Abstract
Objective
To establish the proportion of adolescents among children infected with human immunodeficiency virus (HIV) in Zimbabwe who receive HIV care and support, and what clinic staff perceives to be the main problems faced by HIV-infected children and adolescents.
Methods
In July 2008, we sent a questionnaire to all 131 facilities providing HIV care in Zimbabwe. In it we requested an age breakdown of the children (aged 0–19 years) registered for care and asked what were the two major problems faced by younger children (0–5 years) and adolescents (10–19 years).
Findings
Nationally, 115 (88%) facilities responded. In 98 (75%) that provided complete data, 196 032 patients were registered and 24 958 (13%) of them were children. Of children under HIV care, 33% were aged 0–4 years; 25%, 5–9 years; 25%, 10–14 years; and 17%, 15–19 years. Staff highlighted differences in the problems most commonly faced by younger children and adolescents. For younger children, such problems were malnutrition and lack of appropriate drugs (cited by 46% and 40% of clinics, respectively); for adolescents they concerned psychosocial issues and poor drug adherence (cited by 56% and 36%, respectively).
Conclusion
Interventions for the large cohort of adolescents who are receiving HIV care in Zimbabwe need to target the psychosocial concerns and poor drug adherence reported by staff as being the main concerns in this age group.
ملخص
الغرض
حساب نسبة المراهقين بين الأطفال المصابين بعدوى فيروس العوز المناعي البشري في زيمبابوي ويتلقون الرعاية والدعم الخاصين بالفيروس، وما الذي يعتبره العاملون الإكلينيكيون مشاكل رئيسية تواجه الأطفال والبالغين المصابين بالعدوى.
الطريقة
في تموز/يوليو 2008، أرسل الباحثون استبياناً إلى 131 مرفقاً من المرافق التي تقدم الرعاية للمصابين بفيروس العوز المناعي البشري في زيمبابوي. وطلب الباحثون في الاستبيان تقسيم عمر الأطفال (0-19 سنة) المسجلين في المرفق ويحصلون على خدمات الرعاية، وسأل الباحثون عن أكبر مشكلتين يواجهها صغار الأطفال (في عمر 0-5 سنوات) وأكبر مشكلتين يواجهها المراهقون (في عمر 10-19 سنة).
الموجودات
على الصعيد الوطني، استجاب للاستبيان 115 مرفقاً (بنسبة 88%). قدم 98 مرفقاً (75%) بيانات كاملة، ووجد فيها أن 196032 مريضاً كانوا مسجلين، وأن 24958 (13%) منهم كانوا من الأطفال ومن الأطفال الذين يتلقون الرعاية الخاصة بفيروس العوز المناعي البشري كان 33% منهم في الفئة العمرية 0-4 سنوات؛ و 25% في الفئة العمرية 5-9 سنوات؛ و 25% في الفئة العمرية 10-14 سنة؛ و 17% في الفئة العمرية 15-19سنة. وقد أوضح العاملون في المرافق الاختلافات بين المشاكل الأكثر شيوعاً التي يواجهها صغار الأطفال والمراهقين. فصغار الأطفال يواجهون مشاكل مثل سوء التغذية ونقص الأدوية الملائمة (كما أوردت 46% و 40% من العيادات، على التوالي)؛ أما المراهقون فما يثير القلق هو المشاكل النفسية والاجتماعية وسوء امتثالهم بتلقي الأدوية (كما أوردت 56% و 36% من المرافق، على التوالي).
الاستنتاج
ينبغي أن تستهدف المداخلات الأترابية الواسعة النطاق للمراهقين الذين يتلقون رعاية خاصة بفيروس العوز المناعي البشري الاهتمامات النفسية والاجتماعية وسوء الامتثال للأدوية وفقاً لما أبلغ عنه العاملون في المرافق المقدمة للخدمات حسب اهتمامات هذه الفئة العمرية.
Résumé
Objectif
Déterminer la proportion d'adolescents parmi les enfants infectés par le virus de l'immunodéficience humaine (VIH) au Zimbabwe recevant des soins et un soutien en rapport avec le VIH et ce que le personnel des dispensaires perçoit comme les principaux problèmes rencontrés par les enfants et les adolescents infectés par ce virus.
Méthodes
En juillet 2008, nous avons envoyé un questionnaire à l'ensemble des 131 établissements délivrant des soins liés au VIH au Zimbabwe. Il était demandé dans ce questionnaire de classer par âge les enfants (0–19 ans) enregistrés pour recevoir des soins et d'indiquer les deux principaux problèmes se posant pour les plus jeunes (0–5 ans) et pour les adolescents (10–19 ans).
Résultats
A l'échelle du pays, 115 (88 %) établissements ont répondu. Dans 98 établissements (75 %) ayant fourni des données complètes, 196 032 patients ont été enregistrés, dont 24 958 (13 %) enfants. Parmi les enfants soignés contre le VIH/sida, 33 % étaient âgés de 0 à 4 ans, 25 % de 5 à 9 ans, 25 % de 10 à 14 ans et 17 % de 15 à 19 ans. Le personnel a mis en lumière des différences entre les problèmes qui se posaient le plus fréquemment pour les jeunes enfants et pour les adolescents. Pour les plus jeunes, ces problèmes concernaient la malnutrition et le manque de médicaments adaptés (cités par 46 et 40 % des dispensaires respectivement) ; pour les adolescents, il s'agissait plutôt de problèmes psychosociaux et d'une mauvaise observance du traitement (cités par 56 et 36 % des dispensaires respectivement).
Conclusion
Les interventions destinées à l'importante cohorte d'adolescents qui reçoit des soins liés au VIH au Zimbabwe doivent viser les difficultés psychosociales et les défauts d'observance du traitement rapportés par le personnel en tant que problèmes les plus préoccupants dans cette tranche d'âges.
Resumen
Objetivo
Determinar la proporción de adolescentes que reciben atención y apoyo contra el VIH entre los niños infectados por el virus de la inmunodeficiencia humana (VIH) en Zimbabwe, así como los principales problemas que, en opinión del personal de los dispensarios, afrontan los niños y adolescentes infectados por el virus.
Métodos
En julio de 2008 enviamos un cuestionario a los 131 centros que atienden a pacientes con VIH en Zimbabwe. En él solicitamos un desglose por edades de los niños (de 0 a 19 años) registrados para recibir atención y preguntamos cuáles eran los dos problemas que más afectaban a los niños más pequeños (0–5 años) y a los adolescentes (10–19 años).
Resultados
A nivel nacional, respondieron en total 115 centros (88%). En 98 (75%) que proporcionaron datos completos, había registrados 196 032 pacientes, 24 958 (13%) de los cuales eran niños. De los niños atendidos por ser seropositivos, el 33% tenían entre 0 y 4 años; el 25%, 5–9 años, otro 25%, 10–14 años; y el 17%, 15–19 años. El personal resaltó las diferencias entre los problemas más comunes sufridos por los niños más pequeños y los que afectaban a los adolescentes. Ente los primeros cabe destacar la malnutrición y la falta de medicamentos apropiados (citados por el 46% y el 40% de los dispensarios, respectivamente); y en el caso de los adolescentes, los problemas psicosociales y el escaso seguimiento de la medicación (citados por el 56% y el 36%, respectivamente).
Conclusión
En las intervenciones aplicadas en la extensa cohorte de adolescentes que están recibiendo atención para el VIH en Zimbabwe se debería centrar la atención en los aspectos psicosociales y el cumplimiento del tratamiento, cuestiones identificadas por el personal como los principales motivos de preocupación en ese grupo de edad.
Introduction
Infection with human immunodeficiency virus (HIV) is the leading cause of death in southern Africa, which has the highest prevalence of HIV infection in the world.1 Without interventions, the risk of mother-to-child transmission (MTCT) of HIV is common;2 thus, the adult HIV epidemic in the region has been followed by an epidemic of vertically-acquired HIV infection among children.1 The health-care needs of children are poorly served in most low-income countries,3–5 and adults have been the main targets of HIV-care programmes. However, there is growing recognition of the need for equitable access to antiretroviral therapy (ART) for HIV-infected children as well.
As HIV epidemics in Africa mature, the age profile of children in need of HIV care is changing. The infection appears to progress slowly in one-quarter to one-third of HIV-infected infants. These “slow progressors” may have a median life-expectancy as high as 14–16 years, even without ART.6,7 In countries with severe epidemics of early-onset HIV infection, more and more cases are presenting with clinical symptoms in late childhood or adolescence.8,9 Furthermore, as access to ART and provision of HIV care improve, a greater proportion of HIV-infected infants is likely to survive to adolescence.10
Despite the growing numbers of older children and adolescents who develop symptoms, there has been little focus on providing this group with specialized HIV care.11 In industrialized countries, adolescent medicine is a distinct clinical speciality. However, in resource-poor settings, dedicated health-care services for adolescents are few, and children generally move from paediatric to adult care services at 8–12 years of age.
Zimbabwe has experienced a severe and early-onset HIV epidemic, with the prevalence of HIV infection in adults peaking at 30% in 1997 and subsequently declining to below 15% by 2007. In 2007, an estimated 120 000 children were living with HIV1 and 3.4% of children aged 10 years were HIV-infected long-term survivors following MTCT.12 Within a few years, HIV-related deaths among adolescent long-term survivors are likely to outnumber those among infants.12 However, as is typical for the region, HIV-care programmes routinely report data for only three age categories: 0–4, 5–14 and 15–49 years. Thus, there is no clear age profile of the older children receiving HIV care.
We investigated the number of children receiving HIV care in Zimbabwe to establish the proportion of adolescents (10–19 years of age). We also ascertained clinic staff perceptions of the main problems faced by HIV-infected younger children and adolescents.
Methods
Setting
Zimbabwe is divided into eight provinces and has two major cities – Harare and Bulawayo. ART first became available in the public health sector in 2004 and is now accessible through both primary care and hospital-based HIV services. The therapy is initiated in hospital HIV services, with continuing care decentralized, where possible, to the local primary care level. All clinics providing HIV care are registered with Zimbabwe’s Ministry of Health and Child Welfare and issue a quarterly report of the number of patients registered on ART.
Facility engagement
The Ministry of Health and Child Welfare provided a list of all health-care facilities offering HIV treatment. Permission to conduct a survey was obtained from the Ministry of Health and Child Welfare and from the medical director of each province. No ethical approval was required because the data gathered did not report on individual patients and were considered to be an audit of HIV clinical services.
Each patient who registers at an HIV care service is serially recorded in numbered paper registers. The sex, age and date of registration are recorded at the time of enrolment, and a record of every clinic attendance or death is maintained in the register. Infants exposed to HIV but not diagnosed with HIV infection are not recorded on clinic registers.
In July 2008, we sent each facility a questionnaire requesting a breakdown of the number of registered children into four age groups (0–4 years, 5–9 years, 10–14 years and 15–19 years) and the main modes of HIV testing of patients registered at the clinics. We requested the age at enrolment and the number of patients under active care rather than the number who had ever registered. We did not ask for the number of patients receiving ART.
To determine facility staff’s view of the main problems encountered by children and by adolescents, we asked an open-ended question: “Name two challenges you encounter specifically in relation to looking after young children (aged 0–5 years) and/or adolescents (aged 10–19 years) infected with HIV in your clinic.” We also asked respondents to describe these problems in the context of the local environment.
We sent questionnaires by fax, e-mail, post or personal delivery to all health-care facilities identified with a return deadline of November 2008. Facilities had a financial incentive to complete the questionnaires accurately, and we contacted all those that did not return the questionnaire by the due date to clarify the reason for the lack of response. As a check on the accuracy of the data reported, we visited 24 facilities. Their staff was advised of the visit in advance.
Data analysis
We entered the data into EPI-Info version 3.4 (Centers for Disease Control and Prevention, Atlanta, United States of America) and analysed it using STATA version 10.0 (StataCorp., College Station, USA). We coded and analysed qualitative data using principles of grounded theory.13 Responses to the open-ended question were coded to analyse emerging themes, with statements that cited multiple challenges coded into each applicable category. This meant that any one response could be coded into as many as four separate categories. If a respondent’s answer fell into a single category, it was counted only once.
Results
Characteristics of facilities and respondents
A total of 131 HIV-care facilities were identified. Of these, 115 (88%) responded to the questionnaire (Fig. 1). They comprised 65 (56%) clinics attached to hospitals, 48 (42%) clinics located within primary care facilities and 2 (2%) freestanding HIV-care clinics. In terms of funding, 78 (68%) were government clinics (11 run jointly with city municipal health services), 18 (16%) were municipal city health clinics, 7 (6%) were funded by nongovernmental organizations, 6 (5%) were privately funded, and 6 (5%) were funded through faith-based organizations. The main respondents were nurses-in-charge or clinic matrons (68%), followed by district medical officers or medical superintendents (17%), HIV programme managers or coordinators (13%) and counsellors (2%).
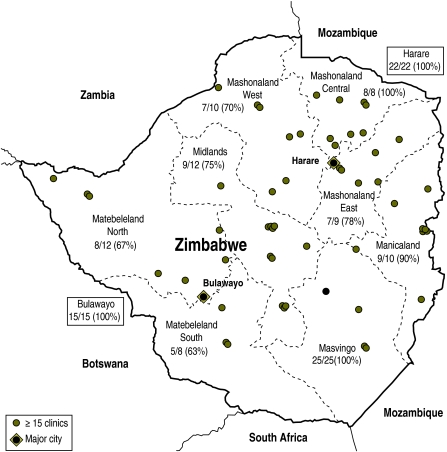
Fig. 1.
Distribution of HIV clinics and response rates by province or city in Zimbabwe, 2008
The locations of the clinics on the map are spatially accurate.
Clinic data from the Zimbabwe Ministry of Health and Child Welfare.
Clinics providing a partial response or none
The lowest response rates were from the western part of the country (Matabeleland North and South provinces). Reasons for non-response were non-receipt of questionnaires by the designated clinic respondent (6 clinics), or inability to return completed forms because of logistical problems with communication and postal services (10 clinics). Of the 115 clinics that provided responses, 17 were unable to provide an age breakdown for children registered in their care. These were fairly equally distributed through all provinces and comprising 12% (26 166) of all patients in HIV care. Of these 17 clinics, 9 provided no numbers of registered patients aged 15–19 years, and 8 provided no numbers of registered patients aged 5–14 years.
Children in HIV-care services
Of the 115 HIV clinics that responded, 98 (75%) provided the requested data as numbers of registered children in the four specified age-groups. These 98 clinics recorded a total of 196 032 registered patients, not all of whom were on ART. Of these patients, 24 958 (13%) were children aged 0–19 years, and this group could be further broken down to 8370 (33%) 0–4 years, 6130 (25%) 5–9 years, 6334 (25%) 10–14 years and 4124 (17%) 15–19 years.
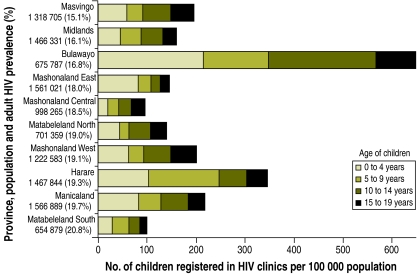
Adult HIV prevalence differed little between provinces; however, the age profile of patients in care did vary more (Fig. 2). Bulawayo had the highest (21%) and Mashonaland Central the lowest (4%) proportion of children aged 0–19 years among all patients (P < 0.001). Bulawayo also had the highest per capita load of adolescent patients (300 per 100 000 population), with 2.8– 8.2 times more adolescents per capita than the other provinces.
Fig. 2.
Numbers of HIV-infected children, per 100 000 population, registered in HIV clinics by age group and province, Zimbabwe, 2008
Population data from the 2002 National Census and prevalence of HIV infection in adults from the Zimbabwe Demographic and Health Survey 2005–2006.17
Modes of HIV testing
Most (55%) respondents reported that children mainly accessed HIV testing in hospital after presenting with an illness. The remainder cited HIV clinics (28%), freestanding services (10%) or private doctors (1%) as routes through which children had accessed HIV testing; only 6% of respondents mentioned primary care services. HIV clinics based in primary care facilities were more likely to have patients who had been tested through freestanding testing services or at primary care clinics than HIV clinics based in hospitals (29% versus 6%, P < 0.008). At the clinic level, diagnosis was often delayed by lack of suitable diagnostic tests and the need for guardian consent before testing.
Problems faced by children and adolescents
Respondents identified major differences in the problems faced by young children and by adolescents (Table 1). Among young children, the most common problems were malnutrition (cited by 46% of clinics); unavailability of drugs, including paediatric formulations (40%); and inconsistent caregiving (37%), which led to erratic clinic attendance. For adolescents, the most common issues were psychosocial problems (56%), including lack of resources to seek help for these issues; erratic drug taking (36%); and lack of disclosure of HIV status (21%). Respondents described the main psychosocial stressors for adolescents as stigma, difficulty in identifying with HIV-negative peers, anxiety about sexual relationships and future planning, and low self-esteem and feelings of hopelessness. These stressors were compounded by having to care for ill relatives and siblings and by being the head of the family.
Table 1. Main problems, as reported by clinic staff, faced by HIV-infected young children and adolescents, Zimbabwe, 2008.
| Problems | No. of clinics (%) |
|
|---|---|---|
|
n = 108 |
n = 113 |
|
| Young children (0–5 years) |
Adolescents (10–19 years) |
|
| Psychosocial issues | 6 (6) | 63 (56) |
| Stigma | 1 | 15 |
| Denial (guardian/patient) | 3 | 4 |
| Schooling | 0 | 12 |
| Emotional and psychological | 1 | 16 |
| Guardian not coping | 1 | 0 |
| Puberty/future planning/peer pressure | 0 | 11 |
| Responsibility for siblings | 0 | 5 |
| Erratic drug taking | 37 (34) | 41 (36) |
| Poor attendance to clinic appointments | 19 | 9 |
| Poor adherence to collected medicines | 18 | 32 |
| Lack of resources (clinic level) | 16 (15) | 37 (33) |
| Paediatric clinical services | 4 | 6 |
| Trained staff | 1 | 1 |
| Space/material resources | 3 | 5 |
| Counselling | 8 | 13 |
| Psychosocial support services | 0 | 12 |
| Lack of disclosure of HIV status | 4 (4) | 24 (21) |
| Malnutrition | 50 (46) | 23 (20) |
| Orphanhood | 23 (21) | 17 (15) |
| Caregiver issues | 40 (37) | 17 (15) |
| Multiple/changing caregivers | 11 | 4 |
| Elderly caregiver | 6 | 1 |
| Ill parent/caregiver | 4 | 0 |
| Unsupportive/negligent caregiver | 19 | 12 |
| Economic difficulties | 22 (20) | 17 (15) |
| Transport fares | 11 | 8 |
| Money for food/medicines/school fees | 11 | 9 |
| Unavailability of drugs | 43 (40) | 13 (12) |
| Paediatric formulations | 19 | 1 |
| Drug shortages | 24 | 12 |
| Sexual health issues | 1(1) | 9 (8) |
| Unsafe sex/rape | 1 | 7 |
| Lack of sexual health services | 0 | 2 |
| Diagnosis of HIV infection | 16 (15) | 9 (8) |
| Lack of guardian consent | 6 | 7 |
| Lack of diagnostic tests | 9 | 0 |
| Lack of counselling and testing | 1 | 2 |
| Presentation with advanced disease | 5 (5) | 3 (3) |
Some problems were common to both age groups: inability to afford transport fares and food and resulting inability to attend clinic appointments; malnutrition; and cessation of treatment to avoid the increased hunger that results from taking ART. Erratic drug taking was another problem for both age groups, but in younger children this was as a direct result of caregivers not bringing the children to the clinic or not consistently giving them medicines, whereas in adolescents it was mainly related to poor adherence to drug regimens. Respondents cited several issues that contributed to poor adherence in adolescents, including delayed disclosure of HIV status, a desire to conform, and attendance at boarding school, where it was not possible to supervise the taking of medicines from guardians.
Discussion
In Zimbabwe in 2008, over 10 000 adolescents (10–19 years of age) were registered in HIV-care services. The adolescents comprised 42% of patients < 20 years of age, and outnumbered those < 5 years of age. To our knowledge, this is the first national report of the burden of adolescents in HIV-care services and of the problems faced by this age group in a high HIV prevalence setting. We achieved a high response rate and captured data from both hospitals and primary care clinics; hence, our results are likely to be reasonably representative of the situation in Zimbabwe.
There has been considerable emphasis on the prevention of HIV infection in adolescents, but much less on the care of those already infected. Africa has an emerging epidemic of adolescent survivors of HIV infection acquired by MTCT in the 1990s.12 Increasing numbers of long-term survivors are presenting for care for the first time during adolescence.8,14 For example, in Harare, underlying HIV/AIDS [acquired immunodeficiency syndrome] is now the most common reason for hospitalization in adolescence.14 There is a 46% prevalence of HIV-related emergency admissions in the age group 15–19 years, and investigations support vertical transmission as the most likely mode of infection in most cases.
In contrast to the well described infant AIDS epidemic, adolescent AIDS has only recently become clinically apparent because of the delay between being infected in infancy and becoming symptomatic in adolescence. Thus, there is relatively little experience in HIV care for this age group. As HIV epidemics mature and prevention of MTCT becomes more widely available and successful, the numbers of HIV-infected infants entering HIV care will decline, while the numbers of already infected children surviving to older ages will continue to increase. Falling prevalence in adults will compound this trend. Thus, our survey findings may presage a regional trend towards increasing median age of children in HIV-care programmes.12 This trend will also be accelerated by the increasing availability of ART for infants and young children, which increases the probability of survival to adolescence, even for fast progressors.15 At present, however, any such regional trends are masked by the standard reporting of HIV data in just three age categories: 0–4, 5–14 and 15–49 years. Accurate monitoring will require a breakdown of the categories to identify adolescents as a separate group.
We found substantial variation among provinces in the age profiles and per capita rates of adolescents receiving HIV care. This was a surprise because the adult HIV epidemic in Zimbabwe has been relatively homogenous, with little variability among geographical areas or between rural and urban zones.16,17 The variability among provinces reported here may be associated with differences in access to HIV-related diagnosis and care for adolescents. This would be consistent with our previous finding that delayed diagnosis, usually following multiple clinical symptoms of HIV infection over a prolonged period, is a major problem in this age group.8,14 Bulawayo, the second largest city in Zimbabwe, has a much greater per capita adolescent case load of HIV infection than any other administrative area. Bulawayo showed early leadership in establishing HIV diagnosis and care services in Zimbabwe and has run a dedicated adolescent HIV testing and care service since 2004. Matabeleland South and Mashonaland East have similar adult prevalence rates but report per capita rates that are less than one-eighth of those found in Bulawayo.18 If Bulawayo provides a better guide to the per capita rates of adolescents receiving HIV care than other administrative areas, then extrapolation of the city’s figures nationally would imply that 34 000 adolescents need HIV care in Zimbabwe. We separately estimated the burden of vertically-acquired HIV infection in adolescents in Zimbabwe to be 43 357. This figure was based on a statistical model that took into account the timing and magnitude of the adult HIV epidemic and on assumptions about MTCT and the survival of infected infants.12,19 Thus, the true number of adolescents with HIV infection may be much higher than the 10 000 currently known to HIV services.
For various reasons, barriers to HIV diagnosis and care are likely to be unusually large for adolescents. HIV testing of older children has not been emphasized because, until recently, long-term survival in the absence of treatment following MTCT was thought to be uncommon.20 This means that HIV testing for young people in Zimbabwe can only be accessed in health-care facilities, with no freestanding HIV-testing services for children less than 16 years of age. Legal barriers to testing young people further hinder this process, because testing people less than 16 years of age requires consent from a legal guardian. High rates of orphanhood result in frequent movement of children between different extended family members, with no clearly defined guardianship.21–23 We found that most children were diagnosed following presentation with acute illness; only a minority accessed testing through primary care or freestanding HIV-testing services.
The primary focus of paediatric HIV-care programmes has been on infants and young children, for whom management challenges are very different from those of HIV-infected adolescents. The major problems reported in this survey for younger children reflect the dependence of younger children on caregivers. The main issues reported for adolescents, however, reflect the physiological and psychological changes that occur during the transition to adulthood.24–27 These issues are likely to affect successful management and are in line with the experience in industrialized countries, where psychosocial concerns (e.g. mental health problems, low self-esteem, lack of social support and poor drug adherence) pose substantial challenges to the successful clinical management of adolescent chronic illness.25,28–31
The lack of services focusing on the specific health needs of HIV-infected adolescents has implications for the individual and for public health. Poor adherents risk developing drug resistance and subsequent treatment failure. This has adverse health consequences for the individual (particularly in settings with limited access to second-line ART); it also carries a risk of HIV transmission. Failure to address emerging sexuality may further increase the risk of unsafe sexual behaviour. Delayed disclosure of HIV diagnosis to children, commonly reported by respondents in this survey, is likely to have an additional impact on both drug adherence and secondary HIV prevention.32,33
This study had some limitations. It did not capture the proportion of patients who were on ART at the time of the survey. Also, some respondents may not have reported data accurately and consistently, and some patients registered in the clinics may not have been under active follow-up. However, we tried to offset these limitations by visiting 24 clinics to verify that reporting instructions were being followed. Another limitation is that the study did not capture infants who were exposed to HIV but had not yet undergone testing; this may partly account for the low proportion of children in the age group 0–4 years. In addition, factors other than access to HIV testing may contribute to the variability among provinces and thus may affect the burden of adolescents currently requiring care. Such factors include fecundity and the prevalence of maternal HIV infection and in the 1990s, the probability of survival to adolescence in those not receiving ART, and the effects on orphanhood and ill health of migration in and out of the country.
The large numbers of adolescents under HIV care in Zimbabwe are consistent with a changing age profile of childhood AIDS in the context of a maturing HIV epidemic. Other countries with similar epidemics are likely to experience the same phenomenon as HIV epidemics mature and PMTCT becomes more widely available. Children’s health needs change markedly with age; thus, standard reporting of HIV data with more age bands would help to inform service planning for older HIV-infected children and adolescents. Given that the burden of older children under HIV care is likely to increase, there will be a growing need for targeted interventions to address adherence and secondary prevention of HIV infection. Researchers and service providers should make a priority of developing and implementing effective strategies for such interventions.
Acknowledgements
We thank all clinic respondents for completing the survey questionnaire and the Ministry of Health and Child Welfare of Zimbabwe for providing information on HIV care services and for permission to conduct the survey.
Funding:
RF and ELC are funded by the Wellcome Trust. The Wellcome Trust had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests:
None declared.
References
- 1.2008 report on the global HIV/AIDS epidemic Geneva: United Nations Programme for HIV/AIDS; 2008.
- 2.De Cock KM, Fowler MG, Mercier E, de Vincenzi I, Saba J, Hoff E, et al. Prevention of mother-to-child HIV transmission in resource-poor countries: translating research into policy and practice. JAMA. 2000;283:1175–82. doi: 10.1001/jama.283.9.1175. [DOI] [PubMed] [Google Scholar]
- 3.Costello A, White H. Reducing global inequalities in child health. Arch Dis Child. 2001;84:98–102. doi: 10.1136/adc.84.2.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duke T, Tamburlini G, Silimperi D, Paediatric Quality Care Group Improving the quality of paediatric care in peripheral hospitals in developing countries. Arch Dis Child. 2003;88:563–5. doi: 10.1136/adc.88.7.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wagstaff A, Bustreo F, Bryce J, Claeson M, WHO-World Bank Child Health and Poverty Working Group Child health: reaching the poor. Am J Public Health. 2004;94:726–36. doi: 10.2105/AJPH.94.5.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marston M, Zaba B, Salomon JA, Brahmbhatt H, Bagenda D, Lutalo T, et al. Estimating the net effect of HIV on child mortality in African populations affected by generalized HIV epidemics. J Acquir Immune Defic Syndr. 2005;38:219–27. doi: 10.1097/00126334-200502010-00015. [DOI] [PubMed] [Google Scholar]
- 7.Stover J, Walker N, Grassly NC, Marston M. Projecting the demographic impact of AIDS and the number of people in need of treatment: updates to the Spectrum projection package. Sex Transm Infect. 2006;82(Suppl 3):iii45–50. doi: 10.1136/sti.2006.020172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrand RA, Luethy R, Bwakura F, Mujuru H, Miller RF, Corbett EL. HIV infection presenting in older children and adolescents: a case series from Harare, Zimbabwe. Clin Infect Dis. 2007;44:874–8. doi: 10.1086/511873. [DOI] [PubMed] [Google Scholar]
- 9.Shisana O, Mehtar S, Mosala T, Zungu-dirwayi N, Rehle T, Dana P, et al. HIV risk exposure among young children. A study of 2 to 9 year olds served by the public health facilities in the Free State, South Africa Cape Town: Human Social Research Council; 2005. [Google Scholar]
- 10.Judd A, Doerholt K, Tookey PA, Sharland M, Riordan A, Menson E, et al. Collaborative HIV Paediatric Study (CHIPS) National Study of HIV in Pregnancy and Childhood (NSHPC) Morbidity, mortality, and response to treatment by children in the United Kingdom and Ireland with perinatally acquired HIV infection during 1996–2006: planning for teenage and adult care. Clin Infect Dis. 2007;45:918–24. doi: 10.1086/521167. [DOI] [PubMed] [Google Scholar]
- 11.Antiretroviral therapy of HIV infection in infants and children in resource-limited settings, towards universal access Geneva: World Health Organization; 2006. [Google Scholar]
- 12.Ferrand RA, Corbett EL, Wood R, Hargrove J, Ndhlovu CE, Cowan FM, et al. AIDS among older children and adolescents in Southern Africa: projecting the time course and magnitude of the epidemic. AIDS. 2009;23:2039–46. doi: 10.1097/QAD.0b013e32833016ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glaser B, Strauss A. The discovery of grounded theory: strategies for qualitative research New York: Aldine; 1967. [Google Scholar]
- 14.Ferrand RA, Bandason T, Musvaire P, Larke N, Nathoo K, Mujuru H, et al. Causes of acute hospitalization during adolescence: the burden and spectrum of HIV-related morbidity in a country with an early-onset and severe HIV epidemic. PLoS Med. 2009 doi: 10.1371/journal.pmed.1000178. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Violari A, Cotton MF, Gibb DM, Babiker AG, Steyn J, Madhi SA, et al. CHER Study Team Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359:2233–44. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gregson S. Evidence for HIV decline in Zimbabwe: a comprehensive review of epidemiological data Geneva: United Nations Programme for HIV/AIDS; 2005. [Google Scholar]
- 17.Zimbabwe Demographic and Health Survey 2005–2006. Harare: Central Statistical Office; 2006.
- 18.Zimbabwe national HIV and AIDS estimates 2007. Harare: Ministry of Health and Child Welfare; 2007.
- 19.Zimbabwe National Health Profile. Harare: Ministry of Health and Child Welfare, National Health Information Unit; 2006.
- 20.Newell ML, Coovadia H, Cortina-Borja M, Rollins N, Gaillard P, Dabis F, Ghent International AIDS Society (IAS) Working Group on HIV Infection in Women and Children Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet. 2004;364:1236–43. doi: 10.1016/S0140-6736(04)17140-7. [DOI] [PubMed] [Google Scholar]
- 21.Nyamukapa CA, Gregson S, Lopman B, Saito S, Watts HJ, Monasch R, et al. HIV-associated orphanhood and children’s psychosocial distress: theoretical framework tested with data from Zimbabwe. Am J Public Health. 2008;98:133–41. doi: 10.2105/AJPH.2007.116038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foster G, Shakespeare R, Chinemana F, Jackson H, Gregson S, Marange C, et al. Orphan prevalence and extended family care in a peri-urban community in Zimbabwe. AIDS Care. 1995;7:3–17. doi: 10.1080/09540129550126911. [DOI] [PubMed] [Google Scholar]
- 23.Howard BH, Phillips CV, Matinhure N, Goodman KJ, McCurdy SA, Johnson CA. Barriers and incentives to orphan care in a time of AIDS and economic crisis: a cross-sectional survey of caregivers in rural Zimbabwe. BMC Public Health. 2006;6:27. doi: 10.1186/1471-2458-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arpadi SM. Growth failure in children with HIV infection. J Acquir Immune Defic Syndr. 2000;25(Suppl 1):S37–42. doi: 10.1097/00042560-200010001-00006. [DOI] [PubMed] [Google Scholar]
- 25.Brown LK, Lourie KJ, Pao M. Children and adolescents living with HIV and AIDS: a review. J Child Psychol Psychiatry. 2000;41:81–96. doi: 10.1017/S0021963099004977. [DOI] [PubMed] [Google Scholar]
- 26.Domek GJ. Social consequences of antiretroviral therapy: preparing for the unexpected futures of HIV-positive children. Lancet. 2006;367:1367–9. doi: 10.1016/S0140-6736(06)68584-X. [DOI] [PubMed] [Google Scholar]
- 27.Buchacz K, Rogol AD, Lindsey JC, Wilson CM, Hughes MD, Seage GR, 3rd, et al. Pediatric AIDS Clinical Trials Group 219 Study Team Delayed onset of pubertal development in children and adolescents with perinatally acquired HIV infection. J Acquir Immune Defic Syndr. 2003;33:56–65. doi: 10.1097/00126334-200305010-00009. [DOI] [PubMed] [Google Scholar]
- 28.Charles M, Noel F, Leger P, Severe P, Riviere C, Beauharnais CA, et al. Survival, plasma HIV-1 RNA concentrations and drug resistance in HIV-1-infected Haitian adolescents and young adults on antiretrovirals. Bull World Health Organ. 2008;86:970–7. doi: 10.2471/BLT.07.050120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferrand RA, Miller RF, Jungmann EA. Management of HIV infection in adolescents attending inner London HIV services. Int J STD AIDS. 2007;18:633–4. doi: 10.1258/095646207781568565. [DOI] [PubMed] [Google Scholar]
- 30.Cameron F. Teenagers with diabetes–management challenges. Aust Fam Physician. 2006;35:386–90. [PubMed] [Google Scholar]
- 31.Chang CW, Yeh CH, Lo FS, Shih YL. Adherence behaviours in Taiwanese children and adolescents with type 1 diabetes mellitus. J Clin Nurs. 2007;16(7B):207–14. doi: 10.1111/j.1365-2702.2005.01541.x. [DOI] [PubMed] [Google Scholar]
- 32.Sarna AAJ, Mahendra V, Rau A, Singh A, Alexander G, Rutenberg N. What factors affect adherence to antiretroviral therapy (ART) in HIV-infected children in India: an exploratory study. Presented at the XVII International AIDS Conference World AIDS Conference, Mexico City, Mexico, 3–8August2008 [Google Scholar]
- 33.Fortenberry JD. Beyond validity and reliability: meaning-in-context of adolescents’ self-reports of sexual behavior. J Adolesc Health. 2009;44:199–200. doi: 10.1016/j.jadohealth.2008.12.018. [DOI] [PubMed] [Google Scholar]