Abstract
Detection of persistent cervical carcinogenic human papillomavirus (HPV) DNA is used as a marker for cervical cancer risk in clinical trials. The authors performed a systematic review and meta-analysis of the association between persistent HPV DNA and high-grade cervical intraepithelial neoplasia (CIN2-3), high-grade squamous intraepithelial lesions (HSIL), and invasive cervical cancer (together designated CIN2-3/HSIL+) to evaluate the robustness of HPV persistence for clinical use. MEDLINE and Current Contents were searched through January 30, 2006. Relative risks (RRs) were stratified by HPV comparison group. Of 2,035 abstracts, 41 studies were eligible for inclusion in the meta-analysis. Over 22,500 women were included in calculation of RRs for persistent HPV DNA detection and cervical neoplasia. RRs ranged from 1.3 (95% confidence interval: 1.1, 1.5) to 813.0 (95% confidence interval: 168.2, 3,229.2) for CIN2-3/HSIL+ versus <CIN2-3/HSIL+; 92% of RRs were above 3.0. Longer durations of infection (>12 months), wider testing intervals, CIN2-3/HSIL+, and use of an HPV-negative reference group were consistently associated with higher RRs. Thus, HPV persistence was consistently and strongly associated with CIN2-3/HSIL+, despite wide variation in definitions and study methods. The magnitude of association varied by duration of persistence and testing interval. Precise definition and standardization of HPV testing, sampling procedure, and test interval are needed for reliable clinical testing. These findings validate HPV persistence as a clinical marker and endpoint.
Keywords: human papillomavirus 16, human papillomavirus 18, longitudinal studies, papillomavirus infections, uterine cervical neoplasms
Carcinogenic human papillomavirus (HPV) infection is necessary for the development of cervical cancer (1–3), the second most common cancer in women worldwide (4). Although carcinogenic HPV types are found in virtually all invasive cervical cancer, with types 16 and 18 being found in approximately 70 percent of cases (5–7), HPV infection is common among young women (8, 9). Most infections become undetectable within 1–2 years (10, 11). Thus, infection alone is not sufficient to cause cervical cancer.
Persistent HPV infections are considered to drive progression of cervical neoplasia to invasive cervical cancer (12). Laboratory evidence shows that continued HPV oncogene expression is critical for the maintenance and progression of cervical neoplasia (13). Several studies have suggested that detection of the same carcinogenic HPV type over time is particularly important for cervical carcinogenesis (14–16). Thus, type-specific persistence of carcinogenic HPV DNA has been used as a surrogate endpoint for risk of cervical cancer in vaccine trials (17, 18) and as a diagnostic marker for high-grade cervical intraepithelial neoplasia (CIN2-3) in cervical cancer screening studies (19, 20).
While HPV persistence is commonly defined as having two or more HPV DNA-positive tests (21, 22), other investigators have evaluated HPV persistence using time to clearance (i.e., duration) (23–25) or proportion of HPV-positive visits (26, 27). Definitions of HPV persistence are further complicated by differences in HPV laboratory detection methods, testing intervals, and HPV categorization and status for analysis (e.g., type-specific vs. non-type-specific HPV persistence; restriction to carcinogenic types, individual types, or overall HPV positivity; baseline HPV status; and clearance requirements). A clearer understanding of how investigators have estimated the association between HPV persistence and high-grade precancer and cancer would provide a basis for interpreting available data and reaching consensus on a standardized definition of HPV persistence for future studies and clinical use. Thus, we performed a systematic review to identify variations in the definitions of HPV persistence within the published literature and to determine the strength of the association between HPV persistence and cervical neoplasia and the influence of study characteristics on this association.
MATERIALS AND METHODS
Data abstraction
We identified relevant studies by searching MEDLINE (via PubMed), Current Contents, and reference lists from eligible articles through January 30, 2006. No language or publication starting-date limitations were imposed. Broad search-term categories included HPV, persistence (e.g., persistent, clearance, duration), and cervical disease (e.g., cervical dysplasia, squamous intraepithelial lesions (SIL), cervical intraepithelial neoplasia (CIN)) (specific search terms available upon request). Peer-reviewed publications that used HPV DNA detection in humans were eligible if they either reported or had calculable relative risks (risk ratios, rate ratios, odds ratios, or hazard ratios, hereafter termed “relative risks”) and corresponding 95 percent confidence intervals for the association between HPV persistence and cervical neoplasia. Posttreatment studies and studies that provided only baseline data on cervical neoplasia were excluded. Cervical outcomes included invasive cervical cancer, CIN2-3, and high-grade SIL (HSIL); low-grade CIN1 or low-grade SIL (LSIL); atypical squamous cells of undetermined significance (ASCUS); or any combination of the above (e.g., any SIL). Cervical cancer, CIN2-3, and HSIL were combined for formal analyses and termed “CIN2-3/HSIL+,” although histologically based diagnoses were examined separately where possible and assessed in study characteristic analyses. Similarly, low-grade CIN and LSIL were combined and termed “CIN1/LSIL.” For simplicity, the term “CIN2” is used to describe both cytologically diagnosed moderate dysplasia and histologically diagnosed CIN2, and “CIN3” describes both cytologically diagnosed severe dysplasia and histologically diagnosed CIN3.
We abstracted relative risks and 95 percent confidence intervals if they were reported or calculated them ourselves for the risk of cervical neoplasia among women with persistent HPV infections as compared with 1) women who were consistently negative for the HPV types used to define persistence (hereafter termed the “HPV-negative referent group”), 2) women who had HPV infections that became undetectable (the “transient HPV referent group”), or 3) a mix of women who were negative or had transient and/or incident HPV infection (the “mixed HPV referent group”). For author-calculated relative risks, 0.5 was added to each of the four interior cells if one of the cells contained zero. For studies with adjusted relative risks, the most adjusted relative risk (i.e., controlled for the largest number of potential confounders) was used for analyses.
Abstracted data included HPV type or group (individual types (e.g., HPV16), overall HPV positivity (any type), combined carcinogenic types), type-specificity (same HPV type or different types across time), HPV detection method, HPV testing interval, baseline HPV status, cervical status (e.g., baseline status, cervical outcome diagnostic method, and severity of lesion), demographic data (e.g., age, human immunodeficiency virus serostatus), and other study characteristics (e.g., sample size, study design, number of visits). Two independent reviewers abstracted and confirmed data from each article to ensure accuracy. Study authors were contacted for clarification of HPV persistence and cervical outcome definitions as needed.
Selection of relative risks
Several articles that were eligible for inclusion were based on the same study population (11, 28–41). In these cases, the article with the largest number of women was used, unless multiple articles could contribute to separate analyses (11, 29–32, 37–39).
In formal analyses, we focused on associations between HPV persistence and CIN2-3/HSIL+. Relative risks for CIN1/LSIL were included only for comparisons with CIN2-3/HSIL+, since CIN1/LSIL reflects active HPV replication and is generally managed clinically by follow-up rather than immediate treatment (42–44). Relative risks for the outcome “any lesion,” which combined lesions with low and high risk for progression to invasion, were not included.
Some studies provided multiple relative risks with differing definitions of HPV persistence or cervical outcome. In these cases, we applied the following decision rules to select one relative risk per study for meta-analyses, thereby maintaining the independence of observations: 1) relative risks for HPV16 persistence were chosen first, followed by HPV18, HPV16/18, other individual carcinogenic types, combined carcinogenic types, and overall HPV (any type); 2) type-specific relative risks were chosen over non-type-specific relative risks; 3) relative risks for CIN2-3/HSIL+ were chosen first, followed by relative risks for cancer only, CIN3 only, and CIN2 only; 4) relative risks based on histologic diagnosis only were chosen first; and 5) HPV persistence definitions involving more HPV-positive visits were chosen over definitions involving fewer HPV-positive visits (e.g., three HPV-positive visits vs. two).
Statistical analyses
Cochran's Q two-sided homogeneity p value (45) was used to assess overall heterogeneity in relative risks. Funnel plots were created by plotting the relative risks by their precision (46). These plots were statistically evaluated for asymmetry, using 1) Begg rank correlation (47), 2) Egger regression (48), and 3) the Duval and Tweedie “trim and fill” method (49), which imputes results that are hypothetically missing due to publication bias. Asymmetry can reflect publication bias, random error, or study characteristics associated with sample size (46). Since nearly 70 percent of relative risks in this review were calculated by us from data provided in the articles, funnel plot asymmetry analyses were also stratified by study purpose (i.e., studies that specifically assessed the relation between HPV persistence and cervical neoplasia vs. those that did not).
Analyses of study characteristics.
To assess variation in the strength of the association between HPV persistence and CIN2-3/HSIL+ by differences across studies, we evaluated key study characteristics using stratified random-effects meta-analysis and restricted maximum likelihood meta-regression. Stratified meta-analysis allows descriptive comparison of summary relative risks across individual categories of a specified study characteristic (e.g., by providing separate summary relative risks for polymerase chain reaction (PCR)-based and non-PCR-based HPV persistence). Restricted maximum likelihood meta-regression formally compares differences in relative risks across study characteristic categories (e.g., ratio of PCR-based to non-PCR-based relative risks) and estimates among-study variance (50). Given inherent differences in comparing persistent infections with HPV-negative, transient, and mixed infections, all analyses were stratified by HPV referent group. We assessed the consistency of study characteristic results across all three referent groups to distinguish true study characteristic effects from random variation.
Analyses of study characteristics focused on relative risks with CIN2-3/HSIL+ as the outcome, given its clinical relevance, and on characteristics related to HPV persistence and cervical status (e.g., HPV testing interval and method of outcome diagnosis). For these analyses, we allowed studies to contribute relative risks to more than one category in order to reduce any potential influence of the decision rules on the distribution of study characteristics. Where studies contributed to more than one category, meta-regression confidence intervals may be influenced by the incomplete independence of observations. We also analyzed disease severity to formally compare relative risks that used CIN2-3/HSIL+ as the outcome with those that used CIN1/LSIL.
Sensitivity analysis.
We performed sensitivity analyses using reasonable alternative decision rules to assess the influence of the above decision rules on the strength and stability of meta-analytic and study characteristic results. The first sensitivity analysis reversed the order of decision rule 1 so that relative risks for overall HPV persistence were chosen first and HPV16 last. The second sensitivity analysis changed decision rule 3 from the choice of CIN2-3/HSIL+ (initially selected for increased estimate stability and because treatment guidelines are based on CIN2 and CIN3 combined) (42, 44) to the choice of cancer only first, followed by CIN3 only, CIN2 only, and then CIN2-3/HSIL+. The sensitivity of the results to these decision rule changes was evaluated by descriptively comparing the homogeneity p value, funnel plots, and Begg and Egger two-sided p values and the magnitude and precision of random-effects summary relative risks. In addition, results of study characteristic analyses in which studies contributed relative risks to more than one category were compared with those with only one relative risk per study.
RESULTS
Descriptive results
Eligible studies.
Over 22,500 women were included in the calculation of relative risks from 41 eligible studies of 37 unique populations identified from 2,035 abstracts. Three studies were clinical trials and 38 were cohort-based, including six nested case-control studies (16, 29–31, 37, 51) and one follow-up of a case-control study (52) (table 1). Most studies required normal cervical diagnosis at study entry, although some included both women with normal diagnoses and a proportion with ASCUS or cervical neoplasia at baseline. Study populations were predominately population- or cervical screening-based and European or North American.
TABLE 1.
Distribution of relative risks for the association between human papillomavirus persistence and cervical neoplasia according to selected study characteristics
| Characteristic | No. of studies | % of studies | Reference no(s). |
| Study design | |||
| Cohort study | 38 | 92.7 | 10, 11, 14, 16, 20, 25, 27, 29–32, 37–39, 51–65, 67–71, 75, 102–104 |
| Clinical trial | 3 | 7.3 | 17, 66, 105 |
| Baseline cervical status* | |||
| Normal | 24 | 60.0 | 10, 16, 17, 29–32, 37–39, 51, 56, 57, 60, 61, 64–68, 71, 75, 102, 104 |
| Normal/ASCUS† | 6 | 15.0 | 11, 54, 58, 59, 62, 70 |
| Normal/lesions | 10 | 25.0 | 14, 20, 25, 27, 52, 53, 63, 69, 103, 105 |
| Population | |||
| Population-based | 5 | 12.2 | 14, 17, 37, 60, 62 |
| Screening-based | 15 | 36.6 | 16, 29–32, 38, 39, 51, 54, 56–59, 61, 75 |
| GYN†/STI†/hospital-based | 9 | 22.0 | 20, 27, 52, 53, 55, 67, 102–104 |
| Other | 12 | 29.3 | 10, 11, 25, 63–71 |
| Continent | |||
| Europe | 20 | 48.8 | 16, 20, 27, 29–32, 37–39, 53–56, 58–60, 69, 75, 102 |
| North America | 16 | 39.0 | 10, 11, 25, 51, 52, 57, 61, 63–68, 70, 71, 105 |
| Other | 5 | 12.2 | 14, 17, 62, 103, 104 |
| Definition of HPV† persistence‡ | |||
| ≥2 HPV-positive visits | 32 | 78.0 | 10, 11, 14, 16, 17, 20, 30–32, 37–39, 51–62, 64–67, 70, 103–105 |
| ≥3 HPV-positive visits | 8 | 19.5 | 11, 29, 62, 67–69, 71, 75 |
| Proportion of HPV-positive visits | 2 | 4.9 | 27, 63 |
| HPV-positive throughout follow-up | 1 | 2.4 | 102 |
| Time to HPV clearance | 1 | 2.4 | 25 |
| Minimum duration of HPV infection (months)§ | |||
| <6 | 12 | 30.0 | 11, 20, 25, 32, 52, 55, 62, 64, 67, 103–105 |
| 6–12 | 18 | 45.0 | 10, 11, 17, 37, 51, 53, 54, 57, 58, 61–63, 65, 66, 68, 70, 71, 75 |
| >12 | 10 | 25.0 | 14, 16, 27, 29, 38, 39, 56, 59, 69, 102 |
| Baseline HPV status¶ | |||
| Positive | 10 | 24.4 | 11, 32, 38, 39, 52, 58, 60, 61, 75, 104 |
| Negative | 2 | 4.9 | 17, 105 |
| Both | 29 | 70.7 | 10, 14, 16, 20, 25, 29–31, 37, 51, 53–57, 59, 61–71, 102, 103 |
| HPV DNA detection method | |||
| Polymerase chain reaction | 33 | 80.5 | 10, 14, 16, 17, 25, 27, 29–31, 37–39, 51–53, 56–60, 62–66, 68–71, 75, 103–105 |
| Hybrid capture | 5 | 12.2 | 20, 32, 54, 55, 102 |
| Other | 3 | 7.3 | 11, 61, 67 |
One study did not evaluate cervical status at baseline (55).
ASCUS, atypical squamous cells of undetermined significance; GYN, gynecology; STI, sexually transmitted infection; HPV, human papillomavirus.
Numbers of studies add up to more than 41 and percentages to more than 100 because some studies (11, 62, 67) used more than one definition of HPV persistence.
Definitions of HPV persistence.
Definitions of HPV persistence varied considerably across studies (table 1). Most studies (78 percent) defined persistence as HPV positivity at two or more time points, whereas others used three or more HPV-positive visits, the proportion of HPV-positive visits, HPV positivity throughout study follow-up, or time to clearance. Persistence was defined as HPV positivity at only two time points in approximately 50 percent of the studies (n = 19) (14, 16, 20, 32, 37–39, 51–62). Consecutive positivity was generally required, although intervening HPV-negative visits were allowed in some studies (11, 25, 27, 62–66). The minimum duration of HPV persistence (i.e., the shortest period of HPV detection necessary for a woman to be classified as having persistent infection) was less than 6 months for 30 percent of studies, 6–12 months for 45 percent, and more than 12 months for 25 percent. The median time between HPV tests was 6 months (range, 2–72 months). Only seven studies clearly identified two or more HPV-positive visits prior to cervical outcome diagnosis (11, 29–31, 58, 62, 67). Most studies (80 percent) used PCR for HPV DNA detection. The hybrid capture assay was the most common non-PCR assay. At baseline, most studies included both HPV-positive and HPV-negative women, although two studies required HPV negativity (5 percent) and nine required HPV positivity (22 percent).
Cervical neoplasia.
A total of 231 relative risks were available for calculation of the association between HPV persistence and cervical neoplasia (see the Web Appendix, which is posted on the Journal's website (http://aje.oxfordjournals.org/)). Seventy-seven relative risks (33 percent) used an HPV-negative referent group, 89 (39 percent) a transient HPV referent group, and 65 (28 percent) a mixed HPV referent group. Forty-one percent used type-specific HPV persistence definitions, and 59 percent used non-type-specific definitions. Twenty relative risks were for HPV16 persistence, two were for HPV18, and 11 were for either HPV16 or HPV18. Only 32 percent of 25 studies with data on CIN2-3/HSIL+ provided information on multiple HPV types, and that information was generally limited to the proportion of women who had multiple types.
CIN2-3/HSIL+ comprised 44 percent of outcomes; CIN1/LSIL, 14 percent; ASCUS, 7 percent; any lesion (CIN/SIL), 25 percent; and any ASCUS or lesion, 10 percent. Outcome diagnosis was based on histology (26 percent), cytology and histology (17 percent), cytology only (55 percent), or colposcopic impression (1 percent) (see Web Appendix).
Six studies provided 17 relative risks from populations including human immunodeficiency virus-positive women (25, 63, 68–71) (see Web Appendix). Relative risks from these studies ranged from 0.98 (95 percent confidence interval (CI): 0.7, 1.4) for LSIL to 18.1 (95 percent CI: 2.6, 125.0) for HSIL (69). Given that human immunodeficiency virus-positive and -negative women have different risks of incident HPV infection and progression to cervical neoplasia due to immunosuppression (72–74), these relative risks were not included in further analyses.
Analytic results
Association between HPV persistence and CIN2-3/HSIL+.
There was notable variation in the magnitude and precision of relative risks for HPV persistence and CIN2-3/HSIL+, which ranged from 1.3 (95 percent CI: 1.1, 1.5) (75) to 813.0 (95 percent CI: 168.2, 3,229.2) (37) (see Web Appendix). There were 13 relative risks for HPV16 persistence (ranging from 2.0 (95 percent CI: 0.14, 28.0) for cytologically diagnosed CIN2 (38) to 94.6 (95 percent CI: 53.4, 167.7) for histologically diagnosed CIN3+ (14)), two relative risks for HPV18 persistence (ranging from 4.5 (95 percent CI: 0.24, 85.1) for cytologically diagnosed CIN2 (38) to 90.6 (95 percent CI: 36.1, 227.4) for histologically diagnosed CIN3+ (14)), and three relative risks for HPV16 and/or HPV18 persistence (ranging from 7.5 (95 percent CI: 0.39, 142.9) for cytologically diagnosed CIN2 (38) to 12.3 (95 percent CI: 2.6, 57.5) for HSIL (62)).
Figure 1 presents a forest plot of 33 relative risks for HPV persistence and CIN2-3/HSIL+ selected through the above-described decision rules for each HPV referent group. All associations were positive, although heterogeneous, and almost all lower 95 percent limits were above 1. Descriptively, associations appeared stronger when women with persistent HPV infections were compared with uninfected women than when they were compared with women with transient infections. Median relative risks were 33.3 for studies with an HPV-negative referent group (homogeneity p < 0.0005), 29.8 for a mixed HPV referent group (homogeneity p < 0.0005), and 14.7 for a transient HPV referent group (homogeneity p = 0.1). Five relative risks required women to have persistent HPV infection (two or more HPV-positive visits) prior to diagnosis of CIN2-3/HSIL+ (11, 29–31, 67); the magnitudes of these relative risks seemed qualitatively similar to those of the other 28 relative risks.
FIGURE 1.
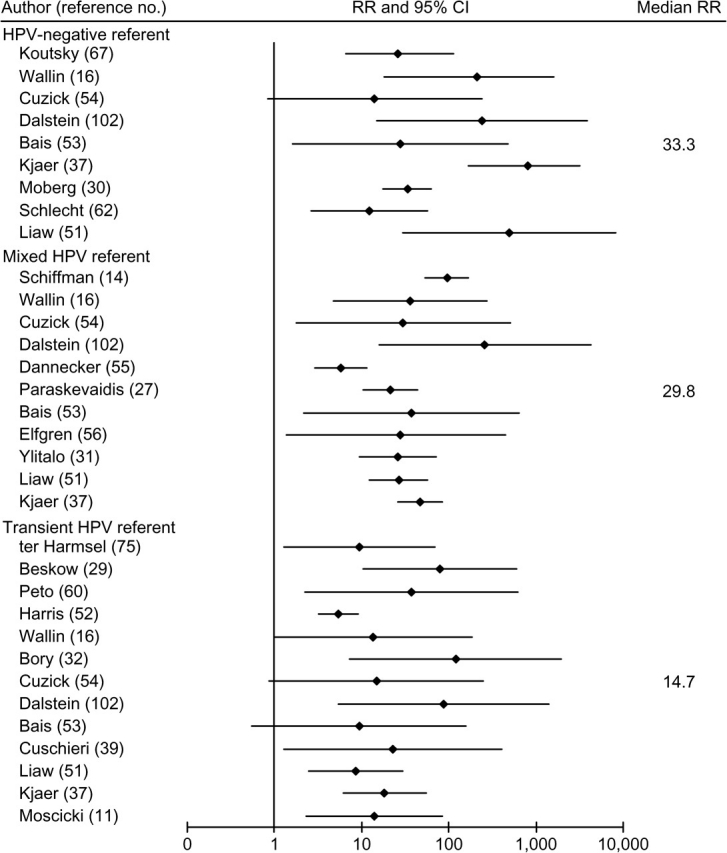
Relative risks (RRs) and 95% confidence intervals (CIs) for associations between human papillomavirus (HPV) persistence and high-grade cervical intraepithelial neoplasia, high-grade squamous intraepithelial lesions, and invasive cervical cancer, by HPV referent group, in the published literature. Results are organized first by outcome diagnosis, then type-specificity, and then baseline cervical status within each HPV referent group.
Only five studies provided relative risks for type-specific HPV persistence and histologically diagnosed CIN2-3 (14, 29, 52, 60, 75) (table 2 and shaded rows in the Web Appendix). These relative risks appeared to be descriptively similar in magnitude to the other relative risks in figure 1. Six studies from five unique study populations in figure 1 provided relative risks based on cytologic, colposcopic, or cytologic/histologic diagnosis of CIN2-3/HSIL+ (30, 31, 37, 39, 56, 62) (table 2). The relative risks generally appeared to be similar to those based solely on histology. Formal analyses of differences by histology and type-specificity were conducted through meta-regression, as presented in table 3. (An enhanced version of table 3 including reference numbers is posted on the Journal's website (http://aje.oxfordjournals.org/) as online table 3.)
TABLE 2.
Selected characteristics of studies contributing to a meta-analysis of human papillomavirus persistence and high-grade cervical intraepithelial neoplasia, high-grade squamous intraepithelial lesions, and invasive cervical cancer
| Authors (ref. no.) | Outcome diagnosis | HPV* type-specificity and grouping | Relative risk† | 95% confidence interval |
| HPV-negative referent group | ||||
| Koutsky et al. (67) | Histology | Non-type-specific overall HPV | 26.0 | 6.5, 112.0 |
| Wallin et al. (16) | Histology | Non-type-specific overall HPV | 213.4 | 18.1, 1,600.0 |
| Cuzick et al. (54) | Histology | Non-type-specific carcinogenic HPV | 14.0 | 0.83, 237.5 |
| Dalstein et al. (102) | Histology | Non-type-specific carcinogenic HPV | 239.9 | 14.8, 3,893.5 |
| Bais et al. (53) | Histology | Carcinogenic HPV‡ | 27.5 | 1.6, 474.6 |
| Kjaer et al. (37) | Not histology only | Type-specific overall HPV | 813.0 | 168.2, 3,229.2 |
| Moberg et al. (30) | Not histology only | Type-specific HPV16 | 33.3 | 17.7, 62.7 |
| Schlecht et al. (62) | Not histology only | Type-specific HPV16/HPV18 | 12.3 | 2.6, 57.5 |
| Liaw et al. (51) | Not histology only | Non-type-specific overall HPV | 497.1 | 29.8, 8,290.2 |
| Mixed HPV referent group | ||||
| Schiffman et al. (14) | Histology | Type-specific HPV16 | 94.6 | 53.4, 167.7 |
| Wallin et al. (16) | Histology | Non-type-specific overall HPV | 36.1 | 4.8, 271.6 |
| Cuzick et al. (54) | Histology | Non-type-specific carcinogenic HPV | 29.8 | 1.8, 507.3 |
| Dalstein et al. (102) | Histology | Non-type-specific carcinogenic HPV | 260.6 | 16.1, 4,229.3 |
| Dannecker et al. (55) | Histology | Non-type-specific carcinogenic HPV | 5.7 | 2.9, 11.3 |
| Paraskevaidis et al. (27) | Histology | Carcinogenic HPV‡ | 21.3 | 10.3, 44.3 |
| Bais et al. (53) | Histology | Carcinogenic HPV‡ | 36.9 | 2.1, 638.2 |
| Elfgren et al. (56) | Not histology only | Type-specific HPV16 | 27.3 | 1.4, 452.0 |
| Ylitalo et al. (31) | Not histology only | Type-specific HPV16 | 25.9 | 9.3, 72.1 |
| Liaw et al. (51) | Not histology only | Non-type-specific overall HPV | 26.8 | 12.4, 57.6 |
| Kjaer et al. (37) | Not histology only | Non-type-specific overall HPV | 46.6 | 25.8, 83.8 |
| Transient HPV referent group | ||||
| ter Harmsel et al. (75) | Histology | Type-specific HPV16 | 9.4 | 1.3, 68.4 |
| Beskow et al. (29) | Histology | Type-specific HPV16 | 79.0 | 10.4, 597.3 |
| Peto et al. (60) | Histology | Type-specific carcinogenic HPV | 37.1 | 2.2, 620.5 |
| Harris et al. (52) | Histology | Type-specific carcinogenic HPV | 5.4 | 3.2, 9.2 |
| Wallin et al. (16) | Histology | Non-type-specific overall HPV | 13.5 | 0.98, 185.5 |
| Bory et al. (32) | Histology | Non-type-specific carcinogenic HPV | 119.1 | 7.4, 1,926.2 |
| Cuzick et al. (54) | Histology | Carcinogenic HPV‡ | 14.7 | 0.87, 249.6 |
| Dalstein et al. (102) | Histology | Non-type-specific carcinogenic HPV | 88.2 | 5.5, 1,427.9 |
| Bais et al. (53) | Histology | Carcinogenic HPV‡ | 9.4 | 0.56, 157.2 |
| Cuschieri et al. (39) | Not histology only | Type-specific carcinogenic HPV | 22.9 | 1.3, 408.1 |
| Liaw et al. (51) | Not histology only | Non-type-specific overall HPV | 8.6 | 2.5, 30.0 |
| Kjaer et al. (37) | Not histology only | Non-type-specific overall HPV | 18.2 | 6.1, 54.3 |
| Moscicki et al. (11) | Not histology only | Non-type-specific carcinogenic HPV | 14.1 | 2.3, 84.5 |
HPV, human papillomavirus.
Relative risks are organized first by outcome diagnosis, then type-specificity, and then baseline cervical status within each HPV referent group.
HPV type-specificity was not specified.
TABLE 3.
Effect of study characteristics on the association between human papillomavirus (HPV) persistence and high-grade cervical intraepithelial neoplasia, high-grade squamous intraepithelial lesions, and invasive cervical cancer, by HPV referent group,* in a meta-analysis of the published literature
| Study characteristic | HPV-negative referent group | Mixed HPV referent group | Transient HPV referent group | ||||||||||||
| No. of studies (homogeneity p value) | Summary effect estimate | Ratio of effect estimates | No. of studies (homogeneity p value) | Summary effect estimate | Ratio of effect estimates | No. of studies (homogeneity p value) | Summary effect estimate | Ratio of effect estimates | |||||||
| RR† | 95% CI† | Ratio of RRs | 95% CI | RR | 95% CI | Ratio of RRs | 95% CI | RR | 95% CI | Ratio of RRs | 95% CI | ||||
| Minimum duration of HPV persistence (months) | |||||||||||||||
| ≤12 | 6 (<0.001) | 63.9 | 7.9, 516.7 | 1.0 | 5 (<0.001) | 21.5 | 7.4, 62.1 | 1.0. | 8 (0.4) | 9.5 | 5.5, 16.4. | 1.0 | |||
| >12 | 2 (0.96) | 223.0 | 39.0, 1,273.3 | 3.3 | 0.13, 84.0 | 5 (0.02) | 50.0 | 18.7, 134.1 | 2.4 | 0.63, 8.9 | 4 (0.7) | 42.9 | 12.3, 149.6 | 4.3 | 1.0, 18.0 |
| HPV testing interval (months) | |||||||||||||||
| ≤6 | 3 (0.2) | 29.0 | 7.6, 110.7 | 1.0 | 3 (0.003) | 18.7 | 4.5, 77.6 | 1.0 | 4 (0.2) | 9.4 | 3.6, 24.3 | 1.0 | |||
| >6–12 | 3 (0.2) | 57.7 | 6.7, 495.9 | 2.3 | 0.34, 15.3 | 3 (0.98) | 27.6 | 13.5, 56.4 | 2.0 | 0.53, 7.9 | 4 (0.4) | 13.0 | 4.8, 35.0 | 2.0 | 0.66, 6.0 |
| >12 | 2 (0.4) | 667.9 | 264.1, 1,689.1 | 31.1 | 11.0, 87.6 | 4 (0.3) | 63.2 | 39.1, 102.0 | 4.2 | 1.4, 12.8 | 3 (0.96) | 17.9 | 6.9, 46.6. | 2.8 | 0.95, 8.1 |
| HPV group | |||||||||||||||
| Overall HPV | 5 (<0.001) | 104.2 | 11.9, 912.1 | 1.0 | 3 (0.5) | 37.8 | 24.0, 59.6 | 1.0 | 4 (0.9) | 13.3 | 6.2, 28.5 | 1.0 | |||
| Carcinogenic types | 5 (0.4) | 30.6 | 17.7, 59.2 | 0.57 | 0.05, 6.6‡ | 5 (0.01) | 20.3 | 6.5, 62.9. | 0.42 | 0.11, 1.8 | 8 (0.1) | 15.4 | 6.2, 38.2 | 1.0 | 0.28, 3.9‡ |
| HPV16/HPV18 | 2 (0.2) | 26.3 | 11.4, 60.8 | 0.35 | 0.02, 7.4‡ | 3 (0.08) | 50.5 | 17.6, 144.8. | 1.4 | 0.37, 5.6 | 3 (0.1) | 15.5 | 3.0, 80.6 | 1.2 | 0.22, 6.5‡ |
| Type-specific persistence | |||||||||||||||
| No | 7 (0.02) | 102.4 | 27.0, 388.1 | 1.0 | 6 (<0.001) | 27.3 | 9.9, 75.1 | 1.0 | 8 (0.7) | 16.0 | 18.4, 30.4 | 1.0 | |||
| Yes | 3 (<0.001) | 74.0 | 5.2, 1,061.1 | 0.77 | 0.07, 8.9‡ | 3 (0.08) | 50.5 | 17.6, 144.8 | 1.9 | 0.42, 8.3 | 5 (0.07) | 14.9 | 4.6, 48.9 | 0.66 | 0.21, 2.0‡ |
| HPV detection method | |||||||||||||||
| Non-PCR† | 3 (0.3) | 36.5 | 9.4, 141.3 | 1.0 | 3 (0.02) | 26.5 | 2.4, 286.1 | 1.0 | 4 (0.5) | 29.9 | 9.0, 99.4 | 1.0 | |||
| PCR | 6 (<0.001) | 99.4 | 15.2, 652.1 | 2.4 | 0.19, 31.5 | 8 (0.06) | 38.5 | 23.9, 62.2 | 3.2 | 0.91, 10.9 | 9 (0.2) | 11.4 | 6.2, 20.9 | 0.37 | 0.09, 1.6 |
| Outcome diagnosis | |||||||||||||||
| Not histology only | 4 (<0.001) | 108.2 | 10.6, 1,107.5 | 1.0 | 4 (0.6) | 35.3 | 23.2, 53.6 | 1.0 | 5 (0.6) | 15.7 | 7.8, 31.8 | 1.0 | |||
| Histology only§ | 5 (0.5) | 49.4 | 17.0, 143.6 | 0.51 | 0.05, 5.4 | 7 (<0.001) | 32.8 | 10.2, 105.3 | 0.94 | 0.25, 3.5 | 9 (0.06) | 18.7 | 7.2, 48.5 | 0.95 | 0.27, 3.4‡ |
| Baseline cervical status | |||||||||||||||
| Normal/lesions | 2 (0.3) | 82.7 | 9.9, 693.0 | 1.0 | 4 (0.01) | 55.3 | 17.5, 174.3 | 1.0 | 3 (0.1) | 10.8 | 2.3, 51.0 | 1.0 | |||
| Normal/ASCUS† | 2 (0.9) | 12.7 | 3.3, 49.3 | 0.16 | 0.004, 6.2 | 1 (NA†) | NA | NA | 2 (0.98) | 14.3 | 3.1, 65.4 | 2.4 | 0.48, 11.8 | ||
| Normal | 5 (<0.001) | 141.0 | 19.8, 1,004.7 | 1.7 | 0.08, 36.5 | 5 (0.8) | 35.4 | 23.5, 53.3 | 0.64 | 0.24, 1.7 | 8 (0.6) | 18.4 | 9.8, 37.7 | 3.1 | 1.4, 6.9 |
| Mean or median age (years) of study population | |||||||||||||||
| ≤35 | 4 (<0.001) | 57.7 | 5.9, 563.9 | 1.0 | 4 (0.4) | 32.5 | 21.5, 49.1 | 1.0 | 4 (0.2) | 9.6 | 4.4, 21.7 | 1.0 | |||
| >35 | 4 (0.4) | 76.6 | 19.1, 308.2 | 1.1 | 0.08, 16.0 | 5 (0.03) | 26.4 | 6.0, 116.8 | 0.58 | 0.16, 2.1 | 6 (0.6) | 20.9 | 7.3, 59.5 | 2.3 | 0.61, 8.6 |
HPV-negative, transient HPV, or mixed.
RR, relative risk; CI, confidence interval; PCR, polymerase chain reaction; ASCUS, atypical squamous cells of undetermined significance; NA, not applicable.
At least one study contributed effect estimates to more than one category. Thus, observations are not independent and 95% confidence intervals may be inaccurate.
One study (54) included one case (out of nine) that had no histologic data available.
Funnel plot asymmetry.
Assessment of funnel plot asymmetry for HPV persistence and CIN2-3/HSIL+ relative risks suggested that there was no notable evidence of asymmetry within the HPV-negative (Begg p = 0.5, Egger p = 0.1) and mixed HPV (Begg p = 0.9, Egger p = 1.0) referent categories. The Duval and Tweedie “trim and fill” imputation method (49) had no effect on these results. Relative risks with a transient HPV referent showed some evidence of funnel plot asymmetry (Begg p = 0.4, Egger p = 0.002). The Duval and Tweedie method imputed data for seven hypothetically missing studies, which reduced the random-effects summary relative risk from 14.4 to 7.2. After stratification of the results by study purpose, all p values were greater than 0.1, which suggests, reduced power notwithstanding, that overall asymmetry is more likely due to study characteristics (described below) than to publication bias.
Evaluation of study characteristics.
Given the small number of studies in each category of a given study characteristic (table 3), study characteristics were evaluated according to consistency in the direction of the ratios of relative risks across HPV referent groups. Associations between HPV persistence and CIN2-3/HSIL+ appeared stronger when persistence was defined as HPV infection lasting for more than 12 months versus 12 months or less (the ratio of relative risks was 3.3 (95 percent CI: 0.13, 84.0) for the HPV-negative referent group, 4.3 (95 percent CI: 1.0, 18.0) for the transient HPV referent group, and 2.4 (95 percent CI: 0.63, 8.9) for the mixed HPV referent group), although the ratios of relative risks were imprecise, as indicated by the wide confidence intervals (table 3). This pattern was supported by trends in the ratios for HPV testing intervals; relative risks were highest for testing intervals of >12 months, followed by >6–12 months and then ≤6 months. However, even the shortest HPV duration and testing intervals produced summary relative risks over 9.0. Other study characteristics assessed (e.g., type-specificity) produced inconsistent results across the three HPV referent groups (table 3). Additional characteristics (e.g., study location) were also inconsistent or were not reported in sufficient detail (e.g., smoking, sexual practices, oral contraceptive use) for us to assess in this review (data not shown). Baseline HPV status, minimum number of visits for HPV persistence, intervening HPV-negative visits, HPV persistence defined prior to the outcome, hand calculation of relative risks, censoring at treatment, health-care provider HPV collection versus self-collection, anatomical sampling location, and time between visits could not be evaluated, since fewer than two relative risks were available for each stratum of the study characteristic.
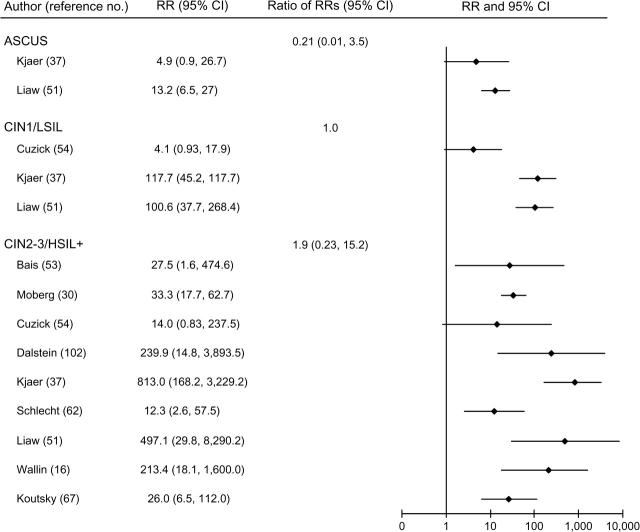
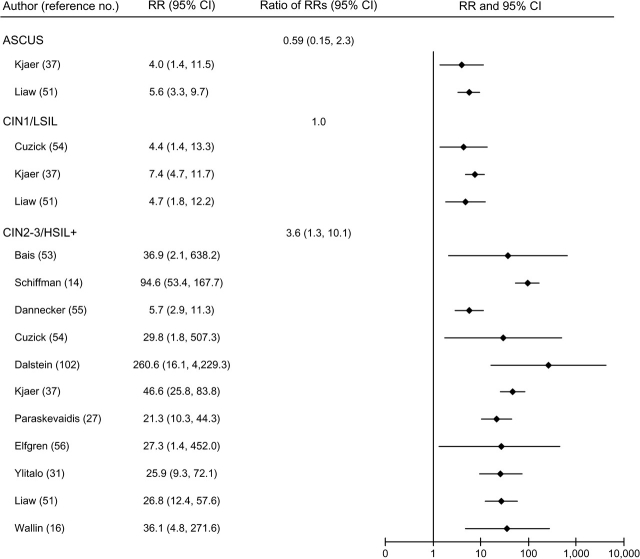
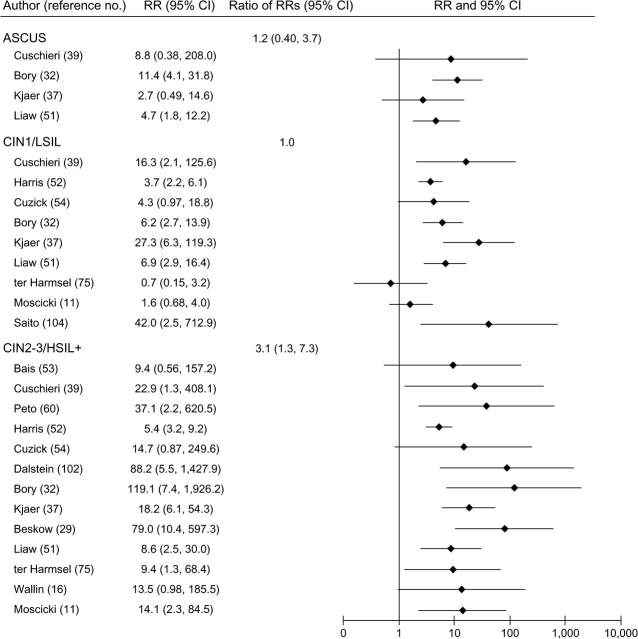
In general, HPV persistence was strongly and positively associated with all grades of cervical neoplasia, although associations appeared stronger for CIN2-3/HSIL+ than for CIN1/LSIL, regardless of HPV referent group (figures 2, 3, and 4).
FIGURE 2.
Relative risks (RRs) and 95% confidence intervals (CIs) for atypical squamous cells of undetermined significance (ASCUS); low-grade cervical intraepithelial neoplasia (CIN1) and low-grade squamous intraepithelial lesions (LSIL); and high-grade cervical intraepithelial neoplasia (CIN2-3), high-grade squamous intraepithelial lesions (HSIL+), and invasive cervical cancer in women with persistent human papillomavirus infection compared with human papillomavirus-negative women in a meta-analysis of the published literature. Some RRs from the same study were included in more than one disease category. Thus, 95% CIs for the ratios of RRs may be inaccurate, since observations were not independent.
FIGURE 3.
Relative risks (RRs) and 95% confidence intervals (CIs) for atypical squamous cells of undetermined significance (ASCUS); low-grade cervical intraepithelial neoplasia (CIN1) and low-grade squamous intraepithelial lesions (LSIL); and high-grade cervical intraepithelial neoplasia (CIN2-3), high-grade squamous intraepithelial lesions (HSIL+), and invasive cervical cancer in women with persistent human papillomavirus infection compared with women with mixed human papillomavirus status in a meta-analysis of the published literature. Some RRs from the same study were included in more than one disease category. Thus, 95% CIs for the ratios of RRs may be inaccurate, since observations were not independent.
FIGURE 4.
Relative risks (RRs) and 95% confidence intervals (CIs) for atypical squamous cells of undetermined significance (ASCUS); low-grade cervical intraepithelial neoplasia (CIN1) and low-grade squamous intraepithelial lesions (LSIL); and high-grade cervical intraepithelial neoplasia (CIN2-3), high-grade squamous intraepithelial lesions (HSIL+), and invasive cervical cancer in women with persistent human papillomavirus infection compared with women with transient human papillomavirus infection in a meta-analysis of the published literature. Some RRs from the same study were included in more than one disease category. Thus, 95% CIs for the ratios of RRs may be inaccurate, since observations were not independent.
Sensitivity analyses.
We conducted sensitivity analyses to evaluate the impact of the hierarchical decision rules used to select relative risks for analyses when multiple relative risks were available for a given study. Meta-analytic results seemed robust to changes in the order of the decision rules regarding HPV group or cervical outcome category within each HPV referent category. For example, changing the order of the HPV group favored in the decision rules did not change the homogeneity, Begg, or Egger p values (p < 0.0005, p = 0.5, and p = 0.1, respectively) for relative risks in the HPV-negative referent group, and use of a summary relative risk was not indicated in either case (data available upon request). Study characteristic analyses also appeared robust to changes in the order of the decision rules and produced similar results when analyses were restricted to one relative risk per study.
DISCUSSION
To our knowledge, this review of 41 studies with over 22,500 women is the first systematic evaluation of the association between HPV persistence and cervical neoplasia. Concerted efforts were made to include all published study results, including translation of foreign-language papers and a full article review for every abstract suggesting data on HPV persistence and cervical neoplasia. All abstracted data were reviewed twice by independent readers to ensure data accuracy. Relative risks were selected through decision rules for analyses of HPV persistence and CIN2-3/HSIL+ to maintain independence of observations. Sensitivity analyses suggested that the results of this meta-analysis were robust to reasonable changes in the decision rules, and funnel plot asymmetry analyses showed little evidence of publication bias.
HPV persistence was strongly and consistently associated with CIN2-3/HSIL+, emphasizing the value of HPV persistence as a clinical marker and as an endpoint in clinical trials and suggesting that sequential HPV DNA testing may be useful for cervical cancer screening programs by identifying women who are at high risk of cervical cancer.
The strength of the association between HPV persistence and cervical neoplasia increased with increasing grade of cervical disease. This trend supports the view that CIN1 represents active, mainly transient HPV infection and has high rates of occurrence and regression among sexually active women (76–79). In contrast, long-term HPV positivity is clearly associated with neoplastic transformation and is thus clinically relevant to cervical cancer and its prevention.
The magnitude of effect for HPV persistence in predicting CIN2-3/HSIL+ varied widely and was partially dependent on the HPV referent group. Comparing women with persistent HPV infections with HPV-negative women produced the highest relative risks because the risk of CIN2-3/HSIL+ approaches zero in HPV-negative women. Comparing women with persistent HPV infections to those with transient infections potentially estimates the effect of HPV persistence beyond exposure to short-term infection. These comparisons produced the weakest relative risks, which may suggest that the risk of CIN2-3/HSIL+ is not zero in women with HPV infections of short duration, as previously shown (18, 80, 81), or that a proportion of women with transient HPV infections actually had persistent infections prior to study initiation and were thus misclassified.
Associations between HPV persistence and cervical neoplasia also appeared stronger among studies with two related characteristics: longer duration of HPV infection (>12 months) and wider HPV testing intervals (>6 months or >12 months). These characteristics may represent a proxy for longer exposure to the carcinogenic effect of HPV oncogene activity, and thus greater likelihood of developing CIN2-3 and invasive cervical cancer. The observed increase in the strength of the association for CIN2-3/HSIL+ with increasing HPV testing interval probably reflects decreased misclassification of both exposure and outcome, since most HPV infections will clear and associated low-grade lesions will regress during the testing interval.
Although the detection of HPV infection at two time points has been criticized as being arbitrary (82), these data clearly demonstrate that two HPV-positive visits are associated with increased risk of CIN2-3/HSIL+. This review confirms that repeated HPV detection does indicate an increased risk of invasive cervical cancer and its precursors, despite differences in HPV persistence definitions, HPV detection techniques and testing intervals, cervical outcome diagnoses, and other study characteristics.
Associations between HPV16 and/or HPV18 persistence and CIN2-3/HSIL+ were consistently positive in formal analyses, albeit with notable heterogeneity (relative risks ranged from 4.5 (95 percent CI: 0.24, 85.1) to 279.7 (95 percent CI: 16.0, 4,894.5)). However, few studies provided sufficient data to obtain HPV16 and HPV18 type-specific associations. Given that even a single detection of HPV16 or HPV18 appears to strongly predict the development of CIN3 and cervical cancer (83, 84), future studies should focus on associations with type-specific persistence.
Analyses of additional study characteristics were inconsistent across HPV referent groups, probably because of random error from sparse data across strata that may have masked true effects. Unreported but potentially important explanatory factors (e.g., sensitivity, specificity, and reproducibility of HPV tests, cytology, colposcopy, and histology) may also explain heterogeneity in associations between HPV persistence and CIN2-3/HSIL+ (85–91).
Although this review focused on the relative risk of CIN2-3/HSIL+ associated with HPV persistence, it is also important to consider the absolute difference in risk of CIN2-3/HSIL+ for women with persistent HPV infection as compared with women without persistent infection (92). To approximate absolute risk differences for the median relative risks in figure 1, we estimated the baseline (unexposed) risk of CIN2-3/HSIL+ for the studies shown in figure 1. Because the unexposed risk was zero in the cohort analyses, we used the nested case-control studies when possible to roughly estimate the median unexposed risks of CIN2-3/HSIL+ (16, 29–31, 37, 51), which were approximately 0.0005, 0.002, and 0.003 for the HPV-negative, mixed, and transient referent groups, respectively. The mean or median duration of follow-up, where stated, was approximately 2–8 years (31, 37). Using these estimates, the median relative risks in figure 1 correspond to risk differences of approximately 2 percent, 6 percent, and 4 percent, respectively (e.g., the risk difference for the HPV-negative group was (33.5 × 0.0005) − 0.0005 = 0.02). Thus, the “numbers needed to treat” (the number of women required to have persistent HPV infection to increase the number of incident CIN2-3/HSIL+ cases by 1) are approximately 60, 15, and 24, respectively. For example, persistent HPV infection results in a rough average of approximately one extra CIN2-3/HSIL+ case in every 60 women followed for about 5 years as compared with HPV-negative women. These estimates, while very approximate, provide some sense of the absolute difference in risk.
Further research would benefit from standardized criteria for the detection and reporting of HPV infections and cervical neoplasia. Over one third of all relative risks used a composite outcome of any cervical lesion/ASCUS as the cervical outcome and thus could not be used to examine the association of HPV persistence by specific grade of neoplasia. Testing intervals also varied widely. Several studies have suggested that the median duration of HPV infection, which may vary by HPV type, is approximately 1 year (10, 24, 57, 93–95). However, the testing interval was generally ≥6 months in these studies, which could have led to overestimation of median duration in those analyses. A recent population-based study from Costa Rica suggests that more than half of all HPV infections clear by 6 months (96). In light of these data and the finding in this systematic review that even testing intervals of ≤6 months produced strong summary relative risks for the association between HPV persistence and CIN2-3/HSIL+, repeat HPV DNA testing at 6 months may be a valuable way to identify women at increased risk of cervical precancer and cancer for clinical purposes. Large studies of persistence and the natural history of precancer are emerging that will continue to inform this issue (e.g., see Plummer et al. (97)).
Testing for persistent HPV DNA may be valuable for cervical cancer screening, particularly given the higher sensitivity of HPV DNA testing as compared with cytology for CIN2-3+ (98, 99) and the underdetection of cervical neoplasia using colposcopy-directed biopsies (100, 101). A reliable definition of HPV persistence would have higher specificity than a single cross-sectional measurement of HPV and higher sensitivity than currently available cytologic screening tests.
In conclusion, this systematic review showed that HPV persistence is strongly associated with CIN2-3/HSIL+. The magnitude of the effect of HPV persistence was stronger with longer duration of infection, wider HPV testing intervals, and higher-grade cervical disease. The overwhelmingly positive associations identified in this review validate the use of HPV persistence as a surrogate endpoint in clinical trials and potentially in cervical cancer screening. With standardization of definitions and testing, detection of persistent HPV DNA can become a valuable marker of CIN2-3 and cancer for clinical and research use.
Supplementary Material
Acknowledgments
Dr. Jill Koshiol's work on this project was supported by the GlaxoSmithKline (GSK)/University of North Carolina (UNC) Center of Excellence from 2004 to 2005 and was directly supported by GSK for a brief period in 2005. Dr. Koshiol completed this work as a Cancer Prevention Fellow at the National Cancer Institute.
The authors thank Danielle Backes, Elizabeth Hodgson, and Dominique Luyts for their considerable aid in collecting and cleaning the data.
All authors participated in designing the study and writing the manuscript. All analyses were conducted by Dr. Jill Koshiol.
Dr. Jill Koshiol was employed part-time by GSK from May 2001 to June 2005. Dr. Jennifer Smith has received grants or consulting fees from GSK. Dr. Charles Poole received partial salary support from the GSK/UNC Center for Excellence and an honorarium for one talk given at GSK. All other authors were full-time employees of GSK while working on this study.
Glossary
Abbreviations
- ASCUS
atypical squamous cells of undetermined significance
- CI
confidence interval
- CIN
cervical intraepithelial neoplasia
- HPV
human papillomavirus
- HSIL
high-grade squamous intraepithelial lesions
- LSIL
low-grade squamous intraepithelial lesions
- PCR
polymerase chain reaction
- SIL
squamous intraepithelial lesions
References
- 1.Bosch FX, Lorincz A, Munoz N, et al. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol. 2002;55:244–65. doi: 10.1136/jcp.55.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.International Agency for Research on Cancer. Lyon, France: International Agency for Research on Cancer; 2007. Human papillomaviruses. (IARC monographs on the evaluation of carcinogenic risks to humans, vol 90) [Google Scholar]
- 3.Muñoz N. Human papillomavirus and cancer: the epidemiological evidence. J Clin Virol. 2000;19:1–5. doi: 10.1016/s1386-6532(00)00125-6. [DOI] [PubMed] [Google Scholar]
- 4.Ferlay J, Bray F, Pisani P, et al. GLOBOCAN 2002. Lyon, France: International Agency for Research on Cancer; 2004. cancer incidence, mortality and prevalence worldwide, version 2.0. (IARC CancerBase no. 5) [Google Scholar]
- 5.Muñoz N, Bosch FX, de Sanjosé S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–27. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 6.Smith JS, Lindsay L, Hoots B, et al. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int J Cancer. 2007;121:621–32. doi: 10.1002/ijc.22527. [DOI] [PubMed] [Google Scholar]
- 7.Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 8.Bosch FX, Muñoz N. The viral etiology of cervical cancer. Virus Res. 2002;89:183–90. doi: 10.1016/s0168-1702(02)00187-9. [DOI] [PubMed] [Google Scholar]
- 9.Burd EM. Human papillomavirus and cervical cancer. Clin Microbiol Rev. 2003;16:1–17. doi: 10.1128/CMR.16.1.1-17.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ho GY, Bierman R, Beardsley L, et al. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med. 1998;338:423–8. doi: 10.1056/NEJM199802123380703. [DOI] [PubMed] [Google Scholar]
- 11.Moscicki AB, Shiboski S, Boroering J, et al. The natural history of human papillomavirus infection as measured by repeated DNA testing in adolescent and young women. J Pediatr. 1998;132:277–84. doi: 10.1016/s0022-3476(98)70445-7. [DOI] [PubMed] [Google Scholar]
- 12.Moscicki AB, Schiffman M, Kjaer S, et al. Chapter 5: updating the natural history of HPV and anogenital cancer. Vaccine. 2006;24(suppl 3):S42–51. doi: 10.1016/j.vaccine.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 13.Snijders PJ, Steenbergen RD, Heideman DA, et al. HPV-mediated cervical carcinogenesis: concepts and clinical implications. J Pathol. 2006;208:152–64. doi: 10.1002/path.1866. [DOI] [PubMed] [Google Scholar]
- 14.Schiffman M, Herrero R, DeSalle R, et al. The carcinogenicity of human papillomavirus types reflects viral evolution. Virology. 2005;337:76–84. doi: 10.1016/j.virol.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 15.Ho GY, Burk RD, Klein S, et al. Persistent genital human papillomavirus infection as a risk factor for persistent cervical dysplasia. J Natl Cancer Inst. 1995;87:1365–71. doi: 10.1093/jnci/87.18.1365. [DOI] [PubMed] [Google Scholar]
- 16.Wallin KL, Wiklund F, Angstrom T, et al. Type-specific persistence of human papillomavirus DNA before the development of invasive cervical cancer. N Engl J Med. 1999;341:1633–8. doi: 10.1056/NEJM199911253412201. [DOI] [PubMed] [Google Scholar]
- 17.Harper DM, Franco EL, Wheeler C, et al. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet. 2004;364:1757–65. doi: 10.1016/S0140-6736(04)17398-4. [DOI] [PubMed] [Google Scholar]
- 18.Mao C, Koutsky LA, Ault KA, et al. Efficacy of human papillomavirus-16 vaccine to prevent cervical intraepithelial neoplasia: a randomized controlled trial. Obstet Gynecol. 2006;107:18–27. doi: 10.1097/01.AOG.0000192397.41191.fb. [DOI] [PubMed] [Google Scholar]
- 19.Kulasingam SL, Hughes JP, Kiviat NB, et al. Evaluation of human papillomavirus testing in primary screening for cervical abnormalities: comparison of sensitivity, specificity, and frequency of referral. JAMA. 2002;288:1749–57. doi: 10.1001/jama.288.14.1749. [DOI] [PubMed] [Google Scholar]
- 20.Perrons C, Brink N, Jalal H, et al. The impact of high risk human papillomavirus testing in an inner London colposcopy clinic. J Med Virol. 2005;76:576–82. doi: 10.1002/jmv.20401. [DOI] [PubMed] [Google Scholar]
- 21.Schiffman M, Kjaer SK. Chapter 2: natural history of anogenital human papillomavirus infection and neoplasia. J Natl Cancer Inst Monogr. 2003;31:14–19. doi: 10.1093/oxfordjournals.jncimonographs.a003476. [DOI] [PubMed] [Google Scholar]
- 22.Woodman CB, Collins S. A critique of cohort studies examining the role of human papillomavirus infection in cervical neoplasia. BJOG. 2002;109:1311–18. doi: 10.1046/j.1471-0528.2002.02008.x. [DOI] [PubMed] [Google Scholar]
- 23.Giuliano AR, Harris R, Sedjo RL, et al. Incidence, prevalence, and clearance of type-specific human papillomavirus infections: The Young Women's Health Study. J Infect Dis. 2002;186:462–9. doi: 10.1086/341782. [DOI] [PubMed] [Google Scholar]
- 24.Molano M, Van den BA, Plummer M, et al. Determinants of clearance of human papillomavirus infections in Colombian women with normal cytology: a population-based, 5-year follow-up study. Am J Epidemiol. 2003;158:486–94. doi: 10.1093/aje/kwg171. [DOI] [PubMed] [Google Scholar]
- 25.Moscicki AB, Ellenberg JH, Crowley-Nowick P, et al. Risk of high-grade squamous intraepithelial lesion in HIV-infected adolescents. J Infect Dis. 2004;190:1413–21. doi: 10.1086/424466. [DOI] [PubMed] [Google Scholar]
- 26.Ahdieh L, Klein RS, Burk R, et al. Prevalence, incidence, and type-specific persistence of human papillomavirus in human immunodeficiency virus (HIV)-positive and HIV-negative women. J Infect Dis. 2001;184:682–90. doi: 10.1086/323081. [DOI] [PubMed] [Google Scholar]
- 27.Paraskevaidis E, Kaponis A, Malamou-Mitsi V, et al. The natural history of HPV infection of the uterine cervix. Long-term observational and histological data. Anticancer Res. 2002;22:1177–81. [PubMed] [Google Scholar]
- 28.Beskow AH, Gyllensten UB. Host genetic control of HPV 16 titer in carcinoma in situ of the cervix uteri. Int J Cancer. 2002;101:526–31. doi: 10.1002/ijc.90010. [DOI] [PubMed] [Google Scholar]
- 29.Beskow AH, Josefsson AM, Gyllensten UB. HLA class II alleles associated with infection by HPV16 in cervical cancer in situ. Int J Cancer. 2001;93:817–22. doi: 10.1002/ijc.1412. [DOI] [PubMed] [Google Scholar]
- 30.Moberg M, Gustavsson I, Gyllensten U. Type-specific associations of human papillomavirus load with risk of developing cervical carcinoma in situ. Int J Cancer. 2004;112:854–9. doi: 10.1002/ijc.20480. [DOI] [PubMed] [Google Scholar]
- 31.Ylitalo N, Josefsson A, Melbye M, et al. A prospective study showing long-term infection with human papillomavirus 16 before the development of cervical carcinoma in situ. Cancer Res. 2000;60:6027–32. [PubMed] [Google Scholar]
- 32.Bory JP, Cucherousset J, Lorenzato M, et al. Recurrent human papillomavirus infection detected with the hybrid capture II assay selects women with normal cervical smears at risk for developing high grade cervical lesions: a longitudinal study of 3,091 women. Int J Cancer. 2002;102:519–25. doi: 10.1002/ijc.10735. [DOI] [PubMed] [Google Scholar]
- 33.Clavel C, Masure M, Levert M, et al. Human papillomavirus detection by the hybrid capture II assay: a reliable test to select women with normal cervical smears at risk for developing cervical lesions. Diagn Mol Pathol. 2000;9:145–50. doi: 10.1097/00019606-200009000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Clavel C, Masure M, Bory JP, et al. Human papillomavirus testing in primary screening for the detection of high-grade cervical lesions: a study of 7932 women. Br J Cancer. 2001;84:1616–23. doi: 10.1054/bjoc.2001.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lorenzato M, Bory JP, Cucherousset J, et al. Usefulness of DNA ploidy measurement on liquid-based smears showing conflicting results between cytology and high-risk human papillomavirus typing. Am J Clin Pathol. 2002;118:708–13. doi: 10.1309/6NXC-V9XD-YM87-8FAE. [DOI] [PubMed] [Google Scholar]
- 36.Kjaer SK, Chackerian B, van den Brule AJ, et al. High-risk human papillomavirus is sexually transmitted: evidence from a follow-up study of virgins starting sexual activity (intercourse) Cancer Epidemiol Biomarkers Prev. 2001;10:101–6. [PubMed] [Google Scholar]
- 37.Kjaer SK, van den Brule AJ, Paull G, et al. Type specific persistence of high risk human papillomavirus (HPV) as indicator of high grade cervical squamous intraepithelial lesions in young women: population based prospective follow up study. BMJ. 2002;325:572. doi: 10.1136/bmj.325.7364.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cuschieri KS, Whitley MJ, Cubie HA. Human papillomavirus type specific DNA and RNA persistence—implications for cervical disease progression and monitoring. J Med Virol. 2004;73:65–70. doi: 10.1002/jmv.20062. [DOI] [PubMed] [Google Scholar]
- 39.Cuschieri KS, Cubie HA, Whitley MW, et al. Persistent high risk HPV infection associated with development of cervical neoplasia in a prospective population study. J Clin Pathol. 2005;58:946–50. doi: 10.1136/jcp.2004.022863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moscicki AB, Palefsky JM, Gonzales J, et al. Colposcopic and histologic findings and human papillomavirus (HPV) DNA test variability in young women positive for HPV DNA. J Infect Dis. 1992;166:951–7. doi: 10.1093/infdis/166.5.951. [DOI] [PubMed] [Google Scholar]
- 41.Moscicki AB, Hills N, Shiboski S, et al. Risks for incident human papillomavirus infection and low-grade squamous intraepithelial lesion development in young females. JAMA. 2001;285:2995–3002. doi: 10.1001/jama.285.23.2995. [DOI] [PubMed] [Google Scholar]
- 42.American College of Obstetricians and Gynecologists. Management of abnormal cervical cytology and histology. (ACOG Practice Bulletin no. 66) Obstet Gynecol. 2005;106:645–64. doi: 10.1097/00006250-200509000-00051. [DOI] [PubMed] [Google Scholar]
- 43.Wright TC, Jr, Cox JT, Massad LS, et al. 2001 consensus guidelines for the management of women with cervical cytological abnormalities. JAMA. 2002;287:2120–9. doi: 10.1001/jama.287.16.2120. [DOI] [PubMed] [Google Scholar]
- 44.Wright TC, Jr, Cox JT, Massad LS, et al. 2001 consensus guidelines for the management of women with cervical intraepithelial neoplasia. Am J Obstet Gynecol. 2003;189:295–304. doi: 10.1067/mob.2003.633. [DOI] [PubMed] [Google Scholar]
- 45.Hardy RJ, Thompson SG. Detecting and describing heterogeneity in meta-analysis. Stat Med. 1998;17:841–56. doi: 10.1002/(sici)1097-0258(19980430)17:8<841::aid-sim781>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 46.Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol. 2000;53:1119–29. doi: 10.1016/s0895-4356(00)00242-0. [DOI] [PubMed] [Google Scholar]
- 47.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. [PubMed] [Google Scholar]
- 48.Egger M, Davey SG, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–63. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 50.Thompson SG, Sharp SJ. Explaining heterogeneity in meta-analysis: a comparison of methods. Stat Med. 1999;18:2693–708. doi: 10.1002/(sici)1097-0258(19991030)18:20<2693::aid-sim235>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 51.Liaw KL, Glass AG, Manos MM, et al. Detection of human papillomavirus DNA in cytologically normal women and subsequent cervical squamous intraepithelial lesions. J Natl Cancer Inst. 1999;91:954–60. doi: 10.1093/jnci/91.11.954. [DOI] [PubMed] [Google Scholar]
- 52.Harris TG, Kulasingam SL, Kiviat NB, et al. Cigarette smoking, oncogenic human papillomavirus, Ki-67 antigen, and cervical intraepithelial neoplasia. Am J Epidemiol. 2004;159:834–42. doi: 10.1093/aje/kwh115. [DOI] [PubMed] [Google Scholar]
- 53.Bais AG, Rebolj M, Snijders PJ, et al. Triage using HPV-testing in persistent borderline and mildly dyskaryotic smears: proposal for new guidelines. Int J Cancer. 2005;116:122–9. doi: 10.1002/ijc.20958. [DOI] [PubMed] [Google Scholar]
- 54.Cuzick J, Szarewski A, Cubie H, et al. Management of women who test positive for high-risk types of human papillomavirus: The HART Study. Lancet. 2003;362:1871–6. doi: 10.1016/S0140-6736(03)14955-0. [DOI] [PubMed] [Google Scholar]
- 55.Dannecker C, Siebert U, Thaler CJ, et al. Primary cervical cancer screening by self-sampling of human papillomavirus DNA in internal medicine outpatient clinics. Ann Oncol. 2004;15:863–9. doi: 10.1093/annonc/mdh240. [DOI] [PubMed] [Google Scholar]
- 56.Elfgren K, Kalantari M, Moberger B, et al. A population-based five-year follow-up study of cervical human papillomavirus infection. Am J Obstet Gynecol. 2000;183:561–7. doi: 10.1067/mob.2000.106749. [DOI] [PubMed] [Google Scholar]
- 57.Hildesheim A, Schiffman MH, Gravitt PE, et al. Persistence of type-specific human papillomavirus infection among cytologically normal women. J Infect Dis. 1994;169:235–40. doi: 10.1093/infdis/169.2.235. [DOI] [PubMed] [Google Scholar]
- 58.Hopman EH, Rozendaal L, Voorhorst FJ, et al. High risk human papillomavirus in women with normal cervical cytology prior to the development of abnormal cytology and colposcopy. BJOG. 2000;107:600–4. doi: 10.1111/j.1471-0528.2000.tb13299.x. [DOI] [PubMed] [Google Scholar]
- 59.Hoyer H, Scheungraber C, Kuehne-Heid R, et al. Cumulative 5-year diagnoses of CIN2, CIN3 or cervical cancer after concurrent high-risk HPV and cytology testing in a primary screening setting. Int J Cancer. 2005;116:136–43. doi: 10.1002/ijc.20955. [DOI] [PubMed] [Google Scholar]
- 60.Peto J, Gilham C, Deacon J, et al. Cervical HPV infection and neoplasia in a large population-based prospective study: the Manchester cohort. Br J Cancer. 2004;91:942–53. doi: 10.1038/sj.bjc.6602049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rosenfeld WD, Rose E, Vermund SH, et al. Follow-up evaluation of cervicovaginal human papillomavirus infection in adolescents. J Pediatr. 1992;121:307–11. doi: 10.1016/s0022-3476(05)81212-0. [DOI] [PubMed] [Google Scholar]
- 62.Schlecht NF, Kulaga S, Robitaille J, et al. Persistent human papillomavirus infection as a predictor of cervical intraepithelial neoplasia. JAMA. 2001;286:3106–14. doi: 10.1001/jama.286.24.3106. [DOI] [PubMed] [Google Scholar]
- 63.Ahdieh L, Muñoz A, Vlahov D, et al. Cervical neoplasia and repeated positivity of human papillomavirus infection in human immunodeficiency virus-seropositive and -seronegative women. Am J Epidemiol. 2000;151:1148–57. doi: 10.1093/oxfordjournals.aje.a010165. [DOI] [PubMed] [Google Scholar]
- 64.Kotloff KL, Wasserman SS, Russ K, et al. Detection of genital human papillomavirus and associated cytological abnormalities among college women. Sex Transm Dis. 1998;25:243–50. doi: 10.1097/00007435-199805000-00005. [DOI] [PubMed] [Google Scholar]
- 65.Smith EM, Ritchie JM, Levy BT, et al. Prevalence and persistence of human papillomavirus in postmenopausal age women. Cancer Detect Prev. 2003;27:472–80. doi: 10.1016/s0361-090x(03)00104-1. [DOI] [PubMed] [Google Scholar]
- 66.Smith EM, Johnson SR, Ritchie JM, et al. Persistent HPV infection in postmenopausal age women. Int J Gynaecol Obstet. 2004;87:131–7. doi: 10.1016/j.ijgo.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 67.Koutsky LA, Holmes KK, Critchlow CW, et al. A cohort study of the risk of cervical intraepithelial neoplasia grade 2 or 3 in relation to papillomavirus infection. N Engl J Med. 1992;327:1272–8. doi: 10.1056/NEJM199210293271804. [DOI] [PubMed] [Google Scholar]
- 68.Aho J, Hankins C, Tremblay C, et al. Genomic polymorphism of human papillomavirus type 52 predisposes toward persistent infection in sexually active women. J Infect Dis. 2004;190:46–52. doi: 10.1086/420787. [DOI] [PubMed] [Google Scholar]
- 69.del Mistro A, Bertorelle R, Franzetti M, et al. Antiretroviral therapy and the clinical evolution of human papillomavirus-associated genital lesions in HIV-positive women. Clin Infect Dis. 2004;38:737–42. doi: 10.1086/381681. [DOI] [PubMed] [Google Scholar]
- 70.Ellerbrock TV, Chiasson MA, Bush TJ, et al. Incidence of cervical squamous intraepithelial lesions in HIV-infected women. JAMA. 2000;283:1031–7. doi: 10.1001/jama.283.8.1031. [DOI] [PubMed] [Google Scholar]
- 71.Gagnon S, Hankins C, Tremblay C, et al. Polymorphism of human papillomavirus type 31 isolates infecting the genital tract of HIV-seropositive and HIV-seronegative women at risk for HIV infection. J Med Virol. 2005;75:213–21. doi: 10.1002/jmv.20259. [DOI] [PubMed] [Google Scholar]
- 72.Massad LS, Ahdieh L, Benning L, et al. Evolution of cervical abnormalities among women with HIV-1: evidence from surveillance cytology in the Women's Interagency HIV Study. J Acquir Immune Defic Syndr. 2001;27:432–42. doi: 10.1097/00126334-200108150-00003. [DOI] [PubMed] [Google Scholar]
- 73.Schuman P, Ohmit SE, Klein RS, et al. Longitudinal study of cervical squamous intraepithelial lesions in human immunodeficiency virus (HIV)-seropositive and at-risk HIV-seronegative women. J Infect Dis. 2003;188:128–36. doi: 10.1086/375783. [DOI] [PubMed] [Google Scholar]
- 74.Strickler HD, Palefsky JM, Shah KV, et al. Human papillomavirus type 16 and immune status in human immunodeficiency virus-seropositive women. J Natl Cancer Inst. 2003;95:1062–71. doi: 10.1093/jnci/95.14.1062. [DOI] [PubMed] [Google Scholar]
- 75.ter Harmsel B, Smedts F, Kuijpers J. Relationship between human papillomavirus type 16 in the cervix and intraepithelial neoplasia. Obstet Gynecol. 1999;93:46–50. doi: 10.1016/s0029-7844(98)00306-8. [DOI] [PubMed] [Google Scholar]
- 76.Cox JT, Schiffman M, Solomon D. Prospective follow-up suggests similar risk of subsequent cervical intraepithelial neoplasia grade 2 or 3 among women with cervical intraepithelial neoplasia grade 1 or negative colposcopy and directed biopsy. Am J Obstet Gynecol. 2003;188:1406–12. doi: 10.1067/mob.2003.461. [DOI] [PubMed] [Google Scholar]
- 77.Kitchener HC, Castle PE, Cox JT. Chapter 7: achievements and limitations of cervical cytology screening. Vaccine. 2006;24(suppl 3):S63–70. doi: 10.1016/j.vaccine.2006.05.113. [DOI] [PubMed] [Google Scholar]
- 78.Melnikow J, Nuovo J, Willan AR, et al. Natural history of cervical squamous intraepithelial lesions: a meta-analysis. Obstet Gynecol. 1998;92:727–35. doi: 10.1016/s0029-7844(98)00245-2. [DOI] [PubMed] [Google Scholar]
- 79.Ostor AG. Natural history of cervical intraepithelial neoplasia: a critical review. Int J Gynecol Pathol. 1993;12:186–92. [PubMed] [Google Scholar]
- 80.Winer RL, Kiviat NB, Hughes JP, et al. Development and duration of human papillomavirus lesions, after initial infection. J Infect Dis. 2005;191:731–8. doi: 10.1086/427557. [DOI] [PubMed] [Google Scholar]
- 81.Woodman CB, Collins S, Winter H, et al. Natural history of cervical human papillomavirus infection in young women: a longitudinal cohort study. Lancet. 2001;357:1831–6. doi: 10.1016/S0140-6736(00)04956-4. [DOI] [PubMed] [Google Scholar]
- 82.Woodman CB, Collins SI, Young LS. The natural history of cervical HPV infection: unresolved issues. Nat Rev Cancer. 2007;7:11–22. doi: 10.1038/nrc2050. [DOI] [PubMed] [Google Scholar]
- 83.Castle PE, Solomon D, Schiffman M, et al. Human papillomavirus type 16 infections and 2-year absolute risk of cervical precancer in women with equivocal or mild cytologic abnormalities. J Natl Cancer Inst. 2005;97:1066–71. doi: 10.1093/jnci/dji186. [DOI] [PubMed] [Google Scholar]
- 84.Khan MJ, Castle PE, Lorincz AT, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst. 2005;97:1072–9. doi: 10.1093/jnci/dji187. [DOI] [PubMed] [Google Scholar]
- 85.Coutlee F, Gravitt P, Kornegay J, et al. Use of PGMY primers in L1 consensus PCR improves detection of human papillomavirus DNA in genital samples. J Clin Microbiol. 2002;40:902–7. doi: 10.1128/JCM.40.3.902-907.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Daniel RW, Ahdieh L, Hayden D, et al. Intra-laboratory reproducibility of human papillomavirus identification in cervical specimens by a polymerase chain reaction-based assay. J Clin Virol. 2000;19:187–93. doi: 10.1016/s1386-6532(00)00142-6. [DOI] [PubMed] [Google Scholar]
- 87.Kornegay JR, Roger M, Davies PO, et al. International proficiency study of a consensus L1 PCR assay for the detection and typing of human papillomavirus DNA: evaluation of accuracy and intralaboratory and interlaboratory agreement. J Clin Microbiol. 2003;41:1080–6. doi: 10.1128/JCM.41.3.1080-1086.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Qu W, Jiang G, Cruz Y, et al. PCR detection of human papillomavirus: comparison between MY09/MY11 and GP5+/GP6+ primer systems. J Clin Microbiol. 1997;35:1304–10. doi: 10.1128/jcm.35.6.1304-1310.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schiffman MH, Bauer HM, Lorincz AT, et al. Comparison of Southern blot hybridization and polymerase chain reaction methods for the detection of human papillomavirus DNA. J Clin Microbiol. 1991;29:573–7. doi: 10.1128/jcm.29.3.573-577.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jeronimo J, Schiffman M. Colposcopy at a crossroads. Am J Obstet Gynecol. 2006;195:349–53. doi: 10.1016/j.ajog.2006.01.091. [DOI] [PubMed] [Google Scholar]
- 91.Stoler MH, Schiffman M. Interobserver reproducibility of cervical cytologic and histologic interpretations: realistic estimates from the ASCUS-LSIL Triage Study. JAMA. 2001;285:1500–5. doi: 10.1001/jama.285.11.1500. [DOI] [PubMed] [Google Scholar]
- 92.Schwartz LM, Woloshin S, Dvorin EL, et al. Ratio measures in leading medical journals: structured review of accessibility of underlying absolute risks. BMJ. 2006;333:1248. doi: 10.1136/bmj.38985.564317.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Franco EL, Villa LL, Sobrinho JP, et al. Epidemiology of acquisition and clearance of cervical human papillomavirus infection in women from a high-risk area for cervical cancer. J Infect Dis. 1999;180:1415–23. doi: 10.1086/315086. [DOI] [PubMed] [Google Scholar]
- 94.Koshiol JE, Schroeder JC, Jamieson DJ, et al. Time to clearance of human papillomavirus infection by type and human immunodeficiency virus serostatus. Int J Cancer. 2006;119:1623–9. doi: 10.1002/ijc.22015. [DOI] [PubMed] [Google Scholar]
- 95.Richardson H, Kelsal G, Tellier P, et al. The natural history of type-specific human papillomavirus infections in female university students. Cancer Epidemiol Biomarkers Prev. 2003;12:485–90. [PubMed] [Google Scholar]
- 96.Schiffman M, Castle PE, Jeronimo J, et al. The natural history and prevention of HPV infection and cervical cancer. Lancet. 2007;370:890–907. doi: 10.1016/S0140-6736(07)61416-0. [DOI] [PubMed] [Google Scholar]
- 97.Plummer M, Schiffman M, Castle PE, et al. A 2-year prospective study of human papillomavirus persistence among women with a cytological diagnosis of atypical squamous cells of undetermined significance or low-grade squamous intraepithelial lesion. J Infect Dis. 2007;195:1582–9. doi: 10.1086/516784. [DOI] [PubMed] [Google Scholar]
- 98.Belinson J, Qiao YL, Pretorius R, et al. Shanxi Province Cervical Cancer Screening Study: a cross-sectional comparative trial of multiple techniques to detect cervical neoplasia. Gynecol Oncol. 2001;83:439–44. doi: 10.1006/gyno.2001.6370. [DOI] [PubMed] [Google Scholar]
- 99.Cuzick J, Clavel C, Petry KU, et al. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int J Cancer. 2006;119:1095–101. doi: 10.1002/ijc.21955. [DOI] [PubMed] [Google Scholar]
- 100.Gage JC, Hanson VW, Abbey K, et al. Number of cervical biopsies and sensitivity of colposcopy. Obstet Gynecol. 2006;108:264–72. doi: 10.1097/01.AOG.0000220505.18525.85. [DOI] [PubMed] [Google Scholar]
- 101.Pretorius RG, Kim RJ, Belinson JL, et al. Inflation of sensitivity of cervical cancer screening tests secondary to correlated error in colposcopy. J Low Genit Tract Dis. 2006;10:5–9. doi: 10.1097/01.lgt.0000192694.85549.3d. [DOI] [PubMed] [Google Scholar]
- 102.Dalstein W, Riethmuller D, Pretet JL, et al. Persistence and load of high-risk HPV are predictors for development of high-grade cervical lesions: a longitudinal French cohort study. Int J Cancer. 2003;106:396–403. doi: 10.1002/ijc.11222. [DOI] [PubMed] [Google Scholar]
- 103.Hawes SE, Critchlow CW, Sow PS, et al. Incident high-grade squamous intraepithelial lesions in Senegalese women with and without human immunodeficiency virus type 1 (HIV-1) and HIV-2. J Natl Cancer Inst. 2006;98:100–9. doi: 10.1093/jnci/djj010. [DOI] [PubMed] [Google Scholar]
- 104.Saito J, Sumiyoshi M, Nakatani H, et al. Dysplasia and HPV infection initially detected by DNA analysis in cytomorphologically normal cervical smears. Int J Gynaecol Obstet. 1995;51:43–8. doi: 10.1016/0020-7292(95)80007-y. [DOI] [PubMed] [Google Scholar]
- 105.Koutsky LA, Ault KA, Wheeler CM, et al. A controlled trial of a human papillomavirus type 16 vaccine. N Engl J Med. 2002;347:1645–51. doi: 10.1056/NEJMoa020586. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.