured to HP and one-third to arginoline. This means that cartilage collagen fibrils are even more densely cross-linked than previously appreciated from their HP content alone (Fig. 8 illustrates the molecular concept). The reported HP content of 18-month bovine articular cartilage ranges from 1.3 to 1.9 mol/mol of collagen (13, 15), which means that 2.6–3.8 of the total six telopeptides/type II collagen molecule (three at each end) are involved in HP linkage. At an equal concentration the modified ketoimine cross-link would account for a further 1.3 to 1.9 telopeptides/molecule, i.e. both forms of cross-link can account for between 3.9 and 5.7 of the six telopeptides theoretically available for cross-linking. As some telopeptide lysines will be missed by lysyl hydroxylase and/or lysyl oxidase, this can account for the remainder.
FIGURE 8.
Illustration of a cross-linking site between registered pairs of collagen molecules staggered by 4D (where D = 234 amino acid residues, the Hodge-Petruska stagger). In the type II collagen molecule, all three telopeptides at both the N-terminal and C-terminal ends form a cross-linking aldehyde from hydroxylysine (hydroxyallysine, see Fig. 7). Just one end is shown. The pyridinol ring illustrates how linkage of two telopeptides to a helical site can form trivalent pyridinoline cross-links, and the vertical dumbbells illustrate divalent (ketoimine) bonds. Such an arrangement at maximum theoretical cross-link occupancy would use all six telopeptides/molecule and result in equimolar trivalent and divalent cross-links each at a stoichiometry of 2 mol/mol of collagen.
The various types of reducible cross-link in collagen disappear from most adult connective tissues, whether their primary formation pathway is from lysine or hydroxylysine aldehydes (22, 23). Several reaction mechanisms in addition to pyridinoline formation from ketoimines have been proposed to account for this disappearance, for example oxidation of aldimines (initial products on the lysine aldehyde pathway) to amides (24), gem-diamine formation (25), and natural reduction. Natural reduction has been ruled out by amino acid analysis after acid hydrolysis without borohydride treatment (26, 27). Oxidation seems a more likely extracellular path in biological systems, and α-amino adipic acid, which has been identified in skin collagen after acid hydrolysis, is proposed to originate from the amide formed by oxidation of the aldimine Δ-HLNL (24).
There is also a precedent for ketoimine oxidation based on the known fate of the glycosylamines that form adventitiously through non-enzymic glycosylation (glycation) of lysine side chains in long-lived proteins. The initial glycosylamine adducts undergo Amadori rearrangement to a ketoimine, which can oxidize to an α,β-dicarbonyl (28, 29). The latter show a high affinity and selectivity in forming additional compounds with the guanido group of arginine (30) or another guanido-containing molecule such as creatine (31). A complex imidazolidine called DOGDIC (32) has been identified among the many advanced glycation end-product (AGE) structures that go on to form from the Amadori adduct between glucose and lysine. Dihydroxyimidazolidines have been identified as major products of α,β-dicarbonyl addition to arginine (30). The results of our present analyses can best be explained if the ketoimine cross-links that fail to form pyridinolines in collagen fibrils undergo a two-step process of slow, spontaneous oxidation followed by the rapid addition of free arginine. The latter is available in tissue fluids. Indeed, concentrations of free arginine are likely to be higher in cartilage matrix than in other tissues because of the high negative fixed charge density of the sulfated proteoglycans. Our results showed no evidence of peptides resulting from the addition of peptidyl arginine side chains to the ketoimines (which would be a further cross-linking reaction) because no peptides larger than the divalent structures plus an M+188 adduct other than the HP-containing peptides were recovered from the type II collagen. Thus, there was no evidence for trivalent cross-linking to a third peptide sequence containing an arginine residue, but only to the free arginine adduct.
The recovery and identification of the linear hexapeptide GLK‡GHR from trypsin-digested cartilage collagen, in which K‡ is a lysine bearing an oxidized hexosylamine that had bound to arginine, supports the proposed mechanism (oxidation to an α,β-dicarbonyl followed by an arginine addition) in cartilage in vivo. Indeed the mass spectral properties of the modified hexapeptide (Fig. 6), which presumably originated from non-enzymic glycation in vivo (23), gave the clue to interpreting the structure of the ketoimine adduct on the divalent cross-linked peptides. The mass spectral evidence that the precursor is the lysine form of the peptide (rather than hydroxylysine) suggests that a lysine side chain in this sequence motif is more readily glycated than hydroxylysine. This lysine (Lys-930) is almost fully hydroxylated in type II collagen, but the equivalent glycated derivative of a hydroxylysine-containing GLK*GHR peptide was not found.
To confirm that this phenomenon was not peculiar to bovine cartilage, we also analyzed pig articular cartilage (from carpal bones) with resulting very similar high levels of the M+188 adduct of the equivalent ketoimine cross-linked type II collagen peptides (results not shown). Analyses of human articular cartilage also revealed the equivalent M+188 peptides from type II collagen but in lower yields and apparently together with related peptides that had lower mass adducts on the ketoimine cross-linking residue. Because the human cartilage was from >30-year-old adults, we suspect that the initial dihydroxy adducts (Fig. 7) can undergo dehydrations or rearrangements of the kind noted for advanced glycation end-products of non-enzymically glycosylated lysine residues (23, 33). We also analyzed type I collagen from bovine meniscus fibrocartilage by similar methods (results not shown) and established that the same M+188 adduct on divalent cross-linked peptides from the N- and C-telopeptide cross-linking sites was a major feature of fibrocartilage type I collagen. So the proposed pathway of oxidation and arginine addition appears to operate in cartilages in general. Whether this is a widespread mechanism of ketoimine maturation in all tissue collagens or is cartilage-specific will require further study. Arginoline is not reducible with sodium borohydride (results not shown) and is likely to be destroyed on acid hydrolysis, so it would be missed by using the earlier techniques of cross-link analysis.
This alternative route of ketoimine maturation is consistent with the results of [14C]lysine labeling of collagen cross-links in vitro (10) and in vivo (9). The kinetics of ketoimine (HLKNL) and pyridinoline (HP) production from [14C]lysine were reported for explant cultures of calf cartilage plugs in vitro (10) monitored for 28 days. By 21–28 days, from a [14C]lysine pulse, HP formation had leveled off, and 60% of the [14C]HLKNL, which peaked at 2–7 days post-pulse, had been converted to HP. But 40% of the peak [14C]HLKNL remained at 28 days. The present findings of a secondary maturation pathway for the fraction of HLKNL unable to react during cartilage development to form HP is consistent with these observations as is the arginoline pathway having slower kinetics than HP condensation. The present findings from fetal and calf cartilage support this concept. Fetal calf cartilage gave essentially only HLKNL in its divalent peptide pool with only trace levels of arginoline, whereas 3-month calf cartilage had both HLKNL and the arginoline adduct. This implies maturation of the ketoimine to arginoline in tissue in vivo with a t½ on the order of weeks but not years. Whether individual chondrocytes can exert some control over this reaction locally in the matrix to close down further trivalent cross-linking, for example (e.g. through a pulse of H2O2 or reactive oxygen species), or whether the reaction is purely adventitious would be important to understand. The material quality of articular cartilage is likely to be influenced by the quantitative balance between the levels of modified divalent and trivalent cross-links and their anatomical distribution profile in a mature collagen network. It is notable that in adult human joints the content of pyridinoline cross-links in type II collagen can vary by a factor of 2 between individuals (in the range of 1 to 2 mol/mol of collagen (14)).
CONCLUSION
The results reveal two maturation pathways for the initial intermolecular ketoimine cross-links in cartilage collagen, condensation to pyridinolines and oxidation and arginine addition. Both pathways involve an oxidative step. Pyridinolines presumably form to the extent permitted stereochemically by the spatial relationships set up between telopeptides and helical sites during the molecular packing of collagen molecules into fibrils (6, 19). On a molar basis, about two-thirds of the divalent cross-links in adult bovine articular cartilage had converted to pyridinoline (HP) and one-third to arginoline. The formation mechanism of the latter and its dihydroxyimidazolidine structure are essentially identical to that of a modified non-enzymically glycated peptide also recovered from the collagen.
Our findings reveal an even higher level of total cross-linking by the lysyl oxidase mechanism in cartilage collagen than previously appreciated. The oxidative status of the matrix could affect the balance between trivalent pyridinolines and divalent cross-links in mature collagen fibrils and thus affect tissue resilience and longevity.
This work was supported, in whole or in part, by National Institutes of Health Grants AR37318 and AR36794 (to D. R. E.).
- HP
- hydroxylysyl pyridinoline
- HLKNL
- hydroxylysino-ketonorleucine
- CB-peptides
- CNBr-cleaved peptides
- IMAC
- immobilized metal ion affinity chromatography
- RP
- reverse phase
- HPLC
- high pressure liquid chromatography
- LC-MS
- liquid chromatography-mass spectroscopy
- MS/MS
- tandem mass spectroscopy.
REFERENCES
- 1.Eyre D. R., Weis M. A., Wu J. J. (2008) Methods 45, 65–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eyre D. R., Paz M. A., Gallop P. M. (1984) Annu. Rev. Biochem. 53, 717–748 [DOI] [PubMed] [Google Scholar]
- 3.Knott L., Bailey A. J. (1998) Bone 22, 181–187 [DOI] [PubMed] [Google Scholar]
- 4.Eyre D. R., Wu J. J. (2005) Top. Curr. Chem. 247, 207–229 [Google Scholar]
- 5.Eyre D. R. (2004) Clin. Orthop. Relat. Res. 427, (suppl.) S118–S122 [DOI] [PubMed] [Google Scholar]
- 6.Eyre D. R. (1980) Science 207, 1315–1322 [DOI] [PubMed] [Google Scholar]
- 7.Eyre D. R., Oguchi H. (1980) Biochem. Biophys. Res. Commun. 92, 403–410 [DOI] [PubMed] [Google Scholar]
- 8.Eyre D. R. (1980) Dev. Biochem. 22, 51–55 [Google Scholar]
- 9.Eyre D. R., Grynpas M. D., Shapiro F. D., Creasman C. M. (1981) Semin. Arthritis Rheum. 11, 46–47 [Google Scholar]
- 10.Ahsan T., Harwood F., McGowan K. B., Amiel D., Sah R. L. (2005) Osteoarthr. Cartil. 13, 709–715 [DOI] [PubMed] [Google Scholar]
- 11.Robins S. P., Duncan A. (1983) Biochem. J. 215, 175–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu J. J., Eyre D. R. (1984) Biochemistry 23, 1850–1857 [DOI] [PubMed] [Google Scholar]
- 13.Eyre D. (1987) Methods Enzymol. 144, 115–139 [DOI] [PubMed] [Google Scholar]
- 14.Eyre D. R., Dickson I. R., Van Ness K. (1988) Biochem. J. 252, 495–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu J. J., Eyre D. R. (1985) Ann. N. Y. Acad. Sci. 460, 520–523 [Google Scholar]
- 16.Muir H., Bullough P., Maroudas A. (1970) J. Bone Joint Surg. Br. 52, 554–563 [PubMed] [Google Scholar]
- 17.Verzijl N., DeGroot J., Thorpe S. R., Bank R. A., Shaw J. N., Lyons T. J., Bijlsma J. W., Lafeber F. P., Baynes J. W., TeKoppele J. M. (2000) J. Biol. Chem. 275, 39027–39031 [DOI] [PubMed] [Google Scholar]
- 18.Eyre D. R., Muir H. (1975) Biochem. J. 151, 595–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu J. J., Woods P. E., Eyre D. R. (1992) J. Biol. Chem. 267, 23007–23014 [PubMed] [Google Scholar]
- 20.Weis M. A., Wilkin D. J., Kim H. J., Wilcox W. R., Lachman R. S., Rimoin D. L., Cohn D. H., Eyre D. R. (1998) J. Biol. Chem. 273, 4761–4768 [DOI] [PubMed] [Google Scholar]
- 21.Weis M. A., Hudson D. M., Kim L., Scott M., Wu J. J., Eyre D. R. (2010) J. Biol. Chem. 285, 2580–2590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bailey A. J., Paul R. G., Knott L. (1998) Mech. Ageing Dev. 106, 1–56 [DOI] [PubMed] [Google Scholar]
- 23.Avery N. C., Bailey A. J. (2005) Scand. J. Med. Sci. Sports 15, 231–240 [DOI] [PubMed] [Google Scholar]
- 24.Bailey A. J., Ranta M. H., Nicholls A. C., Partridge S. M., Elsden D. F. (1977) Biochem. Biophys. Res. Commun. 78, 1403–1410 [DOI] [PubMed] [Google Scholar]
- 25.Davis N. R., Risen O. M., Pringle G. A. (1975) Biochemistry 14, 2031–2036 [DOI] [PubMed] [Google Scholar]
- 26.Bailey A. J., Peach C. M. (1971) Biochem. J. 121, 257–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eyre D. R., Glimcher M. J. (1973) Biochem. J. 135, 393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harding J. J., Ganea E. (2006) Biochim. Biophys. Acta 1764, 1436–1446 [DOI] [PubMed] [Google Scholar]
- 29.Baynes J. W., Thorpe S. R. (1999) Diabetes 48, 1–9 [DOI] [PubMed] [Google Scholar]
- 30.Brock J. W., Cotham W. E., Thorpe S. R., Baynes J. W., Ames J. M. (2007) J. Mass. Spectrom. 42, 89–100 [DOI] [PubMed] [Google Scholar]
- 31.Miyazaki K., Nagai R., Horiuchi S. (2002) J. Biochem. 132, 543–550 [DOI] [PubMed] [Google Scholar]
- 32.Biemel K. M., Reihl O., Conrad J., Lederer M. O. (2001) J. Biol. Chem. 276, 23405–23412 [DOI] [PubMed] [Google Scholar]
- 33.Monnier V. M., Mustata G. T., Biemel K. L., Reihl O., Lederer M. O., Zhenyu D., Sell D. R. (2005) Ann. N. Y. Acad. Sci. 1043, 533–544 [DOI] [PubMed] [Google Scholar]