Summary
Epithelial cells provide the first line of defense against mucosal pathogens, however, their coordination with innate and adaptive immune cells is not well understood. Using mice with conditional gene deficiencies, we found that lymphotoxin (LT) from intestinal innate cells positively for transcription factor RORγt, but not from adaptive T and B cells, was essential for the control of mucosal C. rodentium infection. We demonstrate that the LTβR signaling was required for the regulation of the early innate response against infection. Furthermore, we have revealed that LTβR signals in gut epithelial cells and hematopoietic-derived cells coordinate to protect the host from infection. We further determined that LTβR signaling in intestinal epithelial cells was required for recruitment of neutrophils to the infection site early during infection via production of CXCL1, and CXCL2 chemokines. These results support a model wherein LT from RORγt+ cells signals orchestrate the innate immune response against mucosal microbial infection.
Introduction
The epithelial layer serves not only as a natural barrier against microbial invaders, but is also involved in host defense through its ability to sense mucosal pathogens and mobilize immune cells. However, the pathways that mediate the crosstalk between immune cells and intestinal epithelial cells during mucosal bacterial infection are poorly understood. Citrobacter rodentium (C. rodentium) is a natural mouse extracellular enteric pathogen that mimics human enteropathogenic Escherichia coli (EPEC) and enterohaemorrhagic Escherichia coli (EHEC), all of which use attaching and effacing lesion formation, initially on gut epithelial cells, as a major mechanism of tissue targeting and infection (Mundy et al., 2005). Therefore, this is an ideal model to dissect how immune cells interact with gut epithelial pathogens. Both the innate and adaptive immune systems are involved in control of C. rodentium infection. The adaptive immune components, including CD4+ T cells, B cells and C. rodentium–specific antibodies, have been shown to play an essential role in containing and eradicating the infection (Bry and Brenner, 2004; Maaser et al., 2004; MacDonald et al., 2003; Uren et al., 2005; Vallance et al., 2003). Accordingly, recombination activating gene 1 deficient (Rag1−/−) mice lacking both T and B cells fail to clear C. rodentium infection and eventually die by 3 weeks after infection (Bry and Brenner, 2004; Vallance et al., 2003). However, there are also several innate immune mechanisms in the gut that help to control the infection, such as signals originating from toll-like receptors (TLRs), that bridge innate and adaptive immunity (Gibson et al., 2008; Lebeis et al., 2007).
Membrane-bound lymphotoxin (LT) (LTα1LTβ2), and LIGHT (TNF superfamily member 14, TNFSF14), are members of the TNF family of cytokines. Both LT and LIGHT are primarily expressed on lymphocytes and each can deliver signals through LTβ receptor (LTβR) (Browning, 2008; Ware, 2005). In contrast, LTβR is primary expressed on epithelial, stromal and myeloid cells, but not lymphocytes (Browning, 2008; Ware, 2005), suggesting that it may participate in the communication between lymphocytes and surrounding epithelial and stromal cells. Indeed, LTβR signaling has been shown to be critical for protection against the mucosal pathogen C. rodentium (Spahn et al., 2004), however, the mechanisms underlying the protective role of LTβR remain largely unknown. Most studies have focused on the critical role of LT in the development and maintenance of secondary lymphoid organs and in immune homeostasis (Browning, 2008; Fu and Chaplin, 1999; Ware, 2005). In particular, it has been shown that LT, primarily from B cells, controls the development and maintenance of the lymphoid microstructure of the spleen to support antibody responses (Fu et al., 1998; Gonzalez et al., 1998; Tumanov et al., 2002).
A recent study identified interleukin-22 (IL-22) as an important cytokine for mediating innate protection against C. rodentium infection (Zheng et al., 2008). Both lymphoid tissue inducer-like (LTi-like) cells and a mucosal subset of NK cells that express the NKp46 surface marker (NK-like cells) are able to secrete IL-22 and thus are candidates for mucosal innate defense (Cella et al., 2009; Satoh-Takayama et al., 2008; Takatori et al., 2009; Vivier et al., 2009). These two cell types express the nuclear hormone receptor retinoic acid receptor-related orphan receptor gamma t (RORγt) which is required for their development. Intriguingly, these cell types can also express membrane LT (Cupedo et al., 2009; Luci et al., 2009; Tsuji et al., 2008), however, whether LT on RORγt+ cells is required for host defense against mucosal infection remains unknown.
Both LT and LIGHT are upregulated on T cells after antigen stimulation and involved in Th1- and Th17-mediated immunity (Chiang et al., 2009; Summers-DeLuca et al., 2007; Wang et al., 2009). However, we found that LT but not LIGHT is required for protection against intestinal bacterial infection. Unexpectedly, we reveal that LT from adaptive T and B cells was not essential for protection of the host from mucosal bacterial infection. Instead, LT from RORγt+ innate cells was essential in this early protection. Our data suggest a model according to which LT from innate RORγt+ cells orchestrates intestinal epithelial cells and immune cells via LTβR signaling to trigger innate immune protection during mucosal microbial infection.
Results
LTβR on both radio-resistant and bone marrow-derived cells controls C. rodentium infection
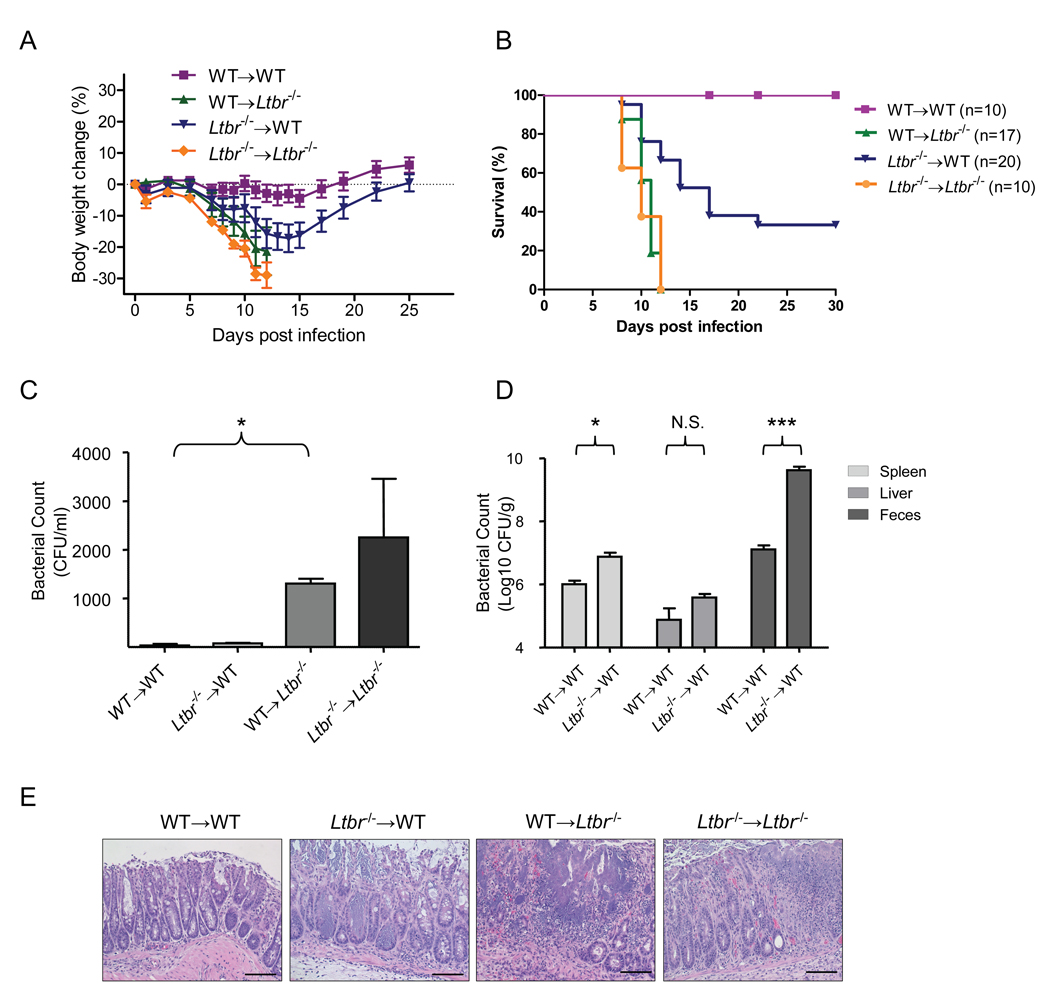
LTβR signaling plays a protective role in host defense against the mucosal pathogen C. rodentium, as all LTβR-deficient mice succumb to infection while all wild type mice survive ((Spahn et al., 2004) and Figure S1). The severity of gut inflammation and tissue injury correlated well with the degree of bacterial load in the host tissues and feces (Figure S1). Due to multiple defects, especially the lack of gut-associated lymphoid tissues in Ltbr−/− mice (Browning, 2008; Fu and Chaplin, 1999; Ware, 2005), it was necessary to dissect the cellular components or signaling pathways that are essential for protection. To define which LTβR-expressing cells are critical for the control of C. rodentium infection, we performed reciprocal bone marrow transfer experiments between WT and Ltbr−/− mice. Mice were orally-infected with C. rodentium five weeks after bone marrow transfer. Ltbr−/− recipients that received bone marrow from either WT or Ltbr−/− mice lost weight substantially during the second week after infection, and died within two weeks post infection (Figures 1A and 1B). WT > Ltbr−/− chimeras showed increased bacterial titers in blood (Figure 1C), suggesting systemic dissemination of C. rodentium. The integrity of the colonic epithelial layer was severely affected in Ltbr−/− recipients compared with WT recipient mice (Figures 1E and S1E). These results suggest a critical role for LTβR signaling on radio-resistant cells for protection. In contrast, Ltbr−/−> WT chimeras showed a less severe phenotype: mice lost a substantial amount of weight 11 to 15 days post infection, displayed increased bacterial titers in feces, and spleen, and exhibited a disorganized colonic epithelial layer (Figure 1). However, 40% of these mice were able to recover and survive the infection (Figures 1A and 1B). Thus, LTβR signaling on bone marrow-derived cells also participates in the control of C. rodentium infection.
Figure 1. LTβR signaling on both bone marrow-derived and radio-resistant stromal cells controls C. rodentium infection.
A–B. Bone marrow cells from WT or Ltbr−/− mice were transferred into lethally-irradiated WT or Ltbr−/− mice respectively (n=5–7/group/experiment). 5 weeks later mice were orally inoculated with C. rodentium. Average body weight change (A) (represent one of three independent experiments with similar results) and survival rates (B) (analyzed from 3 experiments, n=total number of mice analyzed) at the indicated time points are shown. Body weight change in WT > Ltbr−/− and Ltbr−/− > Ltbr−/− chimera mice was significantly different from those of WT > WT chimera mice (**P<0.01) 8 days post infection. Body weight change in Ltbr−/− > WT chimera mice was significantly different from those of the WT > WT chimera mice (*P<0.05) at day 11–15 post infection. C. Bacterial titers in blood at day 6 post infection (n=5). D. Bacterial titers from spleen, liver and feces homogenates cultures at day 11 post infection (n=5). E. WT > Ltbr−/− and Ltbr−/− > Ltbr−/− chimera mice show a severe colon pathology 8 days after infection. H&E staining of representative colons from indicated mice. Original magnification, ×20. Bars=100 µm. C–E Data represent one of three independent experiments. *P<0.05, **P<0.01, ***P<0.001, N.S. – not significant. See also Figure S1.
LTβR on gut epithelial cells and hematopoietic-derived cells coordinate to protect the host
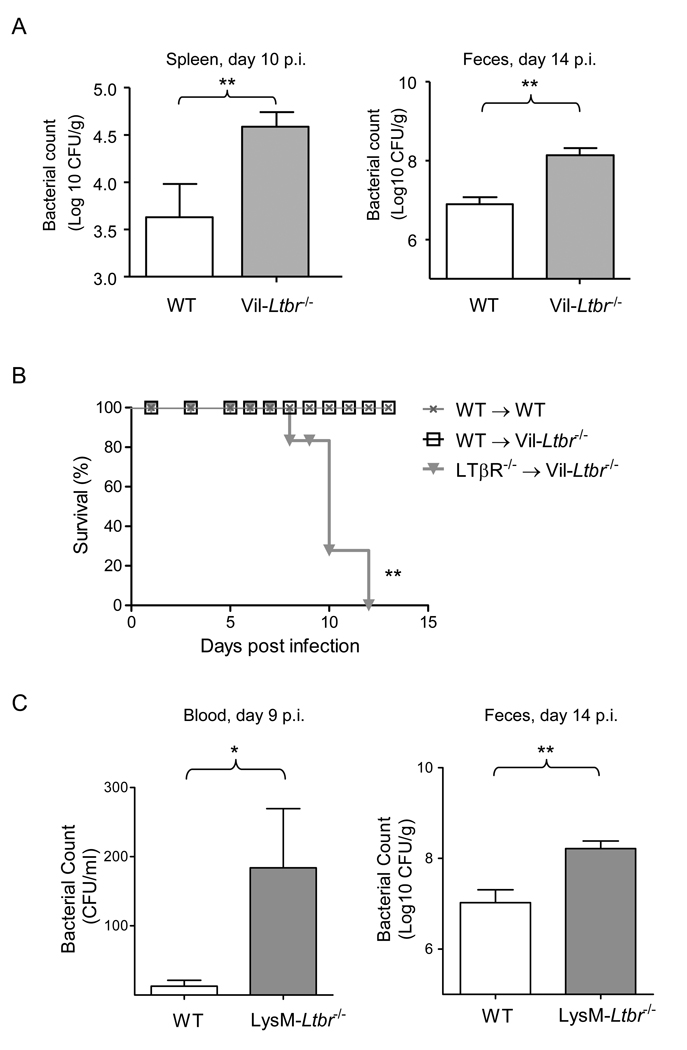
Ltbr−/− mice display multiple defects in the development and maintenance of secondary lymphoid organs that can account for the reduced clearance of bacteria. As LTβR is highly expressed on intestinal epithelium (Browning and French, 2002), we next sought to determine whether the absence of LTβR signaling in gut epithelial cells alone, rather than defective secondary lymphoid organs and tissues, was responsible for the observed phenotype of Ltbr−/− mice. Therefore, we generated mice deficient in LTβR only in intestinal epithelial cells (Figure S2). LTβR-floxed mice were crossed with Villin-Cre transgenic mice (Madison et al., 2002) to generate intestinal epithelial cell-specific, LTβR-deficient (Vil-Ltbr−/−) mice. Efficient deletion of the Ltbr gene was found in epithelial cells from both the small intestine and colon (Figure S2D., and data not shown). These mice were then used to study the role of LTβR on epithelial cells and the interplay between epithelial cells and LT+ immune cells. Vil-Ltbr−/− mice showed a deficiency in clearing C. rodentium infection, and displayed 15–20 times higher bacterial titers in the spleen and feces compared to WT mice at days 10 and 14 post infection (Figure 2A). Thus, LTβR signaling in gut epithelial cells contributes to host defense against a mucosal bacterial pathogen.
Figure 2. LTβR signaling on gut epithelial cells and hematopoietic-derived cells coordinate to protect the host from C. rodentium infection.
A. Bacterial titers in spleen and fecal homogenate cultures from WT and Vil-Ltbr−/− mice at indicated time post infection (n=5). B. Bone marrow cells from Ltbr−/− or WT mice were transferred into lethally-irradiated Vil-Ltbr−/− mice respectively (n=5/group/experiment). 5 weeks after bone marrow re-constitution mice were orally inoculated with C. rodentium. Survival rates at the indicated time points are shown. C. Bacterial titers in blood and fecal homogenate cultures from WT and LysM-Ltbr−/− mice at indicated time points post infection (n=4). *P<0.05, **P<0.001. Data represent one of two independent experiments with similar results. See also Figure S2 for mice generation details.
Intriguingly, although Vil-Ltbr−/− mice displayed an increased pathology in the colon, most of the mice survived the infection raising the possibility that LTβR signaling in other cell types may also contribute to the severity of disease. To define whether LTβR signaling in bone marrow-derived cells cooperates with LTβR signals in gut epithelial cells we transferred bone marrow cells from Ltbr−/− mice to Vil-Ltbr−/− mice. Impressively, Ltbr−/− > Vil-Ltbr−/− bone marrow chimera mice showed severe colon pathology, weight loss, and all died by day 12 after infection (Figure 2B, and data not shown). Thus, LTβR signaling in both gut epithelial cells and hematopoietic-derived cells coordinates for protecting the host against mucosal bacterial infection.
To further define the types of bone marrow-derived cells that contribute to protection against C. rodentium infection, we generated macrophage and neutrophil- specific LTβR deficient mice (LysM-Ltbr−/−) by crossing Ltbr floxed mice with LysM-Cre mice (Clausen et al., 1999) (Figures 2C, S2E, and S2F). Although LysM-Ltbr−/− mice displayed increased bacterial titers in blood, and feces, they were able to survive infection (Figure 2C, and data not shown). This data suggest that LTβR signaling on macrophages and/or neutrophils contributes to bacterial clearance, however it is not essential for the survival of mice after infection. Because the phenotypes of both Vil-LTβR- and LysM-LTβR- deficient mice were less severe than that of complete LTβR deficient mice, it is possible that cooperation of LTβR signaling in several types of bone marrow-derived and radioresistant cells is required for complete protection against mucosal bacterial infection.
Membrane LT, but not LIGHT, is essential for the control of C. rodentium infection
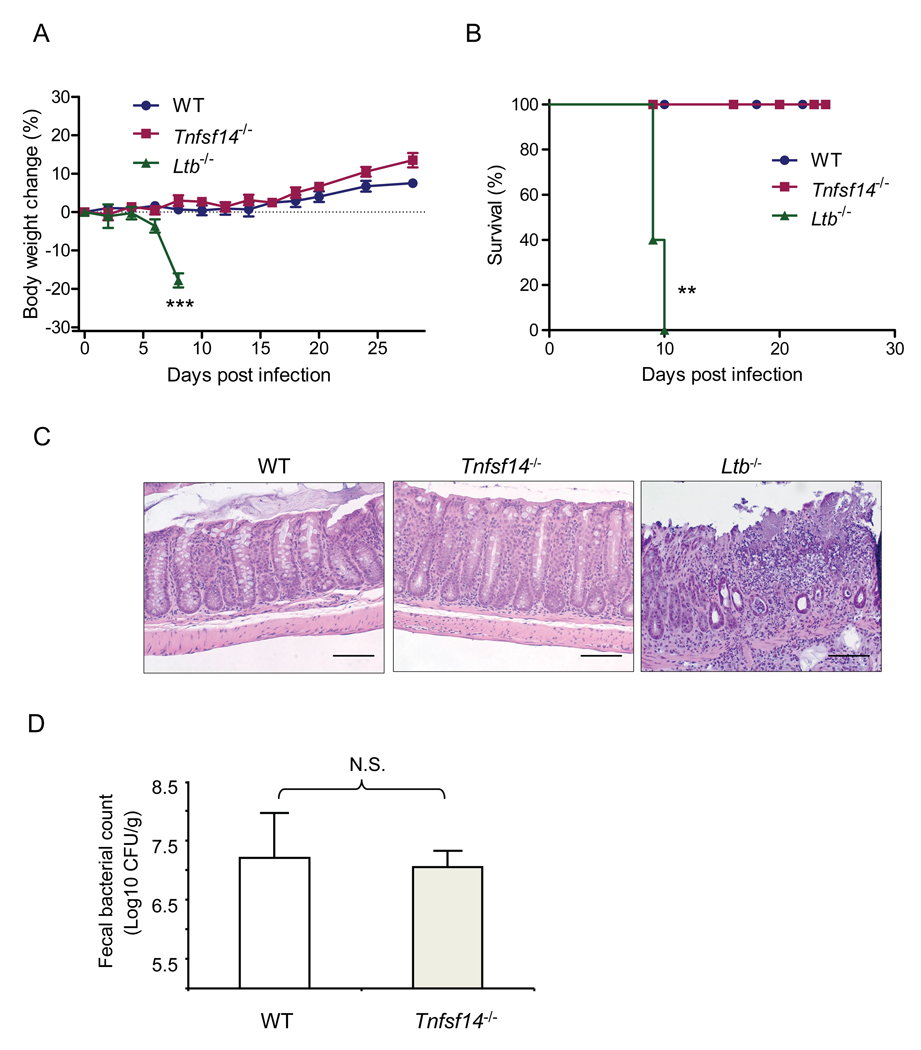
LTβR binds two known ligands, LIGHT (TNFSF14) and membrane LT (LTα1β2), and overexpression of LIGHT on T cells is known to cause gut inflammation (Wang et al., 2004; Ware, 2005). To assess which ligand is essential for the control of C. rodentium infection, we monitored the disease development side by side in Tnfsf14−/−, Ltb−/− and WT mice. WT and Tnfsf14−/− mice showed similar responses, did not lose body weight and all survived the infection. In contrast, Ltb−/− mice lost weight and all died by 10 days after infection (Figures 3A and 3B). The epithelial cell barrier remained intact WT and Tnfsf14−/− mice, while there was severe epithelial cell damage with edema, ulceration, and bacterial abscesses in the colon of Ltb−/− mice (Figure 3C). C. rodentium titers in the feces were similarly low in WT and Tnfsf14−/− mice at 2 weeks after infection (Figure 3D), whereas all Ltb−/− mice already died of overwhelming infection by this time. These results indicate that membrane LT, but not LIGHT, is the major ligand for the LTβR-dependent control of C. rodentium infection.
Figure 3. Membrane LT, but not LIGHT, is essential for the control of C. rodentium infection.
Ltb−/−, Tnfsf14−/− and WT mice (n=5/group/experiment) were orally inoculated with C. rodentium. Survival rates (A) and body weight change (B) are shown at the indicated time points (n=5). **P< 0.01, ***P< 0.001. C. Histological analysis of representative colons of WT, Ltb−/− and Tnfsf14−/− mice at day 8 after inoculation. H&E staining illustrates transmural inflammation, bacterial abscesses, submucosal leukocyte infiltration and edema in Ltb−/− mice, but not in Tnfsf14−/− mice. Original magnification, ×20. Bars=100 µm. D. Normal bacterial titers in feces of Tnfsf14−/− mice at day 14 after C. rodentium infection. All data are representative of two independent experiments. N.S.- not significant.
Lymphotoxin from adaptive T and B cells is not essential for the control of infection
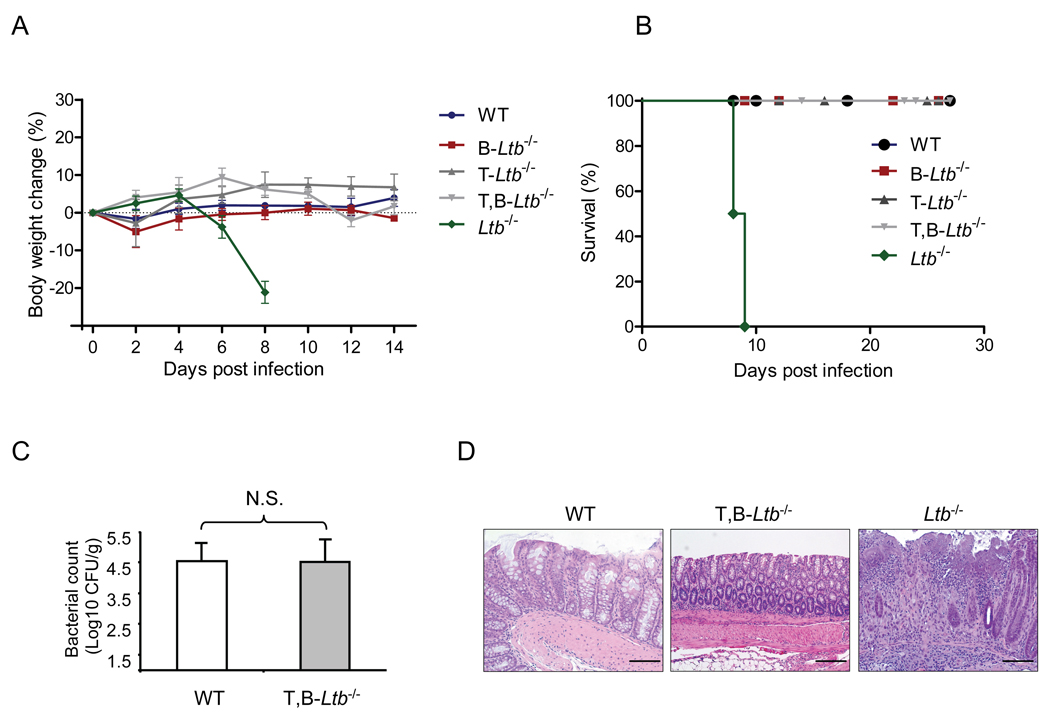
Because T and B cells are the major LT-expressing cells within secondary lymphoid organs, and surface LT is rapidly upregulated on T and B cells after stimulation (Junt et al., 2006; Tumanov et al., 2002), we first tested whether LT-expressing T and/or B cells are required for the control of C. rodentium infection by utilizing mice with conditional inactivation of membrane LT on T cells (T-Ltb−/−), B cells (B-Ltb−/−), or simultaneously on both T and B cells (T,B-Ltb−/−) (Junt et al., 2006; Tumanov et al., 2002). Surprisingly, T-Ltb−/−, B-Ltb−/−− and even T,B-Ltb−/− mice did not lose body weight or display morbidity, and all survived C. rodentium infection (Figures 4A and 4B). Furthermore, fecal titers of C. rodentium in all three types of conditionally deficient mice were similar to that of WT mice 2 weeks after infection (Figure 4C, and data not shown). The colonic epithelial cell layer was intact and showed only minimal pathology in all three conditionally-deficient mice, similar to WT mice, while much more severe colitis was found in Ltb−/− mice (Figure 4D and data not shown). These data collectively demonstrate that membrane LT expressed on adaptive T and/or B cells does not play an important role in the control of C. rodentium infection.
Figure 4. T or B cell- derived lymphotoxin is not essential for bacterial clearance.
A–D. WT, Ltb−/−, and mice with conditional inactivation of LTβ on T, B, or T and B cells were orally infected with C. rodentium. Body weight kinetics (A), survival rates (B), bacterial titers in fecal homogenate cultures at day 14 (C), and histological analysis of representative colons (D) are shown (n=5). All Ltb−/− mice died at day 8–10 post infection, whereas all other mice survived. H&E staining illustrates intact colon epithelial layer in T,B-Ltb−/− mice, compared to severe colon epithelial cell damage, bacterial abscesses and inflammatory cell infiltration in Ltb−/− mice. Original magnification, ×20. Bars=100 µm. Data are representative of two independent experiments.
Lymphotoxin from RORγt+ cells is essential for the control of infection
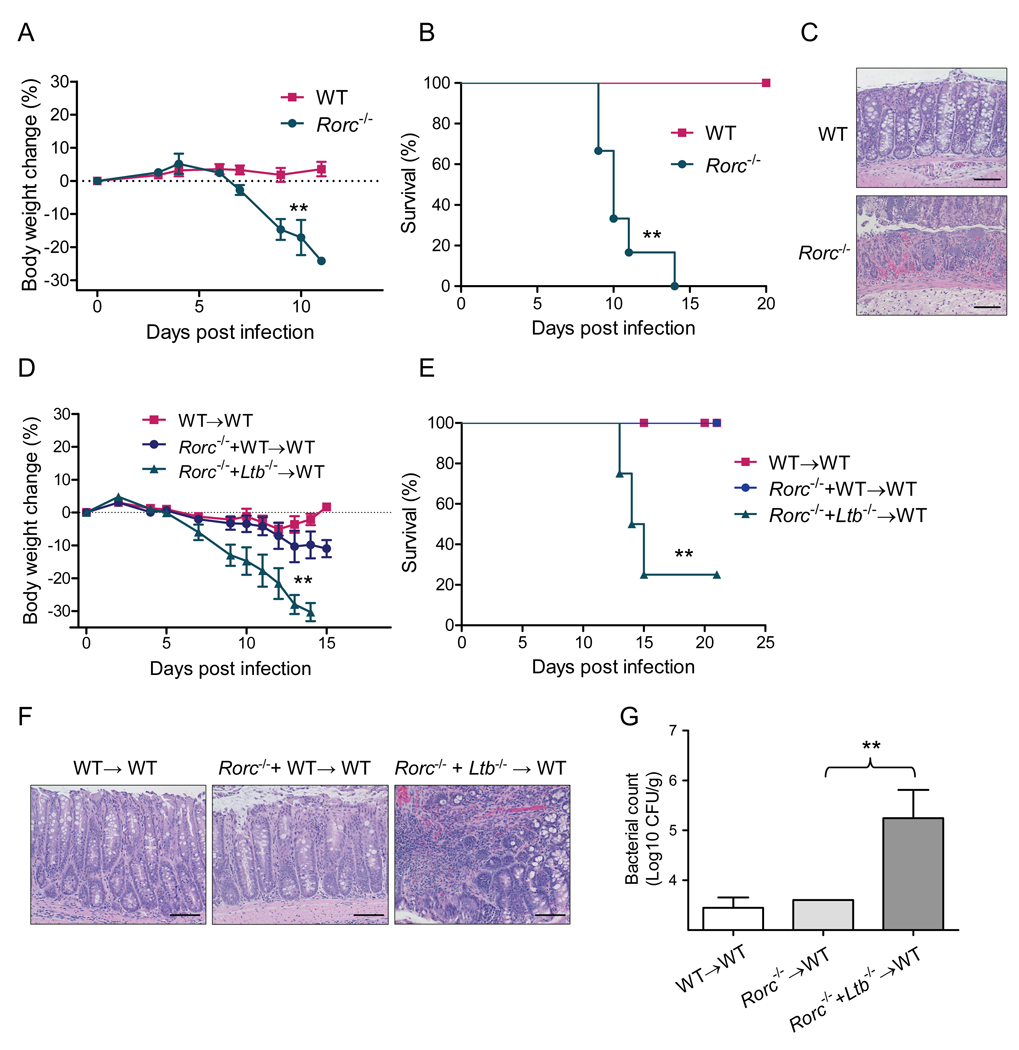
Aside from T and B cells, membrane LT can be expressed on innate RORγt+ cells which include LTi-like cells and NKp46+, NK-like cells (Vivier et al., 2009). Both LTi-like cells and RORγt+ NKp46+ cells produced LTα and LTβ in the gut lamina propria at day 5 after C. rodentium infection (Figure S3A). LT-expressing RORγt+ cells are critical for development of secondary lymphoid organs. Similar to the LT-deficient mice, Rorc−/− mice also lack lymph nodes, Peyer’s patches, and organized secondary lymphoid organs in the gut (Eberl et al., 2004; Sun et al., 2000). To define whether RORγt+ cells are essential for control of mucosal bacterial infection, we orally inoculated Rorc−/− mice with C. rodentium. Impressively, Rorc−/− mice were highly susceptible, lost weight and all died at day 10–12 post infection (Figures 5A and 5B). Histological evaluation of colons revealed severe disruption of the epithelial layer, multifocal necrosis, inflammation and edema (Figure 5C). These data demonstrate the critical role of RORγt+ cells in control of early C. rodentium infection.
Figure 5. Lymphotoxin produced by RORγt+ cells is essential for control of C. rodentium infection.
A–C. RORγt+ cells are essential to control C. rodentium infection. Average body weight change (A), survival rates (B), and histological analysis of representative colons at day 8 post infection (C) are shown (n=5). Bars=50 µm. **P<0.01. D–G. Lymphotoxin provided by RORγt+ cells is essential for control of C. rodentium infection. Lethally irradiated WT mice were reconstituted with 1:1 mixture of bone marrow cells from indicated mice (n=5 mice/group). 5 weeks later mice were orally inoculated with C. rodentium. Average body weight change (D) and survival rates (E) at the indicated time points are shown. F. H&E staining of representative colons from indicated mice. Original magnification, ×20. Bars=100 µm. G. Bacterial titers in spleen at day 13 post infection. **P< 0.01. Data are representative of two independent experiments. See also Figure S5.
To define whether LT from RORγt+ cells is essential for the protection of mice against C. rodentium infection, we transferred a 1:1 mixture of bone marrow cells from Ltb−/− mice and Rorc−/− mice to lethally irradiated WT mice. Bone marrow cells from Rorc−/− mice lack RORγt+ cells, but provide LT on other cell types, whereas bone marrow cells from Ltb−/− mice lack surface LT, but provide RORγt+ cells. Therefore, recipient mice are reconstituted with all LT+ cell populations except those that lack LT on RORγt+ cells. WT mice that received a mixture of bone marrow cells from Rorc−/− + Ltb−/− mice were highly susceptible to infection, lost weight, and 75% of the mice died by day 15 post infection (Figures 5D and 5E). These mice exhibited colon shortening, increased bacterial titers in the spleen, disruption of the epithelial layer, and severe inflammation in the colon compared to control mice (Figures 5F and 5G).
To further prove the role of LT on RORγt+ cells in C. rodentium infection, we analyzed mice with specific inactivation of surface LT on RORγt+ cells (RORγt-Ltb−/− mice). All RORγt-Ltb−/− mice exhibited weight loss, displayed severe colon pathology, had increased bacterial titers in the feces and blood, and died at day 8–12 post infection (Figures S3B–S3F). Overall, these data suggest that LT production by RORγt+ cells, but not by adaptive T and B cells, is essential for the protection of mice against C. rodentium infection.
The LTβR pathway controls early innate immunity against C. rodentium infection
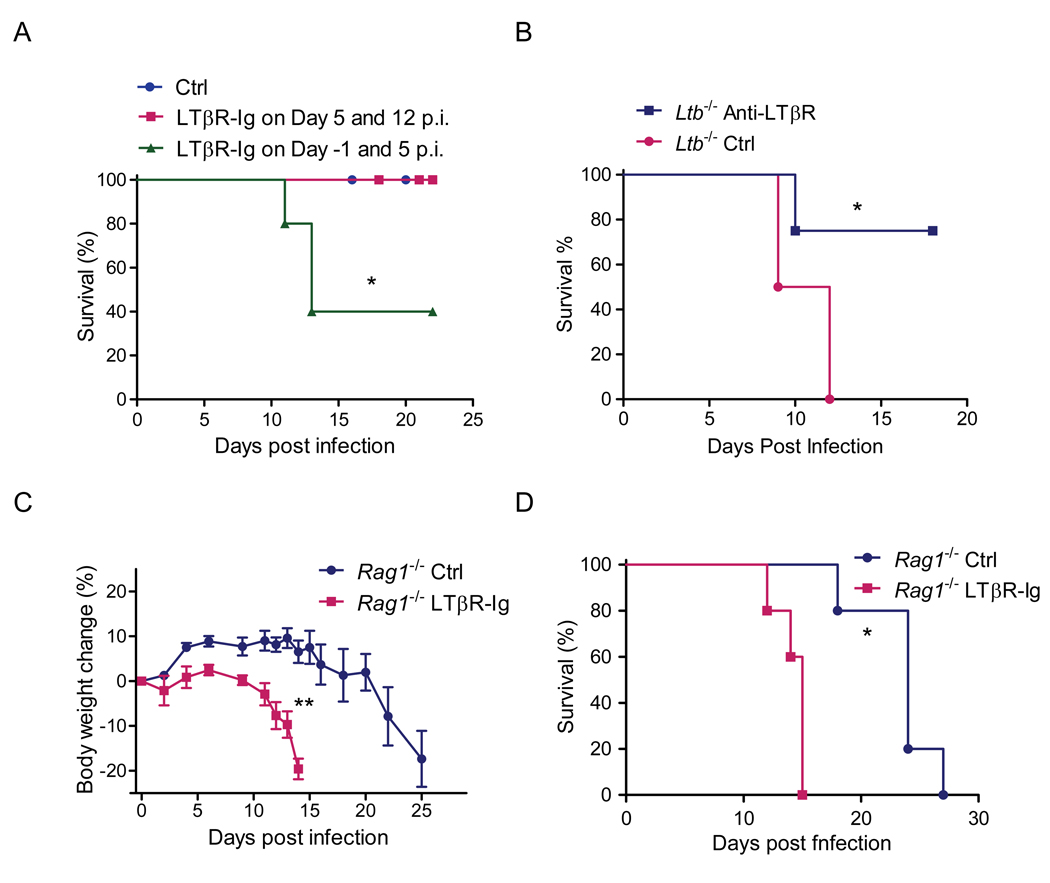
Since LT expressing RORγt+ cells but not LT on adaptive T and B cells was required for protection, we hypothesized that LTβR signaling by innate RORγt+ cells is essential for the early innate phase of the mucosal immune response. Therefore, to define the role of LTβR signaling in the control of early C. rodentium infection in the presence of normal gut-associated lymphoid tissues, we blocked LTβR signaling in WT mice with soluble LTβR-Ig fusion protein. Such blockade by administration of LTβR-Ig fusion protein at days −1 and 5 post infection resulted in 60% mortality Figure 6A). In contrast, mice injected with LTβR-Ig at a later time (days 5 and 12 post infection) all survived infection (Figure 6A). These results suggest that LTβR signaling is crucial in the early stage of C. rodentium infection in the presence of normal lymphoid tissues, likely acting before the generation of adaptive immune responses in the gut.
Figure 6. LTβR pathway controls early innate immunity against C. rodentium infection.
A. WT mice were treated with LTβR-Ig (100 µg per mouse per time, i.p.) or control saline (Ctrl) at indicated time points (n=4). Survival rates are shown. B. Early stimulation of LTβR signaling rescues Ltb−/− mice. Ltb−/− mice were treated with saline (Ctrl) or agonistic LTβR antibody (3C8, 100 µg per mouse per time, i.p.) at the indicated time points. Survival rates are shown. C–D. Inhibition of LTβR signaling during early phases of C. rodentium infection accelerates death of lymphocyte deficient Rag1−/− mice. Rag1−/− mice were treated with saline or LTβR-Ig (100 µg per mouse per time, i.p.) weekly (n=5). Body weight changes (C) and survival rates (D) at indicated time points are shown. N=5, *P<0.05, **P<0.01. All data are representative of two independent experiments.
We next tested whether stimulation of LTβR signaling early in the infection is sufficient to protect mice against lethal C. rodentium challenge by injecting Ltb−/− mice with agonistic LTβR antibody early at day −1, 0, 2, and 4 after infection. Impressively, while all untreated Ltb−/− mice died by day 12 after infection, 75% of anti-LTβR-treated mice survived (Figure 6B and data not shown). Thus, early engagement of LTβR signals is sufficient to induce protection against otherwise lethal infection in LT-deficient mice.
Most previous studies focused on the role of LTβR signaling in the maintenance of organized lymphoid tissues and in the development of adaptive immune responses. However, our data raise the possibility that LTβR signaling might be important for innate responses. To further define whether LTβR signaling by innate RORγt+ cells is critical for the innate immune response during C. rodentium infection, we infected Rag1−/− mice, which lack T and B cells. Rag1−/− mice gradually lost weight and eventually died around 3–4 weeks post infection (Figures 6C and 6D). In contrast, Rag1−/− mice treated early with LTβR-Ig fusion protein lost weight very rapidly, and died within 2 weeks post infection (Figures 6C and 6D). Together, these data suggest that the LTβR signaling pathway by innate LT expressing RORγt+ cells is essential for protecting mice from death during the early phase of C. rodentium infection in the absence of adaptive immunity.
The LTβR pathway controls neutrophil recruitment to protect against bacterial infection
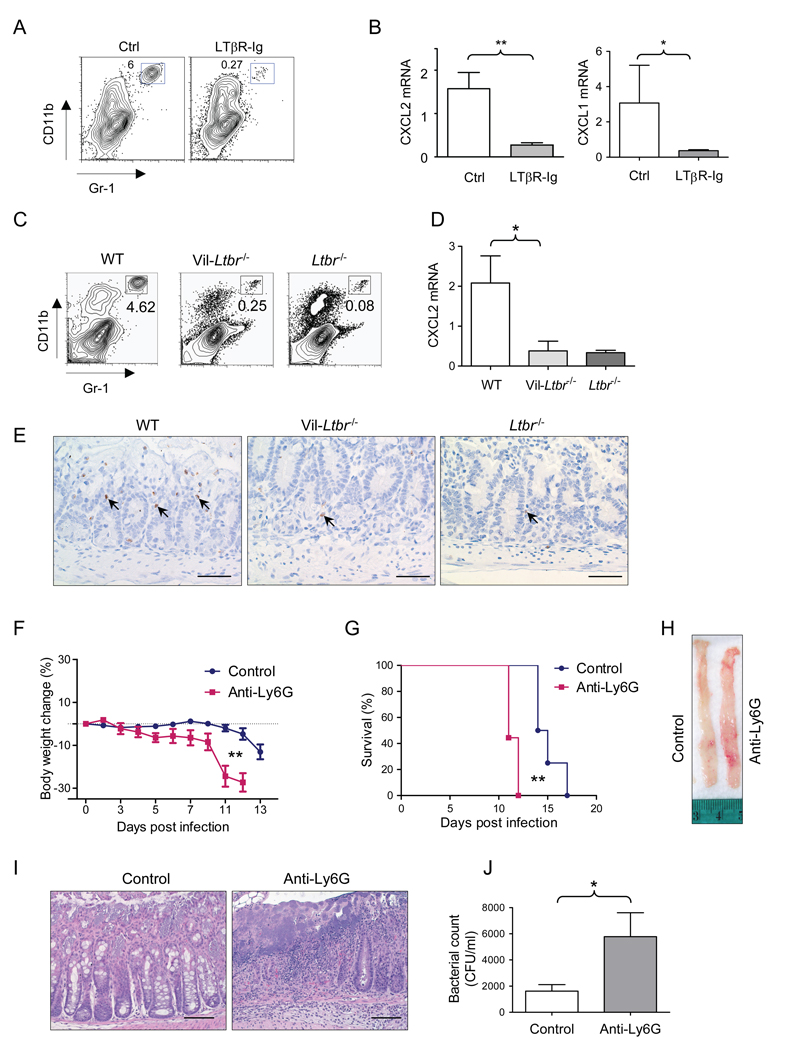
To define the mechanism of LTβR signaling during the innate immune response, we first analyzed the cellular composition of lymphoid cells in the lamina propria of Rag1−/− mice treated with LTβR-Ig protein. Although the total cell number of innate RORγt+ and NKp46+ cell populations were not different between LTβR-Ig treated and control mice (Figure S4A), the number of Gr1+ CD11b+ cells was dramatically reduced in the lamina propria at day 4 after infection (Figure 7A). Gr1+ CD11b+ population represented primarily neutrophils as defined by flow cytometry (CD11b+ Ly6CintLy6Ghi cells) and by anti-myeloperoxidase immunostaining (Figures 7E and S4B).
Figure 7. LTβR pathway controls neutrophil accumulation in the infection site early after infection.
A–B Rag1−/− mice were treated with saline or LTβR-Ig (100 µg i.p..) on day −1, and then orally infected with C. rodentium. 3 days later cecum lamina propria lymphoid cells were collected and stained with CD11b and Gr-1 antibodies (A). The percentages of CD11bhi Gr-1hi cells in the lamina propria of indicated mice are shown. B. CXCL2, and CXCL1 mRNA levels in cecum at day 3 post infection (n=5). C-E. WT, Vil-Ltbr−/− and Ltbr−/− mice were infected orally with C. rodentium. C. The percentages of CD11bhi Gr-1hi neutrophils in the lamina propria at day 4 after C. rodentium infection are shown. D. CXCL2 mRNA expression in colon from WT, Vil-Ltbr−/− and Ltbr−/− at day 4 post infection. *P<0.05, n=5. E. Anti-myeloperoxidase staining of neutrophils in colons of WT, Vil-Ltbr−/− and Ltbr−/− mice at day 4 after infection. Original magnification, ×40. Bars=50 µm. F–J. Neutrophils are essential for innate immune defense against mucosal pathogen. Rag1−/− mice were treated with saline (n=8) or Ly6G antibody (200 µg per mouse per time, i.p., n=9) every 3 days after C. rodentium infection. Body weight change (F) and survival rates (G) at indicated time points after C. rodentium infection are shown (n=8–9). H–I. Colon luminal images (H) and H&E staining (I) of representative colons from indicated mice. Original magnification, ×20. Bars=100 µm. J. Bacterial titers in blood at day 11 post infection (n=4). *P<0.05, **P< 0.01. All data are representative of two independent experiments. See also Figure S4.
To define how LTβR may control neutrophil recruitment to the gut, we analyzed expression of neutrophil recruiting chemokines in Rag1−/− mice treated with LTβR-Ig protein. CXCL1 (KC), and CXCL2 (MIP-2) are two of major principal chemokines that recruit neutrophils following bacterial infection or injury (Lebeis et al., 2007; Ohtsuka et al., 2001; Rakoff-Nahoum et al., 2004). Expression of CXCL1 and CXCL2 was substantially reduced in the ceca of Rag1−/− mice treated with LTβR-Ig, compared to untreated control mice (Figure 7B), and correlated with reduced numbers of neutrophils in the lamina propria at day 4 after infection (Figure 7A).
To further define whether LTβR signaling in intestinal epithelial cells controls early neutrophil recruitment to the colon lamina propria, we analyzed neutrophil numbers in Vil-Ltbr−/− and Ltbr−/− mice following C. rodentium infection. Neutrophil numbers were greatly reduced in the lamina propria of both Vil-Ltbr−/− and Ltbr−/− mice compared to WT mice (Figures 7C, 7E and S4B). The reduced number of neutrophils and lower expression of CXCL1, and CXCL2 chemokines was also found in the colon lamina propria of RORγt-Ltb−/− mice early after infection, as compared to control mice (Figures S3G–S5I). Together these results strongly suggest that LT expression on RORγt+ cells activates LTβR signaling on intestinal epithelial cells to control neutrophil recruitment to the infection site early after mucosal infection.
Finally, to define whether neutrophils are essential for early, innate protection against C. rodentum infection, we depleted neutrophils in Rag1−/− mice. Rag1−/− mice depleted of neutrophils using specific Ly6G antibody, showed accelerated weight loss, increased colon pathology and accelerated mortality after infection, similar to LTβR-Ig treated mice (Figures 7F–7J). Thus, these data indicate that the LTβR pathway controls neutrophil accumulation at the infection site to protect against mucosal bacterial infection.
Discussion
Most studies of LTβR signaling focus on its role in the organization of lymphoid tissues and in the development of adaptive immune responses as lymphoid tissues and adaptive immunity coevolved. Instead, our data suggest that LTβR signaling is important for innate responses. The impaired Th1 cytokine production and DC function in LTβR deficient mice were previously thought to be responsible for the high susceptibility of Ltbr−/− mice to oral C. rodentium infection (Spahn et al., 2004). Unexpectedly, we found that LT from innate RORγt+ cells but not from adaptive T and B cells was essential for protection. Consistently, lymphocyte deficient Rag1−/− mice become more susceptible after LTβR blockade. Furthermore, LTβR signaling in gut epithelial cells and innate cells is required for the early defense against C. rodentium infection, independently of the adaptive immune responses, but dependent upon neutrophils and innate RORγt+ cells. These results support a model wherein LT-expressing RORγt+ cells instruct intestinal epithelial cells, via LTβR signals, to mobilize the innate immune response against microbial infection.
How epithelial cells may coordinate with innate and adaptive immune cells during mucosal infection is poorly understood. The LT-LTβR pathway in the gut provides an interesting model to dissect such interactions. LTβR is expressed, or can be induced, on both bone marrow-derived cells, such as neutrophils, macrophages, DCs and radioresistant cells, including intestinal epithelial cells and other stromal cells (Browning and French, 2002; Ware, 2005). Although the role of LTβR in the production of homeostatic chemokines in secondary lymphoid organs has been demonstrated, the biological function of LTβR on intestinal epithelial cells remained unclear. The generation of mice with conditional inactivation of LTβR in intestinal epithelial cells allowed us to directly define the role of LTβR on the intestinal epithelium. In contrast to mice with complete LTβR deficiency, Vil-Ltbr−/− mice do not show defects in development and organization of secondary lymphoid organs, and display normal DC numbers in secondary lymphoid organs (data not shown). Our data suggest that without LTβR signaling in intestinal epithelial cells in Vil-Ltbr−/− mice, neutrophils could not accumulate rapidly at the infection site, reducing the ability of the host to clear C. rodentium infection. Furthermore, our bone marrow transfer data indicate that additional LTβR signals in hematopoietic-derived cells, such neutrophils and macrophages, coordinate with LTβR signals in intestinal epithelium for the complete control of C. rodentium infection. Furthermore, our data suggest that, in addition to gut epithelial cells, LTβR signaling in other radioresistant stromal cells may contribute to protection, since the phenotype of Vil-Ltbr−/− mice was less severe than in WT>Ltbr−/− chimeras. Identification of additional LTβR expressing cells that contribute to protection will help to further define the role of LTβR in regulation of mucosal immune defense homeostasis.
LTβR can be engaged by at least two known ligands: membrane LT and LIGHT (Wang et al., 2009; Ware, 2005). Both ligands have been implicated in mucosal immune homeostasis (Spahn et al., 2004; Wang et al., 2004). Our previous study showed that expression of LIGHT on T cells in LIGHT-transgenic mice or in a Rag1−/− adoptive transfer model promotes autoimmune inflammation in the gut (Wang et al., 2004). Interestingly, in this study we found a normal response to C. rodentium infection in Tnfsf14−/− mice, as compared to Ltb−/− mice. The reason for this difference is currently unclear, but it is possible that additional defects in the development of gut-associated lymphoid organs and impaired generation of DCs may be responsible for the severe phenotype of Ltb−/− mice. Although both ligands were shown to be expressed on RORγt+ cells in the gut (Luci et al., 2009), different kinetics or expression amounts of LIGHT and LT during infection could be responsible for the distinct phenotypes of bacterial clearance in LT-and LIGHT-deficient mice.
Surface LT is readily detected on T and B cells, especially after activation (Browning, 2008; Fu and Chaplin, 1999; Ware, 2005). To identify the critical LT-expressing cells in our model, we employed mice with conditional inactivation of membrane LT on T or B cells, as previous studies implicated these cells as major LT producers in secondary lymphoid organs (Junt et al., 2006; Tumanov et al., 2002). Unexpectedly, LT deficiency in either T or B cells showed no phenotype. We then generated double-deficient mice that lacked LT on both T and B cells, again, these mice were able to efficiently clear C. rodentium infection, which opened the possibility that LT expression is necessary on innate immune cells such as RORγt+ cells. Innate RORγt+ cells are important for the development of lymphoid tissues in a LT-dependent fashion (Eberl et al., 2004; Sun et al., 2000), however their role in mucosal immunity is poorly defined. To directly address the role of these cells in host defense we have tested the sensitivity of Rorc−/− mice to C. rodentium infection. Our data suggest that RORγt+ innate cells are essential for the mucosal bacterial infection.
LT can be produced by both RORγt+ LTi-like cells and CD3− NKp46+ cells in the gut of naïve mice (Luci et al., 2009; Tsuji et al., 2008). We detected both LTα and LTβ transcripts in both RORγt+ LTi-like cells and RORγt+ NKp46+ cells in the colonic lamina propria early after C. rodentium infection. Our data suggest that the increased mortality of LTβR-Ig treated mice is not due to impaired migration of these cell populations to the lamina propria after infection, but more likely due to the lack of LT activity by those cells. Using Rag1−/− mice and timing of LT blockade, we have shown LT from innate cells is essential for the protection at an early, but not late (>day 5) phase of infection. Furthermore, analysis of mixed bone marrow chimeras and mice with specific inactivation of LT on RORγt+ cells revealed the essential role of LT+ RORγt+ cells in mucosal innate protection. However, which population, RORγt+ LTi-like cells or RORγt+ NKp46+ cells, is more important for protection remains to be determined.
Bacterial invasion of the mucosa is often followed by infiltration of neutrophils that provide early, innate defense against infection (Appelberg, 2007; Lebeis et al., 2007). We found that a lack of LTβR signaling prevented effective recruitment of neutrophils to the infection site early after infection, followed by increased bacterial counts and severe tissue injury. This effect is not simply due to aberrantly organized lymphoid structures in Ltbr−/− mice because short-term blockade of LTβR signals resulted in a delayed neutrophil accumulation at the infection site, thus compromising the early innate immune response. This uncovered role for LTβR in neutrophil recruitment is novel and intriguing since no defect in neutrophil development was reported in either LTβ- or LTβR-deficient mice (Alimzhanov et al., 1997; Futterer et al., 1998). In line with our data, an earlier study using an expression profiling approach hinted at a link between LT signaling and neutrophil function as the expression of several neutrophil-specific genes, such as myeloperoxidase and lactoferrin, were reduced in Lta−/− spleens, compared to WT mice (Shakhov et al., 2000). Our data suggest that reduced LTβR-dependent regulation of neutrophil recruitment after infection can be important for the control of other mucosal bacterial pathogens.
The lack of a proper chemokine milieu is often associated with defective neutrophil recruitment. CXCL1 and CXCL2 are the most potent neutrophil-recruiting chemokines, which are produced by intestinal epithelial cells following bacterial infection or injury and attract neutrophils via CXCR2 (Lebeis et al., 2007; Ohtsuka et al., 2001; Rakoff-Nahoum et al., 2004; Spehlmann et al., 2009). Indeed, we observed reduced CXCL1 and CXCL2 expression in the lamina propria of Rag1−/− mice treated with LTβR-Ig and in mice with conditional inactivation of LTβR on the intestinal epithelium. Thus, our data suggest a unique role for LTβR signaling in regulation of neutrophil recruitment after infection, possibly via a CXCL1 and/or CXCL2-dependent mechanism.
Overall, our data support a model for LTβR-dependent control of the innate immune response to the mucosal bacterial pathogen C. rodentium. Local infection of gut epithelial cells might initially induce chemokines that attract LT+ innate cells from organized lymphoid follicles to the epithelial layer. LT expression on RORγt+ cells triggers LTβR signaling on intestinal epithelial cells to mobilize the early, innate immune response to the mucosal bacterial pathogen. LTβR signaling activates the expression of CXCL1 and CXCL2 chemokines, which promote neutrophil recruitment to the infection site to fight the bacterial pathogen. Contact of RORγt+ cells with LTβR on intestinal epithelial cells may further promote cooperation of various innate immune cells in early defense to invading pathogen before the development of sterilizing adaptive immune responses.
Experimental Procedures
Mice
C57BL/6 and Rag1−/− mice were purchased from Harland Teklad. Ltb−/−, Tnfsf14−/−, and Ltbr−/− mice were backcrossed onto C57BL/6 background 13, 11 or 10 generations, respectively, and maintained under specific pathogen-free conditions as described (Alimzhanov et al., 1997; Futterer et al., 1998; Tamada et al., 2002). Rorc−/− (Sun et al., 2000), Vil-Cre (Madison et al., 2002), LysM-Cre mice (Clausen et al., 1999) (all on C57BL/6 background) were purchased from The Jackson Lab. T-Ltb−/−, B-Ltb−/− and T,B-Ltb−/− mice were intercrossed as previously described (Tumanov et al., 2002; Tumanov et al., 2003). LTβR-floxed mice were generated using CreloxP technology (see Supplemental Materials for details). Vil-Ltbr−/− and LysM-Ltbr−/− − mice were generated by crossing LTβR floxed mice with Vil-Cre or LysM-Cre transgenic mice, respectively. RORγt-Ltb−/− mice were generated by crossing LTβ floxed mice (Tumanov et al., 2002) with RORγt-Cre transgenic mice (Eberl and Littman, 2004). Animal care and use were in accordance with institutional and National Institutes of Health guidelines and all studies were approved by the Animal Care and Use Committee of the University of Chicago.
Bacterial strain and infection of mice
To induce bacterial colitis in mice, mice were orally gavaged with 2×109 cfu C. rodentium strain DBS100 (ATCC 51459; American Type Culture Collection), as previously described (Zheng et al., 2008). Briefly, mice were fasted for 8 h before oral inoculation of C. rodentium culture in a total volume of 0.2ml per mouse. Bacteria were prepared by shaking at 37°C overnight in LB broth. Concentration was assessed by measuring absorbance at OD600. Bacterial culture was serially diluted and plated after each inoculation to confirm the colony-forming units (CFUs) administered. Body weight was assessed before and then frequently during the course of disease.
Tissue collection, histology and colony-forming unit counts
Colons were dissected from the mice and fixed in 10% neutral buffered formalin. Paraffin-embedded tissue sections were stained with H&E to evaluate tissue pathology. Fecal samples were collected and weighted, then homogenized in sterile phosphate-buffered saline. Serially diluted homogenates were plated on MacConkey agar plates (Sigma). C. rodentium colonies were identified as pink colonies after 18–24 h of incubation at 37°C Spleens and livers were aseptically removed and homogenized. Organs colonization was assessed as described for fecal specimens.
LTβR-Ig and anti-LTβR agonist antibody treatment
The LTβR-Ig used in this study has been previously described (Anders et al., 2005). Briefly, cDNA encoding the extracellular domain of murine LTβR was fused with the Fc portion of human IgG, transfected into BHK/VP16 cell, and the supernatant collected. The anti-LTβR agonistic antibody (3C8) was kindly provided by C. Ware (La Jolla Institute for Allergy and Immunology, La Jolla, CA).
Isolation of intraepithelial lymphocytes, lamina propria mononuclear cells and epithelial cells from mouse colon
IELs, LPMCs and colonic epithelial cells were isolated as described (Ivanov et al., 2006), with some modifications. Briefly, mice were killed and colons were removed and placed in ice-cold PBS. The intestine was opened lengthwise, thoroughly washed in ice-cold PBS and cut into 1.5 cm pieces. The pieces were incubated twice in 5 ml of 5 mM EDTA in HBSS for 15–20 min at 37°C with slow rotation (100 rpm). After each incubation, the epithelial cell layer, containing the intraepithelial lymphocytes (IELs), was removed by intense vortexing and passing through a 100mm cell strainer and new EDTA solution was added. After the second EDTA incubation the pieces were washed in HBSS, cut in 1mm2 pieces using razor blades, and placed in 5ml digestion solution contained 2% fetal calf serum, 0.5 mg/ml each of Collagenase D (Sigma) and DNase I (Sigma), and 50 U/ml Dispase (Fisher). Digestion was performed by incubating the pieces at 37°C for 20 min with slow rotation. After the initial incubation, the solution was vortexed intensely and passed through a 40 mm cell strainer. The pieces were collected and placed into fresh digestion solution. Procedure was repeated three times. Supernatants from all three digestions (or from the EDTA treatment for IEL isolation) from a single colon were combined, washed once in cold FACS buffer, resuspended in 10 ml of the 40% fraction of a 40:80 Percoll gradient, and overlaid on 5 ml of the 80% fraction in a 15 ml Falcon tube. Percoll gradient separation was performed by centrifugation for 20 min at 2500 rpm at room temperature. Lamina propria lymphocytes (LPLs) were collected at the interphase of the Percoll gradient, washed once, and resuspended in FACS buffer or T cell medium. The cells were used immediately for experiments.
Flow Cytometry and Antibodies
Flow cytometry analysis was performed on FACSCalibur, FACSCanto, and FACSAria II (BD Biosciences) instruments and analyzed using FlowJo software (Tree Star Inc.). All antibodies were purchased from BD Biosciences or eBiosciences.
RNA isolation and real-time reverse transcriptase PCR
RNA from cells or frozen tissues was isolated using RNeasy Mini Kit (Qiagen). For cDNA synthesis, RNAs were digested with DNase I and reverse transcribed using random primers with AMV Reverse Transcriptase (Promega). The concentration of the target gene was determined using the comparative CT (threshold cycle number at a cross-point between amplification plot and threshold) method and normalized to HPRT and beta-actin. cDNA were amplified using Power Sybr Green PCR master mix (Applied Biosystems) or SSoFast EvaGreen supermix (Bio-Rad) and run on ABI 7300 cycler (Applied Biosystems) or StepOne Plus (Applied Biosystems). PCR primers and probes used: for CXCL1: forward 5`- CCACCCGCTCGCTTCTC, reverse 5`-CACTGACAGCGCAGCTCATT, for CXCL2: forward 5`-ACCAACCACCAGGCTAGA, reverse 5`-GCGTCACACTCAAGCTCT; for LTα: forward 5`-TCCACTCCCTCAGAAGCACT, reverse 5`-AGAGAAGCCATGTCGGAGAA; for LTβ: forward: 5`-TACACCAGATCCAGGGGTTC, reverse: 5`-ACTCATCCAAGCGCCTATGA; for HPRT, forward 5`-TGAAGAGCTACTGTAATGATCAGTCAAC,reverse 5`-AGCAAGCTTGCAACCTTAACCA; for beta actin, forward 5`-TCTTGGGTATGGAATCCTGTGGCA, reverse, ACTCCTGCTTGCTGATCCACATCT
Statistical analysis
Comparisons of data were analyzed by two-tailed Student’s t test using GraphPad Prism 5.0 program. Data from such experiments are presented as mean values ±S.E.M. P< 0.05 was considered significant. For survival curves statistics were done using the log rank (Mantel-Cox) test.
Highlights
Lymphotoxin is required for early protection against mucosal C. rodentium infection
Lymphotoxin from innate mucosal RORγt+ cells is essential for protection
LTβR on both radio-resistant and bone marrow-derived cells controls the infection
LTβR signaling in intestinal epithelial cells recruits neutrophils for host protection
Supplementary Material
Acknowledgements
This research was in part supported by US National Institutes of Health grants AI062026, CA115540 and DK58891 to Y.X.F; by Career Development Award from the Crohn’s and Colitis Foundation (CCFA #2672) to A.V.T., by SFB633 from Deutsche Forschungs gemeinschaft and by MCB Program of the Russian Academy of Sciences. We are grateful to C. Ware (La Jolla Institute for Allergy and Immunology, La Jolla, CA) for 3C8 LTβR antibody. We thank N. Brown, B. Burnette and E. Chang for critical reading of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures:
The authors have no conflicts of financial interest.
References
- Alimzhanov MB, Kuprash DV, Kosco-Vilbois MH, Luz A, Turetskaya RL, Tarakhovsky A, Rajewsky K, Nedospasov SA, Pfeffer K. Abnormal development of secondary lymphoid tissues in lymphotoxin beta-deficient mice. Proc Natl Acad Sci U S A. 1997;94:9302. doi: 10.1073/pnas.94.17.9302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders RA, Subudhi SK, Wang J, Pfeffer K, Fu YX. Contribution of the lymphotoxin beta receptor to liver regeneration. J. Immunol. 2005;175:1295–1300. doi: 10.4049/jimmunol.175.2.1295. [DOI] [PubMed] [Google Scholar]
- Appelberg R. Neutrophils and intracellular pathogens: beyond phagocytosis and killing. Trends Microbiol. 2007;15:87–92. doi: 10.1016/j.tim.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Browning JL. Inhibition of the lymphotoxin pathway as a therapy for autoimmune disease. Immunol Rev. 2008;223:202–220. doi: 10.1111/j.1600-065X.2008.00633.x. [DOI] [PubMed] [Google Scholar]
- Browning JL, French LE. Visualization of lymphotoxin-beta and lymphotoxin-beta receptor expression in mouse embryos. J. Immunol. 2002;168:5079–5087. doi: 10.4049/jimmunol.168.10.5079. [DOI] [PubMed] [Google Scholar]
- Bry L, Brenner MB. Critical role of T cell-dependent serum antibody, but not the gut-associated lymphoid tissue, for surviving acute mucosal infection with Citrobacter rodentium, an attaching and effacing pathogen. J Immunol. 2004;172:433–441. doi: 10.4049/jimmunol.172.1.433. [DOI] [PubMed] [Google Scholar]
- Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz JK, Doherty JM, Mills JC, Colonna M. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang EY, Kolumam GA, Yu X, Francesco M, Ivelja S, Peng I, Gribling P, Shu J, Lee WP, Refino CJ, et al. Targeted depletion of lymphotoxin-alpha-expressing TH1 and TH17 cells inhibits autoimmune disease. Nat Med. 2009;15:766–773. doi: 10.1038/nm.1984. [DOI] [PubMed] [Google Scholar]
- Clausen BE, Burkhardt C, Reith W, Renkawitz R, Forster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999;8:265. doi: 10.1023/a:1008942828960. [DOI] [PubMed] [Google Scholar]
- Cupedo T, Crellin NK, Papazian N, Rombouts EJ, Weijer K, Grogan JL, Fibbe WE, Cornelissen JJ, Spits H. Human fetal lymphoid tissue-inducer cells are interleukin 17-producing precursors to RORC+ CD127+ natural killer-like cells. Nat Immunol. 2009;10:66–74. doi: 10.1038/ni.1668. [DOI] [PubMed] [Google Scholar]
- Eberl G, Littman DR. Thymic origin of intestinal alphabeta T cells revealed by fate mapping of RORgammat+ cells. Science. 2004;305:248–251. doi: 10.1126/science.1096472. [DOI] [PubMed] [Google Scholar]
- Eberl G, Marmon S, Sunshine MJ, Rennert PD, Choi Y, Littman DR. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat Immunol. 2004;5:64. doi: 10.1038/ni1022. [DOI] [PubMed] [Google Scholar]
- Fu YX, Chaplin DD. Development and maturation of secondary lymphoid tissues. Annu Rev Immunol. 1999;17:399–433. doi: 10.1146/annurev.immunol.17.1.399. [DOI] [PubMed] [Google Scholar]
- Fu YX, Huang G, Wang Y, Chaplin DD. B lymphocytes induce the formation of follicular dendritic cell clusters in a lymphotoxin alpha-dependent fashion. J Exp Med. 1998;187:1009–1018. doi: 10.1084/jem.187.7.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futterer A, Mink K, Luz A, Kosco-Vilbois MH, Pfeffer K. The lymphotoxin beta receptor controls organogenesis and affinity maturation in peripheral lymphoid tissues. Immunity. 1998;9:59–70. doi: 10.1016/s1074-7613(00)80588-9. [DOI] [PubMed] [Google Scholar]
- Gibson DL, Ma C, Rosenberger CM, Bergstrom KS, Valdez Y, Huang JT, Khan MA, Vallance BA. Toll-like receptor 2 plays a critical role in maintaining mucosal integrity during Citrobacter rodentium-induced colitis. Cell Microbiol. 2008;10:388–403. doi: 10.1111/j.1462-5822.2007.01052.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez M, Mackay F, Browning JL, Kosco-Vilbois MH, Noelle RJ. The sequential role of lymphotoxin and B cells in the development of splenic follicles. J Exp Med. 1998;187:997–1007. doi: 10.1084/jem.187.7.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Junt T, Tumanov AV, Harris N, Heikenwalder M, Zeller N, Kuprash DV, Aguzzi A, Ludewig B, Nedospasov SA, Zinkernagel RM. Expression of lymphotoxin beta governs immunity at two distinct levels. Eur J Immunol. 2006;36:2061–2075. doi: 10.1002/eji.200626255. [DOI] [PubMed] [Google Scholar]
- Lebeis SL, Bommarius B, Parkos CA, Sherman MA, Kalman D. TLR signaling mediated by MyD88 is required for a protective innate immune response by neutrophils to Citrobacter rodentium. J Immunol. 2007;179:566–577. doi: 10.4049/jimmunol.179.1.566. [DOI] [PubMed] [Google Scholar]
- Luci C, Reynders A, Ivanov II, Cognet C, Chiche L, Chasson L, Hardwigsen J, Anguiano E, Banchereau J, Chaussabel D, et al. Influence of the transcription factor RORgammat on the development of NKp46+ cell populations in gut and skin. Nat Immunol. 2009;10:75–82. doi: 10.1038/ni.1681. [DOI] [PubMed] [Google Scholar]
- Maaser C, Housley MP, Iimura M, Smith JR, Vallance BA, Finlay BB, Schreiber JR, Varki NM, Kagnoff MF, Eckmann L. Clearance of Citrobacter rodentium requires B cells but not secretory immunoglobulin A (IgA) or IgM antibodies. Infect Immun. 2004;72:3315–3324. doi: 10.1128/IAI.72.6.3315-3324.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald TT, Frankel G, Dougan G, Goncalves NS, Simmons C. Host defences to Citrobacter rodentium. Int J Med Microbiol. 2003;293:87–93. doi: 10.1078/1438-4221-00247. [DOI] [PubMed] [Google Scholar]
- Madison BB, Dunbar L, Qiao XT, Braunstein K, Braunstein E, Gumucio DL. Cis elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. J Biol Chem. 2002;277:33275. doi: 10.1074/jbc.M204935200. [DOI] [PubMed] [Google Scholar]
- Mundy R, MacDonald TT, Dougan G, Frankel G, Wiles S. Citrobacter rodentium of mice and man. Cell Microbiol. 2005;7:1697–1706. doi: 10.1111/j.1462-5822.2005.00625.x. [DOI] [PubMed] [Google Scholar]
- Ohtsuka Y, Lee J, Stamm DS, Sanderson IR. MIP-2 secreted by epithelial cells increases neutrophil and lymphocyte recruitment in the mouse intestine. Gut. 2001;49:526–533. doi: 10.1136/gut.49.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Satoh-Takayama N, Vosshenrich CA, Lesjean-Pottier S, Sawa S, Lochner M, Rattis F, Mention JJ, Thiam K, Cerf-Bensussan N, Mandelboim O, et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity. 2008;29:958–970. doi: 10.1016/j.immuni.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Shakhov AN, Lyakhov IG, Tumanov AV, Rubtsov AV, Drutskaya LN, Marino MW, Nedospasov SA. Gene profiling approach in the analysis of lymphotoxin and TNF deficiencies. J Leukoc Biol. 2000;68:151. [PubMed] [Google Scholar]
- Spahn TW, Maaser C, Eckmann L, Heidemann J, Lugering A, Newberry R, Domschke W, Herbst H, Kucharzik T. The lymphotoxin-beta receptor is critical for control of murine Citrobacter rodentium-induced colitis. Gastroenterology. 2004;127:1463. doi: 10.1053/j.gastro.2004.08.022. [DOI] [PubMed] [Google Scholar]
- Spehlmann ME, Dann SM, Hruz P, Hanson E, McCole DF, Eckmann L. CXCR2-dependent mucosal neutrophil influx protects against colitis-associated diarrhea caused by an attaching/effacing lesion-forming bacterial pathogen. J Immunol. 2009;183:3332–3343. doi: 10.4049/jimmunol.0900600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers-DeLuca LE, McCarthy DD, Cosovic B, Ward LA, Lo CC, Scheu S, Pfeffer K, Gommerman JL. Expression of lymphotoxin-alphabeta on antigen-specific T cells is required for DC function. J Exp Med. 2007;204:1071–1081. doi: 10.1084/jem.20061968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Unutmaz D, Zou YR, Sunshine MJ, Pierani A, Brenner-Morton S, Mebius RE, Littman DR. Requirement for RORgamma in thymocyte survival and lymphoid organ development. Science. 2000;288:2369–2373. doi: 10.1126/science.288.5475.2369. [DOI] [PubMed] [Google Scholar]
- Takatori H, Kanno Y, Watford WT, Tato CM, Weiss G, Ivanov II, Littman DR, O'Shea JJ. Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J Exp Med. 2009;206:35–41. doi: 10.1084/jem.20072713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamada K, Ni J, Zhu G, Fiscella M, Teng B, van Deursen JM, Chen L. Cutting edge: selective impairment of CD8+ T cell function in mice lacking the TNF superfamily member LIGHT. J. Immunol. 2002;168:4832–4835. doi: 10.4049/jimmunol.168.10.4832. [DOI] [PubMed] [Google Scholar]
- Tsuji M, Suzuki K, Kitamura H, Maruya M, Kinoshita K, Ivanov II, Itoh K, Littman DR, Fagarasan S. Requirement for lymphoid tissue-inducer cells in isolated follicle formation and T cell-independent immunoglobulin A generation in the gut. Immunity. 2008;29:261–271. doi: 10.1016/j.immuni.2008.05.014. [DOI] [PubMed] [Google Scholar]
- Tumanov A, Kuprash D, Lagarkova M, Grivennikov S, Abe K, Shakhov A, Drutskaya L, Stewart C, Chervonsky A, Nedospasov S. Distinct role of surface lymphotoxin expressed by B cells in the organization of secondary lymphoid tissues. Immunity. 2002;17:239–250. doi: 10.1016/s1074-7613(02)00397-7. [DOI] [PubMed] [Google Scholar]
- Tumanov AV, Grivennikov SI, Shakhov AN, Rybtsov SA, Koroleva EP, Takeda J, Nedospasov SA, Kuprash DV. Dissecting the role of lymphotoxin in lymphoid organs by conditional targeting. Immunol. Rev. 2003;195:106–116. doi: 10.1034/j.1600-065x.2003.00071.x. [DOI] [PubMed] [Google Scholar]
- Uren TK, Wijburg OL, Simmons C, Johansen FE, Brandtzaeg P, Strugnell RA. Vaccine-induced protection against gastrointestinal bacterial infections in the absence of secretory antibodies. Eur J Immunol. 2005;35:180–188. doi: 10.1002/eji.200425492. [DOI] [PubMed] [Google Scholar]
- Vallance BA, Deng W, Jacobson K, Finlay BB. Host susceptibility to the attaching and effacing bacterial pathogen Citrobacter rodentium. Infect Immun. 2003;71:3443–3453. doi: 10.1128/IAI.71.6.3443-3453.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivier E, Spits H, Cupedo T. Interleukin-22-producing innate immune cells: new players in mucosal immunity and tissue repair? Nat Rev Immunol. 2009;9:229–234. doi: 10.1038/nri2522. [DOI] [PubMed] [Google Scholar]
- Wang J, Anders RA, Wu Q, Peng D, Cho JH, Sun Y, Karaliukas R, Kang HS, Turner JR, Fu YX. Dysregulated LIGHT expression on T cells mediates intestinal inflammation and contributes to IgA nephropathy. J Clin Invest. 2004;113:826–835. doi: 10.1172/JCI20096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhu M, Miller M, Fu YX. Immunoregulation by tumor necrosis factor superfamily member LIGHT. Immunol Rev. 2009;229:232–243. doi: 10.1111/j.1600-065X.2009.00762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware CF. Network communications: lymphotoxins, LIGHT, and TNF. Annu Rev Immunol. 2005;23:787–819. doi: 10.1146/annurev.immunol.23.021704.115719. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, de Sauvage FJ, Ouyang W. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.