Abstract
Context
Cocaine dependence, which affects 2.5 million Americans annually, has no FDA approved pharmacotherapy.
Objective
To evaluate the immunogenicity, safety, and efficacy of a novel cocaine vaccine to treat cocaine dependence.
Design
24 week Phase IIb randomized double-blind placebo-controlled trial with efficacy assessed during weeks 8 to 20 and follow-up to week 24.
Setting
Cocaine and opioid dependent persons recruited from 2003–2005 from greater New Haven, CT.
Participants
115 methadone maintained subjects (67% male, 87% Caucasian, aged 18–46) were randomized to vaccine or placebo and 82% completed the trial. Most smoked crack cocaine along with using marijuana (18%), alcohol (10%), and non-prescription opioids (44%).
Intervention
Over 12 weeks 109/115 subjects received five vaccinations of placebo or succinylnorcocaine linked to cholera B protein.
Main Outcome Measure
Semi-quantitative urinary cocaine metabolite levels measured thrice weekly with positive cutoff of 300 ng/ml.
Results
The 38% of vaccinated subjects who attained serum IgG anti-cocaine levels ≥ 43 µg/mL (high IgG) had significantly more cocaine-free urines than those with < 43 µg/mL (low IgG) and the placebo subjects during weeks 9 to 16 (45% vs 35%). The proportion of subjects having a 50% reduction in cocaine use was significantly greater in the high IgG than low IgG subjects (0.53 vs. 0.23) (P<0.04). The most common side effects were injection site induration and tenderness. There were no treatment related serious adverse events, withdrawals, or deaths.
Conclusions
Attaining high (≥ 43 µg/mL) IgG anti-cocaine antibody levels was associated with significantly reduced cocaine use, but only 38% of the vaccinated attained these IgG levels and they had only 2 months of adequate cocaine blockade. Thus, we need improved vaccines and boosters.
Introduction
Cocaine dependence is common, involving one of every three drug related emergency department visits, and has substantial social and economic impacts on those afflicted 1, 2. In 2007 the United States had 2.5 million cocaine dependent people, and only 809,000 of them were treated 3.
The FDA has not approved any pharmacotherapies for cocaine abuse, and behavioral therapies have had a wide range of efficacies with some promise for contingency management 4–10. Experimental animal studies, however, have suggested that high levels of anti-cocaine antibodies can sequester circulating cocaine 11–14 and facilitate inactivation of cocaine by naturally occurring plasma cholinesterases before the drug enters the brain 15. In both animals and humans, reducing cocaine’s entry into the brain by binding antibody reduces cocaine induced euphoria, without causing any direct psychoactive effects or drug-drug interactions associated with other pharmacotherapies 16–18
We tested a cocaine vaccine made by covalently linking succinylnorcocaine (SNC) to cholera B protein (rCTB), adsorbed onto aluminum hydroxide adjuvant. 19–21 The immunogenic carrier, rCTB, has a well established safety record worldwide when used to immunize against cholera 19, 22. In a randomized, double-blind placebo controlled trial involving 34 abstinent cocaine abusers engaged in out-patient treatment, we demonstrated that this vaccine was well tolerated and induced cocaine-specific IgG antibodies in a time and dose dependent manner 23. No serious adverse effects occurred during 12 months follow-up. We also showed a continued safety and immunogenicity profile in a second open-label dose escalation study involving both cocaine abstinent and active users 24. Subjective responses of the vaccinated subjects suggested that the vaccine exerted its expected reduction in euphoria during the time their antibody levels peaked, that is between weeks 12 and 16 after the first inoculation.23, 24
To influence drug seeking behavior, the concentration of anti-cocaine antibody in the blood must attain a target level. Early rat studies with cocaine-CTB vaccine showed that 0.7 mg/ml of high affinity IgG was sufficient to bind 8.7µM of cocaine 11. Since cocaine users can experience pleasure at peak plasma cocaine concentrations as low as approximately 0.5 µM, 25 we hypothesized that the antibody would need to bind and capture this amount of cocaine to slow delivery of typically abused amounts into the brain. Considering the two binding sites on each antibody, we calculated needing 0.28 µM of moderate affinity anti-cocaine antibody in the blood, which equals our target of 43 ug/ml of specific IgG for sub-analyses of efficacy 11, 12, 26.
We also hypothesized distinct patterns of cocaine usage based on subjects’ antibody levels. We have previously shown that 25–30% of vaccinated subjects produce relatively low antibody levels.23, 24 Furthermore, we knew that IgG antibody levels would reach a maximum between weeks 12 and 16, after which IgG antibody levels would begin to fall. Thus, we postulated that starting during week 9 immunized volunteers who made more than 43 ug/ml of anti-cocaine antibodies would use less cocaine than those immunized with the placebo or those who made less than 43 ug/ml following this series of vaccinations.
METHODS
SITE AND POPULATION
Participants meeting DSM IV criteria 27 for cocaine and opioid dependence were enrolled in an out-patient methadone maintenance treatment program in West Haven, Connecticut. We studied methadone maintained subjects because retention in methadone maintenance programs is substantially better than in primary cocaine treatment programs 28 and we needed to retain these volunteers for 12 weeks to complete the vaccination series. We also offered subjects $15 per week to enhance retention. This study was conducted in accordance with Good Clinical Practices and was approved by the institutional review boards of the VA Connecticut Healthcare System and Yale University School of Medicine. All subjects spoke and understood English and gave written informed consent.
PARTICIPANTS
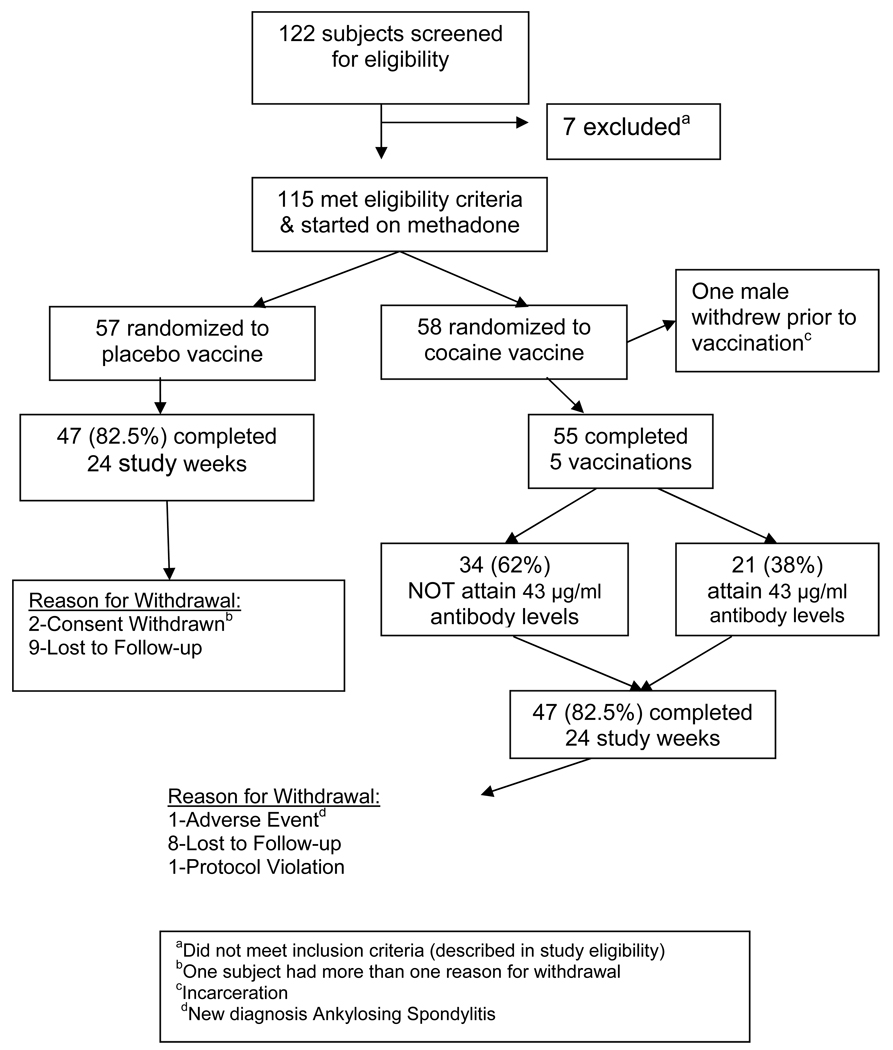
Between October 2003 and April 2005 115 of 122 screened subjects were enrolled and randomized. Figure 1 summarizes subject recruitment and retention. Subjects were excluded for major medical or psychiatric illness, ongoing infection or fever, use of any prescribed psychotropic medications, receipt of blood products within three months of screening, or a history of other vaccinations or corticosteroid use within thirty days. None were transferred from methadone maintenance, but 30 patients had previous methadone maintenance treatment. Eligibility included men or women aged 18 to 55 who met DSM-IV criteria for cocaine and opioid dependence, had positive urine cocaine, and did not have a clinically unstable chronic disease. Enrolled women had to be non-child bearing or willing to use birth control. Race was self-reported at screening to determine whether sufficient numbers would allow ethnic sub-analyses. Complete medical and psychiatric examinations were performed; additional assessments included electrocardiogram, urine toxicology for illicit substances, and blood work including HIV testing. We retained 94 of 115 (82%) subjects during the 24 weeks with equivalent retention in placebo and vaccinated groups (Figure 1).
Figure 1.
Flow chart of study participants
INTERVENTIONS
VACCINATION
The vaccine was made by linking succinylnorcocaine covalently to cholera B (SNC-rCTB). This conjugate was then adsorbed onto aluminum hydroxide adjuvant. The placebo contained saline and aluminum hydroxide. Subjects were randomized to receive either five vaccinations of 360 micrograms of active (SNC-rCTB) or placebo as 0.5 ml intramuscular deltoid injections at weeks 0, 2, 4, 8 and 12. Efficacy assessment began at week 8, when most vaccine responders should have significant IgG anti-cocaine levels 23, 24.
METHADONE MAINTENANCE
Subjects were maintained according to the manufacturer’s and Connecticut’s standard methadone induction and maintenance procedures 29 (Roxane Laboratories; PDR, 1998). Methadone maintenance therapy was initiated 2 weeks prior to the first vaccination and doses stabilized by week 8 of the study at a mean stabilization dose of 83 (SD 16) mg daily. At week 21, subjects were offered either a schedule detoxification or transfer to another methadone treatment program. If detoxified, subjects were tapered on their methadone dose over a six-week (weeks 21–26) period. If transferred, they remained on methadone through week 24 of the study and then transferred. Fifteen of the 114 patients chose the six week detoxification.
COUNSELING
All subjects participated in individual, weekly 30–45 minute cognitive behavioural relapse prevention therapy (CBT) sessions conducted by trained substance abuse counsellors30. During these counselling sessions urine toxicology results were reviewed with the subjects.
SAFETY MONITORING
Medical staff evaluated the vaccination site for erythema, induration, and/or tenderness at one, two, and 48 hours after injection. Subjects were monitored daily for both general health and subjective adverse events. Safety labs that included hematology and clinical chemistries were drawn at baseline and at two week intervals through week twelve and at 16 and 24 weeks. Serious and Non-serious adverse events (AE) possibly related to the vaccine were defined as any new illness or exacerbation of a pre-existing condition occurring after the start of the vaccination series. Medications taken or medical interventions performed after vaccination were evaluated as possible AEs. Serious and non-serious AEs and reasons for study termination were tabulated by treatment group.
OBJECTIVES
The primary objective was to evaluate the efficacy of the vaccine versus placebo in reducing cocaine use. Secondary objectives included confirming vaccine immunogenicity through IgG anti-cocaine antibody levels and evaluating the safety and tolerability of vaccination through a 20 week study and at 24 week follow-up. We also analyzed the relationship between IgG anti-cocaine antibody levels and the utilization of cocaine, as determined by serial thrice-weekly urine samples for cocaine metabolites.
OUTCOME MEASURES
Efficacy Analysis
To compare the numbers of cocaine-free urines each week between vaccinated and placebo control subjects we used intent to treat analysis over two time intervals: one up to week 16 and a second through week 20. This first interval to week 16 corresponds to the time when antibody levels were expected to peak, while from week 16 to 20 antibody levels would then decline. We used a repeated measures assessment (random regression model) to estimate the effect of treatment on the frequency of cocaine-free urines in all subjects during the 16 and 20 week study periods. Only 4% of urine samples were missing due to treatment dropouts; an additional 5% of samples were not collected for missed visits or other reasons during the study.
Exploratory Efficacy Analysis
We compared the weekly rate of cocaine-free urines for placebo controls and two groups of actively vaccinated subjects, who were divided into high and low IgG groups using a cutoff of 43 µg/ml anti-cocaine IgG peak levels. We also compared the high and low IgG groups on the number who had at least half of their urines cocaine-free between weeks 8 to 20, We separately analyzed the 45 vaccinated subjects left after excluding three who dropped out before week 15 and seven who did not use cocaine during weeks 8–20, since anti-cocaine antibodies could not have affected their cocaine use 31, 32.
Quantitative Antibody Measurement
Serum anti-cocaine IgG was measured by an Enzyme-Linked ImmunoSorbent Assay (ELISA). The antigenic target was cocaine conjugated to bovine serum albumin (BSA). Human IgG bound to this antigen was detected with horseradish peroxidase conjugated second antibody and appropriate substrate 23. Nonspecific antibody binding to the carrier alone was subtracted, and each ELISA plate included wells with serially diluted polyclonal human IgG to provide an internal standard. This ELISA’s specificity and reproducibility was validated using serial dilutions of a humanized monoclonal antibody to cocaine, 2E2, from Dr. Andrew Norman at the University of Cincinnati 33
Urine Cocaine Metabolites
Urine was qualitatively tested for benzoylecgonine (BE) at the time of screening, at methadone initiation, and thrice weekly for the duration of the study. Urine BE values of at least 300 ng/ml were considered positive. A pharmacokinetic study in mice has shown that introduction of anti-cocaine antibodies into the circulation did not change BE excretion, despite 15% cross-reactivity 14
Furthermore, quantitative urine BE was available during weeks 8 to 20 allowing us to use the Preston criteria for assessing new uses of cocaine.34 Briefly, Preston criteria separate new uses of cocaine from carry-over of high BE levels by considering BE levels that are above the 300 ng/ml cut-off but are 50% or greater reductions from the urine toxicology taken 2–3 days earlier, as indicating “no new use of cocaine” since the last urine testing. Thus, dropping from 1000 ng/ml of BE on Monday to 400 ng/ml on Wednesday would indicate “no new use of cocaine” rather than another cocaine positive urine on Wednesday. We conducted appropriate sub-analyses of vaccine efficacy using this strategy, but we also noted that, when positive, urine BE levels were typically > 10,000 ng/ml. Since this value is at least 30-fold higher than the cutoff for a positive quantitative urine test, we were further reassured that we did not have false negative urine tests in the vaccinated group due to reduced BE urinary clearance from antibody binding. Studies in experimental animals have also shown that antibody binding of cocaine in vivo does not delay cocaine clearance, reducing the risk of late false positive tests14.
RANDOMIZATION
Subjects were randomized equally to each treatment and all subjects were analyzed. Randomization assignments were securely stored by the pharmacist, who was un-blinded to treatment arms, but research staff, investigators, and subjects were blinded until the database was unlocked in June 2006.
STATISTICAL ANALYSES
Subject Demographics and Adverse Events
Vaccine and placebo groups were compared using Student’s t-test or the non-parametric Mann Whitney U test, depending on data normality, and categorical differences were compared using chi-square or Fisher exact tests.
Vaccine efficacy
Vaccine efficacy was tested by Hierarchical Linear Modeling (HLM) to compare each subject’s fraction of weekly urine samples free of cocaine metabolites 35, 36. The HLM method for modeling repeated measures allows for unbalanced designs with missing data, intra-subject serial correlation, and unequal variance and covariance structures over time35, 36. These analyses were conducted using MIXOR, a program which can implement HLM analyses with ordinal outcome data. These HLM MIXOR analyses produce logit estimates (odds ratios) in a manner similar to logistic regression and statistical significance is given as a Z value, which is independent of degrees of freedom.
SAMPLE SIZE ESTIMATE
Based on previous cocaine pharmacotherapy studies, we postulated that cocaine use would decrease by about 25% in the placebo group and 50% in vaccinated subjects 37. Therefore, to attain a statistical power of 0.8 with an alpha of 0.05, it was anticipated that 60 subjects needed to enroll in each treatment arm. Because the current trial retained over 80% rather than 50% of subjects at 6 months, fewer subjects needed to enroll and 115 rather than 120 subjects were randomized.
RESULTS
Baseline Characteristics, Retention and Opioid Use
The vaccine and placebo subjects were comparable in age, gender and ethnicity, but most were white preventing ethnic sub-analysis. Most subjects (89%) considered smoked cocaine their second drug of choice after opioids including prescription opioids. We found no statistically significant baseline demographic or drug use differences between groups, and treatment retention showed no difference; 82% completed 24 weeks (Table 1 and Figure 1). The full sample of 114 patients showed a significant increase in opioid-free urines from 55% (SEM 0.05) during the first two weeks on methadone maintenance to 68% during week 8 onward on a mean stabilization methadone dose of 83 mg daily (F= 9.9; df = 2,108; P<0.002). The two treatment groups showed no difference in opioid-free urines or methadone dose.
Table 1.
Baseline Clinical Characteristics of Participantsa
| Characteristic | Vaccine | Placebo | P-value |
|---|---|---|---|
| Age (mean ± SD) | 35.6 ± 8.9 | 36.2 ± 9.3 | NS |
| Race [n(%)] | NS | ||
| Caucasian | 53(93) | 46(81) | |
| African-American | 2(3.5) | 8(14) | |
| Mixedb | 2(3.5) | 3(5) | |
| Sex [n(%)] | NS | ||
| Male | 35(61.4) | 41(71.9) | |
| Female | 22(38.6) | 16(28.1) | |
| Employed | 31(54.4) | 33(57.9) | NS |
| Family History of Substance Use | 19(33.3) | 25(43.9) | NS |
| Cocaine History | |||
| Age at first use (mean ± SD) | 22.1±7.8 | 20.9±6.1 | NS |
| Days used/week | |||
| Before vaccine | 3.3+2.9 | 2.9+2.8 | NS |
| First 2 wks after vaccine | 2.2+2.1 | 1.9+2.0 | NS |
| Dimes used per day | |||
| Before vaccine | 3.5+5.8 | 2.4+2.7 | NS |
| First 2 wks after vaccine | 2.3+2.1 | 1.7+1.4 | NS |
| Mean $ amount/day on cocaine | $105.00 | $77.00 | NS |
| Cocaine Cravingc (mean + SD) | 3.8(2.3) | 4.2(2.4) | |
| Route of Use (%)d | |||
| Intranasal | 35(61) | 36(63) | |
| Intravenous | 14(25) | 20(35) | |
| Smoke | 46(81) | 41(72) | |
| Opiate History | |||
| Age at first use (mean ± SD) | 23.1±8.1 | 24.5±12.2 | |
| Amount of Opiates per day | |||
| 1–4 Bags | 5(10.4) | 8(14.5) | |
| 5–9 Bags | 17(35.4) | 15(27.3) | |
| 10–14 Bags | 13(27.1) | 11(20) | |
| 15–19 Bags | 3(6.3) | 6(10.9) | |
| 20–24 Bags | 4(8.3) | 8(14.5) | |
| 25–30 Bags | 2(4.2) | 3(5.4) | |
| > 30 Bags | 1(2.1) | 1(1.8) | |
| Unknown | 3(6.3) | 3(5.4) | |
| Other Substance Used,e | |||
| Alcohol | 8 | 11 | |
| Non-med prescription use of opioids | 27 | 23 | |
| Cannabis | 15 | 20 | |
| Benzodiazapine | 2 | 2 |
N=114; Vaccine (n=57); Placebo (n=57)
Option:Caucasian, African-American, Hispanic, Mixed, other
Assessed by the CSSA (Cocaine Selective Severity Assessment) Maximum score=7
Not mutually exclusive categories
By urine toxicology testing from screening (30 days prior to entry) until study completion (14 weeks)
Cocaine Vaccine Specific IgG Levels
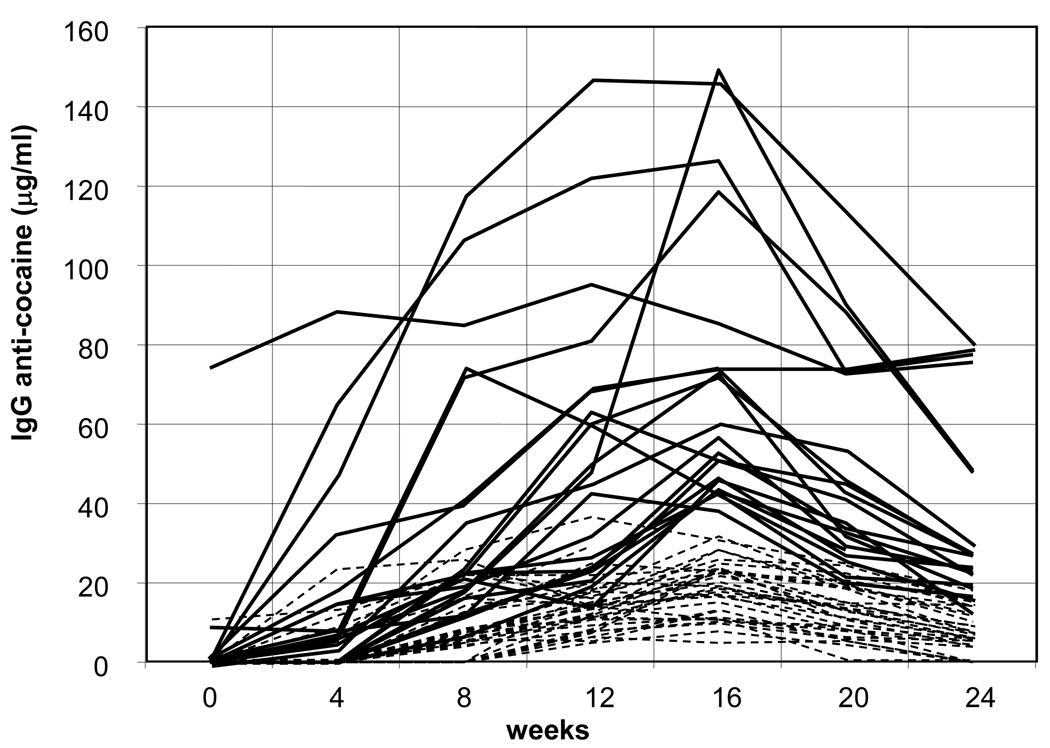
Of the 58 subjects randomized to receive active vaccine, 55 completed the series of five vaccinations and 98% (54/55) made detectable antibody levels. Figure 2 shows individual serum IgG anti-cocaine levels through week 24. Notably, early antibody responders made antibodies as early as week 2; however, many mounted antibody responses only after week 6. One subject had pre-existing high levels of IgG anti-cocaine antibody and maintained them throughout the study. Of those vaccinated, 21 (38%- high IgG group) attained antibody levels greater than 43 µg/ml, whereas, 34 (62%- low IgG group) had levels below this threshold, including one subject who made no antibodies. Only two subjects mounted antibody responses > 43 ug/ml before week 8, and all subjects’ IgG levels declined after week 16.
Figure 2. IgG responses to the Cocaine-CTB vaccine was highly variable.
Among the 55 immunized with the cocaine vaccine (at weeks 0, 2, 4, 8, and 12), 21 (38%) attained levels greater than 43 µg/ml as shown in solid lines. Of those, 3 three were highly responsive subjects, with one having >60 µg/ml of IgG anti-cocaine antibody even before immunization. Two made a vigorous response after the 2nd injection (week 4 samples). Eight subjects made over 43 µg/ml after 3 injections, and 8 required 4 or more injections of antigen to make a response that exceeded 43 µg/ml of anti-cocaine IgG. Antibody responses for the remaining 34 subjects are in dotted lines. The number of subjects at weeks 2, 4, 8, 12, 16, 20, 24 are respectively: 55, 55, 55, 55, 54, 51, 51.
Cocaine-Free Urines by Treatment Group
The frequency of cocaine-free urines did not differ between treatments in an intention to treat analysis at baseline (weeks 1 to 2) (33% vaccinated vs. 25% placebo) (χ2=0.3) or for the full 20 weeks. However, our HLM analyses of weekly cocaine-free urines for both vaccinated and placebo recipients from weeks 1 through 16 showed significantly more cocaine-free urines as the study progressed (placebo by time: Z=5.4, p<0.001; vaccine by time: Z=8.7, p<0.001). As the difference in Z values over time suggest, the frequency of cocaine-free urines rose faster in the vaccinated than placebo group (treatment by time interaction: Z=2.45, p<0.01). However, after we stopped immunizing these subjects and antibody levels began to fall off during the interval between weeks 16 and 24, HLM analyses showed no significant differences in the frequency of cocaine-free urines between the vaccinated and placebo groups.
Cocaine-Free Urines by IgG Response to Vaccine versus Placebo
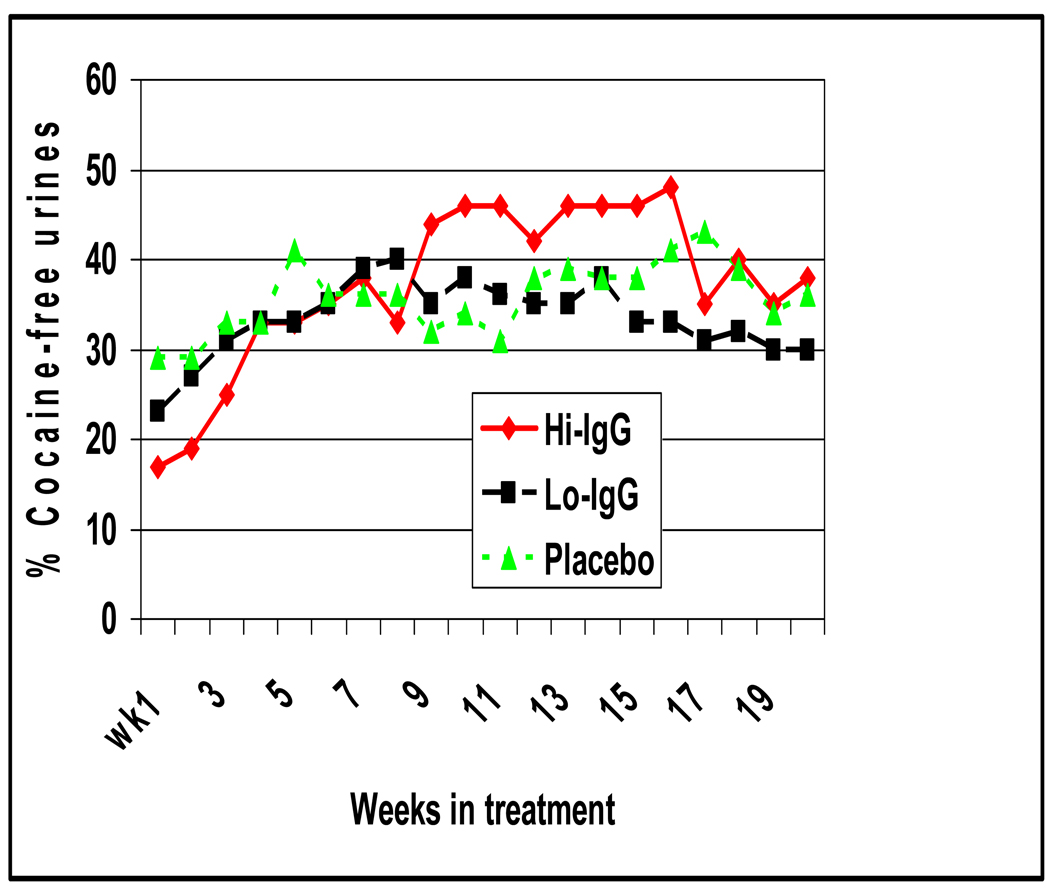
Figure 3 shows the mean rates of cocaine-free urines in the high IgG group (>43µg/ml) and both the placebo and low IgG groups (≤43µg/ml). Standard errors (not shown) at each time point were +/− 3%. Between weeks 9 through 16 cocaine-free urines were significantly greater in the High IgG than the Low IgG and Placebo groups (45% vs 35%; t=2.26; df=110; P<0.03). By week 9 most of the 21 high IgG subjects had antibody levels at or above 43 ug/ml and after week 16 these levels declined to levels found in the low IgG subjects.
Figure 3. Mean weekly cocaine-free urines by medication condition for weeks 1–20.
Green triangles line represents placebo, black squares line active cocaine vaccine recipients with IgG levels below 43 ug/ml (Lo IgG), and red diamonds line active cocaine vaccine recipients with IgG levels > 43 ug/ml (Hi IgG). The x axis is the number of weeks since the first vaccination and the y axis represents 100 times the weekly mean proportion of cocaine-free urines for the three treatment groups. Standard errors are not shown for clarity, but range from +/−5% at any time-point. The Hi IgG group had significantly more cocaine-free urines than the other two groups during weeks 9 to 16 (t=2.26; P<0.03) and a significantly greater increase in cocaine-free urines than the other two groups from weeks 1 to 16 on HLM analysis (Z= 4.8; P<0.01). The three groups did not differ in cocaine-free urines during weeks 17 to 20.
Using HLM analyses of weekly cocaine-free urines through week 16, the high IgG and placebo groups showed significantly more cocaine-free urines as the study progressed (placebo by time: Z=5.4, p<0.001; high IgG by time: Z=9.5, p<0.001), while the low IgG group showed no significant change over time. As the difference in Z values over time suggest, the frequency of cocaine-free urines increased faster in the high IgG group than the placebo and low IgG groups (treatment by time interaction: Z=4.8, p<0.01).
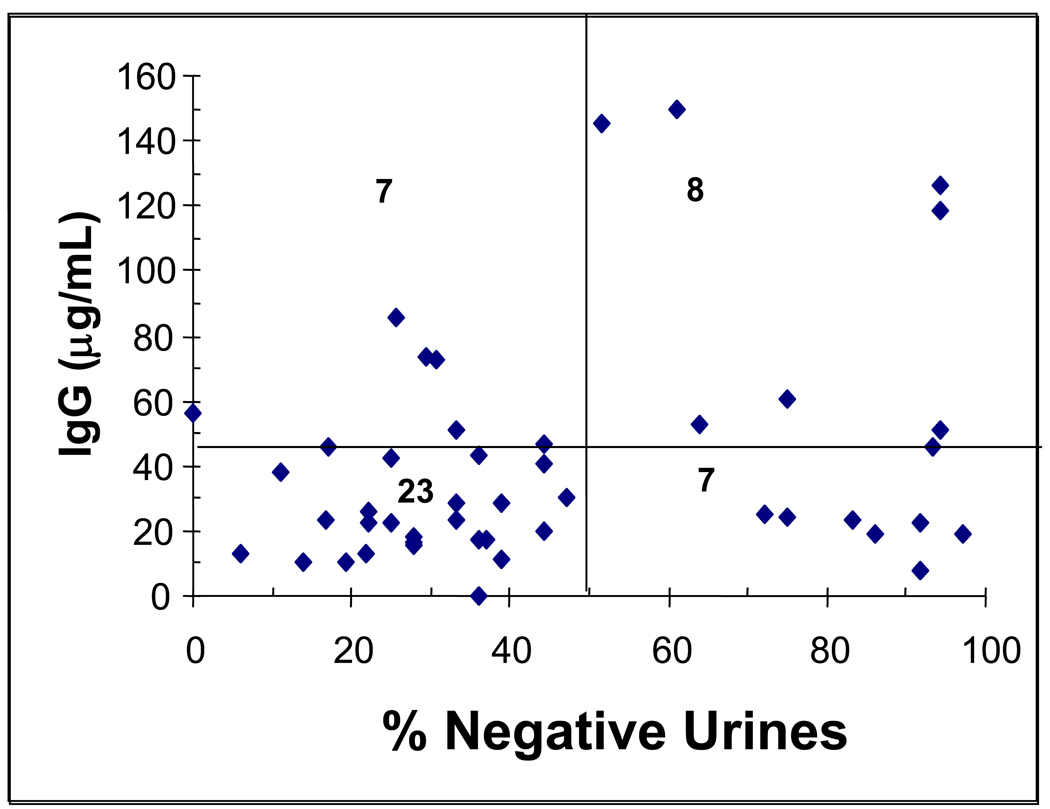
As a stronger test of IgG antibody levels and vaccine efficacy, we then examined the results using the 45 vaccinated subjects who remained after excluding three who dropped out before week 15 and seven who did not use cocaine during weeks 8–20 and therefore did not directly test the vaccine’s efficacy. As shown in Figure 4, the proportion of these subjects whose urinalyses showed no new episodes of cocaine use at least 50% of the time (based on the Preston criteria) was significantly greater in the high IgG as compared to the low IgG subjects (0.53 vs. 0.23) (Fishers test, P<0.04).
Figure 4. Scatter-plot of peak antibody response by % cocaine-negative urines.
Filled diamonds are individual vaccinated subject values for peak antibody response (typically at week 16) plotted against their percentage of cocaine-negative urines using the Preston criteria for new uses of cocaine during weeks 8 through 20. The graph is divided into quadrants by a horizontal line at the 43 ug/ml antibody and a vertical line at 50% cocaine free urines. The numbers in each quadrant represent the number of subjects in each quadrant. The proportion of subjects who had 50% or more cocaine free urines is significantly greater in those with anti-cocaine IgG antibody > 43 ug/ml than those with lower antibody levels (Fishers test, P<0.04).
Adverse Events (AE)
Treatment emergent or associated AEs and subject dropout related to treatment were not significantly different in vaccinated and placebo subjects (Table 2). Dropouts were related to incarceration and loss of housing due to cocaine use. All of the reported AEs were considered mild or moderate in intensity. The more frequent AEs recorded for vaccinated subjects as compared with placebo were: injection site induration (3% versus 0), site tenderness (10% versus 6%), feeling cold (12% versus 7%), hot flashes (19% versus 12%), hyperhydrosis (15% versus 10%) and nausea (14% versus 2%). Treatment-emergent severe AEs included: a toothache and tooth abscess in two placebo subjects, and a vaginal hemorrhage in a vaccinated subject. One vaccinated subject left due to an exacerbation of pre-existing, but initially undiagnosed, spondyloarthropathy.
Table 2.
Summary of Adverse Events (AEs)
| Placebo (N=57) |
Cocaine Vaccine (N=57) |
P-value* | |
|---|---|---|---|
| Number of Subjects with Treatment-Emergent AEs† | 55 (96.5%) | 54 (94.7%) | NS |
| Number of Subjects with Treatment-Associated AEs‡ | 6 (10.5%) | 11 (19.3%) | NS |
| Total Number of Reports of Treatment-Emergent AEs† | 417 | 491 | |
| Total Number of Reports of Treatment-Associated AEs‡ | 9 | 23 | |
| Number of Subjects Discontinued Due to AEs | 0 (0.0%) | 1 (1.8%) | NS |
P-value was based on the Fisher's exact test.
A treatment-emergent AE was defined as either a new illness with onset date on or after the start of vaccination series, or exacerbation of a pre-existing condition after the start of the vaccination series.
Treatment-associated AE was an AE that was possibly, probably, or definitely related to the treatment by the Investigator.
Six serious AEs were all deemed unrelated to the vaccine. The three for vaccinated subjects were: cocaine-related paranoia, molar pregnancy resulting in spontaneous abortion in a 42 year old, and alcoholic pancreatitis. The three for placebo subjects were: hematuria and kidney stones, facial cellulitis, and right forearm cellulitis from self-injecting drugs.
COMMENT
We found that a cocaine specific vaccine significantly reduced cocaine use in those cocaine dependent subjects who attained the target antibody levels of over 43 ug/ml (high IgG group), which animal studies indicate are elevated sufficiently to capture enough circulating cocaine to reduce its euphoric effects. 11,12,26 However, only 38% of those vaccinated attained these levels, and these levels were not attained before week eight and then substantially declined between weeks 16 to 24. Both the active and placebo vaccine groups reduced their cocaine use during the first eight weeks probably due to reducing their illicit opiate use through methadone maintenance and CBT. 37–40 Fifty three percent of the high IgG group was abstinent from cocaine more than half the time as compared with only 23% of the subjects who made lesser quantities of antibody in weeks 8–20.
Although not statistically significant, Figure 3 suggests increased use of cocaine in the high IgG group after week 16 when their antibody levels began to fall relatively rapidly, which is typical of hapten vaccines and unlikely to be related to methadone, since methadone neither enhances nor inhibits antibody responses in humans or experimental animals.41, 42 While this increased use likely reflects an effort to overcome the anti-cocaine antibody blockade, fortunately, cocaine related AEs did not increase (e.g. overdose, hospitalization) during this interval. Optimal treatment will likely require repeated booster vaccinations to maintain appropriate antibody levels. Furthermore, efforts will be needed in order to retain subjects during the initial series of injections since antibody levels rose slowly over the first three months when patients are immunized according to the protocol used in these studies. Other treatments need to be employed during this early treatment period to encourage abstinence. As an example, to retain subjects in this study during the initial slow rise in antibody responses, we enlisted cocaine dependent subjects who were enrolled in a methadone maintenance program. Methadone patients may remain longer in treatment while continuing to use cocaine than would primary cocaine dependent patients, who would drop out. Thus, enhanced retention with methadone maintenance could have resulted in longer durations of continued cocaine use, although the baseline patterns (2–3 days/wk) and amount (2–4 dimes/use) of cocaine use (Table 1) were similar to primary cocaine users4,5,6,8.
This study did not achieve a significant difference in complete abstinence with immunization, and the significant reduction in cocaine use occurred in that minority of patients who attained our target antibody responses (high IgG). Nevertheless, it could be argued that a reduction in cocaine use rather than complete abstinence is therapeutically meaningful. Over half (0.53) of the high IgG group showed no new episodes of cocaine use at least 50% of the time (Preston criteria) and doubled their cocaine-free urines from a baseline below 20% to 45%, as shown in Figure 3. Doubling of cocaine-free urines has been associated with a significant improvement in social functioning as assessed by the Social Adjustment Scale in a meta-analysis of 368 subjects in two 25-week randomized, controlled trials of behavioral abstinence reinforcement in methadone maintained cocaine abusers, a similar population to those in our study.43 Also, in a double-blinded, placebo controlled human laboratory study, actively vaccinated cocaine dependent subjects who achieved antibody levels of ≥22 ug/ml described a 90% reduction in the effects of 50 mg of smoked cocaine as compared with either their pre-vaccination baseline or when compared with subjects whose peak antibody levels were less. This result may reflect a lower dose of cocaine used in this controlled setting than in the outpatient unrestricted conditions of the current study.44 Thus, reducing cocaine use can be therapeutically meaningful and is critically dependent on the achievement of appropriate antibody levels.
The rise and decline over 6 months seen in these subjects with this small molecule conjugate vaccine are similar to the response kinetics found in studies with other recent human conjugate vaccines such as for angiotensin and nicotine18, 45–50. While the long lived antibody responses described in classical immunology would be desirable, the potent adjuvants (e.g., Complete Freund’s Adjuvant) used in experimental animals are too toxic for humans. Using similar numbers of boosters and adjuvants as in our study, both anti-angiotensin and anti-nicotine antibody levels declined over a few months. While it is not clear whether all cocaine abusers will benefit from persistence of high antibody levels, short term blockade of cocaine by the vaccine is likely to have limited efficacy, as does short term opiate blockade by naltrexone18, 51. Thus the goals for future vaccine development will be to increase the proportion of subjects who can attain the desired antibody levels and to extend these periods of abstinence through long term maintenance of these adequate antibody levels. We look forward to extending our promising findings in a broader population of cocaine abusers as we also reach for these future vaccine development goals.
Acknowledgments
The trial was supported by National Institute on Drug Abuse grants 1 R01 DA15477, K05-DA 0454 (TRK), and P50-DA12762 and the Veterans Affairs Mental Illness Research, Education, and Clinical Center (New England MIRECC). Dr. Bridget Martell was provided salary support by the Veteran’s Affairs Office of Research and Development/ Cooperative Studies Program Career Development Award CRCD #733A at the time the research was conducted. Dr. Martell is currently employed as a Medical Director at Pfizer, Inc. Celtic Pharmaceuticals supplied the cocaine-CTB vaccine and gave Dr. Kosten travel fees for consultative services. Celtic had 10% involvement in the design and conduct of the study; 5% involvement in the collection, management, and interpretation of the data (involvement limited to site monitoring). Antibody levels were independently measured by a contract laboratory paid by Celtic and by our laboratory at the Michael E. DeBakey Veterans Affairs Medical Center. Celtic Pharmaceuticals offered some comments prior to initial submission of this manuscript. This work was fully approved by the Yale University Human Investigation Review Committee and the VA CT Healthcare System Institutional Review Board. Drs. Martell and Kosten had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Trial Registration: Protocol ID: NIDA-15477-1
Clinical trials.gov ID: NCT00142857
References
- 1.McLellan AT, Lewis DC, O'Brien CP, Kleber HD. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284:1689–1695. doi: 10.1001/jama.284.13.1689. [DOI] [PubMed] [Google Scholar]
- 2.National Estimates of Drug-related Emergency Department Visits. Substance Abuse and Mental Health Services Administration: Year-End 2005. 2005 http://dawninfo.samhsa.gov. . Updated Last Updated Date.
- 3.Substance Abuse and Mental Health Services Administration (SAMHSA) Summary of Findings from the 2007 National Household Survey on Drug Abuse. Rockville Maryland: Department of Health and Human Services, Substance Abuse and Mental Health Services Administration; 2008. [Google Scholar]
- 4.Silva de Lima M, Garcia de Oliveira Soares G, Alves Pereira Reisser A, Farrell M. Pharmacological treatment of cocaine dependence: a systematic review. Addiction. 2002;97(8):931–949. doi: 10.1046/j.1360-0443.2002.00209.x. [DOI] [PubMed] [Google Scholar]
- 5.Vocci FJ, Jr, Elkashef A. Pharamcotherapy and other treatments for cocaine abuse and dependence. Curr Opinion Psychiatry. 2005;18:265–270. doi: 10.1097/01.yco.0000165596.98552.02. [DOI] [PubMed] [Google Scholar]
- 6.Lima MS, Reisser Lima AAP, Soares BGO, Farrell M. Antidepressants for Cocaine Dependence (Review) Cochrane Database of Systematic Reviews. 2003;(2) doi: 10.1002/14651858.CD002950. [DOI] [PubMed] [Google Scholar]
- 7.Knapp WP, Soares BG, Farrel M, Lima MS. Psychosocial interventions for cocaine and psychostimulant amphetamines related disorders. Cochrane Database Syst Rev. 2007 Jul;18(3) doi: 10.1002/14651858.CD003023.pub2. CD003023. [DOI] [PubMed] [Google Scholar]
- 8.Carroll KM, Fenton LR, Ball SA, et al. Efficacy of disulfiram and cognitive behavior therapy in cocaine-dependent outpatients: a randomized placebo-controlled trial. Archives of General Psychiatry. 2004;61(3):264–272. doi: 10.1001/archpsyc.61.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, Otto MW. A meta-analytic review of psychosocial interventions for substance use disorders. Am J Psychiatry. 2008;165:179–187. doi: 10.1176/appi.ajp.2007.06111851. [DOI] [PubMed] [Google Scholar]
- 10.Silverman K, Chutuape MA, Bigelow GE, Stitzer ML. Voucher-based reinforcement of cocaine abstinence in treatment-resistant methadone patients: effects of reinforcement magnitude. Psychopharmacologia. 1999;146(2):128–138. doi: 10.1007/s002130051098. [DOI] [PubMed] [Google Scholar]
- 11.Fox BS, Kantak KM, Edwards MA, et al. Efficacy of a therapeutic cocaine vaccine in rodent models. Nature Medicine. 1996;2(10):1129–1132. doi: 10.1038/nm1096-1129. [DOI] [PubMed] [Google Scholar]
- 12.Fox BS. Development of a therapeutic vaccine for the treatment of cocaine addiction. Drug and Alcohol Dependence. 1997;48:153–158. doi: 10.1016/s0376-8716(97)00121-x. [DOI] [PubMed] [Google Scholar]
- 13.Carrera MR, Ashley JA, Parsons LH, Wirsching P, Koob GF, Janda KD. Suppression of psychoactive effects of cocaine by active immunization. Nature. 1995;378(6558):727–730. doi: 10.1038/378727a0. [DOI] [PubMed] [Google Scholar]
- 14.Norman AB, Tabet MR, Norman MK, Buesing WR, Pesce AJ, Ball WJ. A chimeric human/murine anticocaine monoclonal antibody inhibits the distribution of cocaine to the brain in mice. J Pharmacol Exp Ther. 2007;320(1):145–153. doi: 10.1124/jpet.106.111781. [DOI] [PubMed] [Google Scholar]
- 15.Lynch TJ, Mattes CE, Singh A, Bradley RM, Brady RO, Dretchen KL. Cocaine Detoxification by Human Plasma Butyrlcholinesterase. Toxicology and Applied Pharmacology. 1997;145:363–371. doi: 10.1006/taap.1997.8187. [DOI] [PubMed] [Google Scholar]
- 16.Nutt DJ. Addiction: Brain mechanisms and their treatment implications. Lancet. 1996;347:31–36. doi: 10.1016/s0140-6736(96)91561-5. [DOI] [PubMed] [Google Scholar]
- 17.Oldendorf WH. Some relationships between addiction and drug delivery to the brain In: Bioavailabilty of Drugs to the brain and blood-brain barrier. NIDA Research Monograph. 1992;120:13–25. [PubMed] [Google Scholar]
- 18.Haney M, Kosten TR. Therapeutic vaccines for substance dependence. Expert Review of Vaccines. 2004;3(1):11–18. doi: 10.1586/14760584.3.1.11. [DOI] [PubMed] [Google Scholar]
- 19.Jertborn M, Svennerholm A-M, Holmgren J. Safety and immunogenicity of an oral recombinant cholera B subunit-whole cell vaccine in Swedish volunteers. Vaccine. 1992;10(2):130–132. doi: 10.1016/0264-410x(92)90030-n. [DOI] [PubMed] [Google Scholar]
- 20.Jerrtborn M, Svennerholm AM, Holmgren J. Immunological memory after immunization with oral cholera B subunit-whole-cell vaccine in Swedish volunteers. Vaccine. 1994;12:1078–1082. doi: 10.1016/0264-410x(94)90176-7. [DOI] [PubMed] [Google Scholar]
- 21.Holmgren J, Czerkinsky C, Lycke N, Svennerholm AM. Strategies for the induciton of immune responses at mucosal surfaces making use of cholera toxin B subunit as immunogen, carrier, and adjuvant. Am J Trop Med Hyg. 1994;50:42–54. [PubMed] [Google Scholar]
- 22.Svennerholm A, Gothefors L, Sack D, Bardhan P, Holmgren J. Local and systemic antibody responses and immunological memory after immunization with cholera B subunit by different routes. Bulletin of the World Health Organization. 1984;62:909–918. [PMC free article] [PubMed] [Google Scholar]
- 23.Kosten TR, Rosen M, Bond J, et al. Human therapeutic cocaine vaccine: safety and immunogenicity. Vaccine. 2002;20(7–8):1196–1204. doi: 10.1016/s0264-410x(01)00425-x. [DOI] [PubMed] [Google Scholar]
- 24.Martell B, Mitchell E, Poling J, Gonsai K, Kosten TR. Vaccine pharmacotherapy for the treatment of cocaine dependence. Biological Psychiatry. 2005;58:158–164. doi: 10.1016/j.biopsych.2005.04.032. [DOI] [PubMed] [Google Scholar]
- 25.Jenkins AJ, Keenan RM, Henningfield JE, Cone EJ. Correlation between pharmacological effects and plasma cocaine concentrations after smoked administration. J Anals Toxicol. 2002;26:382–392. doi: 10.1093/jat/26.7.382. [DOI] [PubMed] [Google Scholar]
- 26.Orson FM, Kinsey BM, Singh RAK, Wu Y, Gardner T, Kosten TR. The future of vaccines in the management of addictive disorders. Current Psychiatry Reports. 2007 doi: 10.1007/s11920-007-0049-z. in press. [DOI] [PubMed] [Google Scholar]
- 27.American Psychiatric Association, ed. Diagnostic and Statistical Manual of Mental Disorders, 4th ed.: DSM-IV. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 28.Ball JC, Corty E, Petroski SP, Bond H, Tommasello A, Graff H. Treatment effectiveness: medical staff and services provided to 2, 394 patients at methadone programs in three states. NIDA Research Monograph. 1987;76:175–181. [PubMed] [Google Scholar]
- 29.Clearinghouse NG. Medication-assisted treatment for opioid addiction in opioid treatment programs: Clinical pharmacotherapy. 2005 [Google Scholar]
- 30.TIP 33: Treatment for Stimulant Use Disorders. http://kap.samhsa.gov/products/manuals/index.htm. http://www.ncbi.nlm.nih.gov/books/bv.fcgi?rid=hstat5.section.57658.
- 31.Golm GT, Halloran ME, Longini IMJ. Semi-parametric models for mismeasured exposure information in vaccine trials. Stat Med. 1998;17(20):2335–2352. doi: 10.1002/(sici)1097-0258(19981030)17:20<2335::aid-sim929>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 32.Halloran ME, Longini IMJ. Using validation sets for outcomes and exposure to infection in vaccine field studies. American Journal Epidemiology. 2001;154(5):391–398. doi: 10.1093/aje/154.5.391. [DOI] [PubMed] [Google Scholar]
- 33.Paula S, Tabet MR, Farr CD, Norman AB, Ball WJJ. Three-dimensional quantitative structure-activity relationship modeling of cocaine binding by a novel human monoclonal antibody. J Med Chem. 2004;47(1):133–142. doi: 10.1021/jm030351z. [DOI] [PubMed] [Google Scholar]
- 34.Preston KL, Silverman K, Schuster CR, Cone EJ. Assessment of cocaine use with quantitative urinalysis and estimation of new uses. Addiction. 1997;92(6):717–727. [PubMed] [Google Scholar]
- 35.Gibbons RD, Hedeker D, Elkin I, et al. Some conceptual and statistical issues in analysis of longitudinal psychiatric data. Application to the NIMH treatment of Depression Collaborative Research Program dataset. Archives of General Psychiatry. 1993;50(9):739–750. doi: 10.1001/archpsyc.1993.01820210073009. [DOI] [PubMed] [Google Scholar]
- 36.Bryk AS, Raudenbush SW. Application of hierarchical linear models to assessing change. Psychological Bulletin. 1987;101:147–158. [Google Scholar]
- 37.Oliveto AH, Feingold A, Schottenfeld R, Jatlow P, Kosten TR. Desipramine in opioid-dependent cocaine abusers maintained on buprenorphine vs methadone. Archives of General Psychiatry. 1999;56(9):812–820. doi: 10.1001/archpsyc.56.9.812. [DOI] [PubMed] [Google Scholar]
- 38.Schottenfeld RS, Pakes JR, Oliveto A, Ziedonis D, Kosten TR. Buprenorphine vs methadone maintenance treatment for concurrent opioid dependence and cocaine abuse. Arch Gen Psychiatry. 1997;54(8):713–720. doi: 10.1001/archpsyc.1997.01830200041006. [DOI] [PubMed] [Google Scholar]
- 39.Kosten T, Oliveto A, Feingold A, et al. Desipramine and contingency management for cocaine and opiate dependence in buprenorphine maintained patients. Drug and Alcohol Dependence. 2003;70:315–325. doi: 10.1016/s0376-8716(03)00032-2. [DOI] [PubMed] [Google Scholar]
- 40.Poling J, Oliveto A, Petry NM, et al. Six-month trial of Bupropion with Contingency Management for Cocaine Dependence in a Methadone-Maintained Population. Archives of General Psychiatry. 2006;63:219–228. doi: 10.1001/archpsyc.63.2.219. [DOI] [PubMed] [Google Scholar]
- 41.McLachlan C, Crofts N, Wodak A, Crowe S. The effects of methadone on immune function among injection drug users: a review. Addiction. 1993 Feb;88(2):257–263. doi: 10.1111/j.1360-0443.1993.tb00809.x. [DOI] [PubMed] [Google Scholar]
- 42.De Waal EJ, Van der Laan JW, Van Loveren H. Effects of prolonged exposure to morphine and methadone on in vivo parameters of immune function in rats. Toxicology. 1998 August 21;129(2–3):201–210. doi: 10.1016/s0300-483x(98)00077-8. [DOI] [PubMed] [Google Scholar]
- 43.Ghitza UE, Epstein DH, Preston KL. Psychosocial Functioning and Cocaine Use during Treatment: Strength of relationship depends on type of urine-testing method. Drug and Alcohol Dependence. 2007 December;911(2–3):169–177. doi: 10.1016/j.drugalcdep.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haney M, Gunderson EW, Collins ED, Foltin RW. Paper presented at: The College on Problems in Drug Dependence. Scottsdale Arizona; 2006. Cocaine vaccine: Smoked cocaine administration in humans. [Google Scholar]
- 45.Boyd B NicVAX (TM) Aid to smoking cessation, nicotine vaccine. Drugs of the Future. 2006;31(3):203–205. [Google Scholar]
- 46.Svensson TH. Active immunization against nicotine for smoking cessation and relapse prevention. International Journal of Neuropsychopharmacology. 2006;9 Supplemental:S55. [Google Scholar]
- 47.Hatsukami DK, Rennard S, Jorenby D, et al. Safety and immunogenicity of a nicotine conjugate vaccine in current smokers. Clinical Pharmacology and Therapeutics. 2005;78(5):456–467. doi: 10.1016/j.clpt.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 48.Hatsukami DK Corporate author UM. Nicotine vaccine: A promising treatment for nicotine addiction. Cancer-Epidemiol-Biomarkers-Prev. 2005;14(11, Pt. 2) 2809S–2810S. [Google Scholar]
- 49.Cornuz J, Klingler K, Mueller P, et al. A therapeutic vaccine for nicotine dependence: Results of a phase I and a randomized phase II study. J-Clin-Oncol. 2005;23(16) Suppl:1008. [Google Scholar]
- 50.Tissot AC, Maurer P, Nussberger J. Effect of immunisation against angiotensin II with CYT006-AngQb on ambulatory blood pressure: a double-blind, randomised, placebo-controlled phase IIa study. Lancet. 2008 Mar 8;371(9615):821–827. doi: 10.1016/S0140-6736(08)60381-5. [DOI] [PubMed] [Google Scholar]
- 51.Kosten TR, Kleber HD. Strategies to improve compliance with narcotic antagonists. American Journal of Drug and Alcohol Abus.e. 1984;10(2):249–266. doi: 10.3109/00952998409002784. [DOI] [PubMed] [Google Scholar]