Abstract
Background
Children with severe asthma have persistent symptoms despite treatment with inhaled corticosteroids (ICSs). The differentiating features of severe asthma in children are poorly defined.
Objective
To identify features of severe versus mild-to-moderate asthma in school-age children using noninvasive assessments of lung function, atopy, and airway inflammation.
Methods
A total of 75 children (median age, 10 years) with asthma underwent baseline characterization including spirometry and lung volume testing, methacholine bronchoprovocation, allergy evaluation, and offline measurement of exhaled nitric oxide (FENO). Twenty-eight were followed longitudinally over 6 months. Participants were assigned to the severe asthma subgroup if they required high-dose ICS plus 2 or more minor criteria.
Results
Children with severe versus mild-to-moderate asthma had more symptoms, greater airway obstruction, more gas trapping, and increased bronchial responsiveness to methacholine. Subjects with severe asthma also had higher concentrations of FENO and significantly greater sensitization to aeroallergens. With long-term study, both the reduction in FEV1 and increase in FENO persisted in the severe versus mild-to-moderate group. Furthermore, despite adjustments in ICS doses, the frequency of exacerbations was significantly higher in subjects with severe (83%) versus mild-to-moderate asthma (43%).
Conclusion
Severe asthma in childhood is characterized by poor symptom control despite high-dose ICS treatment and can be differentiated from mild-to-moderate asthma by measurement of lung function and FENO.
Clinical implications
Clinicians should suspect severe asthma in children with poor response to ICS, airway obstruction, and high FENO.
Keywords: Children, asthma, atopy, nitric oxide, pulmonary function testing
Severe asthma, often termed difficult-to-treat asthma, is identifiable in early childhood and is associated with poor symptom control despite treatment with high doses of inhaled corticosteroids (ICSs).1,2 Conversely, children with mild-to-moderate asthma typically respond to low to moderate doses of ICS.1 Original studies have identified increased biomarkers of inflammation in bronchoalveolar lavage and airway biopsies from children with severe asthma.3–5 Nonetheless, these tests are relatively invasive, and the clinical features that differentiate severe versus mild-to-moderate asthma in children have not been well defined. The purpose of this study was to identify features of severe asthma in school-age children using noninvasive assessments of inflammation, measures of allergic sensitization, and pulmonary function testing.
METHODS
Sample
An unmatched convenience sample of children 6 to 17 years of age treated for asthma at an outpatient pulmonary clinic at Emory University was invited to participate in the study. This clinic was 1 of 10 sites for the National Heart, Lung, and Blood Institute (NHLBI) Severe Asthma Research Program (SARP) and was the only center that recruited pediatric subjects. Participants met standard criteria for a diagnosis of asthma, which included physician diagnosis and at least a 12% increase in FEV1 after bronchodilator administration.6 Children with vocal cord dysfunction, obstructive sleep apnea syndrome, severe sinus disease, or known immunodeficiencies were excluded from participation. Gastroesophageal reflux disease was not a criterion for exclusion, provided it was treated with medications.
Participants were classified as having severe or mild-to-moderate asthma according to criteria developed by the National Institutes of Health/NHLBI SARP based on an American Thoracic Society consensus panel report7 (Table I). Thresholds for high-dose ICS were adjusted for children and defined as ≥440 μg fluticasone equivalent per day for children younger than 12 years and ≥880 μg fluticasone equivalent per day for children 12 to 17 years of age8 (see this article’s Table E1 in the Online Repository at www.jacionline.org). The pharmacy records of all participants were reviewed, and children with 4 or fewer ICS or oral corticosteroid refills in the previous 6 months were excluded from the study.
TABLE I.
Classification of severe and mild-to-moderate asthma in children
| Severe asthma | Mild-to-moderate asthma |
|---|---|
| Physician diagnosis of asthma | Physician diagnosis of asthma |
| ≥ 12% FEV1 reversibility* | ≥ 12% FEV1 reversibility* |
| No significant comorbid conditions, including untreated gastroesophageal reflux, vocal cord dysfunction, and severe sinus disease | |
| Met benchmark for ICS adherence | Met benchmark for ICS adherence |
| Daily low-dose† ICS therapy for 6 months before enrollment | |
| Major criteria (at least 1): | |
| Daily high-dose† ICS and/or daily systemic corticosteroid therapy for 6 months before enrollment | |
| Minor criteria (2 or more): | |
| Treatment with a daily controller medication in addition to ICS | |
| Daily short-acting bronchodilator use‡ | |
| FEV1 < 80% predicted at baseline | |
| ≥ 1 Emergency department visit§ | |
| ≥ 3 Oral corticosteroid bursts§ | |
| History of worsening symptoms with a reduction in corticosteroid dose | |
| History of intubation |
FEV1 % reversal was determined after 2 inhalations of albuterol sulfate delivered via metered-dose inhaler.
High-dose ICSs were defined as ≥440 mcg fluticasone equivalent per day for children younger than 12 years and ≥880 mcg fluticasone equivalent per day for children 12 to 17 years of age.
Daily use defined as 5 of 7 days.
Within 12 months before study enrollment.
From the baseline convenience sample, 40 children (20 with mild-to-moderate asthma, 20 with severe asthma) were asked to participate in a 6-month longitudinal study with repeated assessments. Criteria for longitudinal enrollment included residence within 30 miles of the outpatient clinic, a history of regular outpatient follow-up visits, a reliable caregiver, and willingness to complete 1 outpatient visit per month.
Procedures
Participants underwent detailed characterization consisting of medical history and symptom questionnaires, spirometry, lung volume testing, allergy evaluation, exhaled nitric oxide (FENO) sampling, and methacholine challenge. The longitudinal cohort, with characteristics similar to the larger study sample, underwent spirometry, FENO collection, and symptom monitoring at monthly intervals for 6 months. Asthma exacerbations were monitored and defined as worsening of asthma symptoms that required a prednisone burst, an emergency department visit, or hospitalization.
Symptoms including cough, sputum production, wheeze, chest tightness, shortness of breath, and nocturnal awakenings over the past 3 months before enrollment were scored on a 4-point scale (0 = never, 4 = daily) and summed to obtain a composite symptom score. Spirometry (KoKo PDS; Ferraris, Louisville, Colo) was performed in the presence of daily medication and after a 4-hour and 12-hour withholding of short-acting and long-acting bronchodilators, respectively. Spirometry was repeated 15 minutes after receiving 2 inhalations of albuterol sulfate via metered dose inhaler (90 μg/inhalation) and valved holding chamber and mouthpiece (Aerochamber; Invacare Corp, Elyria, Ohio). The results fulfilled American Thoracic Society criteria for reproducibility9 and were interpreted according to reference standards.10 Lung volumes were measured with a body plethysmograph (MedGraphics Elite Series; Medical Graphics Corporation, St Paul, Minn) and expressed according to reference standards.11 Bronchoprovocation testing was limited to subjects with a baseline FEV1 > 70% predicted12 and was performed using 10 concentrations of methacholine from 0 to 25 mg/mL (Provocholine; Methapharm Inc, Coral Springs, Fla) delivered by a Rosenthal dosimeter (Pulmonary Data Service Instrumentation, Louisville, Colo). Allergy skin prick testing was performed with a standard kit (Multi-Test II; Lincoln Diagnostics, Decatur, Ill) containing tree pollen, grass pollen, ragweed pollen, weed pollen, dog hair, cat epithelium, Alternaria, Cladosporidium, Aspergillus, Dermatophagoides pteronyssinus, Dermatophagoides farinae, cockroach, normal saline, and histamine extracts (Greer Laboratories, Lenoir, NC). The application site was examined 15 minutes after application and considered positive if both a wheal ≥ 3 mm diameter and erythema ≥ 10 mm diameter were present. FENO was collected with a reservoir bag at a fixed exhaled flow rate of 0.35 L/s13,14 and analyzed offline by chemiluminescence (Sievers NOA 280-I; Ionic Instruments, Boulder, Colo).
Statistical analyses
Data were displayed with histogram frequency plots (SPSS Version 13.0; SPSS Inc., Chicago, Ill) and when abnormally distributed were transformed logarithmically. Pearson bivariate correlation was used to test the association of 2 continuous or interval scale variables. Fishers exact tests were used to determine group differences for categorical data and Student t test for continuous data. Analysis of covariance was used to adjust PC20 and FENO data for the potential confounding effects of month of enrollment and allergic sensitization. Longitudinal data were analyzed with repeated-measures ANOVA. Longitudinal FENO data was adjusted by using repeated-measures analysis of covariance to control for the potential confounding effects of month of enrollment and allergic sensitization (serum IgE). Predictors of asthma exacerbations were categorized by occurrence (yes/no) and standardized cut-points (high/low values) and tested by actuarial analysis with Cox regression. A 2-tailed probability of .05 or less was the threshold of significance for all comparisons.
RESULTS
Baseline characterization
Study participants were drawn from a convenience sample of 272 children with asthma treated in the outpatient clinic between October 2003 and March 2006. Of 109 invited to participate (Fig 1), 10 declined, including 7 with features of severe asthma. There were no important differences in the demographic features of those children invited to participate versus those who were not. Of the 99 children enrolled, 17 were excluded, including 13 children not treated with ICS, 2 with Mycoplasma pneumoniae, 1 smoker, and 1 with obstructive sleep apnea and morbid obesity. Seven children, including 5 with severe asthma, were excluded for poor ICS adherence, 2 by admission and 5 by a review of pharmacy records. The features of these children compared with those with acceptable adherence did not differ with regard to age, sex, ethnicity, dose of ICS or prednisone equivalents, FEV1, residual volume (RV):total lung capacity (TLC), serum IgE, PC20, or FENO (data not shown; P > .05 for all).
FIG 1.
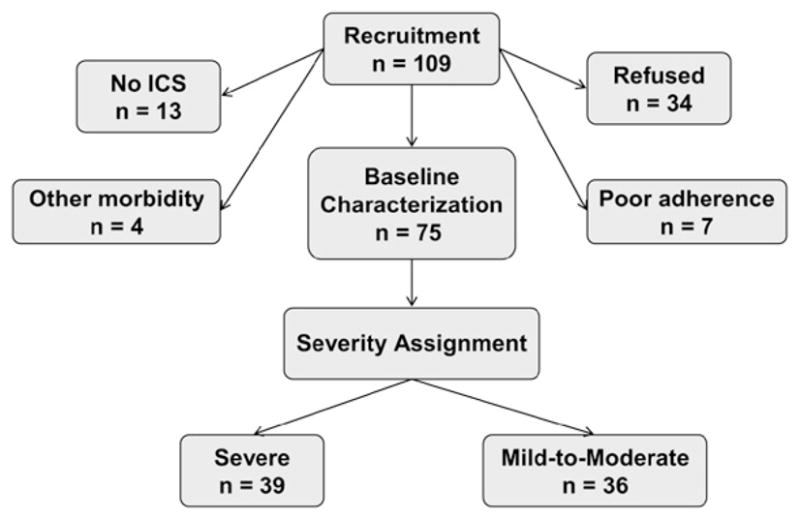
Recruitment schematic.
Children with severe asthma were more likely to be of African American ethnicity; participants with severe and mild-to-moderate asthma had similar age and sex distribution (Table II). Subjects with severe asthma were treated with significantly higher daily doses of ICS (855 ± 257 vs 368 ± 184 mcg fluticasone equivalent/d) yet were more likely to use daily short-acting β-agonists and other controller medications. Children with severe asthma also had greater heath care utilization in the 12 months before enrollment, evidenced by a need for more oral corticosteroid bursts (median 3 vs 1; P < .001) and more emergency department visits for acute asthma exacerbations (median 3 vs 0; P < .001).
TABLE II.
Characteristics of children with asthma*
| Severe (n = 39) | Mild-to-moderate (n = 36) | P | |
|---|---|---|---|
| Age (y) | 10 (6–17) | 10 (6–15) | .119 |
| Ethnicity | |||
| White | 9 (23%) | 26 (72%) | .000 |
| African American | 27 (69%) | 7 (19%) | |
| Other | 3 (8%) | 3 (8%) | |
| Male | 21 (54%) | 18 (50%) | .459 |
| Female | 18 (46%) | 18 (50%) | |
| Age at diagnosis (mo) | 22 (2–144) | 60 (2–156) | .000 |
| Daily daytime symptoms† | 19 (49%) | 6 (17%) | .007 |
| Daily nocturnal symptoms | 11 (28%) | 0 | .030 |
| Atopic dermatitis | 27 (69%) | 15 (42%) | .021 |
| Immediate family history of asthma | 31 (79%) | 19 (53%) | .027 |
| Total hospital admissions | 4 (0–25) | 0 (0–3) | .000 |
| Total intensive care unit admissions | 1 (0–10) | 0 (0–1) | .000 |
| Daily medications | |||
| Short-acting β-agonists‡ | 22 (56%) | 3 (8%) | .000 |
| Long-acting β-agonists§ | 33 (85%) | 23 (64%) | .000 |
| Montelukast (Merck, Whitehouse Station, NJ) | 39 (100%) | 23 (64%) | .041 |
| Prednisone | 8 (21%) | 0 | |
Values represent the frequency (percentage) or the median (range).
Six subjects with mild-to-moderate asthma experienced bronchospasm with daily participation in organized sports.
Three subjects with mild-to-moderate asthma used prophylactic β-agonists before daily sports participation.
All children with long-acting β-agonist use were on fluticasone/salmeterol combination therapy.
Symptoms
Composite symptom scores, including cough, sputum production, chest tightness, wheezing, shortness of breath, and nocturnal awakenings in the 3 months before enrollment, were significantly higher in children with severe versus mild-to-moderate asthma (15 [0–24] vs 8 [3–21]; P = .005). However, in both groups, there were children with reported good asthma control evidenced by symptom occurrence of less than once per month (n = 6 severe; n = 10 mild-to-moderate). Three of these children, all with severe asthma, had abnormal pulmonary function with airway obstruction, possibly indicating poor symptom perception. We found no significant correlation between composite symptom score and lung function (r = −.027; P = .846) or FENO (r = .078; P = .577).
Pulmonary function and lung volumes
FEV1 was lower in children with severe asthma both in the presence of maintenance medication and after bronchodilator withholding, and increased significantly after bronchodilator administration (Table III). Although all participants had a documented history of at least 12% improvement in FEV1 after bronchodilator administration, on study, subjects with severe asthma had a higher frequency of FEV1 reversibility ≥12% despite ICS (41% vs 17%; P =.049). Within the severe asthma group, children with ≥12% FEV1 reversibility had more airway obstruction at baseline (FEV1 69% ± 10% vs 94% ± 21%; P = .001). In both groups, the degree of airway obstruction worsened with advancing age (age vs baseline FEV1, r =−.445, P < .001; see this article’s Fig E1 in the Online Repository at www.jacionline.org). No significant correlations were observed between spirometry variables and symptom scores.
TABLE III.
Spirometry and lung volume measurements*
| Withhold† | Prebronchodilator |
Postbronchodilator |
Percent change |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Severe (n = 39) | Mild-to-moderate (n = 36) | P | Severe (n = 39) | Mild-to-moderate (n = 36) | P | Severe (n = 39) | Mild-to-moderate (n = 36) | P | ||
| Baseline FEV1 | No | 87 ± 20 | 95 ± 12 | .057 | 96 ± 20 | 99 ± 11 | .463 | 13 ± 15 | 6 ± 7 | .029 |
| FVC | Yes | 97 ± 13 | 99 ± 8 | .601 | 102 ± 14 | 101 ± 9 | .884 | 5 ± 9 | 3 ± 4 | .291 |
| FEV1 | Yes | 81 ± 15 | 96 ± 13 | .001 | 92 ± 13 | 102 ± 11 | .009 | 15 ± 13 | 8 ± 6 | .046 |
| FEV1:FVC | Yes | .74 ± .10 | .84 ± .10 | <.001 | .79 ± .09 | .87 ± .08 | .004 | 9 ± 9 | 5 ± 5 | .111 |
| FEF25-75 | Yes | 58 ± 26 | 89 ± 32 | .001 | 78 ± 30 | 106 ± 40 | .011 | 41 ± 37 | 23 ± 17 | .076 |
| TLC | Yes | 97 ± 12 | 100 ± 17 | .397 | 97 ± 12 | 98 ± 8 | .846 | 1 ± 9 | −3 ± 20 | .346 |
| RV | Yes | 121 ± 37 | 96 ± 32 | .028 | 112 ± 35 | 94 ± 45 | .142 | −3 ± 35 | 0.3 ± 30 | .727 |
| RV:TLC | Yes | .26 ± .08 | .21 ± .06 | .041 | .24 ± .07 | .20 ± .09 | .072 | −10 ± 30 | −6 ± 25 | .632 |
FEF25-75, Forced expiratory flow rate at 25% to 75% of FVC.
Values represent the means ± SDs. Prebronchodilator and postbronchodilator values are presented as percentages of predicted values, with the exception of FEV1:FVC and RV:TLC, which are expressed as ratios.
Short-acting and long-acting bronchodilators were withheld 4 and 12 hours before testing, respectively.
Lung overexpansion as measured by an elevated RV to TLC ratio was significantly greater in children with severe versus mild-to-moderate asthma (Table III), and was a result of a higher RV and not lower TLC in the severe group. Albuterol treatment did not significantly change TLC, RV, or RV:TLC in either group. RV:TLC was inversely correlated with FEV1 at baseline (r = −.438; P = .003) and after bronchodilator withholding (r = −.302; P = .044; see this article’s Fig E2 in the Online Repository at www.jacionline.org).
Bronchoprovocation testing
With methacholine bronchoprovocation, 1 child from each group had a positive response to diluent. Five children with mild-to-moderate asthma and 1 child with severe asthma did not react to the highest concentration of methacholine administered (25 mg/mL); this subject with severe asthma was excluded from additional analyses. Mean PC20 was significantly lower in severe asthma (1.69 ± 2.28 vs 10.24 ± 10.47 mg/mL; log-transformed P = .010) and remained different between the 2 groups after controlling for serum IgE and the number of positive skin prick responses (P = .011). PC20 was correlated with FEV1 after bronchodilator withholding (r = .542; P = .003; see this article’s Fig E3 in the Online Repository at www.jacionline.org).
Allergic sensitization
Nearly all the children recruited for this study had significant allergic sensitization; however, this was more profound in children with severe asthma. Atopy, defined as either a serum IgE ≥150 or ≥1 positive skin prick reaction,15 was present in 76% of children with mild-to-moderate asthma and all of the children with severe asthma (P =.012). Seven children with severe asthma and 1 child with mild-to-moderate asthma had serum IgE > 1000 kU/L. Between groups, children with severe asthma had significantly higher serum IgE (821 ± 1066 vs 301 ± 413 kU/L; log-transformed P = .002) and more positive skin prick reactions to aeroallergens (mean 5 ± 3 vs 3 ± 3; P = .009), namely weed mix (34% vs 6%; P =.023), D farinae (79% vs 50%; P = .039), and Dermatophagoides pteryn (86% vs 56%; P = .024). The 2 study groups were not different with regard to animal dander, tree, or mold sensitization. The percentage of eosinophils in peripheral blood was not different between the 2 groups.
Expired nitric oxide
Offline FENO values at baseline were 2-fold higher in children with severe versus mild-to-moderate asthma (16.4 ± 10.2 vs 8.2 ± 4.7 ppb; P < .001) and in the severe group remained high after adjusting the analysis for the potentially confounding effects of serum IgE and allergic sensitization (P =.042; see this article’s Figs E4 and E5 in the Online Repository at www.jacionline.org). Compared with children with mild-to-moderate asthma, subjects with severe asthma had a significantly higher incidence of offline FENO values > 10 ppb (68% vs 22%; P < .001), suggestive of poor asthma control16 and airway inflammation.17 To see whether those children with mild asthma and elevated FENO were possibly undertreated with ICS and thereby had lower levels of lung function and more symptoms, we compared lung function and symptom scores in subjects with mild-to-moderate asthma by using an exhaled FENO of 10 ppb as the cut-point. Lung function was nearly identical in the high versus low FENO groups (FEV1, 94% ± 13% vs 95% ± 12%; P = .792), and composite symptom scores also did not differ (6 [4–10] vs 9 [3–21]; P = .301). In a combined analysis of children in both study groups, FENO did not correlate with serum IgE, the number of positive skin pricks, methacholine PC20, symptom scores, or spirometric variables such as FEV1 (data not shown; P < .05 for all).
Longitudinal assessment of lung function, FENO, and exacerbations
Forty children (20 with mild-to-moderate asthma, 20 with severe asthma) were approached for enrollment in the longitudinal study between October 2003 and September 2005. Of these, 8 (n =3 with severe asthma) did not return for monthly follow-up after the first visit. Four additional children (n = 1 with severe asthma) dropped out of the study after the third visit. Thus, 12 children with mild-to-moderate and 16 with severe asthma completed each of the required visits and are included in the final data analysis.
The children who participated in the longitudinal study had similar features to those in the baseline characterization (data not shown). There were no differences between groups with respect to age (11 ± 3 vs 10 ± 3 years, severe vs mild-to-moderate) or sex (56% vs 42% male), but again there were more African American subjects in the severe asthma group (52% vs 8%; P = .018). By definition, subjects with severe asthma received higher doses of fluticasone at the onset of the study (779 ± 330 vs 242 ± 271 mcg/d; P < .001). ICS doses were titrated as clinically indicated over the 6-month period, and treatment for acute asthma exacerbations (ie, prednisone) was not withheld. Of the 3 children with mild-to-moderate asthma whose ICS doses were increased, the increase was not sufficient to warrant reclassification. None of the subjects with severe asthma required ICS dose increases during the study, because most were already receiving the maximum daily dose of fluticasone equivalents allowed. Two children with severe asthma were taking daily oral prednisone. No subject had a decrease or discontinuation of ICS during the study period.
Pulmonary function
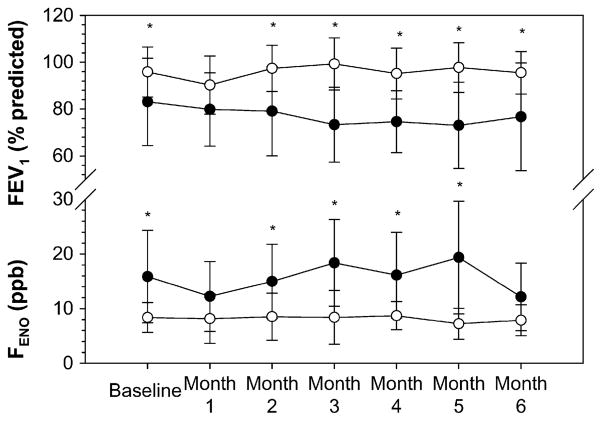
At the start of the study, children with severe asthma had greater airway obstruction (FEV1, 84% ± 18% vs 97% ± 12%; P = .05), lung hyperexpansion (RV:TLC, 0.25 ± 0.05 vs 0.21 ± 0.06; P = .05), and more allergic sensitization (serum IgE 1313 ± 2533 vs 277 ± 310 kU/L; P = .034). Although FEV1 did not differ between groups at month 1 (80% ± 16% vs 90% ± 12%; P = .124), FEV1 % predicted was significantly lower in children with severe asthma at months 2 through 6 (P ≤ .05 for all; Fig 2). The mean difference in FEV1 was also significantly different over time (P = .004) and remained significant after controlling for the month of enrollment (P = .008). Mean FEV1 averaged over 6 months was 79% ± 15% and 95% ± 13% (P =.023) for severe versus mild-to-moderate asthma, respectively.
FIG 2.
Longitudinal FENO and FEV1 data at baseline and over a period of 6 months. Data represent group means (severe asthma is shown in black circles). Group differences are depicted with asterisks (P < .05). Mean differences between groups remain significant over time after controlling for seasonality and serum IgE (repeated-measures ANOVA, P = .005).
Expired nitric oxide
Offline FENO concentrations were relatively higher in children with severe asthma at the start (15.5 ± 8.3 vs 8.7 ± 2.8 ppb; P = .05), and remained higher over the 6-month period of observation (Fig 2). Although FENO was not significantly different between groups at month 1 (12 ± 6 vs 8 ± 5 ppb; P = .133) and month 6 (12 ± 6 vs 8 ± 2 ppb; P = .088), mean differences in FENO over time were significant (P = .001). These differences remained significant after adjusting the analysis for the potentially confounding effects of month of enrollment/seasonality and serum IgE (P = .005). Mean offline FENO averaged over 6 months was 15.1 ± 5.4 vs 9.9 ± 5.8 ppb in children with severe versus mild-to-moderate asthma, respectively (P = .038).
Asthma exacerbations
The frequency of significant acute asthma exacerbations, defined as those requiring a prednisone burst, emergency department visit, or hospitalization for worsening of asthma symptoms, was higher in the severe asthma group (83% vs 43%; P = .031). To identify baseline characteristics associated with a greater frequency of exacerbation during long-term study, we performed actuarial analysis using specific cut-points for clinically relevant characteristics. The following characteristics were tested: (1) African American ethnicity, (2) daily daytime symptom occurrence, (3) daily nocturnal symptom occurrence, (4) serum IgE ≥ 150 kU/L,15 (5) FEV1 reversibility ≥ 12%, (6) PC20 ≤ 16 mg/mL,12 (7) FEV1 < 80% predicted, (8) FEV1:forced vital capacity (FVC) < 0.80, (9) RV:TLC > 0.21, and (10) FENO > 10 ppb.16,17 Significant predictors of asthma exacerbations included baseline FEV1 < 80% predicted (P < .01), FEV1 < 80% predicted after bronchodilator withholding (P < .01), FEV1:FVC < 0.80 (P < .01; Fig 3), RV:TLC > 0.21 (P < .01), African American ethnicity (P < .05), and daily nocturnal symptom occurrence (P < .05).
FIG 3.
Actuarial plot showing the probability of remaining exacerbation-free over the 6-month period study interval for subjects grouped according to high (≥0.80) and low (<0.80) baseline FEV1:FVC. P values were calculated using Cox regression. +, One or more subjects within the group remained exacerbation-free over the duration of the study.
DISCUSSION
When compared with subjects with mild-to-moderate asthma, children with severe asthma have greater airway obstruction, increased markers of allergic sensitization, increased bronchial hyperresponsiveness to methacholine, and lung hyperexpansion. Both the obstructive changes in lung function and elevated FENO persist over time despite adjustments in ICS treatment. Although asthma exacerbations were common in both groups, they were more frequent in children with severe asthma, and were highly associated with baseline markers of airway obstruction. These differences support the idea that unique pathophysiological attributes of a severe asthma phenotype are recognizable in childhood. We would also point out that unlike severe asthma in adults, which appears to include a broad spectrum of phenotypes,18 severe asthma in children is characterized by a relatively narrow spectrum of derangements that includes marked atopy and increased FENO.
The FENO is a noninvasive marker of airway inflammation that is elevated in children with asthma19 and allergic rhinitis20,21 at baseline and during acute exacerbations.16,22 In subjects with mild asthma, FENO correlates with the magnitude of airway eosinophilia23 and bronchial hyper-responsiveness24 and falls in a dose-dependent manner after the initiation of ICS.25 Accordingly, FENO in our study was lower in children with mild-to-moderate asthma treated with lower doses of ICS than in children with severe asthma on twice the amount of daily ICS. Similar findings have been reported by others5,26 and suggest that persistent airway inflammation may be a defining feature of at least a subpopulation of children with severe asthma.27 Although allergic sensitization per se may certainly contribute to FENO in children with asthma via late-phase influx of eosinophils28 and nitric oxide formation29,30 after aeroallergen exposure, our data suggest that atopy cannot solely explain FENO elevations in severe asthma. Rather, it would appear from our data that allergic sensitization is a unique defining feature of severe asthma in children. It may be that the specific mechanisms of asthma and atopy, both related to TH2-mediated IL-4 and IL-5 release, uniquely interact in children with severe asthma to upregulate FENO production.
This study has a number of limitations. Although we monitored ICS use and made dose adjustments as indicated by clinical status in the current study, poor adherence and undertreatment may have influenced our findings.31 However, we submit reduced adherence to ICS per se likely does not account for the findings in this study because ICS adherence is as low or even lower in children with mild-to-moderate asthma as it is in children with severe asthma.32 Corticosteroid insensitivity has been described in patients with difficult-to-control asthma5; however, because we did not conduct a specific corticosteroid-response characterization protocol in the current study, we can make no direct inferences as to the prevalence of corticosteroid insensitivity in the 2 study groups. Furthermore, because the children selected for this study were recruited from an academic referral practice that draws primarily from a tertiary urban children’s hospital, it is possible that some of the phenotypic features of severe asthma that we observed (eg, greater allergic sensitization, higher prevalence of African American ethnicity) may be a result of sample bias and are not representative of the general population of children with asthma. However, a recent study suggested that risk factors for childhood asthma did not differ between nonurban and urban environments.33 In this study and others, children with severe asthma had greater allergic sensitization to aeroallergens and were more likely to be of African American descent.33,34 Population-based studies further suggest disparate trends in asthma prevalence between African American subjects and other ethnicities even after controlling for potentially confounding effects of socioeconomic status and urban residence.35,36 Finally, the sample size used in this study may have been insufficient to identify alternate phenotypes including children with relatively less atopy.
In this characterization study, we have shown that repeated exacerbations, greater allergic sensitization, air-flow obstruction, and increased FENO despite treatment with high-dose ICS are differentiating features of severe versus mild-to-moderate asthma in children. Although severe asthma is certainly a heterogeneous disease, these findings suggest that unlike adults with severe asthma, who are characterized by multiple phenotypes,18,37 children with severe asthma may be predominantly characterized by TH2-mediated patterns of chronic airway inflammation. An important unanswered question is whether this phenotype will change over time. Will the features of the phenotype change over time to the adult pattern of fixed airway obstruction, lower levels of FENO, and less atopy as suggested by large cross-sectional studies in adults with severe asthma18? Alternatively, is severe asthma in adults a more diverse phenotype broadened by inclusion of subjects with new-onset asthma with a unique set of environmental exposures? Long-term cohort studies are necessary to answer these questions and to understand further the progression of severe asthma from childhood to adulthood.
The SARP is a multicenter asthma research group funded by the NHLBI and consisting of the following contributors (Steering Committee members are marked with an asterisk): Brigham and Women’s Hospital: Elliot Israel,* Bruce D. Levy, Gautham Marigowda; Cleveland Clinic Foundation: Serpil C. Erzurum,* Raed A. Dweik, Suzy A. A. Comhair, Abigail R. Lara, Marcelle Baaklini, Daniel Laskowski, Jacqueline Pyle; Emory University: W. Gerald Teague,* Anne M. Fitzpatrick, Eric Hunter; Imperial College School of Medicine: Kian Fan Chung,* Mark Hew, Alfonso Torrego, Sally Meah, Mun Lim; National Jewish Medical and Research Center: Sally E. Wenzel,* Diane Rhodes; University of Pittsburgh: William J. Calhoun,* Renee Folger, Bill T. Ameredes, Melissa P. Clark, Rebecca Z. Wade; University of Virginia: Benjamin Gaston,* John Hunt, Peter Urban; University of Wisconsin: William W. Busse,* Nizar Jarjour, Erin Billmeyer, Cheri Swenson, Gina Crisafi; Wake Forest University: Eugene R. Bleecker,* Deborah Meyers, Wendy Moore, Stephen Peters, Annette Hastie, Gregory Hawkins, Jeffrey Krings, Regina Smith; Washington University in St Louis: Mario Castro,* Leonard Bacharier, Iftikhar Hussain, Jaime Tarsi; Data Coordinating Center: James R. Murphy,* Douglas Curran-Everett; NHLBI: Patricia Noel.
Acknowledgments
We acknowledge the assistance of Lou Ann Brown, PhD, Eric Hunter, BS, Joanne Costolnick, RN, Nicholas Raviele, BS, Wendell Gibson, RRT, Crista Williams, RRT, and Paul Via, MS.
Supported by the National Institutes of Health/National Heart, Lung, and Blood Institute Severe Asthma Research Program (RO1 HL69170).
Abbreviations used
- FENO
Exhaled nitric oxide
- FVC
Forced vital capacity
- ICS
Inhaled corticosteroid
- NHLBI
National Heart, Lung, and Blood Institute
- RV
Residual volume
- SARP
Severe Asthma Research Program
- TLC
Total lung capacity
Footnotes
Disclosure of potential conflict of interest: W. G. Teague is on the speakers’ bureau for Merck. The rest of the authors have declared that they have no conflict of interest.
References
- 1.Szefler SJ, Martin RJ, King SP, Boushey HA, Cherniak RM, Chinchilli VM, et al. Significant variability in response to inhaled corticosteroids for persistent asthma. J Allergy Clin Immunol. 2002;109:410–8. doi: 10.1067/mai.2002.122635. [DOI] [PubMed] [Google Scholar]
- 2.Rasmussen F, Taylor DR, Flannery EM, Cowan JO, Greene JM, Herbison GP, et al. Risk factors for hospital admission for asthma from childhood to young adulthood: a longitudinal population study. J Allergy Clin Immunol. 2002;110:220–7. doi: 10.1067/mai.2002.125295. [DOI] [PubMed] [Google Scholar]
- 3.de Blic J, Tillie-Leblond I, Tonnel A, Jaubert F, Scheinmann P, Gosset P. Difficult asthma in children: an analysis of airway inflammation. J Allergy Clin Immunol. 2004;113:94–100. doi: 10.1016/j.jaci.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 4.Payne DNR, Rogers AV, Adelroth E, Bandi V, Guntupalli KK, Bush A, et al. Early thickening of the reticular basement membrane in children with difficult asthma. Am J Respir Crit Care Med. 2003;167:78–82. doi: 10.1164/rccm.200205-414OC. [DOI] [PubMed] [Google Scholar]
- 5.Payne DNR, Adcock IM, Wilson NM, Oates T, Scallan M, Bush A. Relationship between exhaled nitric oxide and mucosal eosinophilic inflammation in children with difficult asthma, after treatment with oral prednisolone. Am J Respir Crit Care Med. 2001;164:1376–81. doi: 10.1164/ajrccm.164.8.2101145. [DOI] [PubMed] [Google Scholar]
- 6.American Thoracic Society. Lung function testing: selection of reference values and interpretive strategies. Am Rev Respir Dis. 1991;144:1202–18. doi: 10.1164/ajrccm/144.5.1202. [DOI] [PubMed] [Google Scholar]
- 7.American Thoracic Society. Proceedings of the American Thoracic Society Workshop on Refractory Asthma: current understanding, recommendations, and unanswered questions. Am J Respir Crit Care Med. 2000;162:2341–51. doi: 10.1164/ajrccm.162.6.ats9-00. [DOI] [PubMed] [Google Scholar]
- 8.National Asthma Education and Prevention Program. Expert Panel report: guidelines for the diagnosis and management of asthma: update on selected topics 2002. Bethesda (MD): National Heart, Lung, and Blood Institute; 2003. Publication #02-5075. [PubMed] [Google Scholar]
- 9.American Thoracic Society. Standardization of spirometry: 1994 update. Am J Respir Crit Care Med. 1995;152:1107–36. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 10.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–87. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 11.Crapo RO, Morris AH, Gardner RM. Reference spirometric values using techniques and equipment that meet ATS recommendations. Am Rev Respir Dis. 1981;123:659–64. doi: 10.1164/arrd.1981.123.6.659. [DOI] [PubMed] [Google Scholar]
- 12.American Thoracic Society. Guidelines for methacholine and exercise challenge testing: 1999. Am J Respir Crit Care Med. 2000;161:309–29. doi: 10.1164/ajrccm.161.1.ats11-99. [DOI] [PubMed] [Google Scholar]
- 13.European Respiratory Society Task Force. Measurement of exhaled nitric oxide in children, 2001. Eur Respir J. 2002;20:1–15. doi: 10.1183/09031936.02.00293102. [DOI] [PubMed] [Google Scholar]
- 14.American Thoracic Society and the European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171:912–30. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 15.Borish L, Chipps B, Deniz Y, Gujrathi S, Zheng B, Dolan CM. Total serum IgE levels in a large cohort of patients with severe or difficult-to-treat asthma. Ann Allergy Asthma Immunol. 2005;95:247–53. doi: 10.1016/S1081-1206(10)61221-5. [DOI] [PubMed] [Google Scholar]
- 16.Jones SL, Kittleson J, Cowan JO, Flannery EM, Hancox RJ, McLachlan CR, et al. The predictive value of exhaled nitric oxide measurements in assessing changes in asthma control. Am J Respir Crit Care Med. 2001;164:738–43. doi: 10.1164/ajrccm.164.5.2012125. [DOI] [PubMed] [Google Scholar]
- 17.Crater SE, Peters EJ, Martin ML, Murphy AW, Platts-Mills TAE. Expired nitric oxide and airway obstruction in asthma patients with an acute exacerbation. Am J Respir Crit Care Med. 1999;159:806–11. doi: 10.1164/ajrccm.159.3.9805103. [DOI] [PubMed] [Google Scholar]
- 18.The ENFUMOSA Study Group. The ENFUMOSA cross-sectional European multicentre study of the clinical phenotype of chronic severe asthma. Eur Respir J. 2003;22:470–7. doi: 10.1183/09031936.03.00261903. [DOI] [PubMed] [Google Scholar]
- 19.Nelson BV, Sears S, Woods J, Ling CY, Hunt J, Clapper LM, et al. Expired nitric oxide as a marker for childhood asthma. J Pediatr. 1997;130:423–7. doi: 10.1016/s0022-3476(97)70204-x. [DOI] [PubMed] [Google Scholar]
- 20.Brussee JE, Smit HA, Kerkhof M, Koopman LP, Wijga AH, Postma DS, et al. Exhaled nitric oxide in 4-year-old children: relationship with asthma and atopy. Eur Respir J. 2005;25:455–61. doi: 10.1183/09031936.05.00079604. [DOI] [PubMed] [Google Scholar]
- 21.Frank TL, Adisesh A, Pickering AC, Morrison JFJ, Wright T, Francis H, et al. Relationship between exhaled nitric oxide and childhood asthma. Am J Respir Crit Care Med. 1998;158:1032–6. doi: 10.1164/ajrccm.158.4.9707143. [DOI] [PubMed] [Google Scholar]
- 22.Pijnenburg MW, Hofhuis W, Hop WC, De Jongste JC. Exhaled nitric oxide predicts asthma relapse in children with clinical asthma remission. Thorax. 2005;60:215–8. doi: 10.1136/thx.2004.023374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Warke TJ, Fitch PS, Brown V, Taylor R, Lyons JDM, Ennis M, et al. Exhaled nitric oxide correlates with airway eosinophils in childhood asthma. Thorax. 2002;57:383–7. doi: 10.1136/thorax.57.5.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berkman N, Avital A, Breuer R, Bardach E, Springer C, Godfrey S. Exhaled nitric oxide in the diagnosis of asthma: comparison with bronchial provocation tests. Thorax. 2005;60:383–8. doi: 10.1136/thx.2004.031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones SL, Herbison P, Cowan JO, Flannery EM, Hancox RJ, McLachlan CR, et al. Exhaled NO and assessment of anti-inflammatory effects of inhaled steroid: dose-response relationship. Eur Respir J. 2002;60:601–8. doi: 10.1183/09031936.02.00285302. [DOI] [PubMed] [Google Scholar]
- 26.Stirling RG, Kharitonov SA, Campbell D, Robinson DS, Durham SR, Chung KF, et al. Increase in exhaled nitric oxide levels in patients with difficult asthma and correlation with symptoms and disease severity despite treatment with oral and inhaled corticosteroids. Thorax. 1998;53:1030–4. doi: 10.1136/thx.53.12.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Payne DNR, Wilson NM, James A, Hablas H, Agrafioti C, Bush A. Evidence for different subgroups of difficult asthma in children. Thorax. 2001;56:345–50. doi: 10.1136/thorax.56.5.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cieslewicz G, Tomkinson A, Adler A, Duez C, Schwarze J, Takeda K, et al. The late, but not early, asthmatic response is dependent on IL-5 and correlates with eosinophil infiltration. J Clin Invest. 1999;104:301–8. doi: 10.1172/JCI7010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roberts G, Hurley C, Bush A, Lack G. Longitudinal study of grass pollen exposure, symptoms, and exhaled nitric oxide in childhood seasonal allergic asthma. Thorax. 2004;59:752–6. doi: 10.1136/thx.2003.008722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Kluijver J, Evertse CE, Schrumpf JA, van der Veen H, Zwinderman AH, Hiemstra PS, et al. Asymptomatic worsening of airway inflammation during low-dose allergen exposure in asthma: protection by inhaled steroids. Am J Respir Crit Care Med. 2002;166:294–300. doi: 10.1164/rccm.2112097. [DOI] [PubMed] [Google Scholar]
- 31.Robinson DS, Campbell DA, Durham SR, Pfeffer J, Barnes PJ, Chung KF. Systematic assessment of difficult-to-treat asthma. Eur Respir J. 2003;22:478–83. doi: 10.1183/09031936.03.00017003. [DOI] [PubMed] [Google Scholar]
- 32.Wraight JM, Cowan JO, Flannery EM, Town GI, Taylor DR. Adherence to asthma self-management plans with inhaled corticosteroid and oral prednisone: a descriptive analysis. Respirology. 2002;7:133–9. doi: 10.1046/j.1440-1843.2002.00374.x. [DOI] [PubMed] [Google Scholar]
- 33.Higgins PS, Wakefield D, Cloutier MM. Risk factors for asthma and asthma severity in nonurban children in Connecticut. Chest. 2005;128:3846–53. doi: 10.1378/chest.128.6.3846. [DOI] [PubMed] [Google Scholar]
- 34.Gruchalla RS, Pongracic JP, Marshall Evans R, Visness CM, Walter M, Crain EF, et al. Inner City Asthma Study: relationships among sensitivity, allergen exposure, and asthma morbidity. J Allergy Clin Immunol. 2005;115:478–85. doi: 10.1016/j.jaci.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 35.Pearlman DN, Zierler S, Meersman S, Kim HK, Viner-Brown SI, Caron C. Race disparities in childhood asthma: does where you live matter? J Natl Med Assoc. 2006;98:239–47. [PMC free article] [PubMed] [Google Scholar]
- 36.McDaniel M, Paxson C, Waldfogel J. Racial disparities in childhood asthma in the United States: evidence from the National Health Interview Survey, 1997 to 2003. Pediatrics. 2006;117:e868–77. doi: 10.1542/peds.2005-1721. [DOI] [PubMed] [Google Scholar]
- 37.Miranda C, Busacker A, Balzar S, Trudeau J, Wenzel SE. Distinguishing severe asthma phenotypes: role of age at onset and eosinophilic inflammation. J Allergy Clin Immunol. 2004;113:101–8. doi: 10.1016/j.jaci.2003.10.041. [DOI] [PubMed] [Google Scholar]