Abstract
The increased expression of McPIP2;1 (MipC), a root-specific aquaporin (AQP) from Mesembryanthemum crystallinum, under salt stress has suggested a role for this AQP in the salt tolerance of the plant. However, whether McPIP2;1 transports water or another solute and how its activity is regulated are so far unknown. Therefore, wild type (wt) or mutated McPIP2;1 protein was expressed in Xenopus laevis oocytes. Then, the osmotic water permeability (Pf) of the oocytes membrane was assessed by hypotonic challenges. Selectivity of McPIP2;1 to water was determined by radiolabeled glycerol or urea uptake assays. Moreover, swelling and in vitro phosphorylation assays revealed that both water permeation and phosphorylation status of McPIP2;1 were significantly increased by the phosphorylation agonists okadaic acid (OA), phorbol myristate acetate (PMA), and 8-Br-cAMP, and markedly decreased by the inhibitory peptides PKI 14-22 and PKC 20-28, inhibitors of protein kinases A (PKA) and C (PKC), respectively. Substitution of Ser123 or both, Ser123 and Ser282, abolished the water channel activity of McPIP2;1 while substitution of Ser282 only partially inhibited it (51.9% inhibition). Despite lacking Ser123 and/or Ser282, the McPIP2;1 mutant forms were still phosphorylated in vitro, which suggests that phosphorylation may have a dual role on this AQP. Our results indicate that McPIP2;1 water permeability depends completely on Ser123 and is positively regulated by PKA- and PKC-mediated phosphorylation. Regulation of the phosphorylation status of McPIP2;1 may contribute to control water transport through root cells when the plant is subjected to high salinity conditions.
Keywords: Channel/Water, Membrane/Channels, Membrane/Proteins, Organisms/Plant, Organisms/Xenopus, Phosphorylation
Introduction
Aquaporins (AQPs)3 are small integral membrane proteins in the range of 25–34 kDa that form channels through which water and/or small neutral solutes can cross driven by a concentration gradient (1–4). They have been found in microorganisms, animals, and plants and show a highly conserved structure (5). In plants, AQPs are phylogenetically classified into four subfamilies (6): plasma membrane intrinsic proteins (PIPs), tonoplast intrinsic proteins (TIPs), Nodulin26-like intrinsic proteins (NIPs), and small basic intrinsic proteins (SIPs). In addition, members of two new subfamilies, the hybrid intrinsic proteins (HIPs) and the X intrinsic proteins (XIPs) have been found in some plant species (7). Interestingly, by heterologous expression in Xenopus laevis oocytes it has been shown that the PIP, TIP, and NIP subfamilies include at least one isoform transporting another solute in addition to water. Some plant AQPs have been found to transport small neutral solutes such as glycerol (8, 9), urea (10, 11), boric acid (12), silicic acid (13), ammonia (NH3, 14), arsenite (15), or CO2 (16). In accordance with these transport properties and genetic studies, plant AQPs have been implicated in physiological processes such as water absorption by roots, nutrient uptake, tissue expansion, transpiration, photosynthesis, and resistance to salt and water stresses (1, 3, 17–20).
The water channel activity of some plant AQPs has been proved to be affected by phosphorylation. By heterologous expression in X. laevis oocytes it has been shown that the water channel activity of the bean seed tonoplast AQP PvTIP3;1 is enhanced in the presence of cAMP agonists, which stimulate oocyte protein kinase A (PKA, 21). Similarly, the activity of SoPIP2;1 from spinach leaf and GmNodulin26 from soybean root nodule has been shown to increase in the presence of okadaic acid (OA), a protein phosphatases inhibitor, and phorbol myristate acetate (PMA), a protein kinase C (PKC) agonist (22, 23). In these studies, the use of mutants has revealed that Ser262 from GmNodulin26, Ser115 from SoPIP2;1 and Ser7, Ser23, and Ser99 from PvTIP3;1 are important, but not indispensable, for the water channel activity of such AQPs (21–23).
Moreover, several plant AQPs have been shown to be phosphorylated in vivo and in vitro at serine residues within the N- or C-terminal regions. Among them are the bean seed tonoplast AQP PvTIP3;1 (Ser7, 24, 25), the spinach leaf SoPIP2;1 (Ser274 and Ser277, 26), the soybean root nodule GmNodulin26 (Ser262, 27–29), several Maize PIP1s and PIP2s (30) and some Arabidopsis thaliana PIP2 proteins (31). Surprisingly, phosphorylation of the serine residue located in the cytoplasmic loop B close to the first NPA motif has not yet been observed, despite the high degree of conservation of this residue in all plant PIPs and several TIPs that suggest it has an important functional role.
In the ice plant, Mesembryanthemum crystallinum, 17 AQPs have been found so far (32). One of these, McPIP2;1 (MipC), has been shown to be expressed specifically in roots (33), where an essential physiological role for this AQP is implied. It has been shown to be present in all cells of the immature root coinciding with the elongation zone close to the root tip, most strongly in the epidermis. Furthermore, at the subcellular level, McPIP2;1 has been found at the plasma membrane, the vacuole membrane (tonoplast) and other subcellular compartments.4 Besides, McPIP2;1 amounts have been shown to increase in response to salt (33) and osmotic stress.4 The ice plant is a halophyte, it grows and develops in salty soils where it has to succeed in contending the osmotic changes imposed by alterations in the amount of salt. Therefore, a fundamental role for McPIP2;1 in salt tolerance of the ice plant is inferred. However, the activity and regulation of this AQP as a water channel have not yet been investigated.
Here, we show that PKA and PKC phosphorylation pathways positively regulate the water channel activity of McPIP2;1 when expressed in X. laevis oocytes. This AQP transports water very efficiently but not glycerol or urea, classifying it as a water-selective AQP. Incubation of McPIP2;1-injected oocytes with OA, PMA, or 8-Br-cAMP induces an increase in both phosphorylation level and water channel activity of McPIP2;1 expressed at the oocyte membrane. On the contrary, inhibition of PKA and PKC reduces the phosphorylation level and the water permeability of McPIP2;1. At the molecular level, the water permeability of McPIP2;1 depends completely on Ser123 and partially on Ser282 with approximately half of the overall effect.
EXPERIMENTAL PROCEDURES
Heterologous Expression in Xenopus laevis Oocytes
cDNAs encoding McTIP1;2 (19), wild-type (wt) McPIP2;1, or the mutant forms McPIP2;1-S123A, McPIP2;1-S123N, McPIP2;1-S123F, McPIP2;1-S282A, McPIP2;1-S282N, McPIP2;1-S282F, and McPIP2;1-S123/282A were cloned into the pGEM-HE vector. The GlpF and AtNIP6;1F clones were kindly provided by Dr. Chaumont (8) and Dr. Roberts (10), respectively. cRNAs from McTIP1;2 and wt or mutated forms of McPIP2;1 were prepared with the T7 RNA polymerase after linearization with NheI, while cRNAs from GlpF and AtNIP6;1F were prepared with the T3 RNA polymerase after linearization with XbaI, using the mMESSAGE mMACHINE in vitro transcription kit (Ambion, Austin, TX). Defolliculated oocytes were injected with 46.0 nl of DEPC-H2O or the corresponding cRNA (1 μg/μl) using a NANOJECT II automatic injector (Drummond, Broomall, PA). Oocytes were incubated in iso-osmotic (200 mosmol/kg) Barth's solution (10 mm HEPES-NaOH, pH 7.4, 88 mm NaCl, 1 mm KCl, 2.4 mm NaHCO3, 0.33 mm Ca(NO3)2, 0.41 mm CaCl2, 0.82 mm MgSO4) for 4–5 days before swelling assays.
Radiolabeled Solute Uptake by Xenopus laevis Oocytes
Radiolabeled glycerol and urea uptake were performed 4–5 days after injection following a modified method described by Wallace et al. (10). Groups of five oocytes were incubated at room temperature (25 °C) in 0.5 ml of iso-osmotic Barth's solution containing 100 mm unlabeled glycerol and 60 μCi/ml [3H]glycerol (3.0 Ci/mmol, Amersham Biosciences, Buckinghamshire, UK), or 100 mm unlabeled urea and 30 μCi/ml [14C]urea (60.0 mCi/mmol, Amersham Biosciences, Buckinghamshire, UK). After 10 min, oocytes were washed five times with ice-cold iso-osmotic Barth's solution containing 200 mm unlabeled glycerol or 200 mm unlabeled urea. Oocytes were lysed overnight in 2 ml of 2% SDS at room temperature. Isotopic uptake was determined by liquid scintillation counting (LS6500, Beckman Coulter, Fullerton, CA).
Site-directed Mutagenesis
McPIP2;1 potential phosphorylation residues Ser123 or Ser282, or both, were mutated by site-directed mutagenesis (BioS & T, Quebec, CA). Single mutations were made by replacing Ser123 or Ser282 with alanine, asparagine, or phenylalanine. Double mutation was made by replacing both Ser123 and Ser282 with alanine.
Swelling Assays
Swelling assays were performed by the transfer of the oocyte from iso-osmotic to hypo-osmotic (100 mosmol/kg) Barth's solution. After the transfer of the cell, volume changes were recorded with a Hitachi KP-D50 color video camera (Hitachi Denshi, Woodbury, NY) on a Nikon Eclipse TE 300 inverted microscope (Nikon, Mexico). Images were captured at intervals of 10 s for 2 min and digitized by the Image-Pro Plus software (Version 4, Media Cybernetics, Silver Spring, MD). The data were analyzed as proportional change in volume and normalized to the initial volume at time 0. All the assays were performed at room temperature (25 °C). To test the effect of agonists and antagonists of protein phosphorylation, oocytes were incubated for 30 min in iso-osmotic Barth's solution containing either 5 μm OA (okadaic acid, Sigma-Aldrich, Mexico), 10 nm PMA (phorbol 12-myristate 13-acetate, Sigma-Aldrich, Mexico), 0.1% DMSO, 40 μm 8-Br-cAMP (8-bromoadenosine-3′,5′-cyclic-monophosphate; Calbiochem-Novabiochem, La Jolla, CA), 1 μm protein kinase A inhibitor (PKI) 14–22 amide, cell permeable, myristoylated (PKA-14–22-Myr-GRTGRRNAI-NH2, Calbiochem-Novabiochem, La Jolla, CA), or 100 μm PKC inhibitor 20–28, cell permeable, myristoylated (PKC-20–28- Myr-N-FARKGALRQ-NH2; Calbiochem-Novabiochem, La Jolla, CA) before swelling assays. The volume increase values were calculated for each independent set of experiments.
Oocyte Membrane Isolation and Western Blotting Analysis
Oocyte membrane fractions were isolated from DEPC-H2O- or cRNA-injected oocytes following a modified method described by Fetter et al. (34). 20 oocytes were homogenized in 500 μl of ice-cold hypotonic phosphate buffer (10 mm KH2PO4, 5 mm EDTA, 5 mm EGTA, 1 mm phenylmethylsulfonyl fluoride, pH 7.6) with a pestle. All steps were done at 4 °C. Yolk proteins and cellular debris were pelleted at 110 × g for 5 min. Membranes were then pelleted at 20,800 × g for 30 min and resuspended with 50 μl of hypotonic phosphate buffer. Isolated membranes were highly enriched in plasma membrane and contained negligent amounts of endoplasmic reticulum and mitochondria (supplemental Table S1). Protein content in membrane fractions was measured as described by Vera-Estrella et al. (19). Afterward, 50 μg of membrane proteins were precipitated by dilution of the membranes 50-fold in 1:1 ethanol/acetone and incubated overnight at −30 °C. Precipitated proteins were collected at 13,000 × g for 20 min. Pellets were air dried and resuspended with 2.5%-Laemmli sample buffer (35). 20 μg of membrane proteins were heated at 60 °C for 2 min before loading onto a 12.5% polyacrylamide SDS gel. SDS-PAGE-separated proteins were electrophoretically transferred onto nitrocellulose membranes (ECL, Amersham Biosciences, Buckinghamshire, UK). Following transfer, blots were incubated with Ponceau S protein stain (0.1% in 1% acetic acid) for 30 s to check for equal loading/transfer of proteins. Blots were then blocked with TBS (100 mm Tris, 150 mm NaCl) containing 0.02% NaN3 and 5% fat-free powdered milk (Svelty, Nestle, Mexico) for 2 h at room temperature. Blocked membranes were incubated with anti-McPIP2;1 primary antibody raised against a peptide from the extracellular loop C of the protein (1:500, HTI-BioProducts, Ramona, CA,33) at room temperature overnight. Blots were washed three times for 10 min with TBS-T (0.1% Tween 20 in TBS) and incubated with goat anti-rabbit secondary antibody conjugated to horseradish peroxidase (1:3000, Invitrogen, Carlsbad, CA) for 1 h at room temperature. After washing again three times with TBS-T, immunodetection was carried out using the chemiluminescent ECL Western blotting analysis system (GE Healthcare, Buckinghamshire, UK).
In Vitro Phosphorylation and Immunoprecipitation Assays
Isolated oocytes membranes (30 μg of protein) were incubated in a 100-μl reaction mixture containing 24 mm Tris/MES, pH 6.5, 10 mm MgCl2, 0.45 mm EDTA, 2 mm dithiothreitol, 0.55 mm CaCl2, and 1 μl of [γ-32P]ATP (10 μCi/μl) for 45 min at room temperature (25 °C). Afterward, membrane proteins were solubilized with 400 μl of NET-gel buffer (50 mm Tris/HCl, pH 7.5, 150 mm NaCl, 1 mm EDTA, 0.1% Nonidet P-40, 0.25% bovine serum albumin, 0.02% NaN3) and incubated overnight at 4 °C with anti-McPIP2;1 antibodies (1/500 dilution) on a rotating table. Protein A-Sepharose CL-4B (20 μl, Amersham Biosciences, Uppsala) was added and incubation continued for 2 h at 4 °C. Membrane proteins were pelleted at 12,000 × g for 20 s. After two washings with NET-gel buffer, the protein pellet was washed with 10 mm Tris/HCl, pH 7.5, and 0.1% Nonidet P-40, and centrifuged at 12,000 × g for 20 s. The pellet was then resuspended with 20 μl of 2.5%-Laemmli sample buffer and heated at 95 °C for 3 min. Immunoprecipitated proteins were resolved by 12.5% SDS-PAGE. After staining, the gel was dried under vacuum for 3 h. Autoradiography was carried out by exposing the gel to Kodak X-OMAT film at −80 °C for 24 h.
Homology Modeling of McPIP2;1
Sequence alignments of McPIP2;1, SoPIP2;1, ZmPIP2;1, and PvTIP3;1 were done by using CLC Sequence Viewer application (CLC bio, supplemental Figs. S1 and S2). Homology models for McPIP2;1 were generated using the automated modeling server MODWEB (36). The automated software pipeline MODPIPE calculated the comparative models based on the best available templates from the Protein Data Bank (PDB). Resultant models were annotated into the MODBASE database. Modeling evaluation was made by a composite model assessment criterion that depends on the compactness of the model, the sequence identity of the sequence-structure match, and statistical energy Z-scores (37). Homology models deduced from the alignment of McPIP2;1 with the open (PDB code, 2B5F) or the closed conformation (PDB code, 1Z98) of SoPIP2;1 were selected for further use in structural analysis. These two resultant comparative models were predicted to be reliable with a probability of the correct fold larger than 95% according to the model score of 1.0 for each derived from statistical potentials (37). Structural analysis and editing of the three-dimensional structures was performed using the Swiss-PdbViewer 4.0.1 application (38).
Data Analysis
The osmotic water permeability (Pf) of the oocytes plasma membrane was calculated as the change in volume relative to the change in osmolarity of the external media, by the relation,
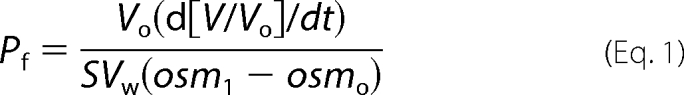 |
where Vo is the initial oocyte volume at time zero, V/Vo is the relative volume, Vw is the partial molar volume of water (18 cm3/mol), S is the initial oocyte surface area, osmi is the osmolarity inside the oocyte, and osmo is the osmolarity of the external medium. The time interval of 60 s was used for the calculations. All average results are presented as mean ± S.E. Statistical significance was evaluated using Student's t test for pairwise comparison and analysis of variance for comparison of several groups of data. A probability level of 0.01 (indicated by asterisks, ***) was considered highly significant. All of the results were obtained from at least three different batches of oocytes.
RESULTS
McPIP2;1 Is a Functional Water Channel
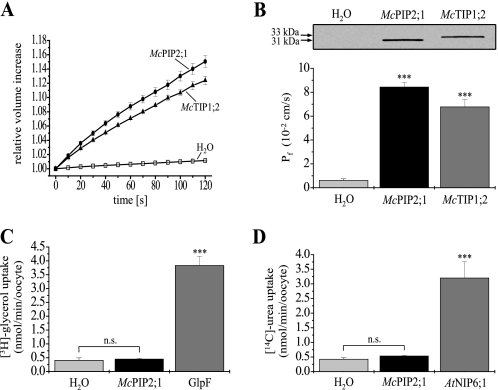
McPIP2;1 and McTIP1;2 from the halophyte M. crystallinum were heterologously expressed in X. laevis oocytes to determine their activity as water channel proteins according to Vera-Estrella et al. (19). The osmotic water permeability (Pf) of the oocytes plasma membrane was assessed by hypotonic swelling assays, recording the volume increase of cells up to 2 min to avoid cell destruction. Within 2 min, oocytes expressing McPIP2;1 showed a 15.1 ± 0.8% increase in cell volume (Fig. 1A, n = 20), relative to initial values, in response to the 100 mosmol/kg osmotic shock imposed. Control oocytes injected with ribonuclease (RNase)-free water did not show any relevant volume change (1.2 ± 0.1%, n = 20, Fig. 1A, p < 0.01 in comparison with cells expressing McPIP2;1 or McTIP1;2). McTIP1;2 injected oocytes were used as a positive control (19). These oocytes exhibited a volume increase of 12.4 ± 0.6% (Fig. 1A; n = 20), relative to initial values, comparable to that previously described by Vera-Estrella et al. (19). From these data, the Pf of the oocytes plasma membrane was calculated based on the initial rate of swelling (60 s) and the imposed hypo-osmotic shock. Oocytes expressing McPIP2;1 and McTIP1;2 had a Pf of 8.43 × 10−2 cm/s and 6.77 × 10−2 cm/s, respectively, which were 14- and 11-fold higher to that of the H2O-injected oocytes (0.61 × 10−2 cm/s, n = 20, Fig. 1B, p < 0.01). The Pf values of the two groups of AQP-injected oocytes were statistically different from each other with a p < 0.01. The expression of McPIP2;1 and McTIP1;2 in the oocytes plasma membrane (Fig. 1B, inset) was confirmed by Western blotting assays of plasma membrane-enriched fractions (supplemental Table S1) using peptide-specific antibodies for each AQP (19, 33). The anti-McPIP2;1 and -McTIP1;2 antibodies recognized specifically polypeptides of 31 and 33 kDa in membrane proteins isolated from oocytes expressing McPIP2;1 and McTIP1;2, respectively. These antibodies recognized no polypeptides in membranes isolated from H2O-injected oocytes (Fig. 1B, inset, left lane). Thus, our results showed that McPIP2;1 and McTIP1;2 were effectively expressed in the oocytes plasma membrane, accounting for the changes in Pf observed.
FIGURE 1.
Determination of the water-selective channel activity of McPIP2;1 in X. laevis oocytes. A, time course of oocyte swelling in response to hypo-osmotic shock. The volume increase of oocytes, relative to initial volumes (set as 1), is plotted as a function of time after transfer of oocytes to the hypo-osmotic medium. McPIP2;1-injected oocytes (black circles) exhibited a significant volume increase as a consequence of water permeability across their membranes. H2O- (white squares) and McTIP1;2- (black up-triangles) injected oocytes were used as negative and positive controls, respectively (n = 20). B, osmotic water permeability (Pf) of oocytes expressing McPIP2;1 or McTIP1;2. Pf values were calculated from the initial (60 s) rate of oocyte swelling (data shown in A) as described under “Experimental Procedures” (n = 20, ***, p < 0.01 compared with H2O-injected oocytes). The immunoblotting detection of McPIP2;1 (31 kDa) and McTIP1;2 (33 kDa) proteins in plasma membrane-enriched fractions isolated from oocytes is shown as an inset (results reproduced in three independent experiments). C and D, radiolabeled solute uptake assays. Oocytes expressing McPIP2;1, GlpF (positive control for glycerol uptake) or AtNIP6;1 (positive control for urea uptake) were incubated for 10 min in an iso-osmotic solution containing [3H]glycerol or [14C]urea. An excess of nonradiolabeled solute was used to facilitate uptake by oocytes. Results are from three different batches of oocytes (n = 15, ***, p < 0.01 compared with H2O-injected oocytes). n.s., not significant difference.
McPIP2;1 Transports Neither Glycerol nor Urea
Sequence analysis of the primary structure of McPIP2;1 showed that the aromatic/arginine (ar/R) constriction pore, proposed to act as a selectivity filter within the channel, is formed by amino acids Phe89, His218, Thr227, and Arg233 (Fig. 2), which are characteristic of orthodox, water-selective AQPs, that have been shown not to be able to transport other small solutes (23, 39, 40). To corroborate the water selectivity of McPIP2;1 we tested the transport of glycerol and urea by radiolabeled solute uptake assays. H2O- or AQP-injected oocytes were incubated for 10 min in iso-osmotic media (200 mosmol/kg) containing [3H]glycerol or [14C]urea along with an excess of cold glycerol or urea, respectively, to induce the uptake of the solutes by the oocytes in favor of their concentration gradient. Oocytes expressing McPIP2;1 showed very low glycerol (0.45 nmol/min/oocyte, Fig. 1C) and urea (0.53 nmol/min/oocyte, Fig. 1D) uptake levels, similar to values showed by H2O-injected oocytes (0.40 and 0.42 nmol/min/oocyte for glycerol and urea, respectively; Fig. 1, C and D). GlpF- and AtNIP6;1-injected oocytes, used as positive controls, showed high uptakes of glycerol (3.83 nmol/min/oocyte, n = 15, Fig. 1C, p < 0.01) and urea (3.2 nmol/min/oocyte, n = 15, Fig. 1D, p < 0.01), respectively, in comparison with H2O-injected oocytes. These results corroborated that McPIP2;1 does not transport glycerol or urea, and thus suggest that McPIP2;1 may be a water-selective channel.
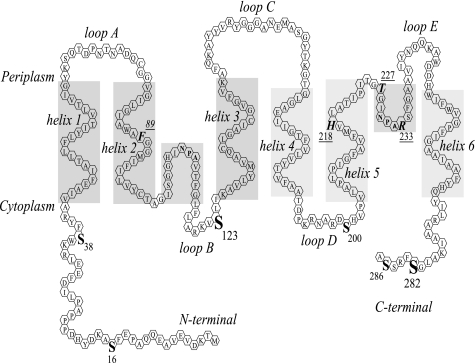
FIGURE 2.
Topological model of a McPIP2;1 monomer. Six transmembrane α-helices (helix 1-helix 6) are connected by five loops (A–E) with N and C termini located in the cytoplasm. The hydrophobic helical domains of loops B and E containing the highly conserved NPA motifs dip halfway into the membrane from opposite sides and create the aqueous pore. Serine residues (S) potentially phosphorylated by either PKA or PKC in the cytoplasmic side at the N- (Ser16, PKC, and Ser38, PKA) and C termini (Ser282, PKC, and Ser286, PKA), and in loops B (Ser123, PKA) and D (Ser200, PKA) are shown. Ser16 is conserved in some PIP1 and PIP2 isoforms. Ser38, Ser123, and Ser200 are conserved in the PIP subfamily. Ser282 is conserved in the PIP2 group. Amino acids Phe89, His218, Thr227, and Arg233 form the aromatic/arginine (ar/R) constriction within the channel pore.
McPIP2;1 Water Permeability Is Regulated by PKA- and PKC-mediated Phosphorylation
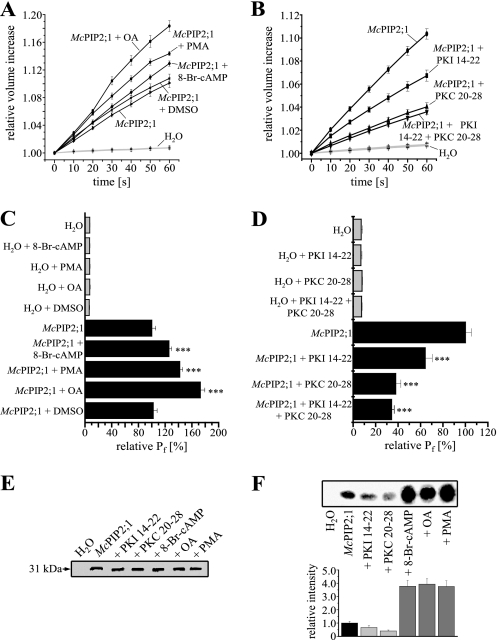
Phosphorylation is a post-translational modification known to affect the water channel activity of some plant AQPs (21–23). Analysis of the primary structure of McPIP2;1 showed six putative phosphorylation serine residues that can be phosphorylated by either PKA or PKC (NetPhos 2.0, Technical University of Denmark, CBX, Fig. 2) located in the cytoplasmic side of the channel at loops B (Ser123, PKA) and D (Ser200, PKA) and the N- (Ser16, PKC and Ser38, PKA) and C termini (Ser282, PKC and Ser286, PKA). The possibility that the Pf may be regulated by the phosphorylation state of the protein was tested by using OA, a specific inhibitor of protein phosphatases type 1A and 2A (41), 8-Br-cAMP, an activator of cAMP-dependent PKA (42) and PMA (22), an activator of PKC, as phosphorylation agonists. Oocytes were incubated for 30 min at room temperature with the agonists prior to swelling assays. Preincubation of oocytes expressing McPIP2;1 with 8-Br-cAMP, OA or PMA resulted in a cell volume increase of 12.9 ± 0.4%, 18.4 ± 0.8%, and 14.4 ± 0.3%, respectively, after 60 s relative to initial values (Fig. 3A, n = 15). According to differences in volume increase, the calculated Pf of the McPIP2;1-injected oocytes increased by 25.7 ± 3.9%, 72.7 ± 6.8%, and 41.9 ± 4.3% in response to 8-Br-cAMP, OA, and PMA, respectively (Fig. 3C, n = 15, p < 0.01 in comparison with no-preincubated McPIP2;1-injected oocytes). H2O-injected oocytes did not show a significant change in volume (Fig. 3A, n = 15, p < 0.01) and consequently in their Pf (Fig. 3C, n = 15, p < 0.01) upon hypo-osmotic shock after pre-incubation with 8-Br-cAMP, OA, or PMA, in comparison with oocytes expressing McPIP2;1. The Pf values shown in Fig. 3C are relative to the Pf of McPIP2;1-injected oocytes in the absence of the agonists (set as 100.0 ± 6.1%, n = 15). Preincubation of H2O- or McPIP2;1-injected oocytes with 0.1% DMSO (OA and PMA solvent) did not alter significantly their volume increase (Fig. 3A) and, accordingly, their Pf (Fig. 3C), indicating that the observed changes were specifically due to the presence of OA or PMA.
FIGURE 3.
Regulation of McPIP2;1 water channel activity by PKA- and PKC-mediated phosphorylation. A and B, time courses of oocytes swelling under hypo-osmotic conditions after pre-incubation with 8-Br-cAMP (40 μm), OA (5 μm), PMA (10 nm), DMSO (0.1%), or peptides PKI 14–22 (1 μm, PKA inhibitor) and PKC 20–28 (100 μm, PKC inhibitor) for 30 min. H2O-injected oocytes were used as negative controls. DMSO was used to dissolve OA and PMA (n = 15). C and D, effect of 8-Br-cAMP, OA, PMA, and peptides PKI 14–22 and PKC 20–28 on the Pf of McPIP2;1-injected oocytes calculated from data in A and B. The osmotic water permeability (Pf) is represented as the relative value of the Pf from McPIP2;1-injected oocytes (set as 100%, n = 15, ***, p < 0.01 compared with no preincubated McPIP2;1-injected oocytes). E, detection of McPIP2;1 (31 kDa) protein in isolated plasma membrane-enriched fractions from McPIP2;1-injected oocytes preincubated with 8-Br-cAMP, OA, PMA, or peptides PKI 14–22 or PKC 20–28 by Western blotting (result representative of three independent experiments). F, effect of 8-Br-cAMP, OA, PMA, and peptides PKI 14–22 and PKC 20–28 on the in vitro phosphorylation of McPIP2;1. Phosphorylated McPIP2;1 protein from isolated oocytes membranes was immunoprecipitated and detected by autoradiography (inset). The relative intensity of the corresponding radiographic band is shown as column graph. These experiments were repeated two times with similar results.
To confirm that McPIP2;1 water permeability was indeed affected by PKA- and PKC-mediated phosphorylation, we decided to use the peptides PKI 14–22 and PKC 20–28, specific inhibitors of cAMP-dependent PKA (43) and PKC (44), respectively, as phosphorylation antagonists. Pre-incubation of oocytes expressing McPIP2;1 for 30 min at room temperature with the peptides PKI 14–22 and PKC 20–28 prior to swelling assays led to volume increases of 6.7 ± 0.5% and 4.0 ± 0.4%, respectively, while incubation with both inhibitory peptides resulted in a 3.6 ± 0.2% volume increase after 60 s relative to initial values (Fig. 3B, n = 15). Thus, the incubation with the kinase inhibitors slowed down the swelling rate of the oocytes expressing McPIP2;1. Hence, according to the observed volume changes, the Pf of the McPIP2;1-injected oocytes (set as 100.0 ± 7.3% in Fig. 3D, n = 15) decreased 35.8% and 62.1% in response to incubation with peptides PKI 14–22 and PKC 20–28, respectively, and a 65.6% in response to incubation with both inhibitory peptides (Fig. 3D, n = 15, p < 0.01 in comparison with no preincubated McPIP2;1-injected oocytes). Preincubation with peptides PKI 14–22 and PKC 20–28 did not alter the volume and consequently the Pf of H2O-injected oocytes after hypo-osmotic shock (Fig. 3, B and D). As shown on the Western blot in Fig. 3E, McPIP2;1 protein expression at the oocyte plasma membrane was not affected by incubation with 8-Br-cAMP, OA, PMA, or the peptides PKI 14–22 and PKC 20–28, corroborating that PKA and PKC phosphorylation pathways are directly involved in the regulation of the water channel activity of McPIP2;1.
To evaluate the phosphorylation status of McPIP2;1 expressed in the oocytes plasma membrane we used isolated plasma membrane-enriched fractions labeled with [γ-32P]ATP. Autoradiographs of labeled oocyte membrane proteins showed that McPIP2;1 polypeptide was constitutively phosphorylated (Fig. 3F, inset). When the oocytes were exposed to 8-Br-cAMP, OA or PMA a 3.8-fold increase in the phosphorylation level of McPIP2;1 was observed (Fig. 3F). However, the phosphorylation level of McPIP2;1 was diminished 0.34- and 0.59-fold by exposure of oocytes to peptides PKI 14–22 or PKC 20–28, respectively (Fig, 3F). These results, together with those showing that McPIP2;1 water permeability was increased by 8-Br-cAMP, OA and PMA, and decreased by kinase inhibitors PKI 14–22 and PKC 20–28, indicate that the water channel activity of McPIP2;1 is positively regulated by PKA- and PKC-mediated phosphorylation.
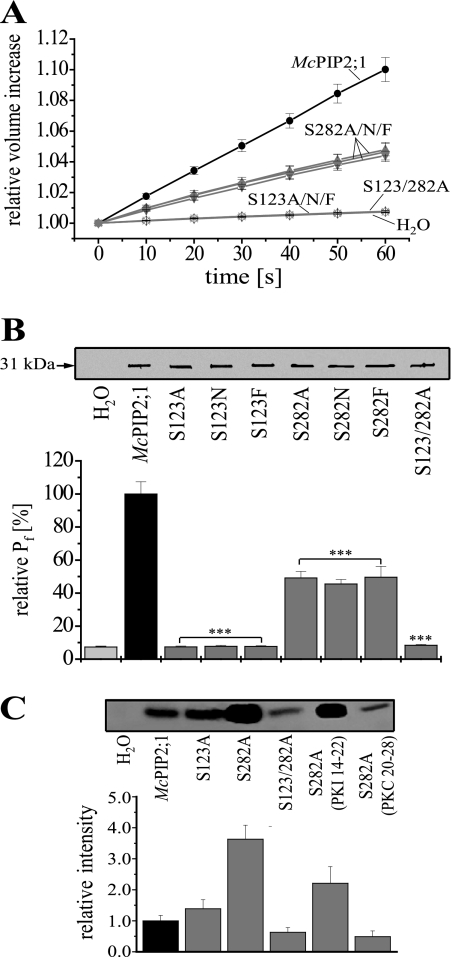
Ser123 and Ser282 Are Required for McPIP2;1 Water Permeability
To further characterize the regulation of the water channel activity of McPIP2;1 by PKA and PKC, the putative phosphorylation sites, Ser123, located in a PKA phosphorylation motif conserved in all plant PIPs, and Ser282, located in a PKC phosphorylation motif conserved in all PIP2 proteins, were modified by site-directed mutagenesis to elucidate their functional role. Each amino acid was replaced by alanine, asparagine, or phenylalanine to abolish phosphorylation at those sites and to discard effects of conformational changes due to the presence of charged residues different to serine. The singly mutated channels McPIP2;1-S123A, McPIP2;1-S123N, McPIP2;1-S123F, McPIP2;1-S282A, McPIP2;1-S282N, McPIP2; 1-S282F and the double mutant McPIP2;1-S123/282A were individually expressed in oocytes and their water permeability assessed under conditions analogous to those described for wt McPIP2;1. After hypo-osmotic shock, the swelling rates of oocytes expressing McPIP2; 1-mutated forms were severely affected. The volume of oocytes expressing any of the McPIP2;1-S282 mutant forms increased a 4.6 ± 0.6% after 60 s relative to initial values (Fig. 4A, n = 15), while the volume of oocytes expressing any of the McPIP2;1-S123 single mutants or the double mutant McPIP2;1-S123/282A did not change after hypo-osmotic shock (Fig. 4A, n = 15). Consequently, the calculated Pf for oocytes expressing the McPIP2;1-S282 single mutants showed a 51.9 ± 4.4% decrease relative to the Pf of wt McPIP2;1-injected oocytes (set as 100% in Fig. 4B, n = 15, p < 0.01), while oocytes expressing the McPIP2;1-S123 single mutants or the double mutant McPIP2;1-S123/282A showed small increases in Pf similar to those observed in water-injected oocytes (7.3 ± 0.6%, n = 15, Fig. 4B, p < 0.01 in comparison with oocytes expressing the wt McPIP2;1). Thus, the absence of Ser123 abolished the water permeability of McPIP2;1 whereas the lacking of Ser282 only partially inhibited it.
FIGURE 4.
Ser123 and Ser282 are required for the water channel activity of McPIP2;1. A, swelling of oocytes expressing wt or mutated McPIP2;1 in time upon hypo-osmotic shock. Oocytes expressing the single mutated channels McPIP2;1-S123A, McPIP2;1-S123N, McPIP2;1-S123F, McPIP2;1-S282A, McPIP2;1-S282N, McPIP2;1-S282F, or the double mutant McPIP2;1-S123/282A were assessed under conditions similar to those described in Fig. 1A for wt McPIP2;1 (n = 15). B, effect of Ser123 and Ser282 on the Pf of McPIP2;1-injected oocytes. Pf was calculated from data shown in A. The Pf is represented as the relative value of the Pf from wt McPIP2;1-injected oocytes (set as 100%, n = 15, p < 0.01 compared with oocytes expressing wt McPIP2;1). The McPIP2;1 mutant forms were detected in plasma membrane-enriched fractions isolated from oocytes by Western blotting (inset, blot representative of three independent experiments). C, influence of Ser123 and/or Ser282 on the in vitro phosphorylation of McPIP2;1 and effect of peptides PKI 14–22 and PKC 20–28 on the phosphorylation level of the McPIP2;1-S282A mutant form. Phosphorylated wt or mutated McPIP2;1 proteins from isolated oocytes membranes were immunoprecipitated and detected by autoradiography (inset). The oocytes injected with McPIP2;1-S282A were incubated for 30 min in the presence of inhibitors prior to membrane isolation. The relative intensity of the radiographic bands is plotted. Assays were repeated two times with similar results.
To demonstrate that the differences in Pf were not a result of a differential expression of the McPIP2;1 mutant forms at the oocytes plasma membrane, the protein levels of the mutant forms were analyzed by Western blotting. As shown in Fig. 4B (inset), similar levels of the 31-kDa polypeptide, corresponding to wt McPIP2;1, were observed in all the membrane proteins isolated from oocytes expressing the wt and the different McPIP2;1 mutant forms. These results confirmed that the amino acid substitutions were responsible of the changes in Pf observed in McPIP2;1 mutants.
In vitro phosphorylation and immunoprecipitation assays showed that mutation of Ser123 did not change the phosphorylation status of McPIP2;1 (Fig. 4C, inset). However, the mutant McPIP2;1-S282A showed a 3.6-fold increase in its phosphorylation level relative to the phosphorylation status of the wt form (Fig. 4C). To further determine the mechanism responsible for the increased phosphorylation observed in the mutant McPIP2;1-S282A, we tested the effect of PKI 14–22 and PKC 20–28, inhibitors of PKA and PKC, respectively, on the phosphorylation status of this mutant (Fig. 4C, inset). The phosphorylation level of McPIP2;1-S282A in the presence of PKI 14–22 and PKC 20–28 was reduced 0.40- and 0.87-fold, respectively (Fig. 4C), suggesting that PKC is mainly involved in the observed increased phosphorylation. Furthermore, the McPIP2;1-S123/282A double mutant showed a 0.37-fold decrease in phosphorylation relative to phosphorylation of the wt form. These results indicated that McPIP2;1 was phosphorylated at sites other than Ser123 or Ser282.
DISCUSSION
The hypothesis that McPIP2;1 may be a water-selective channel regulated by phosphorylation has been tested. Here we show that McPIP2;1 is a functional water-selective channel when expressed in X. laevis oocytes, as proved by the increased water permeability caused by its expression and by its inability to transport small uncharged solutes. We also show that both the phosphorylation status and the water channel activity of McPIP2;1 are up- or down-regulated by activation or inactivation of PKA and PKC phosphorylation pathways. Furthermore, our studies with McPIP2;1 mutated at Ser123 and/or Ser282 revealed that the water channel activity of this AQP is completely dependent on the presence of Ser123 and partially on the presence of Ser282.
Transport Selectivity of McPIP2;1
In this report, we show that McPIP2;1 is a very efficient water transporting AQP as indicated by the 14-fold increase in water permeability across the oocyte plasma membrane mediated by this AQP. Moreover, radiolabeled solute uptake assays showed that McPIP2;1 is unable to transport the small neutral solutes glycerol and urea, which suggests that McPIP2;1 is a water-selective channel. This view is also supported by the aromatic/arginine (ar/R) region of this AQP formed by amino acids Phe89, His218, Thr227, and Arg233. Although threonine is less conserved, the ensemble of such amino acids has been reported to act as a transport selectivity filter (45, 46) and to be highly conserved in orthodox, water-selective AQPs (39). The narrow ar/R region in McPIP2;1 may explain its impermeability to glycerol and urea. Nevertheless, it may be possible that McPIP2;1 transports a solute other than water but not as large as urea or glycerol, i.e. CO2, as it has been suggested for HvPIP2;1 from barley (47).
Regulation of McPIP2;1 Water Channel Activity by PKA and PKC Phosphorylation Pathways
The up-regulation of the water transport activity of McPIP2;1 by PKA- and PKC-mediated phosphorylation is clearly indicated from our results. This was shown by the stimulated water transport activity caused by the phosphorylation agonists 8-Br-cAMP, OA, and PMA and confirmed by the decreased water permeability caused by the PKA (PKI 14–22) and PKC (PKC 20–28) inhibitory peptides. By and large these changes in activity correlated to modifications in the phosphorylation status of McPIP2;1 caused by the same effectors, with 8-Br-cAMP, OA, and PMA inducing the phosphorylation of McPIP2;1 whereas PKA and PKC inhibitory peptides having the opposite effect. The possibility that the changes caused by these treatments were a result of a differential expression of McPIP2;1 was discarded by showing similar levels of protein expression on plasma membrane-enriched fractions isolated from control and phosphorylation effectors-treated oocytes. From these results, we can conclude that McPIP2;1 water permeability is positively regulated by PKA and PKC phosphorylation pathways.
Role of Ser123 and Ser282 on the Water Transport Activity of McPIP2;1
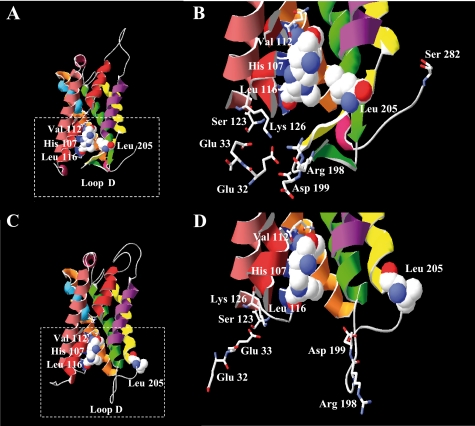
Mutation of Ser123 abolished the water channel activity of McPIP2;1 irrespective of its replacement by alanine (small, non polar), asparagine (uncharged polar) or phenylalanine (large, non polar). This indicates that despite the small or large conformational changes that may be caused by insertion of such amino acids at position 123, the presence of Ser123 is essential for McPIP2;1 water permeability. As described by Törnroth-Horsefield et al. (48), phosphorylation of Ser115 of the spinach AQP SoPIP2;1 (corresponding to Ser123 of McPIP2;1) disrupts the network of hydrogen bonds anchoring loop D to the N terminus, displacing loop D away from the cytoplasmic pore entrance with the consequent opening of the water channel. According to this gating mechanism, the absence of Ser123 in McPIP2;1-S123 mutants would prevent disruption of interactions between loop D and the N terminus, and hence loop D would be kept occluding the channel, which fits well to and may explain the abolishment of water permeability observed in McPIP2;1-S123 mutants.
However, mutation of the serine residue corresponding to Ser123 of McPIP2;1 has been reported to lower, but not to abolish, the water channel activity of the plant AQPs PvTIP3;1 (21), SoPIP2;1 (23), and ZmPIP2;1 (30). We propose that the residual water permeability observed in those mutated AQPs is possibly due to a loose loop D occluding their pore. Homology modeling of McPIP2;1 based on the closed crystal structure of SoPIP2;1 (76% identity, supplemental Fig. S2) revealed that stabilization of loop D beneath the cytoplasmic entrance of the pore, conferred by a network of ionic and hydrogen bond interactions, may involve the connection of Arg198 and Asp199 of loop D to the side chain of Lys126 of loop B according to Törnroth-Horsefield et al. (48, Fig. 5B and supplemental Fig. S1). In addition, Lys126 may form hydrogen bonds to Glu33, which ligates a divalent cation (i.e. Ca2+) also implicated in anchoring of loop D to the N terminus of the AQP. A survey of the sequences of PvTIP3;1, SoPIP2;1, and ZmPIP2;1 revealed the absence of an acidic amino acid at the position corresponding to Glu32 of McPIP2;1 (supplemental Fig. S1). Therefore, the presence of glutamic acid at position 32 (Fig. 5, B and D) confers McPIP2;1 an extra negative charge that may be involved in coordinating the divalent cation or in hydrogen bonding to Lys126, resulting in a stronger stabilization of the anchoring of loop D to the N terminus and, consequently, in a tighter closure of the channel. This may explain the complete inhibition of water permeability in McPIP2;1 when mutated at Ser123, not observed in PvTIP3;1, SoPIP2;1, and ZmPIP2;1 when mutated at the corresponding serine residue. Furthermore, the crystal structure of human AQP5 revealed that Ser83 in loop B (corresponding to Ser123 of McPIP2;1) interacts with the C-terminal α-helix by water-mediated and direct hydrogen bonds anchoring this helix to loop B (49), therefore, a possible structural function of the unphosphorylated form of Ser123 in the gating mechanism of McPIP2;1 is not discarded. Further studies are needed to corroborate these hypotheses. Interestingly, the McPIP2;1-S123A mutant is still phosphorylated in vitro. Thus, phosphorylation of McPIP2;1 at other sites may have an additional role other than controlling water permeability.
FIGURE 5.
Structural models of the closed and open conformations of McPIP2;1. The models were constructed based on the closed and open conformations of the crystal structure of the spinach aquaporin SoPIP2;1. A, side view of the closed conformation of McPIP2;1. Loop D is folded underneath the channel and brings Leu205 close to His107, Val112, and Leu116 (residues shown as van der Waals space-filling spheres) forming a hydrophobic barrier in the entrance of the cytoplasmic vestibule. B, close up view of the frame in A. Loop D is stabilized beneath the cytoplasmic entrance of the channel by the interaction of Arg198 and Asp199 with Lys126 and Glu32 (residues depicted by sticks) of the N-terminal end. C, side view of the open conformation of McPIP2;1. Loop D is displaced from the cytoplasmic vestibule, and Leu205 is moved away from the entrance of the channel. D, close up view of the frame in C. As shown, amino acids of loop D are not connected to amino acids of the N-terminal end, preventing the occluding of the channel by this loop.
Mutation of Ser282 also negatively affected the water channel activity of McPIP2;1, with 51.9% inhibition, which demonstrates the participation of such residue in the water permeability of the AQP. Unexpectedly, the McPIP2;1-S282A mutant protein showed an increased in vitro phosphorylation, respective to the wt form. This result suggests that the absence of Ser282 probably caused a conformational change in which other phosphorylation sites were exposed and hence increased the phosphorylation level of the protein. The phosphorylation level of this mutant form was reduced by PKA and PKC inhibitors, suggesting that both kinases are involved in the observed increase in phosphorylation.
The reduced water permeability observed in McPIP2;1-S282 mutants may be accounted for by a partial opening of the channel. Based on the gating mechanism proposed by Törnroth-Horsefield et al. (48), phosphorylation of Ser282 at the C terminus of McPIP2;1 (corresponding to Ser274 of SoPIP2;1) would disrupt the hydrophobic barrier, consisting of His107, Val112, and Leu116 of loop B and Leu205 of helix 5 (Fig. 5, A and B), that blocks the pore of an adjacent monomer within the AQP tetramer by displacing Leu205 away from the cytoplasmic pore entrance with the consequent opening of the channel (Fig. 5, C and D). In McPIP2;1-S282 mutants, the phosphorylation of Ser123 may be causing the displacement of loop D away from the cytoplasmic pore entrance as described before with the consequent opening of the water channel, however, Leu205 in helix 5 is probably not completely displaced away from the pore entrance because of the absence of Ser282 and it could be partially blocking the water channel, which fits well to and may explain the diminished Pf observed in these mutants. However, it has been reported that mutation of the corresponding serine at the C terminus of SoPIP2;1 (23) and ZmPIP2;1 (30) did not affect the water transport activity of those AQPs when expressed in oocytes. Possibly some particular amino acids, e.g. Glu32 mentioned above, are specifically involved in the gating mechanism of certain plant AQPs, a topic that deserves further investigation.
In addition, the McPIP2;1-S123/282A double mutant showed a complete inhibition of water permeability, corroborating the essentiality of Ser123 for the water transport activity of McPIP2;1. Although significantly decreased, phosphorylation of the double mutant was not completely suppressed, suggesting that phosphorylation of McPIP2;1 may have an additional role other than controlling water permeability. It has been reported that mutation of the A. thaliana AQP AtPIP2;1 at Ser283 (corresponding to Ser285 of McPIP2;1) affects its plasma membrane localization (31). Thus, phosphorylation seems to have a dual role on plant AQPs, controlling their water transport activity and their trafficking to membranes.
Potential Physiological Implications of McPIP2;1 Phosphorylation
Because of the fundamental role of water absorption by plant roots, the functional characterization of McPIP2;1, a root-specific AQP from the halophyte M. crystallinum, is of great interest for the salt tolerance mechanisms of the plant. We show that McPIP2;1 is a highly water permeable channel that is functionally regulated by phosphorylation when expressed in oocytes. Although the physiological relevance of phosphorylation studies in the oocytes heterologous system might be limited there are indirect evidences regarding a physiological role in planta for PIP2 protein phosphorylation at residues corresponding to Ser123 and Ser282 in McPIP2;1 (26, 30, 31, 50).
Regulation of McPIP2;1 gating through phosphorylation/dephosphorylation events would allow plant root cells to rapidly regulate their osmotic water permeability across membranes under environmental stresses such as drought and salinity. Prevention of water loss under these stresses is fundamental to keep plant growth and development. On the other hand, plant recovering and normal growth require substantial water uptake by roots. Although the precise role of McPIP2;1 phosphorylation in M. crystallinum root cells remains to be determined, with this work we began to elucidate the role that McPIP2;1 may have in the osmotic water permeability of root cells and how phosphorylation could be controlling water permeability through them.
In summary, our results show that McPIP2;1 has a water transport activity positively regulated by PKA- and PKC-mediated phosphorylation. Ser123 and Ser282 are required for McPIP2;1 water permeability but only Ser123 is essential suggesting that this amino acid may play a major role in water transport through root cells mediated by this AQP.
Supplementary Material
Acknowledgments
We thank François Chaumont (Institut des Sciences de la Vie, Louvain-la-Neuve, Belgium) for the gift of the GlpF clone and Daniel Roberts (Dept. of Biochemistry and Cellular and Molecular Biology, University of Tennessee, Knoxville, TN) for the AtNIP6;1F clone.
This work was supported by DGAPA IN221308 and CONACYT 57685 (to R. V.-E.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2 and Table S1.
J. C. Amezcua-Romero, unpublished data.
- AQP
- aquaporin
- Pf
- osmotic water permeability
- OA
- okadaic acid
- DMSO
- dimethyl sulfoxide
- PMA
- phorbol 12-myristate 13-acetate
- 8-Br-cAMP
- 8-bromoadenosine-3′,5′-cyclic monophosphate
- PKA
- cAMP-dependent kinase
- PKC
- protein kinase C
- PKI
- protein kinase A inhibitor
- PDB
- Protein Data Bank
- wt
- wild type
- MES
- 4-morpholineethanesulfonic acid.
REFERENCES
- 1.Maurel C., Verdoucq L., Luu D. T., Santoni V. (2008) Annu. Rev. Plant Biol. 59, 595–624 [DOI] [PubMed] [Google Scholar]
- 2.Katsuhara M., Hanba Y. T. (2008) Pflugers Arch. 456, 687–691 [DOI] [PubMed] [Google Scholar]
- 3.Chaumont F., Moshelion M., Daniels M. J. (2005) Biol. Cell 97, 749–764 [DOI] [PubMed] [Google Scholar]
- 4.King L. S., Kozono D., Agre P. (2004) Nat. Rev. Mol. Cell Biol. 5, 687–698 [DOI] [PubMed] [Google Scholar]
- 5.Gonen T., Walz T. (2006) Q. Rev. Biophys. 39, 361–396 [DOI] [PubMed] [Google Scholar]
- 6.Johanson U., Karlsson M., Johansson I., Gustavsson S., Sjövall S., Fraysse L., Weig A. R., Kjellbom P. (2001) Plant Physiol. 126, 1358–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Danielson J. A., Johanson U. (2008) BMC Plant Biol. 8, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gustavsson S., Lebrun A. S., Nordén K., Chaumont F., Johanson U. (2005) Plant Physiol. 139, 287–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biela A., Grote K., Otto B., Hoth S., Hedrich R., Kaldenhoff R. (1999) Plant J. 18, 565–570 [DOI] [PubMed] [Google Scholar]
- 10.Wallace I. S., Roberts D. M. (2005) Biochemistry 44, 16826–16834 [DOI] [PubMed] [Google Scholar]
- 11.Gerbeau P., Güclü J., Ripoche P., Maurel C. (1999) Plant J. 18, 577–587 [DOI] [PubMed] [Google Scholar]
- 12.Takano J., Wada M., Ludewig U., Schaaf G., von Wirén N., Fujiwara T. (2006) Plant Cell 18, 1498–1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma J. F., Tamai K., Yamaji N., Mitani N., Konishi S., Katsuhara M., Ishiguro M., Murata Y., Yano M. (2006) Nature 440, 688–691 [DOI] [PubMed] [Google Scholar]
- 14.Holm L. M., Jahn T. P., Møller A. L., Schjoerring J. K., Ferri D., Klaerke D. A., Zeuthen T. (2005) Pflügers Arch. 450, 415–428 [DOI] [PubMed] [Google Scholar]
- 15.Kamiya T., Tanaka M., Mitani N., Ma J. F., Maeshima M., Fujiwara T. (2009) J. Biol. Chem. 284, 2114–2120 [DOI] [PubMed] [Google Scholar]
- 16.Uehlein N., Lovisolo C., Siefritz F., Kaldenhoff R. (2003) Nature 425, 734–737 [DOI] [PubMed] [Google Scholar]
- 17.Hachez C., Zelazny E., Chaumont F. (2006) Biochim. Biophys. Acta 1758, 1142–1156 [DOI] [PubMed] [Google Scholar]
- 18.Kaldenhoff R., Fischer M. (2006) Biochim. Biophys. Acta 1758, 1134–1141 [DOI] [PubMed] [Google Scholar]
- 19.Vera-Estrella R., Barkla B. J., Bohnert H. J., Pantoja O. (2004) Plant Physiol. 135, 2318–2329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Javot H., Maurel C. (2002) Ann. Bot. 90, 301–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maurel C., Kado R. T., Guern J., Chrispeels M. J. (1995) EMBO J. 14, 3028–3035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guenther J. F., Chanmanivone N., Galetovic M. P., Wallace I. S., Cobb J. A., Roberts D. M. (2003) Plant Cell 15, 981–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johansson I., Karlsson M., Shukla V. K., Chrispeels M. J., Larsson C., Kjellbom P. (1998) Plant Cell 10, 451–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daniels M. J., Yeager M. (2005) Biochemistry 44, 14443–14454 [DOI] [PubMed] [Google Scholar]
- 25.Johnson K. D., Chrispeels M. J. (1992) Plant Physiol. 100, 1787–1795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johansson I., Larsson C., Ek B., Kjellbom P. (1996) Plant Cell 8, 1181–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miao G. H., Hong Z., Verma D. P. S. (1992) J. Cell Biol. 118, 481–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weaver C. D., Roberts D. M. (1992) Biochemistry 31, 8954–8959 [DOI] [PubMed] [Google Scholar]
- 29.Weaver C. D., Crombie B., Stacey G., Roberts D. M. (1991) Plant Physiol. 95, 222–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Wilder V., Miecielica U., Degand H., Derua R., Waelkens E., Chaumont F. (2008) Plant Cell Physiol. 49, 1364–1377 [DOI] [PubMed] [Google Scholar]
- 31.Prak S., Hem S., Boudet J., Viennois G., Sommerer N., Rossignol M., Maurel C., Santoni V. (2008) Mol. Cell Proteomics 7, 1019–1030 [DOI] [PubMed] [Google Scholar]
- 32.Vera-Estrella R., Barkla B. J., Gallardo-Amarillas C., Bohnert H. J., Pantoja O. (2000) Molecular Biology and Physiology of Water and Solute Transport, pp. 339–346, Kluwer Academic/Plenum Publishers, New York [Google Scholar]
- 33.Kirch H. H., Vera-Estrella R., Golldack D., Quigley F., Michalowski C. B., Barkla B. J., Bohnert H. J. (2000) Plant Physiol. 123, 111–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fetter K., van Wilder V., Moshelion M., Chaumont F. (2004) Plant Cell 16, 215–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laemmli U. K. (1970) Nature 227, 680–685 [DOI] [PubMed] [Google Scholar]
- 36.Pieper U., Eswar N., Davis F. P., Braberg H., Madhusudhan M. S., Rossi A., Marti-Renom M. A., Karchin R., Webb B. M., Eramian D., Shen M. Y., Kelly L., Melo F., Sali A. (2006) Nucleic Acids Res. 34, D291–D295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Melo F., Sánchez R., Sali A. (2002) Protein Sci. 11, 430–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guex N., Peitsch M. C. (1997) Electrophoresis 18, 2714–2723 [DOI] [PubMed] [Google Scholar]
- 39.Beitz E., Wu B., Holm L. M., Schultz J. E., Zeuthen T. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 269–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Calamita G., Bishai W. R., Preston G. M., Guggino W. B., Agre P. (1995) J. Biol. Chem. 270, 29063–29066 [DOI] [PubMed] [Google Scholar]
- 41.Haystead T. A., Sim A. T., Carling D., Honnor R. C., Tsukitani Y., Cohen P., Hardie D. G. (1989) Nature 337, 78–81 [DOI] [PubMed] [Google Scholar]
- 42.Hei Y. J., MacDonell K. L., McNeil J. H., Diamond J. (1991) Mol. Pharmacol. 39, 233–238 [PubMed] [Google Scholar]
- 43.Harris T. E., Persaud S. J., Jones P. M. (1997) Biochem. Biophys. Res. Commun. 232, 648–651 [DOI] [PubMed] [Google Scholar]
- 44.Eichholtz T., de Bont D. B., de Widt J., Liskamp R. M., Ploegh H. L. (1993) J. Biol. Chem. 268, 1982–1986 [PubMed] [Google Scholar]
- 45.Sui H., Han B. G., Lee J. K., Walian P., Jap B. K. (2001) Nature 414, 872–878 [DOI] [PubMed] [Google Scholar]
- 46.Fu D., Libson A., Miercke L. J., Weitzman C., Nollert P., Krucinski J., Stroud R. M. (2000) Science 290, 481–486 [DOI] [PubMed] [Google Scholar]
- 47.Hanba Y. T., Shibasaka M., Hayashi Y., Hayakawa T., Kasamo K., Terashima I., Katsuhara M. (2004) Plant Cell Physiol. 45, 521–529 [DOI] [PubMed] [Google Scholar]
- 48.Törnroth-Horsefield S., Wang Y., Hedfalk K., Johanson U., Karlsson M., Tajkhorshid E., Neutze R., Kjellbom P. (2006) Nature 439, 688–694 [DOI] [PubMed] [Google Scholar]
- 49.Horsefield R., Nordén K., Fellert M., Backmark A., Törnroth-Horsefield S., Terwisscha van Scheltinga A. C., Kvassman J., Kjellbom P., Johanson U., Neutze R. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 13327–13332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aroca R., Amodeo G., Fernández-Illescas S., Herman E. M., Chaumont F., Chrispeels M. J. (2005) Plant Physiol. 137, 341–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.