Abstract
Although the family of chimaerin Rac-GAPs has recently gained significant attention for their involvement in development, cancer, and neuritogenesis, little is known about their molecular regulation. Chimaerins are activated by the lipid second messenger diacylglycerol via their C1 domain upon activation of tyrosine kinase receptors, thereby restricting the magnitude of Rac signaling in a receptor-regulated manner. Here we identified a novel regulatory mechanism for β2-chimaerin via phosphorylation. Epidermal growth factor or the phorbol ester phorbol 12-myristate 13-acetate caused rapid phosphorylation of β2-chimaerin on Ser169 located in the SH2-C1 domain linker region via protein kinase Cδ, which retained β2-chimaerin in the cytosol and prevented its C1 domain-mediated translocation to membranes. Furthermore, despite the fact that Ser169 phosphorylation did not alter intrinsic Rac-GAP activity in vitro, a non-phosphorylatable β2-chimaerin mutant was highly sensitive to translocation, and displayed enhanced association with activated Rac, enhanced Rac-GAP activity, and anti-migratory properties when expressed in cells. Our results not only revealed a novel regulatory mechanism that facilitates Rac activation, but also identified a novel mechanism of cross-talk between diacylglycerol receptors that restricts β2-chimaerin relocalization and activation.
Keywords: Diacylglycerol, Phorbol Esters, Protein Kinase C (PKC), Protein Phosphorylation, Protein Translocation, Serine-Threonine Protein Kinase, Signal Transduction, Tyrosine-Protein Kinase (Tyrosine Kinase), Rac, Rac-GAP
Introduction
Diacylglycerol (DAG)2 is a key lipid second messenger generated in membranes upon receptor tyrosine-kinase or G-protein coupled receptor-mediated activation of phospholipase C enzymes. Effectors of DAG contain one or two copies of the C1 domain, a cysteine-rich motif that binds with high affinity to DAG and phorbol esters, natural mimetics of DAG (1, 2). Some of the best characterized DAG receptors include the protein kinase C (PKC) isozymes, a family of at least 10 related Ser/Thr kinases that have key functions in proliferation, differentiation, apoptosis, and other processes relevant to malignant transformation (3). PKCs can be divided into three groups: classical PKCs (cPKC α, βI, βII, and γ), which require DAG and calcium for activation, novel PKCs (nPKC δ, ϵ, η, and θ), which are DAG-dependent but calcium-independent, and DAG/calcium unresponsive atypical PKCs (aPKC ζ and ι). C1 domains in cPKCs and nPKCs mediate membrane translocation of the protein via DAG binding, leading to a conformational change that allows for access to PKC substrates and other PKC-binding proteins (4, 5).
Traditionally, PKCs were thought to be the only receptors for DAG; however, several other C1 domain-containing proteins that bind to and become activated by DAG have also been identified. One such DAG receptor is β2-chimaerin, a member of the chimaerin protein family comprised of α1- (or n-), α2-, β1-, and β2-chimaerin. Chimaerins contain a single C1 domain that binds DAG and phorbol esters with high affinity and mediates translocation to membranes (6, 7). Chimaerins also have a C-terminal GTPase-activating protein (GAP) domain that accelerates the hydrolysis of GTP to GDP of the small G-protein Rac1, thus converting Rac from its active GTP-bound form to an inactive GDP-bound form (8). α2- and β2-chimaerin also have a N-terminal SH2 domain possibly involved in protein-protein interactions. Chimaerins play important roles in neuronal processes, such as axon guidance, growth cone collapse, and dendritic morphology (9–13), and are also important in early zebrafish development and regulation of Rac signaling in the Drosophila retina (14, 15). β2-Chimaerin regulates cell cycle progression, actin cytoskeleton rearrangement, migration, and metastatic dissemination via Rac inactivation (16, 17). Emerging evidence shows that down-regulation of the protein occurs in cancers such as glioma and breast cancer (17–19).
The mechanisms of β2-chimaerin activation and regulation are not completely understood. Previous work revealed that β2-chimaerin is a receptor tyrosine kinase effector and that epidermal growth factor receptor (EGFR) activation causes a phospholipase Cγ/DAG-dependent translocation of β2-chimaerin to the membrane, where it associates with activated Rac and serves as a “brake” that limits the duration and intensity of Rac signaling (20). The crystal structure of β2-chimaerin revealed that the protein exists in a “closed” conformation that occludes the DAG- and Rac-binding sites (21). It has been postulated that translocation of β2-chimaerin to membranes results as a consequence of allosteric interactions with acidic phospholipids in membranes that compete with intramolecular interactions to help release the autoinhibitory conformation and allow binding of DAG to the C1 domain. This consequently allows for a full conformational rearrangement of β2-chimaerin that enables binding to Rac and activation of its Rac-GAP activity.
In this paper we have identified a novel mechanism of cross-talk between DAG effectors involving PKCδ-mediated phosphorylation of β2-chimaerin. This post-translational event prevents membrane relocalization in response to stimuli and may represent a means to down-regulate β2-chimaerin activity, suggesting dual roles for DAG in modulating β2-chimaerin relocalization and activation.
EXPERIMENTAL PROCEDURES
Cell Culture and Transfections
COS-1 and HeLa cells (ATCC) were cultured as described (20). COS-1 cells were transfected using Lipofectamine Plus (Invitrogen). All experiments were performed at least 24 h following transfection.
Western Blot Analysis
The following antibodies were used for Western blot analysis: anti-HA and anti-GST (Covance), anti-Rac1, anti-RhoA, and anti-PKCα (Upstate), anti-PKCδ and anti-phospho-EGFRs (Cell Signaling Technology), anti-PKCϵ (Santa Cruz), and anti-actin and anti-vinculin (Sigma). A polyclonal rabbit anti-phospho-Ser169-β2-chimaerin antibody was generated by FabGennix (Frisco, TX).
Two-dimensional Electrophoresis
COS-1 cells expressing HA-β2-chimaerin adenoviruses (AdV) were serum starved for 24 h and then treated with EGF (100 ng/ml, 5 min). Cell lysates were run on Novex® pH 3–10 IEF gels (Invitrogen) according to the manufacturer's recommendations, then fixed in 12% trichloroacetic acid for 30 min. Following a 10-min incubation in 20% ethanol, the desired gel lane was cut and incubated in 2 ml of 2× SDS sample buffer with 0.5 ml of ethanol for 5 min. The gel strip was inserted into a NuPAGE® Novex 4–12% BisTris gel (Invitrogen) and run in MOPS running buffer. Gels were transferred to nitrocellulose and subjected to Western blotting.
Cellular Radiolabeling
Serum-starved (24 h) COS-1 cells were incubated in phosphate-free Dulbecco's modified Eagle's medium for 4 h prior to the addition of 200 μCi of 32Pi. After 1 h, cells were stimulated with EGF or phorbol 12-myristate 13-acetate (PMA). Cell lysates were incubated with anti-HA antibody pre-conjugated to agarose beads (Santa Cruz) overnight at 4 °C. Beads were washed three times with lysis buffer. Proteins were resolved by 10% SDS-PAGE, transferred to nitrocellulose membranes, and visualized by autoradiography followed by Western blot.
Mass Spectrometry
Recombinant β2-chimaerin protein was diluted in 25 mm NH4HCO3 and 100 mm dithiothreitol, and incubated at 37 °C for 30 min. Iodoacetamide (55 mm) was added for 45 min in the dark. Samples were dialyzed against 50 mm Tris (pH 8.0) for 2 h, then digested with lysine C (0.1 μg/μl) at 37 °C for 18 h. Samples were desalted into 4:1 CH3CN:H2O, 0.1% formic acid using C18 ZipTips. For MALDI-TOF MS, 0.5 μl of the sample was mixed 1:1 with 4 mg/ml of 4-hydroxy-α-cyanocinnamic acid (1:1 CH3CN:H2O, 0.1% trifluoroacetic acid), spotted onto the MALDI target, and allowed to dry. Analyses were performed in the reflectron mode using a PE Biosystems Voyager Elite mass spectrometer.
Site-directed Mutagenesis
Mutagenesis was performed using the QuikChange II site-directed mutagenesis kit (Stratagene) as per the manufacturer's instructions, using pcDNA3-HA-β2-chimaerin or pEGFP-β2-chimaerin (22) as templates.
Generation of AdVs and Infection
AdVs were generated using the AdEasy adenoviral vector system (Stratagene). Generation of LacZ-AdV and β2-chimaerin AdV was described previously (16). S169A-β2-chimaerin AdV was generated similarly. For adenoviral infection, COS-1 or HeLa cells growing in serum-free Dulbecco's modified Eagle's medium were infected with AdVs (multiplicity of infection = 50 plaque-forming units/cell) for 2 or 16 h, respectively.
Isolation of Primary Neurons
Primary mouse cerebellar granule neurons were isolated from P7 FVB mice and cultured as previously described (23).
RNA Interference
21-bp Double-stranded RNAs (120 pmol) (Dharmacon) were transfected into HeLa cells using an Amaxa nucleofector. After 24 h, cells were infected with β2-chimaerin AdV (multiplicity of infection = 100 plaque-forming units/cell, 16 h), serum starved (24 h), then treated with EGF (100 ng/ml, 5 min), lysed, and subjected to SDS-PAGE and Western blot. Targeting sequences used were: Control (CATCGCTGTAGCATCGTCT), PKCα (AATCCTTGTCCAAGGAGGCTG), PKCδ (CCATGAGTTTATCGCCACC), and PKCϵ (GTGGAGACCTCATGTTTCA).
Generation of shRNA Stable Cell Lines
HeLa cell lines stably expressing control or PKCδ-targeted shRNAs were generated using MISSION® Lentiviral Transduction Particles (Sigma). Following lentiviral infection, cells were selected using 1 μg/ml of puromycin. The following lentiviruses were used: NTS control (SHC002V), δ number 1 (TRCN10193), and δ number 2 (TRCN10202).
In Vitro PKCδ Kinase Assays
Recombinant β2-chimaerin (25 ng) was purified from Sf9 cells as described (6) and pretreated with protein phosphatase λ (Calbiochem). For the kinase reaction, β2-chimaerin was incubated for 15 min at 30 °C with recombinant human PKCδ (25 ng) (Calbiochem) in 20 mm HEPES, 10 mm MgCl2, 20 μg of phosphatidylserine vesicles, 100 nm PMA, and 10 μm ATP. GF 109203X was added at 5 μm.
Rac-GTP and Rho-GTP Pull-down Assays
Serum-starved HeLa cells (24 h) were infected with AdVs (multiplicity of infection = 10 plaque-forming units/cell) for 16 h, washed once with serum-free medium, and 2 h later “pulldown” experiments were performed as described (8).
Migration Assays
Cells (1.5 × 104) in Dulbecco's modified Eagle's medium with 0.1% bovine serum albumin were added to the upper wells of a Boyden chamber with polycarbonate membranes (8 μm pores), and FBS was added to the lower chamber. After a 17-h incubation at 37 °C, membranes were fixed and stained with the DiffQuik Stain Set (Dade Behring, Deerfield, IL). Migrated cells were counted by phase microscopy at ×200. All samples were replicated 6 times per experiment. Five fields were counted per sample.
Subcellular Fractionation
Fractionation by ultracentrifugation was carried out as previously described (24).
Fluorescence Microscopy
COS-1 cells were plated on glass coverslips and transfected with pEBG-β2-chimaerin constructs (WT or S169A). After 24 h of incubation in Dulbecco's modified Eagle's medium with 10% FBS, cells were pretreated with GF 109203X (10 μm, 1 h) or vehicle, then treated with PMA (1 μm, 5 min) and fixed in 100% methanol (20 °C, 6 min). Slides were mounted using Fluoromount-G (SouthernBiotech, Birmingham, AL). Images were viewed using a Carl Zeiss LSM 510 confocal laser scanning fluorescence microscope.
In Vitro GAP Assays
Assays were performed using Phosphate Sensor (Invitrogen) and purified recombinant protein as previously described (25).
Co-precipitation with GST-V12Rac1
COS-1 cells co-transfected with pEBG vectors (empty vector or V12Rac1) and HA-β2-chimaerin (WT or S169A) were subjected to GST pulldown as previously described (20).
Statistical Analysis
Data are expressed as mean ± S.E. and analyzed using a Student's t test. A p value of <0.05 was considered statistically significant. All results shown are representative of three independent experiments unless otherwise indicated.
RESULTS
β2-Chimaerin Is Phosphorylated on Ser169 in Response to PMA or EGF
Emerging studies have shown that the chimaerin Rac-GAPs play important roles in development, neuritogenesis, and cancer. Although it is clear that β2-chimaerin and other chimaerins are regulated by phorbol esters and DAG generated by receptors, their regulation by post-translational events is less understood. To determine whether β2-chimaerin is phosphorylated in response to stimuli, we first analyzed if PMA could induce its phosphorylation. COS-1 cells expressing HA-tagged β2-chimaerin were radiolabeled with [32P]orthophosphate, and HA-β2-chimaerin was immunoprecipitated from cells that were stimulated with increasing concentrations of PMA (10 nm to 1 μm). Phosphorylation of β2-chimaerin was robustly induced (Fig. 1A) even at PMA concentrations below those required for translocation and activation of β2-chimaerin (8, 22). Given that EGF also activates β2-chimaerin (20), we examined the effect of EGF treatment on phosphorylation. Like PMA, EGF treatment rapidly and robustly enhanced β2-chimaerin phosphorylation (Fig. 1B). Interestingly, EGF treatment did not induce phosphorylation of the highly homologous α2-chimaerin (data not shown), indicating that these two proteins may be regulated dissimilarly by phosphorylation.
FIGURE 1.
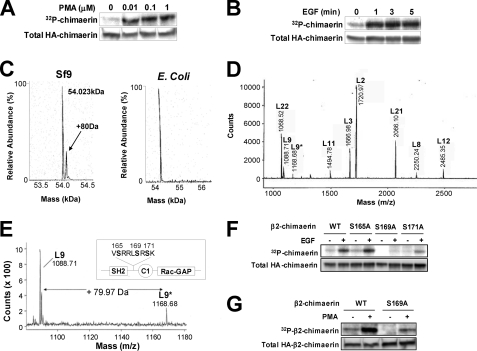
β2-Chimaerin is phosphorylated on Ser169 in response to EGF or PMA. A and B, serum-starved COS-1 cells labeled with 32Pi were treated with PMA (10 min) (A) or EGF (100 ng/ml) (B). Phosphorylation was monitored by autoradiography, and HA-β2-chimaerin expression was assessed by Western blotting. C, recombinant β2-chimaerin purified from Sf9 cells (left) or E. coli (right) was subjected to mass spectroscopy. D, spectrum obtained upon MALDI-TOF mass spectroscopy analysis of Lys-C-digested β2-chimaerin isolated from Sf9 cells. E, mass spectrum of putative phosphopeptide. Inset, localization of the putative phosphorylation sites in β2-chimaerin. F and G, COS-1 cells expressing HA-β2-chimaerin Ser to Ala mutants were labeled with 32Pi and treated with EGF (100 ng/ml, 5 min) (F) or PMA (1 μm, 10 min) (G). Phosphorylation was monitored by autoradiography.
To identify the site(s) of phosphorylation, we used recombinant human β2-chimaerin baculovirally expressed in insect Sf9 cells. Mass spectroscopy analysis of the purified protein revealed the presence of two species, one at the predicted molecular mass of the β2-chimaerin, 54.0 kDa, and a second less abundant species that was 80 Da larger (Fig. 1C, left panel), suggesting that a phosphorylated species was also present. In comparison, β2-chimaerin expressed and purified from Escherichia coli, which lack the ability to perform many post-translational modifications, contained only one species at the predicted molecular weight for β2-chimaerin (Fig. 1C, right panel). Recombinant β2-chimaerin purified from Sf9 insect cells was treated with endoproteinase Lys-C and subjected to MALDI-TOF mass spectroscopy (Fig. 1D). Peptide fragment L9 was observed at m/z 1088.71 and a candidate phosphopeptide fragment, L9*, was detected 79.97 Da above L9 (Fig. 1E), suggesting that the L9 fragment, which comprises amino acids 164–172 of β2-chimaerin, is singly phosphorylated.
The putative phosphopeptide identified by mass spectrometry contained three phosphorylatable residues (Ser165, Ser169, and Ser171), and is located in the linker region between the SH2 and C1 domain (Fig. 1E, inset), a region for which the crystal structure was unable to be resolved (21). This region is not conserved between α2- and β2-chimaerin. To identify the precise site of phosphorylation, site-directed mutagenesis was performed to individually mutate each Ser to Ala. Mutation of Ser169 completely blocked EGF-induced phosphorylation. On the other hand, phosphorylation was still detected upon mutation of Ser165 or Ser171 (Fig. 1F). Thus, Ser169 of β2-chimaerin is the major site of EGF-induced phosphorylation. Mutation of Ser169 also impairs PMA-induced phosphorylation of the protein (Fig. 1G).
A rabbit polyclonal antibody directed toward phospho-Ser169-β2-chimaerin was generated. This antibody was highly specific and showed no cross-reactivity toward the non-phosphorylatable S169A-β2-chimaerin mutant (Fig. 2A). A time course analysis of β2-chimaerin Ser169 phosphorylation in response to EGF or PMA stimulation revealed that phosphorylation is readily detected after 1 min, with maximal phosphorylation occurring at 3–5 min for EGF (Fig. 2A) and 5 min for PMA (Fig. 2B). To determine the extent of EGF-induced Ser169 phosphorylation in cells, we developed a two-dimensional gel electrophoresis approach to separate the non-phosphorylated and phosphorylated fractions of β2-chimaerin. From this analysis, we estimate that ∼40% of β2-chimaerin in cells is phosphorylated on Ser169 in response to EGF (Fig. 2C).
FIGURE 2.
Analysis of β2-chimaerin phosphorylation and stoichiometry of phosphorylation using a phosphospecific antibody. A and B, Ser169 phosphorylation of HA-β2-chimaerin was assessed in COS-1 cells using a phospho-Ser169 specific antibody following EGF (100 ng/ml) (A) or PMA (1 μm) (B) treatments. Top, representative Western blots. Bottom, data relative to t = 0 min presented as mean ± S.E. C, serum-starved COS-1 cells expressing HA-β2-chimaerin AdV were treated with EGF (100 ng/ml, 5 min). Cell lysates were subjected to two-dimensional gel electrophoresis and Western blotting. Left, representative Western blots. Right, analysis of EGF-treated samples expressed as percentage of non-phosphorylated or phosphorylated protein (relative to total protein) expressed as the mean ± S.E. (n = 5). D, primary mouse cerebellar granule neurons were treated with brain-derived neurotrophic factor (BDNF) (100 ng/ml) for the indicated times and assessed for Ser169 phosphorylation of endogenous β2-chimaerin.
We next sought to determine whether endogenous β2-chimaerin is phosphorylated on Ser169. Due to low expression of endogenous β2-chimaerin in most cultured cell lines, we chose to perform this analysis on primary mouse cerebellar granule neurons, as these cells have the highest abundance of β2-chimaerin (26). We utilized brain-derived neurotrophic factor, a TrkB tyrosine kinase receptor ligand, as this growth factor activates Rac and causes DAG production via phospholipase Cγ (23, 27). As shown in Fig. 2D, treatment of primary mouse granule neurons with brain-derived neurotrophic factor led to Ser169 phosphorylation of endogenous β2-chimaerin.
PKCδ Mediates EGF-induced Phosphorylation of β2-Chimaerin on Ser169
Because we found that phosphorylation of β2-chimaerin is induced by low nanomolar concentrations of PMA, we reasoned that PKC might be involved in β2-chimaerin phosphorylation. Moreover, analysis of the sequence surrounding Ser169 of β2-chimaerin revealed that this region is a consensus site for PKC substrate recognition. To test this hypothesis, we utilized the pan-PKC inhibitor GF 109203X (GF), and found it dose dependently inhibited EGF-stimulated phosphorylation (Fig. 3A), suggesting that phosphorylation of β2-chimaerin is indeed PKC mediated.
FIGURE 3.
PKCδ phosphorylates β2-chimaerin on Ser169. A, effect of GF (0–10 μm, 1 h) on EGF-stimulated phosphorylation (100 ng/ml, 5 min) of HA-β2-chimaerin expressed in COS-1 cells, as measured by autoradiography. B, expression of PKC isozymes in HeLa cells following RNA interference; NTS, non-targeting sequence. C, HeLa cells subjected to RNA interference depletion of PKCs were infected with HA-β2-chimaerin AdV, serum starved, and treated with EGF. Top, representative Western blot. Bottom, data expressed as % of NTS (+ EGF) are presented as mean ± S.E. (n = 4), *, p < 0.05. D, expression of PKC isozymes in stable HeLa cell lines created using shRNA lentiviruses directed against PKCδ or a NTS. E, serum-starved cell lines in D infected with HA-β2-chimaerin AdV were treated with EGF. Top, representative Western blot. Bottom, data expressed as % of NTS (+ EGF) presented as mean ± S.E. (n = 3), **, p < 0.01; ***, p < 0.005. F, serum-starved COS-1 cells infected with LacZ or PKCδ AdVs were stimulated with EGF. Top, representative Western blot. Bottom, data expressed relative to LacZ (− EGF) presented as mean ± S.E. (n = 4), *, p < 0.05. G, in vitro phosphorylation of Ser169 of β2-chimaerin by recombinant PKCδ was measured by Western blot. siRNA, small interfering RNA.
To determine which PKC isoform is involved in Ser169 phosphorylation, we first used the inhibitors Go 6970 and rottlerin, which preferentially inhibit cPKCs and PKCδ, respectively. COS-1 cells do not express PKCβI, -βII, or -γ; thus, Go 6970 targets PKCα in these cells. Pretreatment with the PKCδ inhibitor rottlerin dramatically reduced EGF-stimulated phosphorylation of Ser169 on β2-chimaerin, whereas Go 6970 was less effective (supplemental Fig. S1). Thus, PKCδ is likely responsible for β2-chimaerin phosphorylation.
Because pharmacological PKC inhibitors have limited specificity (28), we used small interfering RNA to specifically knockdown individual PKCs in HeLa cells (Fig. 3B). Although PKCα depletion caused partial reduction of Ser169 phosphorylation, depletion of PKCδ completely abolished EGF-stimulated phosphorylation. PKCϵ depletion had no effect on Ser169 phosphorylation (Fig. 3C). To validate these results, we also used 2 shRNA lentiviruses to create HeLa cell lines stably depleted of PKCδ. Significant PKCδ depletion was observed in both cell lines (δ number 1 and δ number 2), whereas a lentivirus expressing a non-targeting sequence (NTS) did not affect PKCδ expression levels (Fig. 3D). EGF-induced phosphorylation was markedly reduced in PKCδ stably depleted cells (Fig. 3E). EGF-mediated phosphorylation of β2-chimaerin was also significantly attenuated in H1299 lung carcinoma cells expressing PKCδ shRNA (supplemental Fig. S2).
To further validate the involvement of PKCδ in β2-chimaerin phosphorylation we overexpressed PKCδ in COS-1 cells and found a significant increase in Ser169 phosphorylation under basal conditions and in response to EGF (Fig. 3F). In vitro kinase assays using purified recombinant proteins also revealed that PKCδ phosphorylates Ser169 of β2-chimaerin in vitro, and that this phosphorylation can be blocked by the PKC inhibitor GF (Fig. 3G). Altogether, these results indicate that PKCδ is involved in β2-chimaerin phosphorylation, probably through direct phosphorylation.
The Non-phosphorylated Form of β2-Chimaerin Has Enhanced Rac-GAP Activity in Cells
We next investigated the functional consequences of Ser169 phosphorylation of β2-chimaerin. EGF causes translocation and activation of β2-chimaerin, and ectopic expression of β2-chimaerin inhibits EGF-mediated Rac1 activation (20). To determine whether phosphorylation had any effect on the Rac-GAP activity of β2-chimaerin, we used AdVs to express both wild-type (WT) and non-phosphorylatable S169A-β2-chimaerin mutants in HeLa cells. A LacZ AdV was used as a control. Importantly, we expressed very low amounts of the proteins to express the WT β2-chimaerin at levels that had little to no effect on Rac1 activation. At this very low level of expression, the S169A-β2-chimaerin mutant markedly inhibited Rac1 activation by EGF as determined by Rac-GTP pulldown assays (Fig. 4A). This indicates that S169A-β2-chimaerin has increased EGF-stimulated Rac1-GAP activity compared with the WT β2-chimaerin protein. Similar results were also seen in T47D breast carcinoma cells (supplemental Fig. S3). A S169E-β2-chimaerin mutant did not accurately mimic phosphorylation (data not shown), as observed for other phosphoproteins (29, 30), which precluded further functional analysis using a phosphomimetic mutant.
FIGURE 4.
S169A-β2-chimaerin has enhanced GAP activity in cells. A and B, serum-starved HeLa cells infected with LacZ, WT, or S169A-β2-chimaerin AdVs were treated with EGF (100 ng/ml, 1 min). Rac1-GTP levels (A) or RhoA-GTP levels (B) were assessed using pulldown assays. Top, results expressed as fold-change relative to LacZ (− EGF) presented as the mean ± S.E. (n = 3). **, p < 0.01. Bottom, representative Western blots. C and D, FBS-directed migration of HeLa cells expressing LacZ, WT-, or S169A-β2-chimaerin was determined using a Boyden chamber. Cells in D also co-expressed V12Rac1 as indicated. Left, representative images of migrated cells. Right, quantification of the number of migrated cells and Western blots showing the expression of chimaerin proteins. Results are expressed as mean ± S.E. (n = 3). ***, p < 0.0001.
To ensure that adenoviral expression of WT or S169A-β2-chimaerin was not interfering with EGFR activation, we looked at levels of phospho-EGFR using site-specific phospho-EGFR antibodies and found no significant differences between cells expressing control, WT, or S169A-β2-chimaerin AdVs (supplemental Fig. S4). We also wanted to determine whether the enhanced GAP activity of S169A-β2-chimaerin was specific for Rac1. We have previously shown that β2-chimaerin has GAP activity toward Rac, but not other Rho GTPases (8). Expression of S169A-β2-chimaerin showed no difference in EGF-stimulated RhoA activation when compared with control or WT-β2-chimaerin expressing cells, indicating that the GAP activity of S169A-β2-chimaerin is specific toward Rac1 (Fig. 4B).
To translate these findings into a functional setting, we assessed cell motility, a Rac-dependent process, using a Boyden chamber. Chemotactic migration toward FBS of HeLa cells infected with LacZ, WT, or S169A-β2-chimaerin AdVs was compared. We found that although expression of WT β2-chimaerin significantly attenuated migration, expression of S169A-β2-chimaerin had an even greater effect and nearly suppressed migration (Fig. 4C). To verify that the effects of S169A-β2-chimaerin on migration were due specifically to Rac inactivation, we utilized a V12Rac1 mutant, a constitutively active form of Rac1. Expression of V12Rac1 in cells reversed the inhibitory effect of both WT and S169A-β2-chimaerin on migration and enhanced the FBS-directed migration of cells to similar levels as LacZ controls (Fig. 4D). Together, these findings demonstrate that the unphosphorylated form of β2-chimaerin has enhanced Rac-GAP activity in cellular models, arguing for a negative role for Ser169 phosphorylation in β2-chimaerin activation.
Phosphorylation of Ser169 Impedes β2-Chimaerin Translocation to Membranes
One of the hallmarks of β2-chimaerin activation is translocation to membranes upon PMA stimulation or DAG generation (20, 22). To begin to account for the enhanced GAP activity of the S169A-β2-chimaerin mutant, we first determined if Ser169 phosphorylation of the β2-chimaerin could impact its subcellular localization. Cells expressing β2-chimaerin and growing in serum were subjected to subcellular fractionation. We observed that β2-chimaerin is roughly equally distributed in the soluble (cytosolic) and insoluble fractions (Fig. 5A). Remarkably, Ser169-phosphorylated β2-chimaerin was almost exclusively localized in the soluble fraction. This suggests that phosphorylated β2-chimaerin is predominantly cytosolic, whereas the activated form of β2-chimaerin found at membranes is not phosphorylated on Ser169.
FIGURE 5.
Phosphorylated β2-chimaerin is localized in the cytosol. A, lysates from COS-1 cells expressing HA-β2-chimaerin were fractionated. Phosphorylation was monitored by Western blot. B, COS-1 cells expressing WT- or P223A-β2-chimaerin were treated with GF (10 μm, 1 h), then with PMA (0–10 μm, 5 min) and fractionated. Chimaerins were detected by Western blot. Numbers below the blot represent the fold-change in protein present in the insoluble fraction with respect to WT, no PMA. C, COS-1 cells expressing the indicated β2-chimaerin mutants were treated with EGF (100 ng/ml, 5 min). Phosphorylation was monitored by Western blot. Left, representative Western blot. Right, data presented relative to WT (+ EGF) expressed as mean ± S.E.
We reasoned that if phosphorylated β2-chimaerin is found in the cytosol, a mutant of β2-chimaerin that is unable to translocate to membranes would have enhanced phosphorylation, whereas a mutant with enhanced membrane association would have reduced levels of phosphorylation. We took advantage of the C1 domain mutant P223A-β2-chimaerin (Pro11 in the C1 domain consensus (31)), which has impaired ability to bind DAG or phorbol esters, whereas still preserving the overall C1 domain structure. This mutant was unable to translocate to membranes upon PMA stimulation (Fig. 5B). We also used the hyperactive mutant I130A-β2-chimaerin. Ile130 stabilizes the closed conformation of β2-chimaerin by interacting with the C1 domain. Mutation of Ile130 “exposes” the C1 domain and enhances association to membranes (21, 25). Striking differences in Ser169 phosphorylation levels of these proteins were seen in COS-1 cells. While P223A-β2-chimaerin had significantly higher phosphorylation levels than WT-β2-chimaerin under basal conditions and in response to EGF, phosphorylation of I130A-β2-chimaerin was barely detectable (Fig. 5C). Thus, subcellular protein localization of β2-chimaerin is key for determining its phosphorylation status.
To further explore whether phosphorylation of Ser169 affects β2-chimaerin relocalization, we analyzed the effect of PKC inhibition on PMA-induced translocation. We predicted that inhibition of Ser169 phosphorylation would favor β2-chimaerin translocation. Interestingly, when cells were treated with PMA in the absence of the PKC inhibitor GF, β2-chimaerin translocation to the insoluble fraction was not detected (Fig. 6A, left). Under this experimental condition a robust increase in phospho-Ser169 levels was also observed. In contrast, GF treatment blocked β2-chimaerin phosphorylation and allowed for PMA-induced translocation, arguing that phosphorylation of Ser169 prevents translocation of the protein.
FIGURE 6.
Phosphorylated β2-chimaerin is unable to translocate to membranes in response to PMA. A, COS-1 cells expressing WT or mutant β2-chimaerin were treated with PMA (1 μm, 5 min) with or without GF (10 μm, 1 h). Cell lysates were fractionated and β2-chimaerin was detected by Western blot. B, GFP-tagged WT- or S169A-β2-chimaerin localization in COS-1 cells in response to PMA (1 μm, 5 min) ± GF (10 μm, 1 h) was monitored by confocal microscopy.
We reasoned that if translocation of β2-chimaerin depends on its phosphorylation status, then the non-phosphorylatable S169A mutant should translocate in response to PMA regardless of whether or not GF is present. Indeed, we found that, unlike WT-β2-chimaerin, S169A-β2-chimaerin translocates upon PMA stimulation both in the absence and presence of GF (Fig. 6A, center), further suggesting that phosphorylation of Ser169 negatively regulates translocation to membranes. To ensure that the ability of the S169A mutant to translocate was driven by specific binding of PMA to the C1 domain of S169A-β2-chimaerin, we also created the S169A/P223A-β2-chimaerin phorbol ester/DAG unresponsive double mutant. Like P223A-β2-chimaerin (Fig. 5B), S169A/P223A-β2-chimaerin also failed to translocate in response to PMA, regardless of the presence or absence of GF (Fig. 6A, right). This indicates that S169A-β2-chimaerin translocation is dependent upon a functional C1 domain.
To recapitulate and verify the fractionation results, we also performed confocal microscopy studies using GFP-tagged chimaerins. As previously shown (22), GFP-β2-chimaerin is predominantly cytoplasmic. Translocation of GFP-β2-chimaerin by PMA in the absence of GF was barely detected (Fig. 6B). Remarkably, GF restored the ability of PMA to translocate GFP-β2-chimaerin. On the other hand, a pronounced translocation of GFP-S169A-β2-chimaerin to the plasma membrane was observed in the absence of GF. These results further establish a negative role for Ser169 phosphorylation in membrane translocation of β2-chimaerin.
The Non-phosphorylatable Form of β2-Chimaerin Has Enhanced Binding to Activated Rac1
To determine whether the enhanced GAP activity of S169A-β2-chimaerin in cells was due to changes in the intrinsic Rac-GAP activity, we performed in vitro GAP assays using purified recombinant Rac1 and β2-chimaerin proteins. GTP hydrolysis was measured using a fluorescent phosphate sensor that detects inorganic phosphate (32). Recombinant S169A-β2-chimaerin had a nearly equal level of GAP activity when compared with WT protein (Fig. 7). As a control, we used recombinant β1-chimaerin, a related chimaerin isoform that lacks the N-terminal autoinhibitory domain. In agreement with previous results (25), β1-chimaerin has markedly higher in vitro GAP activity. These results indicate that the enhanced Rac-GAP activity of S169A-β2-chimaerin in cells is not due to enhanced intrinsic GAP activity of the protein.
FIGURE 7.
S169A-β2-chimaerin does not have enhanced Rac-GAP activity in vitro. In vitro Rac-GAP activity of recombinant chimaerin proteins is shown. Results are expressed as mean ± S.E. (n = 3).
β2-Chimaerin has previously been shown to bind to Rac1 in its active form in response to serum or EGF (8, 20). Given the enhanced GAP activity of the S169A-β2-chimaerin mutant in cells, we hypothesized that this mutant may differentially bind to Rac1. To address this issue we assessed binding to GST-V12Rac1 in COS-1 cells using a GST pulldown assay. Fig. 8A shows that the association of S169A-β2-chimaerin to V12Rac1 in cells is 3-fold higher than WT-β2-chimaerin. We hypothesized that binding of S169A-β2-chimaerin to Rac1 may be due to its enhanced ability to translocate to membranes and hence, enhanced access to the membrane-localized V12Rac1. To test this, we compared V12Rac1 binding of S169A-β2-chimaerin to that of the P223A/S169A-β2-chimaerin double mutant that cannot translocate to membranes. Indeed, we found that binding of S169A/P223A-β2-chimaerin to V12Rac1 is much lower than that of S169A-β2-chimaerin (Fig. 8B). This suggests that the enhanced ability of the S169A mutant to bind to active Rac1 in cells requires a functional C1 domain and is therefore due largely to enhanced association to membranes.
FIGURE 8.
Enhanced association of S169A-β2-chimaerin to V12Rac1. A and B, lysates from COS-1 cells co-expressing GST or GST-V12Rac1 and the indicated β2-chimaerin mutants were subjected to GST pulldown. HA-β2-chimaerin bound to GST beads was detected by Western blot. Left, representative Western blots. Right, data presented relative to WT- (A) or S169A-β2-chimaerin (B) expressed as mean ± S.E. (n = 4–5), **, p < 0.01; ***, p < 0.005.
DISCUSSION
Chimaerins are the only known Rac-GAPs regulated by the lipid second messenger DAG in response to tyrosine kinase receptor activation. Here we have provided the first evidence that β2-chimaerin is phosphorylated in a novel mechanism of DAG receptor cross-talk. EGF and PMA rapidly induce phosphorylation of Ser169 of β2-chimaerin, and PKCδ is implicated in this phosphorylation. Phosphorylation of Ser169 of β2-chimaerin appears to be a means of limiting its access to Rac at the plasma membrane, thereby resulting in reduced activation of β2-chimaerin in cells. A model for this paradigm is presented in Fig. 9. We postulate that rapid EGF-induced phosphorylation of β2-chimaerin may be a mechanism of limiting the strength of and/or spatially restricting the extent of β2-chimaerin activation.
FIGURE 9.
Proposed model for β2-chimaerin regulation by Ser169 phosphorylation. Under resting conditions, β2-chimaerin remains in an inactive, closed conformation in the cytosol. Upon EGFR stimulation, DAG generated via phospholipase Cγ activates PKCδ, which in turn phosphorylates β2-chimaerin on Ser169. Phosphorylated β2-chimaerin is unable to translocate to membranes and remains inactive in the cytosol. On the other hand, non-phosphorylated β2-chimaerin is subject to allosteric activation by DAG and acidic phospholipids in the plasma membrane, where it inactivates Rac.
The current mechanism of activation for β2-chimaerin is derived from the solved crystal structure of the protein (21). β2-Chimaerin exists in an inactive conformation in which extensive intramolecular interactions occlude the DAG/phorbol ester binding site of the C1 domain as well as the Rac interacting site of the GAP domain. Upon DAG generation or PMA treatment, the inactive conformation of β2-chimaerin must undergo an extensive conformational rearrangement that exposes large hydrophobic patches along the length of the protein that are thought to align with the membrane to avoid interactions with polar solvent. This activates the protein by positioning β2-chimaerin at the membrane for interaction with DAG and its effector Rac.
Given that non-phosphorylated β2-chimaerin is the predominant form of the protein found translocated to membranes, we propose that this is the main species that is susceptible to the allosteric activation and conformational rearrangement that serves to activate the protein. Indeed, most of the previous studies of PMA-induced translocation of β2-chimaerin to membranes were done in the presence of the pan-PKC inhibitor GF to avoid phorbol ester ligand binding competition with PKC isoforms. Based on the present studies, we know that treatment with GF blocks PMA-induced phosphorylation of Ser169, and thus most of the protein remains in the non-phosphorylated form and is able to translocate. Furthermore, we have shown here that PMA treatment in the absence of GF is unable to cause translocation of the WT protein, whereas the S169A-β2-chimaerin mutant readily translocates to membranes upon PMA stimulation even in the absence of GF. This is not due to a change in the overall intrinsic ability of the mutant protein to translocate, as S169A-β2-chimaerin translocates to membranes with a similar EC50 of phorbol ester-induced translocation, and as WT-β2-chimaerin in the presence of GF (data not shown). These results strongly suggest that when phosphorylated on Ser169, β2-chimaerin is unable to undergo the allosteric activation and conformational rearrangement needed for full activation. Indeed, this is most likely the reason why S169A-β2-chimaerin is more active in cells: it is more readily available for translocation and hence activation due to a lack of Ser169 phosphorylation. Some Rac inactivation is observed with wild-type β2-chimaerin, as more than 50% of the protein remains non-phosphorylated (Fig. 2C) and therefore available for activation.
One important question is, what is the physiological role of Ser169 phosphorylation of β2-chimaerin. One potential model we envision is that upon receptor tyrosine kinase stimulation, a fraction of β2-chimaerin becomes rapidly phosphorylated on Ser169, and this phosphorylated fraction is unable to translocate to membranes and become activated, thus allowing for Rac1 activation to occur. By limiting the amount of β2-chimaerin in the cell that is available for activation, the strength of β2-chimaerin activation can be controlled; hence, the magnitude of Rac1 activation can also be regulated. Furthermore, this may serve as a mechanism to spatially restrict β2-chimaerin activation to cell compartments where inactivation of Rac is crucial, whereas simultaneously limiting the amount of activated β2-chimaerin available in compartments where Rac activation is essential. This may be especially important for regulating processes in which spatial restriction of Rac activation is important, such as directional migration (33).
The mechanism through which Ser169 phosphorylation precludes translocation has not been identified thus far, but one hypothesis is that phosphorylation of Ser169 may alter interactions with a binding partner that serves to sequester β2-chimaerin away from sites of DAG production. Recent studies have shown that chimaerins interact with Nck1 and -2, multidomain adaptor proteins that may play an important role in linking signaling by the related α2-chimaerin isoform with Ephrin A2 signaling to control neuronal processes (11). The mechanism through which Nck regulates the signaling of chimaerins is not well studied as yet, but preliminary evidence suggests that Nck may bind inactive β2-chimaerin and position it close to membranes, thus bringing it into proximity with sites of DAG generation and facilitating activation.3 If this were the case, β2-chimaerin would also be positioned near potential sites of PKCδ activation, which would in turn preclude activation of β2-chimaerin through phosphorylation. Studies to determine the effect of Ser169 phosphorylation on Nck binding to β2-chimaerin are underway.
Although this study is the first to report Ser phosphorylation of β2-chimaerin, two alternate papers have recently reported Tyr phosphorylation of β2-chimaerin. Phosphorylation at Tyr21 of β2-chimaerin by Src family kinases was reported in response to EGF (34). Like Ser169 phosphorylation, Tyr21 phosphorylation negatively regulates the GAP activity of β2-chimaerin, although the mechanism through which this occurs was not reported. Interestingly, the kinetics of EGF-induced Tyr21 phosphorylation are markedly different from those of Ser169 phosphorylation, with Tyr21 phosphorylation being detectable 5 min post-EGF treatment and increasing up to 30 min following stimulation. This indicates that β2-chimaerin may be regulated in two phases. Initial Ser169 phosphorylation may play a role in limiting the magnitude of β2-chimaerin activation, whereas subsequent Tyr21 phosphorylation may be more important for down-regulation of the activated protein. It is important to note, however, that EGF-induced Tyr21 phosphorylation of β2-chimaerin was only seen in the presence of tyrosine phosphatase inhibitors. Indeed, we did not detect induced Tyr or Thr phosphorylation of β2-chimaerin upon EGF stimulation in the absence of tyrosine phosphatase inhibitors (supplemental Fig. S5), thus suggesting that EGF-induced Tyr21 phosphorylation is probably transient and limited in extent.
Phosphorylation of a second Tyr residue, Tyr153, was recently reported to occur in response to T-cell receptor stimulation via the tyrosine kinase Lck (35). Like Ser169 phosphorylation, Tyr153 phosphorylation negatively regulates Rac-GAP activity of β2-chimaerin, as a non-phosphorylatable mutant enhanced Rac-GAP activity and correspondingly decreased such Rac-mediated T-cell events as actin reorganization in response to immune synapse formation. Interestingly, under the same conditions of T-cell receptor stimulation, Ser169 phosphorylation of β2-chimaerin was not observed (supplemental Fig. S6), indicating that regulation of β2-chimaerin by phosphorylation is largely cell-type and stimulus specific. The authors hypothesize that Tyr153 phosphorylation in T-cells is a mechanism for down-regulation of the protein by promoting dissociation of the activated protein from membranes, although they do not explore the mechanisms through which this dissociation may occur. However, mutation of Tyr153 to Phe leads to PMA-induced translocation of β2-chimaerin to membranes in a manner analogous to that seen for the I130A-β2-chimaerin hyperactive mutant. We would suggest that the T153F-β2-chimaerin mutation also destabilizes intramolecular contacts within the protein, and that this mutant functions differently from the S169A mutation, which we believe does not destabilize the inactive closed conformation of β2-chimaerin, but still has an enhanced ability to translocate to membranes. It has become clear from our study and others that regulation of β2-chimaerin via phosphorylation is a complex process that is most likely cell-type and stimulus-specific, and further experiments will be necessary to determine the relative contributions of each identified phosphorylation site to the activation and/or down-regulation of β2-chimaerin.
The concept of cross-talk between DAG receptors is one that has not been fully appreciated to date. There are currently two known examples of one DAG receptor regulating another. First, RasGRP3, a Ras guanine nucleotide exchange factor expressed in B cells, is phosphorylated by PKCs on Thr133 upon B-cell receptor activation (36, 37). This phosphorylation is essential for activation of RasGRP3 and consequent activation of Ras in response to B-cell receptor stimulation. Second, PKD1–3, Ser/Thr kinases important for mediating such cellular processes as membrane trafficking, migration, and proliferation are also phosphorylated by PKCs (38). Indeed, PKD1 must be phosphorylated by PKC on both Ser744 and Ser748 to relieve autoinhibition and activate the protein (39). In these two cases DAG serves as a dual role in regulating these proteins, first through direct binding to their C1 domains, and second through activation via PKC-mediated phosphorylation. Interestingly, DAG also regulates the localization and hence activation of β2-chimaerin in a reciprocal manner: whereas phosphorylation through PKCδ leads to negative regulation of the protein through restricted localization to the cytosol, ligand binding to DAG is required for translocation of unphosphorylated β2-chimaerin to membranes and activation. The relative concentrations of DAG and PKCδ in any one location of the cell will likely greatly influence the activation status of β2-chimaerin. Furthermore, enhanced PKCδ activation will in turn lead to decreased β2-chimaerin translocation/activation. Thus, it is likely that very tightly regulated mechanisms for controlling relative PKCδ and β2-chimaerin activation exist in cells.
This work identifies for the first time a regulatory mechanism for β2-chimaerin via Ser phosphorylation. In addition to revealing a novel phosphorylation site on β2-chimaerin and determining that phosphorylation on Ser169 negatively regulates activity and localization, we also provide the first evidence for a paradigm of cross-talk between DAG receptors β2-chimaerin and PKCδ. The fact that Rac-GAPs can be regulated by PKCs suggests that the lipid second messenger DAG plays an important role in controlling the activation status of the small GTPase Rac and highlights the complexity of DAG signaling via multiple C1 domain-containing proteins.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grants CA09677 and CA74197.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S6.
F. Colón-González and M. G. Kazanietz, unpublished data.
- DAG
- diacylglycerol
- PKC
- protein kinase C
- GAP
- GTPase-activating protein
- PMA
- phorbol 12-myristate 13-acetate
- AdV
- adenoviruses
- NTS
- non-targeting sequence
- SH2
- Src homology domain 2
- EGFR
- epidermal growth factor receptor
- HA
- hemagglutinin
- GST
- glutathione S-transferase
- MOPS
- 4-morpholinepropanesulfonic acid
- BisTris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol; MALDI-TOF, matrix-assisted laser desorption ionization time-of-flight
- shRNA
- short hairpin RNA
- FBS
- fetal bovine serum
- WT
- wild-type
- GF
- GF 109203X.
REFERENCES
- 1.Blumberg P. M., Delclos K. B., Dunn J. A., Jaken S., Leach K. L., Yeh E. (1983) Ann. N.Y. Acad. Sci. 407, 303–315 [DOI] [PubMed] [Google Scholar]
- 2.Colón-González F., Kazanietz M. G. (2006) Biochim. Biophys. Acta 1761, 827–837 [DOI] [PubMed] [Google Scholar]
- 3.Griner E. M., Kazanietz M. G. (2007) Nat. Rev. Cancer 7, 281–294 [DOI] [PubMed] [Google Scholar]
- 4.Newton A. C. (2003) Biochem. J. 370, 361–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parekh D. B., Ziegler W., Parker P. J. (2000) EMBO J. 19, 496–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caloca M. J., Fernandez N., Lewin N. E., Ching D., Modali R., Blumberg P. M., Kazanietz M. G. (1997) J. Biol. Chem. 272, 26488–26496 [DOI] [PubMed] [Google Scholar]
- 7.Caloca M. J., Garcia-Bermejo M. L., Blumberg P. M., Lewin N. E., Kremmer E., Mischak H., Wang S., Nacro K., Bienfait B., Marquez V. E., Kazanietz M. G. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 11854–11859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caloca M. J., Wang H., Kazanietz M. G. (2003) Biochem. J. 375, 313–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buttery P., Beg A. A., Chih B., Broder A., Mason C. A., Scheiffele P. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 1924–1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi L., Fu W. Y., Hung K. W., Porchetta C., Hall C., Fu A. K., Ip N. Y. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 16347–16352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wegmeyer H., Egea J., Rabe N., Gezelius H., Filosa A., Enjin A., Varoqueaux F., Deininger K., Schnütgen F., Brose N., Klein R., Kullander K., Betz A. (2007) Neuron 55, 756–767 [DOI] [PubMed] [Google Scholar]
- 12.Beg A. A., Sommer J. E., Martin J. H., Scheiffele P. (2007) Neuron 55, 768–778 [DOI] [PubMed] [Google Scholar]
- 13.Hall C., Michael G. J., Cann N., Ferrari G., Teo M., Jacobs T., Monfries C., Lim L. (2001) J. Neurosci. 21, 5191–5202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bruinsma S. P., Cagan R. L., Baranski T. J. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 7098–7103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leskow F. C., Holloway B. A., Wang H., Mullins M. C., Kazanietz M. G. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 5373–5378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Menna P. L., Skilton G., Leskow F. C., Alonso D. F., Gomez D. E., Kazanietz M. G. (2003) Cancer Res. 63, 2284–2291 [PubMed] [Google Scholar]
- 17.Yang C., Liu Y., Leskow F. C., Weaver V. M., Kazanietz M. G. (2005) J. Biol. Chem. 280, 24363–24370 [DOI] [PubMed] [Google Scholar]
- 18.Hoelzinger D. B., Mariani L., Weis J., Woyke T., Berens T. J., McDonough W. S., Sloan A., Coons S. W., Berens M. E. (2005) Neoplasia 7, 7–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yuan S., Miller D. W., Barnett G. H., Hahn J. F., Williams B. R. (1995) Cancer Res. 55, 3456–3461 [PubMed] [Google Scholar]
- 20.Wang H., Yang C., Leskow F. C., Sun J., Canagarajah B., Hurley J. H., Kazanietz M. G. (2006) EMBO J. 25, 2062–2074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Canagarajah B., Leskow F. C., Ho J. Y., Mischak H., Saidi L. F., Kazanietz M. G., Hurley J. H. (2004) Cell 119, 407–418 [DOI] [PubMed] [Google Scholar]
- 22.Caloca M. J., Wang H., Delemos A., Wang S., Kazanietz M. G. (2001) J. Biol. Chem. 276, 18303–18312 [DOI] [PubMed] [Google Scholar]
- 23.Zhou P., Porcionatto M., Pilapil M., Chen Y., Choi Y., Tolias K. F., Bikoff J. B., Hong E. J., Greenberg M. E., Segal R. A. (2007) Neuron 55, 53–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colón-González F., Leskow F. C., Kazanietz M. G. (2008) J. Biol. Chem. 283, 35247–35257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sosa M. S., Lewin N. E., Choi S. H., Blumberg P. M., Kazanietz M. G. (2009) Biochemistry 48, 8171–8178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leung T., How B. E., Manser E., Lim L. (1994) J. Biol. Chem. 269, 12888–12892 [PubMed] [Google Scholar]
- 27.Reichardt L. F. (2006) Philos. Trans. R. Soc. Lond. B Biol. Sci. 361, 1545–1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davies S. P., Reddy H., Caivano M., Cohen P. (2000) Biochem. J. 351, 95–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gómez-del Arco P., Maki K., Georgopoulos K. (2004) Mol. Cell Biol. 24, 2797–2807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang W., Erikson R. L. (1994) Proc. Natl. Acad. Sci. U.S.A. 91, 8960–8963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kazanietz M. G., Wang S., Milne G. W., Lewin N. E., Liu H. L., Blumberg P. M. (1995) J. Biol. Chem. 270, 21852–21859 [DOI] [PubMed] [Google Scholar]
- 32.Brune M., Hunter J. L., Corrie J. E., Webb M. R. (1994) Biochemistry 33, 8262–8271 [DOI] [PubMed] [Google Scholar]
- 33.Ridley A. J., Schwartz M. A., Burridge K., Firtel R. A., Ginsberg M. H., Borisy G., Parsons J. T., Horwitz A. R. (2003) Science 302, 1704–1709 [DOI] [PubMed] [Google Scholar]
- 34.Kai M., Yasuda S., Imai S., Kanoh H., Sakane F. (2007) Biochim. Biophys. Acta 1773, 1407–1415 [DOI] [PubMed] [Google Scholar]
- 35.Siliceo M., Mérida I. (2009) J. Biol. Chem. 284, 11354–11363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aiba Y., Oh-hora M., Kiyonaka S., Kimura Y., Hijikata A., Mori Y., Kurosaki T. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 16612–16617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Teixeira C., Stang S. L., Zheng Y., Beswick N. S., Stone J. C. (2003) Blood 102, 1414–1420 [DOI] [PubMed] [Google Scholar]
- 38.Wang Q. J. (2006) Trends Pharmacol. Sci. O 27, 317–323 [DOI] [PubMed] [Google Scholar]
- 39.Waldron R. T., Rozengurt E. (2003) J. Biol. Chem. 278, 154–163 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.