Abstract
Conditioned place preference (CPP), a commonly used model for studying the role of contextual cues in drug reward and drug seeking, was employed to explore possible behavioral interactions between (±)3,4-methylenedioxymethamphetamine (MDMA; “ecstasy”) and cocaine. On each of four occasions, adult male rats received one of three doses of MDMA (0 mg/kg, 5 mg/kg, 10 mg/kg; administered subcutaneously [s.c.]) combined with one of three doses of cocaine (0 mg/kg, 2.5 mg/kg, 5 mg/kg; administered intraperitoneally [i.p.]), and were then tested in a CPP paradigm. The results showed MDMA-induced CPP at a unit dose of 5 mg/kg, but at the 10 mg/kg dose there was a return to baseline (control) performance levels. For cocaine alone, CPP increased in a linear fashion as the drug dose was increased. Concurrent administration resulted in antagonism of each drug, but there was evidence that this pattern was reversible at higher doses of the respective drugs. These data are instructive insofar as they suggest that the behavioral and neurochemical effects of MDMA and cocaine presented in isolation are dramatically altered when the two drugs are presented in combination.
Keywords: antagonism, cocaine, CPP, MDMA
Although (±)3,4-methylenedioxymethamphetamine (MDMA; “ecstasy”) is an illegal substance, it is gaining global popularity as a recreational drug, especially in youth culture and at all-night dance parties known as “raves” (Clemens et al., 2004; Forsyth, 1996; Green et al., 1995; Peroutka, 1987; Schechter, 1991; Schenk et al., 2003). Although the epidemiology of polydrug use involving MDMA has not been adequately characterized, reports are available which suggest that on occasion MDMA is used in combination with cocaine (e.g., Parrott et al., 2000). Because preclinical studies have focused on the longterm consequences of MDMA pretreatment on subsequent cocaine administration (e.g., Achat-Mendes et al., 2003; Horan et al., 2000), more explicit information on the interactive profile of concomitant exposure to MDMA and cocaine is required.
There are several lines of research that underscore the need to examine possible MDMA/cocaine interactions. Dopamine (DA) has been implicated as mediating the stimulatory effects of cocaine, which acts as a DA transporter inhibitor (blocking presynaptic reuptake and thus increasing DA availability at D1-like and D2-like postsynaptic receptors [Riley, 1995]), therein producing reward by enhancing dopaminergic transmission in the nucleus accumbens [NAcc] (Heidbreder et al., 1996; Kalivas and Duffy, 1993; Pettit et al., 1990). In contrast to cocaine, MDMA activates DA release by stimulating serotonin (5-hydroxytryptamine [5HT]) receptors (Müller et al., 2007), and reverses rather than blocks the DA transporter (DAT) and serotonin transporter (SERT); cf. Rothman and Baumann, 2003. The preferential action of MDMA at the SERT site is of particular significance because manipulations that increase 5-HT function decrease cocaine and amphetamine self-administration (Carroll et al., 1990; Howell and Byrd, 1995; Smith et al., 1986; Wee et al., 2005). In addition, single gene knockout of SERT has been shown to increase the rewarding properties of cocaine, ostensibly because stimulated actions at SERT are at least partially aversive (Uhl et al., 2002), or modulate DA activity (Hall et al., 2004). Paradoxically, and contrary to what has been argued elsewhere (Colado et al., 2004), these data suggest the combined use of MDMA and cocaine in the human population may result in antagonism of the rewarding properties of one or both drugs.
One method for indexing the relative rewarding properties of different psychoactive drugs involves conditioned place preference (CPP). There is general agreement that the CPP model affords a valid assessment of parameters common to drug reward and drug seeking (e.g., Bardo and Bevins, 2000; Cervo et al., 2002; Knapp et al., 2002). The CPP paradigm is based on classical (Pavlovian) conditioning principles, as contextual cues acquire secondary reinforcing (conditioned stimulus [CS]) properties through temporal pairing with a psychoactive drug that functions as an unconditioned stimulus [US] (Calcagnetti and Schechter, 1993). In this model, a drug is administered to an animal immediately before placement in an environment with distinctive contextual stimuli (olfactory, visual, tactile). Place preference is then defined by some measure of preference for one environment over another based on feed-forward conditioning (see Bardo and Blevins, 2000).
MDMA and cocaine, presented separately at low doses, have been shown to elicit CPP in rats (Bardo et al., 1995; Cole et al., 2003; Schechter, 1991). Interestingly, a purportedly neurotoxic dose of MDMA (20 mg/kg, s.c.) administered prior to MDMA CPP testing significantly decreased conditioning at lower doses of the test dose, but not at higher doses of the test dose (Schechter, 1991). More directly related to the rationale that formed the basis for the present investigation, a recent report by Aberg et al. (2007) showed that for adult rats cocaine-induced (10 mg/kg) CPP was diminished by MDMA administered five days prior to CPP training. However, in this study only a single dose of cocaine was tested and there was no attempt to determine the effects of cojoint exposure, which as noted above is the more likely pattern of exposure among young adults. Moreover, the effects of cocaine on MDMA CPP have never been examined. Accordingly, the purpose of this investigation was to systematically characterize the dose-effect pattern produced by the combined administration of both MDMA and cocaine in a CPP preparation, and explicitly determine if synergism occurs when the two drugs are presented concurrently, as some clinical consumption patterns suggest (Block et al., 2002; Gross et al., 2002; Smit et al., 2002), or if antagonism occurs as suggested by the preclinical literature on DA and 5-HT function (cf. Rothman and Bauman, 2003).
METHODS
Animals
Adult male Sprague-Dawley rats (n = 63), weighing ≈ 250–300 g at the start of the experiment, were obtained from a commercial source (Harlan; Houston, TX). All animals were provided standard laboratory rat chow (Teklad; Madison, WI) and tap water ad libitum throughout the course of the experiment, and were housed individually in plastic cages. Animals were not presented with food or water in the test chambers. A 12hr/12hr light-dark cycle was used (lights on at 08:00 hr), with temperature held constant at 23°C in the holding area and test room. Beginning one week prior to the start of the experiment, animals were handled daily to limit any confounding variables caused by handling stress during the conditioning or experimental procedures. Body weights were recorded daily throughout the experiment.
The animal housing and testing facility was approved by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC International), and all animal maintenance, research design, and administration was conducted in accordance with guidelines provided by the Texas A&M University Laboratory Animal Care Committee (ULACC). All aspects of the research were conducted in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (Publication No. 85-23, revised 1985). The health of the animals was monitored by the campus veterinarian throughout the duration of the project.
Apparatus
Place conditioning and testing were conducted in seven 20×60×20 cm wooden shuttle boxes with wooden tilt floors. At each end of each box was a microswitch interfaced to an IBM compatible computer. A BASIC computer program was written to continuously record the number of times and duration the switch was activated via a tilt of the floor.
One compartment of the apparatus had smooth white walls and floor, and the other compartment had black walls with a black sandpaper floor. For conditioning sessions, the boxes were divided into two equal-sized compartments by removable wooden partitions. On test sessions (pretest and posttest), the partitions were removed and a 20×10×5 cm wooden insert was installed 2 cm above the floor to divide the two compartments but allow rats free access to either compartment. In earlier investigations using these chambers, subjects have exhibited a strong preference for the black compartment (Miller et al., 1999; Miller et al., 2000; Miller and Nation, 1997; Smith and Nation, 2003). In an effort to control for this preference, and thereby decrease the likelihood the biased side of the test apparatus would always be the white side, a 40 W light was positioned 50 cm above the black compartment of each apparatus. These seven lamps provided the only illumination in the testing room. Following each conditioning and test session, the apparatus was cleaned with a mild soap solution. The apparatus was located in a sound-resistant room with a 40 dB white noise generator operating continuously. All conditioning and testing sessions were conducted during the light phase of the cycle.
Drugs
The Research Technology Branch of the National Institute on Drug Abuse (NIDA) provided cocaine HCl and MDMA HCl gratis. Cocaine HCl and racemic MDMA [(±)-MDMA HCl] were dissolved in a 0.9% w/v saline vehicle, and the doses were expressed as the salt. Cocaine was administered i.p. and MDMA was administered s.c.; all drugs were delivered in a volume of 1 ml/kg.
Procedure
The CPP experiment started immediately following one week of acclimation to the laboratory environment. The biased CPP procedure employed in this study consisted of a sequence of three phases as outlined by Schechter (1991). The first phase was the preconditioning phase that served to establish a baseline place preference for each rat. On Day 1, animals were transferred in tubs from the colony room to the testing chambers for 20 min in an effort to habituate the animals to transportation and the sound and illumination of the room. Animals were placed in the apparatus and allowed free access between compartments during the 20-min period to overcome freezing effects from exposure to a novel environment. Data recorded from the first day were not used in calculating the preferred side. Between tests, each conditioning apparatus was cleaned thoroughly to eliminate confounding scent cues. On Day 2, animals were placed in the CPP apparatus and allowed free access to either compartment for 15 min in order to establish their baseline preference. Pretest data were measured by the amount of time (min) animals spent in each compartment, and these data were used to determine animal pretest preferences for the white or black compartment.
The conditioning phase consisted of 8 days of repeatedly pairing one specific compartment with an injection of either drug or vehicle. By individually analyzing data from baseline day (Day 2) testing, the most-preferred and least-preferred sides were determined for each rat. On four alternate days (Days 3, 5, 7, and 9), each animal received a daily s.c. injection of its respective combination of MDMA (0, 5, or 10 mg/kg body weight) 25 min before an i.p. cocaine injection (0, 2.5, or 5 mg/kg body weight). The resulting interaction of 3 doses of MDMA × 3 doses of cocaine created 9 groups; 0M (0 mg/kg MDMA) - 0C (0 mg/kg cocaine) (n = 7), 0M - 2.5C (n = 7), 0M - 5C (n = 7), 5M - 0C (n = 7), 5M - 2.5C (n = 7), 5M - 5C(n = 7), 10M - 0C (n = 7), 10M - 2.5C (n = 7), and 10M - 5C (n = 7). The time delay between the MDMA injections and the cocaine injections that were administered immediately prior to testing was based on the slow onset of the physiological effects of MDMA, relative to cocaine (Schechter, 1991).
Animals were confined to the least-preferred compartment (defined as the compartment in which the animal spent the least amount of time on the Day 2 pretest) for 20 min immediately after the injections of MDMA/cocaine combinations. On the other four alternate days (days 4, 6, 8, and 10), all animals received s.c. and i.p. vehicle (saline) injections and were confined to the most-preferred compartment (defined as the compartment in which the animal spent the most amount of time on the Day 2 pretest) for 20 min at the same post-administration time. As noted, all injections were given at a volume of 1 ml/kg vehicle. Animals were run in squads of seven, counterbalanced by drug group assignments and weight.
In the final phase (Day 11), to determine post-conditioned preference, posttest data were obtained using the same procedure as the Day 2 pretest, i.e., in the absence of an injection, subjects were permitted 15 min of free access between the distinctive chambers of the test apparatus. Weight-sensitive flooring in the place preference apparatus recorded the amount of time spent in each chamber.
Statistical Analysis
The pretest data from Day 2 and the posttest data from Day 11 were examined. The conditioning scores in both experiments were defined by the number of min spent on the drug-conditioned compartment on posttest trial minus the number of min spent on the same compartment in the pretest trial. Subject weight was analyzed using a repeated measures analysis of variance (ANOVA) test with daily body weight averages during the testing period serving as the within factor. The behavioral data were analyzed by a 3 doses of MDMA (0 mg/kg, 5 mg/kg, 10 mg/kg) × 3 doses of cocaine (0 mg/kg, 2.5 mg/kg, 5 mg/kg) completely factorial ANOVA. Neuman-Keuls procedure for post hoc analyses was employed for individual comparisons.
During conditioning and prior to testing, one male rat in the 10M - 0C condition exhibited decreased muscle tone, nonresponsiveness, rapid heart rate, shallow breathing, frothing at the mouth, and temporary paralysis. This animal fully recovered and because the data from this rat were not appreciably different from other animals in the 10M - 0C condition, these data were included in the analyses reported here.
RESULTS
Body Weights
Consistent with earlier reports (e.g., Bilsky et al., 1990; Bilsky et al., 1991), there was a systematic trend toward weight loss among animals exposed to 10 mg/kg MDMA. However, the difference in group means was not large (305.6 g for groups receiving 10 mg/kg MDMA compared to 317.6 g for groups receiving 0 mg/kg MDMA). This trend toward weight loss was not evident among animals exposed to cocaine.
Behavioral Data
Despite attempts to limit preferences for the black compartment of the apparatus, the majority of animals in all conditions demonstrated an initial preference for this side of the test chamber during pretesting (Day 2). The conditions did not differ with respect to preference ratios. In terms of the test results, findings from a 3 doses of MDMA (0 mg/kg, 5 mg/kg, 10 mg/kg) × 3 doses of cocaine ANOVA showed the main effects for MDMA dose (F(2,54)=5.43, p<;0.01) and cocaine dose (F(2,54)=3.29, p<0.05) reached an acceptable level for statistical significance. Further examination of the data confirmed that these main effect differences were byproducts of a significant interaction between MDMA and cocaine doses (F(4,54)=7.51, p<0.01). Subsequent comparisons of individual group means showed cocaine administered singularly (at a 0 MDMA dose) produced a standard dose-effect curve; significant differences from saline were evident at both 2.5 mg/kg and 5 mg/kg; p<0.05 (see Figure 1). At a 5 mg/kg dose of MDMA, increasing doses of cocaine resulted in increasing antagonism (declines) of CPP. Interestingly, at the highest dose of MDMA (10 mg/kg) the pattern of antagonism was reversed, i.e., CPP increased as cocaine dose increased. These data suggest a complex pattern wherein cocaine has antagonistic effects at lower doses of MDMA, but this pattern is reversed at higher doses of MDMA.
Figure 1. Cocaine and MDMA effects.
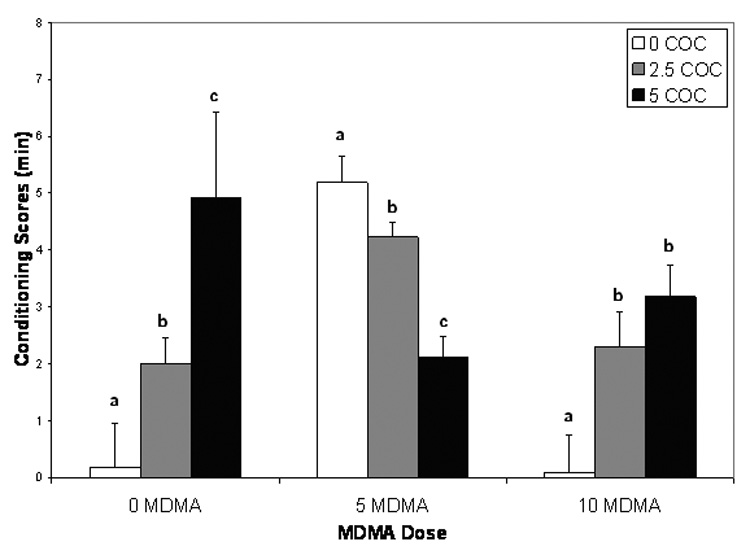
Mean (±SEM) CPP conditioning scores (measured in min) for animals exposed to 0 mg/kg, 2.5 mg/kg, or 5 mg/kg cocaine (COC) across doses of MDMA (0 mg/kg, 5 mg/kg, 10 mg/kg). Groups not possessing a common letter were significantly different (p<0.05).
At a 0 dose of cocaine MDMA reward properties increased at the medium dose (5 mg/kg MDMA) and diminished at the highest dose (10 mg/kg MDMA); Figure 1. Further, it was revealed that although there was a reversal of the reward-decreasing properties of 10 mg/kg MDMA with the addition of cocaine, at the 5 mg/kg dose of cocaine it is clear that MDMA antagonized cocaine CPP relative to the case where cocaine was presented in isolation (at a 0 dose of MDMA).
DISCUSSION
The results of this investigation revealed that when cocaine was presented alone in a CPP paradigm, preference for the drug context increased linearly in a dose-dependent fashion. In contrast, CPP performance for MDMA presented alone was defined by a pattern where place preference significantly increased at a 5 mg/kg dose, but diminished to control levels at a dose of 10 mg/kg MDMA. With respect to combined drug treatments, at a moderate dose of MDMA (5 mg/kg), increases in cocaine dose resulted in a systematic suppression of CPP (Figure 1). However, at an MDMA dose of 10 mg/kg the addition of cocaine reversed the pattern of antagonism and potentiated CPP relative to the case where 10 mg/kg MDMA was presented alone. Further, antagonistic profiles were evident when examining the effects of MDMA on cocaine reward, i.e., at a cocaine dose of 5 mg/kg higher doses of MDMA attenuated CPP relative to the case where the 5 mg/kg cocaine dose was administered singularly.
The finding of a positive linear relation between cocaine dose and cocaine CPP is not surprising given the literature is replete with reliable examples that document the robustness of this effect (see Bardo [1998] for a review). The finding of a curvilinear relation between MDMA dose and CPP performance is both novel and instructive. Previous single-dose CPP investigations of MDMA/cocaine interactions have employed designs aimed at assessing the effects of prior MDMA exposure on cocaine CPP. Achat-Mendes et al. (2003) observed that a single dose of MDMA produced longlasting effects on cocaine-induced reward, but in a similar paradigm Cole et al. (2003) failed to find evidence of any effect of prior MDMA exposure on cocaine CPP. Of course, the limitation of such single dose pretreatment experiments is they do not permit characterization of the full dose-effect function for combined MDMA and cocaine CPP. The importance of this issue is evident from the present study that acutely interacted multiple doses of MDMA and cocaine, revealing noticeably different patterns for MDMA and cocaine when the drugs are administered singularly or in combination in separate groups of animals.
With respect to the antagonistic action between MDMA and cocaine when the drugs are presented concomitantly, a careful inspection of the available, albeit complicated, neurochemical literature is warranted. One line of research reliably demonstrates that MDMA administration enhances serotonergic function (cf. Müller et al., 2007). In this regard, several studies have shown that MDMA increases 5-HT release (e.g., McKenna and Peroutka, 1990; Phillips, 1984; Rattray, 1991; Rothman and Baumann, 2003; Undie and Friedman, 1990). Of particular interest are the findings that show the effects of MDMA on 5-HT and norepinephrine potency (release and transporter inhibition) are selective relative to the impact of the drug on DA kinetics (e.g., Rothman and Baumann, 2003).
A second line of research suggests a much different interaction between MDMA and 5-HT function. Obradovic et al. (1998) examined the effects of repeated twice-daily injections of 20 mg/kg MDMA on inhibitory modulation of glutamate-evoked firing of NAcc cells. In this experiment it was observed that MDMA attenuated inhibitory responses to 5-HT that otherwise occurred. And, numerous, more recent animal investigations have reported that repeated exposure to MDMA at higher doses diminishes 5-HT-mediated changes in a variety of phenomenological behaviors such as exploratory-oriented locomotion (Harkin et al., 2001) and social interaction (Clemens et al., 2004). Consistent with these behavioral effects are findings from studies showing MDMA-based depletion of 5-HT stores across several brain regions (cf. Clemens et al., 2004). The pattern, then, in contrast to the aforementioned MDMA-induced facilitation of 5-HT function, is one of MDMA/5-HT antagonism.
On closer inspection, the data in this area appear more congruent, and indeed actually argue a point of general agreement. Specifically, the bulk of studies that have shown reduced 5-HT activity in MDMA-exposed animals have involved repeated exposure or high levels of the drug, while the enhanced serotonergic consequences of MDMA administration typically are associated with brief exposure to more moderate amounts of MDMA. Indeed, this antagonist/agonist interactive pattern has been revealed clearly in a comprehensive review of the literature by White et al. (1996). In this paper, it is reported that experiments that involve single or limited exposure to MDMA indicate that MDMA increases the levels of 5-HT in the NAcc and several other brain regions that are important for psychostimulant reward. Conversely, it is established by White et al. that repeated or high-dose administration of MDMA results in reduced availability of the neurotransmitter in a subpopulation of neurons that project to the prefrontal cortex, including those that originate in the NAcc. Most likely, this latter effect is due to the well-documented MDMA-induced neurotoxic damage to fine-diameter 5-HT axon terminals (e.g., Armstrong and Noguchi, 2004; Battaglia et al., 1988; Johansen et al., 1991).
The significance of these findings for the present investigation derives from the demonstrated interactions between 5-HT activity and DA activity, each of which is integral to determining stimulant reward efficacy. On the whole, the converging literature in this area is suggestive of an inverse relation between 5-HT and DA (cf. Schechter, 1991). To this end, depletion of 5-HT by medial forebrain bundle lesions with 5,7-dihydroxytryptamine (5,7-DHT) increases the reinforcing efficacy of cocaine, ostensibly by elevating mesocorticolimbic DA levels (Loh and Roberts, 1990). Elsewhere, it has been shown that manipulations that increase 5-HT function decrease cocaine self-administration (Carroll et al., 1990; Glatz et al., 2002; Howell and Byrd, 1995), and there is a negative correlation between psychostimulant potency and binding affinity at the 5-HT transporter (SERT) site (Ritz and Kuhar, 1989). Thus, a compelling case can be made for MDMA/cocaine antagonism, at least at low to moderate levels of the drugs.
With respect to interpreting the present findings, at least in part a case can be made that such perturbations in the pharmacodynamics of 5-HT and/or DA may contribute to some of the effects observed here. Although information on the neurochemical profile of cocaine-induced antagonism of MDMA reward is lacking, it is possible to address other issues relating to our findings such as the biphasic pattern produced by MDMA presented in isolation. Given the inverse relation between 5-HT and DA, an argument can be made that the rewarding effects of MDMA at a low dose (5 mg/kg) are counteracted by a high MDMA dose (10 mg/kg) because the higher dose occasions elevated 5-HT, and consequently lower DA levels and diminished reward potency. Of course, as noted an assertion can be made that high concentrations of MDMA are simply aversive, which is a position compatible with the notion that increasing levels of 5-HT are anhedonic (Hall et al, 2004; Uhl et al., 2002). Whatever the rationale invoked to account for the reward decreasing properties of high levels of MDMA, it is obvious that such negative effects algebraically offset the high conditioned reward value of a 5 mg/kg dose of cocaine (Figure 1).
Further, it is apparent from this investigation that the decreased reward properties of high levels of MDMA are reversible by cocaine co-administration. That is, the addition of either 2.5 and 5 mg/kg cocaine systematically increased 10 mg/kg MDMA CPP relative to a vehicle-only group where CPP values were reduced to control levels. This may derive from the fact that cocaine, a SERT reuptake inhibitor, decreases MDMA-mediated 5-HT release more than MDMA-mediated DA release, thereby increasing overall reward. Of course, larger MDMA doses may result in insurmountable changes to the point that even extremely high doses of cocaine combined with MDMA would engender no rewarding effects. Counterintuitively, it would be predicted that more frequent use or even greater amounts of MDMA would potentiate cocaine effects because of ultimate, reduced 5-HT levels and consequent elevation of DA (Achat-Mendes et al. 2003; Horan et al., 2000).
Acknowledgements
We wish to express our gratitude to Sammy Lee, Clint Tippett, Avanthi Tayi, and Allison Davidson for their advice and assistance with respect to the conduct of the research. Also, thanks to Dr. Anthony Riley and Greg Busse of the Department of Psychology at American University for their assistance in the development of experimental procedures.
This research was supported by United States Public Health Grants DA 13188 and MH 65728
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aberg M, Wade D, Wall E, Izenwasser S. Effect of MDMA (ecstasy) on activity and cocaine conditioned place preference in adult and adolescent rats. Neurotox Teratol. 2007;29:37–46. doi: 10.1016/j.ntt.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achat-Mendes C, Anderson KL, Itzhak Y. Methylphenidate and MDMA adolescent exposure in mice: long-lasting consequences on cocaine-induced reward and psychomotor stimulation in adulthood. Neuropsychopharmacology. 2003;45:106–115. doi: 10.1016/s0028-3908(03)00135-7. [DOI] [PubMed] [Google Scholar]
- Armstrong BD, Noguchi KK. The neurotoxic effects of 3,4-methylenedioxymethamphetamine (MDMA) and methamphetamine on serotonin, dopamine, and GABA-ergic terminals: An in-vitro autoradiographic study in rats. Neurotoxicology. 2004;25:905–914. doi: 10.1016/j.neuro.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Bardo MT. Neuropharmacological mechanisms of drug reward: beyond dopamine in the nucleus accumbens. Crit Rev Neurobiol. 1998;12:37–67. doi: 10.1615/critrevneurobiol.v12.i1-2.30. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology. 2003;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Rowlett JK, Harris MJ. Conditioned place preference using opiate and stimulant drugs: a meta-analysis. Neurosci Biobehav Rev. 1995;19:39–51. doi: 10.1016/0149-7634(94)00021-r. [DOI] [PubMed] [Google Scholar]
- Battaglia G, Yeh SY, Desouza EB. MDMA-induced Neurotoxicity-parameters of degeneration and recovery of brain-serotonin neurons. Pharmacol Biochem Behav. 1998;29:269–274. doi: 10.1016/0091-3057(88)90155-4. [DOI] [PubMed] [Google Scholar]
- Bilsky EJ, Hubbell CL, Delconte JD, Reid LD. MDMA produces a conditioned place preference and elicits ejaculation in male rats – a modulatory role for the endogenous opioids. Pharmacol Biochem Behav. 1991;40:443–447. doi: 10.1016/0091-3057(91)90577-o. [DOI] [PubMed] [Google Scholar]
- Bilsky EJ, Hui YZ, Hubbell CL, Reid LD. Methylenedioxymethamphetamines capacity to establish place preferences and modify intake of an alcoholic beverage. Pharmacol Biochem Behav. 1990;37:633–638. doi: 10.1016/0091-3057(90)90538-s. [DOI] [PubMed] [Google Scholar]
- Block RI, Erwin WJ, Ghoneim MM. Chronic drug use and cognitive impairments. Pharmacol Biochem Behav. 2002;73:491–504. doi: 10.1016/s0091-3057(02)00816-x. [DOI] [PubMed] [Google Scholar]
- Calcagnetti DJ, Schechter MD. Extinction of cocaine-induced place approach in rats – a validation of the biased conditioning procedure. Brain Res Bull. 1993;30:695–700. doi: 10.1016/0361-9230(93)90102-h. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lac ST, Asencio M, Kragh R. Fluoxetine reduces intravenous cocaine self-administration in rats. Pharmacol Biochem Behav. 1990;35:237–244. doi: 10.1016/0091-3057(90)90232-7. [DOI] [PubMed] [Google Scholar]
- Cervo L, Rozio M, Ekalle-Soppo CB, Carovali F, Santangelo E, Samanin R. Stimulation of serotonin (1B) receptors induces conditioned place aversion and facilitates cocaine place conditioning in rats. Psychopharmacology. 2002;163:142–150. doi: 10.1007/s00213-002-1145-8. [DOI] [PubMed] [Google Scholar]
- Clemens KJ, van Niewenhuyzen PS, Li KM, Cornish JL, Hunt GE, McGregor IS. MDMA (“ecstasy”), methamphetamine and their combination: long-term changes in social interaction and neurochemistry in the rat. Psychopharmacology. 2004;173:318–325. doi: 10.1007/s00213-004-1786-x. [DOI] [PubMed] [Google Scholar]
- Colado MI, O’Shea EO, Green AR. Acute and long-term effects of MDMA on cerebral dopamine biochemistry and function. Psychopharmacology. 2004;173:249–263. doi: 10.1007/s00213-004-1788-8. [DOI] [PubMed] [Google Scholar]
- Cole JC, Sumnall HR, O’Shea E, Marsden CA. Effects of MDMA exposure on the conditioned place preference produced by other drugs of abuse. Psychopharmacology. 2003;166:383–390. doi: 10.1007/s00213-002-1374-x. [DOI] [PubMed] [Google Scholar]
- Forsyth AJ. Places and patterns of drug use in the Scottish dance scene. Addiction. 1996;91:511–521. doi: 10.1046/j.1360-0443.1996.9145116.x. [DOI] [PubMed] [Google Scholar]
- Glatz AC, Ehrlich M, Bae RS, Clarke MJ, Quinlan PA, Brown EC, Rada P, Hoebel BG. Inhibition of cocaine self-administration by fluoxetine or D-fenfluramine combined with phentermine. Pharmacol Biochem Behav. 2002;71:197–204. doi: 10.1016/s0091-3057(01)00657-8. [DOI] [PubMed] [Google Scholar]
- Green AR, Cross AJ, Goodwin GM. Review of the pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA or “Ecstasy”) Psychopharmacology. 1995;119:247–260. doi: 10.1007/BF02246288. [DOI] [PubMed] [Google Scholar]
- Gross SR, Barrett SP, Shestowsky JS, Pihl RO. Ecstasy and drug consumption patterns: A Canadian rave population study. Canadian J Psychia. 2002;47:546–551. doi: 10.1177/070674370204700606. [DOI] [PubMed] [Google Scholar]
- Hall FS, Sora I, Drgonova J, Li XF, Goeb M, Uhl GR. Molecular mechanisms underlying the rewarding effects of cocaine. Ann NY Acad Sci. 2004;1025:47–56. doi: 10.1196/annals.1316.006. [DOI] [PubMed] [Google Scholar]
- Harkin A, Connor TJ, Mulrooney J, Kelly JP, Leonard BE. Prior exposure to methylenedioxyamphetamine (MDMA) induces serotonergic loss and changes in spontaneous exploratory and amphetamine-induced behaviors in rats. Life Sci. 2001;68:1367–1382. doi: 10.1016/s0024-3205(00)01039-0. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Thompson AC, Shippenberg TS. Role of extracellular dopamine in the initiation and long term expression of behavioral sensitization to cocaine. J Pharmacol Exp Ther. 1996;278:490–502. [PubMed] [Google Scholar]
- Higgins GA, Fletcher PJ. Serotonin and drug reward: focus on 5-HT2C receptors. Eur J Pharmacol. 2003;480:151–162. doi: 10.1016/j.ejphar.2003.08.102. [DOI] [PubMed] [Google Scholar]
- Horan B, Gardner EL, Ashby CR. Enhancement of conditioned place preference response to cocaine in rats following subchronic administration of 3,4-methylenedioxymethamphetamine (MDMA) Synapse. 2000;35:160–162. doi: 10.1002/(SICI)1098-2396(200002)35:2<160::AID-SYN9>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Howell LL, Byrd LD. Serotonergic modulation of the behavioral effects of cocaine in the squirrel monkey. J Pharmacol Exp Ther. 1995;275:1551–1559. [PubMed] [Google Scholar]
- Johansen PA, Hu XT, White FJ. Relationship between D1 dopamine receptors, adenylate cyclase, and the electrophysiological responses of rat nucleus accumbens neurons. J Neural Transmiss. 1991;86:97–113. doi: 10.1007/BF01250571. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Time course of the extracellular dopamine and behavioral sensitization to cocaine. I. Dopamine axon terminals. J Neurosci. 1993;13:266–275. doi: 10.1523/JNEUROSCI.13-01-00266.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp CM, Printseva B, Cottam N, Kornetsky C. Effects of cue exposure on brain glucose utilization 8 days after repeated cocaine administration. Brain Res. 2002;950:119–126. doi: 10.1016/s0006-8993(02)03011-1. [DOI] [PubMed] [Google Scholar]
- Loh EA, Roberts DCS. Break-points on a progressive ratio schedule reinforced by intravenous cocaine increase following depletion of forebrain serotonin. Psychopharmacology. 1990;101:262–266. doi: 10.1007/BF02244137. [DOI] [PubMed] [Google Scholar]
- McKenna DJ, Peroutka SJ. Neurochemistry and neurotoxicity of 3,4, methylenedioxymethamphetamine (MDMA, “ecstasy”) J Neurochem. 1990;54:14–22. doi: 10.1111/j.1471-4159.1990.tb13277.x. [DOI] [PubMed] [Google Scholar]
- Miller DK, Nation JR. Chronic cadmium exposure attenuates the conditioned reinforcing properties of morphine and fentanyl. Brain Res. 1997;776:162–169. doi: 10.1016/s0006-8993(97)01013-5. [DOI] [PubMed] [Google Scholar]
- Miller DK, Nation JR, Bratton GR. Perinatal exposure to lead attenuates the conditioned reinforcing properties of cocaine in male rats. Pharmacol Biochem Behav. 2000;67:111–119. doi: 10.1016/s0091-3057(00)00303-8. [DOI] [PubMed] [Google Scholar]
- Miller DK, Palme KM, Najvar SA, Caudill SD, Nation JR. Chronic cadmium exposure attenuates conditioned place preference produced by cocaine and other drugs. Pharmacol Biochem Behav. 1999;64:15–20. doi: 10.1016/s0091-3057(99)00095-7. [DOI] [PubMed] [Google Scholar]
- Müller CP, Carey RJ, Huston JP, De Sousza Silva MA. Serotonin and psychostimulant addiction: focus on 5-HT1A receptors. Prog Neurobiol. 2007;81:133–178. doi: 10.1016/j.pneurobio.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Obradovic T, Imel KM, White SR. Repeated exposure to methylenedioxymethamphetamine (MDMA) alters nucleus accumbens neuronal responses to dopamine and serotonin. Brain Res. 1998;785:1–9. doi: 10.1016/s0006-8993(97)01337-1. [DOI] [PubMed] [Google Scholar]
- Parrott AC, Sisk E, Turner JJ. Psychobiological problems in heavy “ecstasy” (MDMA) polydrug users. Drug Alc Depend. 2000;60:105–110. doi: 10.1016/s0376-8716(99)00146-5. [DOI] [PubMed] [Google Scholar]
- Peroutka SJ. Incidence of recreational use of 3,4-methylenedioxymethamphetamine (MDMA, “Ecstasy”) on an undergraduate campus. New Eng J Med. 1987;317:1542–1543. doi: 10.1056/NEJM198712103172419. [DOI] [PubMed] [Google Scholar]
- Pettit HO, Pan HT, Parsons LH, Justice JB. Extracellular concentrations of cocaine and dopamine are enhanced by chronic administration. J Neurochem. 1990;55:798–804. doi: 10.1111/j.1471-4159.1990.tb04562.x. [DOI] [PubMed] [Google Scholar]
- Phillips AG. Brain reward circuitry: a case for separate systems. Brain Res Bull. 1984;12:195–201. doi: 10.1016/0361-9230(84)90189-8. [DOI] [PubMed] [Google Scholar]
- Rattray M. Ecstasy: towards an understanding of the biochemical basis of the actions of MDMA. Essays Biochem. 1991;26:77–87. [PubMed] [Google Scholar]
- Riley AL. Use of drug discrimination learning in behavioral toxicology: classification and characterization of toxins. In: Chang LW, Slikker W Jr, editors. Neurotoxicology: approaches and methods. San Diego, CA: Academic Press; 1995. pp. 309–321. [Google Scholar]
- Ritz MC, Kuhar MJ. Relationship between self-administration of amphetamine and monoamine receptors in brain: comparison with cocaine. J Pharmacol Exp Ther. 1989;248:1010–1017. [PubMed] [Google Scholar]
- Rothman RB, Baumann MH. Monoamine transporters and psychostimulant drugs. Eur J Pharmacol. 2003;479:23–40. doi: 10.1016/j.ejphar.2003.08.054. [DOI] [PubMed] [Google Scholar]
- Schechter MD. Effect of MDMA neurotoxicity upon its conditioned place preference and discrimination. Pharmacol Biochem Behav. 1991;38:539–544. doi: 10.1016/0091-3057(91)90010-y. [DOI] [PubMed] [Google Scholar]
- Schenk S, Gittings D, Johnstone M, Daniela E. Development, maintenance and temporal pattern of self-administration maintained by ecstasy (MDMA) in rats. Psychopharmacology. 2003;169:21–27. doi: 10.1007/s00213-003-1407-0. [DOI] [PubMed] [Google Scholar]
- Smit F, Monshouwer K, Verdurmen J. Polydrug use among secondary school students: combinations, prevalences and risk profiles. Drugs-Education Prevent Pol. 2002;9:355–365. [Google Scholar]
- Smith FL, Yu DS, Smith DG, Leccese AP, Lyness WY. Dietary tryptophan supplements attenuate amphetamine self-administration in the rat. Pharmacol Biochem Behav. 1986;25:849–855. doi: 10.1016/0091-3057(86)90397-7. [DOI] [PubMed] [Google Scholar]
- Smith KR, Nation JR. Developmental exposure to cadmium alters responsiveness to cocaine in the rat. Drug Alc Depend. 2003;72:1–11. doi: 10.1016/s0376-8716(03)00170-4. [DOI] [PubMed] [Google Scholar]
- Uhl GR, Hall FS, Sora I. Cocaine, reward, movement and monoamine transporters. Mol Psychia. 2002;7:21–26. doi: 10.1038/sj.mp.4000964. [DOI] [PubMed] [Google Scholar]
- Undie AS, Freidman E. Stimulation of a dopamine D1 receptor enhances inositol phosphates formation in rat brain. J Pharmacol Exp Ther. 1990;253:987–992. [PubMed] [Google Scholar]
- Wee KG, Anderson MH, Bauman MH, Rothman RB, Blough BE, Woolverton WL. Relationship between the serotonergic activity and reinforcing effects of a series of amphetamine analogs. J Pharmacol Exp Ther. 2005;313:848–854. doi: 10.1124/jpet.104.080101. [DOI] [PubMed] [Google Scholar]
- White SR, Duffy P, Kalivas PW. Methylenedioxymethamphetamine depresses glutamate-evoked neuronal firing and increases extracellular levels of dopamine and serotonin in the nucleus accumbens in vivo. Neuroscience. 1994;62:41–50. doi: 10.1016/0306-4522(94)90313-1. [DOI] [PubMed] [Google Scholar]


