Abstract
We have examined the expression and localization patterns of hyaluronan and hyaluronan-binding chondroitin sulfate proteoglycans in neural stem cells and differentiated neural cells derived from mouse embryonic stem cells. Expression of proteoglycans and hyaluronan was weak in the SSEA1-positive embryonic stem cells but increased noticeably after retinoic acid induction to nestin-positive neural stem cells. After subsequent plating, the hyaluronan-binding chondroitin sulfate proteoglycans aggrecan, neurocan and versican are expressed by cells in both the astrocytic and neuronal lineages. During the time period that hyaluronan was present, it co-localized with each of the hyaluronan-binding proteoglycans studied, and was found to be clearly associated with β-III tubulin-expressing neurons and oligodendrocytes expressing the O4 sulfatide marker. Although proteoglycan expression levels increased to varying degrees following neural differentiation they did not change noticably during the following two weeks in culture, but there was a significant decrease in hyaluronan expression. Our studies therefore demonstrate the expression by neural stem cells and neural cells derived from them of hyaluronan and its associated proteoglycans, thereby providing a necessary foundation for integrating their specific properties into developing strategies for therapeutic applications.
Keywords: embryonic stem cells, neural stem cells, chondroitin sulfate, proteoglycans
1. Introduction
There is considerable interest in the potential use of stem cells for restoration of function in the central nervous system after injury, or as a result of age-related degenerative and disease processes (Cao et al., 2002; Lindvall and Kokaia, 2006; Dimos et al., 2008). It is likely that neural cell lines derived from stem cells, rather than stem cells themselves, will be most useful for purposes of implantation. These cell lines include various types of clonally derived cells that are capable of indefinite replication in tissue culture and differentiation into neural cells (Gottlieb, 2002). Morphogenic signals coordinate major stem cell decisions to regulate the size, shape, and cellular diversity in nervous tissue development (Panchision and McKay, 2002; Muotri and Gage, 2006; Krencik and Zhang, 2006), and there is no doubt that extracellular matrix macromolecules, representing a major component of the biochemical environment of implanted neural cells, will have highly significant effects on their survival and functional properties.
It has now been demonstrated that the approaches for deriving neural cells from mouse embryonic stem (ES) cells are also applicable to human progenitor cells, and it appears that current tissue culture methods can be readily perfected to allow the isolation of highly purified preparations of the major neural cell types. The suitability of stem cells for the preparation of genetically engineered cell lines represents an additional advantage for implantation research, and optimization of transfection protocols has allowed improved genetic manipulation of human embryonic stem cells (Braam et al., 2008).
Proteoglycans and related extracellular matrix components with which they interact (for reviews, see Sugahara and Mikami, 2007; Zimmermann and Dours-Zimmermann, 2008) are obvious candidates for molecules that can be expected to exert a decisive influence on stem cell behavior. These macromolecules would occur both as a result of expression by the stem cells themselves (the subject of this study of hyaluronan and hyaluronan-binding chondroitin sulfate proteoglycans), and as those present in the recipient tissue, which could be expected to result in reciprocal effects.
Hyaluronan is an unsulfated glycosaminoglycan composed of disaccharide repeating units of d-glucuronic acid and N-acetylglucosamine, and has a molecular size of ∼140,000 in brain (Margolis, 1967). The four members of the aggrecan family of hyaluronan-binding chondroitin sulfate proteoglycans contain N-terminal immunoglobulin-like domains followed by tandem repeats that mediate their binding to hyaluronan, and a C-terminal region with epidermal growth factor-like, lectin-like, and complement regulatory protein-like domains. These N- and C-terminal regions have a high degree of amino acid sequence identity, and are separated by a nonhomologous glycosaminoglycan attachment region. In the case of aggrecan (Neame and Barry, 1993) and neurocan (Rauch et al., 1992; Meyer-Puttlitz et al., 1995) binding to hyaluronan has been shown to be stabilized by a 45 kDa link protein that is structurally similar to the N-termini of the proteoglycans, insofar as it has a hyaluronan binding domain and an Ig-like domain that interacts with that of the proteoglycans. A brain-specific link protein (Bral1) has also been identified (Hirakawa et al., 2000), and appears to be involved in the stabilization of versican binding to hyaluronan (Oohashi et al., 2002). In brain and spinal cord, aggrecan, neurocan, versican, hyaluronan and link protein are present in some or all perineuronal nets (Carulli et al., 2007; Galtrey et al., 2008), which are dense extracellular matrix structures that develop around many neuronal cell bodies late in development and are thought to be involved in restricting experience-dependent synaptic plasticity in the adult. In this study we have examined the expression and localization of hyaluronan and its associated chondroitin sulfate proteoglycans in neural stem cells and in differentiated neurons and glia derived from them.
2. Results
Studies were performed using both the W4/129S6 cell line and a mouse embryonic stem cell line (E14Tg2a.4) that does not require a feeder layer. Examination of proteoglycan and hyaluronan expression gave identical results in both cases (for examples of E14 cell results, see Fig. 1, A-C and D-F; Fig. 2, A-C; Fig. 4; supplemental Figs. 1, 2 AC and D-F, and 3, A-C), but unless indicated otherwise, the figures of neurally differentiated cells show results obtained with the W4/129S6 cell line.
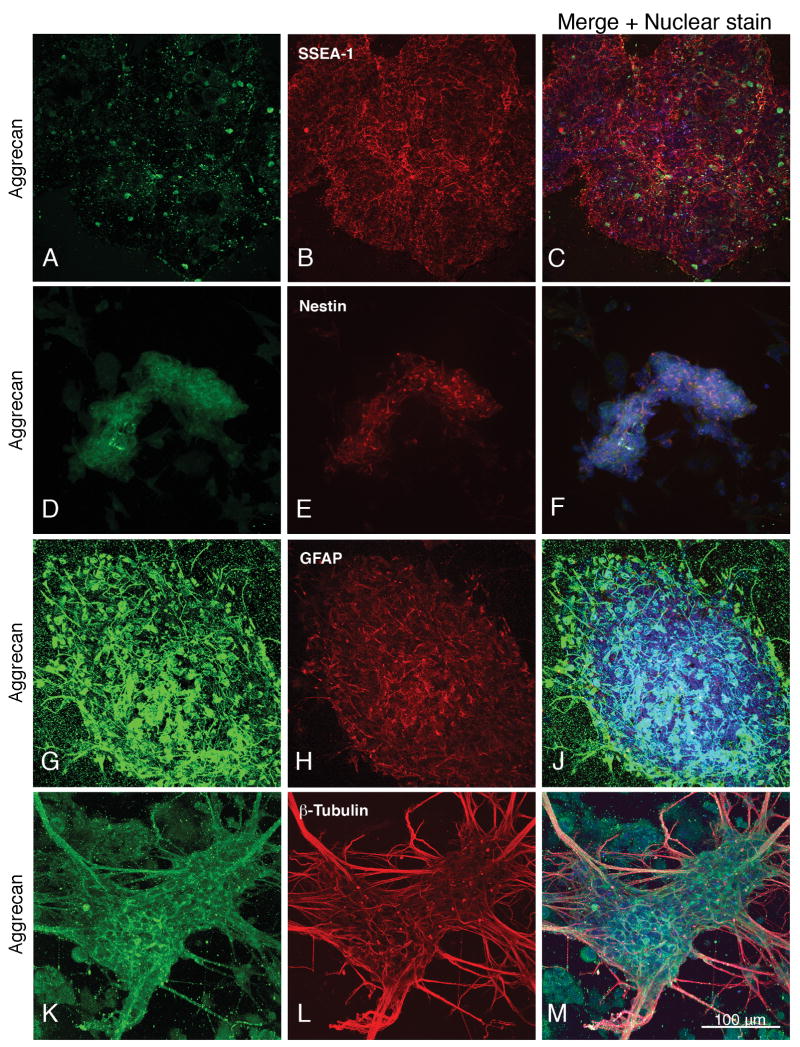
Fig. 1.
Expression of aggrecan in mouse E14T embryonic stem cells (day 0, A-C), and in neural stem cells on the fourth day of retinoic acid treatment during the neural induction period (D-F). Embryonic and neural stem cells are identified by their expression of SSEA-1 and nestin, respectively. Expression of aggrecan by neural cells derived from embryonic stem cells at differentiated day 4 is shown in panels G-J and K-M. Astrocytes and neurons are identified by their expression of GFAP and β-III tubulin. (The punctate labeling occasionally seen in areas where cells are absent represents proteoglycan that is shed or secreted from the cells and adheres to the coverslip.) Nuclear staining with TO-PRO-3 is shown in blue. Magnification: 160×.
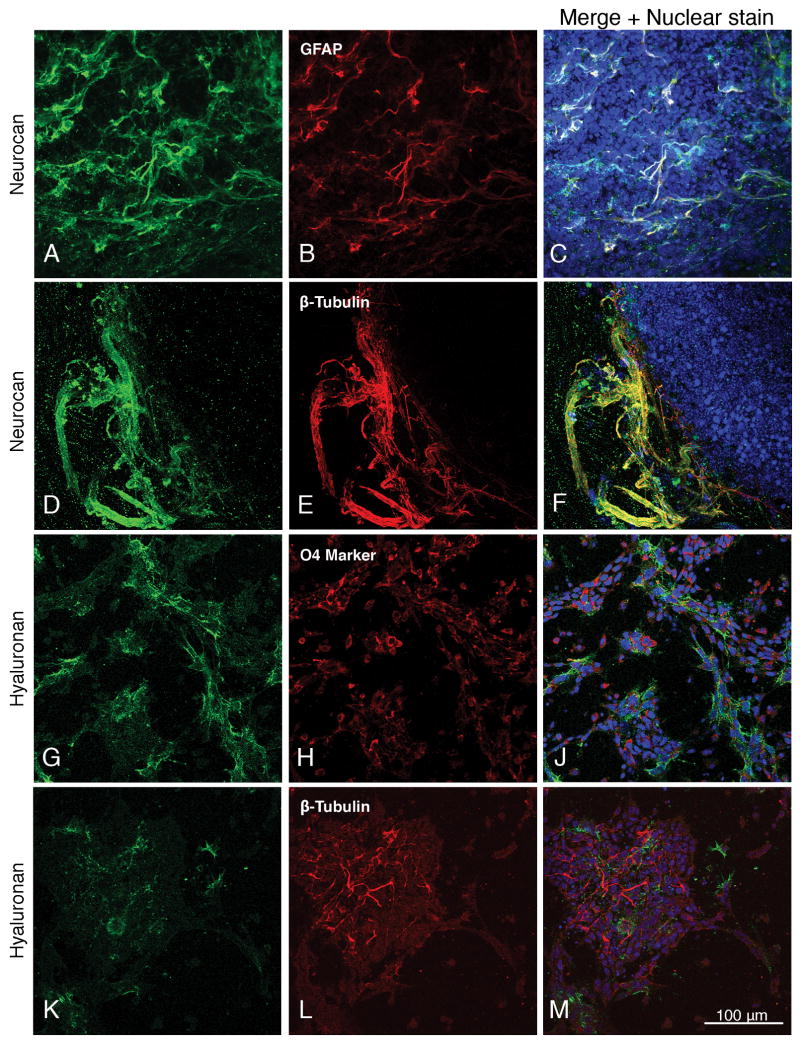
Fig. 2.
A-C and D-F: Expression of neurocan by neural cells derived from embryonic stem cells at differentiated day 4. Astrocytes and neurons are identified by their expression of GFAP and β-III tubulin. Expression of hyaluronan by mouse embryonic stem cells one day after differentiation to oligodendrocytes expressing the O4 sulfatide marker (G-J), and to β-III tubulin-expressing neurons (K-M). Panels A-C show the feeder-independent E14T cell line. Nuclear staining with TO-PRO-3 is shown in blue. Magnification: 160×.
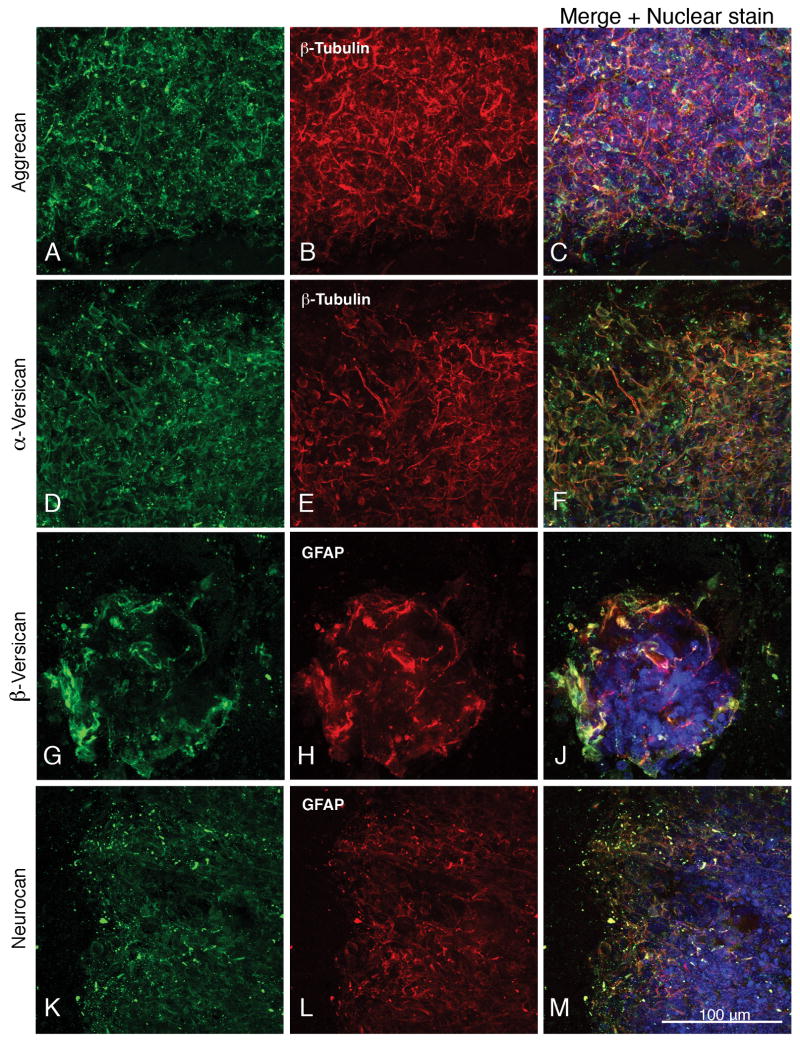
Fig. 4.
Higher magnification images (250×) showing the co-expression of aggrecan, α-versican, β-versican and neurocan with neuronal and astrocytic markers (β-III tubulin and GFAP, respectively). Panels A-C, G-J and K-M show differentiated day 4 cells, and panels D-F show differentiated day 7 cells. Nuclear staining with TO-PRO-3 is shown in blue.
All of the proteoglycans and hyaluronan were expressed to varying extents in the SSEA-positive, nestin-negative embryonic stem cells, but expression of the hyaluronan-binding chondroitin sulfate proteoglycans aggrecan, neurocan, α-versican and β-versican was very weak, and the expression of hyaluronan itself was significantly less than at day 2+ of neural induction (Fig. 1, A-C; supplemental Fig. 1, A, C, and E).
During the second day of the retinoic acid treatment (day 2+), SSEA-1 staining has decreased and nestin staining first appears, but neither tubulin nor GFAP are detectable. Proteoglycan and hyaluronan staining has, however, increased in intensity as compared to the embryonic stem cells (supplemental Fig. 1, B, D, and F). At day 4 of the retinoic acid treatment, only a small proportion of cells still show SSEA-1 staining whereas almost all are strongly nestin positive (Fig. 1, D-F; supplemental Fig. 2, A-C and D-F). There is significant proteoglycan and hyaluronan expression in these neural stem cells (Fig. 1, D-F; supplemental Fig. 2, A-C and D-F) as well as the first appearance of β-tubulin, whereas GFAP is absent (data not shown).
By one day after re-plating (“differentiated day 1” following the retinoic acid neural induction) most cells are strongly nestin-positive and there is similarly strong staining of β-tubulin, the oligodendrocyte O4 marker, and hyaluronan (Fig. 2, G-J and K-M; supplemental Fig. 3, A-C), whereas significant staining of GFAP did not usually appear until days 4-7 of differentiation, at which time nestin has greatly decreased (data not shown). It should also be noted in this connection that the neural induction protocol used in our studies yielded only a small proportion of oligodendrocytes. Hyaluronan expression decreased after day 4-7, and although it was seen at day 4 (supplemental Fig. 2, H and L), there were relatively few GFAP-expressing astrocytes at this time point. We therefore did not find any reliable evidence supporting the expression of hyaluronan by astrocytes, although this possibility cannot be excluded.
Aggrecan, neurocan and versican are expressed by cells in both the astrocytic and neuronal lineages, as shown by their co-expression with GFAP and β-tubulin (Fig. 1, G-J and K-M; Figs. 2-4; supplemental Fig. 3). Aggrecan surrounds GFAP-expressing cells (Fig. 1, G-J) and is present on astrocytic and neuronal processes as well as surrounding neuronal cell bodies (Fig. 1, K-M; Fig. 4, A-C; supplemental Fig. 3, D-F).
Whereas aggrecan is distributed relatively uniformly throughout the cell masses, neurocan is frequently concentrated at the periphery where it co-localizes with astroglial and neuronal processes (Fig. 2, A-C and D-F) and with hyaluronan (supplemental Fig. 2, G-J).
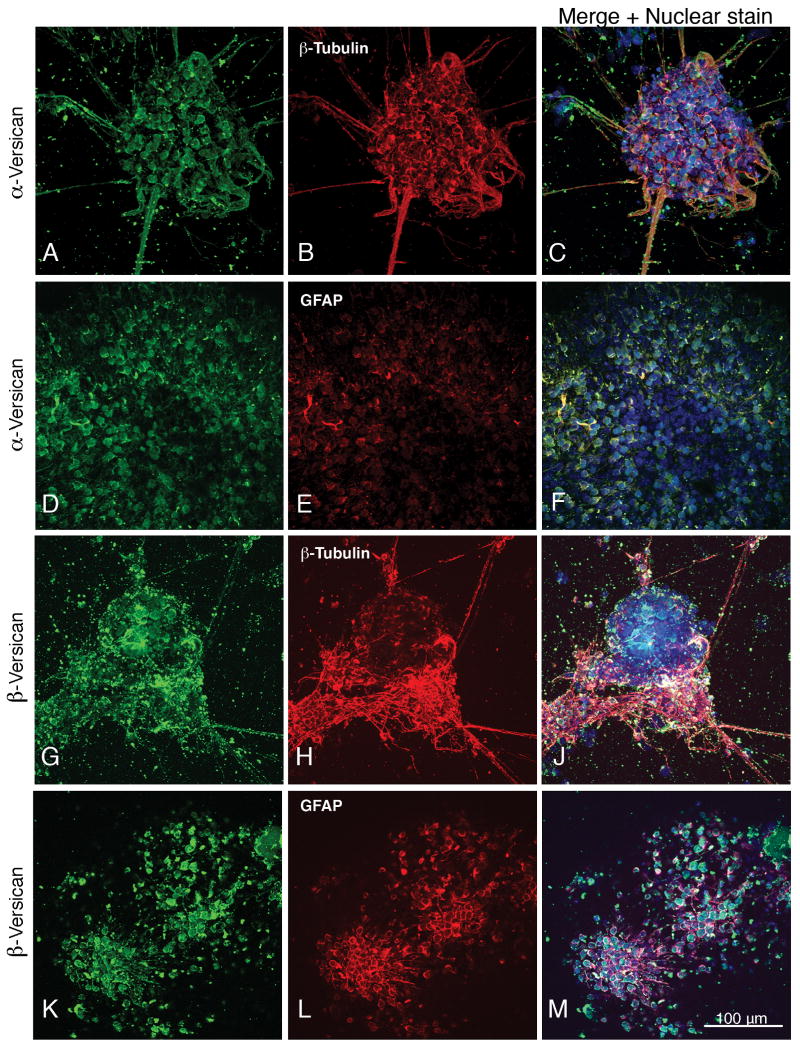
The hyaluronan-binding proteoglycans containing the α and/or β chondroitin sulfate attachment domains of versican (these splice variants are referred to in this report as α-versican and β-versican) co-localized with neuronal (Fig. 3, A-C and supplemental Fig. 3, G-J) and GFAP-expressing astrocyte cell bodies (Fig. 3, D-F and K-M; supplemental Fig. 3, K-M), and were present on neuronal processes (Fig. 3, A-C and G-J).
Fig. 3.
Expression of α-versican and β-versican by neural cells derived from embryonic stem cells at differentiated day 7. Neurons and astrocytes are identified by their expression of β-III tubulin and GFAP. Nuclear staining with TO-PRO-3 is shown in blue. Magnification: 160×.
Cells were examined at 1, 4, 7, 10, and 14 days post-differentiation, and although most of the fields selected to show expression of proteoglycans were from 4- and 7-day cultures, there was no clear trend of increased or decreased proteoglycan expression as a function of time after differentiation. However, as mentioned above, hyaluronan expression decreased with increasing time in the differentiated state.
3. Discussion
Studies of glycoconjugates in neural stem cells and progenitor cells have largely focused on glycolipids and glycoproteins (for a review, see Yanagisawa and Yu, 2007). Although until recently relatively little information has been available on neural stem/progenitor cell proteoglycans and extracellular matrix proteins, microarray studies compared proteoglycan gene expression in acutely isolated human brain A2B5+ progenitor cells to that in an unsorted white matter dissociate, of which they comprise ∼3% of the viable cells (Sim et al, 2006). These studies demonstrated a 5- to 10-fold greater expression of the gene for phosphacan and its transmembrane proteoglycan form (receptor protein-tyrosine phosphatase-ζ/β) in the progenitor cells, as well as less dramatic differences in the expression of other chondroitin sulfate proteoglycans such as neurocan, versican, NG2 and neuroglycan C, and of high-affinity neurocan and phosphacan ligands that we have earlier identified (e.g., tenascin-R, HB-GAM/pleiotrophin, NrCAM, NCAM; Friedlander et al., 1994, Milev et al., 1994, 1995, 1996, 1998b). Other studies have examined the secretion and gene expression of chondroitin sulfate proteoglycans by mouse neural precursor cells grown as neurospheres (Kabos et al., 2004; Ida et al., 2006). However, the neurosphere-forming stem and progenitor cells isolated from fetal tissue used in these studies differ in many significant respects from neural stem cells derived from embryonic stem cells (Shin et al., 2007).
With regard to hyaluronan and the hyaluronan-binding chondroitin sulfate proteoglycans studied here, it has been demonstrated that the full-length versican core protein (versican V0) and the alternative splicing isoform that contains only the β domain (versican V1) have inhibitory effects on neural crest cell migration (Dutt et al., 2006), and there is considerable evidence for signaling by versican to a wide variety of transcriptional pathways (for a review, see Rahmani et al., 2006). It has also been shown that in the developing brain versican is expressed by subsets of interneurons, where it appears to provide a lamina-specific cue for presynaptic maturation (Yamagata and Sanes, 2005). In view of these findings, it may be significant that the level of versican core protein transcript increases gt;10-fold following the differentiation of embryonic stem cells to embryoid bodies (Nairn et al., 2007). There are also distinctive neurobiological characteristics of versican isoforms containing the α or β glycosaminoglycan attachment domains. Isoforms containing the α domain were found to be present at a relatively low level during the late embryonic and early postnatal period, decreased by ∼50% between 1 and 2 weeks postnatal, and then increased steadily in concentration to reach a maximum at 100 days that was 7-fold that present at 10 days postnatal. The opposite pattern is seen with versican isoforms containing the β domain (Milev et al., 1998a). Moreover, versican V1 is expressed in many tissues whereas versican V2 that contains only the α domain is specific to the central nervous system where it is produced by oligodendrocytes, and there are significant differences in the localization of the two isoforms in brain (Zimmermann and Dours-Zimmermann, 2008).
The increase in hyaluronan expression that we observed cytochemically in neural stem cells as compared to the embryonic stem cells from which they were derived is generally consistent with the increase in hyaluronan levels (in this case almost 25-fold) measured in embryoid bodies as compared with embryonic stem cells, and the subsequent decrease observed after differentiation is also in agreement with the ∼45% decrease of hyaluronan recently reported in extraembryonic endoderm as compared to embryoid bodies (Nairn et al., 2007). The low or undetectable expression of hyaluronan by neurons and glia obtained by our procedures after a week or more in the differentiated state suggests that the chondroitin sulfate proteoglycans expressed by these cells generally do not occur in the form of aggregates with hyaluronan.
Based only on the information provided by this initial study demonstrating their expression at various stages of stem cell development, it is too early to draw any specific conclusions concerning the functional roles of hyaluronan and its associated chondroitin sulfate proteoglycans in determining the properties of these cells, and their transition from embryonic stem cell to neural stem cell to differentiated neurons and glia. In the case of neurocan, which we found to be largely associated with neuronal and glial processes or defined layers of cells rather than being distributed more uniformly in the cell mass, this observation perhaps reflects our earlier finding that neurocan inhibits cell adhesion (Friedlander et al., 1994), and may therefore be non-permissive for the formation of certain types of cell aggregates. Because proteoglycans are distinguished by their ability to bind a wide range of growth factors and other proteins, they may also mediate signalling to differentiating stem cells by serving as a reservoir for such molecules, whose effects may be further modulated through a proteoglycan gradient.
Our studies have demonstrated that neural stem cells and neural cells derived from a single known cell type (the embryonic stem cell), after differentiation along neuronal, astroglial, and oligodendroglial pathways, synthesize hyaluronan and the hyaluronan-binding chondroitin sulfate proteoglycans aggrecan, versican and neurocan. Although stem cell-based therapies have shown promise for the repair of spinal cord injury during the subacute post injury period (Karimi-Abdolrezaee et al., 2006), at later time periods this approach has been more problematic due to the presence of a glial scar. The glial scar contains high concentrations of chondroitin sulfate proteoglycans, especially neurocan and versican, that inhibit neurite growth and axonal regeneration (Cafferty et al., 2007; Laabs et al., 2007; Pizzi and Crowe, 2007). For the therapeutic application of neural stem cells it is therefore especially important to have information concerning the expression pattern of chondroitin sulfate proteoglycans and hyaluronan in neural stem cells, and their expression by neurons, astrocytes and oligodendroglia derived from them.
4. Experimental Procedures
Antibodies and probe for hyaluronan
The production and specificity of rabbit antibodies raised to glutathione S-transferase fusion proteins containing sequences from the nonhomologous chondroitin sulfate attachment regions of neurocan (Milev et al., 1996), aggrecan, and the α and β domains of versican (Milev et al., 1998a; Popp et al., 2003) have been described previously.
Antibodies to mouse glial fibrillary acidic protein (GFAP, MAB360), β-III tubulin (MAB1637), and the O4 oligodendrocyte marker (MAB345) were all obtained from Chemicon (Temecula, CA). Secondary antibodies (FITC goat anti-rabbit IgG and Texas Red goat anti-mouse IgG or IgM) were obtained from Jackson ImmunoResearch (West Grove, PA). The MC-480 antibody to SSEA-1 and the Rat-401 antibody to nestin were obtained from the Developmental Studies Hybridoma Bank (Iowa City, IA).
Hyaluronan was detected using a GFP fusion protein containing the hyaluronan binding region of neurocan (Zhang et al., 2004) expressed in HEK 293 FT cells using a cDNA construct provided by Uwe Rauch (University of Lund, Sweden). The specificity of this probe was confirmed by the absence of staining after preincubation with hyaluronan oligosaccharides (Ripellino et al., 1985), or treatment of cells with Streptomyces hyaluronidase (Ripellino et al., 1988).
Cell culture media and other chemicals
Dulbecco's Modified Eagle Medium (DMEM) with high glucose (11960-069), MEM non-essential amino acids (11140-050), MEM sodium pyruvate (11360-070), L-glutamine (25030-081), penicillin-streptomycin (15140-122), and trypsin-EDTA (25300-054) were all from Gibco/Invitrogen (Carlsbad, CA). ES Qualified fetal bovine serum (100-125) was from Gemini Bio-Products (Woodland, CA). Leukemia inhibitory factor (LIF ESGRO, ESG1107) was obtained from Chemicon (Temecula, CA).
Culture of mouse embryonic stem cells
Mouse ES and EMFI cells were obtained from the Alexandra Joyner laboratory (New York University Medical Center) and cultured as described by Matise et al. (2000). Mitomycin C-treated primary mouse embryonic fibroblast (EMFI) feeder cells (Doetschman et al., 1985) were plated on dishes in EMFI medium (DMEM containing 0.1 mM non-essential amino acids, 1 mM sodium pyruvate, 2 mM L-glutamine, 15% heat-inactivated fetal bovine serum, and 50 μg/ml penicillin/streptomycin). The following day, W4/129S6 ES cells (Auerbach et al., 2000) were suspended in ES cell medium (EMFI medium containing ß-mercaptoethanol, 0.1 mM, and leukemia inhibitory factor, 1000 units/ml) and plated on the feeder layer. The ES cells (from a stock used to make knockout mice, and thus demonstrably normal) were propagated for only a limited time (∼1 month) before starting with new frozen stocks to avoid working with karyotypic variants. After 24 h in culture (65-70% confluent) the medium was replaced, and after a further 24 h the cells (90-95% confluent) were plated on 10 cm dishes (Nunc 1-50679) coated with 0.1% bovine skin gelatin (Sigma G9391) and maintained in this manner with a further replating over the following three days.
Culture of feeder-independent mouse embryonic stem cells
The feeder free E14Tg2a.4 mouse embryonic stem cell line was obtained from the Mutant Mouse Regional Resource Center (Davis, CA) and cultured in a manner very similar to that described above, with some minor differences in medium composition, trypsinization and gelatin coating of plates (Brennan and Skarnes, 1999; Skarnes, 2000). Differentiation and subsequent steps were performed exactly as described below for the W4/129S6 cell line.
Neural induction
Neural differentiation of ES cells was performed by the general procedure of Bain et al. (1998) but using the slightly simplified EMFI medium described above. ES cells were detached by mild trypsinization in EMFI medium and placed in suspension culture in dishes (Corning 430591) coated with 0.1% agar (Gibco/BRL M00391B). After 48 h they were transferred to fresh EMFI medium following gravity sedimentation (10 min in 15 ml conical tubes) and replated in the same dish. Two days later they were transferred by sedimentation to EMFI medium containing 0.6 μM all-trans-retinoic acid (Sigma R2625), and the medium was changed after a further two days. (During the 4-day period when cells were cultured in suspension with retinoic acid, the second day is referred to as day 2+, and the fourth and final day of the retinoic acid treatment as day 4+.) At days 2+ and 4+, and nine days after beginning the retinoic acid induction, cells were seeded onto glass coverslips coated with 0.1 mg/ml poly-d-lysine (MW >300,000, Sigma P1024) in a 24-well plate (Corning 3527, 0.5-1 × 106 cells/well). Differentiated cells were maintained for up to two weeks for staining.
Cytochemistry
Cells on coverslips were washed with PBS and fixed for 30 min at room temperature with either 3% paraformaldehyde or, for studies using the hyaluronan probe, with paraformaldehyde containing 0.5% cetylpyridinium chloride. After washing 3 × 5 min with PBS, cells were treated for 1 h at room temperature with 5% BSA in PBS, and for immunocytochemical detection of intracellular markers (e.g., tubulin, GFAP) cells were permeabilized by inclusion of 0.2% Triton X-100 in the BSA blocking solution. Cells were treated overnight at 4°C in a humidified chamber with primary antibodies in BSA blocking solution, washed 3 × 5 min with PBS, and treated for 1 h at room temperature with FITC- or Texas Red-conjugated secondary antibodies in blocking solution. After 3 × 5 min washes with PBS, nuclei were stained by treatment for 30 min at room temperature with TO-PRO-3 (Molecular Probes/Invitrogen, 1 μg/ml in PBS), washed 3 × 5 min with PBS, and the coverslips were mounted on slides using Vectashield (Vector Laboratories, Burlingame, CA). Confocal imaging was performed using a Zeiss LSM 510 microscope.
Supplementary Material
Supplemental Fig. 1. Expression of neurocan, β-versican and hyaluronan by mouse E14T embryonic stem cells (Day 0), and by neural stem cells on the second day of retinoic acid treatment during the neural induction period. SSEA-1 staining of the embryonic stem cells is shown in red, and nuclear staining with TO-PRO-3 is shown in blue. Magnification: 160×.
Supplemental Fig. 2. Co-expression of neurocan (A-C) and hyaluronan (D-F) with nestin in neural stem cells derived from E14T cells, on the fourth day of retinoic acid treatment during the neural induction period. Co-expression of hyaluronan with neurocan and aggrecan at differentiated day 4 is shown in panels G-J and K-M. Nuclear staining with TO-PRO-3 is shown in blue. Magnification: 160×.
Supplemental Fig. 3. Co-expression of hyaluronan, aggrecan, α-versican, and β-versican with neuronal and astrocytic markers (β-III tubulin and GFAP, respectively) at differentiated days 1 (A-C), 4 (G-J) and 7 (D-F, K-M). Nuclear staining with TO-PRO-3 is shown in blue. Panels A-C show the feeder-independent E14T cell line. Magnification: 160×.
Acknowledgments
This research was supported by grants AG022590 and AG024969 from the NIH. The MC-480 antibody to SSEA-1 developed by D. Solter and B.B. Knowles, and the Rat-401 antibody to nestin developed by S. Hockfield, were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by the University of Iowa, Department of Biological Sciences, Iowa City, IA 52242.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Auerbach W, Dunmore JH, Fairchild-Huntress V, Fang Q, Auerbach AB, Huszar D, Joyner AL. Establishment and chimera analysis of 129/SvEv- and C57BL/6-derived mouse embryonic stem cell lines. Biotechniques. 2000;29:1024–8. 1030–1032. doi: 10.2144/00295st04. [DOI] [PubMed] [Google Scholar]
- Bain G, Yao M, Huettner J, Finley M, Gottlieb D. Neuronlike cells derived in culture from P19 embryonal carcinoma and embryonic stem cells. In: Banker G, Goslin K, editors. Culturing Nerve Cells. MIT; Cambridge, MA: 1998. pp. 189–212. [Google Scholar]
- Braam SR, Denning C, van den Brink S, Kats P, Hochstenbach R, Passier R, Mummery CL. Improved genetic manipulation of human embryonic stem cells. Nat Methods. 2008;5:389–92. doi: 10.1038/nmeth.1200. [DOI] [PubMed] [Google Scholar]
- Brennan J, Skarnes WC. Gene trapping in mouse embryonic stem cells. Methods Mol Biol. 1999;97:123–38. doi: 10.1385/1-59259-270-8:123. [DOI] [PubMed] [Google Scholar]
- Cafferty WB, Yang SH, Duffy PJ, Li S, Strittmatter SM. Functional axonal regeneration through astrocytic scar genetically modified to digest chondroitin sulfate proteoglycans. J Neurosci. 2007;27:2176–85. doi: 10.1523/JNEUROSCI.5176-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Q, Benton RL, Whittemore SR. Stem cell repair of central nervous system injury. J Neurosci Res. 2002;68:501–10. doi: 10.1002/jnr.10240. [DOI] [PubMed] [Google Scholar]
- Carulli D, Rhodes KE, Fawcett JW. Upregulation of aggrecan, link protein 1, and hyaluronan synthases during formation of perineuronal nets in the rat cerebellum. J Comp Neurol. 2007;501:83–94. doi: 10.1002/cne.21231. [DOI] [PubMed] [Google Scholar]
- Dimos JT, Rodolfa KT, Niakan KK, Weisenthal LM, Mitsumoto H, Chung W, Croft GF, Saphier G, Leibel R, Goland R, Wichterle H, Henderson CE, Eggan K. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008;321:1218–1221. doi: 10.1126/science.1158799. [DOI] [PubMed] [Google Scholar]
- Doetschman TC, Eistetter H, Katz M, Schmidt W, Kemler R. The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J Embryol Exp Morphol. 1985;87:27–45. [PubMed] [Google Scholar]
- Dutt S, Kléber M, Matasci M, Sommer L, Zimmermann DR. Versican V0 and V1 guide migratory neural crest cells. J Biol Chem. 2006;281:12123–12131. doi: 10.1074/jbc.M510834200. [DOI] [PubMed] [Google Scholar]
- Friedlander D, Milev P, Karthikeyan L, Margolis RK, Margolis RU, Grumet M. The neuronal chondroitin sulfate proteoglycan neurocan binds to the neural cell adhesion molecules Ng-CAM/L1/NILE and N-CAM and inhibits neuronal adhesion and neurite outgrowth. J Cell Biol. 1994;125:669–680. doi: 10.1083/jcb.125.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galtrey CM, Kwok JC, Carulli D, Rhodes KE, Fawcett JW. Distribution and synthesis of extracellular matrix proteoglycans, hyaluronan, link proteins and tenascin-R in the rat spinal cord. Eur J Neurosci. 2008;27:1373–1390. doi: 10.1111/j.1460-9568.2008.06108.x. [DOI] [PubMed] [Google Scholar]
- Gottlieb DI. Large-scale sources of neural stem cells. Annu Rev Neurosci. 2002;25:381–407. doi: 10.1146/annurev.neuro.25.112701.142904. [DOI] [PubMed] [Google Scholar]
- Hirakawa S, Oohashi T, Su WD, Yoshioka H, Murakami T, Arata J, Ninomiya Y. The brain link protein-1 (BRAL1): cDNA cloning, genomic structure, and characterization as a novel link protein expressed in adult brain. Biochem Biophys Res Commun. 2000;276:982–9. doi: 10.1006/bbrc.2000.3583. [DOI] [PubMed] [Google Scholar]
- Ida M, Shuo T, Hirano K, Tokita Y, Nakanishi K, Matsui F, Aono S, Fujita H, Fujiwara Y, Kaji T, Oohira A. Identification and functions of chondroitin sulfate in milieu of neural stem cells. J Biol Chem. 2006;281:5982–5991. doi: 10.1074/jbc.M507130200. [DOI] [PubMed] [Google Scholar]
- Kabos P, Matundan H, Zandian M, Bertolotto C, Robinson ML, Davy BE, Yu JS, Krueger RC., Jr Neural precursors express multiple chondroitin sulfate proteoglycans, including the lectican family. Biochem Biophys Res Commun. 2004;318:955–963. doi: 10.1016/j.bbrc.2004.04.114. [DOI] [PubMed] [Google Scholar]
- Karimi-Abdolrezaee S, Eftekharpour E, Wang J, Morshead CM, Fehlings MG. Delayed transplantation of adult neural precursor cells promotes remyelination and functional neurological recovery after spinal cord injury. J Neurosci. 2006;26:3377–3389. doi: 10.1523/JNEUROSCI.4184-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krencik R, Zhang SC. Stem cell neural differentiation: a model for chemical biology. Curr Opin Chem Biol. 2006;10:592–597. doi: 10.1016/j.cbpa.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Laabs TL, Wang H, Katagiri Y, McCann T, Fawcett JW, Geller HM. Inhibiting glycosaminoglycan chain polymerization decreases the inhibitory activity of astrocyte-derived chondroitin sulfate proteoglycans. J Neurosci. 2007;27:14494–14501. doi: 10.1523/JNEUROSCI.2807-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindvall O, Kokaia Z. Stem cells for the treatment of neurological disorders. Nature. 2006;441:1094–1096. doi: 10.1038/nature04960. [DOI] [PubMed] [Google Scholar]
- Margolis RU. Acid mucopolysaccharides and proteins of bovine whole brain, white matter and myelin. Biochim Biophys Acta. 1967;141:91–102. doi: 10.1016/0304-4165(67)90248-6. [DOI] [PubMed] [Google Scholar]
- Matise MP, Auerbach W, Joyner AL. Production of targeted embryonic stem cell clones. In: Joyner AL, editor. Gene Targeting - A Practical Approach. Oxford University Press; New York: 2000. pp. 101–132. [Google Scholar]
- Meyer-Puttlitz B, Milev P, Junker E, Zimmer I, Margolis RU, Margolis RK. Chondroitin sulfate and chondroitin/keratan sulfate proteoglycans of nervous tissue: Developmental changes of neurocan and phosphacan. Journal of Neurochemistry. 1995;65:2327–2337. doi: 10.1046/j.1471-4159.1995.65052327.x. [DOI] [PubMed] [Google Scholar]
- Milev P, Friedlander DR, Sakurai T, Karthikeyan L, Flad M, Margolis RK, Grumet M, Margolis RU. Interactions of the chondroitin sulfate proteoglycan phosphacan, the extracellular domain of a receptor-type protein tyrosine phosphatase, with neurons, glia, and neural cell adhesion molecules. J Cell Biol. 1994;127:1703–1715. doi: 10.1083/jcb.127.6.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milev P, Meyer-Puttlitz B, Margolis RK, Margolis RU. Complex-type asparagine-linked oligosaccharides on phosphacan and protein-tyrosine phosphatase-ζ/β mediate their binding to neural cell adhesion molecules and tenascin. J Biol Chem. 1995;270:24650–24653. doi: 10.1074/jbc.270.42.24650. [DOI] [PubMed] [Google Scholar]
- Milev P, Maurel P, Häring M, Margolis RK, Margolis RU. TAG-1/axonin-1 is a high-affinity ligand of neurocan, phosphacan/protein tyrosine phosphatase-ζ/β and N-CAM. J Biol Chem. 1996;271:15716–15723. doi: 10.1074/jbc.271.26.15716. [DOI] [PubMed] [Google Scholar]
- Milev P, Maurel P, Chiba A, Mevissen M, Popp S, Yamaguchi Y, Margolis RK, Margolis RU. Differential regulation of expression of hyaluronan-binding proteoglycans in developing brain: aggrecan, versican, neurocan, and brevican. Biochem Biophys Res Commun. 1998a;247:207–12. doi: 10.1006/bbrc.1998.8759. [DOI] [PubMed] [Google Scholar]
- Milev P, Chiba A, Häring M, Rauvala H, Schachner M, Ranscht B, Margolis RK, Margolis RU. High affinity binding and overlapping localization of neurocan and phosphacan/protein-tyrosine phosphatase-ζ/β with tenascin-R, amphoterin, and the heparin-binding growth-associated molecule. J Biol Chem. 1998b;273:6998–7005. doi: 10.1074/jbc.273.12.6998. [DOI] [PubMed] [Google Scholar]
- Muotri AR, Gage FH. Generation of neuronal variability and complexity. Nature. 2006;441:1087–1093. doi: 10.1038/nature04959. [DOI] [PubMed] [Google Scholar]
- Nairn AV, Kinoshita-Toyoda A, Toyoda H, Xie J, Harris K, Dalton S, Kulik M, Pierce JM, Toida T, Moremen KW, Linhardt RJ. Glycomics of proteoglycan biosynthesis in murine embryonic stem cell differentiation. J Proteome Res. 2007;6:4374–4387. doi: 10.1021/pr070446f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neame PJ, Barry FP. The link proteins. Experientia. 1993;49:393–402. doi: 10.1007/BF01923584. [DOI] [PubMed] [Google Scholar]
- Oohashi T, Hirakawa S, Bekku Y, Rauch U, Zimmermann DR, Su WD, Ohtsuka A, Murakami T, Ninomiya Y. Bral1, a brain-specific link protein, colocalizing with the versican V2 isoform at the nodes of Ranvier in developing and adult mouse central nervous systems. Mol Cell Neurosci. 2002;19:43–57. doi: 10.1006/mcne.2001.1061. [DOI] [PubMed] [Google Scholar]
- Panchision DM, McKay RD. The control of neural stem cells by morphogenic signals. Curr Opin Genet Dev. 2002;12:478–87. doi: 10.1016/s0959-437x(02)00329-5. [DOI] [PubMed] [Google Scholar]
- Pizzi MA, Crowe MJ. Matrix metalloproteinases and proteoglycans in axonal regeneration. Exp Neurol Exp Neurol. 2007:496–511. doi: 10.1016/j.expneurol.2006.12.012. [DOI] [PubMed] [Google Scholar]
- Popp S, Andersen JS, Maurel P, Margolis RU. Localization of aggrecan and versican in the developing rat central nervous system. Dev Dyn. 2003;227:143–9. doi: 10.1002/dvdy.10282. [DOI] [PubMed] [Google Scholar]
- Rahmani M, Wong BW, Ang L, Cheung CC, Carthy JM, Walinski H, McManus BM. Versican: signaling to transcriptional control pathways. Can J Physiol Pharmacol. 2006;84:77–92. doi: 10.1139/y05-154. [DOI] [PubMed] [Google Scholar]
- Rauch U, Karthikeyan L, Maurel P, Margolis RU, Margolis RK. Cloning and primary structure of neurocan, a developmentally regulated, aggregating chondroitin sulfate proteoglycan of brain. Journal of Biological Chemistry. 1992;267:19536–19547. [PubMed] [Google Scholar]
- Ripellino JA, Klinger MM, Margolis RU, Margolis RK. The hyaluronic acid binding region as a specific probe for the localization of hyaluronic acid in tissue sections. Application to chick embryo and rat brain. J Histochem Cytochem. 1985;33:1060–1066. doi: 10.1177/33.10.4045184. [DOI] [PubMed] [Google Scholar]
- Ripellino JA, Bailo M, Margolis RU, Margolis RK. Light and electron microscopic studies on the localization of hyaluronic acid in developing rat cerebellum. J Cell Biol. 1988;106:845–855. doi: 10.1083/jcb.106.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin S, Sun Y, Liu Y, Khaner H, Svant S, Cai J, Xu QX, Davidson BP, Stice SL, Smith AK, Goldman SA, Reubinoff BE, Zhan M, Rao MS, Chesnut JD. Whole genome analysis of human neural stem cells derived from embryonic stem cells and stem and progenitor cells isolated from fetal tissue. Stem Cells. 2007;25:1298–1306. doi: 10.1634/stemcells.2006-0660. [DOI] [PubMed] [Google Scholar]
- Sim FJ, Lang JK, Waldau B, Roy N, Schwartz TE, Pilcher WH, Chandross KJ, Natesan S, Merrill JE, Goldman SA. Complementary patterns of gene expression by human oligodendrocyte progenitors and their environment predict determinants of progenitor maintenance and differentiation. Ann Neurol. 2006;59:763–779. doi: 10.1002/ana.20812. [DOI] [PubMed] [Google Scholar]
- Skarnes WC. Gene trapping methods for the identification and functional analysis of cell surface proteins in mice. Meth Enzymol. 2000;3285:592–615. doi: 10.1016/s0076-6879(00)28420-6. [DOI] [PubMed] [Google Scholar]
- Sugahara K, Mikami T. Chondroitin/dermatan sulfate in the central nervous system. Curr Opin Struct Biol. 2007;17:536–545. doi: 10.1016/j.sbi.2007.08.015. [DOI] [PubMed] [Google Scholar]
- Yamagata M, Sanes JR. Versican in the developing brain: lamina-specific expression in interneuronal subsets and role in presynaptic maturation. J Neurosci. 2007;17:536–545. doi: 10.1523/JNEUROSCI.1976-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa M, Yu RK. The expression and functions of glycoconjugates in neural stem cells. Glycobiology. 2007;17:57R–74R. doi: 10.1093/glycob/cwm018. [DOI] [PubMed] [Google Scholar]
- Zhang H, Baader S, Sixt M, Kappler J, Rauch U. Neurocan-GFP fusion protein: a new approach to detect hyaluronan on tissue sections and living cells. J Histochem Cytochem. 2004;52:915–922. doi: 10.1369/jhc.3A6221.2004. [DOI] [PubMed] [Google Scholar]
- Zimmermann DR, Dours-Zimmermann MT. Extracellular matrix of the central nervous system: from neglect to challenge. Histochem Cell Biol. 2008;130:635–653. doi: 10.1007/s00418-008-0485-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. 1. Expression of neurocan, β-versican and hyaluronan by mouse E14T embryonic stem cells (Day 0), and by neural stem cells on the second day of retinoic acid treatment during the neural induction period. SSEA-1 staining of the embryonic stem cells is shown in red, and nuclear staining with TO-PRO-3 is shown in blue. Magnification: 160×.
Supplemental Fig. 2. Co-expression of neurocan (A-C) and hyaluronan (D-F) with nestin in neural stem cells derived from E14T cells, on the fourth day of retinoic acid treatment during the neural induction period. Co-expression of hyaluronan with neurocan and aggrecan at differentiated day 4 is shown in panels G-J and K-M. Nuclear staining with TO-PRO-3 is shown in blue. Magnification: 160×.
Supplemental Fig. 3. Co-expression of hyaluronan, aggrecan, α-versican, and β-versican with neuronal and astrocytic markers (β-III tubulin and GFAP, respectively) at differentiated days 1 (A-C), 4 (G-J) and 7 (D-F, K-M). Nuclear staining with TO-PRO-3 is shown in blue. Panels A-C show the feeder-independent E14T cell line. Magnification: 160×.